Supported molybdenum oxides as effective catalysts for the catalytic fast pyrolysis of lignocellulosic biomass
|
|
|
- Job Barber
- 5 years ago
- Views:
Transcription
1 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 ELECTRONIC SUPPLEMENTARY INFORMATION Supported molybdenum oxides as effective catalysts for the catalytic fast pyrolysis of lignocellulosic biomass Karthick Murugappan, a Calvin Mukarakate, b Sridhar Budhi, b Manish Shetty, a Mark R. Nimlos* b and Yuriy Román- Leshkov* a a Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge MA, yroman@mit.edu b National Renewable Energy Laboratory, Denver West Parkway, Golden, CO , USA. Mark.Nimlos@nrel.gov
2 He/H 2 1g of 10 wt% of MoO 3 /Support Support = TiO 2 or ZrO 2 Catalytic fast pyrolysis T = 500 C Orifice Molecular Beam M + Triple quadrupole mass spectrometer Q1 Q2 Q3 Detector He/Ar 50 mg/boat of pine T = 500 C Free-jet expansion 10-1 Torr 10-5 Torr [Precursor] [Product] + Torr Ar MBMS 10-7 Torr Fig. S1 Schematic of horizontal reactor-mbms set up. Typically, pine boats of 50 mg each were pyrolysed sequentially and flown over 1.0 g of catalyst in a 400 cm 3 min -1 of 50 vol% H 2 -He mixture. Prior to sampling by the MBMS, the H 2 -He gas mixture was further diluted with more He (4000 cm 3 min -1 ). A dilute stream of Argon (40 cm 3 min -1 ) was mixed into the He diluent stream to serve as an internal standard.
3 Fig. S2 Schematic of tandem micropyrolyzer-gcms set up Typically, pine boats of ca. 500 g each were pyrolysed sequentially and the gases were contacted over 40 mg of catalyst in a 197 cm 3 min -1 flow of 71 vol% H 2 -He mixture.
4 Table S1 Products identified by GCMS after the CFP of first pine boat over 10 wt% MoO 3 /TiO 2 in the tandem micropyrolyzer. Retention time/min m/z Compound Structure carbon dioxide O C O propene acetaldehyde methyl-1-propene O 1.94, butene Acetone Furan O O pentene methyl-2-butene ,3-cyclopentadiene cyclopentene Butanone O methylfuran O methyl-1,3-cyclopentadiene methyl-1,3-cyclopentadiene benzene methyl-1,4-Cyclohexadiene methylcyclohexene toluene ethylbenzene p-xylene styrene
5 m-xylene propylbenzene ethyl-3-methyllbenzene ,3,5-trimethylbenzene ethyl-2-methylbenzene ,3,5-trimethylbenzene ,2,3-trimethylbenzene indane indene methyl-3-propylbenzene ethyl-1,4-dimethylbenzene ethyl-2,4-dimethylbenzene (2-methyl-1-propenyl)benzene ,3-dihydro-4-methyl-1H-indene methyl-1H-indene methyl-1H-indene naphthalene ,3-dihydro-1,2-dimethyl-1H-indene 10.71, 10.75, ,3-dimethyl-1H-indene methylnaphthalene methylnaphthalene
6 ethenylnaphthalene ,2-dimethyl-naphthalene ,3-dimethylnaphthalene ,3-dimethylnaphthalene ,6-dimethylnaphthalene ,7-dimethylnaphthalene ,3-dimethylnaphthalene methyl-1,1'-Biphenyl ,4,5-trimethylnaphthalene fluorene diphenylmethane 13.42, methyl-9H-Fluorene phenanthrene ,4,5,7-tetramethylphenanthrene
7 Relative Intensities CO 2 boat 3 boat time(min) boat 1 Fig. S3 Product distribution of CFP of pine over 40 mg of 10 wt% MoO 3 /ZrO 2 in the micropyrolyzer-gcms set up. Reaction conditions: catalyst = 40 mg, biomass = 3 boats of 0.5 mg pine, T = 500 o C, P total = bar (71 vol% H 2 -He)
8 Table S2 Products identified by GCMS after CFP of first pine boat over 10 wt% MoO 3 /ZrO 2 in the tandem micropyrolyzer. Retention time/min m/z Compound Structure ethane carbon dioxide O C O Propene isobutane methyl-1-propene Butane 1.94, butene pentene methyl-2-butene ,3-cyclopentadiene cyclopentene Benzene Toluene ethylbenzene p-xylene m-xylene propylbenzene ethyl-3-methyllbenzene ,3,5-trimethylbenzene ethyl-2-methylbenzene ,3,5-trimethylbenzene
9 ,2,3-trimethylbenzene indane indene methyl-3-propylbenzene ethyl-1,4-dimethylbenzene ethyl-2,4-dimethylbenzene (2-methyl-1-propenyl)benzene ,3-dihydro-4-methyl-1H-indene methyl-1H-indene methyl-1H-indene naphthalene ,3-dihydro-1,2-dimethyl-1H-indene 10.70, ,3-dimethyl-1H-indene methylnaphthalene methylnaphthalene biphenyl ,2-dimethyl-naphthalene ,3-dimethylnaphthalene ,6-dimethylnaphthalene ,7-dimethylnaphthalene
10 ,3-dimethylnaphthalene methyl-1,1'-Biphenyl ,4,5-trimethylnaphthalene fluorene methyl-9H-Fluorene phenanthrene methylphenanthrene methylanthracene
11 (a) H O H Relative Intensities boat 3 boat time(min) boat 1 (b) H O H Relative Intensities TiO 2 MoO 3 /TiO time(min) Fig. S4a GCMS chromatograms of the CFP of 3 pine boats over 40 mg of bare TiO 2 support in the micropyrolyzer-gcms set up. The TiO 2 support shows minimal catalytic activity, and the deoxygenated products decrease in intensity when more pine boats are fed. Fig. S4b Overlay of GCMS spectra of CFP of the 3 rd pine boat over MoO 3 /TiO 2 and support TiO 2 in the micropyrolyzer-gcms set up. The support TiO 2 shows less intense product peaks than MoO 3 /TiO 2.Reaction conditions: catalysts= 40 mg, biomass = 3 boats of 0.5 mg pine, T = 500 o C, P total = bar (71 vol% H 2 -He). The cryo-trap temperature was set to -80 C in both experiments, thereby explaining the absence of alkenes and alkanes.
12 Relative Intensities boat 3 boat time(min) boat 1 Fig. S5 GCMS chromatograms of the CFP of 3 pine boats over 40 mg of bare ZrO 2 support in the micropyrolyzer-gcms set up. The ZrO 2 support shows negligible catalytic activity under the reaction conditions investigated. Reaction conditions: catalyst = 40 mg of ZrO 2, biomass = 3 boats of 0.5 mg pine, T = 500 o C, P total = bar (71 vol% H 2 -He)
13 H O H Relative Intensities boat 4 boat 3 boat time(min) boat 1 Fig. S6 Product distribution of CFP of pine over 40 mg of MoO 3 in the micropyrolyzer-gcms set up. Reaction conditions: catalyst = 40 mg, biomass = 4 boats of 0.5 mg pine, T = 500 o C, P total = bar (71 vol% H 2 -He). The cryo-trap temperature was set to -80 C in this experiment, thereby explaining the absence of alkenes and alkanes.
14 Table S3 Average product yields and selectivity values for the CFP of 3 pine boats over MoO 3 /TiO 2, MoO 3 /ZrO 2 and HZSM-5 in the micropyrolyzer-gcms system. Catalyst MoO 3 /TiO 2 MoO 3 /ZrO 2 HZSM-5 Overall Carbon Yield (%) Aromatic hydrocarbons Olefins Paraffins Oxygenates CO Coke Char Total Aromatic hydrocarbons selectivity (%) Benzene Toluene Xylene Multi-substituted benzenes Naphthalenes Indanes/Indenes Others Olefins selectivity (%) Ethylene Propene Butene Methylpropene Pentene Methylbutene Cyclopentadiene Cyclopentene Cyclohexadiene Others Paraffins selectivity (%) Ethane Butane Dimethylcyclopropane Cyclopentane Isobutane
15 Table S4 Reaction conditions and product distribution from the CFP of biomass over HZSM-5. 1, 2 Catalyst HZSM-5 HZSM-5 Biomass pinewood Hybrid poplar Reactor type micropyrolyzer micropyrolyzer Pyrolysis temperature 550 C 500 C Upgrading temperature 550 C 500 C Biomass: Catalyst ratio Overall Carbon Yield (%) Aromatic hydrocarbons Olefins N.A. c 7.7 Char N.A. c 18.3 Coke N.A. c 26.5 Light gases N.A. c 21.6 Total 89.4 Aromatic hydrocarbons selectivity (%) Benzene Toluene Xylene C 9 aromatics a C 10+ aromatics b Olefins selectivity (%) Ethylene N.A. c 50.5 Propene N.A. c 43.7 Butene N.A. c 5.9 Light gases selectivity (%) CO N.A. c 69.4 CO 2 N.A. c 30.6 a C 9 aromatics: indane, indene, alkylbenzenes b C 10+ aromatics: naphthalenes and higher polyaromatics c N.A. not quantified in the study
16 MoO 3 Intensity (A.U.) MoO 3 /ZrO 2 GCMS spent MoO 3 /ZrO 2 MBMS spent MoO 3 /ZrO 2 fresh ZrO degrees) Fig. S7 Normalised PXRD patterns of the fresh and spent MoO 3 /ZrO 2 catalysts in comparison with fresh ZrO 2 and MoO 3 samples. The spent MoO 3 /ZrO 2 samples from both reactor systems were derived after experiments shown in Fig. 3(b) and S3.
17 MoO 3 /TiO 2 MoO 3 /ZrO 2 Ln Ln(1/(1-X)) y = x y = x Biomass:MoO 3 ratio Fig. S8 Deactivation profiles for MoO 3 /TiO 2 and MoO 3 /ZrO 2 for CFP of pine in the horizontal reactor-mbms set up fitted to a first-order deactivation model.
18 References 1. S. Thangalazhy-Gopakumar, S. Adhikari, R. B. Gupta, M. Tu and S. Taylor, Bioresour. Technol., 2011, 102, K. Wang, P. A. Johnston and R. C. Brown, Bioresour. Technol., 2014, 173,
Selective Hydrogenation of PyGas over Palladium Catalysts
 Selective Hydrogenation of PyGas over Palladium Catalysts S David Jackson Centre for Catalysis Research, Dept of Chemistry, University of Glasgow, Glasgow, UK. Jean-Marc Bader and Gildas Rolland, (Axens),
Selective Hydrogenation of PyGas over Palladium Catalysts S David Jackson Centre for Catalysis Research, Dept of Chemistry, University of Glasgow, Glasgow, UK. Jean-Marc Bader and Gildas Rolland, (Axens),
Supplement of Open burning of rice, corn and wheat straws: primary emissions, photochemical aging, and secondary organic aerosol formation
 Supplement of Atmos. Chem. Phys., 17, 14821 14839, 2017 https://doi.org/10.5194/acp-17-14821-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 4.0 License.
Supplement of Atmos. Chem. Phys., 17, 14821 14839, 2017 https://doi.org/10.5194/acp-17-14821-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 4.0 License.
PETE 203: Properties of oil
 PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
Catalytic Aromatization of Methane
 Catalytic Aromatization of Methane N.I.FAYZULLAYEV* 1, S.M.TUROBJONOV 2 1 Department of Natural Sciences, Division of Chemistry, Samarkand State University, Samarkand, Uzbekistan 2 Tashkent chemistry-technology
Catalytic Aromatization of Methane N.I.FAYZULLAYEV* 1, S.M.TUROBJONOV 2 1 Department of Natural Sciences, Division of Chemistry, Samarkand State University, Samarkand, Uzbekistan 2 Tashkent chemistry-technology
Texas Commission on Environmental Quality INTEROFFICE MEMORANDUM
 Texas Commission on Environmental Quality INTEROFFICE MEMORANDUM To: Lorinda Gardner, Director, R15 Date: Carlos Rubinstein, Texas Border Area Director From: Valerie E. Meyers, Ph.D. Toxicology Section,
Texas Commission on Environmental Quality INTEROFFICE MEMORANDUM To: Lorinda Gardner, Director, R15 Date: Carlos Rubinstein, Texas Border Area Director From: Valerie E. Meyers, Ph.D. Toxicology Section,
General Chemistry Unit 7A ( )
 Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides General Chemistry Unit 7A (2017-2018) 1 2 3 4 Parent Chain: Methane Ethane CH4 C2H6 Propane C3H8
Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides General Chemistry Unit 7A (2017-2018) 1 2 3 4 Parent Chain: Methane Ethane CH4 C2H6 Propane C3H8
Chapter 22. Organic and Biological Molecules
 Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
Organic Chemistry. Saturated Hydrocarbons: The Alkanes. ethane H C C H CH 3 CH 3
 rganic hemistry The classification of chemical compounds in to the general areas of organic and inorganic derives from the use of the "mineral, vegetable and animal" designation by the early workers in
rganic hemistry The classification of chemical compounds in to the general areas of organic and inorganic derives from the use of the "mineral, vegetable and animal" designation by the early workers in
UNSATURATED HYDROCARBONS
 C20 09/16/2013 15:47:28 Page 295 CHAPTER 20 UNSATURATED HYDROCARBONS SOLUTIONS TO REVIEW QUESTIONS 1. The sigma bond in the double bond of ethene is formed by the overlap of two sp 2 electron orbitals
C20 09/16/2013 15:47:28 Page 295 CHAPTER 20 UNSATURATED HYDROCARBONS SOLUTIONS TO REVIEW QUESTIONS 1. The sigma bond in the double bond of ethene is formed by the overlap of two sp 2 electron orbitals
TRANSFORMATION OF ACETONE AND ISOPROPANOL TO HYDROCARBONS USING HZSM-5 CATALYST. A Thesis SEBASTIAN TACO VASQUEZ
 TRANSFORMATION OF ACETONE AND ISOPROPANOL TO HYDROCARBONS USING HZSM-5 CATALYST A Thesis by SEBASTIAN TACO VASQUEZ Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment
TRANSFORMATION OF ACETONE AND ISOPROPANOL TO HYDROCARBONS USING HZSM-5 CATALYST A Thesis by SEBASTIAN TACO VASQUEZ Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment
Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
HYDROCARBON CHARACTERIZATION OF FACE AROMATIC STREAMS
 FINAL REPORT HYDROCARBON CHARACTERIZATION OF FACE AROMATIC STREAMS R. Gieleciak, D. Hager, C. Lay, and C. Fairbridge. CanmetENERGY DEVON Work performed for: CanmetENERGY, Natural Resources Canada; Fuels
FINAL REPORT HYDROCARBON CHARACTERIZATION OF FACE AROMATIC STREAMS R. Gieleciak, D. Hager, C. Lay, and C. Fairbridge. CanmetENERGY DEVON Work performed for: CanmetENERGY, Natural Resources Canada; Fuels
Authorized By: For Intertek Testing Services Ltd., Shanghai. Joanne Li Deputy General Manager
 Applicant: BEIJING FNFOG SECURITY TECHNOLOGY Date: Aug 20, 2018 ROOM 317, BUILD A, NO. 25, YONGTAI ROAD, HAIDIAN DISTRICT, BEIJING Attn: DONG JIANG Sample Description: One (1 ) groups/pieces of submitted
Applicant: BEIJING FNFOG SECURITY TECHNOLOGY Date: Aug 20, 2018 ROOM 317, BUILD A, NO. 25, YONGTAI ROAD, HAIDIAN DISTRICT, BEIJING Attn: DONG JIANG Sample Description: One (1 ) groups/pieces of submitted
Role of products and intermediates in bioethanol conversion to hydrocarbons on H-ZSM-5: A time-resolved study
 Role of products and intermediates in bioethanol conversion to hydrocarbons on H-ZSM-5: A time-resolved study Rakesh Batchu, Vladimir V. Galvita, Konstantinos Alexopoulos, Kristof Van der Borght, Hilde
Role of products and intermediates in bioethanol conversion to hydrocarbons on H-ZSM-5: A time-resolved study Rakesh Batchu, Vladimir V. Galvita, Konstantinos Alexopoulos, Kristof Van der Borght, Hilde
Unsaturated hydrocarbons. Chapter 13
 Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
EARLY CARBONIZATION CHEMISTRY OF FCC DECANT OILS HEATED IN A FLOW REACTOR
 EARLY CARBONIZATION CHEMISTRY OF FCC DECANT OILS HEATED IN A FLOW REACTOR Richard P. Dutta, Kevin Kelleher, and Semih Eser The Energy Institute, Penn State University 29 Academic Projects Bldg, University
EARLY CARBONIZATION CHEMISTRY OF FCC DECANT OILS HEATED IN A FLOW REACTOR Richard P. Dutta, Kevin Kelleher, and Semih Eser The Energy Institute, Penn State University 29 Academic Projects Bldg, University
Organic Chemistry. Organic chemistry is the chemistry of compounds containing carbon.
 Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Jonathan M. Liebmann et al. Correspondence to: John N. Crowley
 Supplement of Atmos. Chem. Phys., 18, 12045 12059, 2018 https://doi.org/10.5194/acp-18-12045-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License.
Supplement of Atmos. Chem. Phys., 18, 12045 12059, 2018 https://doi.org/10.5194/acp-18-12045-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License.
16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond.
 CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
Production of Green Aromatics and Olefins from Lignocellulosic Biomass by Catalytic Fast Pyrolysis: Chemistry, Catalysis, and Process Development
 University of Massachusetts Amherst ScholarWorks@UMass Amherst Open Access Dissertations 5-2012 Production of Green Aromatics and Olefins from Lignocellulosic Biomass by Catalytic Fast Pyrolysis: Chemistry,
University of Massachusetts Amherst ScholarWorks@UMass Amherst Open Access Dissertations 5-2012 Production of Green Aromatics and Olefins from Lignocellulosic Biomass by Catalytic Fast Pyrolysis: Chemistry,
Alkylation process, Feedstocks, reactions, products, catalysts and effect of process variables.
 Alkylation process, Feedstocks, reactions, products, catalysts and effect of process variables. Catalytic Alkylation [1 7] Catalytic alkylation process is used in refineries to upgrade light olefins (produced
Alkylation process, Feedstocks, reactions, products, catalysts and effect of process variables. Catalytic Alkylation [1 7] Catalytic alkylation process is used in refineries to upgrade light olefins (produced
Relative Sensitivity RS Measurements of Gases
 Gas Analysis Relative Sensitivity RS Measurements of Gases Introduction This note describes the main factors that influence the relative sensitivity factor (RSF) of a mass spectrometer and describes how
Gas Analysis Relative Sensitivity RS Measurements of Gases Introduction This note describes the main factors that influence the relative sensitivity factor (RSF) of a mass spectrometer and describes how
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
Gore Amplified Geochemical Imaging SM -
 Gore Amplified Geochemical Imaging SM - The advanced tool for derisking HC exploration FINDING PETROLEUM, Feb 15, 2011 Presented by Dirk Hellwig, W.L. Gore & Associates Today s Menu Innovation Runs through
Gore Amplified Geochemical Imaging SM - The advanced tool for derisking HC exploration FINDING PETROLEUM, Feb 15, 2011 Presented by Dirk Hellwig, W.L. Gore & Associates Today s Menu Innovation Runs through
Author. Abstract. Introduction
 Improved Performance for the Analysis of Aromatics in Gasoline by ASTM Method D5769 Using the Agilent 5973 inert Gas Chromatography/Mass Spectrometry System Application Author James D. McCurry Agilent
Improved Performance for the Analysis of Aromatics in Gasoline by ASTM Method D5769 Using the Agilent 5973 inert Gas Chromatography/Mass Spectrometry System Application Author James D. McCurry Agilent
J. Li et al. Correspondence to: S. D. Xie
 Supplement of Atmos. Chem. Phys., 15, 7945 7959, 2015 http://www.atmos-chem-phys.net/15/7945/2015/ doi:10.5194/acp-15-7945-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Characterization
Supplement of Atmos. Chem. Phys., 15, 7945 7959, 2015 http://www.atmos-chem-phys.net/15/7945/2015/ doi:10.5194/acp-15-7945-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Characterization
CEE 772: Instrumental Methods in Environmental Analysis
 Updated: 11 November 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #19 Mass Spectrometry: Basics (Skoog, Chapt. 11, 26, 27, 28, pp.253 271, 674 693 718 721, 738 739)
Updated: 11 November 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #19 Mass Spectrometry: Basics (Skoog, Chapt. 11, 26, 27, 28, pp.253 271, 674 693 718 721, 738 739)
CEE 772: Instrumental Methods in Environmental Analysis
 Updated: 11 November 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #19 Mass Spectrometry: Basics (Skoog, Chapt. 11, 26, 27, 28, pp.253-271, 674-693 718-721, 738-739)
Updated: 11 November 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #19 Mass Spectrometry: Basics (Skoog, Chapt. 11, 26, 27, 28, pp.253-271, 674-693 718-721, 738-739)
Product Brief. - Hydrocarbons alkanes, alkenes, alkynes, dienes including natural gas, refinery gas, liquified petroleum gas
 Agilent Porous Polymer PLOT Columns: New Products, Expanded Uses, Prices Cut in Half! Product Brief Need improved resolution of small volatile compounds? Didn't try a PLOT column due to high price, short
Agilent Porous Polymer PLOT Columns: New Products, Expanded Uses, Prices Cut in Half! Product Brief Need improved resolution of small volatile compounds? Didn't try a PLOT column due to high price, short
Category Identity Profile
 Category Identity Profile This CIP represents the boundary substance compositions in the Joint Registrations for substances in Category G under the EU legislation REACH 1. It is included in the Lead Registrant
Category Identity Profile This CIP represents the boundary substance compositions in the Joint Registrations for substances in Category G under the EU legislation REACH 1. It is included in the Lead Registrant
Tar measurement by the Solid Phase Adsorption (SPA) method
 Tar measurement by the Solid Phase Adsorption (SPA) method A.J. Grootjes Presented at the 19th European Biomass Conference and Exhibition (EU BC&E), ICC Berlin, Germany (Conference 6-10 June 2011 - Exhibition
Tar measurement by the Solid Phase Adsorption (SPA) method A.J. Grootjes Presented at the 19th European Biomass Conference and Exhibition (EU BC&E), ICC Berlin, Germany (Conference 6-10 June 2011 - Exhibition
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate
 1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
Organic Chemistry. Pre-lab Assignment. Purpose. Background. Experiment 14. Before coming to lab: Hydrocarbons. Read the lab thoroughly.
 Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
CHAPTER 15. Practice exercises 15.1
 CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
Supplement of Secondary formation of nitrated phenols: insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014
 Supplement of Atmos. Chem. Phys., 16, 2139 2153, 2016 http://www.atmos-chem-phys.net/16/2139/2016/ doi:10.5194/acp-16-2139-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Secondary
Supplement of Atmos. Chem. Phys., 16, 2139 2153, 2016 http://www.atmos-chem-phys.net/16/2139/2016/ doi:10.5194/acp-16-2139-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Secondary
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION
 CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
Hydrocarbons from a Renewable Resource with Zeolite Catalyst
 OSAKA GAS FOUNDATION OF INTERNATIONAL CULTURAL EXCHANGE RESEARCH CENTER FOR SCIENCE AND TECHNOLOGY UNIVERSITY OF INDONESIA RESEARCH GRANT PROGRAM FINAL REPORT A Sustainable Production of C 3 Hydrocarbons
OSAKA GAS FOUNDATION OF INTERNATIONAL CULTURAL EXCHANGE RESEARCH CENTER FOR SCIENCE AND TECHNOLOGY UNIVERSITY OF INDONESIA RESEARCH GRANT PROGRAM FINAL REPORT A Sustainable Production of C 3 Hydrocarbons
CHEMISTRY Statistics: 74 pts (74%) 94 pts (94%) 34 pts (34%) 26 (55%) 5 (11%)
 CEMISTRY 314-01 MITERM # 1 answer key bruary 06, 2007 Statistics: Average: 74 pts (74%) ighest: 94 pts (94%) Lowest: 34 pts (34%) Number of students performing at or above average: 26 (55%) Number of students
CEMISTRY 314-01 MITERM # 1 answer key bruary 06, 2007 Statistics: Average: 74 pts (74%) ighest: 94 pts (94%) Lowest: 34 pts (34%) Number of students performing at or above average: 26 (55%) Number of students
Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification
 Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification Milena Nowakowska, Olivier Herbinet, Anthony Dufour, Pierre-Alexandre Glaude
Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification Milena Nowakowska, Olivier Herbinet, Anthony Dufour, Pierre-Alexandre Glaude
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Analysis of Bakken Oil Samples
 Analysis of Bakken Oil Samples conducted by Iowa State University Chemical Instrumentation Facility July-August 2016 A local landowner obtained a small volume of oil from an employee at an undisclosed
Analysis of Bakken Oil Samples conducted by Iowa State University Chemical Instrumentation Facility July-August 2016 A local landowner obtained a small volume of oil from an employee at an undisclosed
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM OUTLINE
 DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM OUTLINE DESCRIPTION OF MECHANISM MECHANISM GENERATION SYSTEM ESTIMATION METHODS MECHANISM EVALUATION LUMPED MECHANISM FOR AIRSHED MODELS UPDATED
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM OUTLINE DESCRIPTION OF MECHANISM MECHANISM GENERATION SYSTEM ESTIMATION METHODS MECHANISM EVALUATION LUMPED MECHANISM FOR AIRSHED MODELS UPDATED
ABSTRACT INTRODUCTION
 Experimental study of the structure of a lean premixed indane/ch 4 /O 2 /Ar flame E. Pousse, P.A. Glaude, R. Fournet, F.Battin-Leclerc Département de Chimie-Physique des Réactions, UMR 763 CNRS Nancy Université,
Experimental study of the structure of a lean premixed indane/ch 4 /O 2 /Ar flame E. Pousse, P.A. Glaude, R. Fournet, F.Battin-Leclerc Département de Chimie-Physique des Réactions, UMR 763 CNRS Nancy Université,
PROCESS TECHNOLOGY- ORGANIC II. 51. Gas phase dehydrogenation of ethyl-benzene to styrene occurs over catalyst based on
 PROCESS TECHNOLOGY- ORGANIC II 51. Gas phase dehydrogenation of ethyl-benzene to styrene occurs over catalyst based on (a) iron oxide, (b) silica alumina, (c) titanium dioxide, (d) sodium silicate, 52.
PROCESS TECHNOLOGY- ORGANIC II 51. Gas phase dehydrogenation of ethyl-benzene to styrene occurs over catalyst based on (a) iron oxide, (b) silica alumina, (c) titanium dioxide, (d) sodium silicate, 52.
Report No. 46. by PARK L. MORSE. January A private report by the PROCESS ECONOMICS PROGRAM PARK, CALIFORNIA STANFORD RESEARCH INSTITUTE I
 Report No. 46 MALEIC ANHYDRIDE by PARK L. MORSE January 1969 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK, CALIFORNIA CONTENTS 1 INTRODUCTION.........................
Report No. 46 MALEIC ANHYDRIDE by PARK L. MORSE January 1969 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK, CALIFORNIA CONTENTS 1 INTRODUCTION.........................
CONVERSION OF CROP OIL TO AROMATICS OVER DOPED ZSM-5 CATALYSTS
 CNVERSIN F CRP IL T ARMATICS VER DPED ZSM-5 CATALYSTS Presented by: Swapnil Fegade Department of Chemical Engineering University of North Dakota Contributors: Swastika Bithi Dr. Brian Tande Dr. Wayne Seames
CNVERSIN F CRP IL T ARMATICS VER DPED ZSM-5 CATALYSTS Presented by: Swapnil Fegade Department of Chemical Engineering University of North Dakota Contributors: Swastika Bithi Dr. Brian Tande Dr. Wayne Seames
Organic Chemistry Worksheets
 Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Table of Physical Properties for Hydrocarbons and Other Compounds of Interest to the Natural Gas and Natural Gas Liquids Industries
 GPA Midstream Standard 2145-16 Table of Physical Properties for Hydrocarbons and Other Compounds of Interest to the Natural Gas and Natural Gas Liquids Industries (Includes data previously published in
GPA Midstream Standard 2145-16 Table of Physical Properties for Hydrocarbons and Other Compounds of Interest to the Natural Gas and Natural Gas Liquids Industries (Includes data previously published in
2. Hydrocarbons. 2.1 Composition of Petroleum
 2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
Zn/H-ZSM-5 zeolite as catalyst for benzene alkylation with isobutane
 Prog. Catal, Vol. 13, No. 1-2, pp. 35-41 (24) Prog. Catal. Zn/H-ZSM-5 zeolite as catalyst for benzene alkylation with isobutane Adriana Urdă *, Ioan Săndulescu, Ioan-Cezar Marcu University of Bucharest,
Prog. Catal, Vol. 13, No. 1-2, pp. 35-41 (24) Prog. Catal. Zn/H-ZSM-5 zeolite as catalyst for benzene alkylation with isobutane Adriana Urdă *, Ioan Săndulescu, Ioan-Cezar Marcu University of Bucharest,
Continuous Improvement in Petroleum/Petrochemical Analysis HP s Family of Innovative PLOT Columns
 Continuous Improvement in Petroleum/Petrochemical Analysis HP s Family of Innovative PLOT Columns Brochure Brief Porous Layer Open Tubular (PLOT) columns have been replacing traditional packed columns
Continuous Improvement in Petroleum/Petrochemical Analysis HP s Family of Innovative PLOT Columns Brochure Brief Porous Layer Open Tubular (PLOT) columns have been replacing traditional packed columns
3. What number would be used to indicate the double bond position in the IUPAC name for CH 3 CH 2 CH=CH CH 3 a. 1 b. 2 c. 3 d.
 Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Pellistor Relative Responses
 Pellistor Relative Responses PELLISTOR RELATIVE RESPONSES SGX Sensortech are often asked for relative response figures when using the company s range of pellistors for non-straight-chain hydrocarbons and
Pellistor Relative Responses PELLISTOR RELATIVE RESPONSES SGX Sensortech are often asked for relative response figures when using the company s range of pellistors for non-straight-chain hydrocarbons and
The characterization by chromatographic methods of petroleum fractions from thermocatalytical processes
 Ovidius University Annals of Chemistry Volume 20, Number 1, pp. 5-10, 2009 The characterization by chromatographic methods of petroleum fractions from thermocatalytical processes Claudia Irina KONCSAG
Ovidius University Annals of Chemistry Volume 20, Number 1, pp. 5-10, 2009 The characterization by chromatographic methods of petroleum fractions from thermocatalytical processes Claudia Irina KONCSAG
Kinetic Characterisation of Zeolite Catalysts Using Cracking, Alkylation and Other Chemical Reactions
 Haldor Topsøe Catalysis Forum Munkerupgaard, 27-28 August 2015 Kinetic Characterisation of Zeolite Catalysts Using Cracking, Alkylation and Other Chemical Reactions Dmitry B. Lukyanov Catalysis & Reaction
Haldor Topsøe Catalysis Forum Munkerupgaard, 27-28 August 2015 Kinetic Characterisation of Zeolite Catalysts Using Cracking, Alkylation and Other Chemical Reactions Dmitry B. Lukyanov Catalysis & Reaction
Change in catalytic activity on acetone conversion to aromatic chemicals using H-ZSM-5
 ISBN 978-979-18962--7 Change in catalytic activity on acetone conversion to aromatic chemicals using H-ZSM-5 Setiadi 1, Slamet 1, Mohammad Nasikin 1, T. Tsutsui 2, T. Kojima 3 1 Jurusan Teknik Kimia, Fakultas
ISBN 978-979-18962--7 Change in catalytic activity on acetone conversion to aromatic chemicals using H-ZSM-5 Setiadi 1, Slamet 1, Mohammad Nasikin 1, T. Tsutsui 2, T. Kojima 3 1 Jurusan Teknik Kimia, Fakultas
CHAPTER HYDROCARBONS. Chapterwise Previous year Qs. (a) Na (b) HCl in H2O (c) KOH in C2H5OH (d) Zn in alcohol. Ans: (c)
 122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
Determination of Hydrocarbon Components in Petroleum Naphthas
 Determination of Hydrocarbon Components in Petroleum Naphthas LECO Corporation; Saint Joseph, Michigan USA Key Words: GC-TOFMS, Petrochemical, Naphtha, Deconvolution, Retention Index 1. Introduction The
Determination of Hydrocarbon Components in Petroleum Naphthas LECO Corporation; Saint Joseph, Michigan USA Key Words: GC-TOFMS, Petrochemical, Naphtha, Deconvolution, Retention Index 1. Introduction The
Chemistry 2202 Unit 3 Test Section 1 &
 Chemistry 2202 Unit 2 Test 2 Section 1 & 2 Page 1 of 6 Chemistry 2202 Unit 3 Test Section 1 & 2-2006 Part 1 Multiple Choice: Complete using the answer form on page 4. (20 points) 1. What is the main idea
Chemistry 2202 Unit 2 Test 2 Section 1 & 2 Page 1 of 6 Chemistry 2202 Unit 3 Test Section 1 & 2-2006 Part 1 Multiple Choice: Complete using the answer form on page 4. (20 points) 1. What is the main idea
CHAPTER 24 Organic Chemistry
 CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
Catalytic Fast Pyrolysis of Biomass in a Bubbling Fluidized Bed Reactor with Gallium Promoted Zsm-5 Catalyst
 University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2012 Catalytic Fast Pyrolysis of Biomass in a Bubbling Fluidized Bed Reactor with Gallium Promoted Zsm-5
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2012 Catalytic Fast Pyrolysis of Biomass in a Bubbling Fluidized Bed Reactor with Gallium Promoted Zsm-5
Transformation of Lower Alkanes into Aromatic. Hydrocarbons over ZSM-5 Zeolites
 Transformation of Lower Alkanes into Aromatic Hydrocarbons over ZSM-5 Zeolites Yoshio ONO*, Hiroyoshi KITAGAWA, and Yoko SENDODA Department of Chemical Engineering, Tokyo Institute of Technology Ookayama,
Transformation of Lower Alkanes into Aromatic Hydrocarbons over ZSM-5 Zeolites Yoshio ONO*, Hiroyoshi KITAGAWA, and Yoko SENDODA Department of Chemical Engineering, Tokyo Institute of Technology Ookayama,
Monomer Analysis. Analysis by Gas Chromatography WASSON - ECE INSTRUMENTATION. Engineered Solutions, Guaranteed Results.
 Monomer Analysis Analysis by Gas Chromatography Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Polymer Grade Monomer Analysis Monomer Analysis Impurities in feedstocks can adversely
Monomer Analysis Analysis by Gas Chromatography Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Polymer Grade Monomer Analysis Monomer Analysis Impurities in feedstocks can adversely
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Kolmetz Handbook of Process Equipment Design BTX EXTRACTION UNIT DESIGN, SIZING AND TROUBLESHOOTING (ENGINEERING DESIGN GUIDELINE)
 Page : 1 of 75 KLM Technology #03-12 Block Aronia, Jalan Sri Perkasa 2 Taman Tampoi Utama 81200 Johor Bahru SOLUTIONS, STANDARDS AND SOFTWARE www.klmtechgroup.com Rev 01- March 2017 Co Author: Rev 01 Yulis
Page : 1 of 75 KLM Technology #03-12 Block Aronia, Jalan Sri Perkasa 2 Taman Tampoi Utama 81200 Johor Bahru SOLUTIONS, STANDARDS AND SOFTWARE www.klmtechgroup.com Rev 01- March 2017 Co Author: Rev 01 Yulis
Chapter Organic Chemistry Basics
 29 Chapter Organic Chemistry Basics 2. A similarity between optical and geometrical isomerism is that [2002] (a) each forms equal number of isomers for a given compound (b) if in a compound one is present
29 Chapter Organic Chemistry Basics 2. A similarity between optical and geometrical isomerism is that [2002] (a) each forms equal number of isomers for a given compound (b) if in a compound one is present
1.3 Reactions of Hydrocarbons
 1.3 Reactions of ydrocarbons All hydrocarbons readily burn in air to give carbon dioxide and water, with the release of large amounts of energy (Figure 1); this chemical reaction accounts for the extensive
1.3 Reactions of ydrocarbons All hydrocarbons readily burn in air to give carbon dioxide and water, with the release of large amounts of energy (Figure 1); this chemical reaction accounts for the extensive
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/330/6008/1222/dc1 Supporting nline Material for Renewable Chemical Commodity Feedstocks from Integrated Catalytic Processing of Pyrolysis ils Tushar P. Vispute, Huiyan
www.sciencemag.org/cgi/content/full/330/6008/1222/dc1 Supporting nline Material for Renewable Chemical Commodity Feedstocks from Integrated Catalytic Processing of Pyrolysis ils Tushar P. Vispute, Huiyan
Thermal decomposition of norbornane. (bicyclo[2.2.1]heptane) dissolved in benzene.
![Thermal decomposition of norbornane. (bicyclo[2.2.1]heptane) dissolved in benzene. Thermal decomposition of norbornane. (bicyclo[2.2.1]heptane) dissolved in benzene.](/thumbs/78/76964261.jpg) Thermal decomposition of norbornane (bicyclo[2.2.1]heptane) dissolved in benzene. Experimental study and mechanism investigation. Olivier HERBINET,* Baptiste SIRJEAN, Frédérique BATIN-LECLERC, René FOURNET
Thermal decomposition of norbornane (bicyclo[2.2.1]heptane) dissolved in benzene. Experimental study and mechanism investigation. Olivier HERBINET,* Baptiste SIRJEAN, Frédérique BATIN-LECLERC, René FOURNET
C11.1 Organic Chemistry Quiz Questions & Answers. Parts 1 & 2; all sets Parts 3 & 4; Sets 1 & 2 only
 C11.1 Organic Chemistry Quiz Questions & Answers Parts 1 & 2; all sets Parts 3 & 4; Sets 1 & 2 only C11.1 Organic Chemistry Part 1 1. Define a mixture. 2. Define crude oil. 3. Define a hydrocarbon. 4.
C11.1 Organic Chemistry Quiz Questions & Answers Parts 1 & 2; all sets Parts 3 & 4; Sets 1 & 2 only C11.1 Organic Chemistry Part 1 1. Define a mixture. 2. Define crude oil. 3. Define a hydrocarbon. 4.
TRANSALKYLATION OF DIISOPROPYLBENZENE WITH BENZENE IN SUPERCRITICAL CARBON DIOXIDE
 TRANSALKYLATION OF DIISOPROPYLBENZENE WITH BENZENE IN SUPERCRITICAL CARBON DIOXIDE J.L. Sotelo, L. Calvo*, D. Capilla, and A. Pérez-Velázquez Departamento de Ingeniería Química, Facultad de Ciencias Químicas,
TRANSALKYLATION OF DIISOPROPYLBENZENE WITH BENZENE IN SUPERCRITICAL CARBON DIOXIDE J.L. Sotelo, L. Calvo*, D. Capilla, and A. Pérez-Velázquez Departamento de Ingeniería Química, Facultad de Ciencias Químicas,
Thermal behaviour/treatment of some vegetable residues. Gas chromatographic analysis of pyrolysis products from pine cone
 Thermal behaviour/treatment of some vegetable residues. Gas chromatographic analysis of pyrolysis products from pine cone Mihai Brebu 1, Cornelia Vasile 1, Jale Yanik 2 1 Petru Poni Institute of Macromolecular
Thermal behaviour/treatment of some vegetable residues. Gas chromatographic analysis of pyrolysis products from pine cone Mihai Brebu 1, Cornelia Vasile 1, Jale Yanik 2 1 Petru Poni Institute of Macromolecular
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
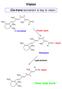 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP012132 TITLE: Fuels Combustion Research: Supercritical Fuel Pyrolysis DISTRIBUTION: Approved for public release, distribution
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP012132 TITLE: Fuels Combustion Research: Supercritical Fuel Pyrolysis DISTRIBUTION: Approved for public release, distribution
Experimental Determination of Pyrolysis Products from Carbon/Resin Ablative Materials
 Experimental Determination of Pyrolysis Products from Carbon/Resin Ablative Materials Hsi-Wu Wong, Jay Peck, and Robin Edwards Aerodyne Research, Inc., Billerica, MA Guillaume Reinisch University of Texas
Experimental Determination of Pyrolysis Products from Carbon/Resin Ablative Materials Hsi-Wu Wong, Jay Peck, and Robin Edwards Aerodyne Research, Inc., Billerica, MA Guillaume Reinisch University of Texas
Unit 12 Organic Chemistry
 Unit 12 Organic Chemistry Day 138 5/5/14 QOD: What is Organic Chemistry? Do Now: True or false? 1. Electrochemical cells generate electricity. 2. Electrons flow from left to right in a battery. 3. Redox
Unit 12 Organic Chemistry Day 138 5/5/14 QOD: What is Organic Chemistry? Do Now: True or false? 1. Electrochemical cells generate electricity. 2. Electrons flow from left to right in a battery. 3. Redox
Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan
 Supporting Information Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan Guangyi Li, a,b Ning Li, a Shanshan Li, a,b Aiqin Wang, a Yu Cong, a Xiaodong Wang a and Tao Zhang a * a State
Supporting Information Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan Guangyi Li, a,b Ning Li, a Shanshan Li, a,b Aiqin Wang, a Yu Cong, a Xiaodong Wang a and Tao Zhang a * a State
Research Article Thermodynamic Equilibrium Analysis of Methanol Conversion to Hydrocarbons Using Cantera Methodology
 Thermodynamics Volume 212, Article ID 1246, 7 pages doi:1.11/212/1246 Research Article Thermodynamic Equilibrium Analysis of Methanol Conversion to Hydrocarbons Using Cantera Methodology Duminda A. Gunawardena
Thermodynamics Volume 212, Article ID 1246, 7 pages doi:1.11/212/1246 Research Article Thermodynamic Equilibrium Analysis of Methanol Conversion to Hydrocarbons Using Cantera Methodology Duminda A. Gunawardena
30. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain your rankings.
 766 hapter 16 Electrophilic Attack on Derivatives of enzene Problems 30. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain
766 hapter 16 Electrophilic Attack on Derivatives of enzene Problems 30. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain
AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES
 JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
Reading Skill Practice
 system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
Production of Benzoic Acid through Catalytic Transformation of Renewable Lignocellulosic Biomass
 CHINESE JOURNAL OF CHEMICAL PHYSICS VOLUME 30, NUMBER 5 OCTOBER 27, 2017 ARTICLE Production of Benzoic Acid through Catalytic Transformation of Renewable Lignocellulosic Biomass Yi-heng Zhang a, Ming-hui
CHINESE JOURNAL OF CHEMICAL PHYSICS VOLUME 30, NUMBER 5 OCTOBER 27, 2017 ARTICLE Production of Benzoic Acid through Catalytic Transformation of Renewable Lignocellulosic Biomass Yi-heng Zhang a, Ming-hui
The SAPRC Chemical Mechanisms
 The SAPR hemical Mechanisms utline William P. L. arter E-ERT, University of alifornia, Riverside December 6, 2006 Major components of the SAPR mechanisms Mechanism generation system Status of SAPR mechanism
The SAPR hemical Mechanisms utline William P. L. arter E-ERT, University of alifornia, Riverside December 6, 2006 Major components of the SAPR mechanisms Mechanism generation system Status of SAPR mechanism
PROCESS ECONOMICS PROGRAM
 Report No. 37 ACETIC ACID by SHIGEYOSHI TAKAOKA March 1968 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 INTRODUCTION........................
Report No. 37 ACETIC ACID by SHIGEYOSHI TAKAOKA March 1968 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 INTRODUCTION........................
Unit 2, Review for Quiz #1: Hydrocarbons
 Unit 2, Review for Quiz #1: Hydrocarbons 1. What is the simplest organic molecule? a) CH 4 c) HCN b) CO 2 d) HC CH 2. Which of the following molecules would be classified as organic? I) CaCO 3 II) C 2
Unit 2, Review for Quiz #1: Hydrocarbons 1. What is the simplest organic molecule? a) CH 4 c) HCN b) CO 2 d) HC CH 2. Which of the following molecules would be classified as organic? I) CaCO 3 II) C 2
Combustible Gas Catalytic Bead (0-100 %LEL) Part No FM Performance Certified 1,4 FM Performance Certified 1
 Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Introduction to GC/MS
 Why Mass Spectrometry? Introduction to GC/MS A powerful analytical technique used to: 1.Identify unknown compounds 2. Quantify known materials down to trace levels 3. Elucidate the structure of molecules
Why Mass Spectrometry? Introduction to GC/MS A powerful analytical technique used to: 1.Identify unknown compounds 2. Quantify known materials down to trace levels 3. Elucidate the structure of molecules
Conversion of methoxy and hydroxyl functionalities of phenolic monomers over zeolites
 Chemical and Biological Engineering Publications Chemical and Biological Engineering 2016 Conversion of methoxy and hydroxyl functionalities of phenolic monomers over zeolites Rajeeva Thilakaratne Iowa
Chemical and Biological Engineering Publications Chemical and Biological Engineering 2016 Conversion of methoxy and hydroxyl functionalities of phenolic monomers over zeolites Rajeeva Thilakaratne Iowa
Supplementary information
 Photosensitized production of functionalized and unsaturated organic compounds at the airsea interface Supplementary information 5 6 7 8 Raluca Ciuraru, Ludovic Fine, Manuela van Pinteren, Barbara D Anna,
Photosensitized production of functionalized and unsaturated organic compounds at the airsea interface Supplementary information 5 6 7 8 Raluca Ciuraru, Ludovic Fine, Manuela van Pinteren, Barbara D Anna,
UNIT (7) ORGANIC COMPOUNDS: HYDROCARBONS
 UNIT (7) RGANI MPUNDS: YDRARBNS rganic chemistry is the study carbon containing compounds. 7.1 Bonding in rganic ompounds rganic compounds are made up of only a few elements and the bonding is almost entirely
UNIT (7) RGANI MPUNDS: YDRARBNS rganic chemistry is the study carbon containing compounds. 7.1 Bonding in rganic ompounds rganic compounds are made up of only a few elements and the bonding is almost entirely
DET REPORT. 1.) CATALYTIC COMBUSTION IONIZATION DETECTION (CCID) - MORE CHARACTERISTICS OF THE SELECTIVITY FOR n PARAFFINS. NO.
 1.) CATALYTIC COMBUSTION IONIZATION DETECTION (CCID) - MORE CHARACTERISTICS OF THE SELECTIVITY FOR n-paraffins. 2.) FINGERPRINT CHROMATOGRAMS OF GASOLINES BY SELECTIVELY DETECTING ALKANE AND OXYGENATE
1.) CATALYTIC COMBUSTION IONIZATION DETECTION (CCID) - MORE CHARACTERISTICS OF THE SELECTIVITY FOR n-paraffins. 2.) FINGERPRINT CHROMATOGRAMS OF GASOLINES BY SELECTIVELY DETECTING ALKANE AND OXYGENATE
CALA Directory of Laboratories
 CALA Directory of Laboratories Membership Number: 2628 Laboratory Name: Caduceon Environmental Laboratories (Richmond Hill) Parent Institution: Caduceon Enterprises Inc. Address: 110 West Beavercreek Rd.
CALA Directory of Laboratories Membership Number: 2628 Laboratory Name: Caduceon Environmental Laboratories (Richmond Hill) Parent Institution: Caduceon Enterprises Inc. Address: 110 West Beavercreek Rd.
Seminar_3. 1. Substituded derivatives of benzene and their nomenclature
 1. Substituded derivatives of benzene and their nomenclature 2. Reactions of arenes. Electrophilic aromatic substitutions 3. Activating substituents. Orientation in the aromatic ring Seminar_3 TEST - Aromatic
1. Substituded derivatives of benzene and their nomenclature 2. Reactions of arenes. Electrophilic aromatic substitutions 3. Activating substituents. Orientation in the aromatic ring Seminar_3 TEST - Aromatic
CHAPTER Identification of side products in the synthesis of MMBC. As shown in the previous chapter, MMBC can be produced with high
 113 CHAPTER 6 IDENTIFICATION OF SIDE PRODUCTS IN THE PRODUCTION OF METHYL 4- (METHOXYMETHYL) BENZENE CARBOXYLATE (MMBC) FROM METHYL 5- (METHOXYMETHYL)-FURAN-2-CARBOXYLATE (MMFC) AND ETHYLENE 6.1 Identification
113 CHAPTER 6 IDENTIFICATION OF SIDE PRODUCTS IN THE PRODUCTION OF METHYL 4- (METHOXYMETHYL) BENZENE CARBOXYLATE (MMBC) FROM METHYL 5- (METHOXYMETHYL)-FURAN-2-CARBOXYLATE (MMFC) AND ETHYLENE 6.1 Identification
4) Interpret in words the equation: P4O10 (s) + 6 H2O (l) 4 H3PO4 (aq)
 CHEM102 Chemistry II Spring 09-10 Mid-term Exam/Faculty of Agricultural Sciences and Technologies Student Registration No: Instructor: Prof.Dr.Hüseyin Oğuz Student Name-Surname: Dept. of Computer Information
CHEM102 Chemistry II Spring 09-10 Mid-term Exam/Faculty of Agricultural Sciences and Technologies Student Registration No: Instructor: Prof.Dr.Hüseyin Oğuz Student Name-Surname: Dept. of Computer Information
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE ADSORPTIVE TRAPPING OF BIO-OIL COMPOUNDS ONTO ACTIVATED CARBON A THESIS SUBMITTED TO THE GRADUATE FACULTY
 UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE ADSORPTIVE TRAPPING OF BIO-OIL COMPOUNDS ONTO ACTIVATED CARBON A THESIS SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE ADSORPTIVE TRAPPING OF BIO-OIL COMPOUNDS ONTO ACTIVATED CARBON A THESIS SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree
Site Specific Conditional Sampler Garfield County, Colorado. VOC Data Summaries. Prepared for
 Site Specific Conditional Sampler Garfield County, Colorado VOC Data Summaries Prepared for Garfield County Public Health 195 West 14 th Street Rifle, Colorado 81650 Prepared by 1901 Sharp Point Dr., Suite
Site Specific Conditional Sampler Garfield County, Colorado VOC Data Summaries Prepared for Garfield County Public Health 195 West 14 th Street Rifle, Colorado 81650 Prepared by 1901 Sharp Point Dr., Suite
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
