The Hei and HeH Photoelectron Spectra of [Fe*?3-C3H5(CO)3X] and [Fe^3-C4H7 (CO) 3 X] ( X = Cl, Br, I) and [CoC3H5(CO)3]
|
|
|
- Leonard Hines
- 5 years ago
- Views:
Transcription
1 The Hei and HeH Photoelectron Spectra of [Fe*?3-C3H5(CO)3X] and [Fe^3-C4H7 (CO) 3 X] ( X = Cl, Br, I) and [CoC3H5(CO)3] Jaap N. Louwen, Jaap Hart, Derk J. Stufkens, and A d Oskam* Anorganisch Chemisch Laboratorium, University of Amsterdam, J. H. van't Hoff Instituut, Nieuwe Achtergracht 166, 1018 W V Amsterdam, The Netherlands Z. Naturforsch. 37b, (1982); received September 29, 1981 Photoelectron Spectra, Iron, Cobalt, Allyl Complexes By means of UV photoelectron spectroscopy (UPS) the electronic structures of [Fe^3.C3H5(CO)3X], [Fe^3-(2-CH3C3H4)(CO)3X] (X = Cl, Br, I) and [Co(C3H5)(CO)3] have been studied. Detailed assignments are possible and surprisingly low ionization energies (as low as 8.19 ev) are found for iodine lone pair type Orbitals. From the spectra a large difference in n backbonding is found between the cobalt and iron complexes. Introduction Allyl complexes, as a class have been known since the late 1950's. They have been extensively studied, mainly because of their relevance to catalytic problems and the fluxionahty most complexes exhibit. Theoretical efforts, both semiemperical [1, 2] and ab initio calculations [3], have mainly been directed towards the platinum metal bis allyl complexes. A number of U V photoelectron spectroscopy (UPS) studies have been devoted to these systems [4^6]. Definite assignments have been given b y Böhm, Gleiter and Batich on the basis of He I/He I I intensity ratios, substitution effects and I N D O calculations, combined with a Greens function approach [2]. Orbitals belonging to ligand type ionization bands are complex combinations of fragment Orbitals. Other UPS studies on allyl complexes are that of Seddon and Green on some Or and Mo complexes [7], where detailed assignments were not possible due to strong overlapping of inonizations bands, and the work of Clark and Green, as well as Van Dam et al., on some bis cyclopentadiene allyl complexes of Ta and N b [8]. The latter studies yielded ionization potentials of about 7.8 ev for the non bonding allyl TI ionization but the position of the bonding TI Orbitals could not be determined with certainty. Thus, as a part of studies performed in this group upon the coordination of unsaturated systems to transition metals [9, 10], we were prompted to investigate a few fairly simple allyl complexes of Fe * Reprint requests to Prof. Dr. Ad Oskam /81/ /$ 01.00/0 and Co. With these complexes detailed assignments are possible since the presence of only one allyl hgand excludes mixing of orbitals on different allyl fragments and, moreover, the other ligands either have low intensity ionization bands in H e l l (the halogens) or do not appear within the upper valence region (CO). Furthermore, comparing the Co and Fe complexes might be instructive with regard to the 7i backbonding capabilities of the allyl ligand, the subject of some discussion [1]. The results should also be of interest because of the possible chemical consequences. For instance, the FeC3Hs(CO)3 moiety survives reduction from Fe(II) to Fe(I) and hence [Fe(C3H5)(C0)3X] is a good starting compound for synthesizing Fe-metal bonds [11, 12]. Also, from a practical point of view, these complexes can easily be sublimed and, at least for the iron complexes, it is known from the literature [13] that no decomposition is to be expected at the temperatures applied ( ~ 5 0 C). Experimental Syntheses were performed by literature methods, for [FeC 3 H5(C0) 3 Cl], [FeC 4 H 7 (C0) 3 Cl], and [FeC3H5(CO)3Br] by reacting the corresponding allyl halide with [Fe 2 (CO) 9 ] [14], for [FeC 3 H 5 (CO) 3 I], [FeC 4 H 7 (CO) 3 I] and [FeC 4 H 7 (CO) 3 I] b y stirring one of the above complexes with the appropriate sodium halide in acetone for 40 min [14], and for the cobalt complex by the action of allyl bromide on [NaCo(CO) 4 ] [15]. Identity and purity were checked b y I R and NMR spectroscopy. U P spectra were recorded on a Perkin-Elmer PS 18 photoelectron spectrometer, equipped with a He I/He II light source. Ar and X e were used as internal calibrants, together with the signal arising from the autoionization of He by H e l l photons. The spectra are given uncorrected for analyzer dependency, which must be taken into account in Dieses Werk wurde im Jahr 2013 vom Verlag Zeitschrift für Naturforschung in Zusammenarbeit mit der Max-Planck-Gesellschaft zur Förderung der Wissenschaften e.v. digitalisiert und unter folgender Lizenz veröffentlicht: Creative Commons Namensnennung 4.0 Lizenz. This work has been digitalized and published in 2013 by Verlag Zeitschrift für Naturforschung in cooperation with the Max Planck Society for the Advancement of Science under a Creative Commons Attribution 4.0 International License.
2 180 J. N. Louwen et al. The He I and H e l l Photoelectron Spectra of [Fer?3-C3H5(CQ)3X] 180 comparing intensity of bands separated by a large energy interval. General Considerations Concerning the Spectra Before discussing the spectra, an inventory of the possible ionizations and several assignment criteria may be set out. oc-^fe* Two other assignment criteria can be used to discriminate between the bonding and nonbonding allyl TI ionizations. Most important is the effect of substitution of a methyl group at the middle C atom of the allyl. This will cause the bonding n orbital to shift considerably, due to the fact that this orbital has its main density on this central C atom while the nonbonding orbital (having a node at this atom) is only inductively affected (as are the other orbitals in the molecule). Fig. 1. Structure of the iron compounds. The molecular geometry of the iron complexes is presented in Fig. 1. The compounds formally exist of an iron 2 + ion (a 3d 6 closed shell configuration) with a halide, an allyl univalent negative ion and three CO ligands within its coordination sphere. From the iron three 3d-type ionizations are to be expected. It is well known that ionizations from transition metal 3 d orbitals show considerable enhancement on going from He I to He II ionization [16]. The halogens will yield three lone pair p type ionizations, two perpendicular to the F e - X bond being nearly degenerate, and one along this bond. In the case of high localization one expects these three ionizations to have high intensity in He I and hardly any in H e l l. The higher CO ionizations will not appear in the valence region, but will be visible, however, in the same region as the higher allyl sigma orbitals (between 12 and 17 ev). Because of their oxygen 2 p participation, ionizations from higher CO orbitals will be enhanced in intensity in the H e l l spectra, especially relative to thectchionizations which are predicted to have lower H e l l intensity. Of utmost importance are the n orbitals on the allyl part and the corresponding ionizations. The three n orbitals are depicted schematically in Fig. 2. The top one, the antibonding orbital, is essentially unfilled and only of importance in connection with possible backbonding towards the allyl moiety. The other two are both formally doubly occupied. Their corresponding ionization bands are characterized b y a H e l l intensity which is considerably lower than that of metal 3 d type ionizations, although mixing with metal d orbitals could result in some enhancement of H e l l intensity. Fig. 2. A plot of the three allyl 71 orbitals. From top to bottom: the antibonding 71* orbital, the non-bonding n and the bonding ji orbitals. Vibrational coupling might also be of some help to dicriminate between the two allyl n ionizations. The TI bonding orbital should have more vibrational coupling with the allyl system than the nonbonding one and this will result in a broader ionization band. The cobalt complex with a formal Co + (3d 8 ) configuration on the metal atom can be treated analogously and needs no further discussion. One further remark has to be made. It is known that these complexes exhibit fluxional behaviour in solution and that throughout the iron series the rates of equilibrium between the endo and exo forms in solution differ to a large extent [17]. In the gas phase, a corresponding behaviour could possibly be reflected in the U P spectra. However, no abnormal broadening of bands, nor differences throughout the series have been observed. Probably, as has been
3 J. N. Louwen et al. The He I and Hell Photoelectron Spectra of [Fer? 3 -C3H5(CQ)3X] 181 noted in similar cases [8], such energy differences are too small to be detected in UPS. J FeCsH8(CO)sCl The Spectra Ionization potentials of the iron and cobalt complexes are given in Table I. [Feri*-CzHb(CO)*Cl] and [Fer)*-CJh(CO)sCl] The spectra depicted in Figs. 3a and 3 b are very much alike, with the exception of the extra a type ionizations around 13.5 ev in the spectrum of [FeC4H7(CO)3Cl] and the band at ev in the spectrum of [FeC3H5(CI)3Cl] which merges with the band at ev in the spectrum of the 2-methyl derivative. At the low energy side of the spectrum one first discerns two ionization bands which clearly enhance in intensity on going from He I to Hell. In view of the criteria outlined in the previous section, these bands have to be assigned to the three 3d type ionizations. Because of its intensity the first band is assigned to ionizations from a degenerate pair of orbitals. The second one, belonging to a single ionization exhibits, in these and the other spectra, stronger enhancement than the first band. Apparently, it is connected with the more loealizedd-orbital. To the higher energy side of these bands are found two sharp intense bands which almost disappear in the Hell spectrum. Undoubtedly these bands belong to ionizations of the halogen lone pair orbitals. From the spectra the band at lower energy seems to belong to the single ionization (metalchlorine bond), a deduction supported by the fact that this band does have some Hell intensity. The two remaining bands in the upper valence region can now easily be assigned to the two allyl He( II) I 20eV Fig. 3a. The Hel/Hell spectrum of [FeC3H5(CO)3Cl]. FeC4H7(C0)3Cl H«KII) i i r 3b. The Hel/HeH spectrum of [FeC4H7(CO)3Cl]. Tab. I. Ionization potentials in electron volts (d = double). Compound Metal 3d Halogen Allyl n.b. b. ffch CO [Fe(CO)3C3H5Cl] 8.96(d) ~ [Fe(CO)3C4H7Cl] 9.15(d) [Fe(CO)3C3H5Br] 8.69(d) [Fe(CO)3C4H7Br] 8.89(d) [Fe(CO)3C3H5I] 9.46(d) ~ [Fe(CO)3C4H7I] 9.15(d) [CO(CO)3C3H5]
4 182 J. N. Louwen et al. The He I and H e l l Photoelectron Spectra of [Fer?3-C3H5(CQ)3X] 182 n ionizations. The two bands can be distinguished by methyl substitution. In [Fe^a-CaHsCCOJaCl] the band at ev, which is hardly affected upon methyl substitution (11.17 ev), must be connected with the nonbonding 7i orbital while the other one at ev is clearly derived from the n bonding orbital because of its large shift to ev in [ F e ^ - O ^ C O ^ C l ]. The remainder of the spectra is complicated, though some features can be discerned. At ~ 13.5 ev some bands are observed which lose much intensity in H e l l. These are assigned to C - H a type ionizations, an assignment which is supported by the He I/He II intensity ratio and b y the fact that methyl substitution seems to add extra ionizations to this region. Next to these can be observed carbon monoxide localized ionizations which are strongly enhanced in intensity in H e l l, due to the oxygen 2 p participation. These have a maximum around 15 ev. 4b. The He I/He II spectrum of [FeC4H7(CO)3Br]. In contrast, the metal 3d-region and the halogen region are now less separated and the single Fe 3 d The spectra of [FeC3Hb(CO)zBr] and ionization band is now visible as either a shoulder [FeCJI^CO^Br] or even a slope on a Br ionization band. In many respects these spectra, as shown in In the H e l l spectrum this band again exhibits Figs. 4 a and 4 b, are similar to those of the chlorine the most dramatic enhancement which results in a compounds. However, in the spectra of the methyl considerable shift of the overall maximum of the substituted derivative the allyl TI ionization bands second band on going from He I to H e l l. do not coincide since the separation between the Because of the strong overlapping the Br lone corresponding ionization energies of the C3H5 compair type band at highest energy can only be plex has now increased, mainly due to destabilizatentatively assigned to an ionization from the single tion of the non bonding Tt orbital. orbital; this would infer a reverse ordering with respect to the Cl compounds. The other main features, i.e. the strong shift of the bonding allyl ionization band upon methyl substitution and the position of the a and CO type ionizations are the same as in the spectra of the Cl complexes. The spectra of[fec3h5(co)zi] [FeC*Hi(CO)zI] Fig. 4a. The Hel/Hell spectrum of [FeC3H5(CO)3Br]. and These spectra are to be found in Figs. 5 a and 5 b. With respect to the spectra of the Cl and Br compounds one now observes a dramatic change in shape. The halogen lone pair ionization bands identified, as are the 3 d orbitals, b y their He I/He II intensity behaviour, have moved to a very low energy indeed (8.36/8.60 ev and 8.19/8.49 ev for the allyl and methyl allyl compounds respectively). The Fe 3 d ionizations are now found at higher en-
5 J. N. Louwen et al. The He I and Hell Photoelectron Spectra of [Fer? 3 -C3H5(CQ)3X] 183 ergies, in contrast to what might have been expected from the inductive effect of iodine compared to that of bromine and chlorine. He(II) ~T" T~ ~20 ev Fig. 5a. The Hel/Hell spectrum of [FeC3H5(CO)3I]. FeC 4 H 7 (C0) 3 Rather, the unexpected shifting of the 3d bands and the very low iodine ionization energies are the result of a different interaction between iodine and metal levels. If one considers the molecule [FeC 3 H5(CO)3X] as consisting of the ions FeC3Hs(CO)3 + and X - we can view the ionizations, especially of Fe 3d and X np electrons, as arising from levels which are combinations of levels on these two fragments. Clearly, upon substituting the halogen the FeC3Hs(CO)3 + levels do not vary, while the X~ levels do. By combining levels, we lower the lowest and raise the upper one. It is now possible to understand the shifts upon iodine substitution by assuming that the I~ levels are above those of FeC3H5(CO)3+ (while the Cl" and Br- levels are below) and this results in extra raising of the I levels, with lowering of Fe 3d levels. The absence of spin-orbit coupling might be surprising. However, because of the low local symmetry of the I~ ion quenching is to be expected, furthermore the possibility of accidently coincident I and Fe 3d levels cannot be fully excluded. The allyl n bands, the a type ionizations and the CO bands are found at the same energies as before, except for the non-bonding allyl n ionization which has again been destabilized. The Hel and Hell spectra of [CoCzHb(CO)z] By comparison with the spectra of the iron complexes, that of the cobalt complex can readily He(II) i i i I T ev 5b. The Hel/Hell spectrum of [FeC4H7(CO)3I]. The possibility that these shifts are due to changes in the molecular structure can be ruled out with some confidence. Crystal structures of the Br and I complex have been published [12] and, although the published structure for the I complex is unfortunately not of particularly high quality, it may be concluded that the structures of the bromine and the iodine complexes do not show basic differences. He<II) 18 I 20 «V Fig. 6. The Hel/Hell spectrum of [CoC3H5(CO)3].
6 184 J. N. Louwen et al. The He I and H e l l Photoelectron Spectra of [Fer? 3 -C 3 H 5 (CQ)3X] 184 be assigned. The first four strongly overlapping bands must be considered as mainly due to Co 3 d ionizations because of their intensity behaviour. Next, one finds the two allyl n bands and the complex grouping of and CO bands. The allyl bands have been considerably destabilized with respect of the corresponding Fe complexes. The maximum of the CO band (at ~ 14 ev) has also shifted about 1 ev, and the CO ionization bands now almost obscure those of the a-type ionizations. Both destabilizations are in agreement with the difference in formal charge on the metal, mainly due to the absence of a halogen atom. Clearly there exists more n backbonding towards the CO ligands in this Co complex than in the Fe complexes. It is therefore of interest that the H e l l intensity of the first band of the spectrum is less than that of the other three d-type ionizations. Evidently this points to mixing of orbitals from the coordinating groups to provide backbonding. There exists, however, no indication concerning the amount of jr-backbonding to the allyl ligand. From the position of the bonding allyl band we can infer that the charge density on the allyl moiety is higher than in the case of the iron complex, though this can be explained b y the decrease in a-donation. An indication of the importance of allyl n backbonding could have been inferred from a possible shift of the first 3 d band upon methyl substitution. Up to now, however, attempts to record the spectrum of the methylated compound have failed. Discussion Within the iron series, donation of charge from the allyl towards the metal is apparent. The energy of the nonbonding allyl n orbital is strongly influenced by the electronegativity of the halogen atom in [FeC3H 5 (CO)3X], whereas the bonding one is not. Apart from the position of the n bonding ionization, the spectrum is not very much influenced b y methyl substitution. The shape of the n* orbital, however, would suggest that any orbital containing some [1] D. A. Brown and A. Owens, Inorg. Chim. Acta 5, 67 (1971). [2] M. C. Böhm and R. Gleiter, Theor. Chim. Acta 57, 315 (1980). [3] M.-M. Rohmer, J. De Muynck, and A. Veillard, n* character, would be destabilized (see Fig. 2). Thus we may conclude that throughout the iron series the allyl-metal interaction is mainly a donating one. The position of the nonbonding orbital seems to be directly influenced b y the charge on the metal, rather than that on the allyl part. This follows from the fact that upon changing the halogen atom in the iron compounds the separation between the non bonding and bonding bands varies. The bonding orbital remains, in fact, at much the same position. The question might be raised as to whether the nonbonding allyl n orbital can be visualized as a combination of two atomic 2 p orbitals. Maybe the actual molecular orbital on the allyl part is more diffuse, which would be in agreement with the results of extended basis GTO-SCF calculations performed on SO2 [18]. As only one cobalt complex has been measured, it is not possible to draw many conclusions. However, it is clear that the actual charge on Co is higher than that on Fe, and backbonding has become an important factor. The allyl orbitals are destabilized with respect to the iron series, due to the excess of charge. It is, unfortunately, not clear whether this results from an increase of backbonding to the allyl part or from a decrease of donation towards the metal. A comparison with the spectra of the bis allyl platinum metal systems [6] is of interest. The results from those studies agree with this one, especially with regard to the energy of the n bonding ionization and the shifts of the allyl ionization energies upon methyl substitution. An important difference is the position of the band, in the region ev throughout the platinum triad, assigned to ionization from a pure combination of allyl nonbonding 71 orbitals. The difference with the ionization energies found for the non bonding Tt orbital in the systems studied can be explained by the interaction of the allyl levels with the metal orbitals of higher energy. Mr. A. Terpstra is thanked for helpful discussions. Theor. Chim. Acta 36, 93 (1973) and references cited therein. [4] D. R. Lloyd and N. Lynaugh, in D. E. Shirley (ed.): Electron Spectroscopy, Proc. of an Int. Conf., Amsterdam, North Holland 1972.
7 J. N. Louwen et al. The He I and Hell Photoelectron Spectra of [Fer? 3 -C3H5(CQ)3X] 185 [5] C. D. Batich, J. Am. Chem. Soc. 98, 7585 (1976). [6] M. C. Böhm, R. Gleiter, and C. D. Batich, Helv. Chim. Acta 63, 990 (1980). [7] J. C. Green and E. A. Seddon, J. Org. Met. Chem. 198, C 61 (1980). [8] a) J. P. Clark and J. C. Green, J. Less Comm. Met. 1977, 31; b) H. van Dam, A. Terpstra, A. Oskam, and J. H. Teuben, Z. Naturforsch. 36b, 420 (1981). [9] H. van Dam and A. Oskam, J. Electron Spectrosc., Relat. Phenom. 13, 273 (1978). [10] H. van Dam, J. N. Louwen, A. Oskam, M. Doran, and I. H. Hillier, ibid. 21, 57 (1980). [11] C. F. Putnik, J. J. Wetter, G. D. Stucky, M. J. D'Aniello, B. A. Sosinsky, J. F. Kirner, and E. L. Mutterties, J. Am. Chem. Soc. 100, 4107 (1978). [12] F. E. Simon and J. W. Lauher, Inorg. Chem. 19, 2338 (1980). [13] A. N. Nesmeyanow, Ju. A. Ustynyuh, I. I. Kritshuya, and J. A. Shchembelov, J. Org. Met. Chem. 14, 395 (1968). [14] H. D. Murdoch and E. Weiss, Helv. Chim. Acta 45, 225 (1962). [15] R. F. Heck and D. S. Breslow, J. Am. Chem. Soc. 83, 1097 (1961). [16] H. van Dam and A. Oskam, Review Transition Metal Chemistry, M. Dekker Inc. Ed. B. N. Figgis, Volume [17] J. W. Faller and M. A. Adams, J. Org. Met. Chem. 170, 71 (1979). [18] J. N. Louwen and A. Oskam, unpublished results.
He(I) and He(II) Photoelectron Spectra of M[Co(CO)4]2 and M[Mn(CO)5]2 Complexes (M = Zn, Cd, and Hg)
![He(I) and He(II) Photoelectron Spectra of M[Co(CO)4]2 and M[Mn(CO)5]2 Complexes (M = Zn, Cd, and Hg) He(I) and He(II) Photoelectron Spectra of M[Co(CO)4]2 and M[Mn(CO)5]2 Complexes (M = Zn, Cd, and Hg)](/thumbs/96/126783682.jpg) He(I) and He(II) Photoelectron Spectra of M[Co(CO)4]2 and M[Mn(CO)5]2 Complexes (M = Zn, Cd, and Hg) Jaap N. Louwen, Ronald R. Andréa, Derk J. Stufkens, and Ad Oskam* Anorganisch Chemisch Laboratorium,
He(I) and He(II) Photoelectron Spectra of M[Co(CO)4]2 and M[Mn(CO)5]2 Complexes (M = Zn, Cd, and Hg) Jaap N. Louwen, Ronald R. Andréa, Derk J. Stufkens, and Ad Oskam* Anorganisch Chemisch Laboratorium,
UV Absorption Spectra of Methyl, Cyclopropyl Ketones. Jabria A. Al-Khafaji and Muthana Shanshal
 V Absorption Spectra of Methyl, Cyclopropyl Ketones Jabria A. Al-Khafaji and Muthana Shanshal Department of Chemistry, College of Science, niversity of Baghdad, Adhamiya, Baghdad, Iraq (Z. Naturforsch.
V Absorption Spectra of Methyl, Cyclopropyl Ketones Jabria A. Al-Khafaji and Muthana Shanshal Department of Chemistry, College of Science, niversity of Baghdad, Adhamiya, Baghdad, Iraq (Z. Naturforsch.
Enthalpies of Mixing for Binary Liquid Mixtures of Monocarbonic Acids and Alcohols
 Enthalpies of Mixing for Binary Liquid Mixtures of Monocarbonic Acids and Alcohols R. H a a s e and R. L o r e n z Institut für Physikalische Chemie, RheinischWestfälische Technische Hochschule, Aachen,
Enthalpies of Mixing for Binary Liquid Mixtures of Monocarbonic Acids and Alcohols R. H a a s e and R. L o r e n z Institut für Physikalische Chemie, RheinischWestfälische Technische Hochschule, Aachen,
Ternary Intercalation Compound of Graphite with Aluminum Fluoride and Fluorine
 Ternary Intercalation Compound of Graphite with Aluminum Fluoride and Fluorine Tsuyoshi Nakajima, Masayuki Kawaguchi, and Nobuatsu Watanabe* Department of Industrial Chemistry, Faculty of Engineering,
Ternary Intercalation Compound of Graphite with Aluminum Fluoride and Fluorine Tsuyoshi Nakajima, Masayuki Kawaguchi, and Nobuatsu Watanabe* Department of Industrial Chemistry, Faculty of Engineering,
Hyperfine Structure in the Rotational Spectrum of Chloroacetonitrile
 Hyperfine Structure in the Rotational Spectrum of Chloroacetonitrile Ilona Merke and Helmut Dreizler Abteilung Chemische Physik im Institut für Physikalische Chemie der Universität Kiel Z. Naturforsch.
Hyperfine Structure in the Rotational Spectrum of Chloroacetonitrile Ilona Merke and Helmut Dreizler Abteilung Chemische Physik im Institut für Physikalische Chemie der Universität Kiel Z. Naturforsch.
Dielectric Study of the Ferroelectric Phase Transition in DMAGaS Crystal
 Dielectric Study of the Ferroelectric Phase Transition in DMAGaS Crystal R. Tchukvinskyi, R. Cach, and Z. Czapla Institute of Experimental Physics, University of Wroclaw, M. Borna 9, 50-204 Wroclaw, Poland
Dielectric Study of the Ferroelectric Phase Transition in DMAGaS Crystal R. Tchukvinskyi, R. Cach, and Z. Czapla Institute of Experimental Physics, University of Wroclaw, M. Borna 9, 50-204 Wroclaw, Poland
Dielectric Spectroscopy of Some Multihydroxy Compound Solutions in Water/Tetrahydrofuran Mixtures
 Dielectric Spectroscopy of Some Multihydroxy Compound Solutions in Water/Tetrahydrofuran Mixtures F. F. Hanna, A. L. G. Saad, A. H. Shafik, R. Biedenkap 3, and M. Stockhausen 3 National Research Centre,
Dielectric Spectroscopy of Some Multihydroxy Compound Solutions in Water/Tetrahydrofuran Mixtures F. F. Hanna, A. L. G. Saad, A. H. Shafik, R. Biedenkap 3, and M. Stockhausen 3 National Research Centre,
Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals.
 Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Introduction. Experimental. The early measurements have been performed at 1.81 T by a multi nuclei frequency-swept CW-spectrometer
 4 Scandium NMR Investigations in Aqueous Solutions E. Haid, D. Köhnlein, G. Kössler, O. Lutz*, W. Messner, K. R. Mohn, G. Nothaft, B. van Rickelen, W. Schich, and N. Steinhauser Physikalisches Institut
4 Scandium NMR Investigations in Aqueous Solutions E. Haid, D. Köhnlein, G. Kössler, O. Lutz*, W. Messner, K. R. Mohn, G. Nothaft, B. van Rickelen, W. Schich, and N. Steinhauser Physikalisches Institut
NQR and NMR Studies of Phase Transitions in R 2 P b [ C u ( N 0 2 ) 6 l (R = K, Rb, Tl, Cs, and NH 4 ) *
 NQR and NMR Studies of Phase Transitions in R 2 P b [ C u ( N 0 2 ) 6 l (R = K, Rb, Tl, Cs, and NH 4 ) * M o t o h i r o M i z u n o 2, M a s a h i k o S u h a r a 3, Tetsuo A s a j i b, and Yoshihiro
NQR and NMR Studies of Phase Transitions in R 2 P b [ C u ( N 0 2 ) 6 l (R = K, Rb, Tl, Cs, and NH 4 ) * M o t o h i r o M i z u n o 2, M a s a h i k o S u h a r a 3, Tetsuo A s a j i b, and Yoshihiro
Constructing a MO of NH 3. Nitrogen AO symmetries are
 Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Chapter 9 Ionic and Covalent Bonding
 Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry. Zachary Chin, Alex Li, Alex Liu
 A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
2. Which of the following salts form coloured solutions when dissolved in water? I. Atomic radius II. Melting point III.
 1. Which pair of elements reacts most readily? A. Li + Br 2 B. Li + Cl 2 C. K + Br 2 D. K + Cl 2 2. Which of the following salts form coloured solutions when dissolved in water? I. ScCl 3 II. FeCl 3 III.
1. Which pair of elements reacts most readily? A. Li + Br 2 B. Li + Cl 2 C. K + Br 2 D. K + Cl 2 2. Which of the following salts form coloured solutions when dissolved in water? I. ScCl 3 II. FeCl 3 III.
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Application of the Pseudo-Spin Model to the Lowest-Temperature Phase Transition of the Mixed Crystal (NH 4 ) 2(1 _ x) K 2x Pb[Cu(N0 2 ) 6 ]
![Application of the Pseudo-Spin Model to the Lowest-Temperature Phase Transition of the Mixed Crystal (NH 4 ) 2(1 _ x) K 2x Pb[Cu(N0 2 ) 6 ] Application of the Pseudo-Spin Model to the Lowest-Temperature Phase Transition of the Mixed Crystal (NH 4 ) 2(1 _ x) K 2x Pb[Cu(N0 2 ) 6 ]](/thumbs/87/96822495.jpg) Application of the Pseudo-Spin Model to the Lowest-Temperature Phase Transition of the Mixed Crystal (NH 4 ) 2(1 _ x) K 2x Pb[Cu(N0 2 ) 6 ] Wolfgang Windsch and Horst Braeter Fachbereich Physik, Universität
Application of the Pseudo-Spin Model to the Lowest-Temperature Phase Transition of the Mixed Crystal (NH 4 ) 2(1 _ x) K 2x Pb[Cu(N0 2 ) 6 ] Wolfgang Windsch and Horst Braeter Fachbereich Physik, Universität
Two-Dimensional NQR Spectroscopy for the Characterization of Crystalline Powders
 Two-Dimensional NQR Spectroscopy for the Characterization of Crystalline Powders P. K ä u p e r 1, V. Göbbels, P. Blümler, a n d B. Blümich Lehrstuhl für Makromolekulare Chemie und Zentrum für Magnetische
Two-Dimensional NQR Spectroscopy for the Characterization of Crystalline Powders P. K ä u p e r 1, V. Göbbels, P. Blümler, a n d B. Blümich Lehrstuhl für Makromolekulare Chemie und Zentrum für Magnetische
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
TT o. Infrared Microwave Double-Resonance Investigations on Trifluoromethyljodide (CF 3 I) a) Experimental. E. Ibisch and U. Andresen. I.
 nfrared Microwave DoubleResonance nvestigations on Trifluoromethyljodide (CF 3 ) E. bisch and U. Andresen Abteilung Chemische Physik im nstitut für Physikalische Chemie der Universität Kiel Z. Naturforsch.
nfrared Microwave DoubleResonance nvestigations on Trifluoromethyljodide (CF 3 ) E. bisch and U. Andresen Abteilung Chemische Physik im nstitut für Physikalische Chemie der Universität Kiel Z. Naturforsch.
Lattice Dynamics of Molybdenum and Chromium
 Lattice Dynamics of Molybdenum and Chromium B. P. Singh, L. P. Pathak, and M. P. Hemkar Department of Physics, University of Allahabad, Allahabad-211002, India Z. Naturforsch. 35a, 230-235 (1980); received
Lattice Dynamics of Molybdenum and Chromium B. P. Singh, L. P. Pathak, and M. P. Hemkar Department of Physics, University of Allahabad, Allahabad-211002, India Z. Naturforsch. 35a, 230-235 (1980); received
Molecular Orbital Theory This means that the coefficients in the MO will not be the same!
 Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Degree of Ionization of a Plasma in Equilibrium *
 Degree of Ionization of a Plasma in Equilibrium * G. ECKER a n d W. KRÖLL Institut für Theoretische Physik der Universität Bochum * * ( Z. Naturforschg. 1 a, 0 3 0 7 [ 1 9 6 6 ] ; received 0 August 1 9
Degree of Ionization of a Plasma in Equilibrium * G. ECKER a n d W. KRÖLL Institut für Theoretische Physik der Universität Bochum * * ( Z. Naturforschg. 1 a, 0 3 0 7 [ 1 9 6 6 ] ; received 0 August 1 9
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics
 Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Rethinking Hybridization
 Rethinking Hybridization For more than 60 years, one of the most used concepts to come out of the valence bond model developed by Pauling was that of hybrid orbitals. The ideas of hybridization seemed
Rethinking Hybridization For more than 60 years, one of the most used concepts to come out of the valence bond model developed by Pauling was that of hybrid orbitals. The ideas of hybridization seemed
Bonding and Physical Properties The Molecular Orbital Theory
 Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
7.1 Ions > Chapter 7 Ionic and Metallic Bonding. 7.1 Ions. 7.2 Ionic Bonds and Ionic Compounds 7.3 Bonding in Metals
 Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and Ionic Compounds 7.3 Bonding in Metals 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY & YOU What
Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and Ionic Compounds 7.3 Bonding in Metals 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY & YOU What
 CHAPTER 6 CHEMICAL BONDING SHORT QUESTION WITH ANSWERS Q.1 Dipole moments of chlorobenzene is 1.70 D and of chlorobenzene is 2.5 D while that of paradichlorbenzene is zero; why? Benzene has zero dipole
CHAPTER 6 CHEMICAL BONDING SHORT QUESTION WITH ANSWERS Q.1 Dipole moments of chlorobenzene is 1.70 D and of chlorobenzene is 2.5 D while that of paradichlorbenzene is zero; why? Benzene has zero dipole
Essential Organic Chemistry. Chapter 1
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 1 Electronic Structure and Covalent Bonding Periodic Table of the Elements 1.1 The Structure of an Atom Atoms have an internal structure consisting
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 1 Electronic Structure and Covalent Bonding Periodic Table of the Elements 1.1 The Structure of an Atom Atoms have an internal structure consisting
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Coordination Chemistry: Bonding Theories. Molecular Orbital Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Kinetic Study of the Acid Hydrolysis of Ethyl Hydrogen Succinate in Binary Solvent Mixtures
 Kinetic Study of the Acid Hydrolysis of Ethyl Hydrogen Succinate in Binary Solvent Mixtures Fayez Y. Khalil and M. T. Hanna Chemistry Department, Faculty of Science, Alexandria University, Alexandria,
Kinetic Study of the Acid Hydrolysis of Ethyl Hydrogen Succinate in Binary Solvent Mixtures Fayez Y. Khalil and M. T. Hanna Chemistry Department, Faculty of Science, Alexandria University, Alexandria,
Chem 101 General Chemistry Practice Final Exam
 Name h = 6.626 x 10-34 J s (Planck s Constant) c = 3.00 x 10 8 m/s (speed of light) R H = 1.097 x 10-7 m -1 (Rydberg Constant) Chem 101 General Chemistry Practice Final Exam Multiple Choice (5 points each)
Name h = 6.626 x 10-34 J s (Planck s Constant) c = 3.00 x 10 8 m/s (speed of light) R H = 1.097 x 10-7 m -1 (Rydberg Constant) Chem 101 General Chemistry Practice Final Exam Multiple Choice (5 points each)
in Halogen-Bonded Complexes
 9 Resonance Assistance and Cooperativity in Halogen-Bonded Complexes Previously appeared as Covalency in Resonance-Assisted Halogen Bonds Demonstrated with Cooperativity in N-Halo-Guanine Quartets L. P.
9 Resonance Assistance and Cooperativity in Halogen-Bonded Complexes Previously appeared as Covalency in Resonance-Assisted Halogen Bonds Demonstrated with Cooperativity in N-Halo-Guanine Quartets L. P.
Chemistry Instrumental Analysis Lecture 11. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 11 Molar Absorptivities Range 0 to 10 5 Magnitude of e depends on capture cross section of the species and probability of the energy-absorbing transition. e
Chemistry 4631 Instrumental Analysis Lecture 11 Molar Absorptivities Range 0 to 10 5 Magnitude of e depends on capture cross section of the species and probability of the energy-absorbing transition. e
Dimerization of Carboxylic Acids in Solution up to High Pressures and Temperatures. 2. Benzoic Acid
 Dimerization of Carboxylic Acids in Solution up to High Pressures and Temperatures. 2. Benzoic Acid R. Barraza*, E. M. Borschel**, and M. Buback** * Departamento de Quimica, Faeultad de Ciencias, Universidad
Dimerization of Carboxylic Acids in Solution up to High Pressures and Temperatures. 2. Benzoic Acid R. Barraza*, E. M. Borschel**, and M. Buback** * Departamento de Quimica, Faeultad de Ciencias, Universidad
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Solution Structures of Pyrophthalones, I Structure and Conformation of l,3-indandionato-2(2-pyridinium)-betai'n a 1 H/ 13 C NMR Approach
 Solution Structures of Pyrophthalones, I Structure and Conformation of l,3-indandionato-2(2-pyridinium)-betai'n a 1 H/ 13 C NMR Approach Erhard T. K. Haupt, Heindirk tom Dieck* Institut für Anorganische
Solution Structures of Pyrophthalones, I Structure and Conformation of l,3-indandionato-2(2-pyridinium)-betai'n a 1 H/ 13 C NMR Approach Erhard T. K. Haupt, Heindirk tom Dieck* Institut für Anorganische
All chemical bonding is based on the following relationships of electrostatics: 2. Each period on the periodic table
 UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
 CHAPTER 6 CHEMICAL BONDING TEXT BOOK EXERCISE Q.1. Select the correct statement. i. An ionic compound A + B - is most likely to be formed when ii. iii. a. the ionization energy of A is high and electron
CHAPTER 6 CHEMICAL BONDING TEXT BOOK EXERCISE Q.1. Select the correct statement. i. An ionic compound A + B - is most likely to be formed when ii. iii. a. the ionization energy of A is high and electron
Organic Chemistry, 7 L. G. Wade, Jr. 2010, Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 16 Aromatic Compounds 2010, Prentice Hall Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 16 Aromatic Compounds 2010, Prentice Hall Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized
Solvation of Coordinatively Saturated Metal Complexes of Nitrogen Ligands
 Solvation of Coordinatively Saturated Metal Complexes of Nitrogen Ligands A. N. Kitaigorodskii and A. N. Belyaev Institute of Chemical Physics. USSR Academy of Sciences. Moscow. USSR Z. Naturforsch. 40a,
Solvation of Coordinatively Saturated Metal Complexes of Nitrogen Ligands A. N. Kitaigorodskii and A. N. Belyaev Institute of Chemical Physics. USSR Academy of Sciences. Moscow. USSR Z. Naturforsch. 40a,
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
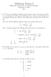 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Electronic structure / bonding in d-block complexes
 LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
Internal Mobilities in Molten (Li, Pb(II))Cl as Remeasured by the Klemm Method
 Internal Mobilities in Molten (Li, Pb(II))Cl as Remeasured by the Klemm Method Teruo H a i b a r a a n d Isao O k a d a Department of Electronic Chemistry, Tokyo Institute of Technology at Nagatsuta, Midori-ku,
Internal Mobilities in Molten (Li, Pb(II))Cl as Remeasured by the Klemm Method Teruo H a i b a r a a n d Isao O k a d a Department of Electronic Chemistry, Tokyo Institute of Technology at Nagatsuta, Midori-ku,
Name CHM 4610/5620 Fall 2017 December 14 FINAL EXAMINATION SOLUTIONS Part I, from the Literature Reports
 Name CHM 4610/5620 Fall 2017 December 14 FINAL EXAMINATION SOLUTIONS Part I, from the Literature Reports I II III IV V VI VII VIII IX X Total This exam consists of several problems. Rough point values
Name CHM 4610/5620 Fall 2017 December 14 FINAL EXAMINATION SOLUTIONS Part I, from the Literature Reports I II III IV V VI VII VIII IX X Total This exam consists of several problems. Rough point values
Part A. Answer all questions in this part.
 Part A Directions (1-20): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Part A Directions (1-20): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
PAPER No. 7: Inorganic chemistry II MODULE No. 5: Molecular Orbital Theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Chapter 4: Bonding in Solids and Electronic Properties. Free electron theory
 Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
TYPES OF SYMMETRIES OF MO s s-s combinations of orbitals: , if they are antibonding. s-p combinatinos of orbitals: CHEMICAL BONDING.
 TYPES OF SYMMETRIES OF MO s s-s combinations of : Orbitals Molecular Orbitals s s Node s s (g) (g) Bonding orbital Antibonding orbital (u) 4 (u) s-s combinations of atomic In the bonding MO there is increased
TYPES OF SYMMETRIES OF MO s s-s combinations of : Orbitals Molecular Orbitals s s Node s s (g) (g) Bonding orbital Antibonding orbital (u) 4 (u) s-s combinations of atomic In the bonding MO there is increased
Selectivity in Ketenimine Cycloadditions. Photoelectron Hel Spectra of Ketenimines
 Selectivity in Ketenimine Cycloadditions. Photoelectron Hel Spectra of Ketenimines Fernando Bernardi and Andrea Bottoni Istituto di Chimica Organica dell'universita, Viale Risorgimento, Bologna, Italy,
Selectivity in Ketenimine Cycloadditions. Photoelectron Hel Spectra of Ketenimines Fernando Bernardi and Andrea Bottoni Istituto di Chimica Organica dell'universita, Viale Risorgimento, Bologna, Italy,
Conjugated Systems. With conjugated double bonds resonance structures can be drawn
 Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Chemistry 2000 Lecture 2: LCAO-MO theory for homonuclear diatomic molecules
 Chemistry 2000 Lecture 2: LCAO-MO theory for homonuclear diatomic molecules Marc R. Roussel January 5, 2018 Marc R. Roussel Homonuclear diatomics January 5, 2018 1 / 17 MO theory for homonuclear diatomic
Chemistry 2000 Lecture 2: LCAO-MO theory for homonuclear diatomic molecules Marc R. Roussel January 5, 2018 Marc R. Roussel Homonuclear diatomics January 5, 2018 1 / 17 MO theory for homonuclear diatomic
Chapter 10 Chemical Bonding II
 Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Answers to Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear Diatomic Molecules
 Answers to Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear Diatomic Molecules 1. The electron configuration for HH is 1ss 1, so HH has 1 valence electron. The electron configuration for
Answers to Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear Diatomic Molecules 1. The electron configuration for HH is 1ss 1, so HH has 1 valence electron. The electron configuration for
1. Following Dalton s Atomic Theory, 2. In 1869 Russian chemist published a method. of organizing the elements. Mendeleev showed that
 20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Molecular Orbital Theory (MOT)
 Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Valence Electrons. How do you find the number of valence electrons in an atom of a representative element?
 Ions Ions Pyrite (FeS 2 ), a common mineral that emits sparks when struck against steel, is often mistaken for gold hence its nickname, fool s gold. Pyrite is an example of a crystalline solid. In this
Ions Ions Pyrite (FeS 2 ), a common mineral that emits sparks when struck against steel, is often mistaken for gold hence its nickname, fool s gold. Pyrite is an example of a crystalline solid. In this
Exam 2 October 29, time finished. Notes on exam format:
 Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 GROUND RULES: a. Closed book; closed notes; no consulting b. allowed: calculator; model set; point group flowchart; ruler c. 180 minute (3 h)
Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 GROUND RULES: a. Closed book; closed notes; no consulting b. allowed: calculator; model set; point group flowchart; ruler c. 180 minute (3 h)
Atoms with a complete outer shell do not react with other atoms. The outer shell is called the valence shell. Its electrons are valence electrons.
 Bonding and the Outer Shell Use this table for reference: http://www.dreamwv.com/primer/page/s_pertab.html Atoms with incomplete shells react with others in a way that allows it to complete the outer shell.
Bonding and the Outer Shell Use this table for reference: http://www.dreamwv.com/primer/page/s_pertab.html Atoms with incomplete shells react with others in a way that allows it to complete the outer shell.
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Ionic Versus Covalent Bonding
 Ionic Versus Covalent Bonding Ionic compounds are formed when electrons are transferred from one atom to another The transfer of electrons forms ions Each ion is isoelectronic with a noble gas Electrostatic
Ionic Versus Covalent Bonding Ionic compounds are formed when electrons are transferred from one atom to another The transfer of electrons forms ions Each ion is isoelectronic with a noble gas Electrostatic
Electronic structure Crystal-field theory Ligand-field theory. Electronic-spectra electronic spectra of atoms
 Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
An Interferometer for Direct Recording of Refractive Index Distributions
 An Interferometer for Direct Recording of Refractive Index Distributions SILAS E. GUSTAFSSON and ROLF A. E. KJELLANDER Department of Physics, Chalmers Institute of Technology, Göteborg, Sweden ( Z. Naturforsch.
An Interferometer for Direct Recording of Refractive Index Distributions SILAS E. GUSTAFSSON and ROLF A. E. KJELLANDER Department of Physics, Chalmers Institute of Technology, Göteborg, Sweden ( Z. Naturforsch.
Organic Chemistry 6 th Edition Paula Yurkanis Bruice. Chapter 1. Electronic Structure and Bonding. Acids and Bases Pearson Education, Inc.
 Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Lecture outline: Section 9. theory 2. Valence bond theory 3. Molecular orbital theory. S. Ensign, Chem. 1210
 Lecture outline: Section 9 Molecular l geometry and bonding theories 1. Valence shell electron pair repulsion theory 2. Valence bond theory 3. Molecular orbital theory 1 Ionic bonding Covalent bonding
Lecture outline: Section 9 Molecular l geometry and bonding theories 1. Valence shell electron pair repulsion theory 2. Valence bond theory 3. Molecular orbital theory 1 Ionic bonding Covalent bonding
Valence Bond Theory Considers the interaction of separate atoms brought together as they form a molecule. Lewis structures Resonance considerations
 CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
4 Diatomic molecules
 s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
NMR and NQR Studies of Borate Glasses*
 NMR and NQR Studies of Borate Glasses* Philip J. Bray and Gary L. Petersen** Department of Physics, Box 1843, Brown University, Providence, Rhode Island 02912 Z. Naturforsch. 53 a, 273-284 (1998); received
NMR and NQR Studies of Borate Glasses* Philip J. Bray and Gary L. Petersen** Department of Physics, Box 1843, Brown University, Providence, Rhode Island 02912 Z. Naturforsch. 53 a, 273-284 (1998); received
Lecture- 08 Emission and absorption spectra
 Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture- 08 Emission and absorption spectra
Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture- 08 Emission and absorption spectra
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
- A CHEMICAL BOND is a strong attractive force between the atoms in a compound. attractive forces between oppositely charged ions
 191 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride
191 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride
11/29/2014. Problems with Valence Bond Theory. VB theory predicts many properties better than Lewis Theory
 Problems with Valence Bond Theory VB theory predicts many properties better than Lewis Theory bonding schemes, bond strengths, bond lengths, bond rigidity however, there are still many properties of molecules
Problems with Valence Bond Theory VB theory predicts many properties better than Lewis Theory bonding schemes, bond strengths, bond lengths, bond rigidity however, there are still many properties of molecules
Unit Five Practice Test (Part I) PT C U5 P1
 Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Test 5: Periodic Table, Ionic, and Molecular Compounds
 Name: - Grade/Group: Subject: Chemistry-7 Teacher: Mrs. Raj Date: Test 5: Periodic Table, Ionic, and Molecular Compounds Directions: Identify the letter of the choice that best completes the statement
Name: - Grade/Group: Subject: Chemistry-7 Teacher: Mrs. Raj Date: Test 5: Periodic Table, Ionic, and Molecular Compounds Directions: Identify the letter of the choice that best completes the statement
15.2 Electrons and Chemical Bonds
 CHAPTER 15: MOLECULES AND COMPOUNDS 15.2 Electrons and Chemical Bonds The discovery of energy levels in the atom solved a 2,000-year-old mystery. The mystery was why elements combined with other elements
CHAPTER 15: MOLECULES AND COMPOUNDS 15.2 Electrons and Chemical Bonds The discovery of energy levels in the atom solved a 2,000-year-old mystery. The mystery was why elements combined with other elements
Chapter 8 Covalent Boding
 Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Partial Periodic Table
 Easily Legible Printed Name: CEM 3311 (300), Fall 2014 Professor Walba First our Exam September 23, 2014 scores: 1) 2) 3) 4) 5) CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CEM 3311 (300), Fall 2014 Professor Walba First our Exam September 23, 2014 scores: 1) 2) 3) 4) 5) CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student,
Ionic Motion of Phenethylammonium Ion in [C 6 H 5 CH 2 CH 2 NH 3 ] 2 PbX 4 (X = C1, Br, I) as Studied by l H NMR
![Ionic Motion of Phenethylammonium Ion in [C 6 H 5 CH 2 CH 2 NH 3 ] 2 PbX 4 (X = C1, Br, I) as Studied by l H NMR Ionic Motion of Phenethylammonium Ion in [C 6 H 5 CH 2 CH 2 NH 3 ] 2 PbX 4 (X = C1, Br, I) as Studied by l H NMR](/thumbs/91/106526978.jpg) onic Motion of Phenethylammonium on in [C 6 H 5 CH CH NH 3 ] Pb 4 ( = C1, Br, ) as Studied by l H NMR Takahiro Ueda, Mariko O m o *, Katsuyuki Shimizu*, Hiroshi Ohki*, and Tsutomu O k u d a * Department
onic Motion of Phenethylammonium on in [C 6 H 5 CH CH NH 3 ] Pb 4 ( = C1, Br, ) as Studied by l H NMR Takahiro Ueda, Mariko O m o *, Katsuyuki Shimizu*, Hiroshi Ohki*, and Tsutomu O k u d a * Department
Stability of Two Superposed Viscoelastic (Walters B') Fluid-Particle Mixtures in Porous Medium
 Stability of Two Superposed Viscoelastic (Walters B') Fluid-Particle Mixtures in Porous Medium Pardeep Kumar Department of Mathematics, Himachal Pradesh University, Summer-Hill, Shimla-171005, India Reprint
Stability of Two Superposed Viscoelastic (Walters B') Fluid-Particle Mixtures in Porous Medium Pardeep Kumar Department of Mathematics, Himachal Pradesh University, Summer-Hill, Shimla-171005, India Reprint
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
The G a m m a Radiolysis of Carbon M o n o x i d e in the Presence of Rare Gases*
 The G a m m a Radiolysis of Carbon M o n o x i d e in the Presence of Rare Gases* B Y S. DONDES, P. HARTECK, a n d H. VON WEYSSENHOFF * * Rensselaer Polytechnic Institute, T r o y, N. Y. (Z. Naturforschg.
The G a m m a Radiolysis of Carbon M o n o x i d e in the Presence of Rare Gases* B Y S. DONDES, P. HARTECK, a n d H. VON WEYSSENHOFF * * Rensselaer Polytechnic Institute, T r o y, N. Y. (Z. Naturforschg.
Covalent Molecules and Lewis Structures Time required: two 50-minute periods
 Mega Molecules, LLC!!!!! Name: Hands-On Science with Molecular Models!! Date:!!!!!!!! Hour: Introduction Covalent Molecules and Lewis Structures Time required: two 50-minute periods To study covalent molecules,
Mega Molecules, LLC!!!!! Name: Hands-On Science with Molecular Models!! Date:!!!!!!!! Hour: Introduction Covalent Molecules and Lewis Structures Time required: two 50-minute periods To study covalent molecules,
Mixed sandwiches of group 3 elements: synthesis and UPS studies of pentamethylcyclopentadienylcyclooctatetraenescandium, -yttrium and -lanthanum
 Journal of Organometallic Chemistry, 350 (1988) 17-23 17 Elsevier Sequoia S.A., Lausanne - Printed in The Netherlands Mixed sandwiches of group 3 elements: synthesis and UPS studies of pentamethylcyclopentadienylcyclooctatetraenescandium,
Journal of Organometallic Chemistry, 350 (1988) 17-23 17 Elsevier Sequoia S.A., Lausanne - Printed in The Netherlands Mixed sandwiches of group 3 elements: synthesis and UPS studies of pentamethylcyclopentadienylcyclooctatetraenescandium,
Lecture 14 Chemistry 362 M. Darensbourg 2017 Spring term. Molecular orbitals for diatomics
 Lecture 14 Chemistry 362 M. Darensbourg 2017 Spring term Molecular orbitals for diatomics Molecular Orbital Theory of the Chemical Bond Simplest example - H 2 : two H atoms H A and H B Only two a.o.'s
Lecture 14 Chemistry 362 M. Darensbourg 2017 Spring term Molecular orbitals for diatomics Molecular Orbital Theory of the Chemical Bond Simplest example - H 2 : two H atoms H A and H B Only two a.o.'s
Chem Spring, 2017 Assignment 5 - Solutions
 Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Chapter 7: Chemical Bonding and Molecular Structure
 Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
Topic 2. Structure and Bonding Models of Covalent Compounds of p-block Elements
 Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Metal Hydrides, Alkyls, Aryls, and their Reactions
 Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Assessment Chapter 5 Pre-Test Chapter: The Periodic Law Use the periodic table below to answer the questions in this Chapter Test.
 Assessment Chapter 5 Pre-Test Chapter: The Periodic Law Use the periodic table below to answer the questions in this Chapter Test. In the space provided, write the letter of the term or phrase that best
Assessment Chapter 5 Pre-Test Chapter: The Periodic Law Use the periodic table below to answer the questions in this Chapter Test. In the space provided, write the letter of the term or phrase that best
