Hygrothermal effects of epoxy resin. Part I: the nature of water in epoxy
|
|
|
- Bryan Paul
- 5 years ago
- Views:
Transcription
1 Polymer 40 (1999) Hygrothermal effects of epoxy resin. Part I: the nature of water in epoxy Jiming Zhou*, James P. Lucas Department of Materials Science and Mechanics, Michigan State University, East Lansing, MI 48824, USA Received 8 July 1998; received in revised form 30 October 1998; accepted 3 November 1998 Abstract This study assesses the nature of sorbed water and the related hygrothermal effects in epoxy resins. This paper is the first of a two-paper series. Three epoxy systems, DGEBA mpda, TGDDM DDS, and Fiberite 934, were used in the investigation. Water sorption was achieved by immersing the materials in distilled water at constant temperature of 45 C, 60 C, 75 C and 90 C for 1530 h. Water absorption and desorption profiles were analyzed to determine the diffusion parameters. Solid state nuclear magnetic resonance (NMR) was conducted to determine the mobility of water in epoxy. The study shows that water molecules bind with epoxy resins through hydrogen bonding. Two types of bound water were found in epoxy resins. The binding types are classified as Type I or Type II bonding, depending on difference in the bond complex and activation energy. The activation energy of Type I and Type II bound water is 10 and 15 kcal/mol, respectively. Type I bonding corresponds to a water molecule which forms a single hydrogen bond with the epoxy resin network. This water molecule possesses a lower activation energy and is easier to remove from the resin. Type II bonding is as a result of a water molecule forming multiple hydrogen bonds with the resin network. This water molecule, therefore, possesses a higher activation energy and is correspondingly harder to remove. Type I bound water is the dominant form of the total amount sorbed water. The amount of Type II bound water depends strongly on the exposure temperature and time. Higher immersion temperature and longer exposure time result in a greater amount of Type II bound water Elsevier Science Ltd. All rights reserved. Keywords: Epoxy; Water absorption and desorption; Hydrogen bonding 1. Introduction Knowledge of the bonding character of water molecules in epoxy resins is the most important and fundamental to understand hygrothermal effects. Even though considerable research has focused on hygrothermal effects [1 7], water diffusion mode and related mechanisms in epoxy resin are still not fully understood. This is because in part to the reality that the hygrothermal effects in epoxy are quite complex. Two mechanistic approaches have emerged that generally characterize the nature of water in epoxy. One is the free volume approach which presumes that water diffuses into epoxy resin and resides in the free volume of the material. For this approach bonding between water molecule and epoxy resin network is deemed insignificant [8,9]. The other approach is the interaction concept that suggests water molecules couple strongly with certain hydrophilic functional groups such as hydroxyl or amine in epoxy resin [7,10 12]. Based on dielectric experiments, Woo and Piggott [8] * Corresponding author. Current address: Delphi Delco Electronics Systems, Advanced Processes, Packaging, and Materials, PO Box 9005, MS D-16, Kokomo, IN , USA. suggested that the water molecules were not bound to polar groups or to hydrogen bonding sites in epoxy resin. Clustering of water molecules in the polymer was reported rather than complete molecular separation. Adamson [7] investigated thermal expansion and swelling by using epoxy resin and graphite/epoxy (Gr/Ep) composites. He determined that some water molecules interacted by forming hydrogen bonds with hydrophilic groups in epoxy resin while other water molecules are retained in free volume of the material. Apicella et al. [12] studied water absorption in glassy epoxy resin and proposed that there are three absorption modes: (i) bulk dissolution of water in the polymer network, (ii) moisture absorption onto the surface of vacuoles which define the excess free volume of the glassy structure, and (iii) hydrogen bonding between polymer hydrophilic groups and water. Jelinski and collaborators [13] investigated the nature of the epoxy water-molecule interaction using quadrupole echo deuterium nuclear magnetic resonance (NMR) spectroscopy. They concluded that: (i) water in epoxy resin was impeded in its movement, (ii) no free water existed, (iii) there was no evidence of tightly bound water, and (iv) sorbed water molecules disruption of the hydrogen-bonded network in the epoxy resin was unlikely. The water molecules migrated from site to site, but /99/$ - see front matter 1999 Elsevier Science Ltd. All rights reserved. PII: S (98)
2 5506 J. Zhou, J.P. Lucas / Polymer 40 (1999) Fig. 1. Chemical structures of DGEBA, mpda, TGDDM and DDS. apparently such a jumping motion did not involve a particular hydrogen exchange mechanism. Although there is considerable agreement among some models, substantial disagreement still persists. No single theory or model exists with sufficient experimental support to explain all hygrothermal phenomena. In this study, water absorption and desorption profiles were analyzed to determine pertinent diffusion parameters. Solid state nuclear magnetic resonance (NMR) was conducted to assess the bonding characteristics of water in epoxy. The aim of this study is to discern the interaction nature of sorbed water and the related hygrothermal effects in epoxy resins. This paper is the first part of a two-paper series. 2. Materials and experimental procedure Three epoxy systems that have been used extensively for high performance polymer matrix composites (PMCs) were used in this study. One epoxy system consists of diglycidyl ether of bisphenol-a (DGEBA, Shell Epon828) and metaphenylene diamine (mdpa) hardener. The other is tetraglycidyl-4,4 0 -diaminodiphenyl methane resin (TGDDM, Ciba Geigy MY720) with 4,4 0 -diaminodiphenyl sulfone (DDS, DuPont) hardener. The third is Fiberite 934 epoxy resin that consists mainly of TGDDM resin, DDS hardener, with small amounts of additives of which the types and concentrations are proprietary. The chemical structures of these resin systems are shown in Fig. 1. Materials for test coupons were prepared using the following procedures. DGEBA resin and 14.5 phr (part per hundred resin by weight) mpda hardener were heated separately to 75 C in an oven until the mpda was melted. Then the epoxy resin and hardener were mixed thoroughly and degassed for 10 min. The degassed mixture was poured into a mold for curing. To cure the resin the following heating sequence was used: 75 C for 2 h followed by 125 C for 2 h, postcured at 180 C for 8 h, and then cooled down to 60 C. Specimens were taken out of the oven after temperature ramped down to 60 C. The temperature rate in all ramping stage was 1.5 C/min. TGDDM resin and 44 phr DDS hardener were heated to 135 C respectively, and then combined and stirred until DDS was melted and a clear brown liquid was obtained. The material was degassed for 15 min. The curing procedure was: 135 C (mixing and degassing) followed by 80 C for 1 h 100 C for 2 h 150 C for 4 h 200 C for 7 h (postcuring) and then cooled down to 60 C. The frozen Fiberite 934 resin was put in a mold and heated to 135 C. The material was degassed for 10 min at 135 C. The curing procedure was 135 C for 2 h followed by 177 C for 4 h, postcured at 190 C for 6 h, and then followed by a 60 C cool down. The temperature ramping rate was the same as that used for the DGEBA mpda systems. After the materials were prepared, infrared (IR) spectroscopy testing was performed to determine the extent of curing. During the curing process, the intensity of the 910 cm 1 wavenumber peak that corresponds to the epoxide group diminishes gradually in the IR spectrum. Infrared results showed that the 910 cm 1 peak of all three epoxy resins vanished therefore confirming that the epoxy systems were fully cured. Incomplete curing in epoxy resin is not desirable for the present study as secondary curing during hygrothermal exposure may render experimental observations ambiguous and promote misinterpretation of results. 3. Results and discussions 3.1. Water absorption The fully cured epoxy resin panels for use in this experiment were cut into samples with dimensions of mm using a fine-grit, diamond-blade saw. The thickness of the water absorption test samples was made small, deliberately, compared to its width and length. The ratio of the edge surface area to the total surface area is only 5%, and as such, edge effects could be ignored and simple onedimensional diffusion model analysis can be applied without incurring significant error. After conditioning samples at 110 C for 24 h to ensure
3 J. Zhou, J.P. Lucas / Polymer 40 (1999) paper and allowed to stand free at ambient lab environment for 2 min. The aforementioned absorption procedure yielded a series of water gain versus time curves. The diffusivity, D, was determined from the initial slope of the percentage water gain M t versus t 1/2 curve [1], D ˆ p 16! h 2 2 Mt2 M t1 p p 2 M m t 2 t 1 where h is the thickness of the specimen, t is exposure time and M m is the maximum water gain (saturation). The activation energy Q and diffusion constant D 0 were obtained by plotting ln D versus 1/T according to the Arrhenius rate equation given, D ˆ D 0 exp Q 3 RT Fig. 2. Water absorption profiles of the three epoxy systems at different temperatures for 1530 h. The symbols are experimental data. complete dryness and the amelioration of residual stresses induced during sample processing and preparation, the specimens were placed into distilled water chambers maintained at constant temperatures of 45 C, 60 C, 75 C, and 90 C. The specimens were weighed periodically using a digital balance with 0.01 mg resolution to determine the percent weight change, and, thus, water uptake. The water gain percentage, M%, was determined from the equation: M% ˆ W W d W d 100% 1 Here, W is the weight of the water-sorbed epoxy specimen and W d is the initial weight of the dry specimen. To ensure the removal of excessive surface (superficial) water, specimens were gently wiped dry using clean, lint-free tissue The water sorption profiles of the three hygrothermalexposed epoxy systems are shown in Fig. 2. At the initial stage water gain is linearly proportional to t 1/2. After exposure for 1530 h, specimens reached full saturation for all hygrothermal conditions. For a given material, the saturation water content (M m ) is virtually the same irrespective of the immersion temperatures. This behavior indicates that water diffusion in the three materials is Fickian in nature. Not unexpectedly, Fiberite 934 and TGDDM DDS systems show similar water gains of 6.95 and 6.80 wt% because of their similar resin chemistry. The DGEBA mpda system exhibited a much lower saturation level (3.35 wt%) compared with to Fiberite 934 and TGDDM DDS systems. The DGEBA mpda system did not show two-stage water absorption behavior as reported by other researchers [9]. This difference may be owing to difference in material preparation since they did not report the degree of cure of the material. The diffusivity D is obtained according to Eq. (2) and the activation energy Q is obtained by plotting ln D(T) versus 1/ T following Eq. (3). All water absorption related parameters of the three epoxy systems are shown in Table Water desorption After exposure in distilled water at temperatures, 45 C, 60 C, 75 C, and 90 C for 1530 h, all specimens reached full saturation, M m. Subsequently, water-saturated specimens were placed into a dry chamber in order to desorb water from the specimens. During desorption testing, specimens Table 1 The diffusion-related parameters of the three epoxy systems: diffusivity D at different exposure temperatures, activation energy Q and maximum water content M m D 45 (mm 2 s 1 ) D 60 (mm 2 s 1 ) D 75 (mm 2 s 1 ) D 90 (mm 2 s 1 ) Q (kcal/mol) M m (%) TGDDM DDS DGEBA mpda Fiberite
4 5508 J. Zhou, J.P. Lucas / Polymer 40 (1999) Fig. 3. Water desorption profiles of the three epoxy systems at 60 C for 1450 h and then 140 C 240 h. The symbols are experimental data and represent the samples with different bath temperatures in water absorption process. are weighed periodically to determine the percent weight change. Each datum of the desorption profile was the average value of three samples. Water desorption profiles of the three epoxy systems are shown in Fig. 3 resulting from thermal soaking at 60 C for 1450 h, and then at 140 C for 240 h. The initial desorption rate of absorbed water was rapid. With time, however, the desorption slowed. Even after long-time desorption at 60 C for 1450 h, it was observed that some residual water remained in the specimens. The retained water could not be expediently removed at 60 C regardless of how long the materials were thermally soaked. Much higher temperatures (i.e., 140 C) had to be used for removing this residual water from the resins. The amounts of retained water of the three epoxy systems exposed at different temperatures are shown in Table 2. The data in Table 2 are taken directly from Fig. 3. The trend suggests that more residual water retention occurs at higher exposure temperature. To investigate the relationship between the amount of the residual water and exposure time, Fiberite 934 resin, immersed in water at 90 C, was taken out at different exposure time and then desorbed at 60 C to determine the amount of residual water. Fig. 4 shows an increase in the amount of retained water with exposure time at the fixed absorption exposure temperature (90 C). The existence of residual water in polymer materials is not an unfamiliar phenomenon as Moy [10], Marsh [14], and Xiang [15] have reported that some of the absorbed water could not be removed unless a higher desorption temperature was used. Moy [10] investigated epoxy water interaction by DSC, IR, NMR spectroscopy, and water absorption/ desorption experiments using TGDDM DDS resin system. He reported a strong hysteresis related to the desorption process indicating residual amounts of water that could only be removed by heating the polymer to temperatures above 100 C. Marsh and Springer [14] reported moisture solubility and diffusion results for epoxy-glass composite. They too showed that the sorbed water could not be fully removed during desorption at temperatures Table 2 The amount of retained water in the three water-saturated epoxies after desorption at 60 C for 1450 h. The trend is that higher immersion temperature induces greater levels of retained water Residual water of sample bathed at 45 C then desorp. at 60 C 1450 h (w%) Residual water of sample bathed at 60 C then desorp. at 60 C 1450 h (w%) Residual water of sample bathed at 75 C then desorp. at 60 C 1450 h (w%) Residual water of sample bathed at 90 C then desorp. at 60 C 1450 h (w%) TGDDM DDS DGEBA mpda Fiberite
5 J. Zhou, J.P. Lucas / Polymer 40 (1999) Fig. 4. The amount of residual water of the epoxy Fiberite 934 changes with exposure time at 90 C. used typically for water absorption. Attempts to remove the residual water in the material at temperatures of 85 C, 100 C and 110 C were unsuccessful. Temperatures in excess of the glass transition temperature (i.e., 120 C) were required to remove the residual water. Previous studies [10,14,15] have suggested the existence of the residual water, but none pursued this matter further with regard to the mechanisms of such occurrence. The present study not only reveals the existence of the residual water but also shows the amount of residual water varies with hygrothermal exposure history. The amount of residual water depends strongly on the exposure time and temperature. As shown in Table 2 and Fig. 4, both higher immersion temperatures and longer immersion times enhance the amount of residual water in the resin Desorption activation energy It is evident that at least two bound water epoxy resin complex exist in the epoxy resins as a result of long-time hygrothermal exposure. Retained water molecules which are easily removed by thermal desorption at lower temperatures are consistent with a so-called Type I bound water complex. Moreover, retained water molecules which are considerablely more difficult to remove by thermal desorption are referred to as a Type II bound water complex. Higher temperatures are required to remove Type II bound water from the epoxy resin network. To determine the activation energies of Type I and Type II sorbed water more detailed desorption experiments were conducted. Saturated TGDDM DDS samples were thermally desorbed to determine diffusivity and activation energy of Type I and Type II bound water. Fig. 5 schematically illustrates the circumstance for the desorption of Type I and Type II bound water. Both fields indicative of the removal of Type I and Type II bound water are illustrated in Fig. 5. The activation energy, Q, for desorption Type I bond water was determined using fully-saturated, M m, specimens as the initial condition of the desorption diffusion process. The activation energy for desorption Type II bound water was determined using specimens that still contained residual water after lowtemperature (75 C) bake out in dry air. The activation energies for both Type I and Type II bound water are shown in Fig. 6. Also, the activation energy for absorption of water in the resin is shown. The results in Fig. 6 show that activation energy necessary to desorb Type II bound water is significantly higher ( 15 kcal/mol). In contrast, the activation energy necessary for desorption of Type I bound water is less ( 10 kcal/mol). It is of interest to note, and perhaps fortuitous, that the difference between Q for Type II and Q for Type I water is approximately 5 kcal/mol which is on order of the energy for hydrogen bonding. Such differences in the activation energies for desorption of Type I and Type II bound water suggest that the binding strengths bound complexes between water molecules and the resin vary NMR test NMR testing provides a sensitive probe to assess the interaction between sorbed water and epoxy resin since the NMR peak width directly reflects the mobility of molecular species. Broad spectral NMR peaks are indicative of tight bonding between interacting species. Although many NMR reports document various aspects of sorbed water in epoxy resins, scant detailed NMR reports were found by the authors on water in epoxy at different hygrothermal stages. In this study NMR was carried out to determine water resin bonding characteristics. A Varian VXR 400 solid state NMR spectroscopy was used for 1 H NMR testing. The Fig. 5. Schematic of water desorption process.
6 5510 J. Zhou, J.P. Lucas / Polymer 40 (1999) Fig. 6. Activation energy of TGDDM DDS system. Q type I was obtained by desorption of saturated specimens at 45 C, 60 C, 75 C and 90 C. Q type II was obtained by desorption of semi-dried specimens at 115 C, 130 C, 145 C and 160 C. For comparison absorption activation energy is also shown. Fig H NMR spectra for TGDDM DDS system. (a) dry epoxy, (b) water-saturated epoxy and (c) free water mixed with dry epoxy. The results indicate that the mobility of sorbed water in epoxy is between free-water and solid states.
7 J. Zhou, J.P. Lucas / Polymer 40 (1999) Fig H NMR spectra of Fiberite 934 show free water changes into impeded sorbed water with time. (a) dry resin mixed with 1% liquid water. Liquid water and sorbed water can be seen clearly. (b) 10 min later, liquid water disappeared and all sorbed water molecules were impeded. resin examined in the NMR tests was ground into small particles where the particle diameter on average was (1 mm. The spin frequency used was 4000 and 6000 Hz. Three 1 H NMR spectra of the TGDDM DDS epoxy system are shown in Fig. 7: (a) dry epoxy sample, (b) watersaturated epoxy sample, and (c) dry epoxy sample with adsorbed water on its surface. The peak width of sorbed water (7b) is broader than that of liquid water (7c) and much narrower than that of tightly bonded hydrogen and hydroxyl group in epoxy resin (7a). The mobility of bound water in epoxy is between free-water (liquid) and solid states. Fig. 8 represents the 1 H NMR spectra of Fiberite 934 (TGDDM DDS) and it indicates how quickly the free water is absorbed and changed into impeded water. Fig. 8a is a spectrum of dry resin which was acquired right after mixing with 1 wt% water. Free water and a small amount of bound water are exhibited. However, after just 10 min, free water molecules abates revealing that sorbed water molecules have become impeded (8b). This result also reveals the rate in which bound water complexes form in epoxy resins. that breaking hydrogen bonding of water requires energy between 5 and 20 kcal/mol [18]. The mobility of sorbed water ranges between that of nearly-free water and the tightly-bonded hydrogen molecules on the backbone. Moreover, the activation energy of the two types of bound water is in the energy range of hydrogen bonding. Therefore, water molecules bond with epoxy resin network through hydrogen bonding. A model of bonding between water and epoxy network is 3.5. Postulation of the two types of bound water The results from this study indicate that water in epoxy resin can be classified into two types of bound water according to their activation energies for desorption and NMR spectral evidence. Bonding for these bound water molecules is not nearly as strong as main-chain bonding ( kcal/ mol) of the polymer network structure [16], but for Type II bound water, it is significantly higher than physical bonding (0.5 2 kcal/mol) denoted by Van der Waals and dipole dipole bonding. Antoon et. al. proposed that the sorbed water was held by hydrogen bonding [17]. Atkins reported Fig. 9. Possible bound water complexes in epoxy network: (a) water molecules form one hydrogen bond with resin network and have lower activation energy, (b) water molecules form more than one hydrogen bonds with resin network and have higher activation energy.
8 5512 J. Zhou, J.P. Lucas / Polymer 40 (1999) proposed in Fig. 9. Fig. 9a presents single hydrogen bonding between water and epoxy network. This corresponds to the Type I bound water which has lower activation energy and is more easily to remove. Fig. 9b shows multi-site interconnective hydrogen bonding model. The bonding complexes of the multi-site/interconnective model corresponds to the Type II bound water which has a higher activation energy and is harder to remove by desorption. Type I bound water diffuses into the epoxy network and breaks the initial interchain Van der Waals force resulting in the increase of chain segment mobility and swelling. As the amount of the Type I bound water is dominant in the water sorption process, it readily plasticizes the epoxy resins. Water molecules which form multi-site interconnective bond complexes (Type II) do not contribute significantly to plasticization of the resin. Moreover, these bond complexes create bridging between chain segments resulting in (pseudo crosslinking) secondary crosslinking. The formation of the Type I bonded water occurs readily in the moisture sorption process, particular at low temperature hygrothermal exposure, however, the impetus to form Type II bonding is substantially less. Longer time and higher temperatures provide the stimulus for the formation of multi-site interconnective bonding. Consequently, the amount of Type II bound water increases with exposure time and temperature. The phenomenon of water retention is not limited to epoxy resins. Residual water or so-called locked-in water has been observed in other polymers [19]. 4. Conclusion The degree of sorbed water interaction in epoxy resins was assessed from the results obtained by water desorption measurement, residual water contents, desorption activation energy analysis, and 1 H NMR studies on the epoxy resins at different hygrothermal stages. Depending on the difference in bond structures and activation energy, the bond states have been classified as Type I or Type II bonding. Type I bonding involves water molecules forming a single hydrogen bond and or dispersion bonding with the epoxy resin network. Type I bound water possesses a thermal desorption activation energy of 9.5 kcal/mol and, thus, is more easily desorbed from the resin. Type I bound water manifested by disrupting the interchain Van der Waals force resulting in the increase of segment mobility. Type I bound water acts as a plasticizer. Type II bonding results from water molecules forming multiple hydrogen bonds with the resin network. The activation energy in Type II bonding is 15.1 kcal/mol. These water molecules possess a higher activation energy and are correspondingly harder to remove from the resin by desorption. Type II bound water molecules do not act as a plasticizer but rather form bridges between structural segments resulting in secondary crosslinking. The amount of Type II bound water strongly depends on the hygrothermal temperature and time history. Higher exposure temperature and longer exposure time result in the formation of a greater amount of Type II bound water. Hygrothermal effects on physical properties such as the T g in epoxy resins will be discussed in Part II of this series. References [1] Loos AC, Springer GS. In: Springer GS, editor. Environmental effects on composite materials, Westport: Technomic Publishing Co, 1981, pp. 34. [2] DeNeve D, Shanahan MER. Polymer 1993;34:5099. [3] Imaz JJ, Rodriguez JL, Rubio A, Mondragon I. J Mater Sci Lett 1991;10:662. [4] Biro DA, Pleizier G, Deslandes Y. Composites Sci and Tech 1993;46:293. [5] Lee MC, Peppas NA. J Applied Polymer Science 1993;47:1349. [6] Zhou J, Lucas JP. Composites Sci and Tech 1995;53:57. [7] Adamson MJ. J Mater Sci 1980;15:1736. [8] Woo M, Piggott M. J Composite Technology Research 1987;9:101. [9] Gupta VB, Drzal LT. J Applied Polymer Science 1985;30:4467. [10] Moy P, Karasz FE. Polymer Engineering and Science 1980;20: 315. [11] Bellenger V, Verdu J. J Mater Sci 1989;24:63. [12] Apicella A, Nicolais L, de Cataldis C. J of Membrane Sci 1985;18:211. [13] Jelinski LW, Dumais JJ, Cholli AL, Ellis TS, Karasz FE. Macromolecules 1985;18:1091. [14] Marsh LL, Lasky R, Seraphim DP, Springer GS. In: Springer GS, editor. Environmental effects on composite materials, 3. Westport: Technomic Publishing Co, pp. 51. [15] Xiang ZD, Jones FR. Proc of the International Conference on Composite Materials ICCM-9, Vol.5, p. 601, Woodhead Publishing Ltd. Zaragoza, Spain, [16] Atkins T, Batich C. MRS Bulletin 1993;28 29:40. [17] Antoon MK, Koenig JL. J of Polym Sci: Polym Phys 1981;19:1567. [18] Atkins PW. Quanta. New York: Oxford University Press, [19] Morgan RJ, Shin EE, Lincoln JE, Choi J, Lee A. Proc of the 28th International SAMPE Technical Conference, 1996:213.
Mechanisms of Moisture absorption by cyanate ester modified epoxy resin matrices: The clustering of water molecules.
 Mechanisms of Moisture absorption by cyanate ester modified epoxy resin matrices: The clustering of water molecules. Sunil K. Karad a,*, Frank R. Jones b a Maharashtra Institute of Technology, Paud Road,
Mechanisms of Moisture absorption by cyanate ester modified epoxy resin matrices: The clustering of water molecules. Sunil K. Karad a,*, Frank R. Jones b a Maharashtra Institute of Technology, Paud Road,
New Developments in Structure/Property Relationships: Relating Fluid Ingress to mechanical and thermal properties
 THE 19 TH INTERNATINAL CNFERENCE N CMPSITE MATERIALS New Developments in Structure/Property Relationships: Relating Fluid Ingress to mechanical and thermal properties W. Tian*, B. Dao and R. Varley CSIR
THE 19 TH INTERNATINAL CNFERENCE N CMPSITE MATERIALS New Developments in Structure/Property Relationships: Relating Fluid Ingress to mechanical and thermal properties W. Tian*, B. Dao and R. Varley CSIR
QUANTITATIVE EVALUATION OF CURING SHRINKAGE IN POLYMERIC MATRIX COMPOSITES
 6 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS QUANTITATIVE EVALUATION OF CURING SHRINKAGE Masahiro KOTANI*, Yoshihiko ARAO*, Jun KOYANAGI**, Hiroyuki KAWADA***, Hiroshi HATTA**, Yuichi ISHIDA****:
6 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS QUANTITATIVE EVALUATION OF CURING SHRINKAGE Masahiro KOTANI*, Yoshihiko ARAO*, Jun KOYANAGI**, Hiroyuki KAWADA***, Hiroshi HATTA**, Yuichi ISHIDA****:
Composites in the Sea: Sorption, Strength and Fatigue
 Composites in the Sea: Sorption, Strength and Fatigue Y. J. Weitsman Mechanical and Aerospace Engineering and Engineering Science Department The University of Tennessee Knoxville, Tennessee 37996-2030,
Composites in the Sea: Sorption, Strength and Fatigue Y. J. Weitsman Mechanical and Aerospace Engineering and Engineering Science Department The University of Tennessee Knoxville, Tennessee 37996-2030,
EVALUATION OF THE COEFFICIENTS OF MOISTURE EXPANSION USING TRANSIENT SIMULATED LAMINATES METHODOLOGY (TSL)
 EVALUATION OF THE COEFFICIENTS OF MOISTURE EXPANSION USING TRANSIENT SIMULATED LAMINATES METHODOLOGY (TSL) Takeshi Takatoya, Kimo Chung, Yi-Jui Wu, and James C. Seferis Polymeric Composites Laboratory,
EVALUATION OF THE COEFFICIENTS OF MOISTURE EXPANSION USING TRANSIENT SIMULATED LAMINATES METHODOLOGY (TSL) Takeshi Takatoya, Kimo Chung, Yi-Jui Wu, and James C. Seferis Polymeric Composites Laboratory,
Effect of water absorption in polymers at low and high temperatures
 JPOL 3406 Polymer 40 (1999) 3433 3441 Effect of water absorption in polymers at low and high temperatures G. Baschek a, G. Hartwig a, *, F. Zahradnik b a Forschungszentrum Karlsruhe, Institut für Materialforschung
JPOL 3406 Polymer 40 (1999) 3433 3441 Effect of water absorption in polymers at low and high temperatures G. Baschek a, G. Hartwig a, *, F. Zahradnik b a Forschungszentrum Karlsruhe, Institut für Materialforschung
Preparation of poly(vinyl alcohol) hydrogels with radiation grafted citric and succinic acid groups
 Radiation Physics and Chemistry 55 (1999) 667±671 www.elsevier.com/locate/radphyschem Preparation of poly(vinyl alcohol) hydrogels with radiation grafted citric and succinic acid groups Hatice BodugoÈ
Radiation Physics and Chemistry 55 (1999) 667±671 www.elsevier.com/locate/radphyschem Preparation of poly(vinyl alcohol) hydrogels with radiation grafted citric and succinic acid groups Hatice BodugoÈ
Cationic Cure of Epoxy Resin by an Optimum Concentration of N-benzylpyrazinium Hexafluoroantimonate
 Macromolecular Research, Vol. 10, No. 1, pp 34-39 (2002) Cationic Cure of Epoxy Resin by an Optimum Concentration of N-benzylpyrazinium Hexafluoroantimonate Jong Keun Lee* and Yusong Choi Department of
Macromolecular Research, Vol. 10, No. 1, pp 34-39 (2002) Cationic Cure of Epoxy Resin by an Optimum Concentration of N-benzylpyrazinium Hexafluoroantimonate Jong Keun Lee* and Yusong Choi Department of
Hygrothermal aging of a filled epoxy resin
 Technical collection Hygrothermal aging of a filled epoxy resin 2007 - Conferences publications E. Brun P. Rain G. Teissédre C. Guillermin S. Rowe 2007 InternationalConferenceon Solid Dielectrics, Winchester,
Technical collection Hygrothermal aging of a filled epoxy resin 2007 - Conferences publications E. Brun P. Rain G. Teissédre C. Guillermin S. Rowe 2007 InternationalConferenceon Solid Dielectrics, Winchester,
Effects of methanol on crystallization of water in the deeply super cooled region
 Effects of methanol on crystallization of water in the deeply super cooled region Ryutaro Souda Nanoscale Materials Center National Institute for Materials Science Japan PHYSICAL REVIEW B 75, 184116, 2007
Effects of methanol on crystallization of water in the deeply super cooled region Ryutaro Souda Nanoscale Materials Center National Institute for Materials Science Japan PHYSICAL REVIEW B 75, 184116, 2007
Measurement techniques
 Measurement techniques 1 GPC GPC = gel permeation chromatography GPC a type of size exclusion chromatography (SEC), that separates analytes on the basis of size. The column used for GPC is filled with
Measurement techniques 1 GPC GPC = gel permeation chromatography GPC a type of size exclusion chromatography (SEC), that separates analytes on the basis of size. The column used for GPC is filled with
POLYAMIDE-6,9 WITH CARBAZOLE
 Chapter 5 POLYAMIDE-6,9 WITH CARBAZOLE CONTENTS 5.1 Introduction 174 5.2 Thermogravimetric Analysis 175 5.3 Differential Scanning Calorimetry 176 5.3.1 Pan Melt Blending 176 5.3.1.1 Melting Temperatures
Chapter 5 POLYAMIDE-6,9 WITH CARBAZOLE CONTENTS 5.1 Introduction 174 5.2 Thermogravimetric Analysis 175 5.3 Differential Scanning Calorimetry 176 5.3.1 Pan Melt Blending 176 5.3.1.1 Melting Temperatures
Measurement and Characterization of the Moisture-Induced Properties of ACF Package
 Measurement and Characterization of the Moisture-Induced Properties of ACF Package Ji-Young Yoon e-mail: koths82@kaist.ac.kr Ilho Kim Soon-Bok Lee Department of Mechanical Engineering, Korea Advanced Institute
Measurement and Characterization of the Moisture-Induced Properties of ACF Package Ji-Young Yoon e-mail: koths82@kaist.ac.kr Ilho Kim Soon-Bok Lee Department of Mechanical Engineering, Korea Advanced Institute
CHAPTER 10. Characteristics of the Surfaces of Biomaterials
 CHAPTER 10 Characteristics of the Surfaces of Biomaterials 10.1 Surface Characteristics Related to Chemical Bonding 10.2 Surface Chemistry Related to Bonding of Biological Molecules 10.3 Porosity 10.4
CHAPTER 10 Characteristics of the Surfaces of Biomaterials 10.1 Surface Characteristics Related to Chemical Bonding 10.2 Surface Chemistry Related to Bonding of Biological Molecules 10.3 Porosity 10.4
Effect of Resin Molecular Architecture on Epoxy Thermoset Mechanical Properties
 Effect of Resin Molecular Architecture on Epoxy Thermoset Mechanical Properties The effect of resin molecular architecture on the small strain elastic constants of diamine-cured epoxy thermosets has been
Effect of Resin Molecular Architecture on Epoxy Thermoset Mechanical Properties The effect of resin molecular architecture on the small strain elastic constants of diamine-cured epoxy thermosets has been
Assessment of moisture absorption in marine GRP laminates with aid of nuclear magnetic resonance imaging
 Assessment of moisture absorption in marine GRP laminates with aid of nuclear magnetic resonance imaging G. Kotsikos 1, A. G. Gibson* 1 and J. Mawella 2 The diffusion of water in an isophthalic polyester
Assessment of moisture absorption in marine GRP laminates with aid of nuclear magnetic resonance imaging G. Kotsikos 1, A. G. Gibson* 1 and J. Mawella 2 The diffusion of water in an isophthalic polyester
ADVANCED MOISTURE MODELING OF POLYMER COMPOSITES
 Proceedings of the 6th Annual ISC Graduate Research Symposium ISC-GRS 22 April 3, 22, Rolla, Missouri ADVANCED MOISTURE MODELING OF POLYMER COMPOSITES ABSTRACT Long term moisture exposure has been shown
Proceedings of the 6th Annual ISC Graduate Research Symposium ISC-GRS 22 April 3, 22, Rolla, Missouri ADVANCED MOISTURE MODELING OF POLYMER COMPOSITES ABSTRACT Long term moisture exposure has been shown
CHAPTER 10. Characteristics of the Surfaces of Biomaterials
 CHAPTER 10 Characteristics of the Surfaces of Biomaterials 10.1 Surface Characteristics Related to Chemical Bonding 10.2 Surface Chemistry Related to Bonding of Biological Molecules 10.3 Porosity 10.4
CHAPTER 10 Characteristics of the Surfaces of Biomaterials 10.1 Surface Characteristics Related to Chemical Bonding 10.2 Surface Chemistry Related to Bonding of Biological Molecules 10.3 Porosity 10.4
3D Thermal-Diffusion Analysis on a Moisture Loaded Epoxy Sample
 Excerpt from the Proceedings of the COMSOL Conference 2010 Boston 3D Thermal-Diffusion Analysis on a Moisture Loaded Epoxy Sample S. Madduri* 1, W. Infantolino 2, and B.G.Sammakia 1 1 Department of Mechanical
Excerpt from the Proceedings of the COMSOL Conference 2010 Boston 3D Thermal-Diffusion Analysis on a Moisture Loaded Epoxy Sample S. Madduri* 1, W. Infantolino 2, and B.G.Sammakia 1 1 Department of Mechanical
Moisture Absorption and Desorption
 Moisture Absorption and Desorption of Composite Materials CHI-HUNG SHEN AND GEORGE S. SPRINGER Department of Mechanical Engineering The University of Michigan Ann Arbor, Michigan 48109 (Received December
Moisture Absorption and Desorption of Composite Materials CHI-HUNG SHEN AND GEORGE S. SPRINGER Department of Mechanical Engineering The University of Michigan Ann Arbor, Michigan 48109 (Received December
Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-a diacrylate
 Polymer Degradation and Stability 66 (1999) 343±347 Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-a diacrylate Mu-Shih Lin*, Ming-Wei Wang, Lon-An Cheng
Polymer Degradation and Stability 66 (1999) 343±347 Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-a diacrylate Mu-Shih Lin*, Ming-Wei Wang, Lon-An Cheng
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
TESTING of AGGREGATES for CONCRETE
 TESTING of AGGREGATES for CONCRETE The properties of the aggregates affect both the fresh and hardened properties of concrete. It is crucial to know the properties of the aggregates to be used in the making
TESTING of AGGREGATES for CONCRETE The properties of the aggregates affect both the fresh and hardened properties of concrete. It is crucial to know the properties of the aggregates to be used in the making
Rule 2. Rule 1. Rule 4. Rule 3. Rule 5. Rule 6. Rule 7. Rule 8
 Rule 1 Follow the directions in your course reader, of your teaching assistant and of your instructor. They are usually much more experienced doing chemistry. Rule 3 When in doubt, ask. This will make
Rule 1 Follow the directions in your course reader, of your teaching assistant and of your instructor. They are usually much more experienced doing chemistry. Rule 3 When in doubt, ask. This will make
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Novel Nanoporous Ferrocenyl Framework for Clean Energy Application Qingquan
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Novel Nanoporous Ferrocenyl Framework for Clean Energy Application Qingquan
CORRELATION BETWEEN HOT PLATE EMISSIVITY AND WAFER TEMPERATURE AT LOW TEMPERATURES
 CORRELATION BETWEEN HOT PLATE EMISSIVITY AND WAFER TEMPERATURE AT LOW TEMPERATURES Tomomi Murakami 1*, Takashi Fukada 1 and Woo Sik Yoo 2 1 WaferMasters Service Factory, 2020-3 Oaza Tabaru, Mashiki, Kamimashiki,
CORRELATION BETWEEN HOT PLATE EMISSIVITY AND WAFER TEMPERATURE AT LOW TEMPERATURES Tomomi Murakami 1*, Takashi Fukada 1 and Woo Sik Yoo 2 1 WaferMasters Service Factory, 2020-3 Oaza Tabaru, Mashiki, Kamimashiki,
CHAPTER 8 ISOLATION AND CHARACTERIZATION OF PHYTOCONSTITUENTS BY COLUMN CHROMATOGRAPHY
 146 CHAPTER 8 ISLATIN AND CHARACTERIZATIN F PHYTCNSTITUENTS BY CLUMN CHRMATGRAPHY 8.1 INTRDUCTIN Column chromatography is an isolation technique in which the phytoconstituents are being eluted by adsorption.
146 CHAPTER 8 ISLATIN AND CHARACTERIZATIN F PHYTCNSTITUENTS BY CLUMN CHRMATGRAPHY 8.1 INTRDUCTIN Column chromatography is an isolation technique in which the phytoconstituents are being eluted by adsorption.
Spectroscopy of Polymers
 Spectroscopy of Polymers Jack L. Koenig Case Western Reserve University WOMACS Professional Reference Book American Chemical Society, Washington, DC 1992 Contents Preface m xiii Theory of Polymer Characterization
Spectroscopy of Polymers Jack L. Koenig Case Western Reserve University WOMACS Professional Reference Book American Chemical Society, Washington, DC 1992 Contents Preface m xiii Theory of Polymer Characterization
Innovative. Technologies. Chemie des Klebens Chemistry of Adhesives. Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013
 Chemie des Klebens Chemistry of Adhesives Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013 Innovative Technologies 1 Overview Chemie des Klebens Chemistry of Adhesives Introduction
Chemie des Klebens Chemistry of Adhesives Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013 Innovative Technologies 1 Overview Chemie des Klebens Chemistry of Adhesives Introduction
Decomposition Mechanism of Epoxy Resin in Nitric Acid for Recycling
 Decomposition Mechanism of Epoxy Resin in Nitric Acid for Recycling Weirong Dang I, Masatoshi Kubouchi I, Shurou Yamamoto I, Hideki Sembokuya I, Kazuyoshi Arai and Ken Tsuda Department of Chemical Engineering,
Decomposition Mechanism of Epoxy Resin in Nitric Acid for Recycling Weirong Dang I, Masatoshi Kubouchi I, Shurou Yamamoto I, Hideki Sembokuya I, Kazuyoshi Arai and Ken Tsuda Department of Chemical Engineering,
Hydrothermal ageing effects on flexural properties of GFRP composite laminates
 Indian Journal of Engineering & Materials Sciences Vol. 20, October 2013, pp. 415-424 Hydrothermal ageing effects on flexural properties of GFRP composite laminates P Sampath Rao* & M Manzoor Hussain Department
Indian Journal of Engineering & Materials Sciences Vol. 20, October 2013, pp. 415-424 Hydrothermal ageing effects on flexural properties of GFRP composite laminates P Sampath Rao* & M Manzoor Hussain Department
Physical and Chemical Reactions during Ion Exchange with TERRA-3000
 Physical and Chemical Reactions during Ion Exchange with TERRA-3000 How does TERRA-3000 work as an ion exchanger and how does it work on silt and colloid particles during treatment? This section looks
Physical and Chemical Reactions during Ion Exchange with TERRA-3000 How does TERRA-3000 work as an ion exchanger and how does it work on silt and colloid particles during treatment? This section looks
Welcome to Organic Chemistry II
 Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Factors Mfecting Moisture Absorption in Polymer Composites Part ll: Influence of External Factors
 Factors Mfecting Moisture Absorption in Polymer Composites Part ll: nfluence of External Factors R. M. V. G. K. RAo MANAS CHANDA. AND N. BALASUBRAMANAN.. Materials Science Division National Aeronautical
Factors Mfecting Moisture Absorption in Polymer Composites Part ll: nfluence of External Factors R. M. V. G. K. RAo MANAS CHANDA. AND N. BALASUBRAMANAN.. Materials Science Division National Aeronautical
Supporting Information: PDMS Nanocomposites for Heat Transfer Enhancement in. Microfluidic Platforms
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information: PDMS Nanocomposites for Heat Transfer Enhancement in Microfluidic
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information: PDMS Nanocomposites for Heat Transfer Enhancement in Microfluidic
An investigation was conducted based on: (1) The study of cure mechanisms and
 Iranian Polymer Journal Available online at: http://journal.ippi.ac.ir 15 (2), 2006, 103-110 Study the Curing Kinetics of DGEBA with Imidazoles and Property-structure Relationships Mousa Ghaemy * and Samaneh
Iranian Polymer Journal Available online at: http://journal.ippi.ac.ir 15 (2), 2006, 103-110 Study the Curing Kinetics of DGEBA with Imidazoles and Property-structure Relationships Mousa Ghaemy * and Samaneh
Lecture #7 In Vivo Water
 Lecture #7 In Vivo Water Topics Hydration layers Tissue relaxation times Magic angle effects Magnetization Transfer Contrast (MTC) CEST Handouts and Reading assignments Mathur-De Vre, R., The NMR studies
Lecture #7 In Vivo Water Topics Hydration layers Tissue relaxation times Magic angle effects Magnetization Transfer Contrast (MTC) CEST Handouts and Reading assignments Mathur-De Vre, R., The NMR studies
Kinetic Isotope Effects
 1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
Supporting Information
 Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
GRIGNARD REACTION Synthesis of Benzoic Acid
 1 GRIGNARD REACTION Synthesis of Benzoic Acid In the 1920 s, the first survey of the acceleration of chemical transformations by ultrasound was published. Since then, many more applications of ultrasound
1 GRIGNARD REACTION Synthesis of Benzoic Acid In the 1920 s, the first survey of the acceleration of chemical transformations by ultrasound was published. Since then, many more applications of ultrasound
TOSYLHYDRAZONE CLEAVAGE OF AN α,β-epoxy KETONE; OXIDATIVE KMnO 4 CLEAVAGE OF AN ALKYNE EXPERIMENT A
 1 EXPERIMENT A EPOXIDATION OF AN α,β-unsaturated KETONE; TOSYLYDRAZONE CLEAVAGE OF AN α,β-epoxy KETONE; OXIDATIVE KMnO 4 CLEAVAGE OF AN ALKYNE The goal of this experiment is the correct assignment of the
1 EXPERIMENT A EPOXIDATION OF AN α,β-unsaturated KETONE; TOSYLYDRAZONE CLEAVAGE OF AN α,β-epoxy KETONE; OXIDATIVE KMnO 4 CLEAVAGE OF AN ALKYNE The goal of this experiment is the correct assignment of the
The Effect of Cure Rate on Glassy Polymer Networks
 The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College 5-2016 The Effect of Cure Rate on Glassy Polymer Networks Kristen M. Van de Voorde Follow this and additional
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College 5-2016 The Effect of Cure Rate on Glassy Polymer Networks Kristen M. Van de Voorde Follow this and additional
Spectroscopy and Chromatography
 Spectroscopy and Chromatography Introduction Visible light is one very small part of the electromagnetic spectrum. The different properties of the various types of radiation depend upon their wavelength.
Spectroscopy and Chromatography Introduction Visible light is one very small part of the electromagnetic spectrum. The different properties of the various types of radiation depend upon their wavelength.
Preface. The Time-Temperature-Transformation (TTT) Cure Diagram of Thermosetting Polymeric Systems
 Editorial With the publication of Vol. 51 the editors and the publisher would like to take this opportunity to thank authors and readers for their collaboration and their efforts to meet the scientific
Editorial With the publication of Vol. 51 the editors and the publisher would like to take this opportunity to thank authors and readers for their collaboration and their efforts to meet the scientific
CURE DEPENDENT CREEP COMPLIANCE OF AN EPOXY RESIN
 CURE DEPENDENT CREEP COMPLIANCE OF AN EPOXY RESIN Daniel J. O Brien and Scott R. White 2 Department of Mechanical and Industrial Engineering, University of Illinois at Urbana- Champaign 206 West Green,
CURE DEPENDENT CREEP COMPLIANCE OF AN EPOXY RESIN Daniel J. O Brien and Scott R. White 2 Department of Mechanical and Industrial Engineering, University of Illinois at Urbana- Champaign 206 West Green,
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Minneapolis Community and Technical College. Separation of Components of a Mixture
 Minneapolis Community and Technical College Chemistry Department Chem1020 Separation of Components of a Mixture Objectives: To separate a mixture into its component pure substances. To calculate the composition
Minneapolis Community and Technical College Chemistry Department Chem1020 Separation of Components of a Mixture Objectives: To separate a mixture into its component pure substances. To calculate the composition
Supporting Information:
 Supporting Information: Visible Light Initiated Thiol-Michael Addition Polymerizations with Coumarin-based Photo-base Generators, Another Photoclick Reaction Strategy Xinpeng Zhang, Weixian Xi, Chen Wang,
Supporting Information: Visible Light Initiated Thiol-Michael Addition Polymerizations with Coumarin-based Photo-base Generators, Another Photoclick Reaction Strategy Xinpeng Zhang, Weixian Xi, Chen Wang,
All measurement has a limit of precision and accuracy, and this must be taken into account when evaluating experimental results.
 Chapter 11: Measurement and data processing and analysis 11.1 Uncertainty and error in measurement and results All measurement has a limit of precision and accuracy, and this must be taken into account
Chapter 11: Measurement and data processing and analysis 11.1 Uncertainty and error in measurement and results All measurement has a limit of precision and accuracy, and this must be taken into account
Questions on Instrumental Methods of Analysis
 Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
IMPROVEMENT IN MECHANICAL PROPERTIES OF MODIFIED GRAPHENE/EPOXY NANOCOMPOSITES
 18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS IMPROVEMENT IN MECHANICAL PROPERTIES OF MODIFIED 1 Introduction Since first successfully separated from graphite by micromechanical cleavage [1], graphene
18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS IMPROVEMENT IN MECHANICAL PROPERTIES OF MODIFIED 1 Introduction Since first successfully separated from graphite by micromechanical cleavage [1], graphene
High-Performance Blend Membranes Composed of An Amphoteric Copolymer Containing Supramolecular Nanosieves for Direct Methanol Fuel Cells
 Electonic Supplementary Information (ESI) for Chemical Communications High-Performance Blend Membranes Composed of An Amphoteric Copolymer Containing Supramolecular Nanosieves for Direct Methanol Fuel
Electonic Supplementary Information (ESI) for Chemical Communications High-Performance Blend Membranes Composed of An Amphoteric Copolymer Containing Supramolecular Nanosieves for Direct Methanol Fuel
Polymer Bulletin 9 by Springer-Verlag 1980
 Polymer Bulletin 3, 391-398 (1980) Polymer Bulletin 9 by Springer-Verlag 1980 Homogeneous Epoxy-Acrylic Interpenetrating Polymer Networks: Preparation and Thermal Properties Alain Dubuisson, Dominique
Polymer Bulletin 3, 391-398 (1980) Polymer Bulletin 9 by Springer-Verlag 1980 Homogeneous Epoxy-Acrylic Interpenetrating Polymer Networks: Preparation and Thermal Properties Alain Dubuisson, Dominique
I. 16. Coloration of Polyethylene Terephthalate (PET) Film by 3MeV Proton Beams
 CYRIC Annual Report 2001 I. 16. Coloration of Polyethylene Terephthalate (PET) Film by 3MeV Proton Beams Matsuyama S., Ishii K., Yamazaki H., Endoh H., Yuki H., Satoh T., Sugihara S., Amartaivan Ts., Tanaka
CYRIC Annual Report 2001 I. 16. Coloration of Polyethylene Terephthalate (PET) Film by 3MeV Proton Beams Matsuyama S., Ishii K., Yamazaki H., Endoh H., Yuki H., Satoh T., Sugihara S., Amartaivan Ts., Tanaka
11/23/ (4) Harmonization Stage 6: <1241> Water Solid Interactions in Pharmaceutical Systems (USP34 NF29 2S) BRIEFING
 BRIEFING 1241 Water Solid Interactions in Pharmaceutical Systems, USP 32 page 759. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
BRIEFING 1241 Water Solid Interactions in Pharmaceutical Systems, USP 32 page 759. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing
 VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing Q. Xiang, M. Xanthos*, S. Mitra and S. H. Patel* Department of Chemical Engineering, Chemistry and Environmental Science
VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing Q. Xiang, M. Xanthos*, S. Mitra and S. H. Patel* Department of Chemical Engineering, Chemistry and Environmental Science
Characterization of water absorbed epoxy insulating coating material used in ZnO varistors by dielectric measurements
 Materials Letters 60 (2006) 114 119 www.elsevier.com/locate/matlet Characterization of water absorbed epoxy insulating coating material used in ZnO varistors by dielectric measurements Shengtao Li a, Lei
Materials Letters 60 (2006) 114 119 www.elsevier.com/locate/matlet Characterization of water absorbed epoxy insulating coating material used in ZnO varistors by dielectric measurements Shengtao Li a, Lei
AC impedance and dielectric spectroscopic studies of Mg 2+ ion conducting PVA PEG blended polymer electrolytes
 Bull. Mater. Sci., Vol. 34, No. 5, August 211, pp. 163 167. c Indian Academy of Sciences. AC impedance and dielectric spectroscopic studies of Mg 2+ ion conducting PVA PEG blended polymer electrolytes
Bull. Mater. Sci., Vol. 34, No. 5, August 211, pp. 163 167. c Indian Academy of Sciences. AC impedance and dielectric spectroscopic studies of Mg 2+ ion conducting PVA PEG blended polymer electrolytes
9. Hydroboration-Oxidation of Alkenes
 9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
Experiment 6: Dehydration of 2-Methylcyclohexanol
 Experiment 6: Dehydration of 2-Methylcyclohexanol Dehydration of 2-Methylcyclohexanol This week's reaction: A B - dehydration of a 2 alcohol to give a mixture of alkene isomers - H 3 PO 4 is a catalyst
Experiment 6: Dehydration of 2-Methylcyclohexanol Dehydration of 2-Methylcyclohexanol This week's reaction: A B - dehydration of a 2 alcohol to give a mixture of alkene isomers - H 3 PO 4 is a catalyst
Chemistry 11. Unit 3 The Physical Properties and Physical Changes of Substances
 Chemistry 11 1 Unit 3 The Physical Properties and Physical Changes of Substances 2 1. Definitions in science Science is the observation, identification, description, experimental investigation, and theoretical
Chemistry 11 1 Unit 3 The Physical Properties and Physical Changes of Substances 2 1. Definitions in science Science is the observation, identification, description, experimental investigation, and theoretical
Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different Proportions
 2015 2 nd International Conference on Material Engineering and Application (ICMEA 2015) ISBN: 978-1-60595-323-6 Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different
2015 2 nd International Conference on Material Engineering and Application (ICMEA 2015) ISBN: 978-1-60595-323-6 Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Pumping Speed in the Drydown Zone
 A Journal of Practical and Useful Vacuum Technology From By Phil Danielson Pumping Speed in the Drydown Zone The extended pumpdown time through the water vapor-dominated drydown zone is a complex process
A Journal of Practical and Useful Vacuum Technology From By Phil Danielson Pumping Speed in the Drydown Zone The extended pumpdown time through the water vapor-dominated drydown zone is a complex process
15NT303E Molecular spectroscopy and its Applications Fifth Semester, (Odd semester)
 . SRM University Faculty of Engineering and Technology Department of Physics and Nanotechnology 15NT303E Molecular spectroscopy and its Applications Fifth Semester, 2017-18 (Odd semester) tailed Session
. SRM University Faculty of Engineering and Technology Department of Physics and Nanotechnology 15NT303E Molecular spectroscopy and its Applications Fifth Semester, 2017-18 (Odd semester) tailed Session
The Kinetics of B-a and P-a Type Copolybenzoxazine via the Ring Opening Process
 The Kinetics of B-a and P-a Type Copolybenzoxazine via the Ring Opening Process Yi-Che Su, Ding-Ru Yei, Feng-Chih Chang Institute of Applied Chemistry, National Chiao-Tung University, Hsin-Chu, Taiwan
The Kinetics of B-a and P-a Type Copolybenzoxazine via the Ring Opening Process Yi-Che Su, Ding-Ru Yei, Feng-Chih Chang Institute of Applied Chemistry, National Chiao-Tung University, Hsin-Chu, Taiwan
Chemistry 213 Practical Spectroscopy
 Chemistry 213 Practical Spectroscopy Dave Berg djberg@uvic.ca Elliott 314 A course in determining structure by spectroscopic methods Different types of spectroscopy afford different information about molecules
Chemistry 213 Practical Spectroscopy Dave Berg djberg@uvic.ca Elliott 314 A course in determining structure by spectroscopic methods Different types of spectroscopy afford different information about molecules
Supporting information for
 Supporting information for High-performance and moisture-stable cellulosestarch nanocomposites based on bioinspired coreshell nanofibers Kasinee Prakobna, 1, 2 Sylvain Galland, 1, 2 and Lars A. Berglund
Supporting information for High-performance and moisture-stable cellulosestarch nanocomposites based on bioinspired coreshell nanofibers Kasinee Prakobna, 1, 2 Sylvain Galland, 1, 2 and Lars A. Berglund
F G C G H H C B B A A DD E E
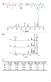 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
Studies on Acoustic Parameters of Ternary Mixture of Dimethyl Acetamide in Acetone and Isobutyl Methyl Ketone using Ultrasonic and Viscosity Probes
 Studies on Acoustic Parameters of Ternary Mixture of Dimethyl Acetamide in Acetone and Isobutyl Methyl Ketone using Ultrasonic and Viscosity Probes ASHOK KUMAR DASH 1, 2 1 Department of Physics,L.N College.Patkura.Kendrapara.Odisha,754134,India,
Studies on Acoustic Parameters of Ternary Mixture of Dimethyl Acetamide in Acetone and Isobutyl Methyl Ketone using Ultrasonic and Viscosity Probes ASHOK KUMAR DASH 1, 2 1 Department of Physics,L.N College.Patkura.Kendrapara.Odisha,754134,India,
DEVELOPMENT OF IMPROVED METHODS FOR CHARACTERISING THE CURE OF COMPOSITE MATERIALS
 20 th International Conference on Composite Materials Copenhagen, 19-24 th July 2015 DEVELOPMENT OF IMPROVED METHODS FOR CHARACTERISING THE CURE OF COMPOSITE MATERIALS Ana Yong 1, 2, Graham D. Sims 1,
20 th International Conference on Composite Materials Copenhagen, 19-24 th July 2015 DEVELOPMENT OF IMPROVED METHODS FOR CHARACTERISING THE CURE OF COMPOSITE MATERIALS Ana Yong 1, 2, Graham D. Sims 1,
BMB/Bi/Ch 173 Winter 2018
 BMB/Bi/Ch 173 Winter 2018 Homework Set 8.1 (100 Points) Assigned 2-27-18, due 3-6-18 by 10:30 a.m. TA: Rachael Kuintzle. Office hours: SFL 220, Friday 3/2 4:00-5:00pm and SFL 229, Monday 3/5 4:00-5:30pm.
BMB/Bi/Ch 173 Winter 2018 Homework Set 8.1 (100 Points) Assigned 2-27-18, due 3-6-18 by 10:30 a.m. TA: Rachael Kuintzle. Office hours: SFL 220, Friday 3/2 4:00-5:00pm and SFL 229, Monday 3/5 4:00-5:30pm.
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cyclic Molecule Aerogels: A Robust Cyclodextrin
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cyclic Molecule Aerogels: A Robust Cyclodextrin
Thin Layer Chromatography
 Thin Layer Chromatography Thin-layer chromatography involves the same principles as column chromatography, it also is a form of solid-liquid adsorption chromatography. In this case, however, the solid
Thin Layer Chromatography Thin-layer chromatography involves the same principles as column chromatography, it also is a form of solid-liquid adsorption chromatography. In this case, however, the solid
Effect of Lime on the Compressibility Characteristics of a Highly Plastic Clay
 Effect of Lime on the Compressibility Characteristics of a Highly Plastic Clay Abstract İnci Süt-Ünver Ph.D. Candidate Istanbul Technical University Istanbul - Turkey Musaffa Ayşen Lav Prof. Dr. Istanbul
Effect of Lime on the Compressibility Characteristics of a Highly Plastic Clay Abstract İnci Süt-Ünver Ph.D. Candidate Istanbul Technical University Istanbul - Turkey Musaffa Ayşen Lav Prof. Dr. Istanbul
NUTC R296. Advanced Moisture Modeling of Polymer Composites. K. Chandrashekhara Curators Professor Department of Mechanical and Aerospace Engineering
 Advanced Moisture Modeling of Polymer Composites by K. Chandrashekhara Curators Professor Department of Mechanical and Aerospace Engineering NUTC R296 A National University Transportation Center at Missouri
Advanced Moisture Modeling of Polymer Composites by K. Chandrashekhara Curators Professor Department of Mechanical and Aerospace Engineering NUTC R296 A National University Transportation Center at Missouri
7.1 Electrolyte and electrolytic solution
 Out-class reading: Levine, pp. 294-310 Section 10.6 solutions of electrolytes Section 10.9 ionic association pp. 512-515 Section 16.6 electrical conductivity of electrolyte solutions. Contents of solution
Out-class reading: Levine, pp. 294-310 Section 10.6 solutions of electrolytes Section 10.9 ionic association pp. 512-515 Section 16.6 electrical conductivity of electrolyte solutions. Contents of solution
A Technical Whitepaper Polymer Technology in the Coating Industry. By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA
 A Technical Whitepaper Polymer Technology in the Coating Industry By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA INTRODUCTION Polymer Technology in the Coating Industry To properly understand
A Technical Whitepaper Polymer Technology in the Coating Industry By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA INTRODUCTION Polymer Technology in the Coating Industry To properly understand
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state
 2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
THE ROLE OF THE EPOXY RESIN: CURING AGENT RATIO ON COMPOSITE INTERFACIAL STRENGTH AND THERMAL PERFORMANCE
 Munich, Germany, 26-30 th June 2016 1 THE ROLE OF THE EPOXY RESIN: CURING AGENT RATIO ON COMPOSITE INTERFACIAL STRENGTH AND THERMAL PERFORMANCE Ross F. Minty 1, James L. Thomason 2 and Liu Yang 3 1 University
Munich, Germany, 26-30 th June 2016 1 THE ROLE OF THE EPOXY RESIN: CURING AGENT RATIO ON COMPOSITE INTERFACIAL STRENGTH AND THERMAL PERFORMANCE Ross F. Minty 1, James L. Thomason 2 and Liu Yang 3 1 University
Chapter 4. Results and Discussion. 4.1 Monomer Syntheses
 Chapter 4 Results and Discussion 4.1 Monomer Syntheses The syntheses of a family of novel, carbazole based methacrylate, dimethacrylate, and acrylate monomers, and subsequent purifications, were successful.
Chapter 4 Results and Discussion 4.1 Monomer Syntheses The syntheses of a family of novel, carbazole based methacrylate, dimethacrylate, and acrylate monomers, and subsequent purifications, were successful.
Simulation of the NMR Second Moment as a Function of Temperature in the Presence of Molecular Motion. Application to (CH 3
 Simulation of the NMR Second Moment as a Function of Temperature in the Presence of Molecular Motion. Application to (CH 3 ) 3 NBH 3 Roman Goc Institute of Physics, A. Mickiewicz University, Umultowska
Simulation of the NMR Second Moment as a Function of Temperature in the Presence of Molecular Motion. Application to (CH 3 ) 3 NBH 3 Roman Goc Institute of Physics, A. Mickiewicz University, Umultowska
Supporting Information
 Supporting Information Nb 2 5 nh 2 as a heterogeneous catalyst with water-tolerant Lewis acid sites Kiyotaka Nakajima, Yusuke Baba, Ryouhei Noma, Masaaki Kitano, Junko N. Kondo, Shigenobu Hayashi, П,*
Supporting Information Nb 2 5 nh 2 as a heterogeneous catalyst with water-tolerant Lewis acid sites Kiyotaka Nakajima, Yusuke Baba, Ryouhei Noma, Masaaki Kitano, Junko N. Kondo, Shigenobu Hayashi, П,*
I690/B680 Structural Bioinformatics Spring Protein Structure Determination by NMR Spectroscopy
 I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
POLARIZATION STABILITY OF AMORPHOUS PIEZOELECTRIC POLYIMIDES
 POLARIZATION STABILITY OF AMORPHOUS PIEZOELECTRIC POLYIMIDES C. PARK*, Z. OUNAIES**, J. SU*, J.G. SMITH JR. AND J.S. HARRISON Advanced Materials and Processing Branch, NASA Langley Research Center, Hampton
POLARIZATION STABILITY OF AMORPHOUS PIEZOELECTRIC POLYIMIDES C. PARK*, Z. OUNAIES**, J. SU*, J.G. SMITH JR. AND J.S. HARRISON Advanced Materials and Processing Branch, NASA Langley Research Center, Hampton
MATERIALS SCIENCE POLYMERS
 POLYMERS 1) Types of Polymer (a) Plastic Possibly the largest number of different polymeric materials come under the plastic classification. Polyethylene, polypropylene, polyvinyl chloride, polystyrene,
POLYMERS 1) Types of Polymer (a) Plastic Possibly the largest number of different polymeric materials come under the plastic classification. Polyethylene, polypropylene, polyvinyl chloride, polystyrene,
New Horizons in Accelerated Stability Modeling-- Tablet Dissolution, Tablet Disintegration and Product Appearance
 New Horizons in Accelerated Stability Modeling-- Tablet Dissolution, Tablet Disintegration and Product Appearance Kenneth C. Waterman, Ph.D. FreeThink Technologies, Inc. Branford, CT ken.waterman@freethinktech.com
New Horizons in Accelerated Stability Modeling-- Tablet Dissolution, Tablet Disintegration and Product Appearance Kenneth C. Waterman, Ph.D. FreeThink Technologies, Inc. Branford, CT ken.waterman@freethinktech.com
Supporting Information for Polybenzimidazolium Salts: A New Class of. Anion-Conducting Polymer
 Supporting Information for Polybenzimidazolium Salts: A ew Class of Anion-Conducting Polymer Owen D. Thomas, Kristen J. W. Y. Soo, Timothy J. Peckham, Mahesh P. Kulkarni and Steven Holdcroft* Department
Supporting Information for Polybenzimidazolium Salts: A ew Class of Anion-Conducting Polymer Owen D. Thomas, Kristen J. W. Y. Soo, Timothy J. Peckham, Mahesh P. Kulkarni and Steven Holdcroft* Department
Properties of Vapors
 Properties of Vapors Topics for Discussion The Pressure/Temperature Relationship Vaporization Condensation Enthalpy Properties of Vapors Topics for Discussion Entropy Properties of Substances Saturated
Properties of Vapors Topics for Discussion The Pressure/Temperature Relationship Vaporization Condensation Enthalpy Properties of Vapors Topics for Discussion Entropy Properties of Substances Saturated
Report AFK0242/18 TABLE OF CONTENTS
 Client: Blue Dent Dental OS: 0161/0211-18 Contact: Rafael Gomes E-mail: rafael@bluedent.com.br Phone: (19) 3563-2222 Address: Rua Joaquim Jorge Port, 1272 City/State: Pirassununga/SP Zip Code: 13636-142
Client: Blue Dent Dental OS: 0161/0211-18 Contact: Rafael Gomes E-mail: rafael@bluedent.com.br Phone: (19) 3563-2222 Address: Rua Joaquim Jorge Port, 1272 City/State: Pirassununga/SP Zip Code: 13636-142
NOTE. Separation of chlorophenols using columns of hydroxyaluminium interlayered clays
 Clay Minerals (1997) 32, 143-147 NOTE Separation of chlorophenols using columns of hydroxyaluminium interlayered clays Clay minerals play an important role in the retention, transport and chemistry of
Clay Minerals (1997) 32, 143-147 NOTE Separation of chlorophenols using columns of hydroxyaluminium interlayered clays Clay minerals play an important role in the retention, transport and chemistry of
Introduction to Engineering Materials ENGR2000 Chapter 14: Polymer Structures. Dr. Coates
 Introduction to Engineering Materials ENGR2000 Chapter 14: Polymer Structures Dr. Coates 14.1 Introduction Naturally occurring polymers Wood, rubber, cotton, wool, leather, silk Synthetic polymers Plastics,
Introduction to Engineering Materials ENGR2000 Chapter 14: Polymer Structures Dr. Coates 14.1 Introduction Naturally occurring polymers Wood, rubber, cotton, wool, leather, silk Synthetic polymers Plastics,
Linear and nonlinear spectroscopy
 Linear and nonlinear spectroscopy We ve seen that we can determine molecular frequencies and dephasing rates (for electronic, vibrational, or spin degrees of freedom) from frequency-domain or timedomain
Linear and nonlinear spectroscopy We ve seen that we can determine molecular frequencies and dephasing rates (for electronic, vibrational, or spin degrees of freedom) from frequency-domain or timedomain
HIGH-TEMPERATURE STEAM-TREATMENT OF PBI AND ITS BLENDS WITH PEEK AND PEKK: A SOLID-STATE NMR STUDY
 HIGH-TEMPERATURE STEAM-TREATMENT OF PBI AND ITS BLENDS WITH PEEK AND PEKK: A SOLID-STATE NMR STUDY Jacqueline C. Pope, Tim Bremner, Janet Blümel,* Texas A&M University, College Station, TX, 77842, Tel.
HIGH-TEMPERATURE STEAM-TREATMENT OF PBI AND ITS BLENDS WITH PEEK AND PEKK: A SOLID-STATE NMR STUDY Jacqueline C. Pope, Tim Bremner, Janet Blümel,* Texas A&M University, College Station, TX, 77842, Tel.
and BP Crosslinked EVA Membranes
 and BP Crosslinked EVA Membranes Chapter 5 Interaction of Chlorinated Hydrocarbons with DCP Summary The interaction of dicumyl peroxide and benzoyl peroxide crosslinked EVA membranes with three chlorinated
and BP Crosslinked EVA Membranes Chapter 5 Interaction of Chlorinated Hydrocarbons with DCP Summary The interaction of dicumyl peroxide and benzoyl peroxide crosslinked EVA membranes with three chlorinated
Characterization and properties of new silicone-containing epoxy resin
 Polymer 41 (2000) 6113 6122 Characterization and properties of new silicone-containing epoxy resin W.J. Wang a,b, L.H. Perng c, *, G.H. Hsiue a, F.C. Chang b a Department of Chemical Engineering, National
Polymer 41 (2000) 6113 6122 Characterization and properties of new silicone-containing epoxy resin W.J. Wang a,b, L.H. Perng c, *, G.H. Hsiue a, F.C. Chang b a Department of Chemical Engineering, National
OHD L-14 METHOD OF TEST FOR DETERMINING THE SPECIFIC GRAVITY AND UNIT WEIGHT OF COMPACTED BITUMINOUS MIXTURES
 Page 1 METHOD OF TEST FOR DETERMINING THE SPECIFIC GRAVITY AND UNIT WEIGHT OF COMPACTED BITUMINOUS MIXTURES I. SCOPE. This method of test covers the procedures for determining the bulk specific gravity
Page 1 METHOD OF TEST FOR DETERMINING THE SPECIFIC GRAVITY AND UNIT WEIGHT OF COMPACTED BITUMINOUS MIXTURES I. SCOPE. This method of test covers the procedures for determining the bulk specific gravity
Free Radical Chlorination
 Free Radical Chlorination Although saturated hydrocarbons are inert to most acidic and basic reagents, they can be halogenated in the presence of a free radical initiator. The process is a chain reaction,
Free Radical Chlorination Although saturated hydrocarbons are inert to most acidic and basic reagents, they can be halogenated in the presence of a free radical initiator. The process is a chain reaction,
Conclusion and Future Work
 Chapter 7 7. Chapter 7 and Future Work Chapter 7 Abstract This chapter gives the details of correlations of the spectroscopic investigation results with those available from other studies and also summarizes
Chapter 7 7. Chapter 7 and Future Work Chapter 7 Abstract This chapter gives the details of correlations of the spectroscopic investigation results with those available from other studies and also summarizes
