Science Lab I Properties of Light
|
|
|
- Duane Rogers
- 6 years ago
- Views:
Transcription
1 Art & Science of Light Fall 2007 Science Lab I Properties of Light Prepared by: Dr. Dharshi Bopegedera
2
3 1 Using the Filtergraph (15 minutes) 1. Turn on the filtergraph, place a card on it and look at the output. Draw a picture of the output accurately (what you have is a graph of transmittance versus wavelength). Draw to scale and be sure to write down the names of the axis of the graph with units. Next to the graph, write down the color on the card. 2. Repeat the above process with 4 different cards. Homework: 1. Based on the above lab experience and the wavelengths of the visible light spectrum given below (see table below), write your own definition for transmittance. 2. Using the library as a resource, write a definition for transmittance (be sure to write down the reference you used in your lab notebook). Are there any differences between your definition and the definition you found in the library? The following wavelengths for the colors in the visible spectrum are provided for your reference. Please note that these are approximate values. Color Wavelength (nm) Violet 420 Blue 470 Green 530 Yellow 580 Orange 620 Red 700 Using the Singerman Color Apparatus (15 minutes) In this activity you will be mixing different colors and observing the resulting color using your eyes as the detector. 1. Turn all three lamps in the color apparatus. Insert red, green and blue color cards into the slits of the color apparatus. 2. Observe and record the individual colors with your naked eye. Also record the additive colors where two or three different colors overlap. 3. Change the intensity (there is an intensity knob on the color apparatus) of the lights and see how it affects the colors. Record your observations. 4. Repeat the above process with other color combinations. Record all observations (time limit 15 minutes).
4 Using the Monochromator with a white light source (10 minutes) 2 Turn on the white light source. Narrow down the slits (there are two of them) so that you see a narrow, clear beam of light coming out from the monochromator. Change the wavelength dial on the monochromator and see the color of the output beam. Assign a wavelength range to each of the seven colors of the visible spectrum. Record your observations. Verifying the Law of Reflection (30 minutes) The law of reflection states that when light is reflected by a surface, the angle of incidence is equal to the angle of reflection. This can be verified by a simple experiment. 1. Using double stick tape, fasten a flat mirror onto a flat surface (a box, a block of wood etc.). 2. Place a ray box on the table, turn it on and get a single ray of light pointing from the box towards the mirror. Make sure you can see the reflected beam of light from the mirror. mirror block of wood Reflected ray normal Ray box 3. Draw a line to mark the position of the mirror and a few carefully placed dots to mark the positions of the incident light ray and the reflected light ray. Remove the mirror and the ray box. 4. Use a ruler to draw lines showing the incident light ray and the reflected light ray. These should be extended to cross the line showing the position of the mirror. At the point where the lines cross, draw a normal to the mirror (this line should be at 90 to the mirror line)
5 5. Use your diagram and a protractor to measure the angle of incidence and the angle of reflection. Verify if the law of reflection is true. If you have extra time you can repeat the experiment with a different angle of incidence. 3 Snell s Law of Refraction (30 minutes) Fill a clear glass with water. Put a pencil in the glass of water at an angle and observe the appearance when you look directly along the part of the pencil that is out of the water. Draw the appearance of the pencil. (Important: Look directly along the part of the pencil that sticks out of the water, not from the side.) What appears to have happened to the pencil? Where does the tip of the pencil appear to be relative to its actual position? When light rays pass from one medium to another, as from air to water, they bend (or refract). The rays of light bend toward the line perpendicular to the surface (normal) when they enter a denser medium (water) from a less-dense medium (air). They bend away from this line when they leave the denser medium. This is called Snell s Law. where n 1 is the index of refraction of medium one n 2 is the index of refraction of medium two θ 1 is the angle of the incident ray θ 2 is the angle of the refracted ray Snell s Law is written as follows: n 1 sin θ 1 = n 2 sin θ 2 medium one with index of refraction = n 1 normal medium two with index of refraction = n 2 The index of refraction of a medium (n) is the ratio of the speed of light in a vacuum to the speed of light in that medium. Index of refraction for water = speed of light in vacuum/speed of light in water
6 4 Let s determine the index of refraction of glass using Snell s law. Set up the experiment as shown in the following diagram. You need a clean sheet of paper, a block of glass, a laser, a protractor, a ruler and a pencil. 1. Lay the glass block on a sheet of paper. With a sharp pencil trace an outline of the block. 2. Place the laser beam so that it moves along line QP towards the glass block. Carefully place dots along this line so that when you remove the laser you can trace its path along QP. 3. Note that the laser beam emerges from the opposite side. Carefully place dots along the emerging laser beam so that when you remove the laser you can trace its path along RS. 4. Remove the laser beam and the block. Draw in lines QP and RS. Where QP crosses the position of the block mark as point O. Similarly, where RS crosses the position of the block mark as point A. Draw in the line OA. Do the following as homework. 1. Draw the line ON, which is the normal to the glass block at point O. Use a protractor to measure the angle correctly. 2. Measure the angle of incidence (θ 1 ) and the angle of refraction (θ 2 ) carefully with a protractor 3. Now calculate sinθ 1 and sinθ Given that the index of refraction of air (n 1 ) is 1.003, calculate the index of refraction of glass (n 2 ) using Snell s law. 5. Now that you know the index of refraction of glass, use this value to calculate the speed of light in glass. 6. Use the library to find the established speed of light in glass. Compare with your value and comment on the accuracy of your work.
7 Kaleidoscope (15 minutes) 5 Hinge two flat mirrors together with masking tape so that they are at 72 to each other. Use a protractor to make sure you have the right angle. Accuracy is important. Place a small object inside the angled mirrors. Count the number of images you can see and record in the following table. Decreasing the angle by 5 each time, continue to record the number of images you get from this system. Record about 5 data points. Angle Number of images What effect does the angle between the mirrors have on the number of images? Using your data, predict what angle would be the best to use if you were to build a Kaleidoscope. Determining the diameter of the Sun (15 minutes) Poke a small hole (pin hole works well) in a note card. Hold the card in the sunlight and find the circular image that is cast on the floor by the hole. As you move the card up and down (towards and away from the ground) the size of the image of the hole will change. The image is that of the Sun. The bigger the distance from the pinhole to he ground, the larger the image. Position the note card so that the image exactly covers a nickel, a dime or a quarter (so you can measure the size of the image accurately). Carefully measure the distance from the pinhole to the image. diameter of the image distance from the image to pinhole = diameter of the Sun distance to the Sun Given that the Sun is approximately 150,000,000 km from the pinhole, calculate the diameter of the Sun. Find the reported diameter of the Sun from a textbook or another resource. Compare your calculated value with this reported value and write a comment.
8 Observing the Fraunhofer lines of the sun (15 minutes) 6 ( The Fraunhofer lines are a set of spectral lines named for the German physicist Joseph von Fraunhofer ( ). The lines were originally observed as dark features in the optical spectrum of the Sun. The English chemist William Hyde Wollaston was in 1802 the first person to note the appearance of a number of dark features in the solar spectrum. In 1814, Fraunhofer independently rediscovered the lines and began a systematic study and careful measurement of the wavelength of these features. In all, he mapped over 570 lines, and designated the principal features with the letters A through K, and weaker lines with other letters. It was later discovered by Kirchoff and Bunsen that each chemical element was associated with a set of spectral lines, and deduced that the dark lines in the solar spectrum were caused by absorption by those elements in the upper layers of the sun. Some of the observed features are also caused by absorption in oxygen molecules in the Earth's atmosphere. The Fraunhofer C-, F-, G'-, and h- lines correspond to the alpha, beta, gamma and delta lines of the Balmer series of emission lines of the hydrogen atom. The D 1 and D 2 lines form the well-known "sodium doublet", the centre wavelength of which ( nm) is given the designation letter "D". Note that there is disagreement in the literature for some line designations; e.g., the Fraunhofer "d" line may refer to the cyan iron line at nm, or alternatively to the yellow helium line (also labelled D 3 ) at nm. Because of their well defined wavelengths, Fraunhofer lines are often used to characterize the refractive index and dispersion properties of optical materials. The major Fraunhofer lines, and the elements they are associated with, are shown in the following table: Designation Element Wavelength (nm) Designation Element Wavelength (nm) y O c Fe Z O F H β A O d Fe B O e Fe C H α G' H γ a O G Fe D 1 Na G Ca D 2 Na h H δ D 3 (or d) He H Ca E 2 Fe K Ca b 1 Mg L Fe b 2 Mg N Fe b 3 Fe P Ti b 4 Fe T Fe b 4 Mg t Ni
n(λ) = c/v(λ). Figure 1: Dispersion curves for some common optical glass types.
 Physics 2310 Lab 2: The Dispersion of Optical Glass Dr. Michael Pierce (Univ. of Wyoming) Based on a lab by Dr. M. Kruger (Univ. of Missouri, Kansas City) Purpose: The purpose of this lab is to introduce
Physics 2310 Lab 2: The Dispersion of Optical Glass Dr. Michael Pierce (Univ. of Wyoming) Based on a lab by Dr. M. Kruger (Univ. of Missouri, Kansas City) Purpose: The purpose of this lab is to introduce
Chapter 4. Dispersion of Glass. 4.1 Introduction. 4.2 Apparatus
 Chapter 4 Dispersion of Glass 4.1 Introduction This experiment will develop skills in choosing a suitable fit for data and plotting the resulting curve. Curve fitting will count for a big chunk of the
Chapter 4 Dispersion of Glass 4.1 Introduction This experiment will develop skills in choosing a suitable fit for data and plotting the resulting curve. Curve fitting will count for a big chunk of the
Dispersion of Glass Introduction Apparatus Theory
 Dispersion of Glass Introduction This experiment will develop skills in aligning and using a spectrometer to measure dispersion of glass, and choosing a suitable fit for data and plotting the resulting
Dispersion of Glass Introduction This experiment will develop skills in aligning and using a spectrometer to measure dispersion of glass, and choosing a suitable fit for data and plotting the resulting
SPECTRUM. Dispersion. This phenomenon can be observed in a lab environment using a
 SPECTRUM Dispersion The phenomenon due to which a polychromatic light, like sunlight, splits into its component colours, when passed through a transparent medium like a glass prism, is called dispersion
SPECTRUM Dispersion The phenomenon due to which a polychromatic light, like sunlight, splits into its component colours, when passed through a transparent medium like a glass prism, is called dispersion
4. Dispersion. The index of refraction of the prism at the input wavelength can be calculated using
 4. Dispersion In this lab we will explore how the index of refraction of a material depends on the of the incident light. We first study the phenomenon of minimum deviation of a prism. We then measure
4. Dispersion In this lab we will explore how the index of refraction of a material depends on the of the incident light. We first study the phenomenon of minimum deviation of a prism. We then measure
THE DIFFRACTION GRATING SPECTROMETER
 Purpose Theory THE DIFFRACTION GRATING SPECTROMETER a. To study diffraction of light using a diffraction grating spectrometer b. To measure the wavelengths of certain lines in the spectrum of the mercury
Purpose Theory THE DIFFRACTION GRATING SPECTROMETER a. To study diffraction of light using a diffraction grating spectrometer b. To measure the wavelengths of certain lines in the spectrum of the mercury
Laboratory #29: Spectrometer
 INDIANA UNIVERSITY, DEPARTMENT OF PHYSICS, P309 LABORATORY Laboratory #29: Spectrometer Goal: Learn to adjust an optical spectrometer, use a transmission grating to measure known spectral lines of mercury,
INDIANA UNIVERSITY, DEPARTMENT OF PHYSICS, P309 LABORATORY Laboratory #29: Spectrometer Goal: Learn to adjust an optical spectrometer, use a transmission grating to measure known spectral lines of mercury,
LIGHT. A beam is made up of several rays. It maybe parallel, diverging (spreading out) or converging (getting narrower). Parallel Diverging Converging
 LIGHT Light is a form of energy. It stimulates the retina of the eye and produces the sensation of sight. We see an object when light leaves it and enters the eye. Objects such as flames, the sum and stars
LIGHT Light is a form of energy. It stimulates the retina of the eye and produces the sensation of sight. We see an object when light leaves it and enters the eye. Objects such as flames, the sum and stars
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
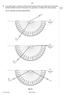 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
Any first year text, sections on atomic structure, spectral lines and spectrometers
 Physics 33 Experiment 5 Atomic Spectra References Any first year text, sections on atomic structure, spectral lines and spectrometers Any modern physics text, eg F.K. Richtmeyer, E.H. Kennard and J.N.
Physics 33 Experiment 5 Atomic Spectra References Any first year text, sections on atomic structure, spectral lines and spectrometers Any modern physics text, eg F.K. Richtmeyer, E.H. Kennard and J.N.
4. Dispersion. The index of refraction of the prism at the input wavelength can be calculated using
 4. Dispersion In this lab we will explore how the index of refraction of a material depends on the of the incident light. We first study the phenomenon of minimum deviation of a prism. We then measure
4. Dispersion In this lab we will explore how the index of refraction of a material depends on the of the incident light. We first study the phenomenon of minimum deviation of a prism. We then measure
AGA5802 Spectroscopy II Prism Gratings Applications
 AGA5802 Spectroscopy II Prism Gratings Applications Bibliography: To Measure the Sky, Kitchin, Lena and others... Prof. Jorge Meléndez 1 Slit Basic components of the Spectrograph Prism or grating Roy &
AGA5802 Spectroscopy II Prism Gratings Applications Bibliography: To Measure the Sky, Kitchin, Lena and others... Prof. Jorge Meléndez 1 Slit Basic components of the Spectrograph Prism or grating Roy &
Ph 3455/MSE 3255 Experiment 2: Atomic Spectra
 Ph 3455/MSE 3255 Experiment 2: Atomic Spectra Background Reading: Tipler, Llewellyn pp. 163-165 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass,
Ph 3455/MSE 3255 Experiment 2: Atomic Spectra Background Reading: Tipler, Llewellyn pp. 163-165 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass,
DISPERSION OF A GLASS PRISM
 PH2 page 1 DISPERSION OF A GLASS PRISM OBJECTIVE The objective of this experiment is to analyze the emission spectrum of helium and to analyze the dispersion of a glass prism by measuring the index of
PH2 page 1 DISPERSION OF A GLASS PRISM OBJECTIVE The objective of this experiment is to analyze the emission spectrum of helium and to analyze the dispersion of a glass prism by measuring the index of
Lab #13: Polarization
 Lab #13: Polarization Introduction In this experiment we will investigate various properties associated with polarized light. We will study both its generation and application. Real world applications
Lab #13: Polarization Introduction In this experiment we will investigate various properties associated with polarized light. We will study both its generation and application. Real world applications
Observation of Atomic Spectra
 Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
EXPERIMENT 09 OBSERVATION OF SPECTRA
 EXPERIMENT 09 OBSERVATION OF SPECTRA INTRODUCTION: In physics, as in very other area of study, one of the most valuable questions a student can learn to ask is, How do they know that? Thus, when you read
EXPERIMENT 09 OBSERVATION OF SPECTRA INTRODUCTION: In physics, as in very other area of study, one of the most valuable questions a student can learn to ask is, How do they know that? Thus, when you read
The Fundamentals of Spectroscopy: Theory BUILDING BETTER SCIENCE AGILENT AND YOU
 The Fundamentals of Spectroscopy: Theory BUILDING BETTER SCIENCE AGILENT AND YOU 1 Agilent is committed to the educational community and is willing to provide access to company-owned material. This slide
The Fundamentals of Spectroscopy: Theory BUILDING BETTER SCIENCE AGILENT AND YOU 1 Agilent is committed to the educational community and is willing to provide access to company-owned material. This slide
Spectroscopy of Various Light Sources: The Interactions between Light and Matter ASTR 170B1, Spring 2017, Lab #2. 1 Introduction.
 Spectroscopy of Various Light Sources: The Interactions between Light and Matter ASTR 170B1, Spring 2017, Lab #2 DUE IN CLASS ON Thursday Sept 28! You CAN work in a group of 2, but you need only turn in
Spectroscopy of Various Light Sources: The Interactions between Light and Matter ASTR 170B1, Spring 2017, Lab #2 DUE IN CLASS ON Thursday Sept 28! You CAN work in a group of 2, but you need only turn in
Spectrometers. Materials: Easy Spectrometer. Old CD Razor Index card Cardboard tube at least 10 inches long
 Spectrometers Overview: Spectrometers (spectroscopes) are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we
Spectrometers Overview: Spectrometers (spectroscopes) are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we
VI. OBSERVATIONS / DATA COLLECTION:
 Lab Write-Up Format THIS OUTLINE WILL HELP YOU TO WRITE OUT YOUR LABS. There may be changes or modifications but all elements must be included in your lab write-up. Each section on your lab paper must
Lab Write-Up Format THIS OUTLINE WILL HELP YOU TO WRITE OUT YOUR LABS. There may be changes or modifications but all elements must be included in your lab write-up. Each section on your lab paper must
The Emission Spectra of Light
 The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
PHY Atomic Spectra
 Page 1 of 6 PHY 124 - Atomic Spectra The purpose of this laboratory is to study transitions between energy levels of the hydrogen atom by observing the spectrum of light emitted when the atoms make transitions
Page 1 of 6 PHY 124 - Atomic Spectra The purpose of this laboratory is to study transitions between energy levels of the hydrogen atom by observing the spectrum of light emitted when the atoms make transitions
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Optics in a Fish Tank Demonstrations for the Classroom
 Optics in a Fish Tank Demonstrations for the Classroom Introduction: This series of demonstrations will illustrate a number of optical phenomena. Using different light sources and a tank of water, you
Optics in a Fish Tank Demonstrations for the Classroom Introduction: This series of demonstrations will illustrate a number of optical phenomena. Using different light sources and a tank of water, you
Atomic Spectra HISTORY AND THEORY
 Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
EXPERIMENT 14. The Atomic Spectrum of Hydrogen
 Name: Laboratory Section: Laboratory Section Date: Partners Names: Grade: Last Revised on March 18, 2003 EXPERIMENT 14 The Atomic Spectrum of Hydrogen 0. Pre-Laboratory Work [2 pts] 1. You will be using
Name: Laboratory Section: Laboratory Section Date: Partners Names: Grade: Last Revised on March 18, 2003 EXPERIMENT 14 The Atomic Spectrum of Hydrogen 0. Pre-Laboratory Work [2 pts] 1. You will be using
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Building your own Pizza-Box Spectroscope. *You will need to bring in a medium- sized sturdy cardboard pizza box, shoe box, or similar from home.
 Building your own Pizza-Box Spectroscope Experimental Notes *You will need to bring in a medium- sized sturdy cardboard pizza box, shoe box, or similar from home. Color, Light, and Atomic Spectroscopy
Building your own Pizza-Box Spectroscope Experimental Notes *You will need to bring in a medium- sized sturdy cardboard pizza box, shoe box, or similar from home. Color, Light, and Atomic Spectroscopy
APAS Laboratory { PAGE } Spectroscopy SPECTROSCOPY
 SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
PHYSICS 122/124 Lab EXPERIMENT NO. 9 ATOMIC SPECTRA
 PHYSICS 1/14 Lab EXPERIMENT NO. 9 ATOMIC SPECTRA The purpose of this laboratory is to study energy levels of the Hydrogen atom by observing the spectrum of emitted light when Hydrogen atoms make transitions
PHYSICS 1/14 Lab EXPERIMENT NO. 9 ATOMIC SPECTRA The purpose of this laboratory is to study energy levels of the Hydrogen atom by observing the spectrum of emitted light when Hydrogen atoms make transitions
PHYS General Physics II Lab The Balmer Series for Hydrogen Source. c = speed of light = 3 x 10 8 m/s
 PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
Lecture 2. In this lecture we will go through the chronological development of the Atomic physics.
 Lecture 2 TITLE: A brief history of the development of structure of atom Page 1 Objectives In this lecture we will go through the chronological development of the Atomic physics. We will find out the thoughts
Lecture 2 TITLE: A brief history of the development of structure of atom Page 1 Objectives In this lecture we will go through the chronological development of the Atomic physics. We will find out the thoughts
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Experiment 24: Spectroscopy
 Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Higher Physics. Particles and Waves
 Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric
Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric
Determination of Cauchy s Contants
 8. Determination of Cauchy s Contants 8.1 Objective: To determine Cauchy s Constants using a prism and spectrometer. Apparatus: Glass prism, spectrometer and mercury vapour lamp. 8. Theory: The wavelength
8. Determination of Cauchy s Contants 8.1 Objective: To determine Cauchy s Constants using a prism and spectrometer. Apparatus: Glass prism, spectrometer and mercury vapour lamp. 8. Theory: The wavelength
Name(s): Date: Course/Section: Spectroscopy
 Name(s): Date: Course/Section: Grade: Spectroscopy Part 1: Visible Light 1. Fill in the table below that summarizes the colors of the lights on the LED array. The table should include the bulb s color,
Name(s): Date: Course/Section: Grade: Spectroscopy Part 1: Visible Light 1. Fill in the table below that summarizes the colors of the lights on the LED array. The table should include the bulb s color,
RAY OPTICS 6. DISPERSION POINTS TO REMEMBER
 Y OPTICS 6. DISPESION POINTS TO EMEMBE. Dispersion : a) The splitting of white light into constituent colours is called dispersion and the band of colours is called spectrum. b) Dispersion of light was
Y OPTICS 6. DISPESION POINTS TO EMEMBE. Dispersion : a) The splitting of white light into constituent colours is called dispersion and the band of colours is called spectrum. b) Dispersion of light was
AP Waves/Optics ~ Learning Guide
 AP Waves/Optics ~ Learning Guide Name: Instructions: Using a pencil, answer the following questions. The guide is marked based on effort, completeness, thoughtfulness, and neatness (not accuracy). Do your
AP Waves/Optics ~ Learning Guide Name: Instructions: Using a pencil, answer the following questions. The guide is marked based on effort, completeness, thoughtfulness, and neatness (not accuracy). Do your
Physics 1C OPTICAL SPECTROSCOPY Rev. 2-AH. Introduction
 Introduction In this lab you will use a diffraction grating to split up light into its various colors (like a rainbow). You will assemble a spectrometer, incorporating the diffraction grating. A spectrometer
Introduction In this lab you will use a diffraction grating to split up light into its various colors (like a rainbow). You will assemble a spectrometer, incorporating the diffraction grating. A spectrometer
Chapter 4. Spectroscopy. Dr. Tariq Al-Abdullah
 Chapter 4 Spectroscopy Dr. Tariq Al-Abdullah Learning Goals: 4.1 Spectral Lines 4.2 Atoms and Radiation 4.3 Formation of the Spectral Lines 4.4 Molecules 4.5 Spectral Line Analysis 2 DR. T. AL-ABDULLAH
Chapter 4 Spectroscopy Dr. Tariq Al-Abdullah Learning Goals: 4.1 Spectral Lines 4.2 Atoms and Radiation 4.3 Formation of the Spectral Lines 4.4 Molecules 4.5 Spectral Line Analysis 2 DR. T. AL-ABDULLAH
Atomic Spectra. Eric Reichwein David Steinberg Department of Physics University of California, Santa Cruz. August 30, 2012
 Atomic Spectra Eric Reichwein David Steinberg Department of Physics University of California, Santa Cruz August 30, 0 Abstract To observe helium spectral lines we used a spectrometer. From a table of known
Atomic Spectra Eric Reichwein David Steinberg Department of Physics University of California, Santa Cruz August 30, 0 Abstract To observe helium spectral lines we used a spectrometer. From a table of known
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
The Spectroscope: New Meanings in Light
 TOPIC 3 The Spectroscope: New Meanings in Light Now that astronomers could gaze deeper into the universe using simple telescopes, they faced a new challenge the stars. Galileo had seen the stars through
TOPIC 3 The Spectroscope: New Meanings in Light Now that astronomers could gaze deeper into the universe using simple telescopes, they faced a new challenge the stars. Galileo had seen the stars through
Atomic emission spectra experiment
 Atomic emission spectra experiment Contents 1 Overview 1 2 Equipment 1 3 Measuring the grating spacing using the sodium D-lines 4 4 Measurement of hydrogen lines and the Rydberg Constant 5 5 Measurement
Atomic emission spectra experiment Contents 1 Overview 1 2 Equipment 1 3 Measuring the grating spacing using the sodium D-lines 4 4 Measurement of hydrogen lines and the Rydberg Constant 5 5 Measurement
NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT. Physics 211 E&M and Quantum Physics Spring Lab #9: Diffraction Spectroscopy
 NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT Physics 211 E&M and Quantum Physics Spring 2018 Lab #9: Diffraction Spectroscopy Lab Writeup Due: Mon/Wed/Thu/Fri, April 30/ May 2/3/4, 2018 Background All
NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT Physics 211 E&M and Quantum Physics Spring 2018 Lab #9: Diffraction Spectroscopy Lab Writeup Due: Mon/Wed/Thu/Fri, April 30/ May 2/3/4, 2018 Background All
Experiment 9. Emission Spectra. measure the emission spectrum of a source of light using the digital spectrometer.
 Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Conceptual Physics. Luis A. Anchordoqui. Department of Physics and Astronomy Lehman College, City University of New York. Lesson VI October 3, 2017
 Conceptual Physics Luis A. Anchordoqui Department of Physics and Astronomy Lehman College, City University of New York Lesson VI October 3, 2017 https://arxiv.org/abs/1711.07445 L. A. Anchordoqui (CUNY)
Conceptual Physics Luis A. Anchordoqui Department of Physics and Astronomy Lehman College, City University of New York Lesson VI October 3, 2017 https://arxiv.org/abs/1711.07445 L. A. Anchordoqui (CUNY)
Physics 1CL OPTICAL SPECTROSCOPY Spring 2010
 Introduction In this lab, you will use a diffraction grating to split up light into the various colors which make up the different wavelengths of the visible electromagnetic spectrum. You will assemble
Introduction In this lab, you will use a diffraction grating to split up light into the various colors which make up the different wavelengths of the visible electromagnetic spectrum. You will assemble
Name Final Exam May 1, 2017
 Name Final Exam May 1, 217 This test consists of five parts. Please note that in parts II through V, you can skip one question of those offered. Some possibly useful formulas appear below. Constants, etc.
Name Final Exam May 1, 217 This test consists of five parts. Please note that in parts II through V, you can skip one question of those offered. Some possibly useful formulas appear below. Constants, etc.
We now realize that the phenomena of chemical interactions, and, ultimately life itself, are to be understood in terms of electromagnetism".
 We now realize that the phenomena of chemical interactions, and, ultimately life itself, are to be understood in terms of electromagnetism". -Richard Feynman Quantum H Atom Review Radia Wave Function (1s):
We now realize that the phenomena of chemical interactions, and, ultimately life itself, are to be understood in terms of electromagnetism". -Richard Feynman Quantum H Atom Review Radia Wave Function (1s):
School. Team Number. Optics
 School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
Pre-lab Quiz/PHYS 224. Your name Lab section
 Pre-lab Quiz/PHYS 224 THE DIFFRACTION GRATING AND THE OPTICAL SPECTRUM Your name Lab section 1. What are the goals of this experiment? 2. If the period of a diffraction grating is d = 1,000 nm, where the
Pre-lab Quiz/PHYS 224 THE DIFFRACTION GRATING AND THE OPTICAL SPECTRUM Your name Lab section 1. What are the goals of this experiment? 2. If the period of a diffraction grating is d = 1,000 nm, where the
Physics 1252 Sec.A Exam #1A
 Physics 1252 Sec.A Exam #1A Instructions: This is a closed-book, closed-notes exam. You are allowed to use a clean print-out of your formula sheet, any scientific calculator, and a ruler. Do not write
Physics 1252 Sec.A Exam #1A Instructions: This is a closed-book, closed-notes exam. You are allowed to use a clean print-out of your formula sheet, any scientific calculator, and a ruler. Do not write
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Sometimes cars that would normally travel at 65 km hour 1 may be limited to about 20 km hour 1 by a cyclist.
 1 Queues of cars often form behind cyclists on narrow, rural roads. Sometimes cars that would normally travel at 65 km hour 1 may be limited to about 20 km hour 1 by a cyclist. (a) Show that 65 km hour
1 Queues of cars often form behind cyclists on narrow, rural roads. Sometimes cars that would normally travel at 65 km hour 1 may be limited to about 20 km hour 1 by a cyclist. (a) Show that 65 km hour
hf = E 1 - E 2 hc = E 1 - E 2 λ FXA 2008 Candidates should be able to : EMISSION LINE SPECTRA
 1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051 FRAUNHOFER DIFFRACTION
 E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051 FRAUNHOFER DIFFRACTION References for Fraunhofer Diffraction 1. Jenkins and White Fundamentals of Optics. Chapters on Fraunhofer diffraction and
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051 FRAUNHOFER DIFFRACTION References for Fraunhofer Diffraction 1. Jenkins and White Fundamentals of Optics. Chapters on Fraunhofer diffraction and
Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I
 Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I Purpose Investigate Kirchhoff s Laws for continuous, emission and absorption spectra Analyze the solar spectrum and identify unknown lines
Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I Purpose Investigate Kirchhoff s Laws for continuous, emission and absorption spectra Analyze the solar spectrum and identify unknown lines
Some Topics in Optics
 Some Topics in Optics The HeNe LASER The index of refraction and dispersion Interference The Michelson Interferometer Diffraction Wavemeter Fabry-Pérot Etalon and Interferometer The Helium Neon LASER A
Some Topics in Optics The HeNe LASER The index of refraction and dispersion Interference The Michelson Interferometer Diffraction Wavemeter Fabry-Pérot Etalon and Interferometer The Helium Neon LASER A
Atomic Spectra. d sin θ = mλ (1)
 Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
high energy state for the electron in the atom low energy state for the electron in the atom
 Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Buy-back points tallied and added: 750 points bought-back. Last Withdrawal date: this friday, Oct 31st.
 Announcements HW #3: Available online now. Due in 1 week, Nov 3rd, 11pm. Buy-back points tallied and added: 750 points bought-back. Last Withdrawal date: this friday, Oct 31st. Evening Observing: next
Announcements HW #3: Available online now. Due in 1 week, Nov 3rd, 11pm. Buy-back points tallied and added: 750 points bought-back. Last Withdrawal date: this friday, Oct 31st. Evening Observing: next
Interference by Wavefront Division
 nterference by Wavefront Division One of the seminal experiments in physics was conducted in 1801 by Thomas Young, an English physicist who cut a small hole in an opaque screen, set a second screen in
nterference by Wavefront Division One of the seminal experiments in physics was conducted in 1801 by Thomas Young, an English physicist who cut a small hole in an opaque screen, set a second screen in
Atomic Theory C &03
 Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Astonomy 62 Lecture #10. Last Time. Applications of Stefan-Boltzmann Law Color Magnitudes Color Index
 Last Time Applications of Stefan-Boltzmann Law Color Magnitudes Color Index Standard Visual Band Filters U B V R I Flux through filter X: F x = 0 F S x d F x F x W x Apparent Color Magnitude: m x,1 m x,2
Last Time Applications of Stefan-Boltzmann Law Color Magnitudes Color Index Standard Visual Band Filters U B V R I Flux through filter X: F x = 0 F S x d F x F x W x Apparent Color Magnitude: m x,1 m x,2
ATOMIC SPECTRA. Objective:
 1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
Higher Physics Particles and Waves 2 Notes
 Higher Physics Particles and Waves 2 Notes Teachers Booklet Learning Outcomes Interference and diffraction This builds on information from N5 Waves and Radiation H Particles and Waves book 1 At the end
Higher Physics Particles and Waves 2 Notes Teachers Booklet Learning Outcomes Interference and diffraction This builds on information from N5 Waves and Radiation H Particles and Waves book 1 At the end
A Wave of Cooperation
 A Wave of Cooperation The students will peer teach in cooperative learning groups some basic topics regarding waves. Materials: Student sheets Note sheets Procedure: 1. Place students into home teams of
A Wave of Cooperation The students will peer teach in cooperative learning groups some basic topics regarding waves. Materials: Student sheets Note sheets Procedure: 1. Place students into home teams of
Lecture 7. Outline. ASTR 111 Section 002. Discuss Quiz 5 Light. Light travels through empty space at a speed of 300,000 km/s
 Lecture 7 ASTR 111 Section 002 Outline Discuss Quiz 5 Light Suggested reading: Chapter 5.1-5.2 and 5.6-5.8 of textbook Light travels through empty space at a speed of 300,000 km/s In 1676, Danish astronomer
Lecture 7 ASTR 111 Section 002 Outline Discuss Quiz 5 Light Suggested reading: Chapter 5.1-5.2 and 5.6-5.8 of textbook Light travels through empty space at a speed of 300,000 km/s In 1676, Danish astronomer
Chemistry 212 ATOMIC SPECTROSCOPY
 Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
DAY LABORATORY EXERCISE: SPECTROSCOPY
 AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
Lab 5: Spectroscopy & the Hydrogen Atom Phy248 Spring 2009
 Lab 5: Spectroscopy & the Hydrogen Atom Phy248 Spring 2009 Name Section Return this spreadsheet to your TA that will use it to score your lab. To receive full credit you must use complete sentences and
Lab 5: Spectroscopy & the Hydrogen Atom Phy248 Spring 2009 Name Section Return this spreadsheet to your TA that will use it to score your lab. To receive full credit you must use complete sentences and
Lab 6: Spectroscopy Due Monday, April 10
 Lab 6: Spectroscopy Due Monday, April 10 The aim of this lab is to provide you with hands-on experience obtaining and analyzing spectroscopic data. In this lab you will be using a spectrograph to obtain
Lab 6: Spectroscopy Due Monday, April 10 The aim of this lab is to provide you with hands-on experience obtaining and analyzing spectroscopic data. In this lab you will be using a spectrograph to obtain
Particles and Waves Homework One (Target mark 13 out of 15)
 Particles and Waves Homework One (Target mark 13 out of 15) Display all answers to 2 significant figures. 1. A car covers a distance of 170m in a time of 18s. Calculate the average speed of the car. 2.
Particles and Waves Homework One (Target mark 13 out of 15) Display all answers to 2 significant figures. 1. A car covers a distance of 170m in a time of 18s. Calculate the average speed of the car. 2.
Physics 476LW Advanced Physics Laboratory Atomic Spectroscopy
 Physics 476LW Atomic Spectroscopy 1 Introduction The description of atomic spectra and the Rutherford-Geiger-Marsden experiment were the most significant precursors of the so-called Bohr planetary model
Physics 476LW Atomic Spectroscopy 1 Introduction The description of atomic spectra and the Rutherford-Geiger-Marsden experiment were the most significant precursors of the so-called Bohr planetary model
Taking Fingerprints of Stars, Galaxies, and Other Stuff. The Bohr Atom. The Bohr Atom Model of Hydrogen atom. Bohr Atom. Bohr Atom
 Periodic Table of Elements Taking Fingerprints of Stars, Galaxies, and Other Stuff Absorption and Emission from Atoms, Ions, and Molecules Universe is mostly (97%) Hydrogen and Helium (H and He) The ONLY
Periodic Table of Elements Taking Fingerprints of Stars, Galaxies, and Other Stuff Absorption and Emission from Atoms, Ions, and Molecules Universe is mostly (97%) Hydrogen and Helium (H and He) The ONLY
OPSE FINAL EXAM Fall 2016 YOU MUST SHOW YOUR WORK. ANSWERS THAT ARE NOT JUSTIFIED WILL BE GIVEN ZERO CREDIT.
 CLOSED BOOK. Equation Sheet is provided. YOU MUST SHOW YOUR WORK. ANSWERS THAT ARE NOT JUSTIFIED WILL BE GIVEN ZERO CREDIT. ALL NUMERICAL ANSERS MUST HAVE UNITS INDICATED. (Except dimensionless units like
CLOSED BOOK. Equation Sheet is provided. YOU MUST SHOW YOUR WORK. ANSWERS THAT ARE NOT JUSTIFIED WILL BE GIVEN ZERO CREDIT. ALL NUMERICAL ANSERS MUST HAVE UNITS INDICATED. (Except dimensionless units like
You Are the Spectrometer! A Look Inside Astronomy's Essential Instrument (Robert B. Friedman & Matthew K. Sharp)
 You Are the Spectrometer! A Look Inside Astronomy's Essential Instrument (Robert B. Friedman & Matthew K. Sharp) Introduction Astronomy is a unique science because unlike many of the other sciences, the
You Are the Spectrometer! A Look Inside Astronomy's Essential Instrument (Robert B. Friedman & Matthew K. Sharp) Introduction Astronomy is a unique science because unlike many of the other sciences, the
PHYSICS 116 SPECTROSCOPY: DETERMINATION OF THE WAVELENGTH OF LIGHT
 Name Date Lab Time Lab TA PHYSICS 116 SPECTROSCOPY: DETERMINATION OF THE WAVELENGTH OF LIGHT I. PURPOSE To use a diffraction grating to investigate the spectra produced by several unknown gas discharge
Name Date Lab Time Lab TA PHYSICS 116 SPECTROSCOPY: DETERMINATION OF THE WAVELENGTH OF LIGHT I. PURPOSE To use a diffraction grating to investigate the spectra produced by several unknown gas discharge
Solution 3: A glass prism deviates the violet light most and the red light least.
 EXERCISE- 6 (A) Question 1: Name three factors on which the deviation produces by a prism depends and state how does it depend on the factors stated by you. Solution 1: The deviation produced by the prism
EXERCISE- 6 (A) Question 1: Name three factors on which the deviation produces by a prism depends and state how does it depend on the factors stated by you. Solution 1: The deviation produced by the prism
LAB 10: OPTICAL MATERIALS AND DISPERSION I
 OPTI 202L - Geometrical and Instrumental Optics Lab LAB 10: OPTICAL MATERIALS AND DISPERSION I 10-1 Measuring the refractive index of a material is one of the most fundamental optical measurements, and
OPTI 202L - Geometrical and Instrumental Optics Lab LAB 10: OPTICAL MATERIALS AND DISPERSION I 10-1 Measuring the refractive index of a material is one of the most fundamental optical measurements, and
Wave - Particle Duality of Light
 Properties of Light Objectives Explain wave-particle duality State the speed of light Describe electromagnetic waves and the electromagnetic spectrum Explain how light interacts with transparent and opaque
Properties of Light Objectives Explain wave-particle duality State the speed of light Describe electromagnetic waves and the electromagnetic spectrum Explain how light interacts with transparent and opaque
DISPERSION VERY SHORT ANSWER QUESTIONS. Two identical prisms made of the same material placed with their based on opposite sides (of the
 DISPERSION VERY SHORT ANSWER QUESTIONS Q-1. What will be the spectrum of sun during a total solar eclipse? Q-2. Why the secondary rainbow is always fainter than the primary rainbow? Q-3. Two identical
DISPERSION VERY SHORT ANSWER QUESTIONS Q-1. What will be the spectrum of sun during a total solar eclipse? Q-2. Why the secondary rainbow is always fainter than the primary rainbow? Q-3. Two identical
EXPERIMENT 17: Atomic Emission
 EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
Unit 2 - Particles and Waves - Part 2
 WAVE-PARTICLE DUALITY Unit - Particles and Waves - Part 8. The photoelectric effect and wave particle duality Photoelectric effect as evidence for the particulate nature of light. Photons of sufficient
WAVE-PARTICLE DUALITY Unit - Particles and Waves - Part 8. The photoelectric effect and wave particle duality Photoelectric effect as evidence for the particulate nature of light. Photons of sufficient
Name: Number: Class: Date: What is light? 1. What is the name for all these types of light? 2. What type of light can humans see?
 Name: Number: Class: Date: What is light? Worksheet 1 1. What is the name for all these types of light? 2. What type of light can humans see? 3. What type of light has the longest wavelength? 4. What types
Name: Number: Class: Date: What is light? Worksheet 1 1. What is the name for all these types of light? 2. What type of light can humans see? 3. What type of light has the longest wavelength? 4. What types
Optical/IR Observational Astronomy Spectroscopy. David Buckley, SALT
 David Buckley, SALT 1 Background is really just monochromatic photometry History 1637 Descartes explained the origin of the rainbow. 1666 Newton s classic experiments on the nature of colour. 1752 Melvil
David Buckley, SALT 1 Background is really just monochromatic photometry History 1637 Descartes explained the origin of the rainbow. 1666 Newton s classic experiments on the nature of colour. 1752 Melvil
ECEN 4606, UNDERGRADUATE OPTICS LAB
 ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 6: Polarization Original: Professor McLeod SUMMARY: In this lab you will become familiar with the basics of polarization and learn to use common optical elements
ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 6: Polarization Original: Professor McLeod SUMMARY: In this lab you will become familiar with the basics of polarization and learn to use common optical elements
Experiment 7: Spectrum of the Hydrogen Atom
 Experiment 7: Spectrum of the Hydrogen Nate Saffold nas2173@columbia.edu Office Hour: Mondays, 5:30-6:30PM INTRO TO EXPERIMENTAL PHYS-LAB 1493/1494/2699 Introduction The physics behind: The spectrum of
Experiment 7: Spectrum of the Hydrogen Nate Saffold nas2173@columbia.edu Office Hour: Mondays, 5:30-6:30PM INTRO TO EXPERIMENTAL PHYS-LAB 1493/1494/2699 Introduction The physics behind: The spectrum of
Name Date: Course number: MAKE SURE TA & TI STAMPS EVERY PAGE BEFORE YOU START EXPERIMENT 14. The Atomic Spectrum of Hydrogen
 Laboratory Section: Last Revised on September 21, 2016 Partners Names: Grade: EXPERIMENT 14 The Atomic Spectrum of Hydrogen 0. Pre-Laboratory Work [2 pts] 1. You will be using a diffraction grating in
Laboratory Section: Last Revised on September 21, 2016 Partners Names: Grade: EXPERIMENT 14 The Atomic Spectrum of Hydrogen 0. Pre-Laboratory Work [2 pts] 1. You will be using a diffraction grating in
PHYSICS LABORATORY III
 T.C. MARMARA UNIVERSITY FACULTY OF ARTS AND SCIENCES PHYSICS DEPARTMENT PHYSICS LABORATORY III DEPARTMENT: NAME: SURNAME: NUMBER: 2 T.C.MARMARA UNIVERSITY PHYSICS DEPARTMENT PHYSICS LABORATORY III MANUAL
T.C. MARMARA UNIVERSITY FACULTY OF ARTS AND SCIENCES PHYSICS DEPARTMENT PHYSICS LABORATORY III DEPARTMENT: NAME: SURNAME: NUMBER: 2 T.C.MARMARA UNIVERSITY PHYSICS DEPARTMENT PHYSICS LABORATORY III MANUAL
Since focal length = focal power
 RAY OPTICS PREVIOUS EAMCET BITS (ENGINEERING ). The two lenses of an achromatic doublet should have : [EAMCET 009 E] ) equal powers ) equal dispersive powers ) equal ratio of their power and dispersive
RAY OPTICS PREVIOUS EAMCET BITS (ENGINEERING ). The two lenses of an achromatic doublet should have : [EAMCET 009 E] ) equal powers ) equal dispersive powers ) equal ratio of their power and dispersive
Atomic Emission and Molecular Absorption Spectra
 Atomic Emission and Molecular Absorption Spectra v062513_6pm Objective: The student will observe the atomic emission spectra of hydrogen using a spectroscope, determine the identity of an unknown metal
Atomic Emission and Molecular Absorption Spectra v062513_6pm Objective: The student will observe the atomic emission spectra of hydrogen using a spectroscope, determine the identity of an unknown metal
Geometrical Optics. = n r. sinθ i
 Name Section Number Lab Partner s Name Geometrical Optics Introduction In geometrical optics, refraction is described by Snell s Law. Refraction refers to the bending of light as it passes from one medium
Name Section Number Lab Partner s Name Geometrical Optics Introduction In geometrical optics, refraction is described by Snell s Law. Refraction refers to the bending of light as it passes from one medium
Experiment #9. Atomic Emission Spectroscopy
 Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Pizza Box Spectrometer Data & Report
 Pizza Box Spectrometer Data & Report Team Name: Members: Section or lab meeting time: Data & Observations: 1. How do you think the grating works? Explain in several sentences. 2. If you were to use your
Pizza Box Spectrometer Data & Report Team Name: Members: Section or lab meeting time: Data & Observations: 1. How do you think the grating works? Explain in several sentences. 2. If you were to use your
Note to 8.13 students:
 Note to 8.13 students: Feel free to look at this paper for some suggestions about the lab, but please reference/acknowledge me as if you had read my report or spoken to me in person. Also note that this
Note to 8.13 students: Feel free to look at this paper for some suggestions about the lab, but please reference/acknowledge me as if you had read my report or spoken to me in person. Also note that this
LIGHT. Question. Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light.
 LIGHT Question Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light. What kind of information can we get from light? 1 Light
LIGHT Question Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light. What kind of information can we get from light? 1 Light
