NEPHAR 305 Pharmaceutical Chemistry I Assist.Prof.Dr. Banu Keşanlı
|
|
|
- Andrew Griffith
- 5 years ago
- Views:
Transcription
1 Physicochemical Properties NEPHAR 305 Pharmaceutical Chemistry I Assist.Prof.Dr. Banu Keşanlı
2 NEPHAR 305 Pharmaceutical Chemistry I Fall 2014 Syllabus 1. Introduction to pharmaceutical-medicinal chemistry 2. Physiochemical Properties 3. Metabolism of Drugs 4. Central nervous system, general and local anasthetics 5. Sedative, hypnotic drugs 6. Tranquilizer, neuroleptics drugs 7. Antidepressant, antiepileptic drugs 8. Muscle relaxant, analeptic drugs 9. Antiparkinson agents, analgesics 10. Analgesics 11. Antitussive, expectorant, mucolytic drugs 2
3 Text Books for NEPHAR 305 Pharmaceutical Chemistry Principles of Medicinal Chemistry, William O. Foye, 6th Ed Lippincott Williams & Wilkins An Introduction to Medicinal Chemistry, Graham L. Patrick, 4th Ed Oxford University Press Farmasötik Kimya Hülya Akgün, Ayla Balkan, A.Altan Bilgin, Ünsal Çalış, Sevim Dalkara, Dilek Demir Erol Hakkı Erdoğan, Mevlüt Ertan, Nesrin Gökhan, Fügen Özkanlı, Erhan Palaska, Selma Saraç Cihat Şafak, Birsen Tozkoparan 2. Baskı, 2004, Hacettepe Üniversitesi Yayınları, Ankara 3
4 Pharmaceutical chemistry - Medicinal chemistry Medicinal chemistry mainly deals with the identification, synthesis and development of new chemical entities suitable for therapeutic use. Studies in pharmacology, toxicology, microbiology, biochemistry, biophysics, molecular biology etc. are necessary Pharmaceutical chemistry is to do with the discovery and development of new and better drugs through organic synthesis, analytical study and some physical characterization. It involves organic synthesis, complete analytical characterization including spectroscopy, identification of physical and chemical properties, computational analysis, combinatorial approach etc. Synthesis of compounds which could show biological activity as a drug Structural identification Study of structure activity relationship Mechanism of action of a drug molecule 4
5 Drug Discovery, Design and Development Pure organic compounds are the chief source of agents for the cure, reduction or the prevention of disease. These drugs could be classified according to their origin: Natural compounds: materials obtained from both plant and animal, e.g. vitamins, hormones, amino acids, antibiotics, alkaloids, glycosides. etc.). Synthetic compounds: either purely synthetic or synthesis of naturally occurring compounds (e.g. morphine, atropine, steroids and cocaine) to reduce their cost. Semi-synthetic compounds: Some compounds either can not be purely synthesized or can not be isolated from natural sources in low cost. Therefore, the natural intermediate of such drugs could be used for the synthesis of a desired product (e.g. semi synthetic penicillins). 5
6 6
7 The average cost to research and develop each successful drug is estimated to be $800 million to $1 billion. Time it takes is years. 7
8 Drugs 8
9 Important Functional Groups on Drugs 1. Alkanes ( C 2 H 2n+2 ) and Alkenes (C 2H2n ) Cannot form ionic, hydrogen or ion-dipole bonds with itself or water, only van der Waals is possible They are not water soluble The larger or more branched the alkyl chains the less hydrophilic or more lipophilic the group becomes Halogenated hydrocarbons (CH 3 F, CCl 4 etc ) are generally less hydrophilic than the alkyl form due to lack of electron deficient region of the halide that prevents water bonding F Br Example: Halotane F C CH F Cl
10 Alcohols OH Group participates in intramolecular hydrogen bonding due to electronegative oxygen and positive hydrogen result in permanent dipole OH also forms hydrogen bonds with water via dipole-dipole interactions. Alcohol solubility decreases with length of hydrocarbon and position of OH on molecule also influences solubility OH CH 3 CH 2 CHCH 2 CH 3 OH CH 3 CH 2 CH 2 CH 2 CH 3 More soluble Less soluble
11 Phenols Hydroxyl group attached directly to the aromatic ring OH ortho OH CH 3 OH OH OH para meta OH Phenol (carbolic acid) o-cresol catechol resorcinol OH HO NHR HO R = H, Noradrenaline R = CH 3 Adrenaline
12 Ethers CH 3 OCH 2 CH 3 CH 3 CH 2 OCH 2 CH 3 Ethylmethylether Diethylether (Ether U.S.P.) OCH 3 Methylphenylether (Anisole) Solubility (g/100gh 2 O Diethylether.8.4 Diisopropylether 0.002
13 Aromatic Hydrocarbons Not isolated single, double bonds but electron clouds above and below the plane of the ring Plays significant role in binding to biological proteins via van der Wall s bonding Tend to form the back bone of drug molecules and their solubility is influenced by the functional group attached Benzene Naphtalene Anthracene Phenanthrene
14 What are drugs and why do we need new ones? Drug is any substance presented for treating, curing or preventing disease in human beings or in animals. It may also be used for making a medical diagnosis or for restoring, correcting, or modifying physiological functions. Activity - pharmaceutical/pharmacological effect on the subject, e.g. analgesic or β-blocker Potency - the quantitative nature of the effect 14
15 What must a drug do other than bind? bladder kidneys bile duct BBB An oral drug must be able to: dissolve survive a range of phs (1.5 to 8.0) survive intestinal bacteria cross membranes survive liver metabolism avoid active transport to bile avoid excretion by kidneys partition into target organ avoid partition into undesired places (e.g. brain, fetus) liver 15
16 Physico-chemical properties in relation to biological action Drug action results from the interaction of drug molecules with either normal or abnormal physiological processes. Drugs normally interact with targets/receptors (which they are proteins, enzymes, cell lipids, or pieces of DNA or RNA). The ability of a chemical compound to show a pharmacologic /therapeutic effect is related to the influence of its various physical and chemical (physicochemical) properties 16
17 Physical-chemical Properties Physical-chemical properties refer to both physical and chemical properties of the drug molecule that may have an effect on its biological activity partition coefficient degree of ionization surface activity isosterism intermolecular forces oxidation-reduction potentials interatomic distances between functional groups stereochemistry Drug molecules should have the required physicochemical properties to be accessible to active sites to have favorable drug receptor interaction 17
18 Structure of a Cell Membrane amphiphilic lipid molecules form a lipid bilayer hydrophilic hydrophobic 18
19 Solubility and Chemical Bonding Lipophilicity ( fat-liking ) is the most important physical property of a drug in relation to its absorption, distribution, potency, and elimination. If an organic drug molecule dissolves fully or partially in a nonaqueous or lipid solvent, the molecule is said to be lipophilic or to have lipophilic character. The term lipophilic or lipid loving is synonymous with hydrophobic or water hating, and these terms may be used interchangeably. Hydrophilic water loving Lipophobic lipid hating Lipophilic..lipid loving Hydrophobic..water hating In order to predict whether a drug will dissolve in water or in lipid solvent, it must be determined whether the molecule and its functional groups can be bond to water or the lipid solvent molecules. THIS IS THE KEY TO SOLUBILITY.
20 The hydrophobic effect If a compound is too lipophilic, it may be insoluble in aqueous media (e.g. gastrointestinal fluid or blood) bind too strongly to plasma proteins and therefore the free blood concentration will be too low to produce the desired effect distribute into lipid bilayers and be unable to reach the inside of the cell (can go to the other lipophilic sites in the body) Conversely, if the compound is too polar, it may not be absorbed through the gut wall due to lack of membrane solubility. So it is important that the lipophilicity of a potential drug molecule is correct - optimized-. 20
21 Partition Coefficient (P) Hydrophobic character of a drug can be measured experimentally by testing drug s relative distribution in octanol/water mixture Hydrophobic molecules dissolve in n-octanol (CH 3 (CH 2 ) 7 OH) Hydrophilic molecules dissolve in aqeous layer P = Concentration of drug in octanol Concentration of drug in aqeous solution Partition coefficient is the ratio of concentrations of a compound in the two immiscible phases a measure of differential solubility of the compound between these two solvents. Drug (aq) P Drug (lipid) Aqueous phase Nonpolar phase 21
22 Partition Coefficient (P) useful in estimating distribution of drugs within the body hydrophobic drugs with high partition coefficients are preferentially distributed to hydrophobic compartments such as lipid bilayers of cells while hydrophilic drugs (low partition coefficients) preferentially are found in hydrophilic compartments such as blood serum * Hydrophobic compounds will high P value * Hydrophilic compounds will have low P value π is a measure of hydrophobicity of a substituent relative to hydrogen 22
23 Calculation steps of Log P for Drugs (i) The molecule is divided into its various groups, functionalities and substituents (ii) Appropriate hydrophilic/lipophilic fragment constants are assigned and summed (iii) Compounds with log Pcalc values greater than +0.5 are considered water insoluble (lipophilic) and those with log Pcalc values less than +0.5 are considered water soluble (hydrophilic). Calculated log P Values for salicylic acid and p-hydroxybenzoic acid: Salicylic acid p-hydroxybenzoic acid Fragment π Value Fragment π Value Phenyl +2.0 Phenyl +2.0 OH -1.0 OH -1.0 COOH OH COOH p-hydroxybenzoic acid COOH IMHB* COOH Salicylic acid OH Sum *IMHB: inter molecular hydrogen bonding Prediction Water insoluble Prediction Water soluble 23
24 Example Calculate log P value for m-chlorobenzamide. π X = log P X log P H π Cl = = 0.71 π CONH 2 = = log P (chlorobenzamide) = log P (benzene) + π Cl + π CONH2 = =
25 Blood clot preventing activity of salicylic acids O OH 9 OH 8.5 pic R1 O R2 OH 7 O logp O Aspirin 25
26 Hydrophilic Groups -COO - -COOH -OH -N + R 3 -CHO -NH 2 -CONH 2 -CONHR -CONRR -COOR Lipophilic Groups -CH 3 -C 2 H 5 -C 3 H 7 -CF 3 -Cl -Br 26
27 Water Solubility and Hydrogen Bonding A stronger and important form of chemical bonding is the dipoledipole bond, specific example of which is the hydrogen bond. N δ δ... H O H H H δ O δ... H S R Hydrogen Bond Hydrogen bonding of an amine to water and a thiol to water
28 Predicting Water Solubility An excellent example of the importance of intramolecular bonding: Tyrosine: Three functional group present: a phenol an amine and a carboxylic acid group. NH 2 HO CH 2 CH COOH Solubility in H 2 O 25 o C
29 Solubility Prediction Example: Examination of the structure of chloramphenicol (indicates the presence of both lipophilic (nonpolar) and hydrophilic (polar) groups and substituents. Hydrophilic Lipophilic Hydrophilic Lipophilic OH O O 2 N CH CH NH C CHCl 2 CH 2 OH Chloramphenicol Hydrophilic The presence of oxygen and nitrogen containing functional groups usually increases water solubility. While lipid solubility is enhanced by nonionizable hydrocarbon chains and ring systems. 29
30 Acidity and Basicity Acidic and/or basic properties of drugs are important in both: 1- Pharmaceutical phase (dosage formulation, etc.) and 2- Pharmacological phases (disposition, structure at target site, etc.). The three aspects of acid-base chemistry: (1) Definitions (2) Recognition of acidic or basic organic functional groups and (3) An estimation of the relative acid/base strength of these groups. Definitions: Acid: An organic compound containing a functional group that can donate a proton (H+) Base: An organic compound that contains a functional group that can accept a proton (H+) 30
31 Recognition of acidic or basic organic functional groups 1- Common acidic organic functional groups Carboxylic acid (-COOH) Phenol (Ar-OH) Sulfonamide (R-SO 2 NH2) Imide (R-CO-NH-CO-R) β-carbonyl group (-CO-CHR-CO-) O O R C + H 2 O R C + H 3 O + O - O H Carboxylic acid R Phenol O H + H 2 O R O - + H 3 O + H R SO 2 NH 3 O H 2 O R SO 2 NH - Sulfonamide R R O N O Imide H N + - H 2 O + R R O O + H 3 O + R NH H 2 O R NH 2 + H 3 O + Anilinium cation 31
32 Recognition of acidic or basic organic functional groups (cont) 2- Common basic organic functional groups Aliphatic 1º (R-NH 2 ), 2º (R 2 NH) and 3º (R 3 N)-amines Heterocyclic amines Aromatic amines (Ar-NH 2 ) R R R N + H 3 O + R N + H+ H 2 O R R Aliphatic amines Aromatic amines NH 2 + H 3 O + + H 2 O NH H 3 O + N N + Heteroaromatic amines R + H 2 O N Pyridine N H Piperidine N NH Imidazole 32
33 Ionization Ionization = protonation or deprotonation resulting in charged molecules About 85% of marketed drugs contain functional groups that are ionized to some extent at physiological ph (ph 1.5 8). The acidity or basicity of a compound plays a major role in controlling: Absorption and transport to site of action Solubility, bioavailability, absorption and cell penetration, plasma binding, volume of distribution Binding of a compound at its site of action un-ionised form involved in hydrogen bonding ionised form influences strength of salt bridges or H-bonds Elimination of compound Biliary and renal excretion CYP P 450 metabolism 33
34 How does ph vary in the body? Fluid ph Aqueous humour 7.2 Blood 7.4 Colon 5-8 Duodenum (fasting) Duodenum (fed) Saliva 6.4 Small intestine 6.5 Stomach (fasting) Stomach (fed) 3-7 Sweat 5.4 Urine The same compound will be ionised to different extents in different parts of the body. This means that, for example, basic compounds will not be so well absorbed in the stomach than acidic compounds since it is generally the unionised form of the drug which diffuses into the blood stream. 34
35 Handerson Hasselbalch Equation For calculating the percentage of drug existing in ionized or unionized form at a given ph *Weak acid K a HA H + + A CH 3 COOH CH 3 COO + H + ph = pk a + log [A- ] [HA] *Weak base BH + K a H + + B NH 4 + H 2 O NH 3 + H 3 O + ph = pk a + log [B] [BH + ] Weak acids: ph > pka ; [A - ] > [HA], (ionized > unionized) Weak bases: ph > pka ; [HA] > [A - ], (unionized > ionized) ph = pka ; [HA] = [A - ], (unionized = ionized) 35
36 Biological Activity vs. ph Biological Activity Acids Bases pk a <2: strong acid;conjugate base is insignificant in water pk a 4-6: weak acid; weak conjugate base pk a 8-10: very weak acid; stronger conjugate base pk a > 12: essentially no acidic properties in water; strong conjugate base 36
37 ph = pk a + log [A- ] [HA] B = A - A = HA 37
38 Ionisation of an acid 2,4-dinitrophenol OH pk a = 4.1 O 80 NO 2 -H+ NO percent % neutral % anion NO 2 NO Neutral: unionized Anion: ionized ph 38
39 Ionisation of an base 4-aminopyridine NH 2 NH H+ percent % neutral % cation N + H pk a = 9.1 N ph 39
40 Steric Factors Bulk, size and the shape of a drug have an influence on its interaction with an enzyme or a receptor Bulky (large) substituent may shield and hinder the ideal interaction between drug and receptor or alternatively may help to orientate a drug for maximum receptor binding, increasing activity Difficult to quantify steric properties Taft s steric factor (ES) Molar refractivity (MR) Verloop steric parameter 40
41 Structural features of drugs and their pharmacological activity Stereochemistry: Space arrangement of the atoms or three-dimensional structure of the molecule. Stereochemistry plays a major role in the pharmacological properties because: (1) Any change in stereospecificity of the drug will affect its pharmacological activity (2) The isomeric pairs have different physical properties (partition coefficient, pka, etc.) and thus differ in pharmacological activity. The following steric factors influence pharmacological activity: Optical and geometric isomerism Conformational isomerism Isosterism and bioisosterism 41
42 Optical and geometric isomerism and pharmacological activity CH 3 CH 3 H H 3 C H CH 3 OH OH 2-Hydroxybutane enantiomers (mirror images can not superimposed) Enantiomers (optical isomers) can have large differences in potency, receptor fit, biological activity, transport and metabolism. For example, levo-phenol has narcotic, analgesic, and antitussive properties, whereas its mirror image, dextro-phenol, has only antitussive activity. 42
43 Bioisosterism and pharmacological activity Bioisosteres are compounds or groups that have near-equal molecular shapes and volumes, approximately the same distribution of electrons, and show similar chemical and physical properties producing broadly similar biological effects. Parameters affected with bioisosteric replacements Size, conformation, inductive and mesomeric effects, polarizability, H-bond formation capacity, pka, solubility,hydrophobicity, reactivity, stability Bioisosteric replacements: Why? Greater selectivity Less side effects Decreased toxicity Improved pharmacokinetics (solubility-hydrophobicity) Increased stability Simplified synthesis 43
44 Classical Bioisosteres Halogen to CN or CF 3 replacements COCH 2 -R (ketone), -COOR (ester) CONHR (amide) for the carbonyl containing compounds 44
45 . Bioisosterism - Carboxylic acid replacements 45
46 Bioisosterism and pharmacological activity E.g. (Antihistamine; A; B and C) CH 2 CH 3 CH 3 CHO CH 2 CH 2 N CHO CH 2 CH 2 N CHO CH 2 CH 2 N CH 2 CH 3 CH 3 A B C Compound A has twice the activity of C, and many times greater than B 46
47 What is QSAR? QSAR (quantitative structure-activity relationships) includes all statistical methods, approach attempts to identify and quantify the physicochemical properties of a drug and to see whether any of these properties has an effect on the drug's biological activity QSAR can be used: To predict the design of new compounds and To reduce the types of chemical process involved in the biological activity. 47
48 Molecular Properties and Their Parameters 48
PHYSIO-CHEMICAL PROPERTIES OF THE DRUG. It is the interaction of the drug with its environment
 PYSIO-CEMICAL PROPERTIES OF TE DRUG It is the interaction of the drug with its environment Reducing the complexity Biological process in drug action Dissolution of drug in gastrointestinal fluids Absorption
PYSIO-CEMICAL PROPERTIES OF TE DRUG It is the interaction of the drug with its environment Reducing the complexity Biological process in drug action Dissolution of drug in gastrointestinal fluids Absorption
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR
 Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Acid-Base Properties
 TAMMAR H. Ali Lecture 1 Course No. 326 Faculty of Pharmacy University Of Al-Muthanna 1 Physicochemical Principles of Drug Action Physicochemical Principles of Drug Action To design better drugs: Molecular
TAMMAR H. Ali Lecture 1 Course No. 326 Faculty of Pharmacy University Of Al-Muthanna 1 Physicochemical Principles of Drug Action Physicochemical Principles of Drug Action To design better drugs: Molecular
Drug Discovery. Zainab Al Kharusi Office: 33-10
 Drug Discovery Zainab Al Kharusi Office: 33-10 Objectives: To understand the processes of Drug Development To be able to develop a plan for drug discovery Reference: Patrick G L. An Introduction to Medicinal
Drug Discovery Zainab Al Kharusi Office: 33-10 Objectives: To understand the processes of Drug Development To be able to develop a plan for drug discovery Reference: Patrick G L. An Introduction to Medicinal
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
 COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS
 2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity
 ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity Absorption/Acid-Base Case (p. 42) Question #1: Drug Cetirizine Clemastin e Functional groups
ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity Absorption/Acid-Base Case (p. 42) Question #1: Drug Cetirizine Clemastin e Functional groups
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 01
 Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Acids and Bases. Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago.
 Acids and Bases Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Drug dissolution can impact buffering capacity of the body Most enzymes require
Acids and Bases Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Drug dissolution can impact buffering capacity of the body Most enzymes require
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
PHYSICOCHEMICAL PROPERTIES OF DRUG ACTION
 PHYSICCHEMICAL PRPERTIES F DRUG ACTIN III Year B.Pharm I Sem Subject: Medicinal Chemistry-I Presented by: Mr. N. Uday Kumar M.Pharm (Ph.D) PHYSICCHEMICAL PRINCIPLES F DRUG ACTIN INTRDUCTIN Drug molecules
PHYSICCHEMICAL PRPERTIES F DRUG ACTIN III Year B.Pharm I Sem Subject: Medicinal Chemistry-I Presented by: Mr. N. Uday Kumar M.Pharm (Ph.D) PHYSICCHEMICAL PRINCIPLES F DRUG ACTIN INTRDUCTIN Drug molecules
Alcohols. Alcohol any organic compound containing a hydroxyl (R-OH) group. Alcohols are an extremely important organic source
 Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Downloaded from
 1 Class XI Chemistry Ch 13: Hydrocarbons TOP Concepts: 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
1 Class XI Chemistry Ch 13: Hydrocarbons TOP Concepts: 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
Organic and Biochemical Molecules. 1. Compounds composed of carbon and hydrogen are called hydrocarbons.
 Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Downloaded from
 Page 1 of 6 AMINES Amines are derivatives of ammonia (NH3), obtained by replacement of 1, 2 or all the 3 hydrogen atoms by alkyl and/or aryl groups. In nature amines are present in - proteins, vitamins,
Page 1 of 6 AMINES Amines are derivatives of ammonia (NH3), obtained by replacement of 1, 2 or all the 3 hydrogen atoms by alkyl and/or aryl groups. In nature amines are present in - proteins, vitamins,
 1 TOP Concepts: Class XI Chemistry Ch 13: Hydrocarbons 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
1 TOP Concepts: Class XI Chemistry Ch 13: Hydrocarbons 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
Hydrogen Bonding & Molecular Design Peter
 Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Carbon Compounds. Chemical Bonding Part 2
 Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
2. Acids and Bases. Grossman, CHE Definitions.
 Grossman, CE 230 2. Acids and Bases. 2.1 Definitions. Brønsted acids are proton donors, and Brønsted bases are proton acceptors. Examples of Brønsted acids: Cl, Br, 2 S 4,, + 2, + 4, 3, C 3 C 2, C 2 CC
Grossman, CE 230 2. Acids and Bases. 2.1 Definitions. Brønsted acids are proton donors, and Brønsted bases are proton acceptors. Examples of Brønsted acids: Cl, Br, 2 S 4,, + 2, + 4, 3, C 3 C 2, C 2 CC
Organic Chemistry. Introduction to Organic Molecules and Functional Groups
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Lecture 1 Solubility and Distribution Phenomena
 Physical Pharmacy Lecture 1 Solubility and Distribution Phenomena Assistant Lecturer in Pharmaceutics Overview Solubility Phenomena Introduction Solute-Solvent Interactions Solubility of gas in liquid
Physical Pharmacy Lecture 1 Solubility and Distribution Phenomena Assistant Lecturer in Pharmaceutics Overview Solubility Phenomena Introduction Solute-Solvent Interactions Solubility of gas in liquid
Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life Lecture Outline Overview: Carbon The Backbone of Biological Molecules Although cells are 70 95% water, the rest consists mostly of carbon-based compounds.
Chapter 4 Carbon and the Molecular Diversity of Life Lecture Outline Overview: Carbon The Backbone of Biological Molecules Although cells are 70 95% water, the rest consists mostly of carbon-based compounds.
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water
 Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
CHEM 4170 Problem Set #1
 CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
Introduction into Biochemistry. Dr. Mamoun Ahram Lecture 1
 Introduction into Biochemistry Dr. Mamoun Ahram Lecture 1 Course information Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 7 th edition Instructors Dr. Mamoun
Introduction into Biochemistry Dr. Mamoun Ahram Lecture 1 Course information Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 7 th edition Instructors Dr. Mamoun
The Basics of General, Organic, and Biological Chemistry
 The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
Alkanes, Alkenes and Alkynes
 Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
HW #3: 14.26, 14.28, 14.30, 14.32, 14.36, 14.42, 14.46, 14.52, 14.56, Alcohols, Ethers and Thiols
 Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Biology - Summer Work - Chapter 4 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Organic chemistry is a science based on the study of
AP Biology - Summer Work - Chapter 4 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Organic chemistry is a science based on the study of
Properties of Amines
 Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
Chemistry 6/15/2015. Outline. Why study chemistry? Chemistry is the basis for studying much of biology.
 Chemistry Biology 105 Lecture 2 Reading: Chapter 2 (pages 20-29) Outline Why study chemistry??? Elements Atoms Periodic Table Electrons Bonding Bonds Covalent bonds Polarity Ionic bonds Hydrogen bonding
Chemistry Biology 105 Lecture 2 Reading: Chapter 2 (pages 20-29) Outline Why study chemistry??? Elements Atoms Periodic Table Electrons Bonding Bonds Covalent bonds Polarity Ionic bonds Hydrogen bonding
Worksheet Chapter 10: Organic chemistry glossary
 Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
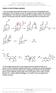 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
1. Which compound would you expect to have the lowest boiling point? A) NH 2 B) NH 2
 MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
Chapter 4. Carbon and the Molecular Diversity of Life
 Lecture Outline Chapter 4 Carbon and the Molecular Diversity of Life Overview: Carbon The Backbone of Life Although cells are 70 95% water, the rest consists of mostly carbon-based compounds. Carbon enters
Lecture Outline Chapter 4 Carbon and the Molecular Diversity of Life Overview: Carbon The Backbone of Life Although cells are 70 95% water, the rest consists of mostly carbon-based compounds. Carbon enters
Introductory Biochemistry
 Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
CHAPTER 2: Structure and Properties of Organic Molecules
 1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
Key ideas: In EAS, pi bond is Nu and undergoes addition.
 Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
AMINES. 3. Secondary When two hydrogen atoms are replaced by two alkyl or aryl groups.
 AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
Glendale Community College, AZ
 Glendale Community College, AZ Mrs. Sandy Gruin n BS in chemistry from Bowling Green State University n MS in Biochemistry from Montana State University n NIH research grant University of Pennsylvania
Glendale Community College, AZ Mrs. Sandy Gruin n BS in chemistry from Bowling Green State University n MS in Biochemistry from Montana State University n NIH research grant University of Pennsylvania
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
The Chemistry and Energy of Life
 2 The Chemistry and Energy of Life Chapter 2 The Chemistry and Energy of Life Key Concepts 2.1 Atomic Structure Is the Basis for Life s Chemistry 2.2 Atoms Interact and Form Molecules 2.3 Carbohydrates
2 The Chemistry and Energy of Life Chapter 2 The Chemistry and Energy of Life Key Concepts 2.1 Atomic Structure Is the Basis for Life s Chemistry 2.2 Atoms Interact and Form Molecules 2.3 Carbohydrates
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Water, water everywhere,; not a drop to drink. Consumption resulting from how environment inhabited Deforestation disrupts water cycle
 Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
AP Chemistry Chapter 22 - Organic and Biological Molecules
 AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
BIOLOGY 101. CHAPTER 4: Carbon and the Molecular Diversity of Life: Carbon: the Backbone of Life
 BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
10/16/17 ACIDS AND BASES, DEFINED WATER IS AMPHOTERIC OUTLINE. 9.1 Properties of Acids and Bases. 9.2 ph. 9.3 Buffers
 ACIDS AND BASES, DEFINED A hydrogen atom contains a proton and an electron, thus a hydrogen ion (H + ) is a proton: Acids: Proton (H + ) transfer between molecules is the basis of acid/base chemistry Ø
ACIDS AND BASES, DEFINED A hydrogen atom contains a proton and an electron, thus a hydrogen ion (H + ) is a proton: Acids: Proton (H + ) transfer between molecules is the basis of acid/base chemistry Ø
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
1) Which of the following represents the breaking of a noncovalent interaction? Topic: The Nature of Noncovalent Interactions
 Multiple Choice Questions 1) Which of the following represents the breaking of a noncovalent interaction? A) hydrolysis of an ester B) dissolving of salt crystals C) ionization of water D) decomposition
Multiple Choice Questions 1) Which of the following represents the breaking of a noncovalent interaction? A) hydrolysis of an ester B) dissolving of salt crystals C) ionization of water D) decomposition
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø
 `1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
PSI Chemistry. 3) How many electron pairs does carbon share in order to complete its valence shell? A) 1 B) 2 C) 3 D) 4 E) 8
 Organic Chemistry HW PSI Chemistry Name I - Organic Introduction 1) Organic chemistry is a science based on the study of A) functional groups. B) vital forces interacting with matter. C) carbon compounds.
Organic Chemistry HW PSI Chemistry Name I - Organic Introduction 1) Organic chemistry is a science based on the study of A) functional groups. B) vital forces interacting with matter. C) carbon compounds.
Lecture 2. The framework to build materials and understand properties
 Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 4: 1. Organic chemistry is the study of carbon compounds 2. Carbon atoms
Chapter 4 Carbon and the Molecular Diversity of Life Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 4: 1. Organic chemistry is the study of carbon compounds 2. Carbon atoms
ADVANCED CHEMISTRY 2
 ADVANCED CHEMISTRY 2 Philip Matthews ±m±l CAMBRIDGE UNIVERSITY PRESS Acknowledgements How to use this book INORGANIC CHEMISTRY 88 Periodicity of physical properties 88.1 Periodicity of ionisation energies
ADVANCED CHEMISTRY 2 Philip Matthews ±m±l CAMBRIDGE UNIVERSITY PRESS Acknowledgements How to use this book INORGANIC CHEMISTRY 88 Periodicity of physical properties 88.1 Periodicity of ionisation energies
and Stereochemistry) PAPER 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) MODULE 4: Applications of Electronic Effects
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Outline. Organic Compounds. Overview: Carbon: The Backbone of Life. I. Organic compounds II. Bonding with Carbon III. Isomers IV.
 Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
12U Biochemistry Unit Test
 1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
4 Carbon and the Molecular Diversity of Life
 4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups
4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups
Slide 1 / 97. Organic Chemistry: Carbon and the Molecular Diversity of Life
 Slide 1 / 97 Organic Chemistry: Carbon and the Molecular Diversity of Life Slide 2 / 97 Organic Chemistry Organic chemistry is the study of carbon compounds Organic compounds range from simple molecules
Slide 1 / 97 Organic Chemistry: Carbon and the Molecular Diversity of Life Slide 2 / 97 Organic Chemistry Organic chemistry is the study of carbon compounds Organic compounds range from simple molecules
BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY-
 BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY- ELEMENTS AND COMPOUNDS - anything that has mass and takes up space. - cannot be broken down to other substances. - substance containing two or more different elements
BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY- ELEMENTS AND COMPOUNDS - anything that has mass and takes up space. - cannot be broken down to other substances. - substance containing two or more different elements
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Chimica Farmaceutica
 Chimica Farmaceutica Drug Targets Why should chemicals, some of which have remarkably simple structures, have such an important effect «in such a complicated and large structure as a human being? The answer
Chimica Farmaceutica Drug Targets Why should chemicals, some of which have remarkably simple structures, have such an important effect «in such a complicated and large structure as a human being? The answer
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
Foundations in Microbiology Seventh Edition
 Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 2 The Chemistry of Biology Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 2 The Chemistry of Biology Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 4 Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life All organisms are composed mostly of chemical structures based on the element carbon. This chapter builds upon information and concepts introduced in
Chapter 4 Carbon and the Molecular Diversity of Life All organisms are composed mostly of chemical structures based on the element carbon. This chapter builds upon information and concepts introduced in
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
ORGANIC CHEMISTRY II for 2 nd year Pharmacy students
 Tishk International University Faculty of Pharmacy ORGANIC CHEMISTRY II for 2 nd year Pharmacy students By Prof. Dr. Faiq H.S. Hussain 2018 2019 Academic Year 1.0 Alkenes and their derivatives 1.1 Nomenclature
Tishk International University Faculty of Pharmacy ORGANIC CHEMISTRY II for 2 nd year Pharmacy students By Prof. Dr. Faiq H.S. Hussain 2018 2019 Academic Year 1.0 Alkenes and their derivatives 1.1 Nomenclature
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Chem 261 Dec 6, 2017
 209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
Module 2 Acids and Bases. Lecture 3 Acids and Bases
 Module 2 Acids and Bases Lecture 3 Acids and Bases 2.1 Concepts A compound is classified as an acid or a base based on certain properties. At present there are several theories which define the concepts
Module 2 Acids and Bases Lecture 3 Acids and Bases 2.1 Concepts A compound is classified as an acid or a base based on certain properties. At present there are several theories which define the concepts
Chapter 15 Amines Amines 15.2 Naming Amines 15.3 Physical Properties of Amines
 Chapter 15 Amines 15.1 Amines 15.2 Naming Amines 15.3 Physical Properties of Amines 1 Amines Amines are organic compounds in which one or more H in ammonia, NH 3, is replaced with alkyl or aromatic groups.
Chapter 15 Amines 15.1 Amines 15.2 Naming Amines 15.3 Physical Properties of Amines 1 Amines Amines are organic compounds in which one or more H in ammonia, NH 3, is replaced with alkyl or aromatic groups.
Introduction to Life Science. BSC 1005 Fall 2011 Homework 1! Connect Due Date: 9/18/ :59PM. Multiple Choice Portion
 Introduction to Life Science BSC 1005 Fall 2011 Homework 1 Connect Due Date: 9/18/2011 11:59PM Instructions Complete this homework assignment as the material is covered in class. You may refer to any of
Introduction to Life Science BSC 1005 Fall 2011 Homework 1 Connect Due Date: 9/18/2011 11:59PM Instructions Complete this homework assignment as the material is covered in class. You may refer to any of
Chapter 2 The Chemistry of Biology. Dr. Ramos BIO 370
 Chapter 2 The Chemistry of Biology Dr. Ramos BIO 370 2 Atoms, Bonds, and Molecules Matter - all materials that occupy space and have mass Matter is composed of atoms. Atom simplest form of matter not divisible
Chapter 2 The Chemistry of Biology Dr. Ramos BIO 370 2 Atoms, Bonds, and Molecules Matter - all materials that occupy space and have mass Matter is composed of atoms. Atom simplest form of matter not divisible
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
