Atomic levers control pyranose ring conformations
|
|
|
- Emory Atkins
- 6 years ago
- Views:
Transcription
1 Proc. Natl. Acad. Sci. USA Vol. 96, pp , July 1999 Biophysics Atomic levers control pyranose ring conformations PIOTR E. MARSZALEK, YUAN-PING PANG, HONGBIN LI, JAMAL EL YAZAL, ANDRES F. OBERHAUSER, AND JULIO M. FERNANDEZ Department of Physiology and Biophysics, Mayo Clinic Cancer Center, Department of Pharmacology, Mayo Foundation, Rochester, MN Edited by Jiri Jonas, University of Illinois at Urbana Champaign, Urbana, IL, and approved May 18, 1999 (received for review March 12, 1999) ABSTRACT Atomic force microscope manipulations of single polysaccharide molecules have recently expanded conformational chemistry to include force-driven transitions between the chair and boat conformers of the pyranose ring structure. We now expand these observations to include chair inversion, a common phenomenon in the conformational chemistry of six-membered ring molecules. We demonstrate that by stretching single pectin molecules (1 3 4-linked -D-galactouronic acid polymer), we could change the pyranose ring conformation from a chair to a boat and then to an inverted chair in a clearly resolved two-step conversion: 4 C 1 i boat i 1 C 4. The two-step extension of the distance between the glycosidic oxygen atoms O 1 and O 4 determined by atomic force microscope manipulations is corroborated by ab initio calculations of the increase in length of the residue vector O 1 O 4 on chair inversion. We postulate that this conformational change results from the torque generated by the glycosidic bonds when a force is applied to the pectin molecule. Hence, the glycosidic bonds act as mechanical levers, driving the conformational transitions of the pyranose ring. When the glycosidic bonds are equatorial (e), the torque is zero, causing no conformational change. However, when the glycosidic bond is axial (a), torque is generated, causing a rotation around COC bonds and a conformational change. This hypothesis readily predicts the number of transitions observed in pyranose monomers with 1a-4a linkages (two), 1a-4e (one), and 1e-4e (none). Our results demonstrate single-molecule mechanochemistry with the capability of resolving complex conformational transitions. Atomic force microscope (AFM) manipulations of single polysaccharide molecules have recently expanded conformational chemistry (1) to include force-driven transitions between the chair and boat conformers of the pyranose ring structure (2). The application of a force to a single molecule will deform it elastically and also induce conformational transitions. Although it is easy to understand the origin of an elastic deformation, the mechanics of the conformational transition is less clear. Pyranose-based sugars have two distinct chair conformations, 4 C 1 and 1 C 4 (3), separated by an energy barrier of 11 kcal mol (4). In addition to the chair conformers, pyranoses have intermediate conformers corresponding to the boat conformation, whose energy is 5 8 kcal mol above the energy of the 4 C 1 chair (5). Thermally driven transitions do occur between these conformers. However, in the absence of an applied force, the most stable conformation of a pyranose is that of the 4 C 1 chair (4 9). Application of a force of 200 pn to polymers of -D-glucopyranose such as amylose drives a conformational change in the pyranose ring that is evident as a sudden elongation of the molecule, marking a prominent enthalpic component of the elasticity of the molecule (2, 10). The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C solely to indicate this fact. PNAS is available online at FIG. 1. A simplified mechanical model of the pyranose ring. An external force (F) is applied to the ring through the oxygen atoms O 1 and O 4 as in an (1a 3 4e)-linked polysaccharide chain (e.g., amylose). The direction of F coincides with the direction of the residue vector O 1 O 4. The force applied to the axial oxygen O 1 has a large torque P1 relative to the pivot point P 1 (P 1 lies on the imaginary axis of rotation passing through C 2 and O 5 ) and promotes a transition of the ring to the boat conformation. The axial bond C 1 O 1 works as a lever arm. The force applied to the equatorial oxygen O 4 has a negligible torque relative to P 2 (P 2 lies on the imaginary axis of rotation that passes through C 3 and C 5 ) because its line of action passes in the vicinity of P 2. Therefore the equatorial bond cannot mediate a conformational transition; however, a force applied to it will strain the ring. (Inset) Definition of torque as the cross product between the distance vector l and the force vector F. This enthalpic component results from an increase in the distance between glycosidic oxygen atoms caused by a forceinduced transition between the chair and boat conformations of the pyranose ring (2, 11). The glycosidic bonds of amylose are disposed in the C1-O1 [axial (a)] and C4-O4 [equatorial (e)] configuration. Inspection of this configuration reveals that a stretching force (whose direction coincides with the direction of the line passing through the backbone oxygen atoms) applied to the axial glycosidic oxygen O1 has a large lever arm relative to the C2-O5 axis and therefore produces torque about this axis, promoting the transition to the boat conformation. The line of action of the force applied to the equatorial oxygen atom O4 passes in the vicinity of the C3-C5 axis and therefore the C4-O4 bond does not produce significant torque about this axis. Fig. 1 illustrates this concept. Typically, ab initio calculations are required to predict the path of the conformational changes of the pyranose ring. However, the simplified mechanical schematic of Fig. 1 does make useful predictions. It is evident from this picture that axial linkages generate torque, whereas equatorial linkages do not. We propose that the torque generated by forces applied to the glycosidic linkages This paper was submitted directly (Track II) to the Proceedings office. Abbreviations: AFM, atomic force microscope; FJC, freely jointed chain; e, equatorial; a, axial. To whom reprint requests should be addressed. fernandez. julio@mayo.edu or piotr@mayo.edu. 7894
2 Biophysics: Marszalek et al. Proc. Natl. Acad. Sci. USA 96 (1999) 7895 does the rotational work necessary to flip the linkage to an extended conformer. To examine the validity of this hypothesis, we compare the extensibility of three different pyranosebased polymers with 1 4 glycosidic linkages: pectin (1a-4a), amylose (1a-4e), and cellulose (1e-4e). We find that, as predicted, the number of conformational transitions reported in force-extension curves of these polymers correlates with the number of glycosidic linkages in the axial orientation. MATERIALS AND METHODS Single-Molecule AFM. Our custom-made AFM apparatus, as well as its mode of operation, is identical to those that we have recently described (2, 12); it is capable of measuring the extensibility of individual molecules. The spring constant of each individual AFM tip was calibrated in solution, by using the equipartition theorem as described by Florin et al. (13). This method gives values for the spring constant of the cantilever that are within 20% of the values obtained by other methods (13). Polysaccharides. The polysaccharides used were: pectin (14), a partially methoxylated -(1 3 4)D-galactouronan (from citrus fruits; Sigma; galactouronic acid content 80%, methoxyl content 9%); amylose, an -(1 3 4)D-glucan (Type III: from potato; Sigma); and methylcellulose, a partially methoxylated -(1 3 4)D-glucan (degree of substitution, ; Sigma). Amylose and methylcellulose are linear homopolymers of glucopyranose, whereas in pectin the galactouronan chain is interrupted by short sequences of -Lrhamnopyranosyl units linked (1 3 2) and contains small amounts of neutral sugar side chains (15). Pectin and methylcellulose were dissolved in water at a concentration of 0.1 1% (wt vol). Amylose was solubilized by wetting with ethanol (100 mg ml), followed by treatment with sodium hydroxide (10%) and heating. The final concentration of amylose was 0.2 1% (wt vol). A layer of polysaccharide molecules was created by drying a drop of these solutions onto glass coverslips followed by extensive rinsing. This procedure leaves a monolayer of polysaccharide molecules tightly adsorbed to the glass surface (16). The measurements were carried out in water or in PBS buffer (pectin). For ring cleavage, the polysaccharide samples were treated with 5 mm sodium metaperiodate (Sigma) for up to 180 min. We cannot exclude the possibility that, on cleavage, the dialdehyde groups react further to form a seven-membered ring. This reaction would occur by the addition of water to one of the carbonyls and the subsequent addition of one of the formed hydroxyl groups to the other aldehyde carbonyl. Another possibility is that an aldehyde carbonyl forms a new six-membered ring by reacting with the free carboxyl group of pectin. However, our experimental observations show that after periodate oxidation, dissimilar polysaccharides such as dextran, amylose, and pectin all show similar force-extension curves with a large decrease in their Kuhn length, to the size of a single bond (2). Hence, it is unlikely that after ring cleavage, new cyclization reaction makes a permanent contribution to pectin elasticity. To pick polysaccharide molecules, an AFM tip was pressed down onto the sample for 1 3 sec and at forces of several nn. A subsequent force-extension measurement typically revealed a complex pattern with many overlapping force peaks. By manipulating the polysaccharide concentration and the force and duration of the tip-sample contact, it was possible to regularly obtain single polysaccharide force-extension curves. Ab Initio Calculations of the Galactouronic Acid Ring Conformations. Different conformations of the galactopyranuronic acid ring were generated by the published procedure (2), with the exception that in the present work the B3LYP 6 31G* method (17) was used to consider the electron correlation effect. Length of the residue vector in the relaxed state of galactouronan (no external force) was obtained from the distance between the two consecutive glycosidic oxygen atoms (O 1 O 4 ) in the most stable pyranose conformer ( 4 C 1 chair). The corresponding lengths of the residue vector in the boat and inverted chair conformations were optimized by ab initio calculations (Table 1). RESULTS AND DISCUSSION Stretched Pectin Monomers Undergo a Two-Step Chair Inversion Reaction. We begin by studying the elasticity of single pectin molecules, which has not been characterized before. In Fig. 2A we show a family of force-extension curves obtained from pectin molecules. The force-extension curves for pectin deviate from the shape predicted for a simple entropic freely jointed chain (FJC) (2, 10, 16, 18 21), and they display two enthalpic extensions at an average force of 300 and 800 pn. The curves superimpose well after being normalized by the length of the molecule, indicating that we stretched single molecules. Both enthalpic extensions are fully reversible (Fig. 3A) and disappear on cleavage of the pyranose rings with periodate (Fig. 3B) (22), confirming the view that they correspond to two separate force-driven transitions of the pyranose ring (2). We characterized these transitions by fitting the data to the modified FJC model of polymer elasticity (10, 20), as exemplified in Fig. 2B, where l 1, l 2, and l 3 correspond to the normalized contour length (monomer size; see Fig. 2 legend) of the polymer before the first transition, after the first transition, and after the second transition, respectively. The first transition was observed at a force of 300 pn and elongated the molecule by nm monomer, (l 2 l 1 ) l 1 corresponding to 9.3% of its total length. The second transition was resolved at 900 pn and elongated the molecule by nm monomer, (l 3 l 2 ) l 2, corresponding to 10% of its total length (for average values, see Table 1). Ab initio calculation of the length of the residue vector O 1 O 4 of the different pectin Table 1. Ab initio calculation of the separation of glycosidic oxygen atoms during chair inversion in galactouronic acid 4 C 1 Boat* 1 C 4 4 C 1 3 boat Boat 3 1 C 4 4 C C 4 Method l 1, Å l 2, Å l 3, Å (l 2 l 1 ) l 1,% (l 3 l 2 ) l 2,% (l 3 l 1 ) l 1,% HF 6-31G* B3LYP 6-31G* AFM data *This is a symmetrical boat with the flagpole hydroxyl at positon C2 and the flagpole hydrogen at position C5 (not shown). Standard error of the mean (n 8).
3 7896 Biophysics: Marszalek et al. Proc. Natl. Acad. Sci. USA 96 (1999) FIG. 2. Force-extension curves for single-pectin molecules reveal a two-step transition. (A) The shape of the curves obtained from short and long molecules clearly deviates from the shape expected for a FJC. The curves reveal two enthalpic extensions that occur at 300 and pn. (B) High-resolution plot of the force-extension relationship for a single pectin molecule. The extension, x, was normalized: x norm 0.45x l c 1, where 0.45 nm corresponds to length of the residue vector O 1 O chair 4 in the relaxed state of the polymer (chair 4 C 1 ; see Table 1), and l c 1 the contour length of the molecule determined from the FJC fit to the nonnormalized data before the first transition (l c nm for this recording). Thin lines are the local fits of the FJC model modified to include the extensibility of the monomers (segment elasticity S E ; 10, 16, 20). l nm (by definition); S E1 was found to be 17,000 pn nm; l 2 was determined to be nm (it corresponds to O 1 O boat 4 ); S E2 was determined to be 24,000 pn nm; l 3 was found to be nm (it corresponds to O 1 O inv-chair 4 ), whereas S E3 was found to be 83,000 pn nm. The length of the Kuhn segment l K was determined from the low force region fit to be 1.81 nm. (Inset) Structure of the -D-galactouronic acid (the pectin monomer). Note that in contrast to -D-glucopyranose that has the C4OO4 bond in the equatorial position, -D-galactopyranuronic acid has the C4OO4 bond in the axial position. ring conformers show that a 4 C 1 3 boat transition elongates the molecule by nm monomer (8.2%, B3LYP 6 31G* method; see Table 1) and that chair inversion (23) further elongates the monomer by 0.06 nm (12.3%, B3LYP 6 31G* method; see Table 1). The ab initio calculations are in good agreement with our experimental observations, suggesting that the two transitions observed in pectin correspond to a sequential chair inversion reaction 4a C 1a i boat i 1e C 4e. The total elongation of the monomer determined experimentally, 20.1%, is slightly shorter than the elongation calculated ab initio: 21.5%. This difference is consistent with the fact that the pectin chain contains a small amount of -L-rhamnopyranosyl units linked (1 3 2) (15), which do not undergo a transition to FIG. 3. Force-extension curves for native (A) and periodateoxidized pectins (B). In A, we demonstrate that the conformational transitions underlying the enthalpic components of pectin elasticity are reversible. The blue trace represents the recording obtained on stretching and the red trace, on relaxing a single pectin. The recording did not show any hysteresis and was separated only to reveal the forward and backward traces. Pulling speed, 0.5 nm ms. (B) The force-extension curve of periodate-oxidized galactouronan (Inset) does not reveal any significant enthalpic elasticity and represents the extensibility of a FJC of very short (l K 0.19 nm) and rigid (S E 70,000 pn nm 1 ) segments. elongated conformers. Significantly, the first transition in pectin occurs at the force that was found to trigger a chairto-boat transition of the pyranose rings in amylose (2). Hence, our AFM measurements clearly resolve a chair inversion reaction, as a two-step conformational transition within the pectin pyranose rings. The Number of Axial Glycosidic Bonds Determines the Number of Force-Induced Conformational Transitions. We can now compare the mechanics of the different glycosidic attachments to a pyranose ring. Fig. 4 compares the three types of linkages. It is clear that forces applied to the 1(equatorial)(e)-4(e) molecule (methylcellulose; Fig. 4C) will not generate any torque about the C2-O5 and C3-C5 axes of rotation (see Fig. 1). By contrast, a force applied to the oxygen O1 in the 1(axial)(a)-4(e) molecule generates torque about the C2-O5 axis of rotation (see Fig. 1) and triggers a single type of conformational transition 4e C 1a i boat, as observed experimentally (Fig. 4B). By contrast, forces applied to O1 and O4 in the 1(a)-4(a) molecule (pectin, Figs. 2 and 4A) generate torques about both the C2-O5 and C3-C5 axes, triggering the two transitions observed experimentally and identified as a chair inversion reaction. These data support the view that, depending on their bond orientation, the glycosidic linkages act as levers generating the torque that does the work necessary to complete conformational transitions of the pyranose ring. However, the precise way in which the work done by the levers is distributed between bond stretching and conformational energy is unknown. We anticipate that through the use of steered molecular dynamics simulations, it will be possible to predict the energy surface describing the pyranose ring tran-
4 Biophysics: Marszalek et al. Proc. Natl. Acad. Sci. USA 96 (1999) 7897 FIG. 4. A comparison of elastic characteristics of pyranose-based polysaccharides that exploit both glycosidic bonds in the axial position (galactouronan, A), one axial and one equatorial bond (amylose, B) and both equatorial bonds (methylcellulose, C). (Right) Conformational transitions that are responsible for the enthalpic extensions of the polymers. The dominant conformers of the pyranose ring are indicated along different fragments of the force extension curves. The extensions in A and B were normalized in the same way as in Fig. 2B (O 1 O chair 4 for amylose is also equal to 0.45 nm; ref. 2); in C the extension was normalized: x norm 0.542x l c nm, where nm corresponds to the length of the residue vector O 1 O chair 4 for the -D-glucopyranose monomer of cellulose (2), and l c is the contour length of methylcellulose determined from the FJC model fit to the data in the whole force region (not shown). sitions that then can be contrasted with the forces measured by AFM. Furthermore, our work shows that by finely calibrating the applied force, it is possible to selectively populate the different conformers allowed by the pyranose ring. Force-Induced Conformational Transitions as Switching Signals. It is likely that the force-driven conformational changes of the pyranose ring have biological significance. For example, pectin ring conformers that are accessible by force could serve as off-on-off switches that modulate biological functions (Fig. 5). Our results contrast the widespread view that the pyranose ring serves as a rigid scaffold for ligand binding. Furthermore, the range of forces required to trigger conformational changes of the pyranose ring are similar to those commonly found in many cellular systems, such as the bacterial and plant cell walls that accommodate high tensile stresses generated during changes in turgor pressure (24, 25). The cell wall is now known to play an active role in the signaling that coordinates plant growth and other functions (26). Hydroxyl and other functional groups attached to the pyranose ring coordinate the binding of lectins (27, 28). The selectivity of lectin binding to polysaccharides is largely determined by the position of the hydroxyl groups of the pyranose ring (28). Our ab initio calculations of the pyranose conformers show that a chair inversion reaction will change the spacing and the orientation of the hydroxyl groups in positions 2 and 3 (Fig. 5). As shown, a forced chair inversion reaction would increase the O2-O3 distance by 0.7 Å and also would change the orientation of the hydroxyls from equatorial to axial. It is very likely that these changes will have a large effect on the affinity of lectin binding (28). Hence, pectin and other polysaccharides may serve as detectors that send biological signals in a narrow force range. Conclusions. Our results support the hypothesis that the conformational transitions of the pyranose ring are driven by the glycosidic bonds that act as mechanical levers. The torque generated by these levers determines the conformers that can be reached by an applied force and hence the number of
5 7898 Biophysics: Marszalek et al. Proc. Natl. Acad. Sci. USA 96 (1999) This work was supported by grants from the National Science Foundation (P.E.M.) and the National Institutes of Health (J.M.F.). FIG. 5. The pyranose ring as an elastic switch. We model the receptor ligand interaction as a bivalent contact where a forced deformation of the ligand (e.g., pectin monomers) changes the affinity of the interaction. In our example, the balls (oxygen atoms O2 and O3) represent epitopes on pectin recognized by a receptor. (A) At zero applied force, the pectin ring is in the 4 C 1 chair conformer and interacts at low affinity with the receptor. The low affinity results from the mismatch between the binding sites and O2-O3 (equatorial and spaced by 2.9 Å, derived from ab initio). (B) A force of 300 pn converts the ring into the boat conformation ( On ) with O2 in the axial position. The O2-O3 distance increases to 3.2 Å, favoring high-affinity binding. (C) At higher forces ( 800 pn), the ring changes its conformation to an inverted chair, 1 C 4, moving O3 to the axial position and further increasing the O2-O3 separation to 3.6 Å. This results in bond failure ( Off ). transitions during the elongation of the molecule. Our experiments also demonstrate the capability of the AFM to manipulate the conformation of single molecules and of resolving complex conformational transitions, opening up the new field of single molecule mechanochemistry. 1. Barton, D. H. R. (1970) Science 169, Marszalek, P. E., Oberhauser, A. F., Pang, Y.-P. & Fernandez, J. M. (1998) Nature (London) 396, Barrows, S.E., Dulles, F. J., Cramer, C. J., French, A. D. & Truhlar, D. G. (1995) Carbohydr. Res. 276, Joshi, N. V. &. Rao, V. S. R. (1979) Biopolymers 18, Dowd, M. K., French, A. D. & Reilly, P. J. (1994) Carbohydr. Res. 264, Brown, G. M. & Levy, H. A. (1965) Science 147, Chu, S. C. C. & Jeffrey, G. A. (1968) Acta Crystallogr. B 24, Brown, G. M. & Levy H. A. (1979) Acta Crystallogr. B 35, Brady, J. W. (1986) J. Am. Chem. Soc. 108, Rief, M., Oesterhelt, F., Heymann, B. & Gaub, H. E. (1997) Science 275, Li, H., Rief, M., Oesterhelt, F., Gaub, H. E., Zhang, X. & Shen J. (1999) Chem. Phys. Lett. 305, Oberhauser, A. F., Marszalek, P. E., Erickson, H. P. & Fernandez, J. M (1998) Nature (London) 393, Florin, E. L., Rief, M., Lehmann, H., Ludwig, M., Dornmair, C., Moy, V. T. & Gaub, H. E. (1995) Biosens. Biolelectron. 10, Barton, D. H. R. & Ollis, W. D. (1979) Comprehensive Organic Chemistry (Pergamon, Oxford), Vol. 5, p Dea, I. C. M. (1993) Industrial Gums. Polysaccharides and Their Derivatives (Academic, New York), p Li, H., Rief, M., Oesterhelt, F. & Gaub, H. E. (1998) Adv. Mater. 3, Becke, A. D. (1993) J. Chem. Phys. 98, Flory, P. J. (1953) Principles of Polymer Chemistry (Cornell Univ. Press, Ithaca, NY). 19. Smith, S. B., Finzi, L. & Bustamante, C. (1992) Science 258, Smith, S. B., Cui, Y. & Bustamante, C. (1996) Science 271, Rief, M., Fernandez, J. M. & Gaub, H. E. (1998) Phys. Rev. Let. 81, Bobbitt, J. M. (1956) in Advances in Carbohydrate Chemistry. eds. Wolfram, M. L. & Tipson, R. S. (Academic, New York) pp Pickett, H. M. & Strauss, H. L. (1970) J. Am. Chem. Soc. 92, Cosgrove, D. J. (1997) Plant Cell 9, Thwaites, J. J. & Mendelson, N. H. (1985) Proc. Natl. Acad. Sci. USA 82, Strauss, E. (1998) Science 282, Drickamer, K. (1997) Structure (London) 5, Weis, W. I. & Drickamer, K. (1996) Annu. Rev. Biochem. 65,
Recently developed single-molecule atomic force microscope
 Stepwise unfolding of titin under force-clamp atomic force microscopy Andres F. Oberhauser*, Paul K. Hansma, Mariano Carrion-Vazquez*, and Julio M. Fernandez* *Department of Physiology and Biophysics,
Stepwise unfolding of titin under force-clamp atomic force microscopy Andres F. Oberhauser*, Paul K. Hansma, Mariano Carrion-Vazquez*, and Julio M. Fernandez* *Department of Physiology and Biophysics,
Single-molecule force spectroscopy on polysaccharides by AFM nanomechanical fingerprint of a- 1,4 -linked polysaccharides
 21 May 1999 Ž. Chemical Physics Letters 305 1999 197 201 Single-molecule force spectroscopy on polysaccharides by AFM ž / nanomechanical fingerprint of a- 1,4 -linked polysaccharides Hongbin Li a,b, Matthias
21 May 1999 Ž. Chemical Physics Letters 305 1999 197 201 Single-molecule force spectroscopy on polysaccharides by AFM ž / nanomechanical fingerprint of a- 1,4 -linked polysaccharides Hongbin Li a,b, Matthias
Single-Molecule Force Spectroscopy on Poly(acrylic acid) by AFM
 2120 Langmuir 1999, 15, 2120-2124 Single-Molecule Force Spectroscopy on Poly(acrylic acid) by AFM Hongbin Li, Bingbing Liu, Xi Zhang,*, Chunxiao Gao, Jiacong Shen, and Guangtian Zou Key Lab for Supramolecular
2120 Langmuir 1999, 15, 2120-2124 Single-Molecule Force Spectroscopy on Poly(acrylic acid) by AFM Hongbin Li, Bingbing Liu, Xi Zhang,*, Chunxiao Gao, Jiacong Shen, and Guangtian Zou Key Lab for Supramolecular
Structural investigation of single biomolecules
 Structural investigation of single biomolecules NMR spectroscopy and X-ray crystallography are currently the most common techniques capable of determining the structures of biological macromolecules like
Structural investigation of single biomolecules NMR spectroscopy and X-ray crystallography are currently the most common techniques capable of determining the structures of biological macromolecules like
Multimedia : Fibronectin and Titin unfolding simulation movies.
 I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
Viscoelasticity of a Single Polymer Chain
 Netsu Sokutei 33 4 183-190 2006 7 10 2006 7 22 Viscoelasticity of a Single Polymer Chain Ken Nakajima and Toshio Nishi (Received July 10, 2006; Accepted July 22, 2006) Atomic force microscopy (AFM) enabled
Netsu Sokutei 33 4 183-190 2006 7 10 2006 7 22 Viscoelasticity of a Single Polymer Chain Ken Nakajima and Toshio Nishi (Received July 10, 2006; Accepted July 22, 2006) Atomic force microscopy (AFM) enabled
Mechanical Proteins. Stretching imunoglobulin and fibronectin. domains of the muscle protein titin. Adhesion Proteins of the Immune System
 Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Stretching lattice models of protein folding
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 2031 2035, March 1999 Biophysics Stretching lattice models of protein folding NICHOLAS D. SOCCI,JOSÉ NELSON ONUCHIC**, AND PETER G. WOLYNES Bell Laboratories, Lucent
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 2031 2035, March 1999 Biophysics Stretching lattice models of protein folding NICHOLAS D. SOCCI,JOSÉ NELSON ONUCHIC**, AND PETER G. WOLYNES Bell Laboratories, Lucent
Single-molecule Stretching Method For Lateral And Normal AFM Levers Calibration
 Single-molecule Stretching Method For Lateral And Normal AFM Levers Calibration Maciej Dendzik 1,, Andrzej Kulik, Fabrizio Benedetti, Piotr E. Marszalek 3 and Giovanni Dietler 1 Centre for Nanometer-Scale
Single-molecule Stretching Method For Lateral And Normal AFM Levers Calibration Maciej Dendzik 1,, Andrzej Kulik, Fabrizio Benedetti, Piotr E. Marszalek 3 and Giovanni Dietler 1 Centre for Nanometer-Scale
Supporting information for
 Supporting information for High-performance and moisture-stable cellulosestarch nanocomposites based on bioinspired coreshell nanofibers Kasinee Prakobna, 1, 2 Sylvain Galland, 1, 2 and Lars A. Berglund
Supporting information for High-performance and moisture-stable cellulosestarch nanocomposites based on bioinspired coreshell nanofibers Kasinee Prakobna, 1, 2 Sylvain Galland, 1, 2 and Lars A. Berglund
Investigation of the effects of fine structure on the nanomechanical properties of pectin
 Investigation of the effects of fine structure on the nanomechanical properties of pectin M. A. K. Williams, 1,2 A. T. Marshall, 1,3 P. Anjukandi, 1 and R. G. Haverkamp 3 1 Institute of Fundamental Sciences,
Investigation of the effects of fine structure on the nanomechanical properties of pectin M. A. K. Williams, 1,2 A. T. Marshall, 1,3 P. Anjukandi, 1 and R. G. Haverkamp 3 1 Institute of Fundamental Sciences,
VIII. Rubber Elasticity [B.Erman, J.E.Mark, Structure and properties of rubberlike networks]
![VIII. Rubber Elasticity [B.Erman, J.E.Mark, Structure and properties of rubberlike networks] VIII. Rubber Elasticity [B.Erman, J.E.Mark, Structure and properties of rubberlike networks]](/thumbs/90/103066845.jpg) VIII. Rubber Elasticity [B.Erman, J.E.Mark, Structure and properties of rubberlike networks] Using various chemistry, one can chemically crosslink polymer chains. With sufficient cross-linking, the polymer
VIII. Rubber Elasticity [B.Erman, J.E.Mark, Structure and properties of rubberlike networks] Using various chemistry, one can chemically crosslink polymer chains. With sufficient cross-linking, the polymer
Loudon Chapter 24 Review: Carbohydrates Jacquie Richardson, CU Boulder Last updated 4/26/2018
 This chapter is about carbohydrates molecules with the general formula of C n( 2O) n, or in other words C n 2nO n. This is a very common formula for sugars and many other natural products. The structure
This chapter is about carbohydrates molecules with the general formula of C n( 2O) n, or in other words C n 2nO n. This is a very common formula for sugars and many other natural products. The structure
Announcements. Homework 3 (Klaus Schulten s Lecture): Due Wednesday at noon. Next homework assigned. Due Wednesday March 1.
 Announcements Homework 3 (Klaus Schulten s Lecture): Due Wednesday at noon. Next homework assigned. Due Wednesday March 1. No lecture next Monday, Feb. 27 th! (Homework is a bit longer.) Marco will have
Announcements Homework 3 (Klaus Schulten s Lecture): Due Wednesday at noon. Next homework assigned. Due Wednesday March 1. No lecture next Monday, Feb. 27 th! (Homework is a bit longer.) Marco will have
Atomic weight = Number of protons + neutrons
 1 BIOLOGY Elements and Compounds Element is a substance that cannot be broken down to other substances by chemical reactions. Essential elements are chemical elements required for an organism to survive,
1 BIOLOGY Elements and Compounds Element is a substance that cannot be broken down to other substances by chemical reactions. Essential elements are chemical elements required for an organism to survive,
Question 1. Identify the sugars below by filling in the table below (except shaded areas). Use the page or a separate sheet
 Question 1. Identify the sugars below by filling in the table below (except shaded areas). Use the page or a separate sheet Sugar name aworth projection(s) of the corresponding pyranose form (Six membered
Question 1. Identify the sugars below by filling in the table below (except shaded areas). Use the page or a separate sheet Sugar name aworth projection(s) of the corresponding pyranose form (Six membered
7 Steroids and sugars
 7 Steroids and sugars Purpose In this chapter you widen your scope on stereochemistry from that of relatively simple compounds to that of more complex and naturally occurring compounds. ne stereochemically
7 Steroids and sugars Purpose In this chapter you widen your scope on stereochemistry from that of relatively simple compounds to that of more complex and naturally occurring compounds. ne stereochemically
25.6 Cyclic Forms of Carbohydrates: Furanose Forms
 .6 Cyclic Forms of Carbohydrates: Furanose Forms Recall from Section 7.8 R R' C + R" Product is a hemiacetal. R" C R R' R C Cyclic emiacetals R C Aldehydes and ketones that contain an group elsewhere in
.6 Cyclic Forms of Carbohydrates: Furanose Forms Recall from Section 7.8 R R' C + R" Product is a hemiacetal. R" C R R' R C Cyclic emiacetals R C Aldehydes and ketones that contain an group elsewhere in
Importance of Carbohydrates
 Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
Mechanical Proteins. Stretching imunoglobulin and fibronectin. domains of the muscle protein titin. Adhesion Proteins of the Immune System
 Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Cross-linking of a charged polysaccharide using polyions as electrostatic staples
 Cross-linking of a charged polysaccharide using polyions as electrostatic staples Sabyasachi Rakshit and Sanjeevi Sivasankar* Department of Physics and Astronomy, Iowa State University, Ames, IA 50011.
Cross-linking of a charged polysaccharide using polyions as electrostatic staples Sabyasachi Rakshit and Sanjeevi Sivasankar* Department of Physics and Astronomy, Iowa State University, Ames, IA 50011.
Single molecule force spectroscopy reveals a highly compliant helical
 Supplementary Information Single molecule force spectroscopy reveals a highly compliant helical folding for the 30 nm chromatin fiber Maarten Kruithof, Fan-Tso Chien, Andrew Routh, Colin Logie, Daniela
Supplementary Information Single molecule force spectroscopy reveals a highly compliant helical folding for the 30 nm chromatin fiber Maarten Kruithof, Fan-Tso Chien, Andrew Routh, Colin Logie, Daniela
V(φ) CH 3 CH 2 CH 2 CH 3. High energy states. Low energy states. Views along the C2-C3 bond
 Example V(φ): Rotational conformations of n-butane C 3 C C C 3 Potential energy of a n-butane molecule as a function of the angle φ of bond rotation. V(φ) Potential energy/kj mol -1 0 15 10 5 eclipse gauche
Example V(φ): Rotational conformations of n-butane C 3 C C C 3 Potential energy of a n-butane molecule as a function of the angle φ of bond rotation. V(φ) Potential energy/kj mol -1 0 15 10 5 eclipse gauche
BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY-
 BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY- ELEMENTS AND COMPOUNDS - anything that has mass and takes up space. - cannot be broken down to other substances. - substance containing two or more different elements
BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY- ELEMENTS AND COMPOUNDS - anything that has mass and takes up space. - cannot be broken down to other substances. - substance containing two or more different elements
CHEMISTRY 332 SUMMER 08 EXAM I June 26-28, 2008
 First Three Letters of Last Name NAME Network ID CHEMISTRY 332 SUMMER 08 EXAM I June 26-28, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed, electronic,
First Three Letters of Last Name NAME Network ID CHEMISTRY 332 SUMMER 08 EXAM I June 26-28, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed, electronic,
25.3 Cyclization of Monosaccharides
 090 CAPTER CARBYDRATES PRBLEM. Determine the identity of each of these carbohydrates: C a) b) c) C C C C C (int: This must be rotated first.). Cyclization of Monosaccharides Up to this point, the structure
090 CAPTER CARBYDRATES PRBLEM. Determine the identity of each of these carbohydrates: C a) b) c) C C C C C (int: This must be rotated first.). Cyclization of Monosaccharides Up to this point, the structure
Measurements of interaction forces in (biological) model systems
 Measurements of interaction forces in (biological) model systems Marina Ruths Department of Chemistry, UMass Lowell What can force measurements tell us about a system? Depending on the technique, we might
Measurements of interaction forces in (biological) model systems Marina Ruths Department of Chemistry, UMass Lowell What can force measurements tell us about a system? Depending on the technique, we might
Many prominent proposals (e.g., see refs. 1 and 2) and
 How important are entropic contributions to enzyme catalysis? J. Villà, M.Štrajbl, T. M. Glennon, Y. Y. Sham, Z. T. Chu, and A. Warshel* Department of Chemistry, University of Southern California, Los
How important are entropic contributions to enzyme catalysis? J. Villà, M.Štrajbl, T. M. Glennon, Y. Y. Sham, Z. T. Chu, and A. Warshel* Department of Chemistry, University of Southern California, Los
Chapter 02 Chemical Composition of the Body
 Chapter 02 Chemical Composition of the Body 1. In an atom, the number of Student: A. Protons always equals the number of neutrons B. Of protons always equals the number of electrons C. Of neutrons always
Chapter 02 Chemical Composition of the Body 1. In an atom, the number of Student: A. Protons always equals the number of neutrons B. Of protons always equals the number of electrons C. Of neutrons always
Chem 310, Organic Chemistry Lab Molecular Modeling Using Macromodel
 Chem 310, Organic Chemistry Lab Molecular Modeling Using Macromodel This is a molecular modeling experiment, and should be written up in your lab notebook just as if it were a normal "wet-chemistry" experiment.
Chem 310, Organic Chemistry Lab Molecular Modeling Using Macromodel This is a molecular modeling experiment, and should be written up in your lab notebook just as if it were a normal "wet-chemistry" experiment.
Wood Chemistry. Cellulose: the Basics. Cellulose: More Basics. PSE 406/Chem E 470. Reducing End Groups. Lecture 5 Cellulose.
 : the Basics PSE 406/Chem E 470 Lecture 5 PSE 406: Lecture 5 1 Linear polymer made up of -d glucopyranose units linked with 1 4 glycosidic bonds. Repeating unit glucose (cellobiose) Glucopyranose units
: the Basics PSE 406/Chem E 470 Lecture 5 PSE 406: Lecture 5 1 Linear polymer made up of -d glucopyranose units linked with 1 4 glycosidic bonds. Repeating unit glucose (cellobiose) Glucopyranose units
Loudon Chapter 24 Review: Carbohydrates CHEM 3331, Jacquie Richardson, Fall Page 1
 CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE. Honors Biology I
 NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
12U Biochemistry Unit Test
 1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
Nature Methods: doi: /nmeth Supplementary Figure 1. Principle of force-distance (FD) curve based AFM.
 Supplementary Figure 1 Principle of force-distance (FD) curve based AFM. (a) FD-based AFM contours the sample surface while oscillating the AFM tip with a sine wave at a frequency of 0.25 khz. Pixel-by-pixel
Supplementary Figure 1 Principle of force-distance (FD) curve based AFM. (a) FD-based AFM contours the sample surface while oscillating the AFM tip with a sine wave at a frequency of 0.25 khz. Pixel-by-pixel
RIGID BODY REFINEMENT OF WATER MEDIATED TRIPLE HELICAL STRUCTURE OF β-d-1,3 XYLAN FROM PALMARIA PALMATA (L.) KUNTZE, RHODOPHYTA)
 Pak. J. Bot., 36(4): 745-750, 2004. RIGID BODY REFINEMENT OF WATER MEDIATED TRIPLE HELICAL STRUCTURE OF β-d-1,3 XYLAN FROM PALMARIA PALMATA (L.) KUNTZE, RHODOPHYTA) NAHEED AKHTAR, SADAF NAEEM, WASEEM AHMED
Pak. J. Bot., 36(4): 745-750, 2004. RIGID BODY REFINEMENT OF WATER MEDIATED TRIPLE HELICAL STRUCTURE OF β-d-1,3 XYLAN FROM PALMARIA PALMATA (L.) KUNTZE, RHODOPHYTA) NAHEED AKHTAR, SADAF NAEEM, WASEEM AHMED
ATOMIC STRUCTURES OF GLUCOSE, FRUCTOSE AND SUCROSE AND EXPLANATION OF ANOMERIC CARBON
 1 ATOMIC STRUCTURES OF GLUCOSE, FRUCTOSE AND SUCROSE AND EXPLANATION OF ANOMERIC CARBON RAJI HEYROVSKA Na Stahlavce 6, 160 00 Praha 6, Czech Republic Email: rheyrovs@hotmail.com Abstract: Presented here
1 ATOMIC STRUCTURES OF GLUCOSE, FRUCTOSE AND SUCROSE AND EXPLANATION OF ANOMERIC CARBON RAJI HEYROVSKA Na Stahlavce 6, 160 00 Praha 6, Czech Republic Email: rheyrovs@hotmail.com Abstract: Presented here
2: CHEMICAL COMPOSITION OF THE BODY
 1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
Proteins polymer molecules, folded in complex structures. Konstantin Popov Department of Biochemistry and Biophysics
 Proteins polymer molecules, folded in complex structures Konstantin Popov Department of Biochemistry and Biophysics Outline General aspects of polymer theory Size and persistent length of ideal linear
Proteins polymer molecules, folded in complex structures Konstantin Popov Department of Biochemistry and Biophysics Outline General aspects of polymer theory Size and persistent length of ideal linear
Material Chemistry KJM 3100/4100. Synthetic Polymers (e.g., Polystyrene, Poly(vinyl chloride), Poly(ethylene oxide))
 Material Chemistry KJM 3100/4100 Lecture 1. Soft Materials: Synthetic Polymers (e.g., Polystyrene, Poly(vinyl chloride), Poly(ethylene oxide)) Biopolymers (e.g., Cellulose derivatives, Polysaccharides,
Material Chemistry KJM 3100/4100 Lecture 1. Soft Materials: Synthetic Polymers (e.g., Polystyrene, Poly(vinyl chloride), Poly(ethylene oxide)) Biopolymers (e.g., Cellulose derivatives, Polysaccharides,
Glucose Anomers. Conformational Analysis by NMR
 Glucose Anomers Conformational Analysis by NMR Glucose in solution remains mostly in the cyclic pyranose form in two conformational anomers shown in the figure above. These two forms interconvert via an
Glucose Anomers Conformational Analysis by NMR Glucose in solution remains mostly in the cyclic pyranose form in two conformational anomers shown in the figure above. These two forms interconvert via an
Supplementary Information
 1 Supplementary Information Figure S1 The V=0.5 Harker section of an anomalous difference Patterson map calculated using diffraction data from the NNQQNY crystal at 1.3 Å resolution. The position of the
1 Supplementary Information Figure S1 The V=0.5 Harker section of an anomalous difference Patterson map calculated using diffraction data from the NNQQNY crystal at 1.3 Å resolution. The position of the
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS
 2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
Other Cells. Hormones. Viruses. Toxins. Cell. Bacteria
 Other Cells Hormones Viruses Toxins Cell Bacteria ΔH < 0 reaction is exothermic, tells us nothing about the spontaneity of the reaction Δ H > 0 reaction is endothermic, tells us nothing about the spontaneity
Other Cells Hormones Viruses Toxins Cell Bacteria ΔH < 0 reaction is exothermic, tells us nothing about the spontaneity of the reaction Δ H > 0 reaction is endothermic, tells us nothing about the spontaneity
Supplementary Information
 Supplementary Information Switching of myosin-v motion between the lever-arm swing and Brownian search-and-catch Keisuke Fujita 1*, Mitsuhiro Iwaki 2,3*, Atsuko H. Iwane 1, Lorenzo Marcucci 1 & Toshio
Supplementary Information Switching of myosin-v motion between the lever-arm swing and Brownian search-and-catch Keisuke Fujita 1*, Mitsuhiro Iwaki 2,3*, Atsuko H. Iwane 1, Lorenzo Marcucci 1 & Toshio
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Dynamical principles in biological processes
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 358 362, February 998 Chemistry Dynamical principles in biological processes E. W. SCHLAG, S.H.LIN, R.WEINKAUF, AND P. M. RENTZEPIS Institute for Physical Theoretical
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 358 362, February 998 Chemistry Dynamical principles in biological processes E. W. SCHLAG, S.H.LIN, R.WEINKAUF, AND P. M. RENTZEPIS Institute for Physical Theoretical
 There are two types of polysaccharides in cell: glycogen and starch Starch and glycogen are polysaccharides that function to store energy Glycogen Glucose obtained from primary sources either remains soluble
There are two types of polysaccharides in cell: glycogen and starch Starch and glycogen are polysaccharides that function to store energy Glycogen Glucose obtained from primary sources either remains soluble
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø
 `1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
The Molecules of Life Chapter 2
 The Molecules of Life Chapter 2 Core concepts 1.The atom is the fundamental unit of matter. 2.Atoms can combine to form molecules linked by chemical bonds. 3.Water is essential for life. 4.Carbon is the
The Molecules of Life Chapter 2 Core concepts 1.The atom is the fundamental unit of matter. 2.Atoms can combine to form molecules linked by chemical bonds. 3.Water is essential for life. 4.Carbon is the
Chap. 2. Polymers Introduction. - Polymers: synthetic materials <--> natural materials
 Chap. 2. Polymers 2.1. Introduction - Polymers: synthetic materials natural materials no gas phase, not simple liquid (much more viscous), not perfectly crystalline, etc 2.3. Polymer Chain Conformation
Chap. 2. Polymers 2.1. Introduction - Polymers: synthetic materials natural materials no gas phase, not simple liquid (much more viscous), not perfectly crystalline, etc 2.3. Polymer Chain Conformation
Supporting Information
 Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Chapter 3 Entropy elasticity (rubbery materials) Review basic thermal physics Chapter 5.1 to 5.5 (Nelson)
 Chapter 3 Entropy elasticity (rubbery materials) Review basic thermal physics Chapter 5.1 to 5.5 (Nelson) Outline: 3.1 Strain, stress and Young modulus 3. Energy density 3.3 Typical stress-strain curve
Chapter 3 Entropy elasticity (rubbery materials) Review basic thermal physics Chapter 5.1 to 5.5 (Nelson) Outline: 3.1 Strain, stress and Young modulus 3. Energy density 3.3 Typical stress-strain curve
FLOW INDUCED MECHANOCHEMICAL ACTIVATION
 FLOW INDUCED MECHANOCHEMICAL ACTIVATION H Magnus Andersson 1, Charles R Hickenboth 2, Stephanie L Potisek 2, Jeffrey S Moore 2, Nancy R Sottos 3 and Scott R White 4 1 Beckman Institute, 2 Department of
FLOW INDUCED MECHANOCHEMICAL ACTIVATION H Magnus Andersson 1, Charles R Hickenboth 2, Stephanie L Potisek 2, Jeffrey S Moore 2, Nancy R Sottos 3 and Scott R White 4 1 Beckman Institute, 2 Department of
Untangling the Mechanics of Entangled Biopolymers
 Untangling the Mechanics of Entangled Biopolymers Rae M. Robertson-Anderson Physics Department University of San Diego students/postdocs: Cole Chapman, PhD Tobias Falzone, PhD Stephanie Gorczyca, USD 16
Untangling the Mechanics of Entangled Biopolymers Rae M. Robertson-Anderson Physics Department University of San Diego students/postdocs: Cole Chapman, PhD Tobias Falzone, PhD Stephanie Gorczyca, USD 16
Supporting Information for: Mechanism of Reversible Peptide-Bilayer. Attachment: Combined Simulation and Experimental Single-Molecule Study
 Supporting Information for: Mechanism of Reversible Peptide-Bilayer Attachment: Combined Simulation and Experimental Single-Molecule Study Nadine Schwierz a,, Stefanie Krysiak c, Thorsten Hugel c,d, Martin
Supporting Information for: Mechanism of Reversible Peptide-Bilayer Attachment: Combined Simulation and Experimental Single-Molecule Study Nadine Schwierz a,, Stefanie Krysiak c, Thorsten Hugel c,d, Martin
AP Biology: Biochemistry Learning Targets (Ch. 2-5)
 Understand basic principles of chemistry. Distinguish between an element and a compound. Describe the structure of an atom. Compare the various types of chemical bonding. Describe what is meant by a covalent
Understand basic principles of chemistry. Distinguish between an element and a compound. Describe the structure of an atom. Compare the various types of chemical bonding. Describe what is meant by a covalent
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS 1. INTRODUCTION Crystals are held together by interatomic or intermolecular bonds. The bonds can be covalent,
3.091 Introduction to Solid State Chemistry Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS 1. INTRODUCTION Crystals are held together by interatomic or intermolecular bonds. The bonds can be covalent,
Xylan Adsorption on Cellulose Model Films & Influence on Bond Strength
 Xylan Adsorption on Cellulose Model Films & Influence on Bond Strength Experiments Conducted in Context of My Master Thesis at the Solid State Physics Institute of TU Graz Siegfried Zöhrer szoehrer@sbox.tugraz.at
Xylan Adsorption on Cellulose Model Films & Influence on Bond Strength Experiments Conducted in Context of My Master Thesis at the Solid State Physics Institute of TU Graz Siegfried Zöhrer szoehrer@sbox.tugraz.at
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Information Figure S1: (a) Initial configuration of hydroxyl and epoxy groups used in the MD calculations based on the observations of Cai et al. [Ref 27 in the
SUPPLEMENTARY INFORMATION Supplementary Information Figure S1: (a) Initial configuration of hydroxyl and epoxy groups used in the MD calculations based on the observations of Cai et al. [Ref 27 in the
Mr. Carpenter s Biology Biochemistry. Name Pd
 Mr. Carpenter s Biology Biochemistry Name Pd Chapter 2 Vocabulary Atom Element Compound Molecule Ion Cohesion Adhesion Solution Acid Base Carbohydrate Monosaccharide Lipid Protein Amino acid Nucleic acid
Mr. Carpenter s Biology Biochemistry Name Pd Chapter 2 Vocabulary Atom Element Compound Molecule Ion Cohesion Adhesion Solution Acid Base Carbohydrate Monosaccharide Lipid Protein Amino acid Nucleic acid
Single-Molecule Measurement of the Strength of a Siloxane Bond
 Langmuir 2008, 24, 1343-1349 1343 Single-Molecule Measurement of the Strength of a Siloxane Bond Peter Schwaderer, Enno Funk, Frank Achenbach, Johann Weis, Christoph Bräuchle, and Jens Michaelis*, Department
Langmuir 2008, 24, 1343-1349 1343 Single-Molecule Measurement of the Strength of a Siloxane Bond Peter Schwaderer, Enno Funk, Frank Achenbach, Johann Weis, Christoph Bräuchle, and Jens Michaelis*, Department
Energy Landscape Distortions and the Mechanical Unfolding of Proteins
 3494 Biophysical Journal Volume 88 May 2005 3494 3501 Energy Landscape Distortions and the Mechanical Unfolding of Proteins Daniel J. Lacks Department of Chemical Engineering, Case Western Reserve University,
3494 Biophysical Journal Volume 88 May 2005 3494 3501 Energy Landscape Distortions and the Mechanical Unfolding of Proteins Daniel J. Lacks Department of Chemical Engineering, Case Western Reserve University,
Carbon and the Molecular Diversity of Life
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity
Carbon and the Molecular Diversity of Life
 Chapter 4 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Carbon and the Molecular Diversity
Chapter 4 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Carbon and the Molecular Diversity
Carbon and the Molecular Diversity of Life
 Chapter 4 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Carbon and the Molecular Diversity
Chapter 4 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Carbon and the Molecular Diversity
SUPPORTING INFORMATION
 Crystallization of calcium carbonate in alginate and xanthan hydrogels Cleo Kosanović 1, Simona Fermani 2, Giuseppe Falini 2, * and Damir Kralj 3, * 1 Meteorological and Hydrological Service, Air Quality
Crystallization of calcium carbonate in alginate and xanthan hydrogels Cleo Kosanović 1, Simona Fermani 2, Giuseppe Falini 2, * and Damir Kralj 3, * 1 Meteorological and Hydrological Service, Air Quality
Carbon and the Molecular Diversity of Life
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity
Size exclusion chromatography of branched polymers: Star and comb polymers
 Macromol. Theory Simul. 8, 513 519 (1999) 513 Size exclusion chromatography of branched polymers: Star and comb polymers Hidetaka Tobita*, Sadayuki Saito Department of Materials Science and Engineering,
Macromol. Theory Simul. 8, 513 519 (1999) 513 Size exclusion chromatography of branched polymers: Star and comb polymers Hidetaka Tobita*, Sadayuki Saito Department of Materials Science and Engineering,
Supporting Information
 Supporting Information Thickness of suspended epitaxial graphene (SEG) resonators: Graphene thickness was estimated using an atomic force microscope (AFM) by going over the step edge from SiC to graphene.
Supporting Information Thickness of suspended epitaxial graphene (SEG) resonators: Graphene thickness was estimated using an atomic force microscope (AFM) by going over the step edge from SiC to graphene.
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Lecture 34 Protein Unfolding Thermodynamics
 Physical Principles in Biology Biology 3550 Fall 2018 Lecture 34 Protein Unfolding Thermodynamics Wednesday, 21 November c David P. Goldenberg University of Utah goldenberg@biology.utah.edu Clicker Question
Physical Principles in Biology Biology 3550 Fall 2018 Lecture 34 Protein Unfolding Thermodynamics Wednesday, 21 November c David P. Goldenberg University of Utah goldenberg@biology.utah.edu Clicker Question
AN AB INITIO STUDY OF INTERMOLECULAR INTERACTIONS OF GLYCINE, ALANINE AND VALINE DIPEPTIDE-FORMALDEHYDE DIMERS
 Journal of Undergraduate Chemistry Research, 2004, 1, 15 AN AB INITIO STUDY OF INTERMOLECULAR INTERACTIONS OF GLYCINE, ALANINE AND VALINE DIPEPTIDE-FORMALDEHYDE DIMERS J.R. Foley* and R.D. Parra Chemistry
Journal of Undergraduate Chemistry Research, 2004, 1, 15 AN AB INITIO STUDY OF INTERMOLECULAR INTERACTIONS OF GLYCINE, ALANINE AND VALINE DIPEPTIDE-FORMALDEHYDE DIMERS J.R. Foley* and R.D. Parra Chemistry
An ESR Study of the Effect of Adsorbed Species on the Radiolysis of Cellulose
 An ESR Study of the Effect of Adsorbed Species on the Radiolysis of Cellulose Matthew O Reilly The Catholic University of America - Chemistry Dept. Senior Comprehensive Paper An ESR Study of the Effect
An ESR Study of the Effect of Adsorbed Species on the Radiolysis of Cellulose Matthew O Reilly The Catholic University of America - Chemistry Dept. Senior Comprehensive Paper An ESR Study of the Effect
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Carbon and Molecular Diversity - 1
 Carbon and Molecular Diversity - 1 Although water is the most abundant compound of living organisms, and the "medium" for the existence of life, most of the molecules from which living organisms are composed
Carbon and Molecular Diversity - 1 Although water is the most abundant compound of living organisms, and the "medium" for the existence of life, most of the molecules from which living organisms are composed
Supporting Information
 Supporting Information Controlled Growth of Ceria Nanoarrays on Anatase Titania Powder: A Bottom-up Physical Picture Hyun You Kim 1, Mark S. Hybertsen 2*, and Ping Liu 2* 1 Department of Materials Science
Supporting Information Controlled Growth of Ceria Nanoarrays on Anatase Titania Powder: A Bottom-up Physical Picture Hyun You Kim 1, Mark S. Hybertsen 2*, and Ping Liu 2* 1 Department of Materials Science
11/14/2016. NaBH 4. Superimposable on mirror image. (achiral) D-ribose. NaBH 4 NaBH 4. Not superimposable on mirror image.
 There are multiple ways to relate the structures of ketoses to aldoses. Reduction of a ketose with NaB 4 forms a new stereocenter and thus forms a mixture of two alditols. An aldopentose forms a chiral
There are multiple ways to relate the structures of ketoses to aldoses. Reduction of a ketose with NaB 4 forms a new stereocenter and thus forms a mixture of two alditols. An aldopentose forms a chiral
Chapter 002 The Chemistry of Biology
 Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Supplementary Figures
 Fracture Strength (GPa) Supplementary Figures a b 10 R=0.88 mm 1 0.1 Gordon et al Zhu et al Tang et al im et al 5 7 6 4 This work 5 50 500 Si Nanowire Diameter (nm) Supplementary Figure 1: (a) TEM image
Fracture Strength (GPa) Supplementary Figures a b 10 R=0.88 mm 1 0.1 Gordon et al Zhu et al Tang et al im et al 5 7 6 4 This work 5 50 500 Si Nanowire Diameter (nm) Supplementary Figure 1: (a) TEM image
Sec. 2.1 Filaments in the cell 21 PART I - RODS AND ROPES
 Sec. 2.1 Filaments in the cell 21 PART I - RODS AND ROPES Sec. 2.1 Filaments in the cell 22 CHAPTER 2 - POLYMERS The structural elements of the cell can be broadly classified as filaments or sheets, where
Sec. 2.1 Filaments in the cell 21 PART I - RODS AND ROPES Sec. 2.1 Filaments in the cell 22 CHAPTER 2 - POLYMERS The structural elements of the cell can be broadly classified as filaments or sheets, where
Ch. 2. Carbon: The Backbone of Life. Organic chemistry is the study of carbon compounds. carbon-based compounds. Molecules of life. cells 70 95% water
 Ch. 2 Chemistry / Water / Carbon BIOL 222 Carbon: The Backbone of Life carbon-based compounds Molecules of life cells 70 95% water rest mostly carbon-based Carbon capable of forming large, complex, and
Ch. 2 Chemistry / Water / Carbon BIOL 222 Carbon: The Backbone of Life carbon-based compounds Molecules of life cells 70 95% water rest mostly carbon-based Carbon capable of forming large, complex, and
Proteins are not rigid structures: Protein dynamics, conformational variability, and thermodynamic stability
 Proteins are not rigid structures: Protein dynamics, conformational variability, and thermodynamic stability Dr. Andrew Lee UNC School of Pharmacy (Div. Chemical Biology and Medicinal Chemistry) UNC Med
Proteins are not rigid structures: Protein dynamics, conformational variability, and thermodynamic stability Dr. Andrew Lee UNC School of Pharmacy (Div. Chemical Biology and Medicinal Chemistry) UNC Med
Catalytic Mechanism of the Glycyl Radical Enzyme 4-Hydroxyphenylacetate Decarboxylase from Continuum Electrostatic and QC/MM Calculations
 Catalytic Mechanism of the Glycyl Radical Enzyme 4-Hydroxyphenylacetate Decarboxylase from Continuum Electrostatic and QC/MM Calculations Supplementary Materials Mikolaj Feliks, 1 Berta M. Martins, 2 G.
Catalytic Mechanism of the Glycyl Radical Enzyme 4-Hydroxyphenylacetate Decarboxylase from Continuum Electrostatic and QC/MM Calculations Supplementary Materials Mikolaj Feliks, 1 Berta M. Martins, 2 G.
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
CHAPTER The calculations are shown for each molecule using values from Table 1.5 in Chapter 1. K eq = B A = B 1-B
 Chapter CPTER. The calculations are shown for each molecule using values from Table.5 in Chapter. 2 C N 2 C 2 N 2 C C-C N 2 C C 2 C C C (a) = CR2 + C = 2. + 0.2 = 2. kcal mol - If + = = NR + G C + G CR2
Chapter CPTER. The calculations are shown for each molecule using values from Table.5 in Chapter. 2 C N 2 C 2 N 2 C C-C N 2 C C 2 C C C (a) = CR2 + C = 2. + 0.2 = 2. kcal mol - If + = = NR + G C + G CR2
3.22 Mechanical Properties of Materials Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 3.22 Mechanical Properties of Materials Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Problem Set
MIT OpenCourseWare http://ocw.mit.edu 3.22 Mechanical Properties of Materials Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Problem Set
et[ailn B:ag;el Research Limited LIBRARY TECHNICAL FILES.
 ja 15 ' 76 et[ailn B:ag;el Research Limited LIBRARY TECHNICAL FILES.,...., rife -+ {-- *b A.- i a e; t V t~ AX ;, A, : d d-. *. -. -1s \ I '~:, - r... -_,-, -...-- _.---.A..h $ I. m THE INSTITUTE OF PAPER
ja 15 ' 76 et[ailn B:ag;el Research Limited LIBRARY TECHNICAL FILES.,...., rife -+ {-- *b A.- i a e; t V t~ AX ;, A, : d d-. *. -. -1s \ I '~:, - r... -_,-, -...-- _.---.A..h $ I. m THE INSTITUTE OF PAPER
1. What is the letter of the alphabet in parentheses that follows EXAM I in the title above? a. a b. b c. c d. d e. e
 HEM 102, EXAM I ( a ) 1. What is the letter of the alphabet in parentheses that follows EXAM I in the title above? a. a b. b c. c d. d e. e 2. Which compound has the most constitutional isomers? a. 2 H
HEM 102, EXAM I ( a ) 1. What is the letter of the alphabet in parentheses that follows EXAM I in the title above? a. a b. b c. c d. d e. e 2. Which compound has the most constitutional isomers? a. 2 H
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Carbon and the Molecular Diversity of Life
 Chapter 4 1 Carbon and the Molecular Diversity of Life PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 4 1 Carbon and the Molecular Diversity of Life PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Phys 450 Spring 2011 Solution set 6. A bimolecular reaction in which A and B combine to form the product P may be written as:
 Problem Phys 45 Spring Solution set 6 A bimolecular reaction in which A and combine to form the product P may be written as: k d A + A P k d k a where k d is a diffusion-limited, bimolecular rate constant
Problem Phys 45 Spring Solution set 6 A bimolecular reaction in which A and combine to form the product P may be written as: k d A + A P k d k a where k d is a diffusion-limited, bimolecular rate constant
Visualizing folding of proteins (1 3) and RNA (2) in terms of
 Can energy landscape roughness of proteins and RNA be measured by using mechanical unfolding experiments? Changbong Hyeon and D. Thirumalai Chemical Physics Program, Institute for Physical Science and
Can energy landscape roughness of proteins and RNA be measured by using mechanical unfolding experiments? Changbong Hyeon and D. Thirumalai Chemical Physics Program, Institute for Physical Science and
BIOLOGY 101. CHAPTER 4: Carbon and the Molecular Diversity of Life: Carbon: the Backbone of Life
 BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
Full wwpdb/emdatabank EM Map/Model Validation Report i
 Full wwpdb/emdatabank EM Map/Model Validation Report i Sep 25, 2018 07:01 PM EDT PDB ID : 6C0V EMDB ID: : EMD-7325 Title : Molecular structure of human P-glycoprotein in the ATP-bound, outwardfacing conformation
Full wwpdb/emdatabank EM Map/Model Validation Report i Sep 25, 2018 07:01 PM EDT PDB ID : 6C0V EMDB ID: : EMD-7325 Title : Molecular structure of human P-glycoprotein in the ATP-bound, outwardfacing conformation
Enzyme function: the transition state. Enzymes & Kinetics V: Mechanisms. Catalytic Reactions. Margaret A. Daugherty A B. Lecture 16: Fall 2003
 Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 Enzyme function: the transition state Catalytic Reactions A B Catalysts (e.g. enzymes) act by lowering the transition state
Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 Enzyme function: the transition state Catalytic Reactions A B Catalysts (e.g. enzymes) act by lowering the transition state
Catalytic Reactions. Intermediate State in Catalysis. Lecture 16: Catalyzed reaction. Uncatalyzed reaction. Enzymes & Kinetics V: Mechanisms
 Enzyme function: the transition state Catalytic Reactions Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 A B Catalysts (e.g. enzymes) act by lowering the transition state
Enzyme function: the transition state Catalytic Reactions Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 A B Catalysts (e.g. enzymes) act by lowering the transition state
BIOCHEMISTRY BIOCHEMISTRY INTRODUCTION ORGANIZATION? MATTER. elements into the order and appearance we now
 BIOCHEMISTRY MR. HULSE BVHS BIOLOGY MATTER Matter - anything that occupies space and has mass Lacked clarity and flow BIOCHEMISTRY INTRODUCTION Biochemistry study of chemical and physiological process
BIOCHEMISTRY MR. HULSE BVHS BIOLOGY MATTER Matter - anything that occupies space and has mass Lacked clarity and flow BIOCHEMISTRY INTRODUCTION Biochemistry study of chemical and physiological process
Exercises for Windows
 Exercises for Windows CAChe User Interface for Windows Select tool Application window Document window (workspace) Style bar Tool palette Select entire molecule Select Similar Group Select Atom tool Rotate
Exercises for Windows CAChe User Interface for Windows Select tool Application window Document window (workspace) Style bar Tool palette Select entire molecule Select Similar Group Select Atom tool Rotate
Models for Single Molecule Experiments
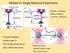 Models for Single Molecule Experiments Binding Unbinding Folding - Unfolding -> Kramers Bell theory 1 Polymer elasticity: - Hooke s law (?) - FJC (Freely joined chain) - WLC (Worm-like chain) 3 Molecular
Models for Single Molecule Experiments Binding Unbinding Folding - Unfolding -> Kramers Bell theory 1 Polymer elasticity: - Hooke s law (?) - FJC (Freely joined chain) - WLC (Worm-like chain) 3 Molecular
