DYNAMIC ANALYSIS OF ADSORPTIVE HEAT TRANSFORMERS: OPTIMAL ADSORBENT
|
|
|
- Spencer Alexander
- 5 years ago
- Views:
Transcription
1 DYNAMIC ANALYSIS OF ADSORPTIVE HEAT TRANSFORMERS: OPTIMAL ADSORBENT Yuriy I. Aristov Boreskov Institute of Catalysis SB RAS Lavrenieva Av., 5, Novosibirsk, , RUSSIA Abstract This paper presents a novel experimental method to study adsorption dynamics under conditions typical for an adsorptive heat transformer. Effect of the adsorbent nature, its grain size, residual non-adsorbable gas, heating rate, local shape of adsorption isobar on the adsorption dynamics and specific cooling (heating) power was studied for promising adsorbents of water (Fuji silica RD, FAM-Z02, SWS-1L). Based on these results some demands to an adsorbent optimal from the dynamic point of view have been discussed. KEYWORDS Adsorptive heat transformers Adsorption kinetics Optimal adsorbent Linear driving force model INTRODUCTION An adsorbent is a key element of adsorptive heat transformers, AHT (heat pumps, chillers and amplifiers) and its proper choice is of prime importance. Intelligent choice of adsorbent should be based on comprehensive analysis that takes into consideration both thermodynamic and dynamic aspects. Thermodynamic requirements to an optimal adsorbent which ensures the best cycle performance under prescribed operating conditions were reported for non-regenerative AHT cycles in [1, 2]. These requirements for a basic cycle reversibly operating at the fixed evaporator temperature T e and condenser temperature T c are based on an universal relation between three cycle temperatures (T e, T c and the minimal desorption temperature T 2 ) [3] T 2 T e =T c 2, that directly comes from the empiric Truton s rule [4] or the Polaniy principle of temperature invariance [5]. The conclusion was drawn that the optimal adsorbent should have a step-like adsorption isobar with the step positioned exactly at T = T 2 (at P = P o (T c )). Because of zero temperature difference between an adsorber and an external heat source the entropy generation is absent and the AHT efficiency can reach the Carnot efficiency [1, 6]. On the other hand, no driving force for heat transfer Kinetic properties of the adsorbent strongly affect the dynamic behaviour of AHT and contribute to the specific power of the unit. This paper gives an overview of the current state of the art in this field and presents a novel approach focused on studying adsorption dynamics under conditions typical for AHT. This approach closely imitates boundary conditions of isobaric stages of AHT when adsorption (desorption) is initiated by a fast drop (jump) of the temperature of a heat exchanger (HE) wall which is in thermal contact with the adsorbent [6-11]. This method allowed experimental modelling the dynamics of isobaric stages of AHT. The effects of adsorbent nature, its grain size, heating rate, residual non-adsorbable gas, etc. on the uptake evolution and AHT specific power were experimentally examined for promising adsorbents (Fuji silica RD, FAM-Z02, SWSs). Applicability of the Linear Driving Force (LDF) model is discussed. Based on this method as well as on literature data some requirements to a dynamically optimal adsorbent and configuration of its layer are considered. THE CURRENT STATE OF THE ART As stated above, the adsorbent kinetic properties can strongly affect the dynamic behaviour of well-designed AHT and its specific power. Common way for taking these complex effects into account is a mathematical modelling of the AHT dynamics. These models are used for describing experimental behaviour of real AHT, predicting the performances, understanding the influence of the adsorbent properties, optimizing AHT operation, etc. Depending on the complexity, the dynamic models may be classified as lumped parameters (LP) and heat and mass transfer (HMT) models. 284
2 The HMT models take into account variation of the adsorbent temperature T a, pressure P and adsorbate concentration w both in time and space. Hence, the governing equations are partial differential equations. The complex and nonlinear character of coupled heat and mass transfer requires sophisticated and time-consuming numerical methods for solving these equations and simulation of adsorbent bed dynamics. Because of this the HMT models are not wide-spread and the most popular are LP models which give a simplified representation of the adsorption process, neglecting any space gradients. The following assumptions are generally made: a) the temperature T a is uniform in the adsorbent layer; b) the refrigerant (adsorbate, working fluid) is distributed in the adsorber uniformly and can be described by the average concentration w; and, c) the intrinsic adsorption is fast and at any time the equilibrium between solid and gas phases are attained. In the LP models only the heat transfer resistance between a HE wall and an adsorbent is considered, while that inside the adsorbent layer is neglected [12]. A common LP model includes three main equations representing the energy balance, mass balance and adsorption equilibrium. Such model was first applied for analysis of adsorptive chillers in [13, 14] and afterwards was replicated in both original or modified forms in many papers [15-22]. Only two dynamic characteristics of an adsorbent are presented in the LP equations: the adsorption rate dw/dt and the coefficient of heat transfer between the adsorbent and the HE wall h. To calculate dw/dt the authors of [13] used the LDF model with the efficient rate constant dw/dt = K(w eq - w) (1) K(T) = 15D ef (T)/R p 2, (2) where D ef (T) = D ef0 exp(-e a /RT) is the temperature dependent effective diffusivity, R p is the radius of a spherical grain, w eq and w are equilibrium and current uptakes. In [13] there was no special justification for applying Eq. (1), just a reference to Glueckauf [23] who first suggested it to simplify a numerical analysis of the sorption dynamics in chromatographic columns. Determination of the effective diffusivity D ef. To measure the water diffusivity (means, D ef0 and E a ) in a silica Fuji type A the authors of [13] applied the well known Isothermal Differential Step (IDS) method [24, 25]. This quasi-equilibrium method is considered to be reliable as it allows obtaining the diffusivity in the case of a non-linear adsorption isotherm [25]. The procedure based on the moderate deviation of the system vapour-adsorbent out of adsorption equilibrium by a small jump of the vapour pressure. The adsorption kinetics was found to follow the Fickian Diffusion (FD) model [13]. The weight change was analyzed to obtain the water diffusivity D ef by fitting of these experimental curves with the theoretical model [24]. So obtained diffusivities were approximated in [13] by the function D ef = D ef0 exp(-e a /RT) with D ef0 = m 2 /s and E a = 42.0 kj/mol. These values allowed successful interpretation of the experimental results of [13] and were used in numerous later simulations [15-22]. Similar values for the Fuji silica type RD were measured by the IDS method in [26]: D ef0 = m 2 /s and E a = 41.5 kj/mol. They are close to those for the silica Fuji type A, because these adsorbents have almost equal pore structure [26]. Determination of the heat transfer coefficient h. The heat transfer resistance 1/h between the HE wall and the adsorbent layer is a rate limiting factor in many AHTs, especially utilizing loose adsorbent grains. The heat transfer coefficient h can be estimated as follows [27]: if the main mechanism of heat transfer from the plate to the grain (or vice versa) is the heat conductance through the layer of a stagnant vapour, then h λ/r р = W/(m 2 К), where λ = W/(m К) is the heat conductivity of water vapour at T = 70 o C. A few experimental studies were dedicated to direct measurements of this important parameter [28]. This value was dependent on the gas nature and pressure and changed for Ar from 35 to 75 W/(m 2 R) and for He from 75 to 200 W/(m 2 R). The authors mentioned that this value increases for smaller grains with higher skeleton heat conductivity and smaller average bed porosity. Probably because of this complexity some confusion does exist in literature: for water vapour the heat transfer coefficient h of 15 to 50 W/(m 2 R) has been used by various authors [13-18, 29-32]. No reliable data on temperature dependence h(t) are available in literature, and in LP models h is as- 285
3 sumed to be constant. For vapours this resistance can be additionally affected by the heat pipe effect which was reported by Zhang and Wang [29] during the isosteric phases of AHT cycle. This creates an additional channel of the transfer due to a convective vapour flux towards colder sites in the adsorbent layer [22, 32] enhancing both heat and mass transfer. Other input parameters should be measured and input in the LP models are the adsorbent specific heat C p (w) and the heat of adsorption H a (w) as well as some apparatus characteristics. If the thermodynamic and dynamic parameters are identified, the evolution of the temperature, pressure and concentration can be obtained by numerical solving a system of ordinary differential and algebraic equations. Hence, even simplified LP models require many experimental parameters to be known. Moreover, a serious obstacle is a reliability of these input parameters: the uncertainty with the h-values has been already mentioned. Let us consider another fundamental discrepancy of the current methodology: the values of D ef and h measured under quasi-equilibrium conditions can be quite different from those at real conditions of AHT cycle. Indeed, for the IDS method the adsorption driving force is a small pressure jump, and thermal effects can be neglected, whereas at common AHTs the adsorption is initiated by a large drop of the temperature of a metal support (HE fins) at a constant pressure over the adsorbent. NEW METHODOLOGY OF STUDYNG AHT DYNAMICS Adsorption dynamics under real conditions of AHT. To avoid the mentioned fundamental discrepancy of the current methodology we suggested [6] a new Large Temperature Jump (LTJ) method to study the dynamics of refrigerant adsorption (desorption) under conditions which almost repeat the isobaric stages of AHT cycle. The measurements were performed with one layer of loose adsorbent grains placed on a metal plate which imitates a HE fin (Fig. 1). Temperature of the plate was changed due to a thermal carrier circulating under the plate. Vapour pressure over the adsorbent was main-tained almost constant by connecting the measuring cell to a large vapour vessel which moderated the pres-sure change. Water uptake was converted from the pressure evolution P(t). This method allows a direct measurement of adsorption dynamics under conditions, which closely simulate the scenario of temperature jump or drop in real AHT. Experimental setup contained three main compartments: the measuring cell, the vapour vessel and the evaporator (Fig. 2). Loose adsorbent grains (mass mg) were placed in one layer on a metal holder. Its temperature could be adjusted and controlled with the accuracy of ±0.1ºC using a heat carrier circuit coupled by a three-way valve (3WV) either to circulating vapor thermal bath 1 or 2. The vapour pressure was measured by an absolute pressure transducer MKS Baratron type 626A (accuracy ±0.01 mbar). More experimental details can be found elsewhere [6, 8, 10, 11]. This set-up was a modification of the constant-volume, variable pressure setup first suggested by Knocke [33] and then applied in [34]. That approach can be called as a Large Pressure Jump (LPJ) method as the driving force for water adsorption was a large jump of the pressure over the adsorbent. Typical adsorption LTJ run was as follows [6]. A monolayer of loose adsorbent grains was heated to the starting temperature of the isobaric adsorption stage T 0 = 60 ºC and evacuated for two hours using a vacuum pump (valves V MC, V 2, V 3 were opened, V A & V 1 was closed). Then the vapour vessel and the measuring cell were connected to the evaporator and the starting pressure of the adsorption process, e.g. P ev (T e = 10 ºC) = 12.4 mbar was set (V 1, V MC & V 3 were open, V 2 & V A were closed). The sample was equilibrated with water vapour for two hours. After that valve V1 was closed and the metal holder was cooled down to T f = 35 ºC by turning 3WV (V MC was open). It initiated the adsorpheat carrier Fig. 1. Schematic of the layer configuration 286
4 tion process and the vapour pressure over the adsorbent was reduced by some mbar, so that the process could be considered as quasi-isobaric. To initiate the desorption run the reverse temperature leap was performed. Data on the pressure evolution P(t) required for calculating the water uptake m H2O (t) were recorded each 1 s by a data acquisition system. P V A Ambient air Gate valve V MC Sorbent granules Vaporvessel Measuring cell V3 V2 Vacuum pump Thermal bath 1 3WV T 3WV V1 Evaporator Thermal bath 2 T f V ev Fig. 2. Schematic diagram of the LTJ kinetic setup T plate/ o C P/ mbar ,5 10,0 9,5 9,0 a b 8,5 8,0 uptake/ (g/100g) c Time/ s Fig. 3. Temperature evolution of metal plate (a), pressure over the sample (b) and the water uptake (c) for cooling (bold symbols) and heating (open symbols) runs for SWS-1L grains (size mm). The solid line is exponential approximation m t = m 0 +(m f - m 0 )(1 exp(- t/τ)) [10] Typical evolution of the temperature of the metal plate, the vapour pressure over the sample and the water uptake are shown on Fig. 3 for the composite SWS-1L ( CaCl 2 in the KSK silica pores ). After initiating the cooling process, the holder reached the final temperature T f for app. 1 min (Fig. 3 a), while the pressure reduction was slower and completed within some tens minutes (Fig. 3 b). Hence, the adsorption took place at almost constant temperature of the holder equal to its final temperature as it was considered in the mathematical model described in [7]. Near-exponential evolution of the uptake w on time was found for both adsorption and desorption runs (Fig. 3 c). The LTJ method was further applied to study effects of adsorbent nature, its grain size, heating rate, residual non-adsorbable gas, etc. on the uptake evolution and AHT specific power. The experiments have been run on loose grains of water adsorbents promising for AHT, namely, Fuji silica RD (Fuji Silysia Ltd.) [26], FAM-Z02 (Mitsubishi Chemical Ltd.) [35] and SWS-1L. 287
5 Adsorbent nature. The new LTJ methodology allows a simple and reliable comparison of various adsorbents to be used in AHTs. For the three mentioned adsorbents evolution of the dimensionless water uptake in time is presented in Fig. 4 a for typical conditions of isobaric adsorption stage. It takes 101, 307 and 453 s to reach 80 % of the equilibrium uptake for silica, SWS-1L and FAM-Z02, respectively. Absolute variation of the uptake was 0.094, and g/g (Fig. 4 b), that gives the specific cooling power of 2.3, 1.4 and 1.1 kw/kg. At short adsorption time all the adsorbents generate almost the same cooling power (Fig. 4) exceeding 2.3 kw/kg. Hence, even simplest configuration of adsorbent bed - a single layer of touched adsorbent grains loosely placed on the metal plate [36] can combine easy realization with practically attractive cooling power. For silica and SWS, the experimental uptake curves were close to exponential, while for FAM a long tail was observed at the uptakes larger than 0.8 (Fig. 4 a). This is probably caused by FAM structural transformations. 1,0 0, m t / m 0,6 0,4 0,2 a 0,0 0,20 m H2O, [g/g dry ] 0,15 0,10 0,05 0, b Time, [s] Fig. 4. Dimensionless (a) and absolute (b) adsorption uptake curves for loose grains mm size, initiated by a temperature jump 60 o C=>35 o C: 1 Fuji RD, 2 SWS 1L, 3 FAM-Z02. P 0 H 2 O = 10.3 mbar Grain size. The size of adsorbent grains can affect simultaneously both heat and mass transfer in the adsorbent bed. Adsorption was faster for smaller grains (Fig. 5). The shape of these kinetic curves was near-exponential over the range 0 90 % of the final uptake while the tail was longer than exponential. The times τ 0.5 and τ 0.8 corresponding to 50 and 80 % of the final uptake were found to be 2 15 min. 1.5mm mm that is reasonable for practice (Fig. 5). For larger grains the ratio τ was close to ( mm mm r ) τ 0.5 r that may indicate an essential contribution of intraparticle vapour diffusion to the total adsorption rate [10]. For smaller SWS grains the time of adsorption became very short and comparable with the time of the holder heating, so that the heat transfer seemed to play a dominant role. Strong acceleration of sorption process with the decrease in the FAM grain size was found in [8], too. Residual air. From practical experience it is known that some amount of a non-adsorbing gas such as air can poison two-phase heat and mass transfer, reducing adsorption rate. The LTJ approach allows careful study of this effect [10]. We found that the presence of air at the partial pressure as low as 0.06 mbar can visibly decrease the rate of water adsorption by SWS-1L (Fig. 6). It was especially significant at the intermediate uptakes 0.3 < m t /m f < 0.9, while at smaller and larger uptakes no influence was revealed (Fig. 6). The reduction of the adsorption rate can be caused by the Stephan flux which effectively sweeps air to the grain surface where it accumulates as an air-rich layer [37]. The transfer of vapour to the surface may then become controlled by the diffusion through this layer. At small uptakes this layer, probably, did not have enough time to form. At large uptakes (m t /m f > 0.95) adsorption rate is low and the Stephan flux was not sufficient to efficiently sweep air to the grain surface, hence, the 288
6 extra-resistance was small. It was not unlikely that the residual air can also accumulate inside the grain pores so that the combined effect of external and internal resistances took place. 1,0 0, m t / m 0,6 0,4 0,2 0, Time, [s] Fig. 5. Adsorption dynamics for loose SWS-1L grains initiated by temperature jump 60 => 35 o C at various grain size: mm, mm and mm. 1,0 0,22 Fig. 6. Inhibition of the adsorption rate for SWS-1L grains mm in the presence of air. 1) P A 0.02 mbar; 1 * ) P A = 0.06 mbar; 2) P A = 0.4 mbar; 3) P A = 1 mbar; 4) P A = 4.7 mbar. Dotted lines exponential approxi- 0,0 mation w 0,8 0,6 0,4 0,2 1 1 * Time, [s] 0,18 0,13 0,09 0,04 0,00 mh2o, [g/g dry ] Experimental kinetic curves were near-exponential m t = m 0 + (m f - m 0 ) (1 exp(-t/τ)). At P A 0.4 mbar the characteristic time of adsorption was τ = τ 0 + BP A, where B = 700 ± 50 s/mbar for the both grain sizes [10, 11]. Similar linear dependences were observed for Fuji RD and FAM-Z02, although an inhibition of the sorption rate was less strong than for SWS (Fig. 7). Hence, a careful removal of nonadsorbable gases from the adsorbent surface, set-up walls and degassing water in the evaporator is strictly obligatory prior a start-up of an AHT τ 0.8, [s] Fig. 7. Characteristic adsorption time τ 0.8 as a function of air pressure for SWS-1L ( mm and o mm), silica Fuji ( mm) and FAM-Z02 ( mm). Dotted lines linear approximation τ = A + B*P P, [mb] Heating rate. For the fast heating run the metal plate became isothermal in s, while for the slow one, it took 300 s and 500 s for the heating and cooling, respectively (Fig. 8). As a result, the uptake changed much slower than for the fast run. Especially large difference was observed at the initial part of uptake curve, which was rather S-shaped than exponential (Fig. 8 c). To analyse such complex kinetics we introduced the characteristic times τ 0.5 and τ 0.9. The τ 0.5 -time was much longer for the slow temperature change, while the τ 0.9 -times were almost equal, especially for cooling mode [6]. Thus, a cooling scenario affects the beginning of uptake curve stronger than its tail. Specific power. For the exponential uptake curves the instantaneous specific power W(t) which is consumed (released) in the evaporator (condenser) is W = H (dm/dt)/m a = W max exp(-t/τ), where H is the latent heat of evaporation of liquid water taken as 2478 J/g, m a is the mass of dry adsorbent, W max = H (m m o )/(τ m a ) is the maximum specific power at t 0. Even for quite large SWS grains (
7 mm) at heating stage it could reach 3.7 kw/kg at the vapour pressure mbar. For water adsorption at low vapour pressure (10-15 mbar) the maximum power was still good ( kw/kg). T plate, o C a 40 1,0 0,8 b P/(P f - P 0 ) 0,6 0, ,2 1,0 m/(m f - m 0 ) 0,8 0,6 0,4 0,2 0,0 c Fig. 8. Temperature evolution of the metal plate (a), the pressure over the sample (b) and the water uptake (c) for different heating scenario: 1 fast and 2 slow [6] Time, s Thus, our LTJ tests with loose adsorbent grains give experimental evidence for the possibility to build quite compact adsorption cooling/heating devices which utilize loose grains of Fuji RD, FAM- Z02 and SWS-1L. One can expect even higher specific power for a compact adsorbent layer prepared with a binder and consolidated with metal surface of heat exchanger [28]. The measured uptake curves were exponential, that can be formally obtained by integrating Eq. (1). On the one hand, this justifies applicability of the LDF model for analyzing isobaric stages of AHT cycles as it was suggested in [13, 14]. On the other hand, the exponential shape of the uptake curves can be derived from Eq. (1) only at K = const but not at K dependent on T as ordered in [13, 23] (Eq. (2)). It means that calculations with LP models for the studied configuration of adsorbent layer can be simplified by using a constant K instead of that introduced by Eq.(1). Indeed, for the configuration of the system adsorbent-he and fast heating conditions discussed above, dynamics of each isobaric stage of AHT cycle can be unambiguously characterized by a single K-value. For particular case, K depends on the boundary temperatures T o, T f and grain size R p and remains constant during adsorption (desorption) stage. Consequently, for fixed cycle this value can be directly measured and then introduced into heat and mass balance equations. Then a simple analytical expression can be easily obtained for the derivative dw/dt. In fortunate fact, a near-constancy of K and, hence, a simple exponential shape of experimental kinetic curves is a result of a complex interference of HMT processes in the system HE wall adsorbent. Although K implicitly depends on D ef and h, no exact knowledge of these coefficients and their temperature dependence is necessary any more for making dynamic calculations. Models based on constant K can be called as super-lumped parameter models. This approach can be generalized to any given configuration of the HE-adsorbent system. Indeed, if the uptake w(t) has been directly measured for the particular configuration (or for representative piece of this configuration), then, the adsorption rate dw/dt can be obtained by a numerical differentiation of the experimental function w(t) and can be immediately input in a LP model instead of Eq. (2) or any other simplification. Mathematical model of the coupled heat and mass transfer under the LTJ run. For deeper understanding a coupled heat and mass transfer in the course of LTJ experiment a detailed HMT model was developed in [7]. A single adsorbent grain in thermal contact with a metal plate subjected to a fast temperature jump/drop at constant pressure was considered. The model took into account coupled heat and mass transfer in the grain with adsorption processes. Heat and mass transfer in a single adsorbent grain was described by detailed energy and mass balance equations [7]. This model was used for analysing the experimental results presented in [6] and for understanding links between dynamics and equilibrium parameters of adsorption process. The local adsorption equilibrium in each point of the 290
8 grain was assumed. The system of differential equations was numerically solved by methods of runs and iterations to obtain distributions of temperature T(r, t) and concentration in gas C w (r,t) and adsorbed w(r, t) phases. Analysis of the experimental data. Good agreement between experimental and calculated uptake curves was obtained with the best fit corresponding to the efficient pore diffusivity D = m 2 /s and the efficient coefficient of heat transfer h = 120 W/(m 2 К). This diffusivity is very close to the Knudsen diffusivity in a straight cylindrical pore of radius r p = 7.5 nm [25] at T = 90 o C = 363 K: D kn = 9700 r p T/µ = m 2 /s. The diffusivity found in [7] is significantly higher than measured by the IDS method under isothermal conditions (T = C) for loose grains of SWS-1L [26]. The coefficient of heat transfer h obtained is also much larger than the one (15-60 W/(m K)) commonly used. These differences in D and h indicated that at large deviations from the equilibrium the transfer of both heat and vapour can be more efficient than under quasi-equilibrium conditions. Possible reason could be an additional channel of the transfer due to a non-uniform temperature distribution that causes convective vapour flux towards colder sites in the system (a so called heat pipe or recondensation effect [22, 32]), which can enhance both heat and mass transfer. The experimental and calculated characteristic times τ с were in close agreement [7], hence the model accounted for major features of the LTJ experiments reported in [6]. In particular, the model confirms the quasi-exponential shape of the uptake curves, and the finding that the adsorption process was slower than the desorption one at the same temperature jump (Fig. 9). Moreover, our numerical calculations showed that the latter conclusion is not general and holds true only for particular conditions of temperature jumps applied in [6]. Fig. 9. Dimensionless uptake vs. time for a concave isobar (taken for SWS-1L): desorption (1) and adsorption (2). 3 adsorption and desorption runs calculated for linear isobar. Jump 90 o C 80 o C [7] The interdependence between the heat and mass transfer can be more clearly seen if present the evolution of the grain state as the average grain temperature T vs. the average pressure inside the grain P w (Fig. 10): A corresponds to the state of the system before the jump 90 о С 80 о С. Line ABD represents the process of the grain cooling which initiates the adsorption of vapour. D corresponds to the state of the system before the jump 80 о С 90 о С. Line DEA represents the process of the grain heating which initiates the desorption of vapour. Fig. 10. Evolution of the average grain temperature T and the average pressure inside the grain P w for the temperature jumps 90 о С 80 о С [7] 291
9 For jump 90 о С 80 о С at t = 0 (point A) the driving force for heat transfer is maximum while the driving force for mass transfer equals 0. This initiates the fast cooling of the grain that reduces the driving force for the heat transfer. Dropping the grain temperature stimulates the adsorption of water molecules first from the gas phase inside the pores. As the adsorption is fast, at short times the diffusional flux of vapour into the grain is not sufficient to compensate the pressure decrease. The pressure difference P - P w generates the driving force for mass transport which is increasing till point B (Fig. 10). The adsorption of water results in the release of the adsorption heat inside the grain which slows down the temperature decrease and so on. Thus, the mass and heat transfer processes are inevitably coupled and strongly affect each other. At longer times, P w is increasing due to the vapour flux from the outside into the grain, until the system reaches the equilibrium at point D. At this point the driving forces for both the heat and mass transfer become zero. Similar evolution was observed during the reverse jump 80 о С 90 о С [7]. a a 4 N, molh2o/molcacl b T, C Fig. 11. Specific heat of an SWS-1L grain (a) as a function of the average temperature (1 desorption, 2 adsorption), isobar of water sorption (b) on SWS-1L at P = 56.5 mbar The calculated specific heat С = dq/dt/м of the grain as a function of the average grain temperature has a break at T С for both the adsorption and desorption runs (see the fragment on Fig. 11a) [7]. It is interesting that at the same temperature there is a break of the isobar w(t) of water sorption on SWS-1L (Fig. 11 b) at 56.5 mbar that is the average pressure during the jump. One can assume that these two breaks are linked. Indeed, during the desorption run the majority of water is desorbed within the temperature range from 80.0 to 85.5 o C, so that the heat needed for desorption is contributed to the large value of C (up to 160 J/(g K)). At T > С the contribution of water desorption becomes much smaller; the C(T) has the break and is slowly approaching the sensible specific heat of the system (SiO 2 +CaCl 2 2H 2 O). During the adsorption run within the temperature range from 90.0 to 85.5 o C there is almost no adsorption (dw/dt is very small), and the average grain temperature changes faster than during reverse desorption run. At T av < 85.5 o C the adsorption starts, because the absolute value of the derivative dw/dt increases. When T av is approaching 90 o C the driving force for the heat transfer proportional to T = (90 o C - T av ) is getting smaller, while [dw/dt] is continuously rising. This concave shape of adsorption isobar is the main reason why the adsorption has longer tail that desorption, that results in larger characteristic times. A linear sorption isobar leads to equal kinetics of adsorption and desorption (Fig. 9, curve 3), while convex isobar shape results in faster adsorption run. The latter has recently been confirmed experimentally [38]. Thus, a correlation between the driving force for heat transfer and the derivative dw/dt which defines the heat demand for desorption or heat release during adsorption, is dominant for overall dynamics in the LTJ runs as well as in real AHT. Since this driving force is gradually reducing, the derivative should decrease when T is approaching T f, too. Hence, d 2 w/dt 2 has to be negative. It means that to shorten the isobaric stages, at desorption run the isobar has to be concave while at adsorption 292
10 run convex (Fig. 12). Ln P w T o des T f ads 4 w T f d 2 w/dt 2 > 0 T o T Fig. 12. Optimal shape of isobars at adsorption (4-1) and desorption (2-3) phases Thus, the dynamics of water adsorption on a single adsorbent grain is closely linked with equilibrium properties of the adsorbent, in particular, with the shape (convex, concave or linear) of the segment of water adsorption isobar between the initial and final temperatures. CONCLUSIONS A novel experimental approach (LTJ) to study adsorption dynamics under conditions typical for AHTs has recently been suggested. It provides a correct methodology to investigate the effect of the adsorbent nature, its grain size, non-adsorbable gas, heating rate, shape of adsorption isobar, etc. on the adsorption dynamics. The main findings are a) near-exponential kinetic curves were found for a majority of adsorption and desorption runs, b) simple configuration of loose adsorbent grains can provide cooling power of kw/kg; b) strong reduction of the adsorption rate in the presence of residual air; c) validity of the LDF model with a fixed rate constant; d) strong link between kinetic and equilibrium parameters. Mathematical modelling indicated that at large deviations from the equilibrium the transfer of both heat and vapour can be more efficient than under quasi-equilibrium conditions. Based on these results some demands to an adsorbent optimal from the dynamic point of view have been discussed. Acknowledgements The author thanks the Russian Foundation for Basic Researches for partial financial support of this study (grants , and ). Nomenclature A constant, s B constant, s/mbar c specific heat, J/(kg C) D diffusivity, m 2 /s Е activation energy, J/mol h heat transfer coefficient, W/(m 2 C) H enthalpy, J/mol K rate constant, 1/s m mass, kg P pressure, mbar r pore radius, m R grain radius, m; universal gas constant, J/(m K) T temperature, K, C t time, s 293
11 w adsorbate concentration, g/g W specific power, kw/kg µ molar mass, g τ characteristic time, s increment Subscripts a adsorbent, activation A air c condenser e evaporator ef effective eq equilibrium f final kn Knudsen max maximum p particle; pore; at constant pressure t temporal 0 initial infinity References 1. I. An optimal sorbent for adsorption heat pumps: thermodynamic requirements and molecular design // Proc. VI Intern. Seminar Heat pipes, heat pumps, refrigerators, Sept. 2005, Minsk, Belorus, pp I. Novel materials for adsorptive heat pumping and storage: screening and nanotailoring of sorption properties // J. Chem. Engn. Japan, 2007, Vol. 40, pp Critoph R. E. Performance limitation of adsorption cycles for solar cooling // Sol. Energy, 1988 Vol. 41, pp Alefeld G., Radermacher R. Heat Conversion Systems, CRC Press, Boca Raton, I., Sharonov V. E., Tokarev M. M. Universal relation between the boundary temperatures of a basic cycle of sorption heat machines // Chem. Engn. Sci., 2008, Vol. 63, pp I., Dawoud B., Glaznev I. S., A. Elyas A. A new methodology of studying the dynamics water sorption/desorption under real operating conditions of adsorption heat pumps: Experiment // Intern. J. Heat and Mass Transfer, 2008 (doi: /j. ijheatmasstransfer ). 7. Okunev B. N., Gromov A. P., Heifets L. I., I. A new methodology of studying the dynamics of water sorption/desorption under real operating conditions of adsorption heat pumps: Modelling of coupled heat and mass transfer // Intern. J. Heat and Mass Transfer, 2008, Vol. 51, pp Dawoud B. On the effect of grain size on the kinetics of water vapour adsorption and desorption into/ from loose pellets of FAM-Z02 under a typical operating condition of adsorption heat pumps // J. Chem. Engn. Japan, Vol. 40, pp Okunev B. N., Gromov A. P., Heifets L. I., I. Dynamics of water sorption on a single adsorbent grain caused by a pressure jump: modelling of coupled heat & mass transfer // Int. J. Heat & Mass Transer (doi: /j.ijheatmasstransfer ). 10. Glaznev I. S., I. Kinetics of water adsorption on loose grains of SWS-1L under isobaric stages of adsorption heat pumps: the effect of residual air // Int. J. Heat&Mass Transfer (accepted). 11. Glaznev I. S., Ovoshchnikov D. S., I. Dynamics of water adsorption on loose grains in the presence of non-adsorbable gas under typical operating conditions of an adsorptive chiller, Cryogenics and Refrigeration // Proc. ICCR 2008, Shanghai, China, April 5-9, 2008, pp Yong L., Sumathy K. Review of mathematical investigation on the closed adsorption heat pump and cooling systems // Renewable and Sustainable Energy Reviews, 2002, Vol. 6, pp Sakoda A., Suzuki M. Fundamental study on solar powered adsorption cooling system // J. Chem. Engn. Japan, 1984, Vol. 17, pp
12 14. Sakoda A., Suzuki M. Simultaneous transport of heat and adsorbate in closed type adsorption cooling system utilizing solar heat // J. Solar Energy Engin., Trans. of the ASME, 1986, Vol. 108, pp Douss N., Meunier F. Effect of operating temperatures on the coefficient of performance of active carbon-methanol systems // J.Heat Recovery Systems & CHP, 1988, Vol. 8, pp Cho S.-H., Kim J.-N. Modelling of a silica gel/water sorption-cooling system // Energy, 1992, Vol. 17, pp Saha B. B., Boelman E. C., Kashiwagi T. Computer simulation of a silica gel-water adsorption refrigeration cycle The influence of operating conditions on cooling output and COP // ASHRAE Transactions: Research, 1995, Vol. 101, pp Saha B. B., Boelman E. C., Kashiwagi T. Computational analysis of an advanced adsorptionrefrigeration cycle // Energy, 1995, Vol. 20, pp Critoph R. E. Forced convection adsorption cycles. // Applied Thermal Engineering, 1998, Vol. 18, pp Cacciola G., Hajji A., Maggio G., Restuccia G. Dynamic simulation of a recuperative adsorption heat pump // Energy, 1993, Vol. 18, pp Sami S.M., Tribes C. An improved model for predicting the dynamic behaviour of adsorption systems // Applied Thermal Engineering, 1996, Vol. 16, pp Wu J. Y., Wang R. Z., Xu Y. X. Dynamic simulation and experiments of a heat regenerative adsorption heat pump // Energy Conversion and Management, 2000, Vol. 41, pp Glueckauf E. Part 10. Formulae for diffusion into spheres and their application to chromatography // Trans. Faraday Soc. 1955, Vol. 51, pp Crank J. Mathematics of Diffusion, Oxford University Press, London, Ruthven D. M. Principles of adsorption and adsorption processes, John Wiley&Sons, New York, I., Tokarev M. M., Freni A., Glaznev I. S., Restuccia G. Kinetics of water adsorption on silica Fuji Davison RD // Microporous & Mesoporous Materials, 2006, Vol. 96, pp Andersson J. Y., Bjurstroem H., Azoulay M., Carlsson B. Experimental and theoretical investigation of the kinetics of the sorption of water vapour by silica gel // J. Chem.Soc., Faraday Trans., 1985, Vol. 1, 81, pp Guilleminot J. J., Gurgel J. M. Heat transfer intensification in adsorbent beds of adsorption thermal devices // Proc. Intern. Solar Energy Conf. ASME, Miami, USA, April 1990, pp Zhang L. Z., Wang L., Momentum and heat transfer in the adsorbent of a waste-heat adsorption cooling system // Energy, 1999, Vol. 24, pp Marletta L., Maggio G., Freni A., Ingrasciotta M., Restuccia G. A non-uniform temperature nonuniform pressure dynamic model of heat and mass transfer in compact adsorbent beds // Int. J. Heat Mass Transfer, 2002, Vol. 45, pp Saha B. B., Chakraborty A., Koyama S., I. A New Generation Cooling Device Employing CaCl 2 -in-silica gel Water System // Intern. J. Heat Mass Transfer, 2008, (accepted). 32. Heifets L. I., Predtechenskaya D. M., Pavlov Yu. V., Okunev B. N. Modelling of dynamic effects in the adsorbent beds. 1. Simple method to estimate thermal conductivity of the composite adsorbent bed (CaCl 2, impregnated into pores of silica gel lattice) // Moscow University Chemistry Bulletin, 2006, Vol. 61, pp Strauss R., Schallenberg K., Knocke K.F. Measurement of the kinetics of water vapour adsorption into solid zeolite layers // Proc. Int. Symp. Solid Sorption Refrigeration, Paris, November 18-21, 1992, pp Dawoud B., I. Experimental study on the kinetics of water vapour sorption on selective water sorbents, silica-gels and alumina under typical operating conditions of sorption heat pumps // Intern. J. Heat & Mass Transfer, 2003, Vol. 46, pp Kakiuchi H. M., Iwade S., Shimooka S., Ooshima K., Yamazaki M. and Takewaki T. Water Vapour Adsorbent FAM-Z02 and Applicability to Adsorption Heat Pump // Kagaku Kogaku Ronbunshu, 2005, Vol. 31, pp Lang R., Roth M., Stricker M. Development of a modular zeolite-water heat pump // Proceeding of International Sorption Heat Pump Conference ISHPC 99, Munich, Germany, 1999, pp Nusselt W. Surface condensation of water vapour // Z. Ver. Deut. Ing., 1916, Vol. 60, pp
13 38. Glaznev I. S., Ovoshchnikov D. S., I. Kinetics of water adsorption under isobaric stages of adsorption heat pumps: the effect of isobar shape // Intern. J. Heat &Mass Transfer, 2008, (submitted). 296
Ammonia sorption on composites CaCl 2 in inorganic host matrix : isosteric chart and its performance
 Ammonia sorption on composites CaCl 2 in inorganic host matrix : isosteric chart and its performance Vasily E. Sharonov 1, Janna V. Veselovskaya 2 and Yury I. Aristov 1 (corresponding author) 1 Boreskov
Ammonia sorption on composites CaCl 2 in inorganic host matrix : isosteric chart and its performance Vasily E. Sharonov 1, Janna V. Veselovskaya 2 and Yury I. Aristov 1 (corresponding author) 1 Boreskov
A Novel Model Considered Mass and Energy Conservation for Both Liquid and Vapor in Adsorption Refrigeration System.
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2016 A Novel Model Considered Mass and Energy Conservation for Both Liquid and
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2016 A Novel Model Considered Mass and Energy Conservation for Both Liquid and
Evaluation of the Characteristic of Adsorption in a Double-Effect Adsorption Chiller with FAM-Z01
 Journal of Materials Science and Chemical Engineering, 2016, 4, 8-19 http://www.scirp.org/journal/msce ISSN Online: 2327-6053 ISSN Print: 2327-6045 Evaluation of the Characteristic of Adsorption in a Double-Effect
Journal of Materials Science and Chemical Engineering, 2016, 4, 8-19 http://www.scirp.org/journal/msce ISSN Online: 2327-6053 ISSN Print: 2327-6045 Evaluation of the Characteristic of Adsorption in a Double-Effect
A generic adsorption heat pump model for system simulations in TRNSYS
 A generic adsorption heat pump model for system simulations in TRNSYS Christian Glück and Ferdinand P. Schmidt Karlsruhe Institute of Technology, Kaiserstraße 12, 76131 Karlsruhe, Germany Phone: +49 721
A generic adsorption heat pump model for system simulations in TRNSYS Christian Glück and Ferdinand P. Schmidt Karlsruhe Institute of Technology, Kaiserstraße 12, 76131 Karlsruhe, Germany Phone: +49 721
Selective water sorbent for solid sorption chiller: experimental results and modelling
 International Journal of Refrigeration 27 (2004) 284 293 www.elsevier.com/locate/ijrefrig Selective water sorbent for solid sorption chiller: experimental results and modelling G. Restuccia a, *, A. Freni
International Journal of Refrigeration 27 (2004) 284 293 www.elsevier.com/locate/ijrefrig Selective water sorbent for solid sorption chiller: experimental results and modelling G. Restuccia a, *, A. Freni
Investigation into NH 3 -MnCl 2 and NH 3 -CaCl 2 Reaction Rates for the Development of a Thermal Transformer
 Investigation into NH 3 -MnCl 2 and NH 3 -CaCl 2 Reaction Rates for the Development of a Thermal Transformer J. Locke, M. King, S. Hassan, R. Baxter, J. Chan, S. Woodward, L. Brady School of Engineering,
Investigation into NH 3 -MnCl 2 and NH 3 -CaCl 2 Reaction Rates for the Development of a Thermal Transformer J. Locke, M. King, S. Hassan, R. Baxter, J. Chan, S. Woodward, L. Brady School of Engineering,
AN OPTIMAL SORBENT FOR ADSORPTION HEAT PUMPS: THERMODYNAMIC REQUIREMENTS AND MOLECULAR DESIGN
 VI Minsk International Seminar Heat Pipes, Heat Pumps, Refrigerators AN OPTIMAL SORBENT FOR ADSORPTION HEAT PUMPS: THERMODYNAMIC REQUIREMENTS AND MOLECULAR DESIGN Yury I. Aristov Boreskov Institute of
VI Minsk International Seminar Heat Pipes, Heat Pumps, Refrigerators AN OPTIMAL SORBENT FOR ADSORPTION HEAT PUMPS: THERMODYNAMIC REQUIREMENTS AND MOLECULAR DESIGN Yury I. Aristov Boreskov Institute of
Evaluation of Performance of Thermal and Electrical Hybrid Adsorption Chiller Cycles with Mechanical Booster Pumps
 Journal of Materials Science and Chemical Engineering, 217, 5, 22-32 http://www.scirp.org/journal/msce ISSN Online: 2327-653 ISSN Print: 2327-645 Evaluation of Performance of Thermal and Electrical Hybrid
Journal of Materials Science and Chemical Engineering, 217, 5, 22-32 http://www.scirp.org/journal/msce ISSN Online: 2327-653 ISSN Print: 2327-645 Evaluation of Performance of Thermal and Electrical Hybrid
INFLUENCES OF SALT HYDRATE MIXTURES AND PORE SIZES ON THE SORPTION HEAT OF COMPOSITE TES MATERIALS. K. Posern, Ch. Kaps
 INFLUENCES OF SALT HYDRATE MIXTURES AND PORE SIZES ON THE SORPTION HEAT OF COMPOSITE TES MATERIALS ABSTRACT K. Posern, Ch. Kaps Bauhaus-University Weimar Coudraystraße 13 C, 99423 Weimar, Germany Tel:
INFLUENCES OF SALT HYDRATE MIXTURES AND PORE SIZES ON THE SORPTION HEAT OF COMPOSITE TES MATERIALS ABSTRACT K. Posern, Ch. Kaps Bauhaus-University Weimar Coudraystraße 13 C, 99423 Weimar, Germany Tel:
Pulsating heat pipe panels
 Pulsating heat pipe panels A.A. Antukh, M.I. Rabetsky, V.E. Romanenkov, L.L. Vasiliev Luikov Heat and Mass Transfer Institute, P. Brovka 15, 220072, Minsk, Belarus Phone/Fax: +375-17-284-21-33, E-mail:
Pulsating heat pipe panels A.A. Antukh, M.I. Rabetsky, V.E. Romanenkov, L.L. Vasiliev Luikov Heat and Mass Transfer Institute, P. Brovka 15, 220072, Minsk, Belarus Phone/Fax: +375-17-284-21-33, E-mail:
Performance investigation of a waste heat driven pressurized adsorption refrigeration cycle
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Performance investigation of a waste heat driven pressurized adsorption rigeration cycle o cite this article: K Habib 2015 IOP
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Performance investigation of a waste heat driven pressurized adsorption rigeration cycle o cite this article: K Habib 2015 IOP
Error in the Estimation of Effective Diffusion Coefficients from Sorption Measurements*
 Error in the Estimation of Effective Diffusion Coefficients from Sorption Measurements* D. BOBOK and E. BESEDOVÁ Department of Chemical and Biochemical Engineering, Faculty of Chemical Technology, Slovak
Error in the Estimation of Effective Diffusion Coefficients from Sorption Measurements* D. BOBOK and E. BESEDOVÁ Department of Chemical and Biochemical Engineering, Faculty of Chemical Technology, Slovak
Influence of water on the total heat transfer in evacuated insulations
 Bavarian Center for Applied Energy Research Influence of water on the total heat transfer in evacuated insulations Ulrich Heinemann ZAE Bayern, Würzburg, Germany ulrich.heinemann@zae.uni-wuerzburg.de 7
Bavarian Center for Applied Energy Research Influence of water on the total heat transfer in evacuated insulations Ulrich Heinemann ZAE Bayern, Würzburg, Germany ulrich.heinemann@zae.uni-wuerzburg.de 7
Energy Procedia 00 (2011) SHC J. Jänchen a, H. Stach b, a*
 Energy Procedia 00 (2011) 000 000 Energy Procedia www.elsevier.com/locate/procedia SHC 2012 Adsorption properties of porous materials for solar thermal energy storage and heat pump applications J. Jänchen
Energy Procedia 00 (2011) 000 000 Energy Procedia www.elsevier.com/locate/procedia SHC 2012 Adsorption properties of porous materials for solar thermal energy storage and heat pump applications J. Jänchen
QUASI STEADY STATE MODEL FOR ADSORPTION COOLING SYSTEMS: AUTOMOTIVE APPLICATIONS
 Proceedings of the ASME 22 6th International Conference on Energy Sustainability & th Fuel Cell Science, Engineering and Technology Conference ESFuelCell22 July 23-26, 22, San Diego, CA, USA ESFuelCell22-9362
Proceedings of the ASME 22 6th International Conference on Energy Sustainability & th Fuel Cell Science, Engineering and Technology Conference ESFuelCell22 July 23-26, 22, San Diego, CA, USA ESFuelCell22-9362
Study of the performance of activated carbon methanol adsorption systems concerning heat and mass transfer
 Applied Thermal Engineering 23 (2003) 1605 1617 www.elsevier.com/locate/apthermeng Study of the performance of activated carbon methanol adsorption systems concerning heat and mass transfer L.W. Wang,
Applied Thermal Engineering 23 (2003) 1605 1617 www.elsevier.com/locate/apthermeng Study of the performance of activated carbon methanol adsorption systems concerning heat and mass transfer L.W. Wang,
Basic Principles of an Adsorption Heat Storage System
 Development of a High Energy Density Sorption Storage System Günter Gartler, Dagmar Jähnig, Gottfried Purkarthofer, Waldemar Wagner AEE-INTEC, A-82 Gleisdorf, Feldgasse 19, Austria Phone: +43/3112/5886/64,
Development of a High Energy Density Sorption Storage System Günter Gartler, Dagmar Jähnig, Gottfried Purkarthofer, Waldemar Wagner AEE-INTEC, A-82 Gleisdorf, Feldgasse 19, Austria Phone: +43/3112/5886/64,
NATIONAL RESEARCH COUNCIL OF ITALY
 NATIONAL RESEARCH COUNCIL OF ITALY Institute for Advanced Energy Technologies Nicola Giordano Thermally driven adsorption heat pumps: recent advancements and future technical challenges Giovanni Restuccia
NATIONAL RESEARCH COUNCIL OF ITALY Institute for Advanced Energy Technologies Nicola Giordano Thermally driven adsorption heat pumps: recent advancements and future technical challenges Giovanni Restuccia
Title Process of Ethanol in Activated Car. Citation Physics Procedia (2015), 69:
 Title Visualization and Measurement of Ad Process of Ethanol in Activated Car Author(s) Asano, Hitoshi; Murata, Kenta; Take Yasushi Citation Physics Procedia (2015), 69: 503-50 Issue Date 2015 URL http://hdl.handle.net/2433/216137
Title Visualization and Measurement of Ad Process of Ethanol in Activated Car Author(s) Asano, Hitoshi; Murata, Kenta; Take Yasushi Citation Physics Procedia (2015), 69: 503-50 Issue Date 2015 URL http://hdl.handle.net/2433/216137
Experimental investigation of the silica gel±water adsorption isotherm characteristics
 Applied Thermal Engineering 21 2001) 1631±1642 www.elsevier.com/locate/apthermeng Experimental investigation of the silica gel±water adsorption isotherm characteristics K.C. Ng a, *, H.T. Chua a,b, C.Y.
Applied Thermal Engineering 21 2001) 1631±1642 www.elsevier.com/locate/apthermeng Experimental investigation of the silica gel±water adsorption isotherm characteristics K.C. Ng a, *, H.T. Chua a,b, C.Y.
Applications of Adsorption Refrigeration Cycle Review
 International Journal of Emerging Research in Management &Technology Research Article May 2015 Applications of Adsorption Refrigeration Cycle Review 1 Bhushan M. Dusane, 2 N. C. Ghuge 1 Department of Mechanical
International Journal of Emerging Research in Management &Technology Research Article May 2015 Applications of Adsorption Refrigeration Cycle Review 1 Bhushan M. Dusane, 2 N. C. Ghuge 1 Department of Mechanical
Apparent diffusivity of water in silica gel and NaX zeolite pellets
 High Temperatures ^ High Pressures, 2001, volume 33, pages 435 ^ 439 15 ECTP Proceedings pages 1003 ^ 1007 DOI:10.1068/htwu90 Apparent diffusivity of water in silica gel and NaX zeolite pellets Jose M
High Temperatures ^ High Pressures, 2001, volume 33, pages 435 ^ 439 15 ECTP Proceedings pages 1003 ^ 1007 DOI:10.1068/htwu90 Apparent diffusivity of water in silica gel and NaX zeolite pellets Jose M
A Review On Solar Adsorption Refrigeration System
 IOSR Journal of Engineering (IOSRJEN) ISSN (e): 2250-3021, ISSN (p): 2278-8719 Vol. 06, Issue 11 (Nov. 2016), V1 PP 07-13 www.iosrjen.org A Review On Solar Adsorption Refrigeration System Prof B. M. Dusane
IOSR Journal of Engineering (IOSRJEN) ISSN (e): 2250-3021, ISSN (p): 2278-8719 Vol. 06, Issue 11 (Nov. 2016), V1 PP 07-13 www.iosrjen.org A Review On Solar Adsorption Refrigeration System Prof B. M. Dusane
III E
 THE AMERICAN SOCIETY OF MECHANICAL ENGINEERS 345 E. 47th St., New York, N.Y. 10017. 9i-M633 The Society shall not be responsible for statements or opinions advanced ii papers or thicussion at meetings
THE AMERICAN SOCIETY OF MECHANICAL ENGINEERS 345 E. 47th St., New York, N.Y. 10017. 9i-M633 The Society shall not be responsible for statements or opinions advanced ii papers or thicussion at meetings
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Diffusion and Adsorption in porous media. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Diffusion and Adsorption in porous media Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Devices used to Measure Diffusion in Porous Solids Modes of transport in
Diffusion and Adsorption in porous media Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Devices used to Measure Diffusion in Porous Solids Modes of transport in
Heat Transfer Analysis of Centric Borehole Heat Exchanger with Different Backfill Materials
 Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Heat Transfer Analysis of Centric Borehole Heat Exchanger with Different Backfill Materials Lei H.Y. and Dai C.S. Geothermal
Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Heat Transfer Analysis of Centric Borehole Heat Exchanger with Different Backfill Materials Lei H.Y. and Dai C.S. Geothermal
Adsorption Equilibrium and Kinetics of H 2 O on Zeolite 13X
 Korean J. Chem. Eng., 8(4), 55-530 (00) Adsorption Equilibrium and Kinetics of H O on Zeolite 3X Young Ki Ryu*, Seung Ju Lee, Jong Wha Kim and Chang-Ha Lee *External Relations Department, Procter & Gamble
Korean J. Chem. Eng., 8(4), 55-530 (00) Adsorption Equilibrium and Kinetics of H O on Zeolite 3X Young Ki Ryu*, Seung Ju Lee, Jong Wha Kim and Chang-Ha Lee *External Relations Department, Procter & Gamble
A New Mass Transfer Model for Cyclic Adsorption and Desorption
 Korean J. Chem. Eng., 63), 40-405 999) SHORT COMMUNICATION A New Mass Transfer Model for Cyclic Adsorption and Desorption Yong-Min Kim* and Sung-Sup Suh Department of Chemical Engineering, Hong-Ik University,
Korean J. Chem. Eng., 63), 40-405 999) SHORT COMMUNICATION A New Mass Transfer Model for Cyclic Adsorption and Desorption Yong-Min Kim* and Sung-Sup Suh Department of Chemical Engineering, Hong-Ik University,
Characterisation of Microporous Materials by Finite Concentration Inverse Gas Chromatography
 Characterisation of Microporous Materials by Finite Concentration Inverse Gas Chromatography Surface Measurement Systems Ltd. Finite concentration IGC SEA is a useful tool for the investigation of surface
Characterisation of Microporous Materials by Finite Concentration Inverse Gas Chromatography Surface Measurement Systems Ltd. Finite concentration IGC SEA is a useful tool for the investigation of surface
Preparation, hydrothermal stability and thermal adsorption storage properties of binderless zeolite beads
 International Journal of Low-Carbon Technologies Advance Access published May 5, 2012 *Corresponding author: jochen.jaenchen@th-wildau. de Preparation, hydrothermal stability and thermal adsorption storage
International Journal of Low-Carbon Technologies Advance Access published May 5, 2012 *Corresponding author: jochen.jaenchen@th-wildau. de Preparation, hydrothermal stability and thermal adsorption storage
Synthesis and adsorption property of hypercross-linked sorbent
 Journal of Scientific & Industrial Research 52 J SCI IND RES VOL 68 JANUARY 29 Vol. 68, January 29, pp. 52-56 Synthesis and adsorption property of hypercross-linked sorbent Li Dongguang*, Zhang Yanli and
Journal of Scientific & Industrial Research 52 J SCI IND RES VOL 68 JANUARY 29 Vol. 68, January 29, pp. 52-56 Synthesis and adsorption property of hypercross-linked sorbent Li Dongguang*, Zhang Yanli and
Complex Compounds Background of Complex Compound Technology
 Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
If there is convective heat transfer from outer surface to fluid maintained at T W.
 Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Adsorption of Polar and Nonpolar Vapors on Selected Adsorbents: Breakthrough Curves and their Simulation
 Adsorption of Polar and Nonpolar Vapors on Selected Adsorbents: Breakthrough Curves and their Simulation Dr. Robert Eschrich Quantachrome GmbH & Co. KG 2018-04-17 Leipziger Symposium on Dynamic Sorption
Adsorption of Polar and Nonpolar Vapors on Selected Adsorbents: Breakthrough Curves and their Simulation Dr. Robert Eschrich Quantachrome GmbH & Co. KG 2018-04-17 Leipziger Symposium on Dynamic Sorption
Investigations on Performance of an Auto-Cascade Absorption Refrigeration System Operating with Mixed Refrigerants
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2016 Investigations on Performance of an Auto-Cascade Absorption Refrigeration
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2016 Investigations on Performance of an Auto-Cascade Absorption Refrigeration
ScienceDirect. Fluid dynamics optimization of a novel isothermal adsorption dehumidification system for solar driven applications
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 48 (2014 ) 628 637 SHC 2013, International Conference on Solar Heating and Cooling for Buildings and Industry September 23-25, 2013,
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 48 (2014 ) 628 637 SHC 2013, International Conference on Solar Heating and Cooling for Buildings and Industry September 23-25, 2013,
A heat transfer correlation for transient vapor uptake of powdered adsorbent embedded onto the fins of heat exchangers
 A heat transfer correlation for transient vapor uptake of powdered adsorbent embedded onto the fins of heat exchangers Item Type Article Authors Li, Ang; Thu, Kyaw; Ismail, Azhar Bin; Ng, Kim Choon Citation
A heat transfer correlation for transient vapor uptake of powdered adsorbent embedded onto the fins of heat exchangers Item Type Article Authors Li, Ang; Thu, Kyaw; Ismail, Azhar Bin; Ng, Kim Choon Citation
Examination Heat Transfer
 Examination Heat Transfer code: 4B680 date: 17 january 2006 time: 14.00-17.00 hours NOTE: There are 4 questions in total. The first one consists of independent sub-questions. If necessary, guide numbers
Examination Heat Transfer code: 4B680 date: 17 january 2006 time: 14.00-17.00 hours NOTE: There are 4 questions in total. The first one consists of independent sub-questions. If necessary, guide numbers
EXECUTIVE SUMMARY. especially in last 50 years. Industries, especially power industry, are the large anthropogenic
 EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
Study of thermal and adsorption characteristics of composite ammonia adsorber with expanded graphite and using nano fluids.
 International Engineering Research Journal Special Edition PGCON-MECH-2017 Study of thermal and adsorption characteristics of composite ammonia adsorber with expanded graphite and using nano fluids. Swapnil
International Engineering Research Journal Special Edition PGCON-MECH-2017 Study of thermal and adsorption characteristics of composite ammonia adsorber with expanded graphite and using nano fluids. Swapnil
INVESTIGATION OF VAPOR GENERATION INTO CAPILLARY STRUCTURES OF MINIATURE LOOP HEAT PIPES
 Minsk International Seminar Heat Pipes, Heat Pumps, Refrigerators Minsk, Belarus, September 8-, INESTIGATION OF APOR GENERATION INTO CAPIARY STRUCTURES OF MINIATURE OOP HEAT PIPES.M. Kiseev, A.S. Nepomnyashy,
Minsk International Seminar Heat Pipes, Heat Pumps, Refrigerators Minsk, Belarus, September 8-, INESTIGATION OF APOR GENERATION INTO CAPIARY STRUCTURES OF MINIATURE OOP HEAT PIPES.M. Kiseev, A.S. Nepomnyashy,
THE EXPERIMENTAL STUDY OF THE EFFECT OF ADDING HIGH-MOLECULAR POLYMERS ON HEAT TRANSFER CHARACTERISTICS OF NANOFLUIDS
 THE EXPERIMENTAL STUDY OF THE EFFECT OF ADDING HIGH-MOLECULAR POLYMERS ON HEAT TRANSFER CHARACTERISTICS OF NANOFLUIDS Dmitriy Guzei 1, *, Maxim Pryazhnikov 1, Andrey Minakov 1,, and Vladimir Zhigarev 1
THE EXPERIMENTAL STUDY OF THE EFFECT OF ADDING HIGH-MOLECULAR POLYMERS ON HEAT TRANSFER CHARACTERISTICS OF NANOFLUIDS Dmitriy Guzei 1, *, Maxim Pryazhnikov 1, Andrey Minakov 1,, and Vladimir Zhigarev 1
Lecture 44: Review Thermodynamics I
 ME 00 Thermodynamics I Lecture 44: Review Thermodynamics I Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R. China Email : liyo@sjtu.edu.cn
ME 00 Thermodynamics I Lecture 44: Review Thermodynamics I Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R. China Email : liyo@sjtu.edu.cn
Thermal conductivity measurement of two microencapsulated phase change slurries
 Thermal conductivity measurement of two microencapsulated phase change slurries Xiaoli Ma (corresponding author), Siddig Omer, Wei Zhang and S. B. Riffat Institute of Sustainable Energy Technology, School
Thermal conductivity measurement of two microencapsulated phase change slurries Xiaoli Ma (corresponding author), Siddig Omer, Wei Zhang and S. B. Riffat Institute of Sustainable Energy Technology, School
High-Pressure Volumetric Analyzer
 High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
First tests on a solid sorption prototype for seasonal solar thermal storage.
 First tests on a solid sorption prototype for seasonal solar thermal storage. Wim van Helden 1, Waldemar Wagner 1, Verena Schubert 1, Christof Krampe-Zadler 2, Henner Kerskes 3, Florian Bertsch 3, Barbara
First tests on a solid sorption prototype for seasonal solar thermal storage. Wim van Helden 1, Waldemar Wagner 1, Verena Schubert 1, Christof Krampe-Zadler 2, Henner Kerskes 3, Florian Bertsch 3, Barbara
Studies on Equilibrium and Dynamic Characteristics of New Adsorption Pairs. Yong Zhong
 Studies on Equilibrium and Dynamic Characteristics of New Adsorption Pairs Yong Zhong This dissertation has been submitted to the University of Warwick in the fulfillment of the requirements for the award
Studies on Equilibrium and Dynamic Characteristics of New Adsorption Pairs Yong Zhong This dissertation has been submitted to the University of Warwick in the fulfillment of the requirements for the award
A Lumped Parameter Heat Transfer Analysis for Composting Processes With Aeration
 A Lumped Parameter Heat Transfer Analysis for Composting Processes With Aeration Akira Nakayama 1 Department of Mechanical Engineering, Kiyohiko Nakasaki Department of Chemical Engineering, Fujio Kuwahara
A Lumped Parameter Heat Transfer Analysis for Composting Processes With Aeration Akira Nakayama 1 Department of Mechanical Engineering, Kiyohiko Nakasaki Department of Chemical Engineering, Fujio Kuwahara
Part I.
 Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Monte Carlo Simulation of Long-Range Self-Diffusion in Model Porous Membranes and Catalysts
 Monte Carlo Simulation of Long-Range Self-Diffusion in Model Porous Membranes and Catalysts Brian DeCost and Dr. Sergey Vasenkov College of Engineering, University of Florida Industrial processes involving
Monte Carlo Simulation of Long-Range Self-Diffusion in Model Porous Membranes and Catalysts Brian DeCost and Dr. Sergey Vasenkov College of Engineering, University of Florida Industrial processes involving
Effect of moisture transfer on heat energy storage in simple layer walls
 Effect of moisture transfer on heat energy storage in simple layer walls C. MAALOUF 1, A.D. TRAN LE 1, M. LACHI 1, E. WURTZ 2, T.H. MAI 1 1-Laboratoire Thermomécanique/GRESPI, Faculté des Sciences, University
Effect of moisture transfer on heat energy storage in simple layer walls C. MAALOUF 1, A.D. TRAN LE 1, M. LACHI 1, E. WURTZ 2, T.H. MAI 1 1-Laboratoire Thermomécanique/GRESPI, Faculté des Sciences, University
A dynamic model of a vertical direct expansion ground heat exchanger
 A dynamic model of a vertical direct expansion ground heat exchanger B. Beauchamp 1, L. Lamarche 1 and S. Kajl 1 1 Department of mechanical engineering École de technologie supérieure 1100 Notre-Dame Ouest,
A dynamic model of a vertical direct expansion ground heat exchanger B. Beauchamp 1, L. Lamarche 1 and S. Kajl 1 1 Department of mechanical engineering École de technologie supérieure 1100 Notre-Dame Ouest,
Mathematical Modelling for Refrigerant Flow in Diabatic Capillary Tube
 Mathematical Modelling for Refrigerant Flow in Diabatic Capillary Tube Jayant Deshmukh Department of Mechanical Engineering Sagar Institute of Research and Technology, Bhopal, M.P., India D.K. Mudaiya
Mathematical Modelling for Refrigerant Flow in Diabatic Capillary Tube Jayant Deshmukh Department of Mechanical Engineering Sagar Institute of Research and Technology, Bhopal, M.P., India D.K. Mudaiya
THERMODYNAMIC ANALYSIS OF ADSORPTION COOLING CYCLE USING CONSOLIDATED COMPOSITE ADSORBENTS - ETHANOL PAIRS
 VOL., NO. 2, OCTOBER 26 ISSN 89-668 26-26 Asian Research Publishing Network (ARPN). All rights reserve. THERMODYNAMIC ANALYSIS OF ADSORPTION COOLING CYCLE USING CONSOLIDATED COMPOSITE ADSORBENTS - ETHANOL
VOL., NO. 2, OCTOBER 26 ISSN 89-668 26-26 Asian Research Publishing Network (ARPN). All rights reserve. THERMODYNAMIC ANALYSIS OF ADSORPTION COOLING CYCLE USING CONSOLIDATED COMPOSITE ADSORBENTS - ETHANOL
Investigations Of Heat Transfer And Components Efficiencies In Two-Phase Isobutane Injector
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering July 208 Investigations Of Heat Transfer And Components Efficiencies In Two-Phase
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering July 208 Investigations Of Heat Transfer And Components Efficiencies In Two-Phase
THERMOCHEMICAL STORAGE OF LOW TEMPERATURE HEAT BY ZEOLITES; SAPO S AND IMPREGNATED ACTIVE CARBON
 THERMOCHEMICAL STORAGE OF LOW TEMPERATURE HEAT BY ZEOLITES; SAPO S AND IMPREGNATED ACTIVE CARBON J. Jänchen a, b, D. Ackermann b, E. Weiler b, H. Stach b and W. Brösicke a a Fachhochschule für Technik
THERMOCHEMICAL STORAGE OF LOW TEMPERATURE HEAT BY ZEOLITES; SAPO S AND IMPREGNATED ACTIVE CARBON J. Jänchen a, b, D. Ackermann b, E. Weiler b, H. Stach b and W. Brösicke a a Fachhochschule für Technik
Heat Transfer of Condensation in Smooth Round Tube from Superheated Vapor
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2016 Heat Transfer of Condensation in Smooth Round Tube from Superheated Vapor
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2016 Heat Transfer of Condensation in Smooth Round Tube from Superheated Vapor
Adsorption Characteristics of Water and Silica. Gel System for Desalination Cycle. Oscar Rodrigo Fonseca Cevallos
 Adsorption Characteristics of Water and Silica Gel System for Desalination Cycle Thesis by Oscar Rodrigo Fonseca Cevallos In Partial Fulfillment of the Requirements For the Degree of Master of Science
Adsorption Characteristics of Water and Silica Gel System for Desalination Cycle Thesis by Oscar Rodrigo Fonseca Cevallos In Partial Fulfillment of the Requirements For the Degree of Master of Science
, an adiabatic expansion machine, an isothermal compression machine at T L
 The Direct Method from Thermodynamics with Finite Speed used for Performance Computation of quasi-carnot Irreversible Cycles IEvaluation of coefficient of performance and power for refrigeration machines
The Direct Method from Thermodynamics with Finite Speed used for Performance Computation of quasi-carnot Irreversible Cycles IEvaluation of coefficient of performance and power for refrigeration machines
A Review on Performance Improvement of Adsorber Bed by Effective Heating and Cooling
 A Review on Performance Improvement of Adsorber Bed by Effective Heating and Cooling 1 Ameya C. Lohokare, 2 V. N. Kapatkar 1,2 Sinhgad College of Engineering Email: 1 ameya.c.lohokare@gmail.com, 2 vnkapatkar.scoe@sinhgad.edu
A Review on Performance Improvement of Adsorber Bed by Effective Heating and Cooling 1 Ameya C. Lohokare, 2 V. N. Kapatkar 1,2 Sinhgad College of Engineering Email: 1 ameya.c.lohokare@gmail.com, 2 vnkapatkar.scoe@sinhgad.edu
Scale-up problems are often perceived as difficult. Here the reaction calorimetry has proven to be
 APPLICATION OF REACTION CALORIMETRY FOR THE SOLUTION OF SCALE-UP PROBLEMS A paper from the RC User Forum Europe, Interlaken, 1995 Francis Stoessel, Ciba AG, Basel, Switzerland. Scale-up problems are often
APPLICATION OF REACTION CALORIMETRY FOR THE SOLUTION OF SCALE-UP PROBLEMS A paper from the RC User Forum Europe, Interlaken, 1995 Francis Stoessel, Ciba AG, Basel, Switzerland. Scale-up problems are often
CHAPTER - 12 THERMODYNAMICS
 CHAPER - HERMODYNAMICS ONE MARK QUESIONS. What is hermodynamics?. Mention the Macroscopic variables to specify the thermodynamics. 3. How does thermodynamics differ from Mechanics? 4. What is thermodynamic
CHAPER - HERMODYNAMICS ONE MARK QUESIONS. What is hermodynamics?. Mention the Macroscopic variables to specify the thermodynamics. 3. How does thermodynamics differ from Mechanics? 4. What is thermodynamic
Salt/Zeolite Composite Materials for Thermochemical Energy Storage Steffen Beckert Roger Gläser
 Salt/Zeolite Composite Materials for Thermochemical Energy Storage Steffen Beckert Roger Gläser Institute of Chemical Technology Universität Leipzig Symposium Dynamische Sorptionsverfahren Leipzig, May
Salt/Zeolite Composite Materials for Thermochemical Energy Storage Steffen Beckert Roger Gläser Institute of Chemical Technology Universität Leipzig Symposium Dynamische Sorptionsverfahren Leipzig, May
A 3 Vapor pressure of volatile liquids
 Versuchsanleitungen zum Praktikum Physikalische Chemie für Anfänger 1 A 3 Vapor pressure of volatile liquids Purpose The purpose of this experiment is to determine the vaporization enthalpy, entropy and
Versuchsanleitungen zum Praktikum Physikalische Chemie für Anfänger 1 A 3 Vapor pressure of volatile liquids Purpose The purpose of this experiment is to determine the vaporization enthalpy, entropy and
Thermal Systems. What and How? Physical Mechanisms and Rate Equations Conservation of Energy Requirement Control Volume Surface Energy Balance
 Introduction to Heat Transfer What and How? Physical Mechanisms and Rate Equations Conservation of Energy Requirement Control Volume Surface Energy Balance Thermal Resistance Thermal Capacitance Thermal
Introduction to Heat Transfer What and How? Physical Mechanisms and Rate Equations Conservation of Energy Requirement Control Volume Surface Energy Balance Thermal Resistance Thermal Capacitance Thermal
Possibilities and Limits for the Determination of. Adsorption Data Pure Gases and Gas Mixtures
 MOF-Workshop, Leipzig, March 2010 Possibilities and Limits for the Determination of Adsorption Data Pure Gases and Gas Mixtures Reiner Staudt Instutut für Nichtklassische Chemie e.v. Permoserstraße 15,
MOF-Workshop, Leipzig, March 2010 Possibilities and Limits for the Determination of Adsorption Data Pure Gases and Gas Mixtures Reiner Staudt Instutut für Nichtklassische Chemie e.v. Permoserstraße 15,
MOLECULAR DYNAMICS SIMULATION ON TOTAL THERMAL RESISTANCE OF NANO TRIANGULAR PIPE
 ISTP-16, 2005, PRAGUE 16 TH INTERNATIONAL SYMPOSIUM ON TRANSPORT PHENOMENA MOLECULAR DYNAMICS SIMULATION ON TOTAL THERMAL RESISTANCE OF NANO TRIANGULAR PIPE C.S. Wang* J.S. Chen* and S. Maruyama** * Department
ISTP-16, 2005, PRAGUE 16 TH INTERNATIONAL SYMPOSIUM ON TRANSPORT PHENOMENA MOLECULAR DYNAMICS SIMULATION ON TOTAL THERMAL RESISTANCE OF NANO TRIANGULAR PIPE C.S. Wang* J.S. Chen* and S. Maruyama** * Department
MOLECULAR SIMULATION OF THE MICROREGION
 GASMEMS2010-HT01 MOLECULAR SIMULATION OF THE MICROREGION E.A.T. van den Akker 1, A.J.H. Frijns 1, P.A.J. Hilbers 1, P. Stephan 2 and A.A. van Steenhoven 1 1 Eindhoven University of Technology, Eindhoven,
GASMEMS2010-HT01 MOLECULAR SIMULATION OF THE MICROREGION E.A.T. van den Akker 1, A.J.H. Frijns 1, P.A.J. Hilbers 1, P. Stephan 2 and A.A. van Steenhoven 1 1 Eindhoven University of Technology, Eindhoven,
 9 th International Conference on Quantitative InfraRed Thermography July 2-5, 2008, Krakow - Poland Application of infrared thermography for validation of numerical analyses results of a finned cross-flow
9 th International Conference on Quantitative InfraRed Thermography July 2-5, 2008, Krakow - Poland Application of infrared thermography for validation of numerical analyses results of a finned cross-flow
Lecture 7. Sorption-Separation Equipment
 Lecture 7. Sorption-Separation Equipment Adsorption - Stirred-tank, slurry operation - Cyclic fixed-bed batch operation - Thermal (temperature)-swing adsorption - Fluidizing bed for adsorption and moving
Lecture 7. Sorption-Separation Equipment Adsorption - Stirred-tank, slurry operation - Cyclic fixed-bed batch operation - Thermal (temperature)-swing adsorption - Fluidizing bed for adsorption and moving
On the effective thermal conductivity of wetted zeolite under the working conditions of an adsorption chiller
 On the effective thermal conductivity of wetted zeolite under the working conditions of an adsorption chiller B. Dawoud, M. Imroz Sohel, A. Freni, S. Vasta, G. Restuccia To cite this version: B. Dawoud,
On the effective thermal conductivity of wetted zeolite under the working conditions of an adsorption chiller B. Dawoud, M. Imroz Sohel, A. Freni, S. Vasta, G. Restuccia To cite this version: B. Dawoud,
S.E. (Chemical Engineering) (Second Semester)EXAMINATION, 2012 THERMODYNAMICS-I (2008 PATTERN) Time : Three Hours Maximum Marks : 100
 Total No. of Questions 12] [Total No. of Printed Pages 7 Seat No. [4162]-189 S.E. (Chemical Engineering) (Second Semester)EXAMINATION, 2012 THERMODYNAMICS-I (2008 PATTERN) Time : Three Hours Maximum Marks
Total No. of Questions 12] [Total No. of Printed Pages 7 Seat No. [4162]-189 S.E. (Chemical Engineering) (Second Semester)EXAMINATION, 2012 THERMODYNAMICS-I (2008 PATTERN) Time : Three Hours Maximum Marks
(Heat capacity c is also called specific heat) this means that the heat capacity number c for water is 1 calorie/gram-k.
 Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Development of Advanced Multi Stage Adsorption Refrigeration Cycles
 Development of Advanced Multi Stage Adsorption Refrigeration Cycles DISSERTATION A.F.M. MIZANUR RAHMAN March 2013 GRADUATE SCHOOL OF BIO-APPLICATIONS AND SYSTEMS ENGINEERING TOKYO UNIVERSITY OF AGRICULTURE
Development of Advanced Multi Stage Adsorption Refrigeration Cycles DISSERTATION A.F.M. MIZANUR RAHMAN March 2013 GRADUATE SCHOOL OF BIO-APPLICATIONS AND SYSTEMS ENGINEERING TOKYO UNIVERSITY OF AGRICULTURE
This document is downloaded from DR-NTU, Nanyang Technological University Library, Singapore.
 This document is downloaded from DR-NTU, Nanyang Technological University Library, Singapore. Title Improved adsorption characteristics data for AQSOA types zeolites and water systems under static and
This document is downloaded from DR-NTU, Nanyang Technological University Library, Singapore. Title Improved adsorption characteristics data for AQSOA types zeolites and water systems under static and
Applied Thermal Engineering
 Applied Thermal Engineering 3 (2) 49e46 Contents lists available at ScienceDirect Applied Thermal Engineering journal homepage: www.elsevier.com/locate/apthermeng Adsorption kinetics of zeolite coatings
Applied Thermal Engineering 3 (2) 49e46 Contents lists available at ScienceDirect Applied Thermal Engineering journal homepage: www.elsevier.com/locate/apthermeng Adsorption kinetics of zeolite coatings
Liquid water is one of the
 Formanski 71 1/07/09 8:57 Page 71 V olume 5 - Number 7 - May 2009 (71-75) Abstract Liquid water is one of the agents responsible for damage of building materials. Therefore determination of its content
Formanski 71 1/07/09 8:57 Page 71 V olume 5 - Number 7 - May 2009 (71-75) Abstract Liquid water is one of the agents responsible for damage of building materials. Therefore determination of its content
Properties of Vapors
 Properties of Vapors Topics for Discussion The Pressure/Temperature Relationship Vaporization Condensation Enthalpy Properties of Vapors Topics for Discussion Entropy Properties of Substances Saturated
Properties of Vapors Topics for Discussion The Pressure/Temperature Relationship Vaporization Condensation Enthalpy Properties of Vapors Topics for Discussion Entropy Properties of Substances Saturated
HEAT AND MASS TRANSFER CHARACTERISTICS OF A ZEOLITE 13X/CACL 2 COMPOSITE ADSORBENT IN ADSORPTION COOLING SYSTEMS
 Proceedings of the ASME 212 6th International Conference on Energy Sustainability & 1th Fuel Cell Science, Engineering and Technology Conference ESFuelCell212 July 23-26, 212, San Diego, CA, USA ESFuelCell212-91246
Proceedings of the ASME 212 6th International Conference on Energy Sustainability & 1th Fuel Cell Science, Engineering and Technology Conference ESFuelCell212 July 23-26, 212, San Diego, CA, USA ESFuelCell212-91246
INTRODUCTION TO CATALYTIC COMBUSTION
 INTRODUCTION TO CATALYTIC COMBUSTION R.E. Hayes Professor of Chemical Engineering Department of Chemical and Materials Engineering University of Alberta, Canada and S.T. Kolaczkowski Professor of Chemical
INTRODUCTION TO CATALYTIC COMBUSTION R.E. Hayes Professor of Chemical Engineering Department of Chemical and Materials Engineering University of Alberta, Canada and S.T. Kolaczkowski Professor of Chemical
QUESTION ANSWER. . e. Fourier number:
 QUESTION 1. (0 pts) The Lumped Capacitance Method (a) List and describe the implications of the two major assumptions of the lumped capacitance method. (6 pts) (b) Define the Biot number by equations and
QUESTION 1. (0 pts) The Lumped Capacitance Method (a) List and describe the implications of the two major assumptions of the lumped capacitance method. (6 pts) (b) Define the Biot number by equations and
S6. (a) State what is meant by an ideal gas...
 IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 3 TEST REVIEW S1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation.
IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 3 TEST REVIEW S1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation.
Design, Testing and Pharmaceutical Applications of a Gas Pressure Controller Device for Solid - Gas Microcalorimetric Titration
 Design, Testing and Pharmaceutical Applications of a Gas Pressure Controller Device for Solid - Gas Microcalorimetric Titration A. Bakri University Joseph Fourier Faculty of Pharmacy Pharmaceutical Engineering
Design, Testing and Pharmaceutical Applications of a Gas Pressure Controller Device for Solid - Gas Microcalorimetric Titration A. Bakri University Joseph Fourier Faculty of Pharmacy Pharmaceutical Engineering
Kinetics of Water Adsorption in Microporous Aluminophosphate Layers for Regenerative Heat Exchangers
 Kinetics of Water Adsorption in Microporous Aluminophosphate Layers for Regenerative Heat Exchangers Hendrik Van Heyden, Gunther Munz, Lena Schnabel, Ferdinand Schmidt, Svetlana Mintova, Thomas Bein To
Kinetics of Water Adsorption in Microporous Aluminophosphate Layers for Regenerative Heat Exchangers Hendrik Van Heyden, Gunther Munz, Lena Schnabel, Ferdinand Schmidt, Svetlana Mintova, Thomas Bein To
Chemical Reaction Engineering Prof. Jayant Modak Department of Chemical Engineering Indian Institute of Science, Bangalore
 Chemical Reaction Engineering Prof. Jayant Modak Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture No. # 26 Problem solving : Heterogeneous reactions Friends, in last few
Chemical Reaction Engineering Prof. Jayant Modak Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture No. # 26 Problem solving : Heterogeneous reactions Friends, in last few
6. (6) Show all the steps of how to convert 50.0 F into its equivalent on the Kelvin scale.
 General Physics I Quiz 8 - Ch. 13 - Temperature & Kinetic Theory July 30, 2009 Name: Make your work clear to the grader. Show formulas used. Give correct units and significant figures. Partial credit is
General Physics I Quiz 8 - Ch. 13 - Temperature & Kinetic Theory July 30, 2009 Name: Make your work clear to the grader. Show formulas used. Give correct units and significant figures. Partial credit is
Principles of Food and Bioprocess Engineering (FS 231) Problems on Heat Transfer
 Principles of Food and Bioprocess Engineering (FS 1) Problems on Heat Transfer 1. What is the thermal conductivity of a material 8 cm thick if the temperature at one end of the product is 0 C and the temperature
Principles of Food and Bioprocess Engineering (FS 1) Problems on Heat Transfer 1. What is the thermal conductivity of a material 8 cm thick if the temperature at one end of the product is 0 C and the temperature
Theoretical and Experimental Studies on Transient Heat Transfer for Forced Convection Flow of Helium Gas over a Horizontal Cylinder
 326 Theoretical and Experimental Studies on Transient Heat Transfer for Forced Convection Flow of Helium Gas over a Horizontal Cylinder Qiusheng LIU, Katsuya FUKUDA and Zheng ZHANG Forced convection transient
326 Theoretical and Experimental Studies on Transient Heat Transfer for Forced Convection Flow of Helium Gas over a Horizontal Cylinder Qiusheng LIU, Katsuya FUKUDA and Zheng ZHANG Forced convection transient
Basic Thermodynamics Module 1
 Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
The study of dynamic process of ORC variable conditions based on control characteristics analysis
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 00 (2017) 000 000 www.elsevier.com/locate/procedia IV International Seminar on ORC Power Systems, ORC2017 13-15 September 2017, Milano,
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 00 (2017) 000 000 www.elsevier.com/locate/procedia IV International Seminar on ORC Power Systems, ORC2017 13-15 September 2017, Milano,
Article Experimental Investigations of Composite Adsorbent 13X/CaCl2 on an Adsorption Cooling System
 Article Experimental Investigations of Composite Adsorbent 13X/CaCl2 on an Adsorption Cooling System Huizhong Zhao 1, *, Shaolong Jia 1, Junfeng Cheng 1, Xianghu Tang 1, Min Zhang 2, Haoxin Yan 1 and Wenting
Article Experimental Investigations of Composite Adsorbent 13X/CaCl2 on an Adsorption Cooling System Huizhong Zhao 1, *, Shaolong Jia 1, Junfeng Cheng 1, Xianghu Tang 1, Min Zhang 2, Haoxin Yan 1 and Wenting
EVALUATION OF THE THERMAL AND HYDRAULIC PERFORMANCES OF A VERY THIN SINTERED COPPER FLAT HEAT PIPE FOR 3D MICROSYSTEM PACKAGES
 Stresa, Italy, 25-27 April 2007 EVALUATION OF THE THERMAL AND HYDRAULIC PERFORMANCES OF A VERY THIN SINTERED COPPER FLAT HEAT PIPE FOR 3D MICROSYSTEM PACKAGES Slavka Tzanova 1, Lora Kamenova 2, Yvan Avenas
Stresa, Italy, 25-27 April 2007 EVALUATION OF THE THERMAL AND HYDRAULIC PERFORMANCES OF A VERY THIN SINTERED COPPER FLAT HEAT PIPE FOR 3D MICROSYSTEM PACKAGES Slavka Tzanova 1, Lora Kamenova 2, Yvan Avenas
Hydrogen Adsorption and Storage on Porous Materials. School of Chemical Engineering and Advanced Materials. Newcastle University United Kingdom
 Hydrogen Adsorption and Storage on Porous Materials K. M. Thomas. School of Chemical Engineering and Advanced Materials H2FC SUPERGEN Conference Birmingham University, 16-18 th December 2013 Newcastle
Hydrogen Adsorption and Storage on Porous Materials K. M. Thomas. School of Chemical Engineering and Advanced Materials H2FC SUPERGEN Conference Birmingham University, 16-18 th December 2013 Newcastle
Chapter 4: Transient Heat Conduction. Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University
 Chapter 4: Transient Heat Conduction Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives When you finish studying this chapter, you should be able to: Assess when the spatial
Chapter 4: Transient Heat Conduction Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives When you finish studying this chapter, you should be able to: Assess when the spatial
Introduction. Monday, January 6, 14
 Introduction 1 Introduction Why to use a simulation Some examples of questions we can address 2 Molecular Simulations Molecular dynamics: solve equations of motion Monte Carlo: importance sampling Calculate
Introduction 1 Introduction Why to use a simulation Some examples of questions we can address 2 Molecular Simulations Molecular dynamics: solve equations of motion Monte Carlo: importance sampling Calculate
Effective Borehole Thermal Resistance of A Single U-Tube Ground Heat Exchanger
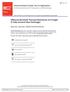 Numerical Heat Transfer, Part A: Applications An International Journal of Computation and Methodology ISSN: 1040-7782 (Print) 1521-0634 (Online) Journal homepage: http://www.tandfonline.com/loi/unht20
Numerical Heat Transfer, Part A: Applications An International Journal of Computation and Methodology ISSN: 1040-7782 (Print) 1521-0634 (Online) Journal homepage: http://www.tandfonline.com/loi/unht20
Kinetic, Thermodynamic and Regeneration Studies for CO 2 Adsorption onto Activated Carbon
 International Journal of Advanced Mechanical Engineering. ISSN 50-334 Volume 4, Number 1 (014), pp. 7-3 Research India Publications http://www.ripublication.com/ijame.htm Kinetic, Thermodynamic and Regeneration
International Journal of Advanced Mechanical Engineering. ISSN 50-334 Volume 4, Number 1 (014), pp. 7-3 Research India Publications http://www.ripublication.com/ijame.htm Kinetic, Thermodynamic and Regeneration
THE CHARACTERISTICS OF BRAZED PLATE HEAT EXCHANGERS WITH DIFFERENT CHEVRON ANGLES
 THE CHARACTERISTICS OF BRAZED PLATE HEAT EXCHANGERS WITH DIFFERENT CHEVRON ANGLES M. Amala Justus Selvam 1, Senthil kumar P. 2 and S. Muthuraman 3 1 Sathyabama University, Tamil Nadu, India 2 K. S. R College
THE CHARACTERISTICS OF BRAZED PLATE HEAT EXCHANGERS WITH DIFFERENT CHEVRON ANGLES M. Amala Justus Selvam 1, Senthil kumar P. 2 and S. Muthuraman 3 1 Sathyabama University, Tamil Nadu, India 2 K. S. R College
(Refer Slide Time: 00:00:43 min) Welcome back in the last few lectures we discussed compression refrigeration systems.
 Refrigeration and Air Conditioning Prof. M. Ramgopal Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No. # 14 Vapour Absorption Refrigeration Systems (Refer Slide
Refrigeration and Air Conditioning Prof. M. Ramgopal Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No. # 14 Vapour Absorption Refrigeration Systems (Refer Slide
