High-throughput, global proteomics assays are
|
|
|
- Josephine Austin
- 5 years ago
- Views:
Transcription
1 Evaluation of the Influence of Amino Acid Composition on the Propensity for Collision-Induced Dissociation of Model Peptides Using Molecular Dynamics Simulations William R. Cannon, a Danny Taasevigen, a Douglas J. Baxter, b and Julia Laskin c a Computational Biology and Bioinformatics Group, Computational and Information Sciences Directorate, Pacific Northwest National Laboratory, Richland, Washington, USA b Molecular Sciences Computing Facility, Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, Washington, USA c Chemical Structure and Dynamics Group, Fundamental Science Directorate Pacific Northwest National Laboratory, Richland, Washington, USA The dynamical behavior of model peptides was evaluated with respect to their ability to form internal proton donor-acceptor pairs using molecular dynamics simulations. The proton donor-acceptor pairs are postulated to be prerequisites for peptide bond cleavage resulting in formation of b and y ions during low-energy collision-induced dissociation in tandem mass spectrometry (MS/MS). The simulations for the polyalanine pentamer Ala 5 H were compared with experimental data from energy-resolved surface induced dissociation (SID) studies. The results of the simulation are insightful into the events that likely lead up to the fragmentation of peptides. Nine-mer polyalanine-based model peptides were used to examine the dynamical effect of each of the 20 common amino acids on the probability to form donor-acceptor pairs at labile peptide bonds. A range of probabilities was observed as a function of the substituted amino acid. However, the location of the peptide bond involved in the donor-acceptor pair plays a critical role in the dynamical behavior. This influence of position on the probability of forming a donor-acceptor pair would be hard to predict from statistical analyses on experimental spectra of aggregate, diverse peptides. In addition, the inclusion of basic side chains in the model peptides alters the probability of forming donor-acceptor pairs across the entire backbone. In this case, there are still more ionizing protons than basic residues, but the side chains of the basic amino acids form stable hydrogen bond networks with the peptide carbonyl oxygens and thus act to prevent free access of mobile protons to labile peptide bonds. It is clear from the work that the identification of peptides from low-energy CID using automated computational methods should consider the location of the fragmenting bond as well as the amino acid composition. (J Am Soc Mass Spectrom 2007, 18, ) 2007 American Society for Mass Spectrometry High-throughput, global proteomics assays are making important contributions to our ability to understand the complex biology of cell populations. A typical approach in global proteomics assays is to identify the protein complement of the cell population by digesting the isolated proteins with trypsin or another protease having high specificity [1 3]. The resulting peptides are then analyzed using chromatographic separation coupled to a tandem mass spectrometer in which the peptides are dissociated into fragments using low-energy collision-induced dissociation (CID) or other ion activation techniques. However, Address reprint requests to Dr. W. R. Cannon, Computational Biology and Bioinformatics Group, Computational and Information Sciences Directorate, Pacific Northwest National Laboratory, P.O. Box 999/MS K5-12, Richland, Washington, 99352, USA. william.cannon@pnl.gov despite recent advances in the statistical sophistication of peptide identification programs [4 10], the relative number of tandem mass spectra identified with a peptide is only in the range of 15% to 20%. Higher mass resolution increases confidence for those peptides that are identifiable with today s programs; however, many spectra are missing peaks due to major ion series fragments and, in this case, increased mass resolution will not necessarily help the identification. For the 80% or so of spectra that remain unidentified with a peptide, it is not clear how many of these spectra can be attributed to post-translational modifications. However, clearly many of the unidentified spectra are due to peptides for which we have limited understanding of their fragmentation behavior and hence are not able to accurately predict their fragmentation patterns. Published online June 20, American Society for Mass Spectrometry. Published by Elsevier Inc. Received April 3, /07/$32.00 Revised June 13, 2007 doi: /j.jasms Accepted June 14, 2007
2 1626 CANNON ET AL. J Am Soc Mass Spectrom 2007, 18, In most peptide identification software, generic fragmentation models are used in which peaks due to members of an ion series, such as the b ion series, are all expected with equal probabilities in the experimental spectra. This is, of course, a convenient assumption computationally, but it is nevertheless a poor assumption. This assumption has been relaxed in a recent report by using fragmentation models in which each member of an ion series is expected with varying probability that depends on the position of the fragmenting bond along the peptide backbone [4]. However, this work does not take into account the amino acid composition of a peptide. Progress has been made in the prediction of composition-specific fragmentation patterns from both the perspective of kinetic modeling [11, 12] and the examination of statistical trends involving amino acid pairs [13 15]. These are important steps toward the development of sequence-specific fragmentation models that should ultimately result in a significant increase in the number of peptides identified by MS/MS. The difficulty of predicting sequence-specific fragmentation patterns lies, of course, in the rich diversity of the underlying chemistry. The state of our knowledge of fragmentation pathways of protonated peptides and the underlying mechanisms have been extensively reviewed [16 20]. Many studies in the last 20 years have lead up to the development of the mobile proton model [21] as a fundamental shared aspect of the several fragmentation pathways. In this model, the proton is initially localized on a basic group such as N-terminal amine or side chain of histidine, lysine, or arginine, but can migrate and exchange with other labile protons within the peptide. Two different fragmentation pathways are thought to be involved in this model. In the charge-remote pathway, the charge is thought to be sequestered on strongly basic groups such as the C-terminal arginine in tryptic peptides. These peptides typically require higher collision energies to observe fragmentation products, which is mainly attributed to substantial rearrangement resulting in unfavorable entropy effects that significantly slow down the dissociation rates [22, 23]. The second pathway involved in the mobile proton model involves a proton that can readily move throughout the peptide. This pathway is often referred to as a charge-directed pathway. Isotopic studies have demonstrated that protons initially located on the N-terminal amine are readily exchanged with labile protons located throughout the peptide [24 27]. Sites of protonation and exchange can include the backbone carbonyl oxygens and amide nitrogens as well as basic side-chain groups. The fragmentation products are thought to result from different protonated forms of the same peptide in which the proton moves from site to site. Hence, the fragmentation mechanism is referred to as charge-directed. Protonation at carbonyl oxygens and amide nitrogens can feasibly occur as the basic groups containing the proton explore different dynamical configurations. In this case, the backbone groups solvate the proton, which is primarily located on the either the N-terminus or lysine and arginine side chains. We postulate that these more basic groups are principally involved in proton migration before fragmentation in a manner that includes solvation by backbone carbonyl oxygens and amide nitrogens. Alternatively, the proton may migrate independent of the basic side chains and N-terminal amine. Although solvation of the proton solely by backbone carbonyls or backbone amide nitrogens could occur, these migration pathways are energetically disfavored relative to migration of the proton as part of more basic the N-terminal amine and the basic side chains of histidine, lysine, and arginine. As a result, we expect that charge-directed fragmentation pathways can be highly influenced by the conformational dynamics of the ground state peptide. Here we report the use of molecular dynamics simulations to understand the configurational properties of ground state model peptides that lead up to the fragmentation events. Based on the postulate that proton migration in charge-directed fragmentation occurs primarily as part of the dynamical solvation of the N- terminal amine and basic side-chain groups, the simulations allow us to characterize and quantitate the configurations that are conducive to proton transfers to backbone groups, which are necessary for subsequent fragmentation. The configurational properties are the probabilities of forming proton donor-acceptor pairs of the protonated N-terminal amino group with each of the backbone oxygens. First, we examine the configurational probabilities of the polyalanine pentamer and compare these structural probabilities with fragmentation probabilities derived from surface induced dissociation (SID) spectra obtained at different collision energies. Subsequently, to test the differential effect of each of the 20 amino acids on the conformations that are conducive to proton transfer to the backbone peptide groups, we use the 9-mer peptide polyalanine as a null or baseline model, and then substitute at position 5 each of the 20 common amino acids. The results are summarized in the form of the probability for forming proton donor-acceptor pairs capable of transferring a proton to each of the backbone peptide groups for each of the peptides examined. A wide variability of the probability of forming donor-acceptor pairs are observed depending on the substituted amino acid and the position of the peptide bond. The variability due to the position of the bond would be hard to predict from statistical analyses on experimental spectra of aggregate, diverse peptides. As expected, introducing a charge via arginine, lysine, or histidine significantly disrupts the pattern for forming donor-acceptor pairs across the peptide backbone. In addition, for these polyalanine derivatives, peptide bonds near the N-terminus are less likely to be involved in donor-acceptor pairs than are peptide bonds near the C-terminus. It is clear from the work presented here that the identification of peptides from low-energy CID using automated computational
3 J Am Soc Mass Spectrom 2007, 18, PROTON DONOR-ACCEPTOR CONFIGURATIONS GAS PHASE PEPTIDES 1627 methods should consider the location of the fragmenting bond as well as the amino acid composition. Methods Computational Methods The pentamer A 5 H, the 9-mer A 9 H, and 19 derivatives of A 9 and were studied. Each of the 9-mer peptides differed by the identity of the amino acid located at position 5. At this position, each of the commonly occurring amino acids was substituted and different tautomeric forms and charge states were considered. This resulted in a total of 25 unique 9-mer peptides. For histidine, there are two neutral isomers in which a proton occupies the site at either the nitrogen (His-D) and nitrogen (His-E), and one protonated form involving protonation at both the or nitrogens. In addition, doubly protonated histidine, lysine and arginine derivatives as well as singly protonated structures were investigated. All other peptides were singly protonated. The acid form of all carboxylic acid groups were used in this study. Extensible Computational Chemistry Environment (ECCE)[28]wasusedforsettingupsimulationsandfor building peptide structures, and the NWChem [29] computational chemistry package was used for the simulations. The initial configuration for each simulation was the extended peptide configuration obtained after 1000 steps of steepest descent energy optimization. Simulations were carried out for 5 ns for each of the peptides. For each simulation, an initial temperature of 500 K was chosen and the velocities were rescaled every 1000 time steps. The leapfrog integration method was used with a2fstime step. A 5.0 nm cutoff was used for determination of all forces and energies with the Amber force field [30]. Configurational distribution functions were determined from the simulations as a function of generalized coordinates. When the coordinate is the radial distance r away from an atomic center, the distribution function is known as the radial distribution function, g(r). The integral of g(r) over the generalized coordinate r from r min to r max is a probability (T) of obtaining a conformation for which r min r r max. For the purpose of examining proton donor-acceptor pairs, we are interested in both the radial distance between donor and acceptor atoms and the angular dependence of hydrogen bond formation. Thus, the configurational distribution function formed in this manner has both the radial and angular dependence, g(r, 1, 2 ). In this case, the generalized coordinate r is the radial distance between the proton donor group and the backbone carbonyl oxygen at position i, and q 1, and 2 are the angles between the donor and acceptor groups as defined in Figure 1. Integration of the distribution function results in the probability of obtaining a configuration with appropriate geometric parameters (r, 1, 2 ) for donating and receiving a proton, Figure 1. Definition of variables used in eqs 1 and 2 for determining hydrogen bonds. The distance between the hydrogen donor and the acceptor oxygen is r (not shown), the angle 1 is the angle between the donor heavy atom, the hydrogen and the acceptor oxygen, and 2 is the angle between the hydrogen, the acceptor oxygen and the carbonyl carbon of the peptide backbone. i (T) 2.min 2.max 1.max 1.min r max g i (r, 1, 2 )drd 1 d 2 0. (1) gi (r, 1, 2 )drd 1 d 2 0 Here r max is the maximum distance away from the acceptor atom that a proton can be transferred from the proton donor to the proton acceptor atom, which we set at 2.75 Å. We use a liberal value of r max because of the high-temperature used in the study. Likewise 1,min and 1,max are the minimum and maximum angles between the donor nitrogen, proton, and acceptor oxygen at which a proton can be transferred from donor to acceptor, and 2,min and 2,max are the minimum and maximum angles between the proton, acceptor oxygen and the carbonyl carbon at which a proton can be transferred from donor to acceptor. In practice, we set 1,min 2,min 90 and 1,min 2,min 180. The result of the integration is the probability of forming a proton donor-acceptor pair at temperature T for backbone oxygen i, i T. The proton donor could be the N-terminal amine as well as the side chains of lysine, arginine, and protonated histidine. In the case of an amino donor group, multiple hydrogen bonds can form between a single pair of donor and acceptor atoms. However, the contribution of each donor is only counted only once. When more than one proton donor is present in the peptide, as is the case in peptides having a total charge of 2, the distribution functions due to the different donors j can be combined into an overall probability of forming a donor-acceptor configuration at backbone carbonyl i using relative weighting factors w j, i (T) j w j 2.max 2.min j 1.max 1.min 2 2 w j 0 0 r max g ij (r, 1, 2 )drd 1 d 2 0 (2) gij (r, 1, 2 )drd 1 d 2 0 When we were simply interested in the statistics of the various conformers of the peptides, the weighting factors used were each 1.0; alternatively, when we were interested in the probability to actually donate a proton we used a Boltzmann density function of the relative gas-phase basicities, w i expg j G Nterm RT. We
4 1628 CANNON ET AL. J Am Soc Mass Spectrom 2007, 18, took the gas-phase basicities as those for the respective individual amino acids [31]. SID Experiments Experiments were performed on the University of Delaware 7T Bruker BioApex FT-ICR Mass Spectrometer equipped with a commercial electrospray source (Analytica, Branford, CT). The system is operated at an indicated base pressure of torr. Penta-alanine was purchased from Sigma (St. Louis, MO) and used without further purification. The sample was dissolved in 50:50 (vol/vol) water-methanol solution containing a small amount of acetic acid (1%). Sample solutions were infused into the ESI source using a Harvard Apparatus (Natick, MA) syringe pump at a flow rate of 10 to 50 L/h. Detailed description of the experimental setup for SID experiments can be found in published work [32] and has been used for the study of related polyalanine peptides [33, 34]. Briefly, the surface was introduced into the ICR cell through the aperture in the rear ICR trapping plate at 90 to the magnetic field. The surface holder was in electrical contact with the back trapping plate. Ions produced in the ESI source were accumulated in the hexapole and extracted after a delay of 0.4 s by a 100 s extraction pulse. The ions were transferred into the ICR cell by a series of lenses. The ion s kinetic energy was varied by floating the hexapole rods, skimmer and hexapole extraction plate using an external power supply. The SID collision energy was determined as a difference between the source offset and the target potentials. Scattered ions produced by surface impact are trapped by applying 7 V potential to both trapping plates. After an additional delay of 1 s the ions were excited for detection by broadband chirp excitation. SAMs were prepared on a solid gold disk soldered onto the surface holder. The surface was thoroughly cleaned in a UV cleaner and immersed ina1mmethanol solution of the FC 12 alkyl thiol (CF 3 (CF 2 ) 9 C 2 H 4 SH) for at least 24 h. Extra layers of the SAMs were removed by four-stage rinsing (10 min ultrasonic cleaning) of the assembly in ethanol. Results and Discussion For the pentamer Ala 5 H, probabilities of forming donoracceptor pairs were determined from the radial and angular distribution function (eq 1) from a 5 ns simulation. For the purpose of demonstration, the radial distribution function g i (r) (no angular dependence) and the convergence of the probabilities of forming donoracceptor pairs based on g(r) are shown in Figure 2. The radial distribution function in this case measures the probability density of a donor-acceptor pair forming at a radial distance r between the donor and acceptor groups defined in Figure 1. In this peptide, the only significant electrostatic interactions to compensate for the charged N-terminal amine are those due to the Figure 2. (a) Radial distribution functions g i (r) of the donoracceptor pairs for the polyalanine pentamer. The radial distribution function counts the relative density of configurations present at a radial distance of r between the hydrogen donor and acceptor, as defined in Figure 1. The carbonyl oxygen for position 1 is disproportionately high when bond angles are not taken into account, and is therefore not shown. (b) Convergence of the radial distribution functions as a function of simulation time. backbone carbonyl oxygens, in which the carbonyl groups compete to orient their dipoles toward the charged N-terminal amine. For example, the carbonyl oxygen associated with peptide bond 4 is shown in the top of Figure 2(solid green line) to have asharp peak just below r 3.0 Å. This peak and similar ones found in the range of 2 to 4 Å are due to configurations involving direct interactions of the carbonyl oxygens with the protonated N-terminal amine. From the distributions, it is apparent that the the C-terminal carbonyl oxygen (5 O) is the most likely acceptor group to be found in a hydrogen bond with the N-terminal amine, followed in decreasing order by the carbonyl oxygens located at positions 4, 3, and 2. The acceptor group labeled 5-OH is the C-terminal hydroxyl group, which competes with the C-terminal carbonyl oxygen for hydrogen bonding with the N-terminal amine. Since the carbonyl oxygen has more favorable electrostatic interactions with the N-terminal amine, the hydroxyl group becomes energetically restrained at a distance 4 to 6 Å away from the N-terminal amine. As a result, proton transfer to the hydroxyl group that would allow for loss of water from the peptide is relatively unlikely. The integration of the radial distribution function g i (r) from r 0 to a maximum distance at which direct interactions are likely to occur gives the probability, i, of formation of the donor-acceptor pair for carbonyl oxygen i and the N-terminal amine. Due to the rela-
5 J Am Soc Mass Spectrom 2007, 18, PROTON DONOR-ACCEPTOR CONFIGURATIONS GAS PHASE PEPTIDES 1629 tively high temperatures used in this study, we chose a maximum value for direct interactions to be 2.75 Å. The plot at the bottom of Figure 2demonstrates that the probabilities converge easily on the nanosecond time scale. The probabilities obtained from the joint radial and angular distribution function (eq 1) behave similarly with the exception that the values are reduced relative to those obtained using only radial dependence since the angular constraint that the hydrogen bond angles be between 1,min 2,min 90 and 1,min 2,min 180 reduces the number of acceptable configurations. The probabilities of forming proton donoracceptor pairs using both radial and angular dependence of hydrogen bond formation, i, at backbone carbonyl oxygens were 0.22, 0.39, 0.45, 0.68, and 0.01 for peptide bond positions i 2 to 4, the C-terminal hydroxyl oxygen and the C-terminal carbonyl oxygen, respectively. Ideally, the relative probabilities of forming donoracceptor pairs at peptide bonds 2 4 can be compared with the experimental relative abundances of b-ion and y-ions formed as a result of peptide bond cleavages at the corresponding positions. However, because of the presence of multiple fragmentation pathways and consecutive fragmentations, the comparison is not entirely straightforward. To demonstrate, Figure 3 (top right inset) shows an experimental SID spectrum of Ala 5 H obtained at 15 ev collision energy. The spectrum is dominated by N-terminal fragments including an almost complete series of b ions (b 2 b 5 ), a 1,a 2,a 3 -NH 3, and a 4 ions. In addition, two abundant C-terminal fragments, y 2 and y 3 ions, are observed in the spectrum. It is important to note that while several fragment ions are formed directly from the protonated precursor, many fragments observed in the spectrum correspond to consecutive fragmentation of the primary fragments. Double-resonance experiments along with RRKM modeling of the experimental data, described in detail elsewhere [34, 35], revealed that b 5,b 4,y 2, and y 3 ions are the four primary fragments of Ala 5 H. All other SID fragments are formed by consecutive fragmentation of these primary product ions. Experimental probabilities of peptide bond cleavages at different positions were obtained from the experimental data by combining each primary product ion with its subsequent fragments. The relative abundance of the protonated precursor, Ala 5 H, and the dependence of the combined abundance of the primary fragments and their consecutive products on the collision energy are shown in the left of Figure 3.Experimentalprobabilitiesofcleavagesat bonds 2 5 of Ala 5 H were determined from the relative abundance of the y 3 (bond 2), y 2 (bond 3), b 4 (bond 4), and b 5 (bond 5) ions. The probabilities of peptide bond cleavages increase with collision energy at collision energies below 15 ev and level off at higher collision energies. Some variations observed at higher collision energies most likely result from multiple pathways of the formation of some consecutive product ions that were difficult to take into account. The overall estimate of experimental fragmentation probabilities for peptide bonds 2 4 and water loss at the C-terminal carbonyl are 17%, 7%, 73%, and 3%, respectively. Water loss from the C-terminus accounts for only 3% of the fragmentation products. From the simulation data, the terminal carbonyl oxygen and hydroxyl oxygen account for 39 and 1% of the total probability for forming donor-acceptor pairs, respectively. This suggests that the terminal hydroxyl oxygen is the group Figure 3. Left: Fragment ion abundances for y 3, y 2, b 4, and b 5 and their respective sequential fragmentation products from the SID spectra of the polyalanine pentamer as a function of collision energy. These fragments represent the dissociation of peptide bonds 2 4 and loss of water from the parent ion (bottom right inset). At collision energies below 17 ev, the parent peptides undergo single fragmentations, while above 17 ev sequential fragmentations can occur. Upper right inset: spectrum from a 15 ev CID spectrum.
6 1630 CANNON ET AL. J Am Soc Mass Spectrom 2007, 18, Figure 4. (a) Radial distribution functions g i (r) of the donoracceptor pairs for the 9-mer polyalanine. The carbonyl oxygen for position 1 is disproportionately high when bond angles are not taken into account, and is therefore not shown. (b) Convergence of the radial distribution functions as a function of simulation time. involved rather than the terminal carbonyl oxygen. However, without a better understanding of the mechanics of water loss from the C-terminus nothing conclusive can be stated. Assuming that water loss occurs from the C-terminal hydroxyl group, the fraction of the total probability estimates for forming proton donor-acceptor pairs for peptide bond positions i 2to 4 and the C-terminal hydroxyl oxygen are 20%, 36%, 42%, and 1%. Although only relative accuracy should be expected from the simulations because the simulations employ classical mechanics and cannot provide information on bond breaking, the rank order of the estimated probabilities are the same as the order of transition-state energy barriers found by Paizs and Suhai using a higher level theory [36]. This provides us with further confidence that the probability estimates of forming donoracceptor pairs are related to fragmentation patterns. For the practical purposes, the important question to be answered is whether probabilities derived from theory and simulations can provide more accurate model fragmentation patterns than generic fragmentation patterns that are currently used. In most current fragmentation patternsthatareusedforidentifyingpeptides[6,8],itis assumed that all ions from an ion series such as b and y ion series occur with the equal abundance. Recent work extended these fragmentation patterns to vary as a function of location of the peptide bond [4]. However, as will be suggested later, both position of the peptide bond and the sequence composition of the peptide are essential for accurate prediction of model spectra. Unfortunately, the influence of position and sequence composition are inseparably intertwined and it is not likely that statistical characterization of fragmentation patterns that do not simultaneously account for both will result in accurate predictions for specific peptides. For nonamer Ala 9 H, the radial distribution function g i (r) and the convergence of the probabilities of forming donor-acceptor pairs are shown in Figure 4. As before, the radial distribution function measures the probability density of a donor-acceptor pair forming at a radial distance r between the donor and acceptor groups defined in Figure 1. In the nonamer polyalanine as in the pentamer, the only significant electrostatic interactions to compensate for the charged N-terminal amine are those due to the backbone carbonyl oxygens. For example, the carbonyl oxygen associated with peptide bond 5 is shown in the top of Figure 4(solid blue line) to have a sharp peak just below r 3.0 Å and a smaller peak just above r 7.0 Å. The peak near r 3.0 Å is due to configurations having a direct interaction of this carbonyl oxygen with the protonated N-terminal amine. Since the peak near r 7.0 Å is at a distance greater than the distance expected for a direct interaction, it is very likely due to a local minima in the potential energy surface in which other carbonyl oxygens directly solvate the N-terminal amine, and carbonyl oxygen 5 is restrained at a distance 7 Åaway. The structure shown in Figure 5 is a snapshot of the trajectory in which the general conformational features involved in solvation of the N-terminal amine can be seen. In this case, the carbonyl oxygen associated with peptide bond 5 directly solvates the N-terminal amine while the carbonyl oxygen associated with position 4 is held at a greater distance from the N-terminal amine. The peptide adopts conformations such that the charge on the N-terminal amine can maximize solvation by the dipoles of the backbone carbonyl groups. The integration of the radial distribution function Figure 5. A representative conformation for polyalanine. The principal interaction that drives the conformations is the solvation of the N-terminal charge by backbone dipoles of the carbonyl oxygen.
7 J Am Soc Mass Spectrom 2007, 18, PROTON DONOR-ACCEPTOR CONFIGURATIONS GAS PHASE PEPTIDES 1631 g i (r) from r 0 to a maximum distance at which direct interactions are likely to occur gives the probability, i, of formation of the donor-acceptor pair for carbonyl oxygen i and the N-terminal amine. The visualization of the simulation (movie) shows that the carbonyl oxygens at positions 6 to 9 spend more time solvating the charge on the N-terminus than the carbonyl oxygens at positions 2 to 4. This behavior is quantified by the time dependence of i inthe bottom plot of Figure 4. In addition, the plot demonstrates that the probabilities converge easily on the nanosecond time scale. From the perspective of fragmentation through an amide oxygen pathway [17], the high probability of forming proton donor-acceptor pairs at carbonyl oxygens in positions 6 to 9 would imply, but not necessitate, that fragmentation will be more likely at peptide bonds near the C-terminus. For other pathways such as those involving protonation of backbone amide nitrogens, a local minima on the pathway between transfer of the proton from the N-terminal amine to the backbone amide is the intermediate protonation of the carbonyl oxygen associate with the peptide bond containing the amide. As a result, kinetics of the protonation of the backbone oxygen will still influence the kinetics of the fragmentation. The results for the probability of forming donoracceptor pairs as a function of r, 1, and 2 for the set of 22 singly protonated peptides studied are summarized intheheatmapshowninfigure6.eachrowoftheheat map represents a derivative of the 9-mer polyalanine peptide in which each of the naturally occurring amino acids are in turn substituted at position 5. There are two tautomeric forms of neutral forms of histidine to consider in which a proton is located on either the nitrogen (His-D) or nitrogen (His-E), and one protonated form of histidine (His 1) in which protons are on both the and nitrogens. The preferred neutral tautomer, His-D or His-E, is highly dependent on the Figure 6. Heat map of probabilities for forming donor-acceptor pairs at backbone positions. Each row represents a derivative of the 9-mer polyalanine in which the amino acid indicated along the left hand side is substituted at position 5. Each column represents a backbone positions in the peptide. Each peptide carries a 1 charge on the N-terminus, with the exception of the Arg, His1, and Lys peptides in which the charge was located on the side chain. The bottom two histograms represent the standard deviation of the probabilities down the respective column for all peptides (middle) and for just those peptides carrying the charge at the N-terminus (bottom). The histogram along the right hand side of the figure represents a sum of the probabilities across each row and indicates the average number of hydrogen bonds formed at 500 K in the simulation when 0 r 2.75 Å, , and
8 1632 CANNON ET AL. J Am Soc Mass Spectrom 2007, 18, localenvironment[37,38].foreachpeptiderepresented in Figure 6the proton donor is the N-terminal amine, except for histidine (His 1), lysine and arginine in which case the proton donor is the respective sidechain. The columns of the heat map represent each position along the peptide backbone from 1 9. Each cell in the heat map then represents the donor-acceptor probability for a specific backbone position for a specific peptide. As can be seen from the heat map, the variability across each row is greater that the variability down any column. Generally, the probability of obtaining a donoracceptor configuration is much higher at positions toward the C-terminus than it is for positions towards the N-terminus. The reason for this is that electrostatic compensation of the 1 charge on the N-terminal amine is most effective when the internal solvation of the charge includes the C-terminal carboxyl group, which can form two donor-acceptor interactions with the N-terminal amine. An obvious conclusion derived from the variability is that position along the backbone significantly effects the formation of proton donoracceptor configurations. This influence of position on the probability of forming a donor-acceptor pair would be hard to predict from statistical analyses on experimental spectra of aggregate, diverse peptides. In addition, each substituted amino acid at position 5 of the 9-mer peptide influences the formation of donor-acceptor configurations across all backbone positions. However, as expected, the variability down columns corresponding to positions 4 and 5 of the peptide, which flank the location of the side-chain of the substituted amino acid, approach the variability observed across the rows. This implies that variability in forming proton donor-acceptor pairs at specific peptide bonds must consider both position and amino acid composition. The bottom two panels of Figure 6 display the standard deviation of i for each backbone position, which measures the variability across all peptides of the probability of forming proton donor-acceptor pairs at position i. The middle panel shows the standard deviation for all peptides while the bottom panel shows the standard deviation for the peptides protonated on the N-terminus (i.e., no lysine, arginine, and protonated histidine containing peptides). The variability is greatest at positions 4 and 5 for N-terminal protonated peptides, as expected. The variability then decreases as one moves away from the position of substitution (position 5) as one might expect, with the exception that the variability is somewhat higher at the C-terminal most position. This variability at positions remote from the site of substitution is due to the conformational dynamics of the peptide such that the hydrogen bond network that solvates the charge is constantly changing. When the peptides containing lysine, arginine, and protonated histidine are considered, the largest impact on the probability of forming acceptor-donor pairs is at positions 6 and 7. This effect is due to the close proximity in space of the positively charged side-chain with the backbone carbonyl groups at positions 6 and 7. Proton donor-acceptor pairs at positions 6 and 7 are favored over the analogous positions 2 and 3 because the former positions also readily allow for simultaneous interaction with the C-terminal acid group. This suggests that statistical training on large data sets of experimental spectra to elucidate fragmentation patterns should separately examine lysine, arginine and histidine-containing peptides in a manner similar to that used by Huang et al. [39]. When comparing the probability of forming donoracceptor pairs at select positions between individual peptides, it must be kept in mind that the results are qualitative. Errors arise principally from two sources, the mathematical model and sampling statistics. The molecular mechanics force field used in the simulations is an imperfect mathematical model of the quantum mechanical force field. Errors arising from statistical sampling can be estimated from the fluctuations in the probability shown over the last 3 ns of the simulations. For the polyalanine simulation, these probabilities can be seen to converge within 0.05 in Figure 4 over the last 3 ns of the simulation. The implication is that small differences in tones of individual cells are not significant. In addition, the probability of forming proton donoracceptor pairs for different amino acids are all discussed below relative to the effect that alanine has at the same position. We do this to control for the influence of position on forming proton donor pairs, which is less attainable in experimental studies. Analogous experimental studies have examined N- versus C-terminal fragmentation propensities within each amino acid. We prefer the comparison to the analogous positions in alanine because there is likely a significant positiondependent effect when comparing the probabilities at the positions flanking the substituted amino acid. As a result, in the discussion below it is possible to have both enhanced probabilities of forming donor-acceptor pairs at both the N- and C-terminal sides of the amino acid. With these caveats in mind, we can compare the probability of forming donor-acceptor pairs for individual peptides on a qualitative basis. For the polyalanine derivatives, proline and methionine containing peptides show an enhanced probability of forming donor-acceptor configurations at the N- terminal side of the substituted amino acid (position 4) relative to alanine. Proline is well known to show enhanced fragmentation on the N-terminal side of the residue [40]. The correlation of the available conformational space of the backbone, dihedral angles of proline with fragmentation behavior has been noted previously by Huang et al. [41]. This is not to say all of the propensity of proline to fragment on the N-terminal side can be attributed to mechanics, however. Proton transfer from the carbonyl oxygen of the peptide bond to the amide nitrogen is likely necessary for fragmentation to occur, and the amide nitrogen of proline has
9 J Am Soc Mass Spectrom 2007, 18, PROTON DONOR-ACCEPTOR CONFIGURATIONS GAS PHASE PEPTIDES 1633 recently been suggested to have a greater gas-phase basicity than previously thought [42]. In contrast, 14 of the peptides show an enhanced probability of forming donor-acceptor configurations at the C-terminal side of the substituted amino acid (position 5) relative to alanine. At 500 K, this series proceeds as follows: Lys His 1 His-E Asn Arg Leu Val Gln Asp Cys Phe Ser Glu Met Lysine, histidine (His1 and His-E), asparagine, and arginine show particularly strong probability of forming donor-acceptor configurations on the C-terminal side of the position of substitution for these model peptides. The propensity for histidine to fragment on its C-terminal side has been reported for doubly protonated tryptic peptides [15, 43] Although not observed in the model peptides described here, we have preliminary evidence from simulations of lysine-terminated 2 tryptic peptides that Asp and Glu also have a tendency to show increased probability of formation of donoracceptor pairs on the C-terminal side of the residue. In these cases, the C-terminal lysine is the proton donor. FromtheheatmapinFigure6,onecanalsocompare the probability of forming donor-acceptor pairs for the carbonyl oxygens of the peptide bonds flanking the substituted amino acid. For example, proline, methionine, and tyrosine all show preference for forming donor acceptor pairs at the N-terminal flanking carbonyl oxygen. Proline has been discussed above. For methionine, whether N- or C-terminal donor-acceptor pairs dominate is sensitive to the angles (Figure 1) used for determining hydrogen bonds. In contrast, for tyrosine the pattern is the same regardless of the hydrogen bond angles used. The major driving force in this case appears to be maximization of hydrogen bonds throughout the low-energy conformations, which entails rotation of the tyrosine side chain out of interaction with neighboring residues. However, the difference between N- and C-terminal donor-acceptor pair formation here does not appear to be large. It would likely be premature to generalize these trends to more complex peptides because we have not examined how these relative probabilities would change as a function of the site of substitution. Moreover, non-model peptides that have diverse amino acid compositions, unlike those studied here, may differ significantly from polyalanine derivatives. The sum of the probabilities across each row of the heat map in Figure 6is the number of donor-acceptor pairs that are formed, on average, in each configuration of the model peptide and is shown on the right-hand side bar graph of Figure 6. Generally, between one and two donor acceptor pairs are formed for each of the peptides at a temperature of 500 K. At lower temperature, this number will likely increase due to the peptide reaching a stable local minima of state space and, conversely, at higher temperatures the peptides are expected to have fewer donor-acceptor pairs. The most surprising observation is that the model peptides containing arginine and protonated histidine contain the fewest donoracceptor pairs. This is especially apparent when comparing the total number of hydrogen bonds for protonated histidine (His 1) to HisD, and HisE, which carry the 1 charge on the N-terminal amine. The reason for this is the geometry requirements for forming donor-acceptor pairs in these species. Typical configurations for the lysine, protonated histidine and arginine peptides having a total charge of 1 are shown in Figure 7. Unlike the protonated N-terminal amino groups and the side-chain amino group of lysine which are roughly spherical and have approximately C 3 symmetry, histidine and arginine are planar and have less than C 2 symmetry. The net result is that the lower symmetry of the latter species results in a lower probability of forming favorable geometries for proton transfer. Likely this geometrical effect also contributes to the gas-phase basicity of these groups. The results for doubly protonated arginine, lysine, andhistidinederivativesareshowninfigure8.thetop Figure 7. Representative conformations of the arginine, protonated histidine, and lysine derivatives of polyalanine carrying a total charge of 1. The principal driving force in these peptides is the solvation of the protonated side-chain moieties by the backbone dipoles of the carbonyl oxygens. Residues 1 4 (left hand side) maximize formation of hydrogen bonded pairs by forming helical structures, while the backbone oxygen of residue 5 and the terminal carboxylate group of residue 9 (right hand side) tend to be principally involved in solvation of the side-chain groups.
10 1634 CANNON ET AL. J Am Soc Mass Spectrom 2007, 18, Figure 8. Heat map as in Figure 6of probabilities for forming donor-acceptor pairs at backbone positions of doubly protonated peptides. The top three rows count side-chain and N-terminal donor-acceptor pairs equally, while the bottom three rows take into account the different gas-phase basicities of the side-chain groups relative to the N-terminus. three rows of the heat map represent the probability of forming proton donor-acceptor pairs, similar to that shown for singly protonated species in Figure 6. In comparison to the singly protonate species, the probability of forming donor-acceptor pairs is increased dramatically as can be seen by the histogram of the average number of donor-acceptor pairs on the right hand side of Figure 8. Each peptide forms in excess of two proton donor-acceptor pairs in comparison to the 1.5 to 2 donor-acceptor pairs formed for the singly protonated species. This increase in number of donor-acceptor pairs occurs primarily at positions 4 to 9, as evidenced by the increased probabilities for forming such pairs at those sites. However, when the gas-phase basicities of the donor side chains are taken into account using eq 2, very different statistics emerge. The bottom three rows of the heat map in Figure 8are adjusted to account for the difference in the proton donating ability of the sidechain groups relative to the protonated N-terminus by weighting the probabilities using gas-phase basicities. Again, the sum of the probabilities across each row of the heat map is the number of donor-acceptor pairs that are formed, on average, in each configuration of the model peptide and is shown on the right-hand side bar graph. As a result, this bar graph summarizes the total number of viable proton donor-acceptor pairs for each peptide. As can be seen, the number of viable donoracceptor pairs is significantly reduced when gas-phase basicities are taken into account, particularly at positions 4 and 5. For lysine, this reduction is dramatic and results in fewer donor-acceptor pairs for the 2 peptide than for the comparable 1 peptide. This non-intuitive behavior is a result of competition of the charged groups to be solvated by the backbone carbonyl oxygens. A representative configuration of the lysine containing peptide is shown in the bottom of Figure 9, in which the protonated amine of the lysine side-chain is seen to be solvated by the backbone carbonyl oxygens. The lysine-containing peptide roughly equally solvates the side-chain amine and the N-terminal amine. The reason for this is likely due to the roughly spherical symmetry of the side-chain amine, which is similar to that of the N-terminal amine. Due to charge charge repulsions between the amines, this also leads to movement of the N-terminal amine to an extended position in which it has little contact with backbone oxygens in 50% of the configurations. This is verified by determination of the individual radial distribution function between each amine group and the respective backbone oxygens (not shown). However, the gas-phase basicity of the side-chain amine is roughly 20 Kcal/mol greater than that for the N-terminal amine. The consequence is that higher collision energies (and a higher simulation temperature) would be required for this peptide to obtain configurations that are conducive to fragmentation relative to the other polyalanine derivatives. In contrast, the protonated side chains of arginine and histidine do not compete as well as the N-terminal amine for solvation by the backbone carbonyl groups. Representative configurations for the histidine and arginine containing peptides are shown in the top and middle of Figure 9, respectively, which can be compared with the typical configurations for the 1 species shown in Figure 7. Unlike the lysine derivative, these peptides form a strand structure in which the N- terminal amine is preferentially solvated over the sidechain groups. In the strand structures, the N-terminal amine is highly solvated by the C-terminal most backbone oxygens. Charge charge repulsion again results in separation of the charged groups such that the distance between the charges is maximized, however in these two cases it is the side-chain groups that are in extended configurations. As a result, the number of viable donor-acceptor pairs is not reduced by gas-phase basicities as greatly as it is for lysine. Conclusions and Future Work The 20 common amino acids show a wide range of probabilities for forming donor-acceptor pairs in model peptides that can lead to fragmentation. However, in peptides that are more complex, these tendencies to form donor-acceptor pairs will be greatly influenced by the specific amino acid composition of the peptide. This makes it difficult to describe these properties as fast and hard rules for single amino acids or even pairs of amino acids. In addition, clearly the location of the fragment-
11 J Am Soc Mass Spectrom 2007, 18, PROTON DONOR-ACCEPTOR CONFIGURATIONS GAS PHASE PEPTIDES 1635 Figure 9. Representative conformations of the doubly protonated arginine, histidine, and lysine derivatives of polyalanine. In these peptides, the protonated amine on the N-terminus competes with the protonated side-chain groups for solvation by the backbone carbonyl oxygens. Due to charge charge repulsion, the two charge groups act to move as far apart from each other as possible. As a result and in contrast to the respective singly protonated species, the peptides tend to form sheet-like structures, which maximally moves the side-chain charge away from the solvated charge in the N-terminal amine and allows formation of as many hydrogen bonded pairs as possible. ing bond along the peptide backbone is critical in understanding behavior leading up to fragmentation, and the use of summary statistics regarding the fragmentation of a pair of amino acids independent of position can potentially lead to significant errors in estimating the propensity of a bond in a specific peptide to undergo fragmentation. The inclusion of basic side chains in a peptide and the charge state of a peptide critically influence the dynamic behavior of the peptide. Not only are basic side chains less likely to give up a proton than the N-terminal amine, but in peptides that would be classified as containing a mobile proton (doubly protonated for the set of peptides studied here), they also block free access of the N-terminal amine to the backbone carbonyl groups. The counter-intuitive conclusion, at least for the model peptides examined here, is that we would expect doubly protonated peptides containing even a single lysine, histidine, or arginine to require more collision energy for fragmentation than singly protonated peptides containing no histidine, lysine, or arginine. This counter-intuitive behavior follows from the competition of the charged moieties for solvation from the backbone groups. This hypothesis is testable with the use of isotope exchange studies at varying collision energies. Ironically, it is the close proximity of side chains of the charge remote amino acids to labile bonds that results in this behavior. On a more general note regarding peptide identification programs, we must understand low-energy fragmentation mechanisms and dynamics better to more accurately predict fragmentation patterns that can be used to increase the number of peptides identified from MS/MS. Both the learning of fragmentation patterns from statistically analyzing large data sets and the use of molecular level simulations of peptides have inherent weaknesses but complementary strengths. The learning of fragmentation patterns from large sets of confidently identified peptides is inherently biased towards the generic fragmentation patterns used by current peptide identification methods. In addition, significant undersampling occurs because it is not experimentally feasible to build a large enough training set that would represent all possible combinations of amino acids at every position in peptide. For example, statistically learning patterns for all 6-mer peptides would require 20 6 peptides to be considered. The use of spectral libraries in which a spectrum due to an unknown peptide is compared with spectra from known peptides is very promising [44 46] but is limited to those peptides that are initially identifiable with current computational methods. In contrast, it is feasible with next generation computers to consider the simulation of a genomes worth of tryptic peptides. In this approach, it would not be necessary to learn all possible fragmentation patterns, but only those that are relevant to the species under study and only for those peptides for which spectral libraries are not available. Unfortunately, molecular simulations using classical mechanics inherently cannot address the transition-state energetics involved in fragmentation, and thus will provide imperfect information. The use of hybrid classical-quantum mechanics simulations that would include transition-state energetics is not practically feasible with current computational hardware due to the processing time. However, the short-comings of both statistical learning and simulations are not fatal to the concept of generating sequencespecific fragmentation patterns for peptide identification because the utility of these approaches will be judged by whether they provide better information than the current state-of-the art technology. Currently, this bar is set rather low due to the generic fragmentation models that are used to evaluate peptide MS/MS spectra. Likely the combination of statistically learning
Lecture 15: Realities of Genome Assembly Protein Sequencing
 Lecture 15: Realities of Genome Assembly Protein Sequencing Study Chapter 8.10-8.15 1 Euler s Theorems A graph is balanced if for every vertex the number of incoming edges equals to the number of outgoing
Lecture 15: Realities of Genome Assembly Protein Sequencing Study Chapter 8.10-8.15 1 Euler s Theorems A graph is balanced if for every vertex the number of incoming edges equals to the number of outgoing
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Solutions In each case, the chirality center has the R configuration
 CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4.
 Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4. Field Desorption 5. MS MS techniques Matrix assisted
Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4. Field Desorption 5. MS MS techniques Matrix assisted
Diastereomeric resolution directed towards chirality. determination focussing on gas-phase energetics of coordinated. sodium dissociation
 Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION
 THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
MS/MS of Peptides Manual Sequencing of Protonated Peptides
 S/S of Peptides anual Sequencing of Protonated Peptides Árpád Somogyi Associate irector CCIC, ass Spectrometry and Proteomics Laboratory SU July 11, 2018 Peptides Product Ion Scan Product ion spectra contain
S/S of Peptides anual Sequencing of Protonated Peptides Árpád Somogyi Associate irector CCIC, ass Spectrometry and Proteomics Laboratory SU July 11, 2018 Peptides Product Ion Scan Product ion spectra contain
Conformational Analysis
 Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Section Week 3. Junaid Malek, M.D.
 Section Week 3 Junaid Malek, M.D. Biological Polymers DA 4 monomers (building blocks), limited structure (double-helix) RA 4 monomers, greater flexibility, multiple structures Proteins 20 Amino Acids,
Section Week 3 Junaid Malek, M.D. Biological Polymers DA 4 monomers (building blocks), limited structure (double-helix) RA 4 monomers, greater flexibility, multiple structures Proteins 20 Amino Acids,
Proton Acidity. (b) For the following reaction, draw the arrowhead properly to indicate the position of the equilibrium: HA + K + B -
 Proton Acidity A01 Given that acid A has a pk a of 15 and acid B has a pk a of 10, then: (a) Which of the two acids is stronger? (b) For the following reaction, draw the arrowhead properly to indicate
Proton Acidity A01 Given that acid A has a pk a of 15 and acid B has a pk a of 10, then: (a) Which of the two acids is stronger? (b) For the following reaction, draw the arrowhead properly to indicate
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Collision Cross Section: Ideal elastic hard sphere collision:
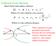 Collision Cross Section: Ideal elastic hard sphere collision: ( r r 1 ) Where is the collision cross-section r 1 r ) ( 1 Where is the collision distance r 1 r These equations negate potential interactions
Collision Cross Section: Ideal elastic hard sphere collision: ( r r 1 ) Where is the collision cross-section r 1 r ) ( 1 Where is the collision distance r 1 r These equations negate potential interactions
Amino Acids and Peptides
 Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Proteomics. November 13, 2007
 Proteomics November 13, 2007 Acknowledgement Slides presented here have been borrowed from presentations by : Dr. Mark A. Knepper (LKEM, NHLBI, NIH) Dr. Nathan Edwards (Center for Bioinformatics and Computational
Proteomics November 13, 2007 Acknowledgement Slides presented here have been borrowed from presentations by : Dr. Mark A. Knepper (LKEM, NHLBI, NIH) Dr. Nathan Edwards (Center for Bioinformatics and Computational
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
BCH 4053 Exam I Review Spring 2017
 BCH 4053 SI - Spring 2017 Reed BCH 4053 Exam I Review Spring 2017 Chapter 1 1. Calculate G for the reaction A + A P + Q. Assume the following equilibrium concentrations: [A] = 20mM, [Q] = [P] = 40fM. Assume
BCH 4053 SI - Spring 2017 Reed BCH 4053 Exam I Review Spring 2017 Chapter 1 1. Calculate G for the reaction A + A P + Q. Assume the following equilibrium concentrations: [A] = 20mM, [Q] = [P] = 40fM. Assume
BENG 183 Trey Ideker. Protein Sequencing
 BENG 183 Trey Ideker Protein Sequencing The following slides borrowed from Hong Li s Biochemistry Course: www.sb.fsu.edu/~hongli/4053notes Introduction to Proteins Proteins are of vital importance to biological
BENG 183 Trey Ideker Protein Sequencing The following slides borrowed from Hong Li s Biochemistry Course: www.sb.fsu.edu/~hongli/4053notes Introduction to Proteins Proteins are of vital importance to biological
Peptides And Proteins
 Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Biomolecules: lecture 9
 Biomolecules: lecture 9 - understanding further why amino acids are the building block for proteins - understanding the chemical properties amino acids bring to proteins - realizing that many proteins
Biomolecules: lecture 9 - understanding further why amino acids are the building block for proteins - understanding the chemical properties amino acids bring to proteins - realizing that many proteins
Chapter 1. Introduction
 1-1 Chapter 1. Introduction 1.1. Background Non covalent interactions are important for the structures and reactivity of biological molecules in the gas phase as well as in the solution phase. 1 It is
1-1 Chapter 1. Introduction 1.1. Background Non covalent interactions are important for the structures and reactivity of biological molecules in the gas phase as well as in the solution phase. 1 It is
Tutorial 1: Setting up your Skyline document
 Tutorial 1: Setting up your Skyline document Caution! For using Skyline the number formats of your computer have to be set to English (United States). Open the Control Panel Clock, Language, and Region
Tutorial 1: Setting up your Skyline document Caution! For using Skyline the number formats of your computer have to be set to English (United States). Open the Control Panel Clock, Language, and Region
B O C 4 H 2 O O. NOTE: The reaction proceeds with a carbonium ion stabilized on the C 1 of sugar A.
 hbcse 33 rd International Page 101 hemistry lympiad Preparatory 05/02/01 Problems d. In the hydrolysis of the glycosidic bond, the glycosidic bridge oxygen goes with 4 of the sugar B. n cleavage, 18 from
hbcse 33 rd International Page 101 hemistry lympiad Preparatory 05/02/01 Problems d. In the hydrolysis of the glycosidic bond, the glycosidic bridge oxygen goes with 4 of the sugar B. n cleavage, 18 from
Introduction to Proteomics: Fragmentation of protonated peptides and manual sequencing
 Introduction to Proteomics: ragmentation of protonated peptides and manual sequencing Árpád Somogyi CCIC SP SU Summer Workshop S/S hod using ESI Ion rap (Bottom up) 1 An alternative strategy for complex
Introduction to Proteomics: ragmentation of protonated peptides and manual sequencing Árpád Somogyi CCIC SP SU Summer Workshop S/S hod using ESI Ion rap (Bottom up) 1 An alternative strategy for complex
Atomic masses. Atomic masses of elements. Atomic masses of isotopes. Nominal and exact atomic masses. Example: CO, N 2 ja C 2 H 4
 High-Resolution Mass spectrometry (HR-MS, HRAM-MS) (FT mass spectrometry) MS that enables identifying elemental compositions (empirical formulas) from accurate m/z data 9.05.2017 1 Atomic masses (atomic
High-Resolution Mass spectrometry (HR-MS, HRAM-MS) (FT mass spectrometry) MS that enables identifying elemental compositions (empirical formulas) from accurate m/z data 9.05.2017 1 Atomic masses (atomic
Identification of Human Hemoglobin Protein Variants Using Electrospray Ionization-Electron Transfer Dissociation Mass Spectrometry
 Identification of Human Hemoglobin Protein Variants Using Electrospray Ionization-Electron Transfer Dissociation Mass Spectrometry Jonathan Williams Waters Corporation, Milford, MA, USA A P P L I C AT
Identification of Human Hemoglobin Protein Variants Using Electrospray Ionization-Electron Transfer Dissociation Mass Spectrometry Jonathan Williams Waters Corporation, Milford, MA, USA A P P L I C AT
Electron Transfer Dissociation of N-linked Glycopeptides from a Recombinant mab Using SYNAPT G2-S HDMS
 Electron Transfer Dissociation of N-linked Glycopeptides from a Recombinant mab Using SYNAPT G2-S HDMS Jonathan P. Williams, Jeffery M. Brown, Stephane Houel, Ying Qing Yu, and Weibin Chen Waters Corporation,
Electron Transfer Dissociation of N-linked Glycopeptides from a Recombinant mab Using SYNAPT G2-S HDMS Jonathan P. Williams, Jeffery M. Brown, Stephane Houel, Ying Qing Yu, and Weibin Chen Waters Corporation,
Exam I Answer Key: Summer 2006, Semester C
 1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
1. Amino Acids and Peptides Structures and Properties
 1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
Mass spectrometry has been used a lot in biology since the late 1950 s. However it really came into play in the late 1980 s once methods were
 Mass spectrometry has been used a lot in biology since the late 1950 s. However it really came into play in the late 1980 s once methods were developed to allow the analysis of large intact (bigger than
Mass spectrometry has been used a lot in biology since the late 1950 s. However it really came into play in the late 1980 s once methods were developed to allow the analysis of large intact (bigger than
Rotamers in the CHARMM19 Force Field
 Appendix A Rotamers in the CHARMM19 Force Field The people may be made to follow a path of action, but they may not be made to understand it. Confucius (551 BC - 479 BC) ( ) V r 1 (j),r 2 (j),r 3 (j),...,r
Appendix A Rotamers in the CHARMM19 Force Field The people may be made to follow a path of action, but they may not be made to understand it. Confucius (551 BC - 479 BC) ( ) V r 1 (j),r 2 (j),r 3 (j),...,r
Physiochemical Properties of Residues
 Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Physiochemical Properties of Residues Various Sources C N Cα R Slide 1 Conformational Propensities Conformational Propensity is the frequency in which a residue adopts a given conformation (in a polypeptide)
Proteins: Characteristics and Properties of Amino Acids
 SBI4U:Biochemistry Macromolecules Eachaminoacidhasatleastoneamineandoneacidfunctionalgroupasthe nameimplies.thedifferentpropertiesresultfromvariationsinthestructuresof differentrgroups.thergroupisoftenreferredtoastheaminoacidsidechain.
SBI4U:Biochemistry Macromolecules Eachaminoacidhasatleastoneamineandoneacidfunctionalgroupasthe nameimplies.thedifferentpropertiesresultfromvariationsinthestructuresof differentrgroups.thergroupisoftenreferredtoastheaminoacidsidechain.
Protein Structure Bioinformatics Introduction
 1 Swiss Institute of Bioinformatics Protein Structure Bioinformatics Introduction Basel, 27. September 2004 Torsten Schwede Biozentrum - Universität Basel Swiss Institute of Bioinformatics Klingelbergstr
1 Swiss Institute of Bioinformatics Protein Structure Bioinformatics Introduction Basel, 27. September 2004 Torsten Schwede Biozentrum - Universität Basel Swiss Institute of Bioinformatics Klingelbergstr
Computational Methods for Mass Spectrometry Proteomics
 Computational Methods for Mass Spectrometry Proteomics Eidhammer, Ingvar ISBN-13: 9780470512975 Table of Contents Preface. Acknowledgements. 1 Protein, Proteome, and Proteomics. 1.1 Primary goals for studying
Computational Methods for Mass Spectrometry Proteomics Eidhammer, Ingvar ISBN-13: 9780470512975 Table of Contents Preface. Acknowledgements. 1 Protein, Proteome, and Proteomics. 1.1 Primary goals for studying
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I Dr. Glen B Legge
 EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
Sequential resonance assignments in (small) proteins: homonuclear method 2º structure determination
 Lecture 9 M230 Feigon Sequential resonance assignments in (small) proteins: homonuclear method 2º structure determination Reading resources v Roberts NMR of Macromolecules, Chap 4 by Christina Redfield
Lecture 9 M230 Feigon Sequential resonance assignments in (small) proteins: homonuclear method 2º structure determination Reading resources v Roberts NMR of Macromolecules, Chap 4 by Christina Redfield
CHEM J-9 June 2014
 CEM1611 2014-J-9 June 2014 Alanine (ala) and lysine (lys) are two amino acids with the structures given below as Fischer projections. The pk a values of the conjugate acid forms of the different functional
CEM1611 2014-J-9 June 2014 Alanine (ala) and lysine (lys) are two amino acids with the structures given below as Fischer projections. The pk a values of the conjugate acid forms of the different functional
NMR study of complexes between low molecular mass inhibitors and the West Nile virus NS2B-NS3 protease
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Viewing and Analyzing Proteins, Ligands and their Complexes 2
 2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
Using Higher Calculus to Study Biologically Important Molecules Julie C. Mitchell
 Using Higher Calculus to Study Biologically Important Molecules Julie C. Mitchell Mathematics and Biochemistry University of Wisconsin - Madison 0 There Are Many Kinds Of Proteins The word protein comes
Using Higher Calculus to Study Biologically Important Molecules Julie C. Mitchell Mathematics and Biochemistry University of Wisconsin - Madison 0 There Are Many Kinds Of Proteins The word protein comes
Identification of proteins by enzyme digestion, mass
 Method for Screening Peptide Fragment Ion Mass Spectra Prior to Database Searching Roger E. Moore, Mary K. Young, and Terry D. Lee Beckman Research Institute of the City of Hope, Duarte, California, USA
Method for Screening Peptide Fragment Ion Mass Spectra Prior to Database Searching Roger E. Moore, Mary K. Young, and Terry D. Lee Beckman Research Institute of the City of Hope, Duarte, California, USA
MS/MS .LQGVRI0606([SHULPHQWV
 0DVV6SHFWURPHWHUV Tandem Mass Spectrometry (MS/MS) :KDWLV0606" Mass spectrometers are commonly combined with separation devices such as gas chromatographs (GC) and liquid chromatographs (LC). The GC or
0DVV6SHFWURPHWHUV Tandem Mass Spectrometry (MS/MS) :KDWLV0606" Mass spectrometers are commonly combined with separation devices such as gas chromatographs (GC) and liquid chromatographs (LC). The GC or
4 Examples of enzymes
 Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Lecture 15: Enzymes & Kinetics. Mechanisms ROLE OF THE TRANSITION STATE. H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl. Margaret A. Daugherty.
 Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Chemical Properties of Amino Acids
 hemical Properties of Amino Acids Protein Function Make up about 15% of the cell and have many functions in the cell 1. atalysis: enzymes 2. Structure: muscle proteins 3. Movement: myosin, actin 4. Defense:
hemical Properties of Amino Acids Protein Function Make up about 15% of the cell and have many functions in the cell 1. atalysis: enzymes 2. Structure: muscle proteins 3. Movement: myosin, actin 4. Defense:
Introduction to Comparative Protein Modeling. Chapter 4 Part I
 Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Introduction to Comparative Protein Modeling Chapter 4 Part I 1 Information on Proteins Each modeling study depends on the quality of the known experimental data. Basis of the model Search in the literature
Chem. 27 Section 1 Conformational Analysis Week of Feb. 6, TF: Walter E. Kowtoniuk Mallinckrodt 303 Liu Laboratory
 Chem. 27 Section 1 Conformational Analysis TF: Walter E. Kowtoniuk wekowton@fas.harvard.edu Mallinckrodt 303 Liu Laboratory ffice hours are: Monday and Wednesday 3:00-4:00pm in Mallinckrodt 303 Course
Chem. 27 Section 1 Conformational Analysis TF: Walter E. Kowtoniuk wekowton@fas.harvard.edu Mallinckrodt 303 Liu Laboratory ffice hours are: Monday and Wednesday 3:00-4:00pm in Mallinckrodt 303 Course
Protein Identification Using Tandem Mass Spectrometry. Nathan Edwards Informatics Research Applied Biosystems
 Protein Identification Using Tandem Mass Spectrometry Nathan Edwards Informatics Research Applied Biosystems Outline Proteomics context Tandem mass spectrometry Peptide fragmentation Peptide identification
Protein Identification Using Tandem Mass Spectrometry Nathan Edwards Informatics Research Applied Biosystems Outline Proteomics context Tandem mass spectrometry Peptide fragmentation Peptide identification
Problem Set 1
 2006 7.012 Problem Set 1 Due before 5 PM on FRIDAY, September 15, 2006. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. For each of the following parts, pick
2006 7.012 Problem Set 1 Due before 5 PM on FRIDAY, September 15, 2006. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. For each of the following parts, pick
L.7. Mass Spectrum Interpretation
 L.7. Mass Spectrum Interpretation Fragmentation reactions Spectrum interpretation Confirmation of ion structural assignment Biomolecule dissociation Fragmentation reactions 1. Fragmentation reactions of
L.7. Mass Spectrum Interpretation Fragmentation reactions Spectrum interpretation Confirmation of ion structural assignment Biomolecule dissociation Fragmentation reactions 1. Fragmentation reactions of
Translation. A ribosome, mrna, and trna.
 Translation The basic processes of translation are conserved among prokaryotes and eukaryotes. Prokaryotic Translation A ribosome, mrna, and trna. In the initiation of translation in prokaryotes, the Shine-Dalgarno
Translation The basic processes of translation are conserved among prokaryotes and eukaryotes. Prokaryotic Translation A ribosome, mrna, and trna. In the initiation of translation in prokaryotes, the Shine-Dalgarno
Resonance assignments in proteins. Christina Redfield
 Resonance assignments in proteins Christina Redfield 1. Introduction The assignment of resonances in the complex NMR spectrum of a protein is the first step in any study of protein structure, function
Resonance assignments in proteins Christina Redfield 1. Introduction The assignment of resonances in the complex NMR spectrum of a protein is the first step in any study of protein structure, function
NPTEL VIDEO COURSE PROTEOMICS PROF. SANJEEVA SRIVASTAVA
 LECTURE-25 Quantitative proteomics: itraq and TMT TRANSCRIPT Welcome to the proteomics course. Today we will talk about quantitative proteomics and discuss about itraq and TMT techniques. The quantitative
LECTURE-25 Quantitative proteomics: itraq and TMT TRANSCRIPT Welcome to the proteomics course. Today we will talk about quantitative proteomics and discuss about itraq and TMT techniques. The quantitative
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION DOI: 10.1038/NCHEM.2720 1 2 3 Tuning underwater adhesion with cation-π interactions Matthew A. Gebbie, Wei Wei, Alex M. Schrader,
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION DOI: 10.1038/NCHEM.2720 1 2 3 Tuning underwater adhesion with cation-π interactions Matthew A. Gebbie, Wei Wei, Alex M. Schrader,
Assessing Intrinsic Side Chain Interactions between i and i + 4 Residues in Solvent-Free Peptides: A Combinatorial Gas-Phase Approach
 10566 J. Phys. Chem. A 2003, 107, 10566-10579 Assessing Intrinsic Side Chain Interactions between i and i + 4 Residues in Solvent-Free Peptides: A Combinatorial Gas-Phase Approach Catherine A. Srebalus
10566 J. Phys. Chem. A 2003, 107, 10566-10579 Assessing Intrinsic Side Chain Interactions between i and i + 4 Residues in Solvent-Free Peptides: A Combinatorial Gas-Phase Approach Catherine A. Srebalus
BMB/Bi/Ch 173 Winter 2018
 BMB/Bi/Ch 173 Winter 2018 Homework Set 8.1 (100 Points) Assigned 2-27-18, due 3-6-18 by 10:30 a.m. TA: Rachael Kuintzle. Office hours: SFL 220, Friday 3/2 4:00-5:00pm and SFL 229, Monday 3/5 4:00-5:30pm.
BMB/Bi/Ch 173 Winter 2018 Homework Set 8.1 (100 Points) Assigned 2-27-18, due 3-6-18 by 10:30 a.m. TA: Rachael Kuintzle. Office hours: SFL 220, Friday 3/2 4:00-5:00pm and SFL 229, Monday 3/5 4:00-5:30pm.
NH 2. Biochemistry I, Fall Term Sept 9, Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter
 Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
Dental Biochemistry EXAM I
 Dental Biochemistry EXAM I August 29, 2005 In the reaction below: CH 3 -CH 2 OH -~ ethanol CH 3 -CHO acetaldehyde A. acetoacetate is being produced B. ethanol is being oxidized to acetaldehyde C. acetaldehyde
Dental Biochemistry EXAM I August 29, 2005 In the reaction below: CH 3 -CH 2 OH -~ ethanol CH 3 -CHO acetaldehyde A. acetoacetate is being produced B. ethanol is being oxidized to acetaldehyde C. acetaldehyde
Final Chem 4511/6501 Spring 2011 May 5, 2011 b Name
 Key 1) [10 points] In RNA, G commonly forms a wobble pair with U. a) Draw a G-U wobble base pair, include riboses and 5 phosphates. b) Label the major groove and the minor groove. c) Label the atoms of
Key 1) [10 points] In RNA, G commonly forms a wobble pair with U. a) Draw a G-U wobble base pair, include riboses and 5 phosphates. b) Label the major groove and the minor groove. c) Label the atoms of
2. Separate the ions based on their mass to charge (m/e) ratio. 3. Measure the relative abundance of the ions that are produced
 I. Mass spectrometry: capable of providing both quantitative and qualitative information about samples as small as 100 pg (!) and with molar masses in the 10 4-10 5 kdalton range A. The mass spectrometer
I. Mass spectrometry: capable of providing both quantitative and qualitative information about samples as small as 100 pg (!) and with molar masses in the 10 4-10 5 kdalton range A. The mass spectrometer
Read more about Pauling and more scientists at: Profiles in Science, The National Library of Medicine, profiles.nlm.nih.gov
 2018 Biochemistry 110 California Institute of Technology Lecture 2: Principles of Protein Structure Linus Pauling (1901-1994) began his studies at Caltech in 1922 and was directed by Arthur Amos oyes to
2018 Biochemistry 110 California Institute of Technology Lecture 2: Principles of Protein Structure Linus Pauling (1901-1994) began his studies at Caltech in 1922 and was directed by Arthur Amos oyes to
Structure of the α-helix
 Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Introduction to the Q Trap LC/MS/MS System
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment ABI Q Trap LC/MS/MS Introduction to the Q Trap LC/MS/MS System The Q Trap
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment ABI Q Trap LC/MS/MS Introduction to the Q Trap LC/MS/MS System The Q Trap
FOCUS: NOVEL APPROACHES TO PEPTIDE AND PROTEIN STRUCTURE
 FOCUS: NOVEL APPROACHES TO PEPTIDE AND PROTEIN STRUCTURE Computational Investigation and Hydrogen/Deuterium Exchange of the Fixed Charge Derivative Tris(2,4,6-Trimethoxyphenyl) Phosphonium: Implications
FOCUS: NOVEL APPROACHES TO PEPTIDE AND PROTEIN STRUCTURE Computational Investigation and Hydrogen/Deuterium Exchange of the Fixed Charge Derivative Tris(2,4,6-Trimethoxyphenyl) Phosphonium: Implications
Protein structure. Protein structure. Amino acid residue. Cell communication channel. Bioinformatics Methods
 Cell communication channel Bioinformatics Methods Iosif Vaisman Email: ivaisman@gmu.edu SEQUENCE STRUCTURE DNA Sequence Protein Sequence Protein Structure Protein structure ATGAAATTTGGAAACTTCCTTCTCACTTATCAGCCACCT...
Cell communication channel Bioinformatics Methods Iosif Vaisman Email: ivaisman@gmu.edu SEQUENCE STRUCTURE DNA Sequence Protein Sequence Protein Structure Protein structure ATGAAATTTGGAAACTTCCTTCTCACTTATCAGCCACCT...
Kinetics and Mechanism for the Formation of b- and y-type Ions from Singly Protonated Peptides on a Microsecond Time Scale: The Arginine Mystery
 Arginine Mystery in Peptide Ion Dissociation Bull. Korean Chem. Soc. 2008, Vol. 29, No. 12 2427 Kinetics and Mechanism for the Formation of b- and y-type Ions from Singly Protonated Peptides on a Microsecond
Arginine Mystery in Peptide Ion Dissociation Bull. Korean Chem. Soc. 2008, Vol. 29, No. 12 2427 Kinetics and Mechanism for the Formation of b- and y-type Ions from Singly Protonated Peptides on a Microsecond
Chapter 4: Amino Acids
 Chapter 4: Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. lipid polysaccharide enzyme 1940s 1980s. Lipids membrane 1960s. Polysaccharide Are energy metabolites and many of
Chapter 4: Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. lipid polysaccharide enzyme 1940s 1980s. Lipids membrane 1960s. Polysaccharide Are energy metabolites and many of
MASS SPECTROMETRY. Topics
 MASS SPECTROMETRY MALDI-TOF AND ESI-MS Topics Principle of Mass Spectrometry MALDI-TOF Determination of Mw of Proteins Structural Information by MS: Primary Sequence of a Protein 1 A. Principles Ionization:
MASS SPECTROMETRY MALDI-TOF AND ESI-MS Topics Principle of Mass Spectrometry MALDI-TOF Determination of Mw of Proteins Structural Information by MS: Primary Sequence of a Protein 1 A. Principles Ionization:
Potentiometric Titration of an Amino Acid. Introduction
 NAME: Course: DATE Sign-Off: Performed: Potentiometric Titration of an Amino Acid Introduction In previous course-work, you explored the potentiometric titration of a weak acid (HOAc). In this experiment,
NAME: Course: DATE Sign-Off: Performed: Potentiometric Titration of an Amino Acid Introduction In previous course-work, you explored the potentiometric titration of a weak acid (HOAc). In this experiment,
Dihedral Angles. Homayoun Valafar. Department of Computer Science and Engineering, USC 02/03/10 CSCE 769
 Dihedral Angles Homayoun Valafar Department of Computer Science and Engineering, USC The precise definition of a dihedral or torsion angle can be found in spatial geometry Angle between to planes Dihedral
Dihedral Angles Homayoun Valafar Department of Computer Science and Engineering, USC The precise definition of a dihedral or torsion angle can be found in spatial geometry Angle between to planes Dihedral
From Amino Acids to Proteins - in 4 Easy Steps
 From Amino Acids to Proteins - in 4 Easy Steps Although protein structure appears to be overwhelmingly complex, you can provide your students with a basic understanding of how proteins fold by focusing
From Amino Acids to Proteins - in 4 Easy Steps Although protein structure appears to be overwhelmingly complex, you can provide your students with a basic understanding of how proteins fold by focusing
I690/B680 Structural Bioinformatics Spring Protein Structure Determination by NMR Spectroscopy
 I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
CHEM 3653 Exam # 1 (03/07/13)
 1. Using phylogeny all living organisms can be divided into the following domains: A. Bacteria, Eukarya, and Vertebrate B. Archaea and Eukarya C. Bacteria, Eukarya, and Archaea D. Eukarya and Bacteria
1. Using phylogeny all living organisms can be divided into the following domains: A. Bacteria, Eukarya, and Vertebrate B. Archaea and Eukarya C. Bacteria, Eukarya, and Archaea D. Eukarya and Bacteria
Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions Van der Waals Interactions
Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions Van der Waals Interactions
MS-based proteomics to investigate proteins and their modifications
 MS-based proteomics to investigate proteins and their modifications Francis Impens VIB Proteomics Core October th 217 Overview Mass spectrometry-based proteomics: general workflow Identification of protein
MS-based proteomics to investigate proteins and their modifications Francis Impens VIB Proteomics Core October th 217 Overview Mass spectrometry-based proteomics: general workflow Identification of protein
Separation of Large and Small Peptides by Supercritical Fluid Chromatography and Detection by Mass Spectrometry
 Separation of Large and Small Peptides by Supercritical Fluid Chromatography and Detection by Mass Spectrometry Application Note Biologics and Biosimilars Author Edgar Naegele Agilent Technologies, Inc.
Separation of Large and Small Peptides by Supercritical Fluid Chromatography and Detection by Mass Spectrometry Application Note Biologics and Biosimilars Author Edgar Naegele Agilent Technologies, Inc.
Supporting Information for: A. Portz 1, C. R. Gebhardt 2, and M. Dürr 1, Giessen, D Giessen, Germany
 Supporting Information for: Real-time Investigation of the H/D Exchange Kinetics of Porphyrins and Oligopeptides by means of Neutral Cluster Induced Desorption/Ionization Mass Spectrometry A. Portz 1,
Supporting Information for: Real-time Investigation of the H/D Exchange Kinetics of Porphyrins and Oligopeptides by means of Neutral Cluster Induced Desorption/Ionization Mass Spectrometry A. Portz 1,
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
Course Notes: Topics in Computational. Structural Biology.
 Course Notes: Topics in Computational Structural Biology. Bruce R. Donald June, 2010 Copyright c 2012 Contents 11 Computational Protein Design 1 11.1 Introduction.........................................
Course Notes: Topics in Computational Structural Biology. Bruce R. Donald June, 2010 Copyright c 2012 Contents 11 Computational Protein Design 1 11.1 Introduction.........................................
Chapter 4. Glutamic Acid in Solution - Correlations
 Chapter 4 Glutamic Acid in Solution - Correlations 4. Introduction Glutamic acid crystallises from aqueous solution, therefore the study of these molecules in an aqueous environment is necessary to understand
Chapter 4 Glutamic Acid in Solution - Correlations 4. Introduction Glutamic acid crystallises from aqueous solution, therefore the study of these molecules in an aqueous environment is necessary to understand
PROTEIN STRUCTURE AMINO ACIDS H R. Zwitterion (dipolar ion) CO 2 H. PEPTIDES Formal reactions showing formation of peptide bond by dehydration:
 PTEI STUTUE ydrolysis of proteins with aqueous acid or base yields a mixture of free amino acids. Each type of protein yields a characteristic mixture of the ~ 20 amino acids. AMI AIDS Zwitterion (dipolar
PTEI STUTUE ydrolysis of proteins with aqueous acid or base yields a mixture of free amino acids. Each type of protein yields a characteristic mixture of the ~ 20 amino acids. AMI AIDS Zwitterion (dipolar
Exam III. Please read through each question carefully, and make sure you provide all of the requested information.
 09-107 onors Chemistry ame Exam III Please read through each question carefully, and make sure you provide all of the requested information. 1. A series of octahedral metal compounds are made from 1 mol
09-107 onors Chemistry ame Exam III Please read through each question carefully, and make sure you provide all of the requested information. 1. A series of octahedral metal compounds are made from 1 mol
Lecture'18:'April'2,'2013
 CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie 2 3 N cysteine (Cys) S oxidation S S 3 N cystine N 3 Lecture'18:'April'2,'2013 Disaccharides+&+Polysaccharides Amino+acids++(26.1926.3)
CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie 2 3 N cysteine (Cys) S oxidation S S 3 N cystine N 3 Lecture'18:'April'2,'2013 Disaccharides+&+Polysaccharides Amino+acids++(26.1926.3)
SRM assay generation and data analysis in Skyline
 in Skyline Preparation 1. Download the example data from www.srmcourse.ch/eupa.html (3 raw files, 1 csv file, 1 sptxt file). 2. The number formats of your computer have to be set to English (United States).
in Skyline Preparation 1. Download the example data from www.srmcourse.ch/eupa.html (3 raw files, 1 csv file, 1 sptxt file). 2. The number formats of your computer have to be set to English (United States).
Translational Biomarker Core
 Translational Biomarker Core Instrumentation Thermo Scientific TSQ Quantum Triple Quadrupole Mass Spectrometers. There are two TSQ Quantum Ultra AM instruments available in the TBC. The TSQ Quantum Ultra
Translational Biomarker Core Instrumentation Thermo Scientific TSQ Quantum Triple Quadrupole Mass Spectrometers. There are two TSQ Quantum Ultra AM instruments available in the TBC. The TSQ Quantum Ultra
12/6/12. Dr. Sanjeeva Srivastava IIT Bombay. Primary Structure. Secondary Structure. Tertiary Structure. Quaternary Structure.
 Dr. anjeeva rivastava Primary tructure econdary tructure Tertiary tructure Quaternary tructure Amino acid residues α Helix Polypeptide chain Assembled subunits 2 1 Amino acid sequence determines 3-D structure
Dr. anjeeva rivastava Primary tructure econdary tructure Tertiary tructure Quaternary tructure Amino acid residues α Helix Polypeptide chain Assembled subunits 2 1 Amino acid sequence determines 3-D structure
Due in class on Thursday Sept. 8 th
 Problem Set #1 Chem 391 Due in class on Thursday Sept. 8 th ame Solutions A note: To help me read quickly, I structure the problem set like an exam. I have made small spaces for answers where I d like
Problem Set #1 Chem 391 Due in class on Thursday Sept. 8 th ame Solutions A note: To help me read quickly, I structure the problem set like an exam. I have made small spaces for answers where I d like
CHMI 2227 EL. Biochemistry I. Test January Prof : Eric R. Gauthier, Ph.D.
 CHMI 2227 EL Biochemistry I Test 1 26 January 2007 Prof : Eric R. Gauthier, Ph.D. Guidelines: 1) Duration: 55 min 2) 14 questions, on 7 pages. For 70 marks (5 marks per question). Worth 15 % of the final
CHMI 2227 EL Biochemistry I Test 1 26 January 2007 Prof : Eric R. Gauthier, Ph.D. Guidelines: 1) Duration: 55 min 2) 14 questions, on 7 pages. For 70 marks (5 marks per question). Worth 15 % of the final
A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of
 Questions with Answers- Amino Acids & Peptides A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of 5.0-6.5 (Questions 1-4) 1. Which
Questions with Answers- Amino Acids & Peptides A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of 5.0-6.5 (Questions 1-4) 1. Which
Dental Biochemistry Exam The total number of unique tripeptides that can be produced using all of the common 20 amino acids is
 Exam Questions for Dental Biochemistry Monday August 27, 2007 E.J. Miller 1. The compound shown below is CH 3 -CH 2 OH A. acetoacetate B. acetic acid C. acetaldehyde D. produced by reduction of acetaldehyde
Exam Questions for Dental Biochemistry Monday August 27, 2007 E.J. Miller 1. The compound shown below is CH 3 -CH 2 OH A. acetoacetate B. acetic acid C. acetaldehyde D. produced by reduction of acetaldehyde
Biochemistry Prof. S. DasGupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture - 06 Protein Structure IV
 Biochemistry Prof. S. DasGupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture - 06 Protein Structure IV We complete our discussion on Protein Structures today. And just to recap
Biochemistry Prof. S. DasGupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture - 06 Protein Structure IV We complete our discussion on Protein Structures today. And just to recap
Chemistry Chapter 22
 hemistry 2100 hapter 22 Proteins Proteins serve many functions, including the following. 1. Structure: ollagen and keratin are the chief constituents of skin, bone, hair, and nails. 2. atalysts: Virtually
hemistry 2100 hapter 22 Proteins Proteins serve many functions, including the following. 1. Structure: ollagen and keratin are the chief constituents of skin, bone, hair, and nails. 2. atalysts: Virtually
Instrumental Analysis. Mass Spectrometry. Lecturer:! Somsak Sirichai
 303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
Conformational Geometry of Peptides and Proteins:
 Conformational Geometry of Peptides and Proteins: Before discussing secondary structure, it is important to appreciate the conformational plasticity of proteins. Each residue in a polypeptide has three
Conformational Geometry of Peptides and Proteins: Before discussing secondary structure, it is important to appreciate the conformational plasticity of proteins. Each residue in a polypeptide has three
Chemical Kinetics. Goal. Objectives
 4 Chemical Kinetics To understand the physical Goal factors that determine reaction rates. bjectives After this chapter, you should be able to: describe the factors that determine reaction rates. identify
4 Chemical Kinetics To understand the physical Goal factors that determine reaction rates. bjectives After this chapter, you should be able to: describe the factors that determine reaction rates. identify
Theoretical study of through-space and through-bond electron transfer within positively charged peptides in the gas phase
 Available online at www.sciencedirect.com International Journal of Mass Spectrometry 269 (2008) 149 164 Theoretical study of through-space and through-bond electron transfer within positively charged peptides
Available online at www.sciencedirect.com International Journal of Mass Spectrometry 269 (2008) 149 164 Theoretical study of through-space and through-bond electron transfer within positively charged peptides
