HL Chemistry. Tuesday August 25th Tuesday, August 25, 15
|
|
|
- Bonnie Wright
- 5 years ago
- Views:
Transcription
1 HL Chemistry Tuesday August 25th 2015
2 Agenda Warm-Up NONE The Atom Project - Use your class time wisely
3 HOMEWORK Questions? The Atom Project -Group Members? DUE August 31st 2015 Atomic Structure Questions DUE August 26th 2015 Topic 12.1 Study Guide HW DUE September 2nd 2015
4 The Atom Project The aim of this project is to learn about the structure of atoms including: Basic structure in terms of protons, neutrons, and electrons and their position in the atom Isotopes, radio-isotopes, and their uses Calculation of relative atomic mass from experimental data The evidence for energy levels and sub-levels within atoms Atomic emission spectroscopy Ionization energy Full electron configuration - using levels and sublevels Solution of problems involving energy levels, with the use of E=hv
5 The Atom Project Your project must meet the following requirements: Describes and explains the structure of atoms, ions and their isotopes in terms of protons, neutrons, and electrons Discusses the various isotopes of the element, their abundance, and their uses Describes the uses of some common radioisotopes Shows how atomic emission spectroscopy provides evidence for energy levels in the atom
6 The Atom Project Your project must meet the following requirements: Explains how to determine the full electron configuration of atoms (including Cr and Cu) Shows how trends ionization energy across the first 20 elements provides evidence to support the existence of energy levels and sub-levels Explains how to solve problems involving energy levels using E=hv Includes a workbook with questions testing the full range of knowledge, with mark-schemes in the back
7 Electrons in atoms Exceptions to the trend Copper (Cu) and Chromium (Cr) Cr [Ar] Cr [Ar] 4s 1 3d 5 Cu[Ar] Cu[Ar] d orbital will fill up before s orbital when it can become half full or completely full
8 Topic 12.1 Electrons in atoms In an emission spectrum, the limit of convergence at higher frequency corresponds to the first ionization energy. Trends in first ionization energy across periods account for the existence of main energy levels and sub levels in atoms. Successive ionization energy data for an element give information that shows relations to electron configurations.
9 Electrons in atoms Nature of science Experimental evidence to support theories - emission spectra provide evidence for the existence of energy levels.
10 Electron Configuration Review Emission spectra and Bohr s theory of the hydrogen atom colour violet blue blue-green red λ/nm transition n = 6 n = 5 n = 4 to n = 2 n = 3 to n = 2 to to n = 2 n = 2
11 Electron Configuration Review Emission spectra and Bohr s theory of the hydrogen atom n=6 n=5 n=4 n=3 n=2 n=1
12 Electron Configuration Review Emission spectra and Bohr s theory of the hydrogen atom Series nf ni Region of EMS Lyman 1 2,3,4,5,... UV Balmer 2 3,4,5,6,... visible and UV Paschen 3 4,5,6,7,... IR
13 Ionization Energy of Hydrogen Emission spectra and Bohr s theory of the hydrogen atom Ionization process for Hydrogen In an atom, the highest possible energy level corresponds to the frequency at which the lines converge By determining the frequency at which the spectral lines can converge (known as the convergence limit) the ionization energy can be calculated. If enough energy is supplied, the one electron in hydrogen atom can be promoted to the th level and would be said to have been ionized to H + Represented by the equation: H(g) > H + (g) + e -
14 Electrons in atoms Emission spectra and ionization Emission spectra provide experimental evidence for the existence of atomic energy levels. Ionization energy is the minimum amount of energy required to remove an electron from a neutral gaseous atom or molecule in its ground state. First ionization energy (IE1): X(g) --> X + (g) + e - Second ionization energy (IE2): X + (g) --> X 2+ (g) + e - Successive ionization energy (IEn): X (n-1)+ (g) --> X n+ (g) + e - With each successive ionization an electron is being removed from an increasingly positive species, so more energy is required. At higher energy, lines of spectra converge, forming a continuum. Outside of this limit the electron is no longer under the influence of the nucleus and has successfully been ionized.
15 Electrons in atoms Ionization energies of first 20 elements Period 1 - Ionization energy increases Period 2 - Ionization energy increases
16 Electrons in atoms Ionization energies of first 20 elements Why is this? As the electrons are removed the nuclear charge increases across a period due to the increased attraction of the nucleus to the valence electrons! The atomic radius also decreases across a period making the electrostatic force even stronger! COOL!
17 Electrons in atoms Ionization energies of first 20 elements Group 1 - Ionization energy decreases Group 2 - Ionization energy decreases
18 Electrons in atoms Ionization energies of first 20 elements Why is this? As we move down a group, more energy levels are added and making the electrostatic force weaker as the distance between the nucleus and valence electrons increases - there is also an increase in electron shielding which further decreases the strength of the electrostatic force between the nucleus and valence electrons COOL!
19 Electrons in atoms Ionization energies of first 20 elements But WAIT! What about the discrepancies to the trend?
20 Electrons in atoms Exceptions to the trend Why is this? - Be has the electron configuration 1s 2 2s 2 - B has the electron configuration 1s 2 2s 2 2p 1 The electrons in the p orbital are higher in energy and further away from the nucleus and require less energy to remove than electrons in the s orbital - Mg has the electron configuration 1s 2 2s 2 2p 6 3s 2 - Al has the electron configuration 1s 2 2s 2 2p 6 3s 2 2p 1
21 Electrons in atoms Exceptions to the trend Why is this? - N has the electron configuration 1s 2 2s 2 2p 3 - O has the electron configuration 1s 2 2s 2 2p 4 Oxygen has a p orbital that is occupied by two electrons where nitrogen only has one electron in the p orbital. This means less energy is required to remove the electron in a half filled orbital due to the fact that like charges repel one another! P orbital Nitrogen P orbital Oxygen
22 Electrons in atoms Exceptions to the trend Why is this? More stable! Less stable! Orbitals that are empty, full, or half-full are more stable and require more energy to remove electrons!
23 Ionization Energy Trends in the Periodic Table of Elements Increasing ionization energy Increasing ionization energy
24 Topic 12.1 Electrons in atoms In an emission spectrum, the limit of convergence at higher frequency corresponds to the first ionization energy. Trends in first ionization energy across periods account for the existence of main energy levels and sub levels in atoms. Successive ionization energy data for an element give information that shows relations to electron configurations.
25 HOMEWORK Questions? The Atom Project -Group Members? DUE August 31st 2015 Atomic Structure Questions - Chapter(s) DUE August 26th 2015 Topic 12.1 Study Guide HW DUE September 2nd 2015
HL Chemistry. Monday August 24th Monday, August 24, 15
 HL Chemistry Monday August 24th 2015 Agenda Warm Up: Ionization Energy of boron Review Topic 12.1 Atomic Structure Calculating Ionization Energy The Atom Project (?) Warm Up You have 5 minutes ONLY! GO!
HL Chemistry Monday August 24th 2015 Agenda Warm Up: Ionization Energy of boron Review Topic 12.1 Atomic Structure Calculating Ionization Energy The Atom Project (?) Warm Up You have 5 minutes ONLY! GO!
HL Chemistry. Thursday August 20th Thursday, August 20, 15
 HL Chemistry Thursday August 20th 2015 Agenda Warm Up: Electron Configuration Practice Review Topic 2.2 Electron Configuration and begin Topic 12.1 Atomic Structure Warm Up You have 10 minutes ONLY! GO!
HL Chemistry Thursday August 20th 2015 Agenda Warm Up: Electron Configuration Practice Review Topic 2.2 Electron Configuration and begin Topic 12.1 Atomic Structure Warm Up You have 10 minutes ONLY! GO!
SL Chemistry. Monday September 21st Monday, September 21, 15
 SL Chemistry Monday September 21st 2015 Agenda Begin Topic 2 - Atomic Structure The Atom Project - Use your class time wisely The Atom Project The aim of this project is to learn about the structure of
SL Chemistry Monday September 21st 2015 Agenda Begin Topic 2 - Atomic Structure The Atom Project - Use your class time wisely The Atom Project The aim of this project is to learn about the structure of
Topic 3: Periodic Trends and Atomic Spectroscopy
 Topic 3: Periodic Trends and Atomic Spectroscopy Introduction Valence Electrons are those in the outer most shell of an element and are responsible for the bonding characteristics of that element. Core
Topic 3: Periodic Trends and Atomic Spectroscopy Introduction Valence Electrons are those in the outer most shell of an element and are responsible for the bonding characteristics of that element. Core
CHAPTER 3 QUESTIONS. Multiple-Choice Questions
 CHAPTER 3 QUESTIONS Multiple-Choice Questions Use the PES spectra below to answer questions 1-4. Relative Number of Electrons 4.98 104 6.84 2.29 1.76 100 10 5 Binding Energy (MJ/mol) 1. What element does
CHAPTER 3 QUESTIONS Multiple-Choice Questions Use the PES spectra below to answer questions 1-4. Relative Number of Electrons 4.98 104 6.84 2.29 1.76 100 10 5 Binding Energy (MJ/mol) 1. What element does
CDO AP Chemistry Unit 5
 1. a. Calculate the wavelength of electromagnetic radiation that has a frequency of 5.56 MHz. b. Calculate the frequency of electromagnetic radiation that has a wavelength equal to 667 nm. 2. Electromagnetic
1. a. Calculate the wavelength of electromagnetic radiation that has a frequency of 5.56 MHz. b. Calculate the frequency of electromagnetic radiation that has a wavelength equal to 667 nm. 2. Electromagnetic
Chapter 2 Atoms and Elements. 2.4 The Atom
 Chapter 2 Atoms and Elements 2.4 The Atom Atoms Dalton s Atomic Theory Are tiny particles of matter. Of an element are similar and different from other elements. Of two or more different elements combine
Chapter 2 Atoms and Elements 2.4 The Atom Atoms Dalton s Atomic Theory Are tiny particles of matter. Of an element are similar and different from other elements. Of two or more different elements combine
Trends in the Periodic Table revisited! SCH4U1 SP04
 Trends in the Periodic Table revisited! SCH4U1 SP04 Factors Affecting the Properties Many of the properties of the elements are related to the force of attraction between the nucleus and the electrons.
Trends in the Periodic Table revisited! SCH4U1 SP04 Factors Affecting the Properties Many of the properties of the elements are related to the force of attraction between the nucleus and the electrons.
Transition Metals. Thursday 09/10/15. Thursday, September 10, 15
 Transition Metals Thursday 09/10/15 Agenda Review assigning oxidation numbers and practice with partners Start topic 13.1 First row d-block elements - Transition Metals Rules for assigning oxidation numbers
Transition Metals Thursday 09/10/15 Agenda Review assigning oxidation numbers and practice with partners Start topic 13.1 First row d-block elements - Transition Metals Rules for assigning oxidation numbers
An element Is a substance that cannot be split into simpler substance. It is composed of discrete particles called atoms.
 digitalteachers.co.ug Atomic structure Inorganic chemistry deals with the physical and chemical properties of the elements of the the periodic table. An element Is a substance that cannot be split into
digitalteachers.co.ug Atomic structure Inorganic chemistry deals with the physical and chemical properties of the elements of the the periodic table. An element Is a substance that cannot be split into
Topic 2 : Atomic Structure
 Topic 2 : Atomic Structure AJC/P2/Q1a 1. The first ionisation energy of aluminium will be lower than that of sulphur, as S has a higher nuclear charge than Al, but the shielding effect is similar for both
Topic 2 : Atomic Structure AJC/P2/Q1a 1. The first ionisation energy of aluminium will be lower than that of sulphur, as S has a higher nuclear charge than Al, but the shielding effect is similar for both
[3.2] The Atom. p in Textbook
![[3.2] The Atom. p in Textbook [3.2] The Atom. p in Textbook](/thumbs/83/88700013.jpg) [3.2] The Atom p. 145 149 in Textbook We will be learning about three different parts of the atom today 1. What makes up an atom 2. Where an atom s mass is found 3. What are isotopes What does the atom
[3.2] The Atom p. 145 149 in Textbook We will be learning about three different parts of the atom today 1. What makes up an atom 2. Where an atom s mass is found 3. What are isotopes What does the atom
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Worksheet 2.1. Chapter 2: Atomic structure glossary
 Worksheet 2.1 Chapter 2: Atomic structure glossary Acceleration (in a mass spectrometer) The stage where the positive ions are attracted to negatively charged plates. Alpha decay The emission of an alpha
Worksheet 2.1 Chapter 2: Atomic structure glossary Acceleration (in a mass spectrometer) The stage where the positive ions are attracted to negatively charged plates. Alpha decay The emission of an alpha
THE UNIVERSITY OF QUEENSLAND DEPARTMENT OF PHYSICS PHYS2041 ATOMIC SPECTROSCOPY
 THE UNIVERSITY OF QUEENSLAND DEPARTMENT OF PHYSICS PHYS2041 ATOMIC SPECTROSCOPY Warning: The mercury spectral lamps emit UV radiation. Do not stare into the lamp. Avoid exposure where possible. Introduction
THE UNIVERSITY OF QUEENSLAND DEPARTMENT OF PHYSICS PHYS2041 ATOMIC SPECTROSCOPY Warning: The mercury spectral lamps emit UV radiation. Do not stare into the lamp. Avoid exposure where possible. Introduction
Atoms and Periodic Properties
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Unit 01 (Chp 6,7): Atoms and Periodic Properties John D. Bookstaver St. Charles Community College
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Unit 01 (Chp 6,7): Atoms and Periodic Properties John D. Bookstaver St. Charles Community College
Unit 1 Part 1 Atomic Structure and The Periodic Table Introduction to Atomic Structure UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE
 UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 1 INTRODUCTION TO ATOMIC STRUCTURE Contents 1. Protons, Neutrons and Electrons 2. Early Models of the Atom 3. Isotopes and Atomic Mass 4. Atoms and Ions
UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 1 INTRODUCTION TO ATOMIC STRUCTURE Contents 1. Protons, Neutrons and Electrons 2. Early Models of the Atom 3. Isotopes and Atomic Mass 4. Atoms and Ions
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Light. Light (con t.) 2/28/11. Examples
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Activity # 2. Name. Date due. Assignment on Atomic Structure
 Activity # 2 10 Name Date Date due Assignment on Atomic Structure NOTE: This assignment is based on material on the Power Point called Atomic Structure, as well as pages 167-173 in the Science Probe textbook.
Activity # 2 10 Name Date Date due Assignment on Atomic Structure NOTE: This assignment is based on material on the Power Point called Atomic Structure, as well as pages 167-173 in the Science Probe textbook.
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
CP/Honors Chemistry Unit 3: Atomic Theory Sections 4.1, 4.2, 4.3
 CP/Honors Chemistry Unit 3: Atomic Theory Sections 4.1, 4.2, 4.3 Subatomic Particles Warm-Up Quiz 1. What are the three subatomic particles? 2. Where are the particles located in the atom? 3. What are
CP/Honors Chemistry Unit 3: Atomic Theory Sections 4.1, 4.2, 4.3 Subatomic Particles Warm-Up Quiz 1. What are the three subatomic particles? 2. Where are the particles located in the atom? 3. What are
8. Which of the following could be an isotope of chlorine? (A) 37 Cl 17 (B) 17 Cl 17 (C) 37 Cl 17 (D) 17 Cl 37.5 (E) 17 Cl 37
 Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
1.1 Atomic structure. The Structure of the Atom Mass Spectrometry Electronic Structure Ionisation Energies
 1.1 Atomic structure The Structure of the Atom Mass Spectrometry Electronic Structure Ionisation Energies a) Protons, neutrons and electrons THE STRUCTURE OF THE ATOM Atoms are made up of three fundamental
1.1 Atomic structure The Structure of the Atom Mass Spectrometry Electronic Structure Ionisation Energies a) Protons, neutrons and electrons THE STRUCTURE OF THE ATOM Atoms are made up of three fundamental
Section 11: Electron Configuration and Periodic Trends
 Section 11: Electron Configuration and Periodic Trends The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 11.01 The Bohr Model of the Atom
Section 11: Electron Configuration and Periodic Trends The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 11.01 The Bohr Model of the Atom
U N I T T E S T P R A C T I C E
 South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
Bell-Ringer. Define the term isotope. [2 marks]
![Bell-Ringer. Define the term isotope. [2 marks] Bell-Ringer. Define the term isotope. [2 marks]](/thumbs/96/127718897.jpg) Bell-Ringer Define the term isotope. [2 marks] Bell-Ringer Define the term isotope. [2 marks] Answer per IB Mark scheme: atoms of the same element/same number of protons/same atomic number that have different
Bell-Ringer Define the term isotope. [2 marks] Bell-Ringer Define the term isotope. [2 marks] Answer per IB Mark scheme: atoms of the same element/same number of protons/same atomic number that have different
Warm-up For sulfur: 1. How many valence electrons does it have? 2. What ion does this typically form? 3. Write the electron configuration for the ion.
 Warm-up For sulfur: 1. How many valence electrons does it have? 2. What ion does this typically form? 3. Write the electron configuration for the ion. Nucleus Contains 99.9% of the mass of an atom Found
Warm-up For sulfur: 1. How many valence electrons does it have? 2. What ion does this typically form? 3. Write the electron configuration for the ion. Nucleus Contains 99.9% of the mass of an atom Found
Name: Date: Atomic Structure 2017 Mrs. Mannion Version 1
 Name: Atomic Structure 2017 1. The mass of a proton is approximately equal to the mass of A) a beta particle B) an electron C) an alpha particle D) a neutron 2. What are the characteristics of a neutron?
Name: Atomic Structure 2017 1. The mass of a proton is approximately equal to the mass of A) a beta particle B) an electron C) an alpha particle D) a neutron 2. What are the characteristics of a neutron?
ATOMIC STRUCTURE. Content
 2 ATOMIC STRUCTURE 2 1 Content 2.1 The nucleus of the atom: neutrons and protons, isotopes, proton and nucleon numbers 2.2 Electrons: electronic energy levels, ionisation energies, atomic orbitals, etranuclear
2 ATOMIC STRUCTURE 2 1 Content 2.1 The nucleus of the atom: neutrons and protons, isotopes, proton and nucleon numbers 2.2 Electrons: electronic energy levels, ionisation energies, atomic orbitals, etranuclear
Ch. 7 The Quantum Mechanical Atom. Brady & Senese, 5th Ed.
 Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
1.1 Atomic Structure Details of the three Sub-atomic (fundamental) Particles
 1.1 Atomic Structure Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1800-1 An atom of
1.1 Atomic Structure Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1800-1 An atom of
1.1 Atomic structure
 1.1 Atomic structure History of the atom The model of the atom has changed as our observations of its behavior and properties have increased. A model is used to explain observations. The model changes
1.1 Atomic structure History of the atom The model of the atom has changed as our observations of its behavior and properties have increased. A model is used to explain observations. The model changes
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A
 CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
SAMPLE PROBLEMS! 1. From which of the following is it easiest to remove an electron? a. Mg b. Na c. K d. Ca
 SAMPLE PROBLEMS! 1. From which of the following is it easiest to remove an electron? a. Mg b. Na c. K d. Ca 2. Which of the following influenced your answer to number one the most? a. effective nuclear
SAMPLE PROBLEMS! 1. From which of the following is it easiest to remove an electron? a. Mg b. Na c. K d. Ca 2. Which of the following influenced your answer to number one the most? a. effective nuclear
Name: Date: Blk: Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed:
 Name: Date: Blk: NOTES: PERIODIC TRENDS Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed: I. ATOMIC RADIUS (Size) Going from
Name: Date: Blk: NOTES: PERIODIC TRENDS Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed: I. ATOMIC RADIUS (Size) Going from
ATOMIC MODELS. Models are formulated to fit the available data. Atom was known to have certain size. Atom was known to be neutral.
 ATOMIC MODELS Models are formulated to fit the available data. 1900 Atom was known to have certain size. Atom was known to be neutral. Atom was known to give off electrons. THOMPSON MODEL To satisfy the
ATOMIC MODELS Models are formulated to fit the available data. 1900 Atom was known to have certain size. Atom was known to be neutral. Atom was known to give off electrons. THOMPSON MODEL To satisfy the
Electrons in Atoms. So why does potassium explode in water? Quantum Mechanics Periodic Trends Chemical Bonding
 Electrons in Atoms So why does potassium explode in water? Quantum Mechanics Periodic Trends Chemical Bonding 12.1 Development of Atomic Models Dalton s Thompson s Rutherford s Bohr s carbon Quantum Model
Electrons in Atoms So why does potassium explode in water? Quantum Mechanics Periodic Trends Chemical Bonding 12.1 Development of Atomic Models Dalton s Thompson s Rutherford s Bohr s carbon Quantum Model
2. gamma, x-ray, U.V., Visible Light, I.R., Microwaves, radio waves.
 CH40S 1. See notes. Atomic Structure Review Key 2. gamma, x-ray, U.V., Visible Light, I.R., Microwaves, radio waves. 3. Violet, indigo, blue, green, yellow, orange, red. 4. Inverse as wavelength increases,
CH40S 1. See notes. Atomic Structure Review Key 2. gamma, x-ray, U.V., Visible Light, I.R., Microwaves, radio waves. 3. Violet, indigo, blue, green, yellow, orange, red. 4. Inverse as wavelength increases,
Basic Concepts of Chemistry Notes for Students [Chapter 8, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 8 Modern Atomic Theory
![Basic Concepts of Chemistry Notes for Students [Chapter 8, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 8 Modern Atomic Theory Basic Concepts of Chemistry Notes for Students [Chapter 8, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 8 Modern Atomic Theory](/thumbs/77/76682727.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 8, page 1] Chapter 8 Modern Atomic Theory This chapter is a continuation of our discussions from Chapter 2 during which we saw how atoms, electrons,
Basic Concepts of Chemistry Notes for Students [Chapter 8, page 1] Chapter 8 Modern Atomic Theory This chapter is a continuation of our discussions from Chapter 2 during which we saw how atoms, electrons,
5.111 Lecture Summary #5 Friday, September 12, 2014
 5.111 Lecture Summary #5 Friday, September 12, 2014 Readings for today: Section 1.3 Atomic Spectra, Section 1.7 up to equation 9b Wavefunctions and Energy Levels, Section 1.8 The Principle Quantum Number.
5.111 Lecture Summary #5 Friday, September 12, 2014 Readings for today: Section 1.3 Atomic Spectra, Section 1.7 up to equation 9b Wavefunctions and Energy Levels, Section 1.8 The Principle Quantum Number.
Chemistry (
 Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
LIGHT AND THE QUANTUM MODEL
 LIGHT AND THE QUANTUM MODEL WAVES Wavelength ( ) - length of one complete wave Frequency ( ) - # of waves that pass a point during a certain time period hertz (Hz) = 1/s Amplitude (A) - distance from the
LIGHT AND THE QUANTUM MODEL WAVES Wavelength ( ) - length of one complete wave Frequency ( ) - # of waves that pass a point during a certain time period hertz (Hz) = 1/s Amplitude (A) - distance from the
Ch4 and Ch5. Atomic History and the Atom
 Ch4 and Ch5 Atomic History and the Atom Ch4.2 What are atoms? Atoms are the smallest part of an element that still has the element s properties. Ch. 4.3 The Atom is Defined 400 B.C. the Greek philosopher
Ch4 and Ch5 Atomic History and the Atom Ch4.2 What are atoms? Atoms are the smallest part of an element that still has the element s properties. Ch. 4.3 The Atom is Defined 400 B.C. the Greek philosopher
Chapter 6 The Atom Study Guide
 Chapter 6 The Atom Study Guide Read pages 118-125 Look at all words in bold or colored print Look at the paragraph summaries on the sides Section 6.1 Fundamental Particles and Forces Vocabulary Definition
Chapter 6 The Atom Study Guide Read pages 118-125 Look at all words in bold or colored print Look at the paragraph summaries on the sides Section 6.1 Fundamental Particles and Forces Vocabulary Definition
1.1 The Fundamental Chemistry of life
 1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
- 1 - Core Chemistry Topic 2 Atomic Structure
 - 1 - Core Chemistry REVIEW a model for matter (10 mins) 1. On your own take 5 minutes to construct a concept map organizing what you know about the structure of matter. 2. Take 5 more minutes and share
- 1 - Core Chemistry REVIEW a model for matter (10 mins) 1. On your own take 5 minutes to construct a concept map organizing what you know about the structure of matter. 2. Take 5 more minutes and share
Chapter 5. Arrangement of Electrons in Atoms
 Chapter 5 Arrangement of Electrons in Atoms Light Dual Nature of Light: Light can act like, and as particles. Light is one type of which is a form of Energy that has wavelike behaviour Other types of em
Chapter 5 Arrangement of Electrons in Atoms Light Dual Nature of Light: Light can act like, and as particles. Light is one type of which is a form of Energy that has wavelike behaviour Other types of em
SCH3U- R. H. KING ACADEMY ATOMIC STRUCTURE HANDOUT NAME:
 Particle Theory of Matter Matter is anything that has and takes up. All matter is made up of very small. Each pure substance has its of particle, from the particles of other pure substances. Particles
Particle Theory of Matter Matter is anything that has and takes up. All matter is made up of very small. Each pure substance has its of particle, from the particles of other pure substances. Particles
Different energy levels
 Different energy levels In the microscopic world energy is discrete www.cgrahamphysics.com Review Atomic electrons can only exist in certain discrete energy levels Light is made of photons When e s lose
Different energy levels In the microscopic world energy is discrete www.cgrahamphysics.com Review Atomic electrons can only exist in certain discrete energy levels Light is made of photons When e s lose
Test Bank for General Chemistry Atoms First 2nd Edition by John E. McMurry and Robert C. Fay
 Test Bank for General Chemistry Atoms First 2nd Edition by John E. McMurry and Robert C. Fay Link download full: https://digitalcontentmarket.org/download/test-bank-for-general-chemistry-atoms-f irst-2nd-edition-by-mcmurry-and-fay/
Test Bank for General Chemistry Atoms First 2nd Edition by John E. McMurry and Robert C. Fay Link download full: https://digitalcontentmarket.org/download/test-bank-for-general-chemistry-atoms-f irst-2nd-edition-by-mcmurry-and-fay/
2. Why do all elements want to obtain a noble gas electron configuration?
 AP Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Electron Configuration Metal, Nonmetal or Semi-metal Metalloid)? Group 1, Period 1 Group 11,
AP Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Electron Configuration Metal, Nonmetal or Semi-metal Metalloid)? Group 1, Period 1 Group 11,
7.10: History of the Periodic Table
 7.10: History of the Periodic Table Dmitri Mendeleev given credit for the first periodic table in 1869 Grouped elements with similar chemical & physical properties in rows according to atomic mass He emphasized
7.10: History of the Periodic Table Dmitri Mendeleev given credit for the first periodic table in 1869 Grouped elements with similar chemical & physical properties in rows according to atomic mass He emphasized
Unit 3. The Atom & Modern Atomic Theory
 Unit 3 The Atom & Modern Atomic Theory Theories of the Atom Early Models & Thoughts: Democritus Matter is made up of tiny particles called atoms. Smallest unit that retains the identity of the element
Unit 3 The Atom & Modern Atomic Theory Theories of the Atom Early Models & Thoughts: Democritus Matter is made up of tiny particles called atoms. Smallest unit that retains the identity of the element
Unit 2 - Electrons and Periodic Behavior
 Unit 2 - Electrons and Periodic Behavior Models of the Atom I. The Bohr Model of the Atom A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The
Unit 2 - Electrons and Periodic Behavior Models of the Atom I. The Bohr Model of the Atom A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The
What are the isotopes of hydrogen? H, 2 H, and 3 H
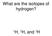 What are the isotopes of hydrogen? 1 H, 2 H, and 3 H The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a
What are the isotopes of hydrogen? 1 H, 2 H, and 3 H The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a
Name: Per: Date: Teacher: Official Class: Chemistry. Unit 1: The Atom
 Unit 1: The Atom The following pages are practice questions for this unit, and will be submitted for homework! You must complete: Unit Vocabulary ALL QUESTIONS What is an Atom? ALL QUESTIONS Calculating
Unit 1: The Atom The following pages are practice questions for this unit, and will be submitted for homework! You must complete: Unit Vocabulary ALL QUESTIONS What is an Atom? ALL QUESTIONS Calculating
Chapter 9: Electrons and the Periodic Table
 C h e m i s t r y 1 2 C h 9 : E l e c t r o n s a n d P e r i o d i c T a b l e P a g e 1 Chapter 9: Electrons and the Periodic Table Work on MasteringChemistry assignments What we have learned: Dalton
C h e m i s t r y 1 2 C h 9 : E l e c t r o n s a n d P e r i o d i c T a b l e P a g e 1 Chapter 9: Electrons and the Periodic Table Work on MasteringChemistry assignments What we have learned: Dalton
4 Periodic Trends. 1.Atomic Radii (AR) 2.Ionization Energy (IE) 3.Ionic Radii (IR) 4.Electronegativity (EN) Periodic Trends > Types of Periodic Trends
 Periodic Trends > Types of Periodic Trends 4 Periodic Trends 1.Atomic Radii (AR) 2.Ionization Energy (IE) 3.Ionic Radii (IR) 4.Electronegativity (EN) 1 of 31 Periodic Trends > Trends in Atomic Size The
Periodic Trends > Types of Periodic Trends 4 Periodic Trends 1.Atomic Radii (AR) 2.Ionization Energy (IE) 3.Ionic Radii (IR) 4.Electronegativity (EN) 1 of 31 Periodic Trends > Trends in Atomic Size The
Physics 1C Lecture 29B
 Physics 1C Lecture 29B Emission Spectra! The easiest gas to analyze is hydrogen gas.! Four prominent visible lines were observed, as well as several ultraviolet lines.! In 1885, Johann Balmer, found a
Physics 1C Lecture 29B Emission Spectra! The easiest gas to analyze is hydrogen gas.! Four prominent visible lines were observed, as well as several ultraviolet lines.! In 1885, Johann Balmer, found a
Atomic Spectroscopy. Objectives
 Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Periodic Trends. Atomic Radius: The distance from the center of the nucleus to the outer most electrons in an atom.
 Periodic Trends Study and learn the definitions listed below. Then use the definitions and the periodic table provided to help you answer the questions in the activity. By the end of the activity you should
Periodic Trends Study and learn the definitions listed below. Then use the definitions and the periodic table provided to help you answer the questions in the activity. By the end of the activity you should
Problems with the atomic model?
 Modern Atomic Theory- Electronic Structure of Atoms DR HNIMIR-CH7 Where should (-) electrons be found? Problems with the atomic model? First, a Little About Electromagnetic Radiation- Waves Another Look
Modern Atomic Theory- Electronic Structure of Atoms DR HNIMIR-CH7 Where should (-) electrons be found? Problems with the atomic model? First, a Little About Electromagnetic Radiation- Waves Another Look
Problems with the Wave Theory of Light (Photoelectric Effect)
 CHEM101 NOTES Properties of Light Found that the wave theory could not work for some experiments e.g. the photovoltaic effect This is because the classic EM view of light could not account for some of
CHEM101 NOTES Properties of Light Found that the wave theory could not work for some experiments e.g. the photovoltaic effect This is because the classic EM view of light could not account for some of
THE STRUCTURE OF ATOMS. ATOMS Atoms consist of a number of fundamental particles, the most important ones are...
 Atomic Structure THE STRUCTURE OF ATOMS ATOMS Atoms consist of a number of fundamental particles, the most important ones are... Mass / kg Charge / C Relative mass Relative Charge PROTON NEUTRON ELECTRON
Atomic Structure THE STRUCTURE OF ATOMS ATOMS Atoms consist of a number of fundamental particles, the most important ones are... Mass / kg Charge / C Relative mass Relative Charge PROTON NEUTRON ELECTRON
i. This is the best evidence for the fact that electrons in an atom surround the nucleus in certain allowed energy levels or orbitals ii.
 Atomic Structure I. The Atom A. Atomic theory: Devised in 1807 by John Dalton, states that all matter is made up of a small number of different kinds of atoms that are indivisible and indestructible but
Atomic Structure I. The Atom A. Atomic theory: Devised in 1807 by John Dalton, states that all matter is made up of a small number of different kinds of atoms that are indivisible and indestructible but
Quantitative chemistry Atomic structure Periodicity
 IB chemistry Units 1-3 review Quantitative chemistry Significant figures The mole- be able to convert to number of particles and mass Finding empirical and molecular formulas from mass percentage States
IB chemistry Units 1-3 review Quantitative chemistry Significant figures The mole- be able to convert to number of particles and mass Finding empirical and molecular formulas from mass percentage States
where n = (an integer) =
 5.111 Lecture Summary #5 Readings for today: Section 1.3 (1.6 in 3 rd ed) Atomic Spectra, Section 1.7 up to equation 9b (1.5 up to eq. 8b in 3 rd ed) Wavefunctions and Energy Levels, Section 1.8 (1.7 in
5.111 Lecture Summary #5 Readings for today: Section 1.3 (1.6 in 3 rd ed) Atomic Spectra, Section 1.7 up to equation 9b (1.5 up to eq. 8b in 3 rd ed) Wavefunctions and Energy Levels, Section 1.8 (1.7 in
Observation information obtained through the senses; observation in science often involves measurement
 Review Sheet Unit 1: The Atom Chemistry the study of the composition of matter and the changes matter undergoes Scientific Method Scientific method a logical, systematic approach to the solution of a scientific
Review Sheet Unit 1: The Atom Chemistry the study of the composition of matter and the changes matter undergoes Scientific Method Scientific method a logical, systematic approach to the solution of a scientific
CHM 111 Unit 7 Sample Questions
 Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
Unit 1. Electronic Structure page 1
 Unit 1 Electronic Structure Section 1. Learning Outcomes Practice Questions Answers Electronic Structure Electromagnetic spectrum / calculations Electron configuration / Periodic Table Electronic Structure
Unit 1 Electronic Structure Section 1. Learning Outcomes Practice Questions Answers Electronic Structure Electromagnetic spectrum / calculations Electron configuration / Periodic Table Electronic Structure
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Electronic Structure of Atoms
 Electronic Structure of Atoms Electrons inhabit regions of space known as orbitals. Heisenberg Uncertainty Principle NOTE Impossible to define with absolute precision, at the same time, both the position
Electronic Structure of Atoms Electrons inhabit regions of space known as orbitals. Heisenberg Uncertainty Principle NOTE Impossible to define with absolute precision, at the same time, both the position
Part A. Answer all questions in this part.
 Part A Directions (1-20): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Part A Directions (1-20): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Chapter 5. Periodicity and the Electronic Structure of Atoms
 Chapter 5 Periodicity and the Electronic Structure of Atoms Electron Spin experiments by Stern and Gerlach showed a beam of silver atoms is split in two by a magnetic field the experiment reveals that
Chapter 5 Periodicity and the Electronic Structure of Atoms Electron Spin experiments by Stern and Gerlach showed a beam of silver atoms is split in two by a magnetic field the experiment reveals that
CHEMISTRY - KIRSS 2E CH.3 - ATOMIC STRUCTURE: EXPLAINING THE PROPERTIES OF ELEMENTS
 !! www.clutchprep.com CONCEPT: THE NATURE OF LIGHT Visible light represents a small portion of the continuum of radiant energy known as. The visible light spectrum ranges from to. Its wave properties of
!! www.clutchprep.com CONCEPT: THE NATURE OF LIGHT Visible light represents a small portion of the continuum of radiant energy known as. The visible light spectrum ranges from to. Its wave properties of
The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006.
 The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006. The first ionization energy (IE 1 ) is the minimum energy required to remove
The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006. The first ionization energy (IE 1 ) is the minimum energy required to remove
3/30/2015. Third energy level. Second energy level. Energy absorbed. First energy level. Atomic nucleus. Energy released (as light)
 Chapter 2 An Introduction Chemistry Lecture 2: Energy Levels and Chemical Bonding Electrons are always moving Outside the nucleus in atomic orbitals Maybe usually Average distance from nucleus (size of
Chapter 2 An Introduction Chemistry Lecture 2: Energy Levels and Chemical Bonding Electrons are always moving Outside the nucleus in atomic orbitals Maybe usually Average distance from nucleus (size of
Periodic Trends. Name: Class: Date: ID: A. Matching
 Name: Class: Date: Periodic Trends Matching Match each item with the correct statement below. a. electronegativity f. periodic law b. ionization energy g. atomic mass c. atomic radius h. period d. metal
Name: Class: Date: Periodic Trends Matching Match each item with the correct statement below. a. electronegativity f. periodic law b. ionization energy g. atomic mass c. atomic radius h. period d. metal
Which order of statements represents the historical development of the atomic model? A) C D A B B) C D B A C) D B A C D) D B C A
 1. The mass of a proton is approximately equal to the mass of A) an electron B) a neutron C) an alpha particle D) a beta particle 2. What is the number of electrons in an atom that has 20 protons and 17
1. The mass of a proton is approximately equal to the mass of A) an electron B) a neutron C) an alpha particle D) a beta particle 2. What is the number of electrons in an atom that has 20 protons and 17
2. Atomic Structure and Periodic Table Details of the three Sub-atomic (fundamental) Particles
 2. Atomic Structure and Periodic Table Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 Electron Orbitals 1/184-1
2. Atomic Structure and Periodic Table Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 Electron Orbitals 1/184-1
1.1 Atomic Structure Details of the three Sub-atomic (fundamental) Particles
 1.1 Atomic Structure Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1840-1 An atom of
1.1 Atomic Structure Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1840-1 An atom of
Edexcel Chemistry A-level
 Edexcel Chemistry A-level Topic 1 - Atomic Structure and Periodic Table Flashcards What was stated in Dalton s atomic theory? (4) What was stated in Dalton s atomic theory? Atoms are tiny particles made
Edexcel Chemistry A-level Topic 1 - Atomic Structure and Periodic Table Flashcards What was stated in Dalton s atomic theory? (4) What was stated in Dalton s atomic theory? Atoms are tiny particles made
Goals for Today. Clarify some Rydberg Concepts Absorption vs. emission
 Note: Due to recent changes the exam 2 material for these slides ends at Ionization Energy Exceptions. You can omit Lewis Structures through General Formal Charge Rules. CH301 Unit 2 QUANTUM NUMBERS AND
Note: Due to recent changes the exam 2 material for these slides ends at Ionization Energy Exceptions. You can omit Lewis Structures through General Formal Charge Rules. CH301 Unit 2 QUANTUM NUMBERS AND
SCH4C Practice WS Unit 1
 Name: Class: Date: SCH4C Practice WS Unit 1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The special band of light waves that the human eye can detect
Name: Class: Date: SCH4C Practice WS Unit 1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The special band of light waves that the human eye can detect
Contents. Atomic Structure. 2(a) The Periodic Table. 2(b) Amount of Substance. 2(c) 2(d) Bonding and Structure. Enthalpy Changes.
 1 C h e m i s t r y Contents 2(a) 2(b) 2(c) 2(d) 2(e) Atomic Structure The Periodic Table Amount of Substance Bonding and Structure Enthalpy Changes 3 14 18 28 39 2 C h e m i s t r y 2(a) Atomic Structure
1 C h e m i s t r y Contents 2(a) 2(b) 2(c) 2(d) 2(e) Atomic Structure The Periodic Table Amount of Substance Bonding and Structure Enthalpy Changes 3 14 18 28 39 2 C h e m i s t r y 2(a) Atomic Structure
Optical Spectroscopy and Atomic Structure. PHYS 0219 Optical Spectroscopy and Atomic Structure 1
 Optical Spectroscopy and Atomic Structure PHYS 0219 Optical Spectroscopy and Atomic Structure 1 Optical Spectroscopy and Atomic Structure This experiment has four parts: 1. Spectroscope Setup - Your lab
Optical Spectroscopy and Atomic Structure PHYS 0219 Optical Spectroscopy and Atomic Structure 1 Optical Spectroscopy and Atomic Structure This experiment has four parts: 1. Spectroscope Setup - Your lab
Chapter 4. Spectroscopy. Dr. Tariq Al-Abdullah
 Chapter 4 Spectroscopy Dr. Tariq Al-Abdullah Learning Goals: 4.1 Spectral Lines 4.2 Atoms and Radiation 4.3 Formation of the Spectral Lines 4.4 Molecules 4.5 Spectral Line Analysis 2 DR. T. AL-ABDULLAH
Chapter 4 Spectroscopy Dr. Tariq Al-Abdullah Learning Goals: 4.1 Spectral Lines 4.2 Atoms and Radiation 4.3 Formation of the Spectral Lines 4.4 Molecules 4.5 Spectral Line Analysis 2 DR. T. AL-ABDULLAH
: the smallest particle that has the properties of an element. In, this Greek philosopher suggested that the universe was made of.
 Notes: ATOMS AND THE PERIODIC TABLE Atomic Structure: : the smallest particle that has the properties of an element. From the early concept of the atom to the modern atomic theory, scientists have built
Notes: ATOMS AND THE PERIODIC TABLE Atomic Structure: : the smallest particle that has the properties of an element. From the early concept of the atom to the modern atomic theory, scientists have built
Name: Block: Date: Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom.
 Name: Block: Date: Chemistry 11 Trends Activity Assignment Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom. Ionic Radius: the distance from the center
Name: Block: Date: Chemistry 11 Trends Activity Assignment Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom. Ionic Radius: the distance from the center
Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/ Atomic Symbol
 2 Atomic tructure Details of the three ub-atomic (fundamental) articles article osition Relative Mass Relative Charge roton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1840-1 Behaviour of beams
2 Atomic tructure Details of the three ub-atomic (fundamental) articles article osition Relative Mass Relative Charge roton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1840-1 Behaviour of beams
Atomic Theory. H. Cannon, C. Clapper and T. Guillot Klein High School
 Atomic Theory Unit 3 Development of the Atomic Theory 1. Where is the mass of the atom concentrated? 2. What is located in the nucleus? 3. What is the negative particle that orbits the nucleus? 4. What
Atomic Theory Unit 3 Development of the Atomic Theory 1. Where is the mass of the atom concentrated? 2. What is located in the nucleus? 3. What is the negative particle that orbits the nucleus? 4. What
Unit Two Test Review. Click to get a new slide. Choose your answer, then click to see if you were correct.
 Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
In addition. The Atom. Atomic Theory Atomic Number and Atomic Mass. In a neutral atom: Number of Protons = Number of Electrons
 Atomic Theory Chemistry 11 The Atom The concept of a discrete unit that makes up all matter has been around for centuries. These ideas were based on philosophical reasoning rather than experimentation
Atomic Theory Chemistry 11 The Atom The concept of a discrete unit that makes up all matter has been around for centuries. These ideas were based on philosophical reasoning rather than experimentation
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET
 EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET 1.) Look at the EM spectrum below to answer this question. As you move across the visible light spectrum from red to violet (A) Does the wavelength
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET 1.) Look at the EM spectrum below to answer this question. As you move across the visible light spectrum from red to violet (A) Does the wavelength
CHM 100 CHEMISTRY MAN & ENVIRONMENT Atoms and Elements Sample Test
 CHM 100 CHEMISTRY MAN & ENVIRONMENT Atoms and Elements Sample Test Multiple Choice: Identify the choice that best completes the statement or answers the question. 1. Which of these elements has two valence
CHM 100 CHEMISTRY MAN & ENVIRONMENT Atoms and Elements Sample Test Multiple Choice: Identify the choice that best completes the statement or answers the question. 1. Which of these elements has two valence
Unit 02 Review: Atomic Theory and Periodic Table Review
 Practice Multiple Choice Questions Unit 02 Review: Atomic Theory and Periodic Table Review 1. The number of neutrons in an atom of radioactive C 14 is: a) 6 c) 8 b) 12 d) 14 2. When a radioactive nucleus
Practice Multiple Choice Questions Unit 02 Review: Atomic Theory and Periodic Table Review 1. The number of neutrons in an atom of radioactive C 14 is: a) 6 c) 8 b) 12 d) 14 2. When a radioactive nucleus
3.1 Hydrogen Spectrum
 3.1 Hydrogen Spectrum Light is electromagnetic radiation that can be produced at different energy levels. High energy light has a short wavelength (λ) and a high frequency (ƒ, ν) (gamma rays, x-rays, ultraviolet).
3.1 Hydrogen Spectrum Light is electromagnetic radiation that can be produced at different energy levels. High energy light has a short wavelength (λ) and a high frequency (ƒ, ν) (gamma rays, x-rays, ultraviolet).
11. The bright-line spectra produced by four elements are represented in the diagram below.
 1. Which substance can not be broken down by a chemical change? A) ammonia B) ethanol C) propanal D) zirconium 2. Which particle has no charge? A) electron B) neutron C) positron D) proton 3. Which phrase
1. Which substance can not be broken down by a chemical change? A) ammonia B) ethanol C) propanal D) zirconium 2. Which particle has no charge? A) electron B) neutron C) positron D) proton 3. Which phrase
