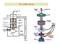
~1 V ~20-40 V. Electron collector PLASMA. Ion extraction optics. Ionization zone. Mass Resolving section Ion detector. e - ~20 V Filament Heater
|
|
|
- Loren Grant
- 6 years ago
- Views:
Transcription
1 RGAs and Leak detectors [Note that standard Ion Implanters are just overgrown RGAs!] RGAs or Residual Gas Analyzers are also known as Mass Spectrum Analyzers. These can sometimes be upgraded to also include energy analysis in which case they are known as Mass and Energy analyzers.) While the portion of the RGA that is used to perform the mass analysis differs from tool to tool there are many similarities between all RGAs. The basic system looks like: ~1 V ~0-40 V PLASMA Electron collector Ionization zone Ion extraction optics e - e - e - Mass Resolving section Ion detector ~0 V Filament Heater Filament Bias ~70-10 V We will go through the ionization part of the RGA first. The plasma produces a large variety of chemical species many of which are neutrals. To examine these neutrals we must be able to apply some well characterized forces to them. The simplest thing to do is to ionize the molecules and then use electromagnetic forces to push them around in the mass resolving section. [NOTE: if you turn off the ionization region than the only thing that you can look at is ions coming out of the plasma. This a useful variant to standard RGAs.] To ionize the neutrals we use fast electrons. These electrons are created by first heating a filament or wire that is known to give off large numbers of electrons at high temperatures. Common materials used for this is Tungsten, Thoriated-Tungsten (1-5% Thorium), or Lanthanum-hexa-Boride (LaB 6 ). Thoriated-Tungsten is perhaps the most common of these materials. This is because of it very high emission coefficient, higher than plain Tungsten, and it does not suffer the same material poisoning problems found with LaB 6 emitters. [Note that LaB6 emitters are common in other applications.] To heat it, a current is drawn through the filament. Often the bias is on the order of 0 V and perhaps an amp of current but this depends on the size and type of the filament. The
2 typical temperature of the filament at typical operating conditions is 1600 to 1800 C. This is in color a very bright red to a white. When the filament reaches this temperature the electrons in the filament have sufficient energy to overcome the binding energies and will leave the surface with a fraction of an ev of energy. (1 ev is energy gained by dropping an item charged to one electron charge through a voltage of of one volt.) Because we wish to ionize the gas in the ionization region, and this often takes 10s of ev in energy, we need to accelerate the emitted electrons. We do this with a bias voltage that is typically in the range of 70 to 10 V. By using a grounding cage around the ionization region, we assure that most of the electrons enter the region with energy near the bias voltage (times e ). This bias voltage is chosen because the maximum in the ionization cross section for most gases is near 100 ev. It is very important to note that the maximum varies both in magnitude and peak location. Thus we will get different numbers of ions from different gases see Figure below Species A Impact energy Cross section (cm ) Species B Energy (ev) If we can determine the number of ions produced per neutral gas molecule we can determine the partial pressure of each of the species in the plasma. If our electrons suffer a number of collisions as they cross the ionization region than we will have a large range of energies at which the ionization collisions might take place. This would make it very difficult to determine the ratios of the densities of different species. Thus we need to make sure that the pressure in the ionization region is low enough that the mean-free path for ALL collisions is long compared to the total distance traveled by the electrons. Often at process conditions the background pressure it too high for this reuirement thus we often have a pin hole entrance aperture and use a high vacuum pump to drop the the RGA to pressures near 10 µtorr. It is important to note that the HV pump is most often a Turbo-molecular pump because of those pumps pump most gases at the same rate and they do not add significant amounts of contaminates. [Ion implanters do not have this limitation. There we simply want to create as many ions of a specific species as possible. We are not trying to preserve density ratios.] IN ADDITION TO IONIZING THE GAS, THIS SECTION ALSO BREAKS UP THE GAS INTO DAUGHTER SPECIES. THIS IS KNOWN AS CRACKING AND WE WILL TALK ABOUT IT DURING THE NEXT CLASS PERIOD.
3 We have now created ions which exist at densities that small compared to the densities of the original gases. This ratio is of course set by the ionization cross section of the gas and the cracking pattern of the gas. (Some species that are stable as neutrals are not stable as ions and immediately fall apart.) We now need to get the ions to our mass analysis region. To do this we accelerate the ion to approximately 0 ev using electrostatic ion optics. [For implanters this is usually ~0 kev. This is because we can get more ions/area through the system at higher energies.] In its simplest form this amounts to a negatively biased plate with a hole in the center. Note that the rest of the RGA sits at this same potential Thus when the ions enter the mass resolver and detector we do not disturb the orbit of the ions with this electrostatic field. At this point we enter the mass resolution system. In reality this is a velocity resolution system we use the fact that mass, energy and velocity are intertwined and we have set the energy using the ion optics. While there are numerous types of velocity analyzers, two are most commonly used. The first uses a static magnetic field or magnetic sector we will go through the math on this one in a moment while the second uses a uadrupole electric field containing both dc and rf parts. The later of these two systems is the most common in good RGAs. However, the analysis of how they operate is beyond the scope of the class. The Magnetic sector systems are not typically used in good RGAs BUT they are the mainstay of ion implantation mass resolution systems. We will go through the math on how these systems work here. The euation of motion for a single particle of charge and mass m is given by the Lorentz force law: m d v ( E+ v B). Cases : E 0; B B0z dv ( E+ v B) m v B 0 z m Thus we find dvx m vb y 0 dvy m vb x 0 dvz 0 To solve this set of euations, we must separate the components of the velocity. This is simple to do by differentiating the euations again and substituting to give.
4 dvx dvy dvz dvy m B m B 0 0 dvx m B m B v x v y These second order euations are of course are easily solved as ± iω ct vx vx0e ± iω ct v v e m B y y0 where 0 vz vz0 Now taking into account the original coupled first order euations we find vx cos( t + ϕ) vy sin( t + ϕ) vz vz0 Integrating a second time we find, x sin( t + ϕ)+ x0 sin( ϕ) ω ω c y cos( t + ϕ)+ y0 cos( ϕ) z v t + z z0 0 c rc v It is easy to see that rc 0 is the radius of a circular orbit around the magnetic field line; it is known as the Larmor radius or the cyclotron radius. Further we note that the positively charged particles orbit in a left-hand orbit while the negatively charged particles orbit in a right-hand orbit. Now for our system the velocity is set by energy we have given the ion with the electrostatic accelerator. Thus we find that the particle travels in a circular orbit with a radius of
5 v rc 0 ( Va m ) 1 / / B / m ( mva ) 1 / / B Thus we can change the radius by either adjusting the magnetic field strength or by adjusting the accelerating voltage. Often some combination is used. By setting up out analyzer as thus: We can eliminate all ions which do not match the reuired Larmour radius. At this point we need to detect the ions that have made it all the way through the system. The simplest detection scheme is to use a Faraday cup. This amount to a cup that simply collects all of the ions, and we measure the associated current. The next thing that one might use is an electron multiplier note that these are also used on photomultiplier tubes to measure photons. There are two main types the chain and the channeltron. Both make use of materials which produce significant uantities of secondary electrons when struck by either energetic ions or energetic electrons. These items greatly increase our currents allowing use to measure single ion events. (Electron multiplier reuire high vacuum for a number reasons Add drawings of chain and Channeltron tubes. We can change our detection system to include electrostatic retarding optics. By varying the potential on our retarding optics, we can stop some of the ions from entering the detection area. By measuring the current as a function of the retarding potential we can determine the energy distribution of the ions entering the detector thereby allowing us to also measure the energy.
6 Cracking patterns
ICPMS Doherty Lecture 1
 ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
Chapter 13: Partial Pressure Analysis
 Chapter 13: Partial Pressure Analysis Previously several types of sub-atmospheric pressure gauge were described. All of these gauges share one common feature: they report the total gas pressure. Partial
Chapter 13: Partial Pressure Analysis Previously several types of sub-atmospheric pressure gauge were described. All of these gauges share one common feature: they report the total gas pressure. Partial
Experiment V Motion of electrons in magnetic field and measurement of e/m
 Experiment V Motion of electrons in magnetic field and measurement of e/m In Experiment IV you observed the quantization of charge on a microscopic bead and measured the charge on a single electron. In
Experiment V Motion of electrons in magnetic field and measurement of e/m In Experiment IV you observed the quantization of charge on a microscopic bead and measured the charge on a single electron. In
Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
 ESS The RGA freenotes Theory page 1 of 14 RGA Theory Notes Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
ESS The RGA freenotes Theory page 1 of 14 RGA Theory Notes Courtesy of ESS and TheRGA web pages part of a series of application and theory notes for public use which are provided free of charge by ESS.
Ratio of Charge to Mass (e/m) for the Electron
 Objective: In this experiment you will determine the ratio of charge to mass (e/m) of the electron, by measuring the deflecting of electrons as they move through a magnetic field. Apparatus: e/m apparatus
Objective: In this experiment you will determine the ratio of charge to mass (e/m) of the electron, by measuring the deflecting of electrons as they move through a magnetic field. Apparatus: e/m apparatus
Chapter 23 Electric Potential. Copyright 2009 Pearson Education, Inc.
 Chapter 23 Electric Potential Units of Chapter 23 Electric Potential Energy and Potential Difference Relation between Electric Potential and Electric Field Electric Potential Due to Point Charges Potential
Chapter 23 Electric Potential Units of Chapter 23 Electric Potential Energy and Potential Difference Relation between Electric Potential and Electric Field Electric Potential Due to Point Charges Potential
Copyright 2008, University of Chicago, Department of Physics. Experiment VI. Gamma Ray Spectroscopy
 Experiment VI Gamma Ray Spectroscopy 1. GAMMA RAY INTERACTIONS WITH MATTER In order for gammas to be detected, they must lose energy in the detector. Since gammas are electromagnetic radiation, we must
Experiment VI Gamma Ray Spectroscopy 1. GAMMA RAY INTERACTIONS WITH MATTER In order for gammas to be detected, they must lose energy in the detector. Since gammas are electromagnetic radiation, we must
Huashun Zhang. Ion Sources. With 187 Figures and 26 Tables Э SCIENCE PRESS. Springer
 Huashun Zhang Ion Sources With 187 Figures and 26 Tables Э SCIENCE PRESS Springer XI Contents 1 INTRODUCTION 1 1.1 Major Applications and Requirements 1 1.2 Performances and Research Subjects 1 1.3 Historical
Huashun Zhang Ion Sources With 187 Figures and 26 Tables Э SCIENCE PRESS Springer XI Contents 1 INTRODUCTION 1 1.1 Major Applications and Requirements 1 1.2 Performances and Research Subjects 1 1.3 Historical
Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002
 Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002 More Quantum Physics We know now how to detect light (or photons) One possibility to detect
Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002 More Quantum Physics We know now how to detect light (or photons) One possibility to detect
EXPERIMENT 2-6. e/m OF THE ELECTRON GENERAL DISCUSSION
 Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Observation of Atomic Spectra
 Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
The illumination source: the electron beam
 The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The Measurement of e/m
 MSCD/UCD Physics Laboratories Lab II e/m The Measurement of e/m PURPOSE The objectives of this experiment are to measure the ratio between the charge and the mass of electrons, and then to find the mass
MSCD/UCD Physics Laboratories Lab II e/m The Measurement of e/m PURPOSE The objectives of this experiment are to measure the ratio between the charge and the mass of electrons, and then to find the mass
Harris: Quantitative Chemical Analysis, Eight Edition
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 21: MASS SPECTROMETRY CHAPTER 21: Opener 21.0 Mass Spectrometry Mass Spectrometry provides information about 1) The elemental composition of
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 21: MASS SPECTROMETRY CHAPTER 21: Opener 21.0 Mass Spectrometry Mass Spectrometry provides information about 1) The elemental composition of
Application Note GA-301E. MBMS for Preformed Ions. Extrel CMS, 575 Epsilon Drive, Pittsburgh, PA I. SAMPLING A CHEMICAL SOUP
 Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Extrel Application Note
 Extrel Application Note Real-Time Plasma Monitoring and Detection of Trace H 2 O and HF Species in an Argon Based Plasma Jian Wei, 575 Epsilon Drive, Pittsburgh, PA 15238. (Presented at the 191st Electrochemical
Extrel Application Note Real-Time Plasma Monitoring and Detection of Trace H 2 O and HF Species in an Argon Based Plasma Jian Wei, 575 Epsilon Drive, Pittsburgh, PA 15238. (Presented at the 191st Electrochemical
Quadrupole Mass Spectrometry Concepts. Mass spectrometers for residual gas analysis: Intermediate Level Users Guide
 Quadrupole Mass Spectrometry Concepts Mass spectrometers for residual gas analysis: Intermediate Level Users Guide What does Residual Gas Analysis allow us to do? RGA is the examination of the molecular
Quadrupole Mass Spectrometry Concepts Mass spectrometers for residual gas analysis: Intermediate Level Users Guide What does Residual Gas Analysis allow us to do? RGA is the examination of the molecular
THE FRANCK-HERTZ EXPERIMENT
 Rice University Physics 332 THE FRANCK-HERTZ EXPERIMENT I. INTRODUCTION... 2 II. THEORETICAL CONSIDERATIONS... 3 III. MEASUREMENT AND ANALYSIS... 5 Revised July 1991 I. Introduction By the early part of
Rice University Physics 332 THE FRANCK-HERTZ EXPERIMENT I. INTRODUCTION... 2 II. THEORETICAL CONSIDERATIONS... 3 III. MEASUREMENT AND ANALYSIS... 5 Revised July 1991 I. Introduction By the early part of
Physics 201: Experiment #1 Franck-Hertz Experiment
 Physics 201: Experiment #1 Franck-Hertz Experiment Carl Adams Winter 2004 Purpose To demonstrate that electrons colliding with gaseous mercury atoms lose energy in discrete amounts. Nobel Prize Winner
Physics 201: Experiment #1 Franck-Hertz Experiment Carl Adams Winter 2004 Purpose To demonstrate that electrons colliding with gaseous mercury atoms lose energy in discrete amounts. Nobel Prize Winner
Lecture 22 Ion Beam Techniques
 Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Charge to Mass Ratio of The Electron
 Introduction Charge to Mass Ratio of The Electron The electron was first discovered by Sir J.J. Thomson in 1897 at the Cavendish Laboratory in Cambridge, England. His experimental apparatus is not very
Introduction Charge to Mass Ratio of The Electron The electron was first discovered by Sir J.J. Thomson in 1897 at the Cavendish Laboratory in Cambridge, England. His experimental apparatus is not very
Using a Microwave Interferometer to Measure Plasma Density Mentor: Prof. W. Gekelman. P. Pribyl (UCLA)
 Using a Microwave Interferometer to Measure Plasma Density Avital Levi Mentor: Prof. W. Gekelman. P. Pribyl (UCLA) Introduction: Plasma is the fourth state of matter. It is composed of fully or partially
Using a Microwave Interferometer to Measure Plasma Density Avital Levi Mentor: Prof. W. Gekelman. P. Pribyl (UCLA) Introduction: Plasma is the fourth state of matter. It is composed of fully or partially
Homework 2: Forces on Charged Particles
 Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Nova 600 NanoLab Dual beam Focused Ion Beam IITKanpur
 Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
MAGNETIC DEFLECTION. OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field.
 MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
Robert A. Meger Richard F. Fernster Martin Lampe W. M. Manheimer NOTICE
 Serial Number Filing Date Inventor 917.963 27 August 1997 Robert A. Meger Richard F. Fernster Martin Lampe W. M. Manheimer NOTICE The above identified patent application is available for licensing. Requests
Serial Number Filing Date Inventor 917.963 27 August 1997 Robert A. Meger Richard F. Fernster Martin Lampe W. M. Manheimer NOTICE The above identified patent application is available for licensing. Requests
Proportional Counters
 Proportional Counters 3 1 Introduction 3 2 Before we can look at individual radiation processes, we need to understand how the radiation is detected: Non-imaging detectors Detectors capable of detecting
Proportional Counters 3 1 Introduction 3 2 Before we can look at individual radiation processes, we need to understand how the radiation is detected: Non-imaging detectors Detectors capable of detecting
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
The Q Machine. 60 cm 198 cm Oven. Plasma. 6 cm 30 cm. 50 cm. Axial. Probe. PUMP End Plate Magnet Coil. Filament Cathode. Radial. Hot Plate.
 1 The Q Machine 60 cm 198 cm Oven 50 cm Axial Probe Plasma 6 cm 30 cm PUMP End Plate Magnet Coil Radial Probe Hot Plate Filament Cathode 2 THE Q MACHINE 1. GENERAL CHARACTERISTICS OF A Q MACHINE A Q machine
1 The Q Machine 60 cm 198 cm Oven 50 cm Axial Probe Plasma 6 cm 30 cm PUMP End Plate Magnet Coil Radial Probe Hot Plate Filament Cathode 2 THE Q MACHINE 1. GENERAL CHARACTERISTICS OF A Q MACHINE A Q machine
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
Instrumental Analysis. Mass Spectrometry. Lecturer:! Somsak Sirichai
 303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
EXPERIMENT 14. The Franck-Hertz Experiment. E 1 E 2 = hf (1)
 EXPERIMENT 14 The Franck-Hertz Experiment The Franck-Hertz experiment is one of the best demonstrations to show the existence of discrete energy states in an atom as suggested by Niels Bohr. In this experiment,
EXPERIMENT 14 The Franck-Hertz Experiment The Franck-Hertz experiment is one of the best demonstrations to show the existence of discrete energy states in an atom as suggested by Niels Bohr. In this experiment,
EXPERIMENT 5. The Franck-Hertz Experiment (Critical Potentials) Introduction
 EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
Gas discharges. Current flow of electric charge. Electric current (symbol I) L 26 Electricity and Magnetism [3] examples of electrical discharges
![Gas discharges. Current flow of electric charge. Electric current (symbol I) L 26 Electricity and Magnetism [3] examples of electrical discharges Gas discharges. Current flow of electric charge. Electric current (symbol I) L 26 Electricity and Magnetism [3] examples of electrical discharges](/thumbs/87/96343871.jpg) L 26 Electricity and Magnetism [3] Electric circuits what conducts electricity what doesn t t conduct electricity Current voltage and resistance Ohm s s Law Heat in a resistor power loss Making simple
L 26 Electricity and Magnetism [3] Electric circuits what conducts electricity what doesn t t conduct electricity Current voltage and resistance Ohm s s Law Heat in a resistor power loss Making simple
Fundamentals of Mass Spectrometry. Fundamentals of Mass Spectrometry. Learning Objective. Proteomics
 Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
MS Goals and Applications. MS Goals and Applications
 MS Goals and Applications 3 Several variations on a theme, three common steps Form gas-phase ions choice of ionization method depends on sample identity and information required Separate ions on basis
MS Goals and Applications 3 Several variations on a theme, three common steps Form gas-phase ions choice of ionization method depends on sample identity and information required Separate ions on basis
The e/m Ratio of the Electron
 OBJECTIVE The e/m Ratio of the Electron To study the behavior of a charged particle in the presence of a potential difference. To study the behavior of a charged particle moving in a magnetic field. To
OBJECTIVE The e/m Ratio of the Electron To study the behavior of a charged particle in the presence of a potential difference. To study the behavior of a charged particle moving in a magnetic field. To
Basic physics Questions
 Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Metal Deposition. Filament Evaporation E-beam Evaporation Sputter Deposition
 Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
QPR No. 68 RADIO PHYSICS I. PHYSICAL ELECTRONICS. Prof. W. B. Nottingham J. L. Coggins B. L. Blackford L. E. Sprague RESEARCH OBJECTIVES
 RADIO PHYSICS I. PHYSICAL ELECTRONICS RESEARCH OBJECTIVES Prof. W. B. Nottingham J. L. Coggins B. L. Blackford L. E. Sprague 1. Theory of Energy Conversion Electronics The effectiveness of energy conversion
RADIO PHYSICS I. PHYSICAL ELECTRONICS RESEARCH OBJECTIVES Prof. W. B. Nottingham J. L. Coggins B. L. Blackford L. E. Sprague 1. Theory of Energy Conversion Electronics The effectiveness of energy conversion
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
CHARGE TO MASS RATIO FOR THE ELECTRON
 CHARGE TO MASS RATIO FOR THE ELECTRON OBJECTIVE: To measure the ratio of the charge of an electron to its mass. METHOD: A stream of electrons is accelerated by having them "fall" through a measured potential
CHARGE TO MASS RATIO FOR THE ELECTRON OBJECTIVE: To measure the ratio of the charge of an electron to its mass. METHOD: A stream of electrons is accelerated by having them "fall" through a measured potential
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Direct-Current Accelerator
 Nuclear Science A Teacher s Guide to the Nuclear Science Wall Chart 1998 Contemporary Physics Education Project (CPEP) Chapter 11 Accelerators One of the most important tools of nuclear science is the
Nuclear Science A Teacher s Guide to the Nuclear Science Wall Chart 1998 Contemporary Physics Education Project (CPEP) Chapter 11 Accelerators One of the most important tools of nuclear science is the
This lab was adapted from Kwantlen University College s Determination of e/m lab.
 e /m: Charge to Mass Ratio of the Electron This lab was adapted from Kwantlen University College s Determination of e/m lab. Purpose To determine the charge to mass ratio of the electron, e /m, using Helmholtz
e /m: Charge to Mass Ratio of the Electron This lab was adapted from Kwantlen University College s Determination of e/m lab. Purpose To determine the charge to mass ratio of the electron, e /m, using Helmholtz
Chemistry Instrumental Analysis Lecture 35. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 35 Principle components: Inlet Ion source Mass analyzer Ion transducer Pumps Signal processor Mass analyzers Quadrupole Time of Flight Double Focusing Ion
Chemistry 4631 Instrumental Analysis Lecture 35 Principle components: Inlet Ion source Mass analyzer Ion transducer Pumps Signal processor Mass analyzers Quadrupole Time of Flight Double Focusing Ion
KE = 1 2 mv2 = ev. (1)
 The e/m ratio Objective To measure the electronic charge-to-mass ratio e/m, by injecting electrons into a magnetic field and examining their trajectories. We also estimate the magnitude of the earth s
The e/m ratio Objective To measure the electronic charge-to-mass ratio e/m, by injecting electrons into a magnetic field and examining their trajectories. We also estimate the magnitude of the earth s
5-A / 9, WEA, Sat Nagar, Karol Bagh New Delhi Web:
 Keshaw Classes IIT/JEE Medical Classes 5-A 11028 / 9, WEA, Sat Nagar, Karol Bagh New Delhi-110005 Mob:9910915514,9953150192 Ph:011-45660510 E-mail : keshawclasses@gmail.com Web:www.keshawclasses.com MODERN
Keshaw Classes IIT/JEE Medical Classes 5-A 11028 / 9, WEA, Sat Nagar, Karol Bagh New Delhi-110005 Mob:9910915514,9953150192 Ph:011-45660510 E-mail : keshawclasses@gmail.com Web:www.keshawclasses.com MODERN
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
PHYS 3446 Lecture #15
 PHYS 3446 Lecture #15 Monday, Oct. 30, 2006 Dr. 1. Particle Accelerators Electro-static Accelerators Cyclotron Accelerators Synchrotron Accelerators 2. Elementary Particle Properties Forces and their relative
PHYS 3446 Lecture #15 Monday, Oct. 30, 2006 Dr. 1. Particle Accelerators Electro-static Accelerators Cyclotron Accelerators Synchrotron Accelerators 2. Elementary Particle Properties Forces and their relative
- A spark is passed through the Argon in the presence of the RF field of the coil to initiate the plasma
 THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
 Cambridge International Examinations Cambridge International Advanced Level *6106210292* PHYSICS 9702/42 Paper 4 A2 Structured Questions May/June 2014 2 hours Candidates answer on the Question Paper. No
Cambridge International Examinations Cambridge International Advanced Level *6106210292* PHYSICS 9702/42 Paper 4 A2 Structured Questions May/June 2014 2 hours Candidates answer on the Question Paper. No
A fluorescent tube is filled with mercury vapour at low pressure. After mercury atoms have been excited they emit photons.
 Q1.(a) A fluorescent tube is filled with mercury vapour at low pressure. After mercury atoms have been excited they emit photons. In which part of the electromagnetic spectrum are these photons? What is
Q1.(a) A fluorescent tube is filled with mercury vapour at low pressure. After mercury atoms have been excited they emit photons. In which part of the electromagnetic spectrum are these photons? What is
Sample Examination Questions
 Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Mass Spectrometry. What is Mass Spectrometry?
 Mass Spectrometry What is Mass Spectrometry? Mass Spectrometry (MS): The generation of gaseous ions from a sample, separation of these ions by mass-to-charge ratio, and measurement of relative abundance
Mass Spectrometry What is Mass Spectrometry? Mass Spectrometry (MS): The generation of gaseous ions from a sample, separation of these ions by mass-to-charge ratio, and measurement of relative abundance
Mass Spectrometry in MCAL
 Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
General Physics II Summer Session 2013 Review Ch - 16, 17, 18
 95.104 General Physics II Summer Session 2013 Review Ch - 16, 17, 18 A metal ball hangs from the ceiling by an insulating thread. The ball is attracted to a positivecharged rod held near the ball. The
95.104 General Physics II Summer Session 2013 Review Ch - 16, 17, 18 A metal ball hangs from the ceiling by an insulating thread. The ball is attracted to a positivecharged rod held near the ball. The
Physics Important Terms and their Definitions
 Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
MAGNETISM LAB: The Charge-to-Mass Ratio of the Electron
 Physics 7B Charge-to-mass: e/m p. 1 NAME: DL SECTION NUMBER: GSI: LAB PARTNERS: MAGNETISM LAB: The Charge-to-Mass Ratio of the Electron Introduction In this lab you will explore the motion of a charged
Physics 7B Charge-to-mass: e/m p. 1 NAME: DL SECTION NUMBER: GSI: LAB PARTNERS: MAGNETISM LAB: The Charge-to-Mass Ratio of the Electron Introduction In this lab you will explore the motion of a charged
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW
 ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces
ELEMENT2 High Resolution- ICP-MS INSTRUMENT OVERVIEW Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces
Deuterium Gas Analysis by Residual Gas Analyzer
 Journal of Physics: Conference Series Deuterium Gas Analysis by Residual Gas Analyzer To cite this article: B K Das et al 2012 J. Phys.: Conf. Ser. 390 012009 View the article online for updates and enhancements.
Journal of Physics: Conference Series Deuterium Gas Analysis by Residual Gas Analyzer To cite this article: B K Das et al 2012 J. Phys.: Conf. Ser. 390 012009 View the article online for updates and enhancements.
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Plasma Physics Prof. V. K. Tripathi Department of Physics Indian Institute of Technology, Delhi
 Plasma Physics Prof. V. K. Tripathi Department of Physics Indian Institute of Technology, Delhi Module No. # 01 Lecture No. # 22 Adiabatic Invariance of Magnetic Moment and Mirror Confinement Today, we
Plasma Physics Prof. V. K. Tripathi Department of Physics Indian Institute of Technology, Delhi Module No. # 01 Lecture No. # 22 Adiabatic Invariance of Magnetic Moment and Mirror Confinement Today, we
Franck-Hertz Experiment
 Franck-Hertz Experiment Introduction: In 1914, James Franck and Gustav Hertz discovered in the course of their investigations an energy loss in distinct steps for electrons passing through mercury vapor,
Franck-Hertz Experiment Introduction: In 1914, James Franck and Gustav Hertz discovered in the course of their investigations an energy loss in distinct steps for electrons passing through mercury vapor,
(Refer Slide Time 00:09) (Refer Slide Time 00:13)
 (Refer Slide Time 00:09) Mass Spectrometry Based Proteomics Professor Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology, Bombay Mod 02 Lecture Number 09 (Refer
(Refer Slide Time 00:09) Mass Spectrometry Based Proteomics Professor Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology, Bombay Mod 02 Lecture Number 09 (Refer
MAGNETIC DEFLECTION. OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field.
 MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
MOCK cet paper II 2012 (PHYSICS)
 MOCK cet paper II 2012 (PHYSICS) 1. The equations of two sound waves are given by Y 1 = 3 sin 100πt and Y 2 = 4 Sin 150 πt. The ratio of the intensities of sound produced in the medium is 1)1:2 2) 1:4
MOCK cet paper II 2012 (PHYSICS) 1. The equations of two sound waves are given by Y 1 = 3 sin 100πt and Y 2 = 4 Sin 150 πt. The ratio of the intensities of sound produced in the medium is 1)1:2 2) 1:4
Multicusp Sources for Ion Beam Lithography Applications
 LBL-3 6645 UC-406 Multicusp Sources for Ion Beam Lithography Applications K.N. Leung, P. H e n, W.B. Kunkel, Y. Lee, L. Perkins, D. Pickard, M. Sarstedt, M. Weber, and M.D. Williams Accelerator and Fusion
LBL-3 6645 UC-406 Multicusp Sources for Ion Beam Lithography Applications K.N. Leung, P. H e n, W.B. Kunkel, Y. Lee, L. Perkins, D. Pickard, M. Sarstedt, M. Weber, and M.D. Williams Accelerator and Fusion
1. The diagram shows the electric field lines produced by an electrostatic focussing device.
 1. The diagram shows the electric field lines produced by an electrostatic focussing device. Which one of the following diagrams best shows the corresponding equipotential lines? The electric field lines
1. The diagram shows the electric field lines produced by an electrostatic focussing device. Which one of the following diagrams best shows the corresponding equipotential lines? The electric field lines
Chemistry 311: Topic 3 - Mass Spectrometry
 Mass Spectroscopy: A technique used to measure the mass-to-charge ratio of molecules and atoms. Often characteristic ions produced by an induced unimolecular dissociation of a molecule are measured. These
Mass Spectroscopy: A technique used to measure the mass-to-charge ratio of molecules and atoms. Often characteristic ions produced by an induced unimolecular dissociation of a molecule are measured. These
Chapter 33 - Electric Fields and Potential. Chapter 34 - Electric Current
 Chapter 33 - Electric Fields and Potential Chapter 34 - Electric Current Electric Force acts through a field An electric field surrounds every electric charge. It exerts a force that causes electric charges
Chapter 33 - Electric Fields and Potential Chapter 34 - Electric Current Electric Force acts through a field An electric field surrounds every electric charge. It exerts a force that causes electric charges
Question 11.1: Find the
 Question 11.1: Find the (a) maximum frequency, and (b) minimum wavelength of X-rays produced by 30 kv electrons. Potential of the electrons, V = 30 kv = 3 10 4 V Hence, energy of the electrons, E = 3 10
Question 11.1: Find the (a) maximum frequency, and (b) minimum wavelength of X-rays produced by 30 kv electrons. Potential of the electrons, V = 30 kv = 3 10 4 V Hence, energy of the electrons, E = 3 10
Chapter 7 Plasma Basic
 Chapter 7 Plasma Basic Hong Xiao, Ph. D. hxiao89@hotmail.com www2.austin.cc.tx.us/hongxiao/book.htm Hong Xiao, Ph. D. www2.austin.cc.tx.us/hongxiao/book.htm 1 Objectives List at least three IC processes
Chapter 7 Plasma Basic Hong Xiao, Ph. D. hxiao89@hotmail.com www2.austin.cc.tx.us/hongxiao/book.htm Hong Xiao, Ph. D. www2.austin.cc.tx.us/hongxiao/book.htm 1 Objectives List at least three IC processes
Fig. 2.1 I =... A [2] Suggest why it would be impossible for overhead cables carrying an alternating current to float in the Earth s magnetic field.
![Fig. 2.1 I =... A [2] Suggest why it would be impossible for overhead cables carrying an alternating current to float in the Earth s magnetic field. Fig. 2.1 I =... A [2] Suggest why it would be impossible for overhead cables carrying an alternating current to float in the Earth s magnetic field.](/thumbs/78/78653113.jpg) 1 (a) Fig. 2.1 shows a horizontal current-carrying wire placed in a uniform magnetic field. I region of uniform magnetic field wire Fig. 2.1 The magnetic field of flux density 0.070 T is at right angles
1 (a) Fig. 2.1 shows a horizontal current-carrying wire placed in a uniform magnetic field. I region of uniform magnetic field wire Fig. 2.1 The magnetic field of flux density 0.070 T is at right angles
MAGNETIC DEFLECTION. OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field.
 MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
Franck-Hertz Experiment in Neon/Hg
 Franck-Hertz Experiment in Neon/Hg Equipment Franck-Hertz tube (Ne or Hg) & Operating Unit Analog oscilloscope Many Banana cables; 1 BNC cables; 2 BNC-Banana Plug connector Graphing paper Theory This experiment
Franck-Hertz Experiment in Neon/Hg Equipment Franck-Hertz tube (Ne or Hg) & Operating Unit Analog oscilloscope Many Banana cables; 1 BNC cables; 2 BNC-Banana Plug connector Graphing paper Theory This experiment
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Stellar Astrophysics: The Interaction of Light and Matter
 Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Particle physics experiments
 Particle physics experiments Particle physics experiments: collide particles to produce new particles reveal their internal structure and laws of their interactions by observing regularities, measuring
Particle physics experiments Particle physics experiments: collide particles to produce new particles reveal their internal structure and laws of their interactions by observing regularities, measuring
SAM Teachers Guide Atoms, Excited States, and Photons
 SAM Teachers Guide Atoms, Excited States, and Photons Overview This activity focuses on the ability of atoms to store energy and re emit it at a later time. Students explore atoms in an ʺexcitedʺ state
SAM Teachers Guide Atoms, Excited States, and Photons Overview This activity focuses on the ability of atoms to store energy and re emit it at a later time. Students explore atoms in an ʺexcitedʺ state
Charge to Mass Ratio of The Electron
 Physics Topics Charge to Mass Ratio of The Electron If necessary, review the following topics and relevant textbook sections from Serway / Jewett Physics for Scientists and Engineers, 9th Ed. Electric
Physics Topics Charge to Mass Ratio of The Electron If necessary, review the following topics and relevant textbook sections from Serway / Jewett Physics for Scientists and Engineers, 9th Ed. Electric
Interface (backside) & Extraction Lens
 Plasma Interface Interface (backside) & Extraction Lens Extraction Lens (-2000 volts) ION OPTICS Tip of the sampler cone is positioned to be in the region of maximum ionization Ions no longer under control
Plasma Interface Interface (backside) & Extraction Lens Extraction Lens (-2000 volts) ION OPTICS Tip of the sampler cone is positioned to be in the region of maximum ionization Ions no longer under control
RGA modelling and simulation to include pressure dependence in the ion source
 RGA modelling and simulation to include pressure dependence in the ion source Jeyan Sreekumar, Boris Brkic, Tom Hogan and Steve Taylor Mass Spectrometry Group Department of Electrical Engineering and Electronics
RGA modelling and simulation to include pressure dependence in the ion source Jeyan Sreekumar, Boris Brkic, Tom Hogan and Steve Taylor Mass Spectrometry Group Department of Electrical Engineering and Electronics
Physics 228. Momentum and Force Kinetic Energy Relativistic Mass and Rest Mass Photoelectric Effect Energy and Momentum of Photons
 Physics 228 Momentum and Force Kinetic Energy Relativistic Mass and Rest Mass Photoelectric Effect Energy and Momentum of Photons Lorentz Transformations vs. Rotations The Lorentz transform is similar
Physics 228 Momentum and Force Kinetic Energy Relativistic Mass and Rest Mass Photoelectric Effect Energy and Momentum of Photons Lorentz Transformations vs. Rotations The Lorentz transform is similar
EXPERIMENT NO. 2. Electrostatic and Magnetic Deflection of Electrons in a Cathode Ray Tube
 EXPERIMENT NO. Electrostatic and Magnetic Deflection of Electrons in a Cathode Ray Tube Part A. Motion of electrons in an electric field: Introduction The heart of an oscilloscope is the cathode-ray tube
EXPERIMENT NO. Electrostatic and Magnetic Deflection of Electrons in a Cathode Ray Tube Part A. Motion of electrons in an electric field: Introduction The heart of an oscilloscope is the cathode-ray tube
Electron beam scanning
 Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
6.5 Optical-Coating-Deposition Technologies
 92 Chapter 6 6.5 Optical-Coating-Deposition Technologies The coating process takes place in an evaporation chamber with a fully controlled system for the specified requirements. Typical systems are depicted
92 Chapter 6 6.5 Optical-Coating-Deposition Technologies The coating process takes place in an evaporation chamber with a fully controlled system for the specified requirements. Typical systems are depicted
CBSE Sample Paper 1. Question 4 What are the maximum and minimum values of power factor in a LCR circuit and under what conditions?
 CBSE Sample Paper General Instruction:. Answer all questions. Internal choices are provided for some questions 3. Question numbers to 8 are very short answer questions and carry mark each. 4. Question
CBSE Sample Paper General Instruction:. Answer all questions. Internal choices are provided for some questions 3. Question numbers to 8 are very short answer questions and carry mark each. 4. Question
20.2 Ion Sources. ions electrospray uses evaporation of a charged liquid stream to transfer high molecular mass compounds into the gas phase as MH n
 20.2 Ion Sources electron ionization produces an M + ion and extensive fragmentation chemical ionization produces an M +, MH +, M +, or M - ion with minimal fragmentation MALDI uses laser ablation to transfer
20.2 Ion Sources electron ionization produces an M + ion and extensive fragmentation chemical ionization produces an M +, MH +, M +, or M - ion with minimal fragmentation MALDI uses laser ablation to transfer
The Larmor Formula (Chapters 18-19)
 2017-02-28 Dispersive Media, Lecture 12 - Thomas Johnson 1 The Larmor Formula (Chapters 18-19) T. Johnson Outline Brief repetition of emission formula The emission from a single free particle - the Larmor
2017-02-28 Dispersive Media, Lecture 12 - Thomas Johnson 1 The Larmor Formula (Chapters 18-19) T. Johnson Outline Brief repetition of emission formula The emission from a single free particle - the Larmor
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
1P22/1P92 Exam Review Problems 2013 Friday, January 14, :03 AM. Chapter 20
 Exam Review Problems 2011 Page 1 1P22/1P92 Exam Review Problems 2013 Friday, January 14, 2011 10:03 AM Chapter 20 True or false? 1 It's impossible to place a charge on an insulator, because no current
Exam Review Problems 2011 Page 1 1P22/1P92 Exam Review Problems 2013 Friday, January 14, 2011 10:03 AM Chapter 20 True or false? 1 It's impossible to place a charge on an insulator, because no current
hν' Φ e - Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous?
 Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
 Dual Nature of Matter and Radiation 9. The work function of a certain metal is 3.3 J. Then the maximum kinetic energy of photoelectrons emitted by incident radiation of wavelength 5 A is- ).48 ev ).4 ev
Dual Nature of Matter and Radiation 9. The work function of a certain metal is 3.3 J. Then the maximum kinetic energy of photoelectrons emitted by incident radiation of wavelength 5 A is- ).48 ev ).4 ev
