You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
|
|
|
- Brianne Small
- 5 years ago
- Views:
Transcription
1 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 17, 2014 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so you can find ones you can answer and show me what you know, rather than just a few questions that may be testing the one thing you forgot. I recommend you look the exam over and answer the questions you are sure of first, then go back and try to figure out the rest. Also make sure to look at the point totals on the questions as a guide to help budget your time. You must have your answers written in PERMAET ink if you want a regrade!!!! This means no test written in pencil or ERASABLE IK will be regraded. Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process. FIALLY, DUE T SME UFRTUATE REET IIDETS YU ARE T ALLWED T ITERAT WIT YUR ELL PE I AY WAY. IF YU TU YUR ELL PE DURIG TE EXAM YU WILL GET A "0" MATTER WAT YU ARE DIG WIT TE PE. PUT IT AWAY AD LEAVE IT TERE!!!
2 Page Points Total (24) (18) (31) (14) (18) (26) (23) (26) (21) (20) (16) (19) (13) (13) (12) (4) (298)
3 Student onor ode As a student of The University of Texas at Austin, I shall abide by the core values of the University and uphold academic integrity. (Your signature)
4 ompound pk a ydrochloric acid -l -7 Protonated alcohol R ydronium ion arboxylic acids Ammonium ion β-dicarbonyls 3 R - 4 R 2 R' Primary ammonium β-ketoesters β-diesters Water Alcohols Acid chlorides Aldehydes Ketones Esters R R R 2 R 2 R 2 R 2 2 R' 2 R' l R 2 R' R' Terminal alkynes R 25 LDA -(i- 3 7 ) 2 40 Terminal alkenes R 2 44 Alkanes
5 Pg 1 (24) 1. (2 pts) What is the most important question in chemistry? 2. (14 points) Suppose a relative of yours is having an MRI. In no more than four sentences, explain to them what is happening when they have the MRI scan. We wil be looking for a minimum of 7 key points here. 3. (8 points) Draw the two most important resonance contributing structures of the amide shown below. Be sure to show all lone pairs and formal charges. You do not have to draw arrows on this one.
6 Aromatic Insect Lifecycle: Mor Phosis I put this here to help you relax. You will do better on the exam in a relaxed frame of mind. (If the above equation made you laugh or even smile, you may be a chem nerd, but nobody has to find out.)
7 Pg 2 (18) 4. (1 pt. each) ere are a number of statements regarding conjugation and light absorption/emission. Do not second guess yourself, this is not meant to be tricky! heck the appropriate box to indicate whether the statement is true or false. True False A. When using molecular orbital theory, it is best to think of electron density as being like waves, since it is described mathematically using wave equations. B. When molecules absorb light, electrons are excited from a bonding to an antibonding molecular orbital. If a substance absorbs blue light, it will appear blue to our eyes. D. The larger the number of pi bonds involved in a conjugated molecule (a "piway") the smaller the energy gap between the higest occupied pi molecular orbital and the lowest unoccupied molecular orbital. E. A reaction is said to be under kinetic control if the ratio of products is dependent on the relative energies of the products. F. A reaction is said to be under thermodynamic control if the ratio of products is dependent on the relative energies of the products. G. Fluorescence occurs when a photon is emitted as an electron relaxes from an antibonding molecular orbital back to a bonding molecular orbital in a molecule that has absrbed light.. Phosphorescence occurs when a chemical reaction generates a product in an excited state. I. Phosphorescence occurs when an electron must flip its spin before relaxing back to a bonding molecular orbital while emitting a photon. J. The light from a green laser will go through your finger, while the light from a red laser is absorbed. 5. (8 points) Aromaticity is a term that refers to molecules with characteristic pi systems. A theorist named ückel helped to derive several criteria that can be used to determine if a molecule is aromatic. List all four of these criteria:
8 Pg 3 (31) 6. (15 points) Draw a circle around all of the molecules below that can be considered aromatic. 7. (16 points) For each pair of molecules, circle the one that is more acidic. A. or B. 3 or F 3. or D. or E. or F. or G.. or or
9 Pg 4 (14) Signature 8. (9 points) n the lines provided, state the hybridization state of the atom indicated by the arrow (5 points) n the lines provided, state the atomic orbital that contains the lone pair of electrons indicated by the arrow. 3 3
10 Pg 5 (18) 10. (2 pts each) In each of the boxes over an arrow, write the minimum number of equivalents of the specified reagent required to carry out the reaction shown to completion. If only a catalytic amount is needed, write "AT". ote: You must assume the carbonyl compound starting material is initially present in an amount of 1.0 equivalent. A) B) 3 equivalents a 1) 2) equivalents mild 3 * (racemic) 3 a * (racemic) 3 ) D) E) 3 3 1) 1) 1) 2) equivalents LDA equivalents LDA mild 3 equivalents LDA 3 * 3 (racemic) * * 2) 3) mild 3 equivalents (racemic) F) l 2 3 G) excess 2 equivalents 2 S 4 ) 1) 2) mild 3 equivalents LDA * (racemic)
11 Pg 6 (26) 11. (26 pts) omplete the mechanism for the following Michael reaction. Be sure to show arrows to indicate movement of all electrons, write all lone pairs, all formal charges, and all the products for each step. Remember, I said all the products for each step. IF A EW IRAL ETER IS REATED I A ITERMEDIATE R TE PRDUTS, MARK IT WIT A ASTERISK AD LABEL AS "RAEMI" IF RELEVAT. I TE BX BY EA SET F ARRWS, WRITE WI F TE 4 MEAISTI ELEMETS IS IDIATED I EA STEP F YUR MEAISM (For example, "Add a proton"). Michael Reaction Equivalent 3 3 tautomerization 3 Products
12 Signature Pg 7 (23) 12. (23 pts) omplete the mechanism for the following Dieckmann reaction. Be sure to show arrows to indicate movement of all electrons, write all lone pairs, all formal charges, and all the products for each step. Remember, I said all the products for each step. IF A EW IRAL ETER IS REATED I A ITERMEDIATE R TE PRDUTS, MARK IT WIT A ASTERISK AD LABEL AS "RAEMI" IF RELEVAT. I TE BX BY EA SET F ARRWS, WRITE WI F TE 4 MEAISTI ELEMETS IS IDIATED I EA STEP F YUR MEAISM (For example, "Add a proton") Products
13 Pg 8 (26) 13. (3 or 5 pts each) For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk (*) and if a racemic mixture is produced, you must write "racemic" under your structure. If an E,Z mixture is produced as the result of a dehydration step, write "E,Z mixture", but you only have to draw one isomer, not both. These directions are different than you may have seen before, and are intended to make it easier for you. You should read them again so you know what we want. 1) 0.5 eq. LDA 2) 3 (mild acid no heat) 1) DIBAL 2) 2 1) 0.5 eq. aet 2) 3 (mild acid no heat) 3 3 / 1) 3 Br 2) 3 1) l 2) 3
14 Pg 9 (21) 13. (3 or 5 pts each) For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk (*) and if a racemic mixture is produced, you must write "racemic" under your structure. If an E,Z mixture is produced as the result of a dehydration step, write "E,Z mixture", but you only have to draw one isomer, not both. These directions are different than you may have seen before, and are intended to make it easier for you. You should read them again so you know what we want. 1) cat. a 2) 3 with heating cat. a (o acid or heat) 1.0 eq. LDA 1) 1.0 eq. LDA 2) 1.0 eq. 3) 3 with heating Et Et 1) cat. a 2) 3 with heating
15 Pg 10 (20) 13. (3 or 5 pts each) For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk (*) and if a racemic mixture is produced, you must write "racemic" under your structure. If an E,Z mixture is produced as the result of a dehydration step, write "E,Z mixture", but you only have to draw one isomer, not both. These directions are different than you may have seen before, and are intended to make it easier for you. You should read them again so you know what we want. 2) 1) 1.0 eq.aet 3) 3 (mild acid, no heat) 3 1) 1.0 eq.aet 2) 3) 3 (stronger acid, with heat) 1) 1.0 eq.aet Br 2) 3) 3 (very strong acid with heat) 4) More heat 1) 1.0 eq. LDA 2) l 3) LiAl 4 4) 2 TIE TIS ow many stereoisomers are possible for this product?
16 Pg 11 (16) 14. (16 pts) Using any reagents turn the starting material into the indicated product. All carbon atoms must come from the starting materials. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to right "Racemic" where appropriate. Remember, all of the carbons of the product must come from the given starting materials.?
17 Pg 12 (19) 15. (19 pts) Using any reagents turn the starting material into the indicated product. All carbon atoms must come from the starting materials. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to right "Racemic" where appropriate. Remember, all of the carbons of the product must come from the given starting materials.? Racemic *
18 Pg 13 (13) 16. (13 pts) Using any reagents turn the starting material into the indicated product. All carbon atoms must come from the starting materials. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to right "Racemic" where appropriate. Remember, all of the carbons of the product must come from the given starting materials.? * Racemic
19 Pg 14 (13) 17. (13 pts) Using any reagents turn the starting material into the indicated product. All carbon atoms must come from the starting materials. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to right "Racemic" where appropriate. This is the hardest synthesis problem I have ever put on an exam. SAVE IT UTIL TE ED Remember, all of the carbons of the product must come from the given starting materials.?
20 Pg 15 (12) 18. Below are listed some key relationships that are useful when analyzing the absorbance and fluorescencce properties of molecules. E = hc/λ E = energy h = Planck's constant c = the speed of light λ = the wavelength of light olors of the rainbow, listed with the wavelengths of light correspoding to those colors Ultraviolet light Violet Blue Green Yellow range Red Infrared light < 380 nm 380 nm 470 nm 520 nm 585 nm 650 nm 700 nm > 700 nm Lycopene β-arotene Select the statement that is true: A) Lycopene has 13 pi bonds in conjugation, while β-carotene has 11 pi bonds in conjugation B) Lycopene has 11 pi bonds in conjugation, while β-carotene has 11 pi bonds in conjugation ) Lycopene has 11 pi bonds in conjugation, while β-carotene has 9 pi bonds in conjugation D) Lycopene has 13 pi bonds in conjugation, while β-carotene has 9 pi bonds in conjugation Tomatoes have lycopene and carrots have β-carotene. Tomatoes are red while carrots are orange because: A) Lycopene absorbs more violet wavelengths compared to β-carotene B) Lycopene absorbs more red wavelengths compared to β-carotene ) Lycopene absorbs more yellow wavelengths compared to β-carotene D) Lycopene absorbs more infrared wavelengths compared to β-carotene Based on your understanding of the light absorbance process, which must be true A) The energy difference between the highest occupied pi molecular orbital and the lowest unoccupied pi molecular orbital is larger for lycopene compared with β-carotene. B) The energy difference between the highest occupied pi molecular orbital and the lowest unoccupied pi molecular orbital is smaller for lycopene compared with β-carotene. ) The energy difference between the lowest occupied pi molecular orbital and the highest unoccupied pi molecular orbital is larger for lycopene compared with β-carotene. D) The energy difference between the lowest occupied pi molecular orbital and the highest unoccupied pi molecular orbital is smaller for lycopene compared with β-carotene. :
21 Pg 16 (4) In addition to molecules with long pi-ways, other types of molecules absorb visible light and are highly colored. For example, many aromatic molecules are used as dyes in our clothes and in nature. For many aromatic molecules, the absorbance of visible wavelengths derives from excitation of an electron in a lone pair on an atom attached to the ring, to the lowest unoccupied pi molecular orbital of the ring. This type of absorbance is often called an n to π* absorbance in which the n refers to the non-bonding lone pair, and the π* (pronounced pi-star ) refers to the lowest unoccupied pi molecule orbital, which is an antibonding pi molecular orbital, hence the π* designation. Below is the parent structure of the so-called anthocyanine molecule responsible for the blue color of blueberries. otice the atoms bonding to the aromatic rings, those have lone pairs of electrons that can be involved in the visible region absorbance. R R 1 R 2 R, R 1, R 2, and R 3 can be various entities including alkyl groups or even carbohydrates R 3 Anthocyanins found in blueberries The absorbance in molecules such as lycopene or β-carotene is often referred to as π to π* because it corrresponds to an electron excited from the highest occupied π orbital to an empty antiboding π* molecular orbital. Based on your understanding of the light absorbance process, which must be true: A) The n to π* energy difference, corresponding to absorbance in the blue and green regions of the spectrum, is larger for blueberry anthocyanins compared with the π to π* energy difference in lycopene or β-carotene. B) The n to π* energy difference, corresponding to absorbance in the blue and green regions of the spectrum, is smaller for blueberry anthocyanins compared with the π to π* energy difference in lycopene or β-carotene. ) The n to π* energy difference, corresponding to absorbance in the orange and red regions of the spectrum, is larger for blueberry anthocyanins compared with the π to π* energy difference in lycopene or β-carotene D) The n to π* energy difference, corresponding to absorbance in the orange and red regions of the spectrum, is smaller for blueberry anthocyanins compared with the π to π* energy difference in lycopene or β-carotene.
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 23, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 23, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 3rd Midterm April 18, 2013 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 3rd Midterm April 18, 2013 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 25, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 25, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 30 Dr. Brent Iverson nd Midterm March 7, 008 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 30 Dr. Brent Iverson nd Midterm March 7, 008 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 22, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 22, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 2nd Midterm March 23, 2017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 2nd Midterm March 23, 2017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310M/318M Dr. ent Iverson Final exam December 8, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): SIGATURE: hemistry 310M/318M Dr. ent Iverson Final exam December 8, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): IGATURE: hemistry 320M/328M Dr. Brent Iverson 2nd Midterm ctober 25, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): IGATURE: hemistry 320M/328M Dr. Brent Iverson 2nd Midterm ctober 25, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320N Dr. Brent Iverson 3rd Midterm April 20, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 320N Dr. Brent Iverson 3rd Midterm April 20, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 3rd Midterm November 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 3rd Midterm November 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 2nd Midterm ctober 25, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 2nd Midterm ctober 25, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed.
AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed.
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 1st Midterm Feb. 14, 2013 (Sorry about the Valentine's day exam!) Please print the first three letters of your last name in the three boxes Please
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 1st Midterm Feb. 14, 2013 (Sorry about the Valentine's day exam!) Please print the first three letters of your last name in the three boxes Please
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm September 29, 2011 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): SIGATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm September 29, 2011 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
Homework Problem Set 11 Iverson CH320N Due Friday, May 5
 omework Problem Set 11 Iverson 320 Due Friday, May 5 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson Problem Set 11 April 28, 2017 Please print the first three letters of your last name in the three
omework Problem Set 11 Iverson 320 Due Friday, May 5 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson Problem Set 11 April 28, 2017 Please print the first three letters of your last name in the three
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 AME (Print): SIGATURE: hemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Score: Homework Problem Set 6 Iverson CH320N Due Friday, March 10. NAME (Print): Chemistry 320N Dr. Brent Iverson 6th Homework March 3, 2017
 omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): Chemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 SIGNATURE:
 AME (Print): SIGATURE: hemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): SIGATURE: hemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: hemistry 30N Dr. Brent Iverson st Midterm Feb. 9, 05 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but there
NAME (Print): SIGNATURE: hemistry 30N Dr. Brent Iverson st Midterm Feb. 9, 05 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but there
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson 1st Midterm Feb. 22, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson 1st Midterm Feb. 22, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson Final Exam May 14, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson Final Exam May 14, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 AME (Print): SIGATUE: hemistry 0M/8M Dr. Brent Iverson 1st Midterm September 8, 017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATUE: hemistry 0M/8M Dr. Brent Iverson 1st Midterm September 8, 017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AM (Print): SIGATUR: hemistry 320 Dr. ent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed. Take
AM (Print): SIGATUR: hemistry 320 Dr. ent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed. Take
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 ME (Print): SIGTURE: hemistry 320 Dr. rent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
ME (Print): SIGTURE: hemistry 320 Dr. rent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): Chemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 SIGNATURE:
 AME (Print): SIGATURE: hemistry 0M/8M Dr. Brent Iverson st Midterm September 7, 08 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 0M/8M Dr. Brent Iverson st Midterm September 7, 08 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 18, 2009 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 18, 2009 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final December 16, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final December 16, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Homework Problem Set 7 Iverson CH320M/328M Due Friday, Nov. 2
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. Brent Iverson 7th omework October 29, 2018 Please print the first three letters of your last name in the three boxes 1 Score: 2 1. For the following reactions,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. Brent Iverson 7th omework October 29, 2018 Please print the first three letters of your last name in the three boxes 1 Score: 2 1. For the following reactions,
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. ent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. ent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 0M/8M Dr. Brent Iverson st Midterm September 7, 0 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 0M/8M Dr. Brent Iverson st Midterm September 7, 0 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 4, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 4, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 1, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 1, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Score: NAME (Print): Chemistry 320N Dr. Brent Iverson 3rd Homework February 3, 2017 SIGNATURE:
 omework Problem Set 3 Iverson 30N Due Friday February 10 NAME (Print): SIGNATURE: hemistry 30N Dr. ent Iverson 3rd omework February 3, 017 Please print the first three letters of your last name in the
omework Problem Set 3 Iverson 30N Due Friday February 10 NAME (Print): SIGNATURE: hemistry 30N Dr. ent Iverson 3rd omework February 3, 017 Please print the first three letters of your last name in the
Note: You must have your answers written in blue or black pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 30M/38M Dr. Brent Iverson st Midterm Sept. 8, 005 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 30M/38M Dr. Brent Iverson st Midterm Sept. 8, 005 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 23, 2006 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 23, 2006 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson Final December 15, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson Final December 15, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): Chemistry 610A/618A Dr. Brent Iverson 2nd Exam Oct. 29, 2003 SIGNATURE:
 NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final exam December 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final exam December 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Homework 5 Organic Chemistry MCAT Review Summer 2012 Brent Iverson
 omework 5 rganic Chemistry MCAT Review Summer 2012 ent Iverson 1 1. For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk
omework 5 rganic Chemistry MCAT Review Summer 2012 ent Iverson 1 1. For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk
2 h; 240 points please print clearly Dr. Kathleen Nolta Signature UM ID # Problem Points Score GSI I 42 II 44 III 40 IV 43 V 31 VI 40 Total 240
 hemistry 10 First ame Final Examination Last ame h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I II III 0 IV V 1 VI 0
hemistry 10 First ame Final Examination Last ame h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I II III 0 IV V 1 VI 0
2 h; 240 points Signature UM ID # Problem Points Score GSI I 42 II 45 III 35 IV 46 V 36 VI 36 Total 240
 hemistry 10 First ame Final Examination Last ame June 5, 01 please print clearly h; 0 points Signature UM ID # PRIT TE FIRST LETTER F YUR LAST AME ERE: Problem Points Score GSI I II 5 III 5 IV V VI Total
hemistry 10 First ame Final Examination Last ame June 5, 01 please print clearly h; 0 points Signature UM ID # PRIT TE FIRST LETTER F YUR LAST AME ERE: Problem Points Score GSI I II 5 III 5 IV V VI Total
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chapter 14: Dienes and Conjugation. Topics Dienes: Naming and Properties. Conjugation. 1,2 vs 1,4 addition and the stability of the allyl cation
 rganic hemistry otes by Jim Maxka hapter 14: Dienes and onjugation Topics Dienes: aming and Properties onjugation 1,2 vs 1,4 addition and the stability of the allyl cation Diels Alder eaction Simple rbital
rganic hemistry otes by Jim Maxka hapter 14: Dienes and onjugation Topics Dienes: aming and Properties onjugation 1,2 vs 1,4 addition and the stability of the allyl cation Diels Alder eaction Simple rbital
UNIT TWO BOOKLET 1. Molecular Orbitals and Hybridisation
 DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT TWO BOOKLET 1 Molecular Orbitals and Hybridisation In the inorganic unit we learned about atomic orbitals and how they could be used to write the electron
DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT TWO BOOKLET 1 Molecular Orbitals and Hybridisation In the inorganic unit we learned about atomic orbitals and how they could be used to write the electron
1.5 h; 100 points Signature UM ID # Problem Points Score GSI I 17 II 20 III 20 IV 19 V 24 Total
 hemistry 10 First ame First Examination Last ame May 0, 01 please print clearly 1.5 h; 100 points ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 17 II 0 III 0 IV 19 V
hemistry 10 First ame First Examination Last ame May 0, 01 please print clearly 1.5 h; 100 points ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 17 II 0 III 0 IV 19 V
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHE 232 Organic Chemistry II Exam 4 Name: KEY
 CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
Identifying Functional Groups. Why is this necessary? Alkanes. Why is this so important? What is a functional group? 2/1/16
 Identifying Functional Groups The Key to Survival Why is this so important? ver and over again, you will be asked to do reactions, the details to which you will receive in lecture and via your textbook.
Identifying Functional Groups The Key to Survival Why is this so important? ver and over again, you will be asked to do reactions, the details to which you will receive in lecture and via your textbook.
PLEASE DO NOT WRITE ON THE EXAM (EVEN YOUR NAME) UNTIL TOLD TO START! Student ID # CHEM 8A, Organic Chemistry EXAM 1 (300 points)
 PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
CHEM 234, Spring 2016 COMPLETE THIS SECTION : Up to TWO POINTS will be removed for incorrect/missing information!
 EM 234, Spring 2016 FIAL EXAM MPLETE TIS SETI : Up to TW PITS will be removed for incorrect/missing information! Ian R. Gould PRITED FIRST AME PRITED LAST AME a Mg Person on your LEFT (or Empty or Aisle)
EM 234, Spring 2016 FIAL EXAM MPLETE TIS SETI : Up to TW PITS will be removed for incorrect/missing information! Ian R. Gould PRITED FIRST AME PRITED LAST AME a Mg Person on your LEFT (or Empty or Aisle)
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Ch 14 Conjugated Dienes and UV Spectroscopy
 Ch 14 Conjugated Dienes and UV Spectroscopy Conjugated Systems - Conjugated systems have alternating single and double bonds. For example: C=C C=C C=C and C=C C=O - This is not conjugated because the double
Ch 14 Conjugated Dienes and UV Spectroscopy Conjugated Systems - Conjugated systems have alternating single and double bonds. For example: C=C C=C C=C and C=C C=O - This is not conjugated because the double
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
Chemistry 14C Spring 2016 Final Exam Part B Page 1
 hemistry 14 Spring 2016 Final Exam Part B Page 1 In lecture we discussed the possibility that the first cells may have been formed in boiling mud puddles, which have been shown (in the lab) to produce
hemistry 14 Spring 2016 Final Exam Part B Page 1 In lecture we discussed the possibility that the first cells may have been formed in boiling mud puddles, which have been shown (in the lab) to produce
Dr. Steven Pedersen February 27, Chemistry 3B. Midterm 1
 r. Steven Pedersen February 7, 017 Student name: ASWER KEY Chemistry 3B Midterm 1 Student I: (Also include your SI in the top left corner of each page) Student signature: Problem 1 Problem Problem 3 Problem
r. Steven Pedersen February 7, 017 Student name: ASWER KEY Chemistry 3B Midterm 1 Student I: (Also include your SI in the top left corner of each page) Student signature: Problem 1 Problem Problem 3 Problem
Homework Problem Set 10 Iverson CH320M/CH328M Due Monday, December 10
 omework Problem Set 0 Iverson 30M/38M ue Monday, ecember 0 NME (Print): SIGNTURE: hemistry 30M/38M r. rent Iverson 0th omework ecember 3, 08 Please print the first three letters of your last name in the
omework Problem Set 0 Iverson 30M/38M ue Monday, ecember 0 NME (Print): SIGNTURE: hemistry 30M/38M r. rent Iverson 0th omework ecember 3, 08 Please print the first three letters of your last name in the
Exam 1 February 16, 2006 Professor Rebecca Hoenigman
 EM 3311 Spring 2006 Exam 1 February 16, 2006 Professor Rebecca oenigman Average Score = 55 igh Score = 96 Low Score = 13 I pledge to uphold the U onor ode: Signature Name (printed) Last four digits of
EM 3311 Spring 2006 Exam 1 February 16, 2006 Professor Rebecca oenigman Average Score = 55 igh Score = 96 Low Score = 13 I pledge to uphold the U onor ode: Signature Name (printed) Last four digits of
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
 Chem 201 Page 7 of 14 SECTION II: To be graded manually (Total value 42) Answers must be written in non-erasable ink to be considered for re-grading! Show all your work for full marks. QUESTION 15 VALUE
Chem 201 Page 7 of 14 SECTION II: To be graded manually (Total value 42) Answers must be written in non-erasable ink to be considered for re-grading! Show all your work for full marks. QUESTION 15 VALUE
CHAPTER 23 HW: ENOLS + ENOLATES
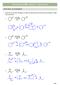 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
**YOU ARE NOT ALLOWED TO TAKE SPARE COPIES OF THIS EXAM FROM THE TESTING ROOM**
 EM 24, Spring 2017 Midterm #2 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST Answer Key Person on your LEFT (or Empty or Aisle) Person on
EM 24, Spring 2017 Midterm #2 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST Answer Key Person on your LEFT (or Empty or Aisle) Person on
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
Infrared Spectroscopy
 Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Problem Points Score GSI I 30 II 21 III 28 IV 30 V 31 Total 140
 hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
stereoselection view Product 1 with its enantiomer
 . ( points) A. Complete the following reactions as directed. (a) Cl C1 C i) major product DBU C C C C C Page 1 ii) Draw a ewman Projection for the conformation of the C1-C bond indicated that specifically
. ( points) A. Complete the following reactions as directed. (a) Cl C1 C i) major product DBU C C C C C Page 1 ii) Draw a ewman Projection for the conformation of the C1-C bond indicated that specifically
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10
 Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Chem 341 Organic Chemistry I Final Exam December 12, 2007 N A M E K E Y
 Chem 341 rganic Chemistry I Final Exam December 12, 2007 N A M E K E Y Top 10 signs you ve had Chem 341: 10. You can read the ingredients list on the back of your shampoo bottle and understand what s in
Chem 341 rganic Chemistry I Final Exam December 12, 2007 N A M E K E Y Top 10 signs you ve had Chem 341: 10. You can read the ingredients list on the back of your shampoo bottle and understand what s in
Problems. 8. Stereochemical Analysis Physical Properties, Forces of Interaction Resonance, Curved Arrows, Formal Charge, Polarity 15
 hem 201 Midterm Exam Winter, 2017 Beauchamp Name Problems Points 1. Functional Group Nomenclature (1 large structure) 30 2. Degrees of Unsaturation & Functional Groups (many different functional groups)
hem 201 Midterm Exam Winter, 2017 Beauchamp Name Problems Points 1. Functional Group Nomenclature (1 large structure) 30 2. Degrees of Unsaturation & Functional Groups (many different functional groups)
**YOU ARE NOT ALLOWED TO TAKE SPARE COPIES OF THIS EXAM FROM THE TESTING ROOM**
 EM 234, Spring 2018 Final Exam Ian R. Gould MPLETE TIS SETI : Up to TW PITS will be removed for incorrect/missing information! PRITED FIRST AME PRITED LAST AME Person on your LEFT (or Empty or Aisle) Person
EM 234, Spring 2018 Final Exam Ian R. Gould MPLETE TIS SETI : Up to TW PITS will be removed for incorrect/missing information! PRITED FIRST AME PRITED LAST AME Person on your LEFT (or Empty or Aisle) Person
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
CHEMISTRY 31 Name: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds:
 CEMISTRY 31 ame: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds: (1S,3S)-1-bromo-3-butylcyclopentane 3,4-diethyl-2,2-dimethyloctane 1-cyclopropyl-2-methylcyclobutane
CEMISTRY 31 ame: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds: (1S,3S)-1-bromo-3-butylcyclopentane 3,4-diethyl-2,2-dimethyloctane 1-cyclopropyl-2-methylcyclobutane
I. (42 points) (a) Label the indicated sites (circles and oval box) with the appropriate stereochemical designation.
 I. ( points) ame Page A. etamethasone is a powerful anti-inflammatory drug that comes with unfortunate immunosupressive properties. It is often prescribed as a topical cream that can be used to alleviate
I. ( points) ame Page A. etamethasone is a powerful anti-inflammatory drug that comes with unfortunate immunosupressive properties. It is often prescribed as a topical cream that can be used to alleviate
2.0 h; 240 points please print clearly Dr. Kathleen Nolta Signature. Problem Points Score GSI I 42 II 35 III 36 IV 46 V 42 VI 39 Total 240
 hemistry 10 irst ame inal Examination Last ame.0 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # Problem Points core GI I II 5 III IV V VI 9 Total 0 Precision in drawing counts. heck
hemistry 10 irst ame inal Examination Last ame.0 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # Problem Points core GI I II 5 III IV V VI 9 Total 0 Precision in drawing counts. heck
CHEM 231 (Davis) Organic Chemistry FINAL EXAM May 15, YOUR NAME (Last, First, M.I.) DISCUSSION SECTION #53 (5 Points)
 CHEM 231 (Davis) rganic Chemistry FINAL EXAM May 15, 2006 YUR NAME (Last, First, M.I.) DISCUSSIN SECTIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and
CHEM 231 (Davis) rganic Chemistry FINAL EXAM May 15, 2006 YUR NAME (Last, First, M.I.) DISCUSSIN SECTIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:!
 CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
Chemistry /002 Exam 1 Version Green. The Periodic Table
 Name: Last First MI Chemistry 233-001/002 Exam 1 Version Green Fall 2018 Dr. J. sbourn Instructions: The first 13 questions of this exam should be answered on the provided Scantron. You must use a pencil
Name: Last First MI Chemistry 233-001/002 Exam 1 Version Green Fall 2018 Dr. J. sbourn Instructions: The first 13 questions of this exam should be answered on the provided Scantron. You must use a pencil
1.5 h; 140 points please print clearly Dr. Ginger Shultz Dr. Kathleen Nolta. Problem Points Score GSI I 32 II 27 III 32 IV 25 V 24 Total 140
 hemistry 10 Third Examination First ame Last ame 1.5 h; 10 points please print clearly Dr. Ginger hultz Dr. Kathleen olta ignature UM D # PRT TE FRT LETTER F YUR LAT AME ERE: Problem Points core G 7 V
hemistry 10 Third Examination First ame Last ame 1.5 h; 10 points please print clearly Dr. Ginger hultz Dr. Kathleen olta ignature UM D # PRT TE FRT LETTER F YUR LAT AME ERE: Problem Points core G 7 V
Organic Chemistry II (CHE ) Final Examination April 30, Name (Print legibly):
 rganic hemistry II (E 232-002) Final Examination April 30, 2007 Name (Print legibly): (last) (first) PLEASE observe the following: You are allowed to have scratch paper (provided by me), and a simple model
rganic hemistry II (E 232-002) Final Examination April 30, 2007 Name (Print legibly): (last) (first) PLEASE observe the following: You are allowed to have scratch paper (provided by me), and a simple model
Midterm #1 Chem 3A - Fall 2013 Sept. 7, :00 8:30 pm. Name SID
 Midterm #1 Chem 3A - Fall 2013 Sept. 7, 2013 7:00 8:30 pm ame SID Including the title page, there should be 5 total questions spread over 7 pages (printed on both sides). The back of the last page may
Midterm #1 Chem 3A - Fall 2013 Sept. 7, 2013 7:00 8:30 pm ame SID Including the title page, there should be 5 total questions spread over 7 pages (printed on both sides). The back of the last page may
5. Stereochemical Analysis. 7. Dipole Moments and Inductive versus Resonance Effects. 8. Types of isomers from a given formula. 9. Physical Properties
 hem 201 Sample Midterm Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Lewis Structures, Resonance, Formal harge 3. yclohexane onformations, 2 substituents, ewman
hem 201 Sample Midterm Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Lewis Structures, Resonance, Formal harge 3. yclohexane onformations, 2 substituents, ewman
