Laboratory studies of UV emissions from proton impact on N 2 : The Lyman Birge Hopfield band system for aurora analysis
|
|
|
- Gregory Berry
- 5 years ago
- Views:
Transcription
1 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116,, doi: /2010ja016103, 2011 Laboratory studies of UV emissions from proton impact on N 2 : The Lyman Birge Hopfield band system for aurora analysis Joseph M. Ajello, 1 Rao S. Mangina, 1 Douglas J. Strickland, 2 and Dariusz Dziczek 3 Received 9 September 2010; revised 14 December 2010; accepted 3 January 2011; published 19 April [1] We have measured the emission cross sections of the Lyman Birge Hopfield (LBH) a 1 P g X 1 S g + band system and several atomic nitrogen (N I) multiplets (1200, 1243, 1493 Å) by H + (proton) impact on N 2 over an impact energy range of 1 7 kev. The peak proton impact induced emission cross section of the LBH band system ( Å) was measured to be 5.05 ± cm 2 at 7 kev. To the best of our knowledge, the present LBH emission cross sections are reported for the first time in the far ultraviolet (FUV) wavelength range of Å. The proton energy range in this study, when coupled with previously published kev proton excited emissions of N I multiplets, provides a wide energy range of emission cross sections for proton energy loss transport codes. This energy range includes the peak cross section and the energy range for Born scaling. The reported measurements lead to an important component of monoenergetic yields for proton FUV auroral emission. Such yields, based on emission cross sections and transport modeling, allowed for convenient comparison of emission efficiencies between proton and electron aurora. In addition, we have measured the HLya, LBH, and N I multiplet emission cross sections for H 2 + and H 3 + ion impact on N 2 at 5 kev and found that the magnitude of H Ly a emission cross section, s em (Ly a), follows in the order of impact ion mass H 3 + >H 2 + >H +. Citation: Ajello, J. M., R. S. Mangina, D. J. Strickland, and D. Dziczek (2011), Laboratory studies of UV emissions from proton impact on N 2 : The Lyman Birge Hopfield band system for aurora analysis, J. Geophys. Res., 116,, doi: /2010ja Introduction [2] Earth, Titan, Triton, and Pluto have nitrogen bearing atmospheres in our solar system. Their emergent far ultraviolet ((FUV) Å) airglow and auroral spectra contain a wealth of information on solar driven and particledriven processes involving N 2. The only way to get a global, instantaneous picture of the energetic particles input over the auroral oval is through spectral imaging. Imaging the proton aurora is an excellent probe for investigating magnetospheric substorms and magnetosphere ionosphere coupling processes [Rees, 1989; Samson et al., 1992; Deehr and Lummerzheim, 2001; Immel et al., 2002; Mende et al., 2000]. For instance, atomic H emissions resulting from excited H atoms produced within the incident proton beam are a unique signature of proton precipitation. The Lyman Birge Hopfield (LBH) band is one of the most important molecular emissions of excited nitrogen in the FUV. It is always found and can be produced due to impact excitation 1 Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California, USA. 2 Computational Physics, Inc., Springfield, Virginia, USA. 3 Institute of Physics, Nicolaus Copernicus University, Torun, Poland. Copyright 2011 by the American Geophysical Union /11/2010JA of protons and electrons in the aurora and airglow observations of nitrogen bearing atmospheres of Earth [e.g., Bishop and Feldman, 2003; Strickland et al., 2001], Titan [e.g., Ajello et al., 2008; Sittler et al., 2009] and Triton [e.g., Broadfoot et al., 1989] and is expected at Pluto [Stern et al., 2008]. [3] Recently, NASA s Imager for Magnetopause to Aurora Global Exploration (IMAGE) spacecraft globally imaged proton aurora for the first time and made a remarkable set of observations, leading to a breakthrough in understanding the origin of a peculiar and puzzling type of aurora, seen as bright spots in the Earth s atmosphere and called dayside proton auroral spots [Frey et al., 2002]. They are now known to occur when fractures appear in the Earth s magnetic field, allowing particles emitted from the Sun to pass through and collide with molecules in the atmosphere. This result has opened up a new area of research to observe dayside proton aurora, and use those observations to know where and how the cracks in the magnetic field are formed and how long the cracks remain open. That makes it a powerful tool to study the entry of the solar wind into the Earth s magnetosphere. [4] The 2003 launch of the Defense Meteorological Satellite Program (DMSP) F16 satellite, which contains, among its suite of sensors, Special Sensor Auroral Particle Sensor (SSJ/5) and Special Sensor Ultraviolet Spectrographic Imager 1of21
2 (SSUSI) [e.g., Paxton et al., 1992a, 1992b] made it possible, for the first time, to undertake extensive statistical studies of auroral output at far ultraviolet (FUV) wavelengths as a function of auroral input of energetic electrons and ions [Knight et al., 2008; J. T. Correira et al., A downward revision of a recently reported proton auroral LBH emission efficiency, submitted to Journal of Geophysical Research, 2010]. Independently, FUV can also be used to infer the solar energy input [e.g., Strickland et al., 2007] and also determine characteristics of auroral particles that deposit energy in the thermosphere [e.g., Strickland et al., 1993; Germany et al., 1994a, 1994b; Lummerzheim et al., 1991]. [5] As observed in many satellite missions (e.g., by the Global Ultraviolet Imager (GUVI)) aboard NASA s Thermosphere Ionosphere Mesosphere Energetics and Dynamics (TIMED) [e.g., Christensen et al., 2003], FUV on IMAGE [Mende et al., 2000], SSUSI on DMSP, High Throughput Imaging Echelle Spectrograph (HiTIES) [McWhirter et al., 2003], and the Ultraviolet and Visible Imaging and Spectrograph Imaging (UVISI) on the Midcourse Space Experiment (MSX) [e.g., Strickland et al., 2001] the auroral emission spectrum spanning the FUV to visible region is dominated by (1) N 2 LBH (within Å), LBH S (in the shorter wavelength range Å), and LBH L (in the longer wavelength Å); (2) H Ly a (1216 Å), H a (6563 Å), and H b (4861 Å); (3) O I multiplets (989, 1027, 1304, 1356 Å); (4) first positive band (B 3 P g + A 3 S u + ) heads of N 2 at 6545 Å, 7534 Å, N I lines ( Å), first negative band (B 2 S u + X 2 S g + ) heads of N 2 + at 3914 and 4278 Å, and N 2 second positive band (C 3 P u B 3 P g )ofn 2 at 3371 Å. It is known that energetic protons and electrons do not interact in the same way within the atmosphere [Basu et al., 1993; Strickland et al., 1993; Galand et al., 1997; Galand and Richmond, 2001; Galand and Lummerzheim, 2004; Knight et al., 2008], one difference being higher LBH emission efficiency for proton aurora. While current usage of cross sections leads to 50% higher efficiency, Knight et al. found even greater values with some downward revision recommended by Correira et al. (submitted manuscript, 2010) (also see section 4). Therefore, it is crucial to separate the electron and proton components of the auroral precipitation in order to correctly assess ionospheric conductivities, heating, and composition changes. This is one of the important scientific issues of polar aeronomy, as stated by Coupling, Energetic, and Dynamics of Atmospheric Regions (CEDAR)/Phase III. In previous proton workshops (1994, 1999), it was realized that many of the available excitation cross sections for these auroral emissions due to proton impact from their thresholds to 40 kev (i.e., typical average peak energy observed in the proton aurora) are still poorly known or undetermined and thus become a limiting factor for accurate determination of charged and neutral particle interactions with species in Earth s and outer space atmospheres. [6] Much of current knowledge about the relative importance of proton versus electron precipitation has come from particle measurements and their use in developing statistical models. Extensive use has been made of DMSP SSJ/4 based models by Hardy et al. [1985, 1989] (electrons and protons). These models illustrate a displacement of the statistical ovals from one another that can result in pure or nearly pure proton precipitation in the vicinity and south of the electron equatorial boundary and most prevalent during premidnight hours. The Space Environment Monitor (SEM) on NOAA POES and TIROS satellites [Evans and Greer, 2000; Raben et al., 1995] complements SSJ/4 with measurements to much higher energies (to 100s of kev in contrast to an upper limit of 30 kev for SSJ/4). Such measurements demonstrate that significant energy flux can reside above 30 kev during active times [e.g., Coumans et al.,2002].maps of proton precipitation similar to the Hardy maps but for higher energies may be seen by Fang et al. [2007] (SEM on POES) and by Codrescu et al. [1997] (SEM on TIROS). [7] LBH emissions result from collisions of N 2 with energetic electrons, combined neutral H and protons, as well as from secondary electrons produced by ionizing events [Knight et al., 2008]. Knight et al. [2008] reported unexpectedly high efficiencies of LBH emission for proton precipitation, higher than predicted by current models. The earliest measurements of LBH excitation by H + impact on N 2 were obtained by energy loss spectroscopy (from 20 to 120 kev by Schowengerdt and Park [1970] and from 0.6 to 4 kev by Moore [1972]). As shown by Moore, the comparison of excitation cross sections of these two measurements do not agree in magnitude so as to join them together to form a single cross section curve for obtaining missing cross section values. At the present time, the continued absence of measured proton impact emission cross sections for the LBH band system of N 2 and the need of these data for modeling prompted this laboratory study of LBH and N I emission cross sections of N 2, which is complementary to our ongoing studies of electron impact emissions of species of interest to Earth and outer space atmospheres [Ajello et al., 2010]. Our Jet Propulsion Laboratory (JPL) team recently remeasured the cross sections for electron excited LBH emissions of N 2 more accurately [Young et al., 2010] and revised our earlier measurements of Ajello and Shemansky [1985, hereafter AS85]. It is important to compare peak electron and proton emission cross sections in the energy regions where auroral particles have high energy fluxes. The energy range of interest for proton impact fluorescence extends from 1 kev to 100 kev. The key to the energy range is to get to high enough energies to measure beyond cross section maxima to the Born region ( 100 kev), so cross sections can be reasonably estimated using the Born approximation to a few hundred kev. We make a first step in this program by measuring the LBH cross sections from 1 to 7 kev, the region of the emission cross section maximum. We measured and analyzed the UV spectrum, which is calibrated by standard techniques developed in this laboratory [Liu et al., 1995]. The absolute emission cross sections of the LBH band system show strong efficiency of proton excitation of LBH band system compared to electron impact LBH emissions. [8] In this paper, we describe our new laboratory facility in section 2: (1) ion source, (2) collision chamber wherein the proton neutral (i.e., N 2 ) gas collisions occur orthoganally in a crossed beams geometry, and (3) UV spectrometer with a detector observing the fluorescence on the third perpendicular axis. We present the calibrated spectral intensities in the FUV as a function of proton energy to determine emission cross sections in section 3. We discuss the application of the results to the aurora and summarize current 2of21
3 Figure 1. The experimental system comprising a Coultron ion beam source, an electrostatic Kimball electron gun (EG5), and a collision chamber designed to cross the proton (or electron) beam with a beam of N 2 from a capillary array source. The projectile (hydrogen ions or electron) beams and the input axis of the UV spectrometer are all perpendicular to the N 2 gas beam. The ion beam is at 90 to the spectrometer axis and at 36 to the electron gun axis. knowledge of H + +N 2 emission cross sections over the energy range of kev in section 4. Finally, we summarize in section Experiment [9] We have built a state of the art crossed beams apparatus that can be used for systematic emission studies of either proton atom/molecule or electron atom/molecule collisions. A schematic of the present experimental arrangement of the crossed beams apparatus is shown in Figure 1. The apparatus mainly consists of two high vacuum chambers, a high energy resolution electron source, a Colutron ion source, an Acton 0.2m UV spectrometer, two Faraday cups (one for electron beam and the other for ion beam current measurement), and a capillary array to flow in target gas beam. The capillary array of N 2 source, comprising a bundle of holes 8 ± 3 microns in diameter with an aspect ratio of 6 10:1, was manufactured by Lenox Laser Corporation. The main vacuum chamber (0.35 m diam 0.25 m height) is pumped by a combination of Varian V 3KT 2000 l/s turbo molecular pump and dual stage 24 ft 3 /m rotary vane pump (Varian SD 700), and Colutron ion source chamber is pumped separately with a 500 l/s Varian 550 turbo molecular pump backed by an oil sealed mechanical pump. A base pressure of torr in the main chamber and torr in the ion source chamber is attained within a couple days of pumping. Since the mass selection aperture at the end of the ion source separates the two vacuum chambers (see section 2.1), differential pumping of ion chamber is required to maintain a vacuum of better than torr in the ion source region while flowing the target gas into the main collision chamber ( torr). Measurements of the fluorescence induced by ion or electron molecule collisions are controlled by a personal computer (PC) executing custom software developed in our laboratory (see section 2.2) The Ion Source [10] The Colutron ion source, which can be baked up to 200 C, consists of a DC discharge cell, heat sink, an ion acceleration electrode, an Einzel lens for focusing the ion beam, a E B velocity filter for selection of a desired projectile ion (e.g., H + or H + 2 or H + 3 ), a set of beam steering electrodes, a beam transporting cylindrical electrode with a rectangular exit aperture at its end, and finally a decelerator that allows provision for ion beam energies from 10 ev to 500 ev. The gas discharge cell contains a tungsten filament and an anode with a 20 microns aperture for ion extraction, which are encapsulated in a quartz minichamber with an inlet for H 2 gas to flow into the cell through a variable leak valve. The velocity filter consists of a magnet, a pair of electrostatic deflection plates, and a set of shims. The electric field plates are mounted between the magnet poles to produce a uniform electric field (E) perpendicular to the magnetic field (B) to achieve a desired E B configuration. The shims are wired separately so that each one s voltage is 3of21
4 adjusted independently enabling the electric field in the filter so as to achieve cylindrical symmetry for optimum collimation of the proton beam. The magnetic field is provided by two electromagnets with combined maximum field of 0.3 T. High magnetic and electric fields increases the dispersion of the ion beam and improves the resolution. In the hydrogen discharge, three types of ions, H +,H + 2,H + 3, coexist with different concentrations based on the pressure in the cell and electric field configuration. In our measurements, we found their fluxes in the sequence of H + 2 >H + 3 >H +, based on total Faraday cup current. As long as the dissociation degree is limited, the H + 2 ion production in the discharge cell is consistently higher than of the other two ions, and the formation of H + 3 occurs mostly due to exothermic nonresonant charge exchange reaction by H + 2 +H 2 H H. For a chosen ion kinetic energy E i, the accelerated ion beam contains all three ions but with different velocities as defined by v =(2qE i /M) 1/2, where M is the ion mass with charge q, velocity ratios H + /H + n of 1.0, 1.41, 1.73 for H + n =H +,H + 2, and H + 3, respectively. The velocity filter acts as a mass filter for ions with equal kinetic energy. We observed that the focusing property of the velocity filter has very little effect with the change in magnet current as compared to the change in electric field. Beam characterization was done by keeping the electric field of the velocity filter constant and scanning the magnet current. Voltages on a set of beam focusing and beam steering electrostatic lenses placed before the velocity filter were adjusted to maximize the ion beam transmission through the ion beam transporting 8 long cylindrical electrode (grounded guard tube), and focus onto a scintillating beam collector screen placed at the entrance of the Faraday cup. We observed three beam spots of H +,H + 2,H + 3 ions at a distance of about 10 mm apart from each other on the screen. After optimum tuning of the ion gun electrical and magnetic settings for maximum beam currents of well separated hydrogen ions, we designed a suitable mass selection aperture (3 mm width 10 mm height) and placed it at the end of the cylindrical electrode just before the ion molecule interaction region. The beam spot of about 3 mm for each ion was obtained after dispersion. The present size of the mass selection aperture is sufficient to block the paths of all other unwanted ions that are deflected off axis by the velocity filter. The ion beam current is measured with a programmable Keithley Source Measure Unit (Model 2612A) controlled by data acquisition software (see section 2.2). It also provides the bias voltages of the Faraday cup and secondary electron suppressor necessary to ensure that the primary beam current is fully accounted and no secondary electrons or ions from Faraday cup enter into the interaction region. Without the decelerator section of the ion source, the projectile ion energy can be varied from 0.1 to 10 kev with beam currents of 0.2 to 10 ma depending on the ion selection and its energy The Data Acquisition System [11] Experimental data collection system is managed by a PC running custom software developed using National Instruments programming environment, which controls and monitors important experimental parameters and performs fluorescence data acquisition. The data acquisition system (DAS) performs repetitive wavelength scans to minimize systematic errors associated with long term drifts in the experimental conditions. [12] Wavelength positions were changed in small increments by rotating the spectrometer s diffraction grating driven by a stepper motor with a microstepping controller/ driver (SilverMax, QuickSilver Controls, Inc.) directly controlled by the data acquisition software. The accuracy of the positioning (better than 0.02 Å) significantly exceeded the requirements following from the selected spectral resolution of the measurements. At each preselected stationary wavelength position, the fluorescence photon signal from the ion or electron interaction with the target molecule is recorded by counting pulses produced by photon detector with a digital counter for a fixed dwell time. The photon counter and the accurate gating dwell timer (50 ns resolution) are formed of counters, a time base generator and digital outputs of a National Instruments PCI 6602 Counter/Timer board (with use of simple external fast logic gates). [13] DAS also monitors and records data on the most crucial experimental parameters such as the background pressure in the collision chamber and the proton beam current collected with the two element Faraday Cup. The chamber pressure is related to the number flux of target molecules effusing from the capillary array in the crossed beams mode and to the target gas number density in the swarm mode. Recording of the chamber pressure facilitates accounting for the variation of the density of target molecules in the region of interaction in given experimental conditions. A Bayard Alpert ion gauge controlled by a Varian XGS 600 unit is used to monitor the pressure. Similarly, recorded instantaneous values of the ion beam current make possible accounting for its fluctuations at the stage of data analysis. The emission spectra accumulated over a number of scans are corrected for long term drifts, background signal contribution, and intensity calibration of the spectrometer (see section 2.3) at the stage of data analysis The Optical System UV Calibration [14] For calibration of the crossed beams apparatus and UV spectrometer, a six element electrostatic electron gun of Kimball Physics Model 5A is used. The performance of the present high energy resolution electron gun was described by James et al. [1997]. In the present crossed beams experimental arrangement, an energy selected electron beam collides orthogonally with a target gas beam of molecules effusing through the capillary array into the collision chamber at a constant flow rate controlled by a Varian variable leak valve. With gas flow into the collision region, the main chamber pressure raised 2 orders of magnitude from 10 7 to 10 5 torr and the ion source chamber pressure by 3 orders from 10 9 to 10 6 torr. The background N 2 gas pressure was maintained at torr during the measurements. We have measured the low resolution (4Å FWHM) UV electron induced fluorescence spectra under nearly optically thin conditions using a 0.2 m Acton VM 502 UV spectrometer coupled to the collision chamber. The spectrometer is capable of operating at resolving power of l/dl = 200 at 1000 Å with a variety of detectors from the extreme ultraviolet (EUV) to the near IR ( nm). The single channel detector is a Hamamatsu pulse counting R6836P photomultiplier tube with magnesium fluoride window and mm photocathode of cesium telluride for wavelengths from 1150 to 3200 Å with less than 5c/s dark noise counts. The holographic grating used with this spectrometer is a MgF 2 concave 1200 grooves/mm grating 4of21
5 Figure 2. The sensitivity of the spectrometer and photomultiplier optical system as a function of wavelength obtained as described by Liu et al. [1995]. with a horizontal aperture ratio of f/4.5. The fast optical system results in a wide field of view of the collision region ( 10 deg square). The UV photon signal is detected by the spectrometer with an optic axis at 90 to the plane defined by the crossed ion or electron beam and N 2 target molecular beam. The interaction volume is approximately 2 mm 3. The relative inverse sensitivity calibration data was obtained by measuring the intensities of emissions from electron impact excitation of H 2 in the 1100 to 1700 Å range, and comparing them to the modeled H 2 intensities [Liu et al., 1995; Jonin et al., 2000; Ajello et al., 2005]. The resulting calibration sensitivity and inverse sensitivity data is shown in Figure 2. Peak sensitivity occurs near 1200 Å, the blaze wavelength for the MgF 2 concave 1200 grooves/mm grating. The curve was smoothly fitted by a cubic spline to the measured calibration points to correct the measured spectra. The calibration curve so obtained was applied in correcting the measured fluorescence spectrum from ion molecule collisions. [15] There are two main spatial regions within the collision chamber that are responsible for exciting the H + N 2 FUV spectrum. We show in Figure 3 a typical set of three calibrated spectra at 5 kev proton energy that accompanied each cross section measurement at the five energies studied: 1, 2, 3, 5 and 7 kev. We measure in the order: (1) a background (shown in green in Figure 3), (2) a swarm spectrum in black (background subtracted), (3) a crossedbeams spectrum (X beam) in red (background subtracted), and (4) a dot spectrum that is the difference between the cross beam and swarm spectra. The ratio of the photon signals at H Ly a (and the LBH bands) in crossed beams and swarm modes is 6, indicating that about 83% of the signal in the crossed beams mode arises from the interaction region and 17% from the background N 2 gas. The relative crossed beams and dot spectra are identical. The improvement in signal to noise ratio (S/N) with a capillary array is dramatic. The axial length of the proton beam within the optical field of view of the UV spectrometer is 3 cm on each side of the capillary array and the exit aperture distance from the guard tube to the capillary is 5 cm. Within this path length and with the low N 2 gas number densities (number densities < cm 3 ) present in both chambers we expect insignificant conversion of the proton beam to a fast H beam by electron capture collisions Emission for H +,H + + 2, and H 3 Impact on N 2 [16] As discussed in sections 2.2 and 2.3, we measured the fluorescence UV emissions of N 2 induced by H + impact at ion energies of 1 7 kev and by H + 2, and H + 3 at 5 kev in the crossed beams collision experimental geometry. The crossed beam emission spectrum was recorded by scanning the grating from 1100 to 1600 Å at a spectral resolution of 4.0 Å FWHM for the present experiment. The wavelength increment was 0.8 Å, and the integration time for each data point was 5 s per scan, and the data was accumulated over a number of scans, typically 20, for each wavelength range. The signal was found to be linear with N 2 background gas pressure over the range of torr. The polarization of the radiation is negligible for the N 2 transitions at 1 10 kev excitation energy. [17] When the hydrogen discharge cell was in operation, the background gas pressure in the Colutron chamber increased from 10 8 torr (with an N 2 gas load in the main chamber) to 10 6 torr due to neutral hydrogen gas leaking out through the 20 micron aperture on the anode. Since only the mass selection aperture mounted at the end of the long cylindrical ion beam transporting tube separates the two vacuum chambers, neutral hydrogen gas, which leaks out 5of21
6 Figure 3. The data set comparison of three calibrated laboratory spectra from three modes of operation: (1) background spectrum in green with the Colutron gas pressure of torr and a collision chamber gas pressure of torr from H 2, consisting of a single emission from H Ly a; (2) a swarm gas spectrum in black at torr; (3) a crossed beams (X beam) spectrum in red at torr; and (4) a dot spectrum in blue that is the difference between the crossed beams spectrum and swarm spectrum and represents the emission from the interaction volume only. Each spectrum was measured at 1.5 ma Faraday cup proton current and a Colutron gas pressure of torr. The crossed beams spectrum and swarm spectrum were each modified by the subtraction of the background spectrum in green. through the anode aperture, can easily flow into the main chamber. This results in weak H Ly a background emission from ion + H 2 collisions as shown in Figure 3, which contaminates the emission from ion + N 2 collisions. In order to subtract the background contribution from H and H 2 emissions to the N 2 emission spectrum, we measured the emissions due to ion collisions with background H 2 in the main chamber for all three H +,H 2 +, and H 3 + projectile ions in the same wavelength range without flowing N 2 into the main chamber. We found no evidence for Lyman and Werner bands above the background level. We observed that the background contribution to the total signal comes only from H Ly a emission due to ion + H 2 collisions, and we subtracted the background signal from the N 2 spectrum as discussed in sections 2.2 and 2.3. [18] We show in Figure 4 a comparison of the proton impact spectra of N 2 in the swarm and crossed beams mode. The calibrated spectra of e,h +,H 2 +, and H 3 + impact on N 2 are shown in Figures 5, 6, 7, and 8. We show in Figure 5 the proton induced fluorescence spectra of N 2 at 7 kev with identification numbers for each feature. We compare electron impact and proton impact crossed beams fluorescence emission spectra in Figure 6. Finally in Figure 7 we show emission spectra from H +, H + 2, and H + 3 projectile impact on N 2 ; and in Figure 8 the emission cross sections of the LBH band system, the NI multiplets and H Ly a as a function of proton energy from 1 to 100 kev for this work and other published results The LBH Glow Correction [19] Figure 4 shows the emission spectra of H + (5 kev) + N 2 FUV observed in the crossed beams and swarm modes. The latter thermodynamic state of the N 2 gas arises inside the chamber for a uniformly filled gas chamber at 300 K. Ideally, an infinitely long proton beam in a uniformly filled chamber will produce a cylindrically uniform glow region, where the emission and excitation rates per unit length of the beam are equal. On the other hand the small interaction volume observed in the crossed beams mode produces a nearly spherically symmetric glow pattern. The two proton impact fluorescence spectra shown in Figure 4 are nearly identical and are an important result in correcting for drift of excited a 1 P g state N 2 molecules out of the 10 wide field of view (FOV), since the cylindrically symmetric glow pattern can be calculated analytically for a given lifetime [Ajello, 1970]. The identification of the FUV 6of21
7 Figure 4. A comparison of 5 kev proton impact induced fluorescence spectra in swarm and crossedbeams mode. spectral features are discussed in section 3. Note that the ratio of H Ly a (peak intensity and integrated) to the LBH bands are the same. The difference in integrated intensities between the LBH bands and the N I multiplets is 12%. [20] One of the complicating factors for the shape of the glow profile about a cylindrically symmetric (swarm mode) is the long lifetime of the a 1 P g state, which leads to significant drift of the excited molecule away from the original point of interaction. The a 1 P g state is metastable and decays to the ground gerade state (X 1 S g + ) via electric quadrupole and magnetic dipole transitions (v = 0 through 6 or predissociates for v > 6). When viewing on center toward Figure 5. A 7 kev proton impact induced FUV fluorescence spectrum with 25 identified features listed in Table 1 with cross sections. The N 2 background gas pressure was torr, Faraday current was 1.5 ma, and discharge pressure was 300 m torr. 7of21
8 Figure 6. A comparison of a 5 kev proton impact induced fluorescence spectrum with a 100 ev electronimpact induced fluorescence spectrum measured, using the same experimental setup. the optic axis directly at the proton beam the long lifetime of the N 2 (a 1 P g ) state resulted in a significant portion of these excited species will drift out of the FOV of the detector before radiating. The minimum ray height of the glow at the edge of the 11 vertical field of view is 2.8 cm radial from the proton beam; and the minimum ray height of the glow at the edge of the 13 horizontal field of view is 3.1 cm radial from the proton beam. The loss of excited a 1 P g metastable molecules from the field of view needs to be quantified to provide absolute emission cross sections in this experiment. Absolute electron impact emission cross section studies for metastable species rely on a LBH emission glow model to extrapolate signal outside the FOV. Two metastable species have been studied by this method in our laboratory: the LBH band system (AS85) from direct excitation of N 2 and the nm atomic O 5 S emission [Kanik et al., 2003] from dissociative excitation of O 2. The lifetime of the a 1 P g state has been, until now, uncertain, with experiments and theory reporting values that typically range from 54 ms to 170 ms. The uncertainty in lifetime combined can lead to considerable uncertainty in the absolute emission cross section using the glow model. For example, for the proton apparatus used in this experiment the percentage of LBH glow observed could potentially vary between 51 to 84% for the range of lifetimes from 55 to 170 ms. [21] We have recently remeasured the glow profile in a separate experiment to be described in a future publication (J. M. Ajello, private communication, 2010) at the University of Colorado in the same apparatus that measured the 1356 Å glow profile of the O 5 S state from dissociative excitation of O 2 [Kanik et al., 2003]. The lifetime was found to be 55 ms within 30% in agreement with the work of Marinelli et al. [1988]. We use that result to quantify the correction to the crossed beams spectra studied here by correcting the observed intensities by a factor of 1.16 to account for emissions outside of the field of view. We measured all spectra in the crossed beams mode and utilized the same correction factor. The crossed beams mode is preferential because of the approximate 6:1 improvement in signal to noise ratio (S/N) over the swarm mode. 3. Results and Discussion 3.1. Proton Emission s [22] We have studied at low spectral resolution the proton impact induced fluorescence spectrum of N 2 in order to provide emission cross sections needed to model proton aurora. The principal ion molecule processes we have studied for emissions are: H þ ð1 7 kev H þ ð1 7 kev ÞþN 2! H þ þ N 2 * a 1 P g! H þ þ N 2 þ h LBH ; by excitation ð1þ ÞþN 2! H þ þ N 2 * ðrepulsiveþ! H þ þ N þ N þ h ; by excitation ð2þ 1200;1493A...: H þ ð1 7 kevþþn 2! H o* þ N þ 2! H o þ N þ 2 þ h Ly ; by electron capture: [23] To obtain integrated emission cross sections for each atomic and molecular feature, we have measured laboratory spectra at five energies: 1, 2, 3, 5 and 7 kev. We show in Figure 5 the calibrated spectrum at 7 kev proton impact energy over the wavelength range of Å. There are 25 identified features numbered from 1 through 25. We show in Figure 6 for comparison a 100 ev electron impact induced ð3þ 8of21
9 Figure 7. An overview of H +,H + 2, and H + 3 FUV fluorescence spectrum with 25 identified features listed in Table 2 with emission cross sections. 9of21
10 Figure 8. The FUV emission cross sections of N 2 by H + impact from 1 to 100 kev: JPL from 1 to 7 kev and published kev of the H Ly a; N I multiplets at 1200, 1243, and 1493 Å; and the N 2 (a 1 P g X 1 S + g ) LBH band system which extends from 1262 to 2600 Å on a log log plot. The published data references can be found by Avakyan et al. [1998]. fluorescence spectrum from 1100 to 1600 Å with a 5 kev proton impact fluorescence spectrum measured from the same instrument. The two sets of features are identical except for the H Ly a feature in the proton spectrum at Å. All other features in both spectra are the prominent members of the LBH band system and N I multiplets. [24] The features in Figure 5 are identified in Table 1 along with the proton emission cross sections for 1 7 kev normalized to the value of the H Ly a (2p 1s) emission cross section found in the review by Avakyan et al. [1998], following a small needed correction to the measured intensity from cascade loss described below (similar to LBH bands already discussed). Atoms in the metastable 2s state are deactivated at the walls after they pass through the observation region. The review work of Avakyan et al. [1998] is key to providing the absolute cross sections of this experimental study. The recommended cross sections of Avakyan et al., include cascade, and represent a true emission cross section, s em = s ex + s cascade. The Avakyan et al. recommended H Ly a proton emission cross sections include direct excitation of the 2p state and cascade from higher lying ns, nd states (Balmer series emitting states with long lifetimes > 15 ns for cascade excitation versus a short lifetime of 1.5 ns for direct excitation). The distribution of H Ly a emission cross section data points in the review by Avakyan et al. summarizing the results of the various sets of experimenters indicates an uncertainty of about 15% for s em. [25] The recommended cross sections used in the review by Avakyan et al. [1998] have been synthesized from several authors and included corrections. For example, the H Ly a proton emission cross sections for absolute calibration of the emission cross section of the features in the 1 7 kev spectra were measured by two sets of authors: Van Zyl and Neumann [1988], who studied low energy collisions of H + with N 2, and by Birely and McNeal [1971]. Higher energy emission proton cross sections beyond 10 kev are provided by Dahlberg et al. [1967] for the Avakyan et al. review. [26] Van Zyl and Neumann [1988] calculated H + +N 2 cascade cross sections, s cascade, contributions from ns and nd states to be nearly constant at about 20 25% of s em from 1 to 10 kev. Their earlier measurements of Ha and Hb were the basis for separating the three separate Balmer series components [Van Zyl and Neumann, 1980]. The H Ly a cascade and emission cross section values are both given in Table 1 for 1 7 kev. The uncertainties in this cascade calculation are estimated to be [Van Zyl and Gealy, 1987] a combination of about 15% in Ha and Hb cross sections and another 10% in determining the separate ns,np 2s,2p emission components from models. On the basis of the work of Van Zyl and coworkers we can conclude that cascade loss does not contribute significantly to the observed H Ly a intensity in this proton induced fluorescence experiment over the path length of 2.8 cm (3.1 cm) (20 cm) from the vertical (horizontal) (along optic axis) interaction region to the edge of the field of view(s) or along the optic axis and can be corrected to a first approximation. In this experiment there is a correction of 10% to the measured H Ly a intensity owing to the long lifetime of the H(ns, n > 2) states (lifetime > 150 ns) produced by the process of electron capture in equation (3), most of the H Ly a photons from the ns 2p 1s Balmer series cascade excitation will escape from the 5.6 cm (6.2) square FOV and 20 cm length along 10 of 21
11 Table 1. The Proton Impact Induced Fluorescence Spectrum Identifications and Emission s of N2 From 1150 to 1600 Å Feature Number Start (Å) Peak (Å) Peak Theory/ Observed (Å) End (Å) Wavelength Identification a 1 kev 2 kev 3 kev 5 kev 7 kev NI( 2 D o 2 D NI( 2 P o 2,4 P) NI( 2 D o 4 P) NI( 2 D o 2 P) NI( 2 D o 4 F) NI( 2 D o 4 P, 2 F) NI( 2 D o 2 P) NI( 2 D o 2 P) NI( 2 D o 2 P) NI(g 4 S o 4 P) NI(g 4 S o 4 P) NI( 2 P o 2,4 P, 2,4 F) NI(g 4 S o 4 P) NI(g 4 S o 4 P) NI(g 4 S o 4 P) H Ly a 186.0/40 b 416.0/88 b 498.0/101 b 535.0/110 b 537/120 b NI( 2 P o 2 D) NI( 2 P o 2 D) NI( 2 P o 2 D) NI( 2 P o 2 P) NI( 2 P o 2 P) NI( 2 P o 2 P) NI( 2 D o 2 D) a(6,0) Total Total Total Total Total NII( 3 D o 3 P) b (1,12) LBH LBH LBH LBH LBH c 4(0,12) Non LBH Non LBH Non LBH Non LBH Non LBH NI,II Total Total Total Total Total NI,II b(1,11) LBH LBH LBH LBH LBH a(5,0) Non LBH Non LBH Non LBH Non LBH Non LBH of 21
12 Table 1. (continued) Feature Number Start (Å) Peak (Å) Peak Theory/ Observed (Å) End (Å) Wavelength Identification a 1 kev 2 kev 3 kev 5 kev 7 kev b (1,12) Total Total Total Total Total c 4(0,13) NI( 2 P o 2 D) LBH LBH LBH LBH LBH NI( 2 P o 2 D) a(6,1) Non LBH Non LBH Non LBH Non LBH Non LBH a(4,0) Total Total Total Total Total b(1,12) NI( 2 P o 2 P) LBH LBH LBH LBH LBH NI( 2 P o 2 P) Non LBH Non LBH Non LBH Non LBH Non LBH a(5,1) Total Total Total Total Total NI,II LBH LBH LBH LBH LBH Non LBH Non LBH Non LBH Non LBH Non LBH a(6,2) a(3,0) a(5,2) a(2,0) a(6,3) a(3,1) NI( 2 P o 2 D) Total Total Total Total Total a(4,2) a(1,0) LBH LBH LBH LBH LBH Non LBH Non LBH Non LBH Non LBH Non LBH a(5,3) a(2,1) a(6,4) a(3,2) 1450 a(0,0) a(4,3) a(1,1) 12 of 21
13 Table 1. (continued) Feature Number Start (Å) Peak (Å) Peak Theory/ Observed (Å) End (Å) Wavelength Identification a 1 kev 2 kev 3 kev 5 kev 7 kev a(5,4) a(2,2) a(6,5) Total Total Total Total Total 1493 a(3,3) NI( 2 D o 2 P) LBH LBH LBH LBH LBH 1501 a(0,1) Non LBH Non LBH Non LBH Non LBH Non LBH a(4,4) a(1,2) a(5,5) a(2,3) a(0,2) a(4,5) a(1,3) a(5,6) 1585 a(2,4) 1592 a(6,7) Totals LBH emission cross section (37.6% of band system) Totals LBH emission cross section (full band system) Total non LBH emission cross section cm cm cm cm cm cm cm cm cm cm cm cm cm cm cm 2 a NIST atomic wavelength tables, Ajello and Shemansky [1985], and J. M. Ajello (private communication, 2010). b HLya cascade cross section from Van Zyl and Neumann [1988]. 13 of 21
14 the optic axis in our experiment [Van Zyl and Gealy, 1987]. We have calculated the escape loss of cascade producing 3s, 4s and 5s atoms from our FOV for 1 7 kev. For 3s atoms moving perpendicular to the optic axis of the spectrometer we find that for the 3s state the maximum percentage of Balmer a cascade observed occurs at 1 kev with 25% at 1 kev and dropping to 10% at 7 kev. For the cascade production by 4s and 5s states the percentage observed drops to less than 10% at all energies. In contrast, only a small amount of the nd 2p 1s Balmer series will escape for the nd series (n 3). The nd states have a shorter lifetime of about a factor of 10 for the same n value (e.g., 15 ns for the H(3d) state). We find that all the cascade emission from 3d (Ha) is observed at 1 kev and nearly 65% of the cascade emission from 5d (Hg) at 1 kev. Dawson and Loyd [1977] and Loyd and Dawson [1975] have shown the ratio of excitation cross sections for electron capture (cross section ratio of 3s/3d states) described by equation (3) to vary from 0.8 to 1.4 (near unity to a first approximation) over the proton energy range of kev. Outside of the range of 1 10 kev Hughes et al. [1970] have found the 3s,3p,3d excitation cross section ratios from 5 to 200 kev to vary over a much larger range. The nd series members for 3 n 6 contribute approximately half the total cascade cross section HLya as determined by Van Zyl and Neumann [1980, 1988] and are mainly observable in this experiment [Van Zyl et al., 1989]. We correct in approximate fashion for the drift loss of ns and nd atoms by assuming all the nd atoms are observed and all the ns atoms are lost. For example the H(3d 2p), cascade transition has an e folding distance to radiate Balmer a, of less than 3 cm for fast H atoms of the range of 1 7 kev. [27] The feature identifications are partly based on highresolution FUV spectra from e + N 2 collisions in the wavelength range of Å (J. M. Ajello, private communication, 2010) and partly based on AS85 for the wavelength range of Å. The relative intensities of the LBH bands (v, v ) are measured to be proportional to Franck Condon factors given by AS85. We observe 57% of the LBH band system from 1262 Å to 1600 Å. The proton emission cross section of the remaining portion of the band system is estimated as by AS85 using Franck Condon factors. The strongest features for each progression are feature 24, the (0,2) band (blended with the 4,5 band) at 1555 Å for the v = 0 progression, feature 19, the (1,1) band (blended with the (5,4) band) at 1464 Å for the v = 1 progression, feature 13, the (2,0) band (blended with the (6,2) band) at 1356 Å for the v = 2 progression, feature 14 the (3,0) band (blended with the (5,2) band) at 1383 Å for the v =3 progression and feature 11, the (4,0) band (blended with some N I multiplets and a b(1,12) band) at 1325 Å for the v = 4 progression. The peak proton impact induced emission cross section of the LBH band system ( Å) was measured in this experiment over the energy range of 1 7 kev to be 5.05 ± cm 2 at 7 kev. We compare this proton impact induced emission cross section to the peak electron impact induced emission cross section of the LBH band system. The electron impact induced emission cross section of the LBH band system is 6.3 ± cm 2 at 100 ev given by Young et al. [2010] is used to normalize cross sections of other energies. The peak electron impact induced emission cross section, based on the measured relative electron excitation function by Young et al. [2010], occurs at 20 ev with an extrapolated value of 1.74 ± cm 2. A comparison of proton and electron impact induced emission cross sections indicates a greater efficiency for LBH emission production from protons than electrons at the respective peak particle energies by at least a factor of about three. [28] Direct excitation of the LBH band system results from the transition X 1 S + g a 1 P g. The overlapping of the nearby a X 1 S + g and w 1 D u vibronic levels, are coupled to the a 1 P g state via exceptionally weak dipole allowed infrared transitions, and can produce significant but slow cascade either radiatively or by collision induced electronic transitions (CIET) [Eastes and Dentamaro, 1996;Eastes, 2000a, 2000b]. The lifetime of the slow cascade states are greater than 1 ms and slow cascade is not observed in this experiment, since collisional deactivation of these longlived metastables occurs at the walls. Additionally, the fast cascade to the a 1 P g state results primarily from the c S u state [Shemansky et al., 1995; Filippelli et al., 1984] but its contribution has been shown to be less than 1%. Thus, neither fast nor slow cascade makes important contributions to the measured LBH proton impact induced emission cross sections (<5%). We have previously estimated cascade contribution to the electron impact emission cross section for slow cascade from a and w states to be significant with a ratio of slow cascade to direct excitation to be 0.3 at 20 ev [Ajello et al., 2010; Young et al., 2010] H + 2 and H + 3 Emission s [29] To our knowledge, the UV emission cross sections from collisions of H + 2 and H + 3 molecular ions with N 2 have never been studied before. A recent study in the visible of the Balmer series members Hb and Hg over the energy range of 3 10 kev has been performed by Lee and Lin [2002] using a similar Colutron source. For the H + 2 ion beam the collision processes with N 2 are described by the following: H þ 2 H þ 2 H þ 2 ð 1 7 kev ÞþN 2! H þ 2 þ N 2* a 1 P g! H þ 2 þ N 2 þ h LBH ; ð4þ H þ 2 ð 1 7 kev ÞþN 2! H þ þ H þ N 2 * a 1 P g! H þ ð5þ þ H þ N 2 þ h LBH ; H þ 2 H þ 2 ð 1 7 kev ÞþN 2! H þ 2 þ N 2* ðrepulsiveþ! H þ þ H þ N þ N þ h 120;149:3nm;...: ; ð 1 7 kev ÞþN 2! H þ þ H þ N 2 * ðrepulsiveþ! H þ þ H þ N þ N þ h 120;149:3nm;...: ; ð 1 7 kev ÞþN 2! H o* þ H þ N þ 2! Ho þ H þ N þ 2 þ h Ly ; ð 1 7 kev ÞþN 2! H o* þ H þ þ N 2! H o þ H þ þ N 2 þ h Ly ; where in equations (8) and (9) the N 2 + neutral or N 2 ion may further dissociate. [30] The emission cross sections of the process H 2 + and H 3 + +N 2 at 5 kev ion energy are listed in Table 2 along ð6þ ð7þ ð8þ ð9þ 14 of 21
15 with a comparison to the H + emission cross section also at 5 kev from Table 1. The emission spectrum is shown in Figure 7. The only previous experiment on H + 2 N 2 was performed by Van Zyl et al. [1964, 1967] who measured the H Ly a emission cross section for H + 2 N 2 collisions usinganiondrift tube technique and found values nearly identical to those in Table 2 for H + and H + 2. [31] It is interesting to compare the H Ly a cross sections for the three projectiles and note that the emission cross sections (s em ) at 5 kev vary in the order s em (H + 3 )> s em (H + 2 )>s em (H + ) in the same order as found by Lee and Lin [2002] for Ha and Hb. We find the H Ly a emission cross section ratios, for H + 3,H + 2,H + ions, to be 1.50:1.46:1, respectively, compared to the same order of 1.52 > 1.38 > 1 for Hb (4861 Å) [Lee and Lin, 2002]. On the other hand + we find the LBH cross sections to be largest for the H 2 projectile ion. Lee and Lin have done a thorough job in explaining a theoretical understanding of the intermediate molecular states involved in the collisional excitation and charge transfer processes responsible for the emissions. We have not yet studied other energies besides our reported 5 kev energy Emission s of N I Multiplets From 1 to 130 kev [32] We show in Figures 8 a semilog plot of the previously published work of collisions of H + with N 2 as reviewed by Avakyan et al. [1998] along with the results from this paper. The uncertainty of these values is about 20% for energies between 10 and 100 kev. The uncertainty of our work is the root sum square uncertainty of (1) 10% in the relative FUV calibration, (2) 20% uncertainty of the absolute calibration of H Ly a, (3) 5% uncertainty in the H cascade model contribution to drift loss outside of the field of view from long lived ns and nd states, (4) 10% uncertainty in the LBH swarm model accounting for drift loss of excited a 1 P g molecules outside of the field of view prior to radiating from the use of the 55ms lifetime, (5) 12% uncertainty in the use of a cross beam approximation to a swarm experiment model, (6) 5% uncertainty in Faraday cup current loss to grounded shield, (7) 3% uncertainty to pressure drift, and (8) 10% uncertainty in measured areas from blended feature overlap and S/N statistical uncertainty. The root sum square uncertainty of our reported LBH and N I emission cross sections is 30%. [33] The only work performed previously on the N I multiplets was from kev. This work was performed by Dahlberg et al. [1967]. In their review, Avakyan et al. [1998] renormalized the published data by newer cross sections for H Ly a from H + +N 2 collisions. The protonexcited H Ly a emission cross section has been measured over the energy range from 120 ev 120 kev by the combined work of Van Zyl and Neumann [1988] and Dahlberg et al. [1967]. Our results are the first for proton energies below 10 kev for the N I multiplets. This region (1 10 kev) contains the peak value of the LBH and H Ly a emission cross section whereas the N I multiplets have peak cross sections in the kev energy range. We now have for the first time a complete set of proton impact cross sections for the energy range of kev for the N I multiplets. We plan to extend the energy range of LBH emission cross sections in similar fashion to higher and lower energies to access both the threshold and Born energy regions. 4. The Auroral Application [34] Proton auroral models make use of three classes of cross sections, namely proton impact, H impact, and electron impact (the latter to address secondary electron impact excitation and ionization). A full set of primary (proton and H) impact cross sections for N 2,O 2, and O is needed to describe the degradation with altitude of these primary particles. The result is altitude profiles of particle fluxes versus energy (and pitch angle unless integrated over this variable) for proton, H, and secondary electrons. For models solving linear transport equations for these three particles [Basu et al., 1993; Strickland et al., 1993; Galand and Lummerzheim, 2004] (the latter joining together the models of Galand et al. [1997] and Lummerzheim and Lilensten [1994]), volume excitation and ionization rates follow from integrations of the product of appropriate cross sections with these fluxes. Monte Carlo models [e.g., Gérard et al., 2000, 2001; Solomon, 2001; Fang et al., 2004] obtain these rates by scoring events during the random walk process. It is thus clear that our new measurements, specific to a given FUV emission feature, help quantify part, but not all emissions. The secondary electron component, in general, does not lack the needed cross section for its quantification. The H atom component, on the other hand, is usually the most uncertain among the three components due to a general lack of laboratory cross section measurements that are more difficult to perform than those for proton impact. This is the case for LBH that led to the need for some type of cross section specification in the work reported by Strickland et al. [1993]. As reported in that paper, an estimate was provided by B. Van Zyl (private communication, 1989). Another key feature lacking H impact measurements is OI 1356 Å (impact on O being more important than on O 2 ). In this case, proton impact on O is unimportant due to the transition being spin forbidden. While we have no current plans for H impact measurements, plans are being formulated for extending current work to energies into the Born region for proton impact on N 2,O 2 and O. [35] For remote sensing applications, the value of the new cross sections being reported is twofold: first to compare with existing representations in models (e.g., with the proton impact LBH cross section reported by Strickland et al. [1993]) and second to provide cross sections previously unspecified. Key features that this pertains to are NI 1200 Å and NI 1493 Å that may comprise portions of instrument bands containing Ly a and LBH. Such bands can be found on SSUSI and GUVI in their imaging modes and in the case of LBH, applies to the band designated as LBHS (S is for short) whose band limits are 1400 Å and 1530 Å. Still lacking are H impact cross sections for these features that, as a first approximation, can be set equal to their proton counterparts. Another approach is to allow the relative strength between H and proton cross sections to reflect that of a feature where both components have previously been specified (e.g., for LBH as discussed above). In the near future, simple scaling techniques such as these can be replaced with anticipated H impact cross sections from extension of our current work. 15 of 21
Contribution of proton precipitation to space-based auroral FUV observations
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003ja010321, 2004 Contribution of proton precipitation to space-based auroral FUV observations M. Galand Center for Space Physics, Boston University,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003ja010321, 2004 Contribution of proton precipitation to space-based auroral FUV observations M. Galand Center for Space Physics, Boston University,
Optical Emissions from Proton Aurora
 Sodankylä Geophysical Observatory Publications (2003) 92:1 5 Optical Emissions from Proton Aurora D. Lummerzheim 1, M. Galand 2, and M. Kubota 3 1 Geophysical Institute, University of Alaska, Fairbanks,
Sodankylä Geophysical Observatory Publications (2003) 92:1 5 Optical Emissions from Proton Aurora D. Lummerzheim 1, M. Galand 2, and M. Kubota 3 1 Geophysical Institute, University of Alaska, Fairbanks,
Assessment of the Azimuthal Homogeneity of the Neutral Gas in a Hall Effect Thruster using Electron Beam Fluorescence
 Assessment of the Azimuthal Homogeneity of the Neutral Gas in a Hall Effect Thruster using Electron Beam Fluorescence IEPC-2015-91059 / ISTS-2015-b-91059 Presented at Joint Conference of 30th International
Assessment of the Azimuthal Homogeneity of the Neutral Gas in a Hall Effect Thruster using Electron Beam Fluorescence IEPC-2015-91059 / ISTS-2015-b-91059 Presented at Joint Conference of 30th International
Calibration of Particle Instruments in Space Physics
 SR-007 September 2007 Calibration of Particle Instruments in Space Physics Editors Martin Wüest INFICON Ltd, Balzers, Principality of Liechtenstein David S. Evans Space Environment Center, NOAA, Boulder
SR-007 September 2007 Calibration of Particle Instruments in Space Physics Editors Martin Wüest INFICON Ltd, Balzers, Principality of Liechtenstein David S. Evans Space Environment Center, NOAA, Boulder
Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
 ESS The RGA freenotes Theory page 1 of 14 RGA Theory Notes Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
ESS The RGA freenotes Theory page 1 of 14 RGA Theory Notes Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
AURORA: GLOBAL FEATURES
 AURORA: GLOBAL FEATURES Jean-Claude Gérard LPAP Université de Liège OUTLINE - collisional processes involved in the aurora - remote sensing of auroral electron energy - Jupiter - Saturn MOP meeting - 2011
AURORA: GLOBAL FEATURES Jean-Claude Gérard LPAP Université de Liège OUTLINE - collisional processes involved in the aurora - remote sensing of auroral electron energy - Jupiter - Saturn MOP meeting - 2011
Visualization of Xe and Sn Atoms Generated from Laser-Produced Plasma for EUV Light Source
 3rd International EUVL Symposium NOVEMBER 1-4, 2004 Miyazaki, Japan Visualization of Xe and Sn Atoms Generated from Laser-Produced Plasma for EUV Light Source H. Tanaka, A. Matsumoto, K. Akinaga, A. Takahashi
3rd International EUVL Symposium NOVEMBER 1-4, 2004 Miyazaki, Japan Visualization of Xe and Sn Atoms Generated from Laser-Produced Plasma for EUV Light Source H. Tanaka, A. Matsumoto, K. Akinaga, A. Takahashi
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Application Note GA-301E. MBMS for Preformed Ions. Extrel CMS, 575 Epsilon Drive, Pittsburgh, PA I. SAMPLING A CHEMICAL SOUP
 Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Spectroscopy for planetary upper atmospheres きょくたん
 Spectroscopy for planetary upper atmospheres きょくたん Spectrum of Venus atmosphere Spectrum of Jupiter and Io Figure 1. An EUV spectrum measured by Hisaki spacecraft. The spectrograph mixes spatial and spectral
Spectroscopy for planetary upper atmospheres きょくたん Spectrum of Venus atmosphere Spectrum of Jupiter and Io Figure 1. An EUV spectrum measured by Hisaki spacecraft. The spectrograph mixes spatial and spectral
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997
 The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
In-Situ vs. Remote Sensing
 In-Situ vs. Remote Sensing J. L. Burch Southwest Research Institute San Antonio, TX USA Forum on the Future of Magnetospheric Research International Space Science Institute Bern, Switzerland March 24-25,
In-Situ vs. Remote Sensing J. L. Burch Southwest Research Institute San Antonio, TX USA Forum on the Future of Magnetospheric Research International Space Science Institute Bern, Switzerland March 24-25,
CHAPTER 3 The Experimental Basis of Quantum Theory
 CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
Lecture 0. NC State University
 Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Solar-terrestrial coupling evidenced by periodic behavior in geomagnetic indexes and the infrared energy budget of the thermosphere
 GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L05808, doi:10.1029/2007gl032620, 2008 Solar-terrestrial coupling evidenced by periodic behavior in geomagnetic indexes and the infrared energy budget of the thermosphere
GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L05808, doi:10.1029/2007gl032620, 2008 Solar-terrestrial coupling evidenced by periodic behavior in geomagnetic indexes and the infrared energy budget of the thermosphere
A Survey of Spacecraft Charging Events on the DMSP Spacecraft in LEO
 A Survey of Spacecraft Charging Events on the DMSP Spacecraft in LEO Phillip C. Anderson Space Science Applications Laboratory The Aerospace Corporation PO Box 92957 M2/260 Los Angeles, CA 90009-2957 ph:
A Survey of Spacecraft Charging Events on the DMSP Spacecraft in LEO Phillip C. Anderson Space Science Applications Laboratory The Aerospace Corporation PO Box 92957 M2/260 Los Angeles, CA 90009-2957 ph:
ICPMS Doherty Lecture 1
 ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
Joule heating and nitric oxide in the thermosphere, 2
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010ja015565, 2010 Joule heating and nitric oxide in the thermosphere, 2 Charles A. Barth 1 Received 14 April 2010; revised 24 June 2010; accepted
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010ja015565, 2010 Joule heating and nitric oxide in the thermosphere, 2 Charles A. Barth 1 Received 14 April 2010; revised 24 June 2010; accepted
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Sparks in Gases: Line Spectra
 Lecture 11 February 4, Chapter 3 The Particlelike Properties of Electromagnetic Radiation Sparks in Gases: Line Spectra This is one of the oldest tools available for the investigation of atoms and radiation.
Lecture 11 February 4, Chapter 3 The Particlelike Properties of Electromagnetic Radiation Sparks in Gases: Line Spectra This is one of the oldest tools available for the investigation of atoms and radiation.
Experimental Studies of Ion Beam Neutralization: Preliminary Results
 Experimental Studies of Ion Beam Neutralization: Preliminary Results N. Ding, J. Polansky, R. Downey and J. Wang Department of Astronautical Engineering University of Southern California Los Angeles, CA
Experimental Studies of Ion Beam Neutralization: Preliminary Results N. Ding, J. Polansky, R. Downey and J. Wang Department of Astronautical Engineering University of Southern California Los Angeles, CA
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
EXPERIMENT 5. The Franck-Hertz Experiment (Critical Potentials) Introduction
 EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
The Photoelectric Effect and the Quantization of Light
 The Photoelectric Effect and the Quantization of Light INTRODUCTION When a light with a sufficiently high frequency shines on a metal plate, electrons are ejected from the plate. This effect is known as
The Photoelectric Effect and the Quantization of Light INTRODUCTION When a light with a sufficiently high frequency shines on a metal plate, electrons are ejected from the plate. This effect is known as
Chapter 8 Geospace 1
 Chapter 8 Geospace 1 Previously Sources of the Earth's magnetic field. 2 Content Basic concepts The Sun and solar wind Near-Earth space About other planets 3 Basic concepts 4 Plasma The molecules of an
Chapter 8 Geospace 1 Previously Sources of the Earth's magnetic field. 2 Content Basic concepts The Sun and solar wind Near-Earth space About other planets 3 Basic concepts 4 Plasma The molecules of an
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Global auroral conductance distribution due to electron and proton precipitation from IMAGE-FUV observations
 Annales Geophysicae () : 9 SRef-ID: -7/ag/--9 European Geosciences Union Annales Geophysicae Global auroral conductance distribution due to electron and proton precipitation from IMAGE-FUV observations
Annales Geophysicae () : 9 SRef-ID: -7/ag/--9 European Geosciences Union Annales Geophysicae Global auroral conductance distribution due to electron and proton precipitation from IMAGE-FUV observations
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
and another with a peak frequency ω 2
 Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
High Resolution Optical Spectroscopy
 PHYS 3719 High Resolution Optical Spectroscopy Introduction This experiment will allow you to learn a specific optical technique with applications over a wide variety of phenomena. You will use a commercial
PHYS 3719 High Resolution Optical Spectroscopy Introduction This experiment will allow you to learn a specific optical technique with applications over a wide variety of phenomena. You will use a commercial
Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas
 Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas U Fantz 1,2, S Briefi 2, D Rauner 1,2 and D Wünderlich 1 1 Max-Planck-Institut für Plasmaphysik, Boltzmannstr. 2, 85748
Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas U Fantz 1,2, S Briefi 2, D Rauner 1,2 and D Wünderlich 1 1 Max-Planck-Institut für Plasmaphysik, Boltzmannstr. 2, 85748
vacuum analysis plasma diagnostics surface science gas analysis
 Hiden EQP Systems High Sensitivity Mass and Energy Analysers for Monitoring, Control and Characterisation of Ions, Neutrals and Radicals in Plasma. vacuum analysis surface science gas analysis plasma diagnostics
Hiden EQP Systems High Sensitivity Mass and Energy Analysers for Monitoring, Control and Characterisation of Ions, Neutrals and Radicals in Plasma. vacuum analysis surface science gas analysis plasma diagnostics
Experiment objectives: measure the ratio of Planck s constant to the electron charge h/e using the photoelectric effect.
 Chapter 1 Photoelectric Effect Experiment objectives: measure the ratio of Planck s constant to the electron charge h/e using the photoelectric effect. History The photoelectric effect and its understanding
Chapter 1 Photoelectric Effect Experiment objectives: measure the ratio of Planck s constant to the electron charge h/e using the photoelectric effect. History The photoelectric effect and its understanding
Neutron Transport Calculations Using Monte-Carlo Methods. Sean Lourette Fairport High School Advisor: Christian Stoeckl
 Neutron Transport Calculations Using Monte-Carlo Methods Sean Lourette Fairport High School Advisor: Christian Stoeckl Laboratory for Laser Energetics University of Rochester Summer High School Research
Neutron Transport Calculations Using Monte-Carlo Methods Sean Lourette Fairport High School Advisor: Christian Stoeckl Laboratory for Laser Energetics University of Rochester Summer High School Research
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Two-electron systems
 Two-electron systems Laboratory exercise for FYSC11 Instructor: Hampus Nilsson hampus.nilsson@astro.lu.se Lund Observatory Lund University September 12, 2016 Goal In this laboration we will make use of
Two-electron systems Laboratory exercise for FYSC11 Instructor: Hampus Nilsson hampus.nilsson@astro.lu.se Lund Observatory Lund University September 12, 2016 Goal In this laboration we will make use of
Extrel Application Note
 Extrel Application Note Real-Time Plasma Monitoring and Detection of Trace H 2 O and HF Species in an Argon Based Plasma Jian Wei, 575 Epsilon Drive, Pittsburgh, PA 15238. (Presented at the 191st Electrochemical
Extrel Application Note Real-Time Plasma Monitoring and Detection of Trace H 2 O and HF Species in an Argon Based Plasma Jian Wei, 575 Epsilon Drive, Pittsburgh, PA 15238. (Presented at the 191st Electrochemical
1P22/1P92 Exam Review Problems 2013 Friday, January 14, :03 AM. Chapter 20
 Exam Review Problems 2011 Page 1 1P22/1P92 Exam Review Problems 2013 Friday, January 14, 2011 10:03 AM Chapter 20 True or false? 1 It's impossible to place a charge on an insulator, because no current
Exam Review Problems 2011 Page 1 1P22/1P92 Exam Review Problems 2013 Friday, January 14, 2011 10:03 AM Chapter 20 True or false? 1 It's impossible to place a charge on an insulator, because no current
Titan s Atomic and Molecular Nitrogen Tori
 s Atomic and Molecular Nitrogen Tori H.T. Smith a, R.E. Johnson a, V.I. Shematovich b a Materials Science and Engineering, University of Virginia, Charlottesville, VA 9 USA b Institute of Astronomy, RAS,
s Atomic and Molecular Nitrogen Tori H.T. Smith a, R.E. Johnson a, V.I. Shematovich b a Materials Science and Engineering, University of Virginia, Charlottesville, VA 9 USA b Institute of Astronomy, RAS,
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
Overview: Astronomical Spectroscopy
 Overview: Astronomical Spectroscopy or How to Start Thinking Creatively about Measuring the Universe Basic Spectrograph Optics Objective Prism Spectrometers - AESoP Slit Spectrometers Spectrometers for
Overview: Astronomical Spectroscopy or How to Start Thinking Creatively about Measuring the Universe Basic Spectrograph Optics Objective Prism Spectrometers - AESoP Slit Spectrometers Spectrometers for
A simple electric thruster based on ion charge exchange
 A simple electric thruster based on ion charge exchange IEPC-2007-35 Presented at the 30 th International Electric Propulsion Conference, Florence, Italy Joe Khachan and Lachlan Blackhall University of
A simple electric thruster based on ion charge exchange IEPC-2007-35 Presented at the 30 th International Electric Propulsion Conference, Florence, Italy Joe Khachan and Lachlan Blackhall University of
INTRODUCTION. As shown in Figure 1a, the phenomena of lightningmesosphere-ionosphere
 ABSTRACT Intense, transient quasi-electrostatic (QE) fields, which exist above the thunderclouds following a positive cloud-to-ground lightning discharge, can produce an upward travelling runaway electron
ABSTRACT Intense, transient quasi-electrostatic (QE) fields, which exist above the thunderclouds following a positive cloud-to-ground lightning discharge, can produce an upward travelling runaway electron
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
PHYS General Physics II Lab The Balmer Series for Hydrogen Source. c = speed of light = 3 x 10 8 m/s
 PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
Photoelectric Effect
 Photoelectric Effect The ejection of electrons from a surface by the action of light striking that surface is called the photoelectric effect. In this experiment, as you investigate the photoelectric effect,
Photoelectric Effect The ejection of electrons from a surface by the action of light striking that surface is called the photoelectric effect. In this experiment, as you investigate the photoelectric effect,
A gas-filled calorimeter for high intensity beam environments
 Available online at www.sciencedirect.com Physics Procedia 37 (212 ) 364 371 TIPP 211 - Technology and Instrumentation in Particle Physics 211 A gas-filled calorimeter for high intensity beam environments
Available online at www.sciencedirect.com Physics Procedia 37 (212 ) 364 371 TIPP 211 - Technology and Instrumentation in Particle Physics 211 A gas-filled calorimeter for high intensity beam environments
-X 2 Σ g + Xianming Liu and Donald Shemansky. Space Environment Technologies. C.P. Malone, P. V. Johnson, J. M. Ajello, and I.
 Experimental and Theoretical Investigations of the Radiative Properties of N 2 Singlet-ungerade States for Modeling Cassini UVIS Observations of Titan the c 1 Σ u + -X 2 Σ g + Band System Xianming Liu
Experimental and Theoretical Investigations of the Radiative Properties of N 2 Singlet-ungerade States for Modeling Cassini UVIS Observations of Titan the c 1 Σ u + -X 2 Σ g + Band System Xianming Liu
Experiment 3 1. The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado
 Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
is the minimum stopping potential for which the current between the plates reduces to zero.
 Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy. Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy
 Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Scintillation Detector
 Scintillation Detector Introduction The detection of ionizing radiation by the scintillation light produced in certain materials is one of the oldest techniques on record. In Geiger and Marsden s famous
Scintillation Detector Introduction The detection of ionizing radiation by the scintillation light produced in certain materials is one of the oldest techniques on record. In Geiger and Marsden s famous
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
Proportional Counters
 Proportional Counters 3 1 Introduction 3 2 Before we can look at individual radiation processes, we need to understand how the radiation is detected: Non-imaging detectors Detectors capable of detecting
Proportional Counters 3 1 Introduction 3 2 Before we can look at individual radiation processes, we need to understand how the radiation is detected: Non-imaging detectors Detectors capable of detecting
Ionization Rates for from Solar Proton Events
 Ionization Rates for 1963-2005 from Solar Proton Events Charles H. Jackman E-mail: Charles.H.Jackman@nasa.gov Phone: 301-614-6053 Code 613.3 Laboratory for Atmospheres NASA Goddard Space Flight Center
Ionization Rates for 1963-2005 from Solar Proton Events Charles H. Jackman E-mail: Charles.H.Jackman@nasa.gov Phone: 301-614-6053 Code 613.3 Laboratory for Atmospheres NASA Goddard Space Flight Center
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
Atomic Spectra HISTORY AND THEORY
 Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
Study of Electron Energy and Angular Distributions and Calculations of X-ray, EUV Line Flux and Rise Times
 J. Astrophys. Astr. (1987) 8, 263 270 Study of Electron Energy and Angular Distributions and Calculations of X-ray, EUV Line Flux and Rise Times Ranjna Bakaya, Sunil Peshin, R. R. Rausaria & P. N. Khosa
J. Astrophys. Astr. (1987) 8, 263 270 Study of Electron Energy and Angular Distributions and Calculations of X-ray, EUV Line Flux and Rise Times Ranjna Bakaya, Sunil Peshin, R. R. Rausaria & P. N. Khosa
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Cassini observations of the thermal plasma in the vicinity of Saturn s main rings and the F and G rings
 GEOPHYSICAL RESEARCH LETTERS, VOL. 32, L14S04, doi:10.1029/2005gl022690, 2005 Cassini observations of the thermal plasma in the vicinity of Saturn s main rings and the F and G rings R. L. Tokar, 1 R. E.
GEOPHYSICAL RESEARCH LETTERS, VOL. 32, L14S04, doi:10.1029/2005gl022690, 2005 Cassini observations of the thermal plasma in the vicinity of Saturn s main rings and the F and G rings R. L. Tokar, 1 R. E.
Radionuclide Imaging MII Detection of Nuclear Emission
 Radionuclide Imaging MII 3073 Detection of Nuclear Emission Nuclear radiation detectors Detectors that are commonly used in nuclear medicine: 1. Gas-filled detectors 2. Scintillation detectors 3. Semiconductor
Radionuclide Imaging MII 3073 Detection of Nuclear Emission Nuclear radiation detectors Detectors that are commonly used in nuclear medicine: 1. Gas-filled detectors 2. Scintillation detectors 3. Semiconductor
Atomic Spectra. d sin θ = mλ (1)
 Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Information about the T9 beam line and experimental facilities
 Information about the T9 beam line and experimental facilities The incoming proton beam from the PS accelerator impinges on the North target and thus produces the particles for the T9 beam line. The collisions
Information about the T9 beam line and experimental facilities The incoming proton beam from the PS accelerator impinges on the North target and thus produces the particles for the T9 beam line. The collisions
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
hν' Φ e - Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous?
 Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
1 AT/P5-05. Institute of Applied Physics, National Academy of Sciences of Ukraine, Sumy, Ukraine
 1 AT/P5-05 H - Ion Source with Inverse Gas Magnetron Geometry for SNS Project V.A. Baturin, P.A. Litvinov, S.A. Pustovoitov, A.Yu. Karpenko Institute of Applied Physics, National Academy of Sciences of
1 AT/P5-05 H - Ion Source with Inverse Gas Magnetron Geometry for SNS Project V.A. Baturin, P.A. Litvinov, S.A. Pustovoitov, A.Yu. Karpenko Institute of Applied Physics, National Academy of Sciences of
Time of Flight Mass Spectroscopy and Velocity Map Imaging
 Time of Flight Mass Spectroscopy and Velocity Map Imaging Geet Ghanshyam January 26, 2013 Velocity map imaging (VMI) is used to study the dynamics of various dissociative electron attachment (DEA) processes
Time of Flight Mass Spectroscopy and Velocity Map Imaging Geet Ghanshyam January 26, 2013 Velocity map imaging (VMI) is used to study the dynamics of various dissociative electron attachment (DEA) processes
Chapter 4 Spectroscopy
 Chapter 4 Spectroscopy The beautiful visible spectrum of the star Procyon is shown here from red to blue, interrupted by hundreds of dark lines caused by the absorption of light in the hot star s cooler
Chapter 4 Spectroscopy The beautiful visible spectrum of the star Procyon is shown here from red to blue, interrupted by hundreds of dark lines caused by the absorption of light in the hot star s cooler
The Performance of the EUV Spectroscope (EXCEED) Onboard the SPRINT-A Mission
 The Performance of the EUV Spectroscope (EXCEED) Onboard the SPRINT-A Mission K. Yoshioka, G. Murakami, A. Yamazaki, K. Uemizu, T. Kimura (ISAS/JAXA), I. Yoshikawa, K. Uji (Univ. Tokyo) F. Tsuchiya, and
The Performance of the EUV Spectroscope (EXCEED) Onboard the SPRINT-A Mission K. Yoshioka, G. Murakami, A. Yamazaki, K. Uemizu, T. Kimura (ISAS/JAXA), I. Yoshikawa, K. Uji (Univ. Tokyo) F. Tsuchiya, and
Line Spectra. 6 The line spectrum of hydrogen includes no X-ray frequencies because
 TOPI 27 Line Spectra 1 The table below gives the energies of the six lowest levels of the hydrogen atom: Leveln 2 3 nergy/j -2.2 x 10-'8-5.3 X 10-'9-2.4 X 10-'9 Level 11 4 5 6 nergy/j -1.3 x JO-'9-8.0
TOPI 27 Line Spectra 1 The table below gives the energies of the six lowest levels of the hydrogen atom: Leveln 2 3 nergy/j -2.2 x 10-'8-5.3 X 10-'9-2.4 X 10-'9 Level 11 4 5 6 nergy/j -1.3 x JO-'9-8.0
[2] (b) An electron is accelerated from rest through a potential difference of 300 V.
![[2] (b) An electron is accelerated from rest through a potential difference of 300 V. [2] (b) An electron is accelerated from rest through a potential difference of 300 V.](/thumbs/89/98791036.jpg) 1 (a) In atomic physics electron energies are often stated in electronvolts (ev) Define the electronvolt. State its value in joule.. [2] (b) An electron is accelerated from rest through a potential difference
1 (a) In atomic physics electron energies are often stated in electronvolts (ev) Define the electronvolt. State its value in joule.. [2] (b) An electron is accelerated from rest through a potential difference
Radioactivity. Lecture 6 Detectors and Instrumentation
 Radioactivity Lecture 6 Detectors and Instrumentation The human organs Neither humans nor animals have an organ for detecting radiation from radioactive decay! We can not hear it, smell it, feel it or
Radioactivity Lecture 6 Detectors and Instrumentation The human organs Neither humans nor animals have an organ for detecting radiation from radioactive decay! We can not hear it, smell it, feel it or
Internal Charging Hazards in Near-Earth Space during Solar Cycle 24 Maximum: Van Allen Probes Measurements
 Internal Charging Hazards in Near-Earth Space during Solar Cycle 24 Maximum: Van Allen Probes Measurements T. Mulligan Skov, J.F. Fennell, J.L. Roeder, J.B. Blake, and S.G. Claudepierre The Aerospace Corporation,
Internal Charging Hazards in Near-Earth Space during Solar Cycle 24 Maximum: Van Allen Probes Measurements T. Mulligan Skov, J.F. Fennell, J.L. Roeder, J.B. Blake, and S.G. Claudepierre The Aerospace Corporation,
Multicusp Sources for Ion Beam Lithography Applications
 LBL-3 6645 UC-406 Multicusp Sources for Ion Beam Lithography Applications K.N. Leung, P. H e n, W.B. Kunkel, Y. Lee, L. Perkins, D. Pickard, M. Sarstedt, M. Weber, and M.D. Williams Accelerator and Fusion
LBL-3 6645 UC-406 Multicusp Sources for Ion Beam Lithography Applications K.N. Leung, P. H e n, W.B. Kunkel, Y. Lee, L. Perkins, D. Pickard, M. Sarstedt, M. Weber, and M.D. Williams Accelerator and Fusion
Imaging the Earth from the Moon FUV Imaging of the Earth s Space Weather. Dr. Larry J. Paxton (office)
 Imaging the Earth from the Moon FUV Imaging of the Earth s Space Weather Dr. Larry J. Paxton 240 228 6871 (office) Larry.paxton@jhuapl.edu Making Observations of the Earth from the Moon Makes Sense Once
Imaging the Earth from the Moon FUV Imaging of the Earth s Space Weather Dr. Larry J. Paxton 240 228 6871 (office) Larry.paxton@jhuapl.edu Making Observations of the Earth from the Moon Makes Sense Once
Calibration of Ocean Colour Sensors
 Dr. A. Neumann German Aerospace Centre DLR Remote Sensing Technology Institute Marine Remote Sensing What is Calibration, why do we need it? Sensor Components Definition of Terms Calibration Standards
Dr. A. Neumann German Aerospace Centre DLR Remote Sensing Technology Institute Marine Remote Sensing What is Calibration, why do we need it? Sensor Components Definition of Terms Calibration Standards
LAAPD Performance Measurements in Liquid Xenon
 LAAPD Performance Measurements in Liquid Xenon David Day Summer REU 2004 Nevis Laboratories, Columbia University Irvington, NY August 3, 2004 Abstract Performance measurements of a 16mm diameter large
LAAPD Performance Measurements in Liquid Xenon David Day Summer REU 2004 Nevis Laboratories, Columbia University Irvington, NY August 3, 2004 Abstract Performance measurements of a 16mm diameter large
Volume Production of D - Negative Ions in Low-Pressure D 2 Plasmas - Negative Ion Densities versus Plasma Parameters -
 Volume Production of D - Negative Ions in Low-Pressure D 2 Plasmas - Negative Ion Densities versus Plasma Parameters - Osamu Fukumasa and Shigefumi Mori Department of Electrical and Electronic Engineering,
Volume Production of D - Negative Ions in Low-Pressure D 2 Plasmas - Negative Ion Densities versus Plasma Parameters - Osamu Fukumasa and Shigefumi Mori Department of Electrical and Electronic Engineering,
Reference literature. (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters )
 September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 Q1. (a) The diagram below shows a narrow beam of electrons produced by attracting electrons emitted from a filament wire to a metal plate which has a small hole in it. (i) Why
PhysicsAndMathsTutor.com 1 Q1. (a) The diagram below shows a narrow beam of electrons produced by attracting electrons emitted from a filament wire to a metal plate which has a small hole in it. (i) Why
The ISIS Penning H - SPS and Diagnostic Developments at RAL
 The ISIS Penning H - SPS and Diagnostic Developments at RAL D.C. Faircloth 1, A.P. Letchford 1, J. Pozimski 1+2, M.O. Whitehead 1, T. Wood 1, S. Jolly 2, P. Savage 2, M. Haigh 3, J. Morrison 3, I. Yew
The ISIS Penning H - SPS and Diagnostic Developments at RAL D.C. Faircloth 1, A.P. Letchford 1, J. Pozimski 1+2, M.O. Whitehead 1, T. Wood 1, S. Jolly 2, P. Savage 2, M. Haigh 3, J. Morrison 3, I. Yew
Plasma Spectroscopy in ISTTOK
 Plasma Spectroscopy in ISTTOK J. Figueiredo 1, R. B. Gomes 1, T. Pereira 1, H. Fernandes 1, A. Sharakovski 2 1 Associação EURATOM/IST, Centro de Fusão Nuclear, IST, 1049-001 Lisboa, Portugal 2 Association
Plasma Spectroscopy in ISTTOK J. Figueiredo 1, R. B. Gomes 1, T. Pereira 1, H. Fernandes 1, A. Sharakovski 2 1 Associação EURATOM/IST, Centro de Fusão Nuclear, IST, 1049-001 Lisboa, Portugal 2 Association
IPBI-TN June 30, 2004
 Spray Electron Beam for Tests of Linear Collider Forward Calorimeter Detectors in SLAC End Station A R. Arnold UMass Amherst, Amherst MA 01003 T. Fieguth Stanford Linear Accelerator Center Menlo Park,
Spray Electron Beam for Tests of Linear Collider Forward Calorimeter Detectors in SLAC End Station A R. Arnold UMass Amherst, Amherst MA 01003 T. Fieguth Stanford Linear Accelerator Center Menlo Park,
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Energy loss of alpha particles - Prelab questions
 Energy loss of alpha particles - Prelab questions 1. Write down the decay path from 226 Ra to 206 Pb. Show the intermediate nuclides and the nuclear reactions which cause each transformation (α/β ± decay).
Energy loss of alpha particles - Prelab questions 1. Write down the decay path from 226 Ra to 206 Pb. Show the intermediate nuclides and the nuclear reactions which cause each transformation (α/β ± decay).
Introduction to the Q Trap LC/MS/MS System
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment ABI Q Trap LC/MS/MS Introduction to the Q Trap LC/MS/MS System The Q Trap
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment ABI Q Trap LC/MS/MS Introduction to the Q Trap LC/MS/MS System The Q Trap
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
~1 V ~20-40 V. Electron collector PLASMA. Ion extraction optics. Ionization zone. Mass Resolving section Ion detector. e - ~20 V Filament Heater
 RGAs and Leak detectors [Note that standard Ion Implanters are just overgrown RGAs!] RGAs or Residual Gas Analyzers are also known as Mass Spectrum Analyzers. These can sometimes be upgraded to also include
RGAs and Leak detectors [Note that standard Ion Implanters are just overgrown RGAs!] RGAs or Residual Gas Analyzers are also known as Mass Spectrum Analyzers. These can sometimes be upgraded to also include
Calibrating the Thermal Camera
 1 of 5 4/19/2012 5:33 AM from photonics.com: 12/01/2009 http://www.photonics.com/article.aspx?aid=40679 Calibrating the Thermal Camera As thermal cameras gain ground in the commercial market, testing becomes
1 of 5 4/19/2012 5:33 AM from photonics.com: 12/01/2009 http://www.photonics.com/article.aspx?aid=40679 Calibrating the Thermal Camera As thermal cameras gain ground in the commercial market, testing becomes
Experiment 4 Radiation in the Visible Spectrum
 Experiment 4 Radiation in the Visible Spectrum Emission spectra can be a unique fingerprint of an atom or molecule. The photon energies and wavelengths are directly related to the allowed quantum energy
Experiment 4 Radiation in the Visible Spectrum Emission spectra can be a unique fingerprint of an atom or molecule. The photon energies and wavelengths are directly related to the allowed quantum energy
Relationship between production and extraction of D - /H - negative ions in a volume negative ion source
 J. Plasma Fusion Res. SERIES, Vol. 8 (2009) Relationship between production and extraction of D - /H - negative ions in a volume negative ion source Takahiro Nakano, Shigefumi Mori, Yasushi Tauchi, Wataru
J. Plasma Fusion Res. SERIES, Vol. 8 (2009) Relationship between production and extraction of D - /H - negative ions in a volume negative ion source Takahiro Nakano, Shigefumi Mori, Yasushi Tauchi, Wataru
Kaonic nitrogen and hydrogen from DEAR
 Kaonic nitrogen and hydrogen from DEAR C. Curceanu (Petrascu) LNF INFN, Via. E. Fermi 40, 00044 Frascati (Roma), Italy On behalf on the DEAR Collaboration 1. DEAR scientific program DEAR (DAΦNE Exotic
Kaonic nitrogen and hydrogen from DEAR C. Curceanu (Petrascu) LNF INFN, Via. E. Fermi 40, 00044 Frascati (Roma), Italy On behalf on the DEAR Collaboration 1. DEAR scientific program DEAR (DAΦNE Exotic
high energy state for the electron in the atom low energy state for the electron in the atom
 Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Update on Periodicities in Saturn s Magnetosphere
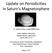 Update on Periodicities in Saturn s Magnetosphere J.F. Carbary & the Cassini/MIMI Team Johns Hopkins University Applied Physics Laboratory Laurel, MD 20723 Presented at Saturn Periodicities Workshop 2
Update on Periodicities in Saturn s Magnetosphere J.F. Carbary & the Cassini/MIMI Team Johns Hopkins University Applied Physics Laboratory Laurel, MD 20723 Presented at Saturn Periodicities Workshop 2
Dept. of Physics, MIT Manipal 1
 Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
PHYSICAL VAPOR DEPOSITION OF THIN FILMS
 PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
