Solution 3: A glass prism deviates the violet light most and the red light least.
|
|
|
- Amberlynn Jones
- 5 years ago
- Views:
Transcription
1 EXERCISE- 6 (A) Question 1: Name three factors on which the deviation produces by a prism depends and state how does it depend on the factors stated by you. Solution 1: The deviation produced by the prism depends on the following four factors: (a) The angle of incidence - As the angle of incidence increases, first the angle of deviation decreases and reaches to a minimum value for a certain angle of incidence. By further increasing the angle of incidence, the angle of deviation is found to increase. (b) The material of prism (i.e., on refractive index) - For a given angle of incidence, the prism with a higher refractive index produces a greater deviation than the prism which has a lower refractive index. (c) Angle of prism- Angle of deviation increases with the increase in the angle of prism. (d) The colour or wavelength of light used- Angle of deviation increases with the decrease in wavelength of light. Question 2: How does the deviation produced by a triangular prism depend on the colours (or wavelengths) of light incident on it? Solution 2: The deviation caused by a prism increases with the decrease in the wavelength of light incident on it. Question 3: Which colour of white light is deviated by a glass prism the most and which the least? Solution 3: A glass prism deviates the violet light most and the red light least.
2 Question 4: Define the term dispersion of light. Solution 4: The phenomenon of splitting of white light by a prism into its constituent colours is known as dispersion of light. Question 5: Explain the cause of dispersion of white light through a prism. Solution 5: When white light is incident on the first surface of a prism and enters in glass, light of different colours due to different speeds in glass, is refracted or deviated through different angles. Thus the dispersion of white light into its constituent colours takes place at the first surface of prism. Thus the cause of dispersion is the change in speed of light with wavelength or frequency. Question 6: Explain briefly, with the help of a neat labelled diagram, how white light gets dispersed by a prism. Solution 6: When white light is incident on the first surface of a prism and enters in glass, light of different colours due to different speeds in glass, is refracted or deviated through different angles. Thus the dispersion of white light into its constituent colours takes place at the first surface of prism. On the second surface, only refraction takes place and different colours are deviated through different angles. As a result, the colours get further separated on refraction at the second surface (violet being deviated the most and red the least).
3 Question 7: The diagram shown below in Fig shows the path taken by a narrow beam of yellow monochromatic light passing through an equiangular glass prism. Now the yellow light is replaced by a narrow beam of white light incident at the same angle. Draw another diagram to show the passage of white light through the prism and label it to show the effect of prism on the white light. Solution 7: Question 8: How does the speed of light in glass change on increasing the wavelength of light? Solution 8: Speed of light increases with increase in the wavelength. Question 9: Which colour of white light travels (a) fastest (b) slowest, in glass? Solution 9: Red colour travels fastest and Blue colour travels slowest in glass.
4 Question 10: Fig 6.12 shows a thin beam of white light from a source S striking on one face of a prism. (a) Complete the diagram to show effect of prism on the beam and to show what is seen on the screen. (b) A slit is placed in between the prism and the screen to pass only the light of green colour. What will you then observe on the screen? (c) What conclusion do you draw from the observation in part (b) above? Solution 10: (a) Constituent colours of white light are seen on the screen after dispersion through the prism. (b) When a slit is introduced in between the prism and screen to pass only the light of green colour, only green light is observed on the screen. (c) From the observation, we conclude that prism itself produces no colour. Question 11: (a) If a monochromatic beam of light undergoes minimum deviation through an equiangular prism, how does the beam pass through the prism, with respect to its base? (b) If white light is used in same way as in part (a) above, what change is expected in the emergent beam? (c) what conclusion do you draw about the nature of white light in part (b)? Solution 11: (a) If a monochromatic beam of light undergoes minimum deviation through an equi-angular prism, then the beam passes parallel to the base of prism.
5 (b) White light splits into its constituent colours i.e., spectrum is formed. (c) We conclude that white light is polychromatic. Question 12: What do you understand by the term spectrum? Solution 12: The colour band obtained on a screen on passing white light through a prism is called the spectrum. Question 13: A ray of white light is passed through a glass prism and spectrum is obtained on a screen. (a) Name the seven colours of the spectrum in order. (b) Dow the colours have the same width in the spectrum? (c) which of the colour of the spectrum of white light deviates (i) the most, (ii) the least? Solution 13: (a) Violet, Indigo, Blue, Green, Yellow, Orange, Red. (b) No, different colours have different widths in the spectrum. (c) (i) Violet colour is deviated the most. (ii) Red colour is deviated the least. Question 14: The wavelengths for the light of red and blue colours are roughly m and m respectively. (a) Which colour has the greater speed in vacuum? (b) Which colour has the greater speed in vacuum? Solution 14: (a) In vacuum, both have the same speeds. (b) In glass, red light has a greater speed.
6 Question 15: (i) Draw a diagram to show the splitting of white light by a prism into its constituent colour. (ii) Draw another diagram to show how the colours of spectrum of white light can be combined to give the effect of white light. Solution 15: Color of light is related to its wavelength. Question 16: Complete the ray diagram given below to show the nature of light produced on the screen. Solution 16: 0 0 i A to 8000 A ii. 400 nm to 800 nm Question 17: Name the subjective property of light related to its wavelength. Solution 17: (i) (ii) For blue light, approximate wavelength=4800 A For red light, approximate wavelength=8000 A Question 18: What is the range of wavelength of the spectrum of white light in (i) A, (ii) nm? Solution 18: Seven prominent colours of the white light spectrum in order of their increasing frequencies: Red, Orange, Yellow, Green, Blue, Indigo, Violet
7 Question 19: Write the approximate wavelengths for (i) blue and (ii) red light. Solution 19: Green, Yellow orange and red have wavelength longer than blue light. MULTIPLE CHOICE TYPE: Question 1: When a white light ray falls on a prism, the ray at its first surface suffers. (a) no refraction (b) only dispersion (c) only deviation (d) both deviation and dispersion Solution 1: Both deviation and dispersion. Hint: When a white light ray falls on the first surface of a prism, light rays of different colours due to their different speeds in glass get refracted (or deviated) through different angles. Thus, the dispersion of white light into its constituent colours takes place at the first surface of prism. Question 2: In the spectrum of white light by a prism, the colour at the extreme end opposite to the base of prism is: (a) violet (c) red (b) yellow (d) blue Solution 2: The colour of the extreme end opposite to the base of the prism is red. Hint: The angle of deviation decreases with the increase in wavelength of light for a given angle of incidence. Since the red light has greatest wavelength, it gets deviated the least and is seen on the extreme end opposite to the base of prism.
8 10 = 8 9 NUMERICALS: Question 1: Calculate the frequency of yellow light of wavelength 550nm. The speed of light is m/s -1 Solution 1: Given, wavelength λ = 550 nm = m Speed of light, c = m/s We know that, Frequency = h h Or, frequency = HZ Question 2: The frequency range of visible light is from 3.75 x Hz to 7.5 x Hz. Calculate its wavelength range. Take speed of light = 3 x 10 8 m/s -1 Solution 2: Speed of light, c = 3 x 10 8 m/s Frequency range = 3.75 x Hz to 7.5 x Hz. Speed of light = frequency x wavelength For frequency = 3.75 x Hz Λ = = m / s Hz m 8000 A For frequency =7.5 x Hz Λ = = m / s m 4000A Hz Wavelength range = 4000 A to 8000 A
9 EXERCISE. 6 B Question 1: (a) Give a list of at least five radiations, in order of their increasing frequencies, which make up the complete electromagnetic spectrum. (b) Which of the radiation mentioned in answer to part (a) has the highest penetrating power? Solution 1: (a) Five radiations, in the order of their increasing frequencies are: Infrared waves, Visible light, Ultraviolet, X-rays and Gamma rays. (b) Gamma rays have the highest penetrating power. Question 2: (a) Arrange the following radiations in the order of their increasing wavelength: X-rays, infrared rays, ratio waves, gamma ray and micro waves? (b) Which radiation is used for satellite communication? Solution 2: (a) Gamma rays, X-rays, infrared rays, micro waves, radio waves. (b) Microwave is used for satellite communication. Question 3: A wave has a wavelength of 0.01 A. (a) Name the wave. (b) State its one property different from light. Solution 3: (a) Gamma ray. (b) Gamma rays have strong penetrating power. Question 4: (a) Name the high energetic invisible electro-magnetic wave which helps in the study of structure of crystals. (b) State one more use of the wave named in part (a). Solution 4: (a) X-rays are used in the study of crystals. (b) It is also used to detect fracture in bones.
10 Question 5: What is the range of the wavelength of the following electromagnetic waves? (a) Gamma rays, (b) X-rays, (c) Ultraviolet (d) Visible, (e) Infrared, (f) Micro waves and (g) Radio waves. Solution 5: (a) (b) (c) (d) (e) (f) (g) Gamma rays-wavelength shorter than 0.1 A X-rays-wavelength range 0.1 A to 100 A Ultraviolet rays-wavelength range 100 A to 4000 A Visible light-wavelength range 4000 A to 8000 A Infrared radiations-wavelength range 8000 A to 10 7 A Microwaves-wavelength range 10 7 A to A Radio waves-wavelength above A Question 6: Give the range of wavelength of the electromagnetic waves visible to us. Solution 6: 4000 A to 8000 A. Question 7: Name the region beyond (i) the red end and (ii) the violet end, of the spectrum. Solution 7: (i) Infrared (ii) Ultraviolet Question 8: What do you understand by the invisible spectrum? How will you investigate the existence of radiation beyond the red and violet ends of the spectrum? Solution 8: The part of spectrum beyond the red and the violet ends is called the invisible spectrum as our eyes do not respond to the spectrum beyond the red and the violet extremes.
11 If the different radiations from the red part of the spectrum to the violet end and beyond it, are made incident on the silver-chloride solution, it is observed that from the red to the violet end, the solution remains unaffected. However just beyond the violet end, it turns violet and finally it becomes dark brown. Thus there exist certain radiations beyond the violet end of the spectrum, which are chemically more active than the visible light, called ultraviolet radiations. Now, if a thermometer with a blackened bulb is moved from the violet end towards the red end, it is observed that there is a slow rise in temperature, but when it is moved beyond the red region, a rapid rise in temperature is noticed. It means that the portion of spectrum beyond the red end has certain radiations which produce a strong heating effect, but they are not visible. These radiations are called the infrared radiations. Question 9: State the approximate range of wavelength associated with (i) the ultraviolet rays, (ii) the visible light and (iii) infrared rays. Solution 9: (i)ultraviolet rays-wavelength range 100 A to 4000 A (ii)visible light-wavelength range 4000 A to 8000 A (iii)infrared radiations-wavelength range 8000 A to 10 7 A Question 10: Name the radiations of wavelength just (i) longer than 8 x 10-7 m, (ii) Shorter than 4 x 10-7 m. Solution 10: (i)infrared radiations are longer than 8 x 10-7 m. (ii) ultraviolet radiations are shorter than 4 x 10-7 m. Question 11: Name two electromagnetic waves of frequency greater than that of violet light. State one use of each. Solution 11: X-rays and ultraviolet rays are electromagnetic waves of frequency greater than that of violet light.
12 Uses: X-rays are used for studying atomic arrangement in crystals. Ultraviolet rays are used for detecting the purity of gems, eggs, ghee etc. Question 12: Give one use each of (i) microwaves, (ii) ultraviolet radiations, (iii) infrared radiations, and (iv) gamma rays. Solution 12: (i)microwaves are used for satellite communication. (ii)ultraviolet radiations are used for detecting the purity of gems, eggs, ghee etc. (iii)infrared radiations are used in remote control of television and other gadgets. (iv)gamma rays are used in medical science to kill cancer cells. Question 13: Name the rays or waves (i) of highest frequency, (ii) used for taking photographs in dark, (iii) produced by the changes in the nucleus of an atom, (iv) of wavelength nearly 0.1 nm. Solution 13: (i)gamma rays are of highest frequency. (ii)infrared rays are used for taking photographs in dark. (iii)gamma rays are produced by the changes in the nucleus of an atom. (iv)x-rays are of wavelength nearly 0.1 nm. Question 14: Two waves A and B have wavelength 0.01 A and 9000 A respectively. (a) Name the two waves. (b) Compare the speeds of these waves when they travel in vacuum. Solution 14: (a) A- Gamma rays, B-infrared radiations (b) Ratio of speeds of these waves in vacuum is 1:1 as all electromagnetic waves travel with the speed of light in vacuum.
13 Question 15: Name two sources, each of infrared radiations and ultraviolet radiations. Solution 15: All heated bodies such as a heated iron ball, flame, fire etc., are the sources of infrared radiations. The electric arc and sparks give ultraviolet radiations. Question 16: What are infrared radiations? How are they detected? State one use of these radiations. Solution 16: Infrared radiations are the electromagnetic waves of wavelength in the range of 8000A to 10 7 A. Detection: If a thermometer with a blackened bulb is moved from the violet end towards the red end, it is observed that there is a slow rise in temperature, but when it is moved beyond the red region, a rapid rise in temperature is noticed. It means that the portion of spectrum beyond the red end has certain radiations which produce a strong heating effect, but they are not visible. These radiations are called the infrared radiations. Use: The infrared radiations are used for therapeutic purposes by doctors. Question 17: What are ultraviolet radiations? How are they detected? State one use of these radiations. Solution 17: The electromagnetic radiations of wavelength from 100A to 4000A are called the ultraviolet radiations. Detection: If the different radiations from the red part of the spectrum to the violet end and beyond it, are made incident on the silver-chloride solution, it is observed that from the red to the violet end, the solution remains unaffected. However just beyond the violet end, it first turns violet and finally it becomes dark brown. Thus there exist certain radiations beyond the violet end of the spectrum, which are chemically more active than visible light, called ultraviolet radiations. Use: Ultraviolet radiations are used for sterilizing purposes.
14 Question 18: Name three properties of ultraviolet radiations which are similar to visible light. Solution 18: (a) Ultraviolet radiations travel in a straight line with a speed of 3 x 10 8 m in air (or vacuum). (b) They obey the laws of reflection and refraction. (c) They affect the photographic plate. Question 19: Give two properties of ultraviolet radiations which differ from the visible light. Solution 19: (a) Ultraviolet radiations produce fluorescence on striking a zinc sulphide screen. (b) They cause health hazards like cancer on the body. Question 20: Mention three properties of infrared radiations similar to the visible light. Solution 20: (a) Infrared radiations travel in straight line as light does, with a speed equal to 3 x 10 8 m/s in vacuum. (b) They obey the laws of reflection and refraction. (c) They do not cause fluorescence on zinc sulphide screen. Question 21: Give two such properties of infrared radiations which are not true for visible light. Solution 21: (a) Infrared radiations are invisible. (b) They do not affect the ordinary photographic film.
15 Question 22: Explain the following: (i) Infrared radiations are used for photography in fog. (ii) Infrared radiations are used as signals during war (iii) the photographic darkrooms are provided with infrared lamps. (iv) A rock salt prism is used instead of a glass prism to obtain the infrared spectrum. (v) A quartz is required for obtaining the spectrum of the ultraviolet light. (vi) Ultraviolet bulbs have a quartz envelope instead of glass Solution 22: (i) Infrared radiations are used in photography in fog because they are not much scattered by the atmosphere, so they can penetrate appreciably through it. (ii) Infrared radiations are used as signals during the war as they are not visible and they are not absorbed much in the medium. (iii) Infrared lamps are used in dark rooms for developing photographs since they do not affect the photographic film chemically, but they provide some visibility. (iv) Infrared spectrum can be obtained only with the help of a rock-salt prism since the rocksalt prism does not absorb infrared radiations whereas a glass prism absorbs them. (v) A quartz prism is used to obtain the spectrum of the ultraviolet radiations as they are not absorbed by quartz, whereas ordinary glass absorbs the ultraviolet light. (vi) Ultraviolet bulbs have a quartz envelope instead of glass as they are not absorbed by quartz, whereas ordinary glad absorbs the ultraviolet light. MULTIPLE CHOICE TYPE: Question 1: The most energetic electromagnetic radiations are: (a) Microwaves (b) ultraviolet waves (c) X-rays (d) gamma rays Solution 1: Gamma rays
16 Question 2: The source of ultraviolet light is: (a) electric bulb (b) red hot iron ball (c) sodium vapour lamp (d) carbon arc-lamp Solution 2: Carbon arc-lamp Question 3: A radiations A is focused by a proper device on the bulb of a thermometer. Mercury in the thermometer shows a rapid increase. The radiations A is: (a) infrared radiation (b) visible light (c) ultraviolet radiation (d) X-rays Solution 3: Infrared radiation Hint: Infrared radiations produce strong heating effect. NUMERICALS Question 1: An electromagnetic wave has a frequency of 500 MHz and a wavelength of 60 cm. (a) Calculate the velocity of the wave. (b) name the medium through which it is travelling Solution 1: (a) Frequency = 500MHz = 500 x 10 6 Hz Wavelength = 60 cm = 0.6 m Velocity of wave = frequency x wavelength = 500x 10 6 x 0.6 = 3 x 10 8 m/s (b) Electromagnetic wave is travelling through air.
17 Question 2: The wavelength of X-rays is 0.01 A. Calculate its frequency. Solution 2: Wavelength = 0.01A = 0.01 x m Speed of X-rays = 3 x 10 8 m/s Speed of light = frequency x wavelength C = ℷ = = ℷ / = Hz EXERCISE. 6 (C) Question 1: What is meant by scattering of light? Solution 1: When white light from sun enters the earth's atmosphere, the light gets scattered i.e., the light spreads in all directions by the dust particles, free water molecules and the molecules of the gases present in the atmosphere. This phenomenon is called scattering of light. Question 2: How does the intensity of scattered light depends on the wavelength of incident light? State conditions when this dependence holds. Solution 2: The intensity of scattered light is found to be inversely proportional to the fourth power of wavelength of light. This relation holds when the size of air molecules is much smaller than the wavelength of the light incident. Question 3: When sunlight enters the earth s atmosphere, state which colour of light is scattered the most and which the least. Solution 3: Violet colour is scattered the most and red the least as the intensity of scattered light is found to be inversely proportional to the fourth power of wavelength of light.
18 Question 4: The danger signal is red. Why? Solution 4: Since the wavelength of red light is the longest in the visible light, the light of red colour is scattered the least by the air molecules of the atmosphere and therefore the light of red colour can penetrate to a longer distance. Thus red light can be seen from the farthest distance as compared to other colours of same intensity. Hence it is used for danger signal so that the signal may be visible from the far distance. Question 5: How would the sky appear when seen from the space (or moon)? Give reason for your answer. Solution 5: On the moon, since there is no atmosphere, therefore there is no scattering of sun light incident on the moon surface. Hence to an observer on the surface of moon (space), no light reaches the eye of the observer except the light directly from the sun. Thus the sky will have no colour and will appear black to an observer on the moon surface. Question 6: What characteristic property of light is responsible for the blue colour of the sky? Solution 6: Scattering property of light is responsible for the blue colour of the sky as the blue colour is scattered the most due to its short wavelength. Question 7: The colour of sky, in direction other than of the sun, is blue. Explain. Solution 7: As the light travels through the atmosphere, it gets scattered in different directions by the air molecules present in its path. The blue light due to its short wavelength is scattered more as compared to the red light of long wavelength. Thus the light reaching our eye directly from sun is rich in red colour, while the light reaching our eye from all other directions is the scattered blue light. Therefore, the sky in direction other than in the direction of sun is seen blue.
19 Question 8: Why does the sun appear red at sunrises and sunset? Solution 8: At the time of sunrise and sunset, the light from sun has to travel the longest distance of atmosphere to reach the observer. The light travelling from the sun loses blue light of short wavelength due to scattering, while the red light of long wavelength is scattered a little, so is not lost much. Thus blue light is almost absent in sunlight reaching the observer, while it is rich in red colour. Question 9: The sky at noon appears white. Give reason. Solution 9: At noon, the sun is above our head, so we get light rays directly from the sun without much scattering of any particular colour. Further, light has to travel less depth of atmosphere; hence the sky is seen white. Question 10: The clouds are seen white. Explain Solution 10: The clouds are nearer the earth surface and they contain dust particles and aggregates of water molecules of sizes bigger than the wavelength of visible light. Therefore, the dust particles and water molecules present in clouds scatter all colours of incident white light from sun to the same extent and hence when the scattered light reaches our eye, the clouds are seen white. MULTIPLE CHOICE TYPE: Question 1: In white light of sun, maximum scattering by the air molecular present in the earth s atmosphere is for: (a) red colour (c) green colour (b) yellow colour (d) blue colour Solution 1: Blue colour Hint: When light of certain frequency falls on that atom or molecule, this atom or molecule responds to the light, whenever the size of the atom or molecule comparable to the wavelength of light. The sizes of nitrogen and oxygen molecules in atmosphere are comparable to the wavelength of blue light. These molecules act as scattering centers for scattering of blue light. This is also the reason that we see the sky as blue.
SPECTRUM. Dispersion. This phenomenon can be observed in a lab environment using a
 SPECTRUM Dispersion The phenomenon due to which a polychromatic light, like sunlight, splits into its component colours, when passed through a transparent medium like a glass prism, is called dispersion
SPECTRUM Dispersion The phenomenon due to which a polychromatic light, like sunlight, splits into its component colours, when passed through a transparent medium like a glass prism, is called dispersion
SNC2D PHYSICS 4/27/2013. LIGHT & GEOMETRIC OPTICS L What Is Light? (P ) What Is Light? What Is Light?
 SNC2D PHYSICS LIGHT & GEOMETRIC OPTICS L What Is Light? (P.380-391) What Is Light? For centuries, scientists have tried to understand the nature of light and its properties. Some of these properties are
SNC2D PHYSICS LIGHT & GEOMETRIC OPTICS L What Is Light? (P.380-391) What Is Light? For centuries, scientists have tried to understand the nature of light and its properties. Some of these properties are
4.2 Properties of Visible Light Date: (pages )
 4.2 Properties of Visible Light Date: (pages 144-149) Visible light is a mixture of all the colours of the rainbow. A prism refracts light separating the colours. A second prism can recombine the colours
4.2 Properties of Visible Light Date: (pages 144-149) Visible light is a mixture of all the colours of the rainbow. A prism refracts light separating the colours. A second prism can recombine the colours
Name Class Date. What two models do scientists use to describe light? What is the electromagnetic spectrum? How can electromagnetic waves be used?
 CHAPTER 16 12 SECTION Sound and Light The Nature of Light KEY IDEAS As you read this section, keep these questions in mind: What two models do scientists use to describe light? What is the electromagnetic
CHAPTER 16 12 SECTION Sound and Light The Nature of Light KEY IDEAS As you read this section, keep these questions in mind: What two models do scientists use to describe light? What is the electromagnetic
The Electromagnetic Spectrum
 The Electromagnetic Spectrum 1 of 19 Boardworks Ltd 2016 The Electromagnetic Spectrum 2 of 19 Boardworks Ltd 2016 Detecting waves beyond the visible spectrum 3 of 19 Boardworks Ltd 2016 Invisible light
The Electromagnetic Spectrum 1 of 19 Boardworks Ltd 2016 The Electromagnetic Spectrum 2 of 19 Boardworks Ltd 2016 Detecting waves beyond the visible spectrum 3 of 19 Boardworks Ltd 2016 Invisible light
DISPERSION VERY SHORT ANSWER QUESTIONS. Two identical prisms made of the same material placed with their based on opposite sides (of the
 DISPERSION VERY SHORT ANSWER QUESTIONS Q-1. What will be the spectrum of sun during a total solar eclipse? Q-2. Why the secondary rainbow is always fainter than the primary rainbow? Q-3. Two identical
DISPERSION VERY SHORT ANSWER QUESTIONS Q-1. What will be the spectrum of sun during a total solar eclipse? Q-2. Why the secondary rainbow is always fainter than the primary rainbow? Q-3. Two identical
Chapter 17, Electromagnetic Waves Physical Science, McDougal-Littell, 2008
 SECTION 1 (PP. 553-558): ELECTROMAGNETIC WAVES HAVE UNIQUE TRAITS. Georgia Standards: S8P4a Identify the characteristics of electromagnetic and mechanical waves; S8P4d Describe how the behavior of waves
SECTION 1 (PP. 553-558): ELECTROMAGNETIC WAVES HAVE UNIQUE TRAITS. Georgia Standards: S8P4a Identify the characteristics of electromagnetic and mechanical waves; S8P4d Describe how the behavior of waves
Light is an important form of energy for all of us
 What is Light? Light is an important form of energy for all of us it allows us to see plants rely on light for photosynthesis many chemical reactions produce light life on Earth would not exist without
What is Light? Light is an important form of energy for all of us it allows us to see plants rely on light for photosynthesis many chemical reactions produce light life on Earth would not exist without
Properties of Waves. Before You Read. What are the features of a wave?
 Properties of Waves Textbook pages 134 143 Before You Read Section 4.1 Summary In this section, you will find out about waves, such as water waves, sound waves, and radio waves. On the lines below, list
Properties of Waves Textbook pages 134 143 Before You Read Section 4.1 Summary In this section, you will find out about waves, such as water waves, sound waves, and radio waves. On the lines below, list
Name Date Class _. Please turn to the section titled The Nature of Light.
 Please turn to the section titled The Nature of Light. In this section, you will learn that light has both wave and particle characteristics. You will also see that visible light is just part of a wide
Please turn to the section titled The Nature of Light. In this section, you will learn that light has both wave and particle characteristics. You will also see that visible light is just part of a wide
Electromagnetic Waves. Electromagnetic Spectrum. Electromagnetic Spectrum. Electromagnetic Waves. CH 27-Physics (B) Fall, 2010
 Electromagnetic Waves Electromagnetic Spectrum CH 27-Physics (B) Fall, 2010 Electric and magnetic fields always exist When ever one is. the other is The fields can exist in a... They are at. o to each
Electromagnetic Waves Electromagnetic Spectrum CH 27-Physics (B) Fall, 2010 Electric and magnetic fields always exist When ever one is. the other is The fields can exist in a... They are at. o to each
Planetary Science: Investigations 9-10 I-Check Quiz STUDY GUIDE- ANSWER KEY Name HR Date
 1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
Chapter 4 - Light. Name: Block:
 Chapter 4 Notes: Light Name: Block: Properties of Waves Waves are a repeating disturbance or movement that energy through matter or space without causing any displacement of material Features of a wave:
Chapter 4 Notes: Light Name: Block: Properties of Waves Waves are a repeating disturbance or movement that energy through matter or space without causing any displacement of material Features of a wave:
Waves. Electromagnetic. No medium required. Can travel in a vacuum (empty space).
 Electromagnetic Waves Made up of vibrating electric and magnetic fields. Carry energy. Move in the form of both a wave and a particle. No medium required. Can travel in a vacuum (empty space). Demonstrate
Electromagnetic Waves Made up of vibrating electric and magnetic fields. Carry energy. Move in the form of both a wave and a particle. No medium required. Can travel in a vacuum (empty space). Demonstrate
Unit 3: Optics Chapter 4. Properties of Light
 Unit 3: Optics Chapter 4 Properties of Light There are many types of light sources... Fluorescence Incandescence Electric Bioluminescence Chemiluminescence Combustion The Nature of Light Pythagoras A Greek
Unit 3: Optics Chapter 4 Properties of Light There are many types of light sources... Fluorescence Incandescence Electric Bioluminescence Chemiluminescence Combustion The Nature of Light Pythagoras A Greek
Sound and Light. Light
 Sound and Light Light What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement or a D if you
Sound and Light Light What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement or a D if you
ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM When white light passes through a prism, it spreads out into a rainbow of colours, with red at one end and
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM When white light passes through a prism, it spreads out into a rainbow of colours, with red at one end and
RADIATION and the EM Spectrum
 RADIATION and the EM Spectrum Radioactivity is the of high-energy particles and/or of energy from a substance as a result of of its atoms. There are several types of radiation. Radiation from the sun is
RADIATION and the EM Spectrum Radioactivity is the of high-energy particles and/or of energy from a substance as a result of of its atoms. There are several types of radiation. Radiation from the sun is
By Dr. Hany Farid, Dartmouth College
 Why Is the Sky Blue? Why Is the Sky Blue? By Dr. Hany Farid, Dartmouth College Gas molecules in the atmosphere scatter, in all directions, the short wavelength light that appears blue to us. Longer wavelength
Why Is the Sky Blue? Why Is the Sky Blue? By Dr. Hany Farid, Dartmouth College Gas molecules in the atmosphere scatter, in all directions, the short wavelength light that appears blue to us. Longer wavelength
Physics 30: Chapter 5 Exam Wave Nature of Light
 Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Grade 8 Science: Unit 3-Optics Chapter 4: Properties of Light
 Grade 8 Science: Unit 3-Optics Chapter 4: Properties of Light Key Terms: Microscope, telescope, amplitude, crest, energy, force, frequency, hertz, medium, transverse wave, trough, wave, wavelength, reflection,
Grade 8 Science: Unit 3-Optics Chapter 4: Properties of Light Key Terms: Microscope, telescope, amplitude, crest, energy, force, frequency, hertz, medium, transverse wave, trough, wave, wavelength, reflection,
Planetary Science: Investigations 9-10 I-Check Quiz STUDY GUIDE Name HR Date
 1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
National 3 Waves and Radiation
 What is a wave? National 3 Waves and Radiation 1. Wave Properties The basic definition Waves are a way of transporting energy from one place to another. They do this through some form of vibration. We
What is a wave? National 3 Waves and Radiation 1. Wave Properties The basic definition Waves are a way of transporting energy from one place to another. They do this through some form of vibration. We
The Electromagnetic Spectrum
 Physics 25 Chapter 24 Dr. Alward Electromagnetic Waves Electromagnetic (EM) waves consist of traveling electric and magnetic disturbances. One source of electromagnetic waves are electric charges oscillating
Physics 25 Chapter 24 Dr. Alward Electromagnetic Waves Electromagnetic (EM) waves consist of traveling electric and magnetic disturbances. One source of electromagnetic waves are electric charges oscillating
HUMAN EYE AND THE COLOURFUL WORLD
 HUMAN EYE AND THE COLOURFUL WORLD Class: 10 (Boys) Sub: PHYSICS NOTES The Human Eye: The human eye is a sensitive sense organ and acts like a camera, which enable us to capture the colourful picture of
HUMAN EYE AND THE COLOURFUL WORLD Class: 10 (Boys) Sub: PHYSICS NOTES The Human Eye: The human eye is a sensitive sense organ and acts like a camera, which enable us to capture the colourful picture of
Fluorescence. Incandescence. Electric. Bioluminescence Chemiluminescence. Combustion
 Fluorescence Incandescence Electric Bioluminescence Chemiluminescence Combustion Pythagoras A Greek philosopher Believed light was beams of tiny particles The eyes could detect these particles and see
Fluorescence Incandescence Electric Bioluminescence Chemiluminescence Combustion Pythagoras A Greek philosopher Believed light was beams of tiny particles The eyes could detect these particles and see
Focusing on Light What is light? Is it a particle or a wave? An age-old debate that has persisted among scientists is related to the question, "Is
 Focusing on Light What is light? Is it a particle or a wave? An age-old debate that has persisted among scientists is related to the question, "Is light a wave or a stream of particles?" Very noteworthy
Focusing on Light What is light? Is it a particle or a wave? An age-old debate that has persisted among scientists is related to the question, "Is light a wave or a stream of particles?" Very noteworthy
A sound wave needs a medium through which it is transmitted. (MS-PS4-2)
 Title: Visible Light: Why is the Sky Blue? Time: 40 min Grade level: 4 th and 5 th Synopsis: In an effort to teach wavelength and frequency in the electromagnetic spectrum, students will look at the visible
Title: Visible Light: Why is the Sky Blue? Time: 40 min Grade level: 4 th and 5 th Synopsis: In an effort to teach wavelength and frequency in the electromagnetic spectrum, students will look at the visible
Chapter 17 Practice Questions KEY
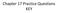 Chapter 17 Practice Questions KEY 1. Long wavelength Medium wavelength Short wavelength 1. Long wavelength Radio, Microwave Medium wavelength Infrared, Visible, Ultraviolet Short wavelength X ray, gamma
Chapter 17 Practice Questions KEY 1. Long wavelength Medium wavelength Short wavelength 1. Long wavelength Radio, Microwave Medium wavelength Infrared, Visible, Ultraviolet Short wavelength X ray, gamma
The Nature of Light. We have a dual model
 Light and Atoms Properties of Light We can come to understand the composition of distant bodies by analyzing the light they emit This analysis can tell us about the composition as well as the temperature
Light and Atoms Properties of Light We can come to understand the composition of distant bodies by analyzing the light they emit This analysis can tell us about the composition as well as the temperature
Electromagnetic Radiation (EMR)
 Electromagnetic Radiation (EMR) It is kind of energy with wave character ( exactly as sea waves ) that can be characterized by : Wavelength ( ) : The distance between two identical points on the wave.
Electromagnetic Radiation (EMR) It is kind of energy with wave character ( exactly as sea waves ) that can be characterized by : Wavelength ( ) : The distance between two identical points on the wave.
P5 Revision Questions
 P5 Revision Questions Part 2 Question 1 How can microwaves be used to communicate? Answer 1 Sent from transmitter, received and amplified by satellite in space, re-transmitted back to earth and picked
P5 Revision Questions Part 2 Question 1 How can microwaves be used to communicate? Answer 1 Sent from transmitter, received and amplified by satellite in space, re-transmitted back to earth and picked
Being a Physicist Unit 5. Summary Sheets. Gleniffer High School
 Being a Physicist Unit 5 Summary Sheets Gleniffer High School 0 Experiences & Outcomes I can explain how sound vibrations are carried by waves through air, water and other materials SCN 2-11a By exploring
Being a Physicist Unit 5 Summary Sheets Gleniffer High School 0 Experiences & Outcomes I can explain how sound vibrations are carried by waves through air, water and other materials SCN 2-11a By exploring
Wave - Particle Duality of Light
 Properties of Light Objectives Explain wave-particle duality State the speed of light Describe electromagnetic waves and the electromagnetic spectrum Explain how light interacts with transparent and opaque
Properties of Light Objectives Explain wave-particle duality State the speed of light Describe electromagnetic waves and the electromagnetic spectrum Explain how light interacts with transparent and opaque
DISPERSION AND SPECTRA CHAPTER 20
 CHAPTER 20 DISPERSION AND SPECTRA 20.1 DISPERSION As mentioned earlier, the refractive index of a material depends slightly on the wavelength of light. The relation between the two may be approximately
CHAPTER 20 DISPERSION AND SPECTRA 20.1 DISPERSION As mentioned earlier, the refractive index of a material depends slightly on the wavelength of light. The relation between the two may be approximately
Higher Physics. Particles and Waves
 Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric
Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric
Ch Guided Reading Sound and Light
 Name Date Hour Chapter 15 Answer Key Ch.15-18 Guided Reading Sound and Light 1. Compare the speed of sound as it travels within a liquid, a solid, and a gas. Why does the speed of sound differ? Sound travels
Name Date Hour Chapter 15 Answer Key Ch.15-18 Guided Reading Sound and Light 1. Compare the speed of sound as it travels within a liquid, a solid, and a gas. Why does the speed of sound differ? Sound travels
Name: COMBINED SCIENCE Topics 4, 5 & 6 LEARNING OUTCOMES. Maintain a record of your progress Use the booklet to guide revision
 Name: COMBINED SCIENCE Topics 4, 5 & 6 LEARNING OUTCOMES Maintain a record of your progress Use the booklet to guide revision Close the Gap Contemporary record of the Topics / Learning outcomes that I
Name: COMBINED SCIENCE Topics 4, 5 & 6 LEARNING OUTCOMES Maintain a record of your progress Use the booklet to guide revision Close the Gap Contemporary record of the Topics / Learning outcomes that I
Chapter 33: ELECTROMAGNETIC WAVES 559
 Chapter 33: ELECTROMAGNETIC WAVES 1 Select the correct statement: A ultraviolet light has a longer wavelength than infrared B blue light has a higher frequency than x rays C radio waves have higher frequency
Chapter 33: ELECTROMAGNETIC WAVES 1 Select the correct statement: A ultraviolet light has a longer wavelength than infrared B blue light has a higher frequency than x rays C radio waves have higher frequency
School. Team Number. Optics
 School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
UNIT-5 EM WAVES UNIT-6 RAY OPTICS
 UNIT-5 EM WAVES 2 Marks Question 1. To which regions of electromagnetic spectrum do the following wavelengths belong: (a) 250 nm (b) 1500 nm 2. State any one property which is common to all electromagnetic
UNIT-5 EM WAVES 2 Marks Question 1. To which regions of electromagnetic spectrum do the following wavelengths belong: (a) 250 nm (b) 1500 nm 2. State any one property which is common to all electromagnetic
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
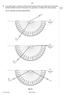 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
ELECTROMAGNETIC WAVES
 ELECTROMAGNETIC WAVES VERY SHORT ANSWER QUESTIONS Q-1. Light of uniform intensity shines perpendicularly on a totally absorbing surface, fully illuminating the surface. If the area of the surface is decreased,
ELECTROMAGNETIC WAVES VERY SHORT ANSWER QUESTIONS Q-1. Light of uniform intensity shines perpendicularly on a totally absorbing surface, fully illuminating the surface. If the area of the surface is decreased,
Sunlight is a combination of light-waves of various frequencies. Some
 96 The Electromagnetic Spectrum r e a d i n g Sunlight is a combination of light-waves of various frequencies. Some of the frequencies can be seen and some cannot be seen by the human eye. The reading
96 The Electromagnetic Spectrum r e a d i n g Sunlight is a combination of light-waves of various frequencies. Some of the frequencies can be seen and some cannot be seen by the human eye. The reading
Topic 5 Practice Test
 Base your answers to questions 1 and 2 on the diagram below, which represents the greenhouse effect in which heat energy is trapped in Earth's atmosphere 1. The Earth surface that best absorbs short-wave
Base your answers to questions 1 and 2 on the diagram below, which represents the greenhouse effect in which heat energy is trapped in Earth's atmosphere 1. The Earth surface that best absorbs short-wave
Which type of electromagnetic wave has a wavelength longer than that of yellow light? A. Infrared radiation C. X-rays B. Gamma Rays D.
 Which type of electromagnetic wave has a wavelength longer than that of yellow light? A. Infrared radiation C. X-rays B. Gamma Rays D. UV Rays Science Starter! 10.14-15.13! THE UNIVERSE AND ELECTROMAGNETIC
Which type of electromagnetic wave has a wavelength longer than that of yellow light? A. Infrared radiation C. X-rays B. Gamma Rays D. UV Rays Science Starter! 10.14-15.13! THE UNIVERSE AND ELECTROMAGNETIC
Republic of Somaliland. Somaliland National Examination Board. Form Four. Physics Examination. June 2009 TIME 2 HOURS
 Name..... Total Score School... Roll No... Republic of Somaliland Form Four Physics Examination June 2009 TIME 2 HOURS Plus 10 minutes for reading through the paper Total time 2 Hours and 10 Minutes INSTRUCTIONS
Name..... Total Score School... Roll No... Republic of Somaliland Form Four Physics Examination June 2009 TIME 2 HOURS Plus 10 minutes for reading through the paper Total time 2 Hours and 10 Minutes INSTRUCTIONS
Light is an electromagnetic wave (EM)
 What is light? Light is a form of energy. Light travels in a straight line Light speed is 3.0 x 10 8 m/s Light is carried by photons Light can travel through a vacuum Light is a transverse wave Light is
What is light? Light is a form of energy. Light travels in a straight line Light speed is 3.0 x 10 8 m/s Light is carried by photons Light can travel through a vacuum Light is a transverse wave Light is
The Nature of Light and Matter 1 Light
 The Nature of Light and Matter 1 Light ASTR 103 4/06/2016 1 Basic properties: The Nature of Light Light travels in a straight line. Most surfaces reflect light. Amount of reflection depends on the medium.
The Nature of Light and Matter 1 Light ASTR 103 4/06/2016 1 Basic properties: The Nature of Light Light travels in a straight line. Most surfaces reflect light. Amount of reflection depends on the medium.
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Spectroscopy Minneapolis Community and Technical College v.10.17
 Spectroscopy Minneapolis Community and Technical College v.10.17 Objective: To observe, measure and compare line spectra from various elements and to determine the energies of those electronic transitions
Spectroscopy Minneapolis Community and Technical College v.10.17 Objective: To observe, measure and compare line spectra from various elements and to determine the energies of those electronic transitions
SPECTROSCOPY PRELAB. 2) Name the 3 types of spectra and, in 1 sentence each, describe them.
 NAME: SPECTROSCOPY PRELAB 1) What is a spectrum? 2) Name the 3 types of spectra and, in 1 sentence each, describe them. a. b. c. 3) Use Wien s law to calculate the surface temperature of the star Alnilam
NAME: SPECTROSCOPY PRELAB 1) What is a spectrum? 2) Name the 3 types of spectra and, in 1 sentence each, describe them. a. b. c. 3) Use Wien s law to calculate the surface temperature of the star Alnilam
Shell Atomic Model and Energy Levels
 Shell Atomic Model and Energy Levels (higher energy, deeper excitation) - Radio waves: Not absorbed and pass through tissue un-attenuated - Microwaves : Energies of Photos enough to cause molecular rotation
Shell Atomic Model and Energy Levels (higher energy, deeper excitation) - Radio waves: Not absorbed and pass through tissue un-attenuated - Microwaves : Energies of Photos enough to cause molecular rotation
Being a Chemist. Summary Sheets. Gleniffer High School
 Being a Chemist Summary Sheets Gleniffer High School 0 State that the light year is a measure of astronomical distance State the speed at which light travels Give examples of the relative distance between
Being a Chemist Summary Sheets Gleniffer High School 0 State that the light year is a measure of astronomical distance State the speed at which light travels Give examples of the relative distance between
PARTICLES AND WAVES CHAPTER 29 CONCEPTUAL QUESTIONS
 CHAPTER 29 PARTICLES AND WAVES CONCEPTUAL QUESTIONS 1. REASONING AND SOLUTION A monochromatic light source emits photons of a single frequency. According to Equation 29.2, the energy, E, of a single photon
CHAPTER 29 PARTICLES AND WAVES CONCEPTUAL QUESTIONS 1. REASONING AND SOLUTION A monochromatic light source emits photons of a single frequency. According to Equation 29.2, the energy, E, of a single photon
SECTION 3 & 4 LIGHT WAVES & INFORMATION TRANSFER
 SECTION 3 & 4 LIGHT WAVES & INFORMATION TRANSFER Light Waves Light is a type of energy that travels as waves. Light is different than other waves because it does not need matter to travel. Light waves
SECTION 3 & 4 LIGHT WAVES & INFORMATION TRANSFER Light Waves Light is a type of energy that travels as waves. Light is different than other waves because it does not need matter to travel. Light waves
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!)
 NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
Electromagnetic Waves
 ELECTROMAGNETIC RADIATION AND THE ELECTROMAGNETIC SPECTRUM Electromagnetic Radiation (EMR) THE ELECTROMAGNETIC SPECTRUM Electromagnetic Waves A wave is characterized by: Wavelength (λ - lambda) is the
ELECTROMAGNETIC RADIATION AND THE ELECTROMAGNETIC SPECTRUM Electromagnetic Radiation (EMR) THE ELECTROMAGNETIC SPECTRUM Electromagnetic Waves A wave is characterized by: Wavelength (λ - lambda) is the
APAS Laboratory { PAGE } Spectroscopy SPECTROSCOPY
 SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
Note on Posted Slides
 Note on Posted Slides These are the slides that I intended to show in class on Tue. Apr. 1, 2014. Since it is April 1 st, there is an April Fools Day joke in here one of the slides is complete nonsense
Note on Posted Slides These are the slides that I intended to show in class on Tue. Apr. 1, 2014. Since it is April 1 st, there is an April Fools Day joke in here one of the slides is complete nonsense
GRADE 9: Physical processes 4. UNIT 9P.4 6 hours. The electromagnetic spectrum. Resources. About this unit. Previous learning.
 GRADE 9: Physical processes 4 The electromagnetic spectrum UNIT 9P.4 6 hours About this unit This unit is the fourth of seven units on physical processes for Grade 9. The unit is designed to guide your
GRADE 9: Physical processes 4 The electromagnetic spectrum UNIT 9P.4 6 hours About this unit This unit is the fourth of seven units on physical processes for Grade 9. The unit is designed to guide your
Topic 8: Beyond Light
 Topic 8: Beyond Light The Sun is the most abundant source of direct natural light on the Earth. There are other forms of energy, invisible, that are also supplied by this source. The tiny band of visible
Topic 8: Beyond Light The Sun is the most abundant source of direct natural light on the Earth. There are other forms of energy, invisible, that are also supplied by this source. The tiny band of visible
Class XII Chapter 8 Electromagnetic Waves Physics
 Question 8.1: Figure 8.6 shows a capacitor made of two circular plates each of radius 12 cm, and separated by 5.0 cm. The capacitor is being charged by an external source (not shown in the figure). The
Question 8.1: Figure 8.6 shows a capacitor made of two circular plates each of radius 12 cm, and separated by 5.0 cm. The capacitor is being charged by an external source (not shown in the figure). The
9/16/08 Tuesday. Chapter 3. Properties of Light. Light the Astronomer s Tool. and sometimes it can be described as a particle!
 9/16/08 Tuesday Announce: Observations? Milky Way Center movie Moon s Surface Gravity movie Questions on Gravity from Ch. 2 Ch. 3 Newton Movie Chapter 3 Light and Atoms Copyright (c) The McGraw-Hill Companies,
9/16/08 Tuesday Announce: Observations? Milky Way Center movie Moon s Surface Gravity movie Questions on Gravity from Ch. 2 Ch. 3 Newton Movie Chapter 3 Light and Atoms Copyright (c) The McGraw-Hill Companies,
Frequency: the number of complete waves that pass a point in a given time. It has the symbol f. 1) SI Units: Hertz (Hz) Wavelength: The length from
 Frequency: the number of complete waves that pass a point in a given time. It has the symbol f. 1) SI Units: Hertz (Hz) Wavelength: The length from the one crest of a wave to the next. I. Electromagnetic
Frequency: the number of complete waves that pass a point in a given time. It has the symbol f. 1) SI Units: Hertz (Hz) Wavelength: The length from the one crest of a wave to the next. I. Electromagnetic
Wavelength (λ)- Frequency (ν)- Which of the following has a higher frequency?
 Name: Unit 5- Light and Energy Electromagnetic Spectrum Notes Electromagnetic radiation is a form of energy that emits wave-like behavior as it travels through space. Amplitude (a)- Wavelength (λ)- Which
Name: Unit 5- Light and Energy Electromagnetic Spectrum Notes Electromagnetic radiation is a form of energy that emits wave-like behavior as it travels through space. Amplitude (a)- Wavelength (λ)- Which
Light The EM Spectrum
 Light The EM Spectrum 1 Spectrum of Electromagnetic Radiation Region Wavelength (Angstroms) Wavelength (centimeters) Frequency (Hz) Energy (ev) Radio > 10 9 > 10 < 3 x 10 9 < 10-5 Microwave 10 9-10 6 10-0.01
Light The EM Spectrum 1 Spectrum of Electromagnetic Radiation Region Wavelength (Angstroms) Wavelength (centimeters) Frequency (Hz) Energy (ev) Radio > 10 9 > 10 < 3 x 10 9 < 10-5 Microwave 10 9-10 6 10-0.01
Atomic Theory C &03
 Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
hf = E 1 - E 2 hc = E 1 - E 2 λ FXA 2008 Candidates should be able to : EMISSION LINE SPECTRA
 1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
PRINCIPLES OF REMOTE SENSING. Electromagnetic Energy and Spectral Signatures
 PRINCIPLES OF REMOTE SENSING Electromagnetic Energy and Spectral Signatures Remote sensing is the science and art of acquiring and analyzing information about objects or phenomena from a distance. As humans,
PRINCIPLES OF REMOTE SENSING Electromagnetic Energy and Spectral Signatures Remote sensing is the science and art of acquiring and analyzing information about objects or phenomena from a distance. As humans,
ASTRONOMY. Chapter 5 RADIATION AND SPECTRA PowerPoint Image Slideshow
 ASTRONOMY Chapter 5 RADIATION AND SPECTRA PowerPoint Image Slideshow FIGURE 5.1 Our Sun in Ultraviolet Light. This photograph of the Sun was taken at several different wavelengths of ultraviolet, which
ASTRONOMY Chapter 5 RADIATION AND SPECTRA PowerPoint Image Slideshow FIGURE 5.1 Our Sun in Ultraviolet Light. This photograph of the Sun was taken at several different wavelengths of ultraviolet, which
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
ELECTROMAGNETIC SPECTRUM All waves travel the SAME speed (the speed of light) 300,000 km/sec (186,000 miles/sec) in a vacuum
 ELECTROMAGNETIC SPECTRUM All waves travel the SAME speed (the speed of light) 300,000 km/sec (186,000 miles/sec) in a vacuum 10 4 Hz 10 6 Hz 10 8 Hz 10 12 Hz 10 14 Hz 10 16 Hz 10 18 Hz 1 million 1 trillion
ELECTROMAGNETIC SPECTRUM All waves travel the SAME speed (the speed of light) 300,000 km/sec (186,000 miles/sec) in a vacuum 10 4 Hz 10 6 Hz 10 8 Hz 10 12 Hz 10 14 Hz 10 16 Hz 10 18 Hz 1 million 1 trillion
This watermark does not appear in the registered version - Laser- Tissue Interaction
 S S d Laser- Tissue Interaction Types of radiation ionizing radiation Non - ionizing radiation You may click on any of the types of radiation for more detail about its particular type of interaction
S S d Laser- Tissue Interaction Types of radiation ionizing radiation Non - ionizing radiation You may click on any of the types of radiation for more detail about its particular type of interaction
progressive electromagnetic wave
 LECTURE 11 Ch17 A progressive electromagnetic wave is a self-supporting, energy-carrying disturbance that travels free of its source. The light from the Sun travels through space (no medium) for only 8.3
LECTURE 11 Ch17 A progressive electromagnetic wave is a self-supporting, energy-carrying disturbance that travels free of its source. The light from the Sun travels through space (no medium) for only 8.3
The Sine Wave. You commonly see waves in the environment. Light Sound Electricity Ocean waves
 The Sine Wave Mathematically, a function that represents a smooth oscillation For example, if we drew the motion of how the weight bobs on the spring to the weight we would draw out a sine wave. The Sine
The Sine Wave Mathematically, a function that represents a smooth oscillation For example, if we drew the motion of how the weight bobs on the spring to the weight we would draw out a sine wave. The Sine
Physics 201. Professor P. Q. Hung. 311B, Physics Building. Physics 201 p. 1/3
 Physics 201 p. 1/3 Physics 201 Professor P. Q. Hung 311B, Physics Building Physics 201 p. 2/3 What are electromagnetic waves? Electromagnetic waves consist of electric fields and magnetic fields which
Physics 201 p. 1/3 Physics 201 Professor P. Q. Hung 311B, Physics Building Physics 201 p. 2/3 What are electromagnetic waves? Electromagnetic waves consist of electric fields and magnetic fields which
Sound Waves. Sound waves are caused by vibrations and carry energy through a medium
 Chapter 16 Sound Waves Sound waves are caused by vibrations and carry energy through a medium An example of a compressional wave Waves can spread out in all directions Their speed depends on its medium
Chapter 16 Sound Waves Sound waves are caused by vibrations and carry energy through a medium An example of a compressional wave Waves can spread out in all directions Their speed depends on its medium
Optics in a Fish Tank Demonstrations for the Classroom
 Optics in a Fish Tank Demonstrations for the Classroom Introduction: This series of demonstrations will illustrate a number of optical phenomena. Using different light sources and a tank of water, you
Optics in a Fish Tank Demonstrations for the Classroom Introduction: This series of demonstrations will illustrate a number of optical phenomena. Using different light sources and a tank of water, you
10.1 Properties of Light
 10.1 Properties of Light Every time you see, you are using light. You can t see anything in complete darkness! Whether you are looking at a light bulb, or a car, or this book, light brings information
10.1 Properties of Light Every time you see, you are using light. You can t see anything in complete darkness! Whether you are looking at a light bulb, or a car, or this book, light brings information
Atoms and Radiation electromagnetic radiation Radiation electromagnetic
 Atoms and Radiation The information about astronomical objects (planets, stars, galaxies) can be obtained by studying the electromagnetic radiation emitted by those objects. Astronomers use the laws of
Atoms and Radiation The information about astronomical objects (planets, stars, galaxies) can be obtained by studying the electromagnetic radiation emitted by those objects. Astronomers use the laws of
Activity 2: Physics and the Visual Arts
 Why? An appreciation for and understanding of the physical processes that underpin the visual arts can be satisfying for an artist and lead to production of some unique pieces such as ferrosculptures:
Why? An appreciation for and understanding of the physical processes that underpin the visual arts can be satisfying for an artist and lead to production of some unique pieces such as ferrosculptures:
ELECTROMAGNETIC WAVES
 UNIT V ELECTROMAGNETIC WAVES Weightage Marks : 03 Displacement current, electromagnetic waves and their characteristics (qualitative ideas only). Transverse nature of electromagnetic waves. Electromagnetic
UNIT V ELECTROMAGNETIC WAVES Weightage Marks : 03 Displacement current, electromagnetic waves and their characteristics (qualitative ideas only). Transverse nature of electromagnetic waves. Electromagnetic
Chapter 26: Properties of Light
 Lecture Outline Chapter 26: Properties of Light This lecture will help you understand: Electromagnetic Waves The Electromagnetic Spectrum Transparent Materials Opaque Materials Seeing Light The Eye Electromagnetic
Lecture Outline Chapter 26: Properties of Light This lecture will help you understand: Electromagnetic Waves The Electromagnetic Spectrum Transparent Materials Opaque Materials Seeing Light The Eye Electromagnetic
EXPERIMENT 17: Atomic Emission
 EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
Higher -o-o-o- Past Paper questions o-o-o- 3.4 Spectra
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Build and Use a Simple Spectroscope
 Build and Use a Simple Spectroscope Subject Area: Physical Sciences Grade Level: 9 12 Overview In this activity students will build a spectroscope to analyze the composition of light. Our scope is inexpensive,
Build and Use a Simple Spectroscope Subject Area: Physical Sciences Grade Level: 9 12 Overview In this activity students will build a spectroscope to analyze the composition of light. Our scope is inexpensive,
RAY OPTICS 6. DISPERSION POINTS TO REMEMBER
 Y OPTICS 6. DISPESION POINTS TO EMEMBE. Dispersion : a) The splitting of white light into constituent colours is called dispersion and the band of colours is called spectrum. b) Dispersion of light was
Y OPTICS 6. DISPESION POINTS TO EMEMBE. Dispersion : a) The splitting of white light into constituent colours is called dispersion and the band of colours is called spectrum. b) Dispersion of light was
Space Physics Questions CfE
 Space Physics Questions CfE 1) Write down the definitions of the following: a) Moon b) Planet c) Sun d) Star e) Solar System f) Exoplanet g) Galaxy h) Universe. 2) What is cosmology the study of? 3) a)
Space Physics Questions CfE 1) Write down the definitions of the following: a) Moon b) Planet c) Sun d) Star e) Solar System f) Exoplanet g) Galaxy h) Universe. 2) What is cosmology the study of? 3) a)
Phys 100 Astronomy (Dr. Ilias Fernini) Review Questions for Chapter 5
 Phys 100 Astronomy (Dr. Ilias Fernini) Review Questions for Chapter 5 MULTIPLE CHOICE 1. What is the wavelength of the longest wavelength light visible to the human eye? a. 400 nm b. 4000 nm c. 7000 nm
Phys 100 Astronomy (Dr. Ilias Fernini) Review Questions for Chapter 5 MULTIPLE CHOICE 1. What is the wavelength of the longest wavelength light visible to the human eye? a. 400 nm b. 4000 nm c. 7000 nm
Chapter 25. Electromagnetic Waves
 Chapter 25 Electromagnetic Waves EXAM # 3 Nov. 20-21 Chapter 23 Chapter 25 Powerpoint Nov. 4 Problems from previous exams Physics in Perspective (pg. 836 837) Units of Chapter 25 The Production of Electromagnetic
Chapter 25 Electromagnetic Waves EXAM # 3 Nov. 20-21 Chapter 23 Chapter 25 Powerpoint Nov. 4 Problems from previous exams Physics in Perspective (pg. 836 837) Units of Chapter 25 The Production of Electromagnetic
Answer Key for Exam C
 Answer Key for Exam C 1 point each Choose the answer that best completes the question. Read each problem carefully and read through all the answers. Take your time. If a question is unclear, ask for clarification
Answer Key for Exam C 1 point each Choose the answer that best completes the question. Read each problem carefully and read through all the answers. Take your time. If a question is unclear, ask for clarification
Answer Key for Exam B
 Answer Key for Exam B 1 point each Choose the answer that best completes the question. Read each problem carefully and read through all the answers. Take your time. If a question is unclear, ask for clarification
Answer Key for Exam B 1 point each Choose the answer that best completes the question. Read each problem carefully and read through all the answers. Take your time. If a question is unclear, ask for clarification
VISIBLE LIGHT. L 32 Light and Optics [2] Seeing through the window. Windows behaving as mirrors. Seeing through a window
![VISIBLE LIGHT. L 32 Light and Optics [2] Seeing through the window. Windows behaving as mirrors. Seeing through a window VISIBLE LIGHT. L 32 Light and Optics [2] Seeing through the window. Windows behaving as mirrors. Seeing through a window](/thumbs/86/94409665.jpg) L 32 Light and Optics [2] Measurements of the speed of light The bending of light refraction Total internal reflection Dispersion Dispersion Rainbows Atmospheric scattering Blue sky and red sunsets Mirrors
L 32 Light and Optics [2] Measurements of the speed of light The bending of light refraction Total internal reflection Dispersion Dispersion Rainbows Atmospheric scattering Blue sky and red sunsets Mirrors
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Properties of Electromagnetic Radiation Chapter 5. What is light? What is a wave? Radiation carries information
 Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
2) The number of cycles that pass through a stationary point is called A) wavelength. B) amplitude. C) frequency. D) area. E) median.
 Chemistry Structure and Properties 2nd Edition Tro Test Bank Full Download: http://testbanklive.com/download/chemistry-structure-and-properties-2nd-edition-tro-test-bank/ Chemistry: Structure & Properties,
Chemistry Structure and Properties 2nd Edition Tro Test Bank Full Download: http://testbanklive.com/download/chemistry-structure-and-properties-2nd-edition-tro-test-bank/ Chemistry: Structure & Properties,
11/18/2010. Only part of the spectrum we can see. A rainbow of colors, each corresponding to a different wavelength.
 The sun is the source of energy to heat the Earth s surface. Solar energy makes it s way to Earth by an energy transfer mechanism called radiation. Energy transferred this way travels outwards in all directions
The sun is the source of energy to heat the Earth s surface. Solar energy makes it s way to Earth by an energy transfer mechanism called radiation. Energy transferred this way travels outwards in all directions
 E.M.WAVES 1. Taller the antenna longer is the coverage of television broadcast. Justify this statement with the help of a figure. 2.If v g, v x v m represents the speed of gamma rays, X-rays microwaves
E.M.WAVES 1. Taller the antenna longer is the coverage of television broadcast. Justify this statement with the help of a figure. 2.If v g, v x v m represents the speed of gamma rays, X-rays microwaves
Unit 5 Lesson 1 Images from Space. Copyright Houghton Mifflin Harcourt Publishing Company
 Florida Benchmarks SC.8.N.4.2 Explain how political, social, and economic concerns can affect science, and vice versa. SC.8.E.5.10 Assess how technology is essential to science for such purposes as access
Florida Benchmarks SC.8.N.4.2 Explain how political, social, and economic concerns can affect science, and vice versa. SC.8.E.5.10 Assess how technology is essential to science for such purposes as access
