Physics 221B Spring 2012 Notes 42 Scattering of Radiation by Matter
|
|
|
- Jason Blankenship
- 5 years ago
- Views:
Transcription
1 Physics 221B Spring 2012 Notes 42 Scattering of Radiation by Matter 1. Introduction In the previous set of Notes we treated the emission and absorption of radiation by matter. In these Notes we turn to the scattering of radiation by matter. As before, the material system in question can be almost anything (atom, molecule, nucleus, etc), but when it is necessary to be specific we shall for simplicity assume that it is a single-electron atom. The formalism is easily extended to other types of material systems. We shall see that in a certain sense the emission and absorption of radiation are special cases of the scattering of radiation, in which the frequency of the initial photon is close to a resonance frequency of the material system (the Einstein frequency connecting two energy eigenstates). In such cases it is partly a matter of preference how one should view the process, but in some respects the scattering point of view is more fundamental and elegant. 2. Applications The applications of the theory developed in these Notes are very broad. Almost any situation in which radiation passes through a gas affords an example of the physics we shall describe here. For example, in infrared and Raman scattering experiments radiation is passed through an molecular gas and the absorption spectrum or scattering cross section is measured, providing a primary source of information about the structure of molecules. Another example involves radiation passing through the Earth s atmosphere. The atmosphere is mostly transparent to sunlight, but there is some scattering, especially at the higher (blue) frequencies. This is Rayleigh scattering, which we shall consider later. The higher frequencies are more strongly scattered because they are closer to certain resonances in the ultraviolet. As we shall see, the cross section for Rayleigh scattering rises rapidly as a function of frequency as we approach a resonance. This is the reason the sky is blue: When we look at a part of the sky away from the sun, the blue light we see is sunlight scattered by the air along our line of sight. The Earth in turn radiates thermal radiation in the infrared that must pass through the atmosphere to reach outer space where it escapes. This radiation has a frequency range that covers the vibrational transitions of most simple molecules, so it is strongly scattered by several types of molecules such as the greenhouse gases CO 2, H 2 O and CH 4. Thus an infrared photon leaving the Earth s surface must diffuse through the atmosphere in a random walk, a process that is much slower than passing straight through. The greenhouse gases act like a blanket, keeping the Earth warm.
2 2 Notes 42: Scattering of Radiation The oxygen and nitrogen that are the major components of the atmosphere do not scatter infrared radiation, because they are homonuclear diatomic molecules which because of symmetry do not have a permanent electric dipole moment. Notice that the atmosphere makes it easy for radiation to get in at optical frequencies, and difficult to get back out at infrared frequencies. There are many examples in astrophysics where the scattering or absorption of radiation by matter is important. For example, sunlight passing through the cooler outer layers of the sun s atmosphere produces an absorption spectrum (the dark Fraunhoffer lines) that give information about the chemical species occurring in the sun and the physical conditions in its atmosphere. Similar information is available for other stars. For another example, transport of energy by radiation is important in the interior of stars, in which photons undergo repeated (Thomson) scattering by free electrons in the plasma and slowly diffuse upward toward the surface. In many stars such as the sun this radiative transport dominates the energy transport at certain radii (at other radii convective transport is dominant). The interaction of photons with matter is also important in nuclear physics. In the Mössbauer effect, for example, gamma ray photons emitted in the decay of a nucleus are used to excite other nuclei, lifting them from their ground state into an excited state. The resonance is very narrow, however, and the photon can be shifted out of resonance by a Doppler shift involving only small velocities. The Mössbauer effect provided the first clear experimental demonstration of the red shift that photons suffer on climbing out of a gravitational field. This effect implies that clocks run at different rates in different regions of a gravitational field. It is one of the conceptual corner stones of general relativity. 3. The Scattering Problem We shall consider the reaction A+γ B +γ, (1) in which an atom in state A interacts with an incident photon γ, which leaves the atom in state B and produces the scattered photon γ. We will write λ = (kµ), λ = (k µ ) for the modes of the incident and scattered photons, where µ is a polarization index, as described in Sec We will assume that λ λ, since otherwise there is no scattering. If A = B, the scattering is elastic (the initial and final atomic states are the same), and by conservation of energy we have ω = ω, where ω and ω are the frequencies of the photons with modes λ and λ. If A B, the scattering is inelastic, and we have ω ω. In this case the final atomic state B may either be higher or lower in energy than the initial state A, depending on circumstances. There is one photon in both the initial and final states. We will write the initial state i and the final state n in a variety of ways, i = A a λ 0 = A λ = Aλ, n = B a λ 0 = B λ = Bλ. (2)
3 Notes 42: Scattering of Radiation 3 We use the index n to label a variable final state, as we did in our presentation of time-dependent perturbation theory in Notes 32; as usual, we will have to sum over collections of final states to get physically relevant probabilities. The energies of the initial and final states are E i = E A + hω, E n = E B + hω, where E A and E B are the energies of the two atomic states, so the Einstein frequency connecting the initial and final states is where ω BA = (E B E A )/ h. ω ni = E n E i h (3) = ω BA +ω ω, (4) The problem will be to compute the differential cross section dσ/dω, where Ω refers to the direction of the outgoing photon (in mode λ ). The differential cross section is a function of the modes of the initial and final photons λ and λ and the two atomic states A and B. 4. The Transition Amplitude Vanishes at First Order We will take the Hamiltonian for the interaction of the matter with the radiation to be H = H 0 +H 1 +H 2, where H 0 = p2 2m +U(x)+ λ hω λ a λ a λ, (5a) H 1 = e mc [p A(x)+S B(x)], (5b) H 2 = e2 2mc 2A(x)2. The potential U(x) allows us to describe any single-electron atom (not only hydrogen). The transition amplitude in first order time-dependent perturbation theory is given by Eq. (32.32), which we reproduce here: (5c) c (1) n (t) = 2 i h eiωnit/2( sinω ni t/2 ) n H 1 i. (6) ω ni This equation is written in the general notation of Notes 32. For the present application, we identify states i and n with the states in Eq. (2), so that ω ni is given by Eq. (4), and we identify H 1 with the term H 1 in Eq. (5b). We ignore H 2 in Eq. (5c) since for the moment we are only working to first order. Then the matrix element in Eq. (6) becomes n H 1 i = e mc Bλ [p A(x)+S B(x)] Aλ. (7) The fields A and B have Fourier series (40.20) and (40.22) in terms of the modes of the field. If we write out these series, suppressing all factors except the creation and annihilation operators, then they have the structure, A,B = λ 1 ( aλ1...a λ 1 ), (8)
4 4 Notes 42: Scattering of Radiation where we are careful to use the index λ 1 as a dummy variable of summation, so as not to confuse it with the indices λ and λ of the incident and scattered photons. Now focusing on the field part of the matrix element (7), we see that the annihilation operator a λ1 does not contribute to the transition amplitude, because it acts on a state with a single photon, Aλ, and either produces a state with zero photons (if λ 1 = λ) or annihilates it (if λ 1 λ). In either case, the resulting state is orthogonal to the state Bλ, and gives zero when the scalar product is taken. Similarly, the creation operator a λ 1 in the Fourier series (8) acts on the single photon state Aλ and produces a two-photon state, which is necessarily orthogonal to any one-photon state such as Bλ. Again, the field part of the scalar product vanishes. Thus we find n H 1 i = 0, (9) and there is no contribution to the scattering amplitude at first order of time-dependent perturbation theory. We must go to second-order to find a nonvanishing contribution. 5. Second-Order Time-Dependent Perturbation Theory This is the first time we have had an application of second-order time-dependent perturbation theory, so we shall return to the formalism of Notes 32 and develop the theory at second order, using the notation of those Notes. In particular, the Hamiltonian is H = H 0 +H 1, without any term H 2 as we have in Eq. (5c) above. The second-order contribution to the transition amplitude c n (t) in the interaction picture is given by Eq. (32.30) in the general case in which H 1 in the Schrödinger picture is allowed to depend on time. For our scattering application, H 1 is time-independent, so we can do the time integrations appearing in Eqs. (32.27). The integral in the first order term in Eq. (32.27a) was done already in Sec. 32.7, with the result shown in Eq. (32.32) and reproduced above in Eq. (6). As for the integrals in the second order term in Eq. (32.27b), we first do the t integration, finding, c (2) n (t) = 1 (i h) 2 t 0 dt k e iω nkt ( e iω kit 1 ) n H 1 k k H 1 i. (10) iω ki Recall that the sum on k is a sum over intermediate states that came from the insertion of a resolution of the identity between the two Hamiltonian factors in Eq. (32.27b). We combine the phase factors in Eq. (10), using the identity, and then we do the t integration, c (2) n (t) = 1 (i h) 2 t 0 dt k ω nk +ω ki = ω ni, (11) 1 (e iωnit e iω nkt ) n H 1 k k H 1 i iω ki = 1 1 [( e iωnit 1 ) ( e iω nk t 1 )] (i h) 2 n H 1 k k H 1 i. (12) iω ki iω ni iω nk k
5 Notes 42: Scattering of Radiation 5 Of the two major time-dependent factors in the square brackets in Eq. (12), the first one, e iωnit 1 iω ni = 2e iωnit/2 sinω nit/2 ω ni, (13) is the same time-dependent factor that appears in the first order term c (1) n (t) shown in Eq. (6). Notice that this factor is independent of k and can be taken out of the sum in Eq. (12). When squared to produce a probability, this factor produces a quantity proportional to time multiplied times a function of frequency that gets narrower as time gets longer, where sin 2 ωt/2 ω 2 = π 2 t t(ω), (14) lim t(ω) = δ(ω). (15) t See Eqs. (32.42) and (32.43). The fact that the result is proportional to time is essential for getting a transition rate (a probability per unit time), and the emerging δ-function in the limit t is necessary for energy conservation, as explained in Notes 32. In fact, as explained in Sec , we do not really have to take t to, it need only be long enough for certain initial transients to die away. These transients are related to the artificiality of the initial conditions, and are nonphysical. The initial conditions for the scattering problem considered in these Notes contain an artificial element, as did the initial conditions in the potential scattering problem considered in Sec That is, we are assuming that at t = 0 the atom is known to be in the state A and the field consists of a single photon in mode λ. This mode is a plane wave that fills up all of space, including the region occupied by the atom. It would be impossible to set up such initial conditions experimentally, since there is no way for a light wave to get into the region occupied by the atom without interacting with it. A more realistic set of initial conditions would be a wave packet made up out of single photon states that initially is remote from the atom and not interacting with it. To treat such wave packets, however, would require a more sophisticated formalism than we are using here, so we will stick with the initial conditions we have chosen. The price we pay, however, is the appearance of initial transients without physical significance. The second major time-dependent term inside the square brackets in Eq. (12) is just such a transient. This term, when squared, does not produce something proportional to time, but rather just gives bounded oscillations that go to zero in the limit t after we divide by time. Thus, they do not contribute to the transition rate. This is because the frequency ω nk occurring in that term is not independent of k. The same is true for the cross terms that arise when the sum of the two major time-dependent terms in Eq. (12) is squared. Thus, if all we are interested in is the transition rate and not the initial transients, we can drop the second major time-dependent term in Eq. (12). The result is an effective transition amplitude at second order that is proportional to the timedependent factor (13), the same factor that occurs at first order. Combining the first and second
6 6 Notes 42: Scattering of Radiation order terms, we obtain an effective transition amplitude, c eff n (t) = 2 i h eiωnit/2( sinω ni t/2 )[ n H 1 i + ω ni k ] n H 1 k k H 1 i, (16) E i E k where we have taken the denominator ω ki appearing in Eq. (12) and written it as (E i E k )/ h. The result is the energy denominator seen in the second order contribution in Eq. (16). Recall that we had such energy denominators also in second-order bound state perturbation theory (see Notes 21). We drop the zeroth order contribution δ ni to the transition amplitude since for our scattering problem n i (since λ λ ). 6. The Photon Scattering Amplitude at Second Order For our application the notation H 1 of Notes 32 and Sec. 5 must be replaced by H 1 +H 2, given by Eqs. (5). In particular, we must make this replacement in the transition amplitude (16). For example, the first order matrix element in Eq. (16) is replaced by n H 1 +H 2 i. (17) The Hamiltonian in Eq. (5) is ordered in powers of the fine structure constant α 1/137, as is most easily seen in atomic units where e = m = h = 1 and 1/c = α. Thus, the H 1 term in the matrix element (17) is of order α. This term, however, vanishes, as we found in Sec. 4. To compute the transition amplitude to order α 2, we must keep the H 2 term in the matrix element (17), and interpret the H 1 in the second order term of Eq. (16) as the H 1 in Eq. (5b), dropping H 2 since this will contribute only at third or fourth order of α. Thus, the effective transition amplitude for our scattering problem, valid through order α 2, is c eff n (t) = 2 i h eiωnit/2( sinω ni t/2 )[ n H 2 i + ω ni k n H 1 k k H 1 i ], (18) E i E k where now H 1 and H 2 are given by Eqs. (5) and states i and n are given by Eqs. (2). One term involves H 2 taken in first order perturbation theory, and the other involves H 1 taken twice at second order perturbation theory. 7. H 2 in First-Order Perturbation Theory We begin with the term n H 2 i = e2 2mc 2 Bλ A(x) 2 Aλ. (19) Squaring the Fourier series (40.20) for A, we obtain a product series with the structure, A(x) 2 λ 1λ 2 (a λ1...a λ 1 ) (a λ2...a λ 2 ), (20)
7 Notes 42: Scattering of Radiation 7 where we suppress all factors except the creation and annihilation operators. This allows us to concentrate on the photon part of the matrix element (19). We see that there are four types of productsofcreationandannihilationoperatorsthatoccurina 2. Theproductofthetwoannihilation operators a λ1 a λ2 does not contribute to the transition amplitude, since it acts on the single photon state Aλ producing zero. Likewise, the product of the two creation operators a λ 1 a λ 2 does not contribute, since it acts on Aλ creating a three-photon state that is orthogonal to the one-photon state Bλ. The product of the annihilation operator times the creation operator, a λ1 a λ 2, does contribute, however, as long as λ 1 = λ and λ 2 = λ. That is, the photon destroyed by a λ1 must be the incident photon, and the one created by a λ 2 must be the scattered photon. Taking into account the other factors in the Fourier series, this product of operators corresponds to the product of polarization vectors ˆǫ λ ˆǫ λ and the product of phase factors eik x ik x. Similarly, the product of the creation operator times the annihilation operator a λ 1 a λ2 contributes as long at λ 1 = λ and λ 2 = λ, and in this case the product of polarization vectors is ˆǫ λ ˆǫ λ and the product of phase factors is e ik x+ik x. Thus out of the double infinite series (20) for A 2, only two terms contribute to the transition amplitude, and both have the same product of polarization vectors and the same phase factors. The contributions of these two terms are equal. It is now easy to do the photon part of the matrix element, leaving only an atomic matrix element. The result is n H 2 i = 2 e2 2π hc 2 1 2mc 2 V (ˆǫ ˆǫ ) B e i(k k ) x A, (21) ωω where the leading factor of 2 comes from the fact that we have two identical terms, and where we have made the abbreviations, ˆǫ = ˆǫ λ, ˆǫ = ˆǫ λ. In the following we shall use the dipole approximation, in which e i(k k ) x = 1. As explained in Notes 41, this approximation is equivalent to a/λ 1, where a is the size of the atom and λ is the wave length of the radiation. This is an excellent approximation for atoms at optical frequencies, where the ratio a/λ is less than This causes the atomic matrix element in Eq. (21) to become simply B A = δ BA. We see that the H 2 -term only contributes to elastic scattering. Altogether, the matrix element becomes n H 2 i = ( e2 h ) 2π 1 m V (ˆǫ ˆǫ )δ BA. (22) ωω We have written the result in such a form that the first factor can be dropped if we are working in atomic units.
Physics 221B Spring 2018 Notes 42 Scattering of Radiation by Matter
 Copyright c 218 by Robert G. Littlejohn Physics 221B Spring 218 Notes 42 Scattering of Radiation by Matter 1. Introduction In the previous set of notes we treated the emission and absorption of radiation
Copyright c 218 by Robert G. Littlejohn Physics 221B Spring 218 Notes 42 Scattering of Radiation by Matter 1. Introduction In the previous set of notes we treated the emission and absorption of radiation
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015)
 Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Thursday, November 1st.
 Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Physics 221B Spring 2018 Notes 34 The Photoelectric Effect
 Copyright c 2018 by Robert G. Littlejohn Physics 221B Spring 2018 Notes 34 The Photoelectric Effect 1. Introduction In these notes we consider the ejection of an atomic electron by an incident photon,
Copyright c 2018 by Robert G. Littlejohn Physics 221B Spring 2018 Notes 34 The Photoelectric Effect 1. Introduction In these notes we consider the ejection of an atomic electron by an incident photon,
Lecture 10. Lidar Effective Cross-Section vs. Convolution
 Lecture 10. Lidar Effective Cross-Section vs. Convolution q Introduction q Convolution in Lineshape Determination -- Voigt Lineshape (Lorentzian Gaussian) q Effective Cross Section for Single Isotope --
Lecture 10. Lidar Effective Cross-Section vs. Convolution q Introduction q Convolution in Lineshape Determination -- Voigt Lineshape (Lorentzian Gaussian) q Effective Cross Section for Single Isotope --
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Physics 221B Spring 2018 Notes 33 Time-Dependent Perturbation Theory. H = H 0 +H 1 (t), (1)
 Copyright c 218 by Robert G. Littlejohn Physics 221B Spring 218 Notes 33 Time-Dependent Perturbation Theory 1. Introduction Time-dependent perturbation theory applies to Hamiltonians of the form H = H
Copyright c 218 by Robert G. Littlejohn Physics 221B Spring 218 Notes 33 Time-Dependent Perturbation Theory 1. Introduction Time-dependent perturbation theory applies to Hamiltonians of the form H = H
B2.III Revision notes: quantum physics
 B.III Revision notes: quantum physics Dr D.M.Lucas, TT 0 These notes give a summary of most of the Quantum part of this course, to complement Prof. Ewart s notes on Atomic Structure, and Prof. Hooker s
B.III Revision notes: quantum physics Dr D.M.Lucas, TT 0 These notes give a summary of most of the Quantum part of this course, to complement Prof. Ewart s notes on Atomic Structure, and Prof. Hooker s
The Sun. How are these quantities measured? Properties of the Sun. Chapter 14
 The Sun Chapter 14 The Role of the Sun in the Solar System > 99.9% of the mass Its mass is responsible for the orderly orbits of the planets Its heat is responsible for warming the planets It is the source
The Sun Chapter 14 The Role of the Sun in the Solar System > 99.9% of the mass Its mass is responsible for the orderly orbits of the planets Its heat is responsible for warming the planets It is the source
Time dependent perturbation theory 1 D. E. Soper 2 University of Oregon 11 May 2012
 Time dependent perturbation theory D. E. Soper University of Oregon May 0 offer here some background for Chapter 5 of J. J. Sakurai, Modern Quantum Mechanics. The problem Let the hamiltonian for a system
Time dependent perturbation theory D. E. Soper University of Oregon May 0 offer here some background for Chapter 5 of J. J. Sakurai, Modern Quantum Mechanics. The problem Let the hamiltonian for a system
Addition of Opacities and Absorption
 Addition of Opacities and Absorption If the only way photons could interact was via simple scattering, there would be no blackbodies. We ll go into that in much more detail in the next lecture, but the
Addition of Opacities and Absorption If the only way photons could interact was via simple scattering, there would be no blackbodies. We ll go into that in much more detail in the next lecture, but the
Molecular spectroscopy
 Molecular spectroscopy Origin of spectral lines = absorption, emission and scattering of a photon when the energy of a molecule changes: rad( ) M M * rad( ' ) ' v' 0 0 absorption( ) emission ( ) scattering
Molecular spectroscopy Origin of spectral lines = absorption, emission and scattering of a photon when the energy of a molecule changes: rad( ) M M * rad( ' ) ' v' 0 0 absorption( ) emission ( ) scattering
Review: Properties of a wave
 Radiation travels as waves. Waves carry information and energy. Review: Properties of a wave wavelength (λ) crest amplitude (A) trough velocity (v) λ is a distance, so its units are m, cm, or mm, etc.
Radiation travels as waves. Waves carry information and energy. Review: Properties of a wave wavelength (λ) crest amplitude (A) trough velocity (v) λ is a distance, so its units are m, cm, or mm, etc.
eigenvalues eigenfunctions
 Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Optical Lattices. Chapter Polarization
 Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
Light. October 14, ) Exam Review 2) Introduction 3) Light Waves 4) Atoms 5) Light Sources
 Light October 14, 2002 1) Exam Review 2) Introduction 3) Light Waves 4) Atoms 5) Light Sources Waves You know of many types of waves water, sound, seismic, etc A wave is something oscillating back and
Light October 14, 2002 1) Exam Review 2) Introduction 3) Light Waves 4) Atoms 5) Light Sources Waves You know of many types of waves water, sound, seismic, etc A wave is something oscillating back and
Astronomy Chapter 12 Review
 Astronomy Chapter 12 Review Approximately how massive is the Sun as compared to the Earth? A. 100 times B. 300 times C. 3000 times D. 300,000 times E. One million times Approximately how massive is the
Astronomy Chapter 12 Review Approximately how massive is the Sun as compared to the Earth? A. 100 times B. 300 times C. 3000 times D. 300,000 times E. One million times Approximately how massive is the
6. Interstellar Medium. Emission nebulae are diffuse patches of emission surrounding hot O and
 6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
Lecture Outline: Spectroscopy (Ch. 4)
 Lecture Outline: Spectroscopy (Ch. 4) NOTE: These are just an outline of the lectures and a guide to the textbook. The material will be covered in more detail in class. We will cover nearly all of the
Lecture Outline: Spectroscopy (Ch. 4) NOTE: These are just an outline of the lectures and a guide to the textbook. The material will be covered in more detail in class. We will cover nearly all of the
PAPER No. : 8 (PHYSICAL SPECTROSCOPY) MODULE No. : 5 (TRANSITION PROBABILITIES AND TRANSITION DIPOLE MOMENT. OVERVIEW OF SELECTION RULES)
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8 and Physical Spectroscopy 5 and Transition probabilities and transition dipole moment, Overview of selection rules CHE_P8_M5 TABLE
Subject Chemistry Paper No and Title Module No and Title Module Tag 8 and Physical Spectroscopy 5 and Transition probabilities and transition dipole moment, Overview of selection rules CHE_P8_M5 TABLE
PTYS 214 Spring Announcements. Midterm 3 next Thursday!
 PTYS 214 Spring 2018 Announcements Midterm 3 next Thursday! 1 Previously Habitable Zone Energy Balance Emission Temperature Greenhouse Effect Vibration/rotation bands 2 Recap: Greenhouse gases In order
PTYS 214 Spring 2018 Announcements Midterm 3 next Thursday! 1 Previously Habitable Zone Energy Balance Emission Temperature Greenhouse Effect Vibration/rotation bands 2 Recap: Greenhouse gases In order
What happens when light falls on a material? Transmission Reflection Absorption Luminescence. Elastic Scattering Inelastic Scattering
 Raman Spectroscopy What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman, Fluorescence and IR Scattering Absorption
Raman Spectroscopy What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman, Fluorescence and IR Scattering Absorption
van Quantum tot Molecuul
 10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
Atoms. Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks
 Atoms Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). Radiation from atoms and molecules
Atoms Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). Radiation from atoms and molecules
NPTEL/IITM. Molecular Spectroscopy Lectures 1 & 2. Prof.K. Mangala Sunder Page 1 of 15. Topics. Part I : Introductory concepts Topics
 Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Lecture 14 - Radiative equilibrium and the atmospheric greenhouse effect
 We now have most of the tools we will need to begin to study energy balance on the earth. It will be a balance between incoming sunlight energy and outgoing energy emitted by the earth. We will look at
We now have most of the tools we will need to begin to study energy balance on the earth. It will be a balance between incoming sunlight energy and outgoing energy emitted by the earth. We will look at
IR Spectrography - Absorption. Raman Spectrography - Scattering. n 0 n M - Raman n 0 - Rayleigh
 RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
Modern Physics for Scientists and Engineers International Edition, 4th Edition
 Modern Physics for Scientists and Engineers International Edition, 4th Edition http://optics.hanyang.ac.kr/~shsong Review: 1. THE BIRTH OF MODERN PHYSICS 2. SPECIAL THEORY OF RELATIVITY 3. THE EXPERIMENTAL
Modern Physics for Scientists and Engineers International Edition, 4th Edition http://optics.hanyang.ac.kr/~shsong Review: 1. THE BIRTH OF MODERN PHYSICS 2. SPECIAL THEORY OF RELATIVITY 3. THE EXPERIMENTAL
Physics 221A Fall 1996 Notes 19 The Stark Effect in Hydrogen and Alkali Atoms
 Physics 221A Fall 1996 Notes 19 The Stark Effect in Hydrogen and Alkali Atoms In these notes we will consider the Stark effect in hydrogen and alkali atoms as a physically interesting example of bound
Physics 221A Fall 1996 Notes 19 The Stark Effect in Hydrogen and Alkali Atoms In these notes we will consider the Stark effect in hydrogen and alkali atoms as a physically interesting example of bound
2m 2 Ze2. , where δ. ) 2 l,n is the quantum defect (of order one but larger
 PHYS 402, Atomic and Molecular Physics Spring 2017, final exam, solutions 1. Hydrogenic atom energies: Consider a hydrogenic atom or ion with nuclear charge Z and the usual quantum states φ nlm. (a) (2
PHYS 402, Atomic and Molecular Physics Spring 2017, final exam, solutions 1. Hydrogenic atom energies: Consider a hydrogenic atom or ion with nuclear charge Z and the usual quantum states φ nlm. (a) (2
i~ ti = H 0 ti. (24.1) i = 0i of energy E 0 at time t 0, then the state at afuturetimedi ers from the initial state by a phase factor
 Chapter 24 Fermi s Golden Rule 24.1 Introduction In this chapter, we derive a very useful result for estimating transition rates between quantum states due to time-dependent perturbation. The results will
Chapter 24 Fermi s Golden Rule 24.1 Introduction In this chapter, we derive a very useful result for estimating transition rates between quantum states due to time-dependent perturbation. The results will
The greenhouse effect
 16 Waves of amplitude of 1 m roll onto a beach at a rate of one every 12 s. If the wavelength of the waves is 120 m, calculate (a) the velocity of the waves (b) how much power there is per metre along
16 Waves of amplitude of 1 m roll onto a beach at a rate of one every 12 s. If the wavelength of the waves is 120 m, calculate (a) the velocity of the waves (b) how much power there is per metre along
Fundamentals of Spectroscopy for Optical Remote Sensing. Course Outline 2009
 Fundamentals of Spectroscopy for Optical Remote Sensing Course Outline 2009 Part I. Fundamentals of Quantum Mechanics Chapter 1. Concepts of Quantum and Experimental Facts 1.1. Blackbody Radiation and
Fundamentals of Spectroscopy for Optical Remote Sensing Course Outline 2009 Part I. Fundamentals of Quantum Mechanics Chapter 1. Concepts of Quantum and Experimental Facts 1.1. Blackbody Radiation and
Exercises 16.3a, 16.5a, 16.13a, 16.14a, 16.21a, 16.25a.
 SPECTROSCOPY Readings in Atkins: Justification 13.1, Figure 16.1, Chapter 16: Sections 16.4 (diatomics only), 16.5 (omit a, b, d, e), 16.6, 16.9, 16.10, 16.11 (omit b), 16.14 (omit c). Exercises 16.3a,
SPECTROSCOPY Readings in Atkins: Justification 13.1, Figure 16.1, Chapter 16: Sections 16.4 (diatomics only), 16.5 (omit a, b, d, e), 16.6, 16.9, 16.10, 16.11 (omit b), 16.14 (omit c). Exercises 16.3a,
K20: Temperature, Heat, and How Heat Moves
 K20: Temperature, Heat, and How Heat Moves Definition of Temperature Definition of Heat How heat flows (Note: For all discussions here, particle means a particle of mass which moves as a unit. It could
K20: Temperature, Heat, and How Heat Moves Definition of Temperature Definition of Heat How heat flows (Note: For all discussions here, particle means a particle of mass which moves as a unit. It could
Chapter 3. Electromagnetic Theory, Photons. and Light. Lecture 7
 Lecture 7 Chapter 3 Electromagnetic Theory, Photons. and Light Sources of light Emission of light by atoms The electromagnetic spectrum see supplementary material posted on the course website Electric
Lecture 7 Chapter 3 Electromagnetic Theory, Photons. and Light Sources of light Emission of light by atoms The electromagnetic spectrum see supplementary material posted on the course website Electric
Non-stationary States and Electric Dipole Transitions
 Pre-Lab Lecture II Non-stationary States and Electric Dipole Transitions You will recall that the wavefunction for any system is calculated in general from the time-dependent Schrödinger equation ĤΨ(x,t)=i
Pre-Lab Lecture II Non-stationary States and Electric Dipole Transitions You will recall that the wavefunction for any system is calculated in general from the time-dependent Schrödinger equation ĤΨ(x,t)=i
Theory of optically thin emission line spectroscopy
 Theory of optically thin emission line spectroscopy 1 Important definitions In general the spectrum of a source consists of a continuum and several line components. Processes which give raise to the continuous
Theory of optically thin emission line spectroscopy 1 Important definitions In general the spectrum of a source consists of a continuum and several line components. Processes which give raise to the continuous
Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory
 Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory 1. Introduction Bound state perturbation theory applies to the bound states of perturbed systems,
Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory 1. Introduction Bound state perturbation theory applies to the bound states of perturbed systems,
Module I: Electromagnetic waves
 Module I: Electromagnetic waves Lectures 10-11: Multipole radiation Amol Dighe TIFR, Mumbai Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation
Module I: Electromagnetic waves Lectures 10-11: Multipole radiation Amol Dighe TIFR, Mumbai Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation
Conclusion. 109m Ag isomer showed that there is no such broadening. Because one can hardly
 Conclusion This small book presents a description of the results of studies performed over many years by our research group, which, in the best period, included 15 physicists and laboratory assistants
Conclusion This small book presents a description of the results of studies performed over many years by our research group, which, in the best period, included 15 physicists and laboratory assistants
Section 5 Time Dependent Processes
 Section 5 Time Dependent Processes Chapter 14 The interaction of a molecular species with electromagnetic fields can cause transitions to occur among the available molecular energy levels (electronic,
Section 5 Time Dependent Processes Chapter 14 The interaction of a molecular species with electromagnetic fields can cause transitions to occur among the available molecular energy levels (electronic,
Example: model a star using a two layer model: Radiation starts from the inner layer as blackbody radiation at temperature T in. T out.
 Next, consider an optically thick source: Already shown that in the interior, radiation will be described by the Planck function. Radiation escaping from the source will be modified because the temperature
Next, consider an optically thick source: Already shown that in the interior, radiation will be described by the Planck function. Radiation escaping from the source will be modified because the temperature
1. The most important aspects of the quantum theory.
 Lecture 5. Radiation and energy. Objectives: 1. The most important aspects of the quantum theory: atom, subatomic particles, atomic number, mass number, atomic mass, isotopes, simplified atomic diagrams,
Lecture 5. Radiation and energy. Objectives: 1. The most important aspects of the quantum theory: atom, subatomic particles, atomic number, mass number, atomic mass, isotopes, simplified atomic diagrams,
24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability ellipsoid. Selection rules.
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability
Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability
Vibrational Spectroscopy
 Vibrational Spectroscopy In this part of the course we will look at the kind of spectroscopy which uses light to excite the motion of atoms. The forces required to move atoms are smaller than those required
Vibrational Spectroscopy In this part of the course we will look at the kind of spectroscopy which uses light to excite the motion of atoms. The forces required to move atoms are smaller than those required
Wednesday, September 8, 2010 Infrared Trapping the Greenhouse Effect
 Wednesday, September 8, 2010 Infrared Trapping the Greenhouse Effect Goals to look at the properties of materials that make them interact with thermal (i.e., infrared, or IR) radiation (absorbing and reemitting
Wednesday, September 8, 2010 Infrared Trapping the Greenhouse Effect Goals to look at the properties of materials that make them interact with thermal (i.e., infrared, or IR) radiation (absorbing and reemitting
Theoretical Photochemistry WiSe 2017/18
 Theoretical Photochemistry WiSe 2017/18 Lecture 7 Irene Burghardt (burghardt@chemie.uni-frankfurt.de) http://www.theochem.uni-frankfurt.de/teaching/ Theoretical Photochemistry 1 Topics 1. Photophysical
Theoretical Photochemistry WiSe 2017/18 Lecture 7 Irene Burghardt (burghardt@chemie.uni-frankfurt.de) http://www.theochem.uni-frankfurt.de/teaching/ Theoretical Photochemistry 1 Topics 1. Photophysical
From Last Time Pearson Education, Inc.
 From Last Time Light: Absorption, Emission, Transmission, Reflection, and Scattering c=λ x f E=h x f Light (electromagnetic radiation) extends from gamma rays (high E, high f, small λ) to radio waves (small
From Last Time Light: Absorption, Emission, Transmission, Reflection, and Scattering c=λ x f E=h x f Light (electromagnetic radiation) extends from gamma rays (high E, high f, small λ) to radio waves (small
The Photoelectric Effect
 Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Physics 9e/Cutnell. correlated to the. College Board AP Physics 2 Course Objectives
 correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
Order of Magnitude Astrophysics - a.k.a. Astronomy 111. Photon Opacities in Matter
 1 Order of Magnitude Astrophysics - a.k.a. Astronomy 111 Photon Opacities in Matter If the cross section for the relevant process that scatters or absorbs radiation given by σ and the number density of
1 Order of Magnitude Astrophysics - a.k.a. Astronomy 111 Photon Opacities in Matter If the cross section for the relevant process that scatters or absorbs radiation given by σ and the number density of
Physics 221B Spring 2018 Notes 41 Emission and Absorption of Radiation
 Copyright c 2018 by Robert G. Littlejohn Physics 221B Spring 2018 Notes 41 Emission and Absorption of Radiation 1. Introduction In these notes we will examine some simple examples of the interaction of
Copyright c 2018 by Robert G. Littlejohn Physics 221B Spring 2018 Notes 41 Emission and Absorption of Radiation 1. Introduction In these notes we will examine some simple examples of the interaction of
PHYSICS 359E: EXPERIMENT 2.2 THE MOSSBAUER EFFECT: RESONANT ABSORPTION OF (-RAYS
 PHYSICS 359E: EXPERIMENT 2.2 THE MOSSBAUER EFFECT: RESONANT ABSORPTION OF (-RAYS INTRODUCTION: In classical physics resonant phenomena are expected whenever a system can undergo free oscillations. These
PHYSICS 359E: EXPERIMENT 2.2 THE MOSSBAUER EFFECT: RESONANT ABSORPTION OF (-RAYS INTRODUCTION: In classical physics resonant phenomena are expected whenever a system can undergo free oscillations. These
Radiative Transfer and Molecular Lines Sagan Workshop 2009
 Radiative Transfer and Molecular Lines Sagan Workshop 2009 Sara Seager Trent Schindler Trent Schindler MIT Lecture Contents Overview of Equations for Planetary Atmospheres Radiative Transfer Thermal Inversions
Radiative Transfer and Molecular Lines Sagan Workshop 2009 Sara Seager Trent Schindler Trent Schindler MIT Lecture Contents Overview of Equations for Planetary Atmospheres Radiative Transfer Thermal Inversions
Module 4 : Third order nonlinear optical processes. Lecture 28 : Inelastic Scattering Processes. Objectives
 Module 4 : Third order nonlinear optical processes Lecture 28 : Inelastic Scattering Processes Objectives In this lecture you will learn the following Light scattering- elastic and inelastic-processes,
Module 4 : Third order nonlinear optical processes Lecture 28 : Inelastic Scattering Processes Objectives In this lecture you will learn the following Light scattering- elastic and inelastic-processes,
Chapter 5 Light and Matter: Reading Messages from the Cosmos
 Chapter 5 Light and Matter: Reading Messages from the Cosmos 5.1 Light in Everyday Life Our goals for learning How do we experience light? How do light and matter interact? How do we experience light?
Chapter 5 Light and Matter: Reading Messages from the Cosmos 5.1 Light in Everyday Life Our goals for learning How do we experience light? How do light and matter interact? How do we experience light?
HOMEWORK - Chapter 4 Spectroscopy
 Astronomy 10 HOMEWORK - Chapter 4 Spectroscopy Use a calculator whenever necessary. For full credit, always show your work and explain how you got your answer in full, complete sentences on a separate
Astronomy 10 HOMEWORK - Chapter 4 Spectroscopy Use a calculator whenever necessary. For full credit, always show your work and explain how you got your answer in full, complete sentences on a separate
COX & GIULI'S PRINCIPLES OF STELLAR STRUCTURE
 COX & GIULI'S PRINCIPLES OF STELLAR STRUCTURE Extended Second Edition A. Weiss, W. Hillebrandt, H.-C. Thomas and H. Ritter Max-Planck-lnstitut fur Astrophysik, Garching, Germany C S P CONTENTS PREFACE
COX & GIULI'S PRINCIPLES OF STELLAR STRUCTURE Extended Second Edition A. Weiss, W. Hillebrandt, H.-C. Thomas and H. Ritter Max-Planck-lnstitut fur Astrophysik, Garching, Germany C S P CONTENTS PREFACE
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours.
 Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Atoms, Molecules and Solids (selected topics)
 Atoms, Molecules and Solids (selected topics) Part I: Electronic configurations and transitions Transitions between atomic states (Hydrogen atom) Transition probabilities are different depending on the
Atoms, Molecules and Solids (selected topics) Part I: Electronic configurations and transitions Transitions between atomic states (Hydrogen atom) Transition probabilities are different depending on the
Lecture 5: Greenhouse Effect
 /30/2018 Lecture 5: Greenhouse Effect Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature atmosphere Wien s Law Shortwave and Longwave Radiation
/30/2018 Lecture 5: Greenhouse Effect Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature atmosphere Wien s Law Shortwave and Longwave Radiation
Electrodynamics II: Lecture 9
 Electrodynamics II: Lecture 9 Multipole radiation Amol Dighe Sep 14, 2011 Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation Outline 1 Multipole
Electrodynamics II: Lecture 9 Multipole radiation Amol Dighe Sep 14, 2011 Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation Outline 1 Multipole
Lattice Vibrations. Chris J. Pickard. ω (cm -1 ) 200 W L Γ X W K K W
 Lattice Vibrations Chris J. Pickard 500 400 300 ω (cm -1 ) 200 100 L K W X 0 W L Γ X W K The Breakdown of the Static Lattice Model The free electron model was refined by introducing a crystalline external
Lattice Vibrations Chris J. Pickard 500 400 300 ω (cm -1 ) 200 100 L K W X 0 W L Γ X W K The Breakdown of the Static Lattice Model The free electron model was refined by introducing a crystalline external
Blackbody Radiation. A substance that absorbs all incident wavelengths completely is called a blackbody.
 Blackbody Radiation A substance that absorbs all incident wavelengths completely is called a blackbody. What's the absorption spectrum of a blackbody? Absorption (%) 100 50 0 UV Visible IR Wavelength Blackbody
Blackbody Radiation A substance that absorbs all incident wavelengths completely is called a blackbody. What's the absorption spectrum of a blackbody? Absorption (%) 100 50 0 UV Visible IR Wavelength Blackbody
ATS150 Global Climate Change Spring 2019 Candidate Questions for Exam #1
 1. How old is the Earth? About how long ago did it form? 2. What are the two most common gases in the atmosphere? What percentage of the atmosphere s molecules are made of each gas? 3. About what fraction
1. How old is the Earth? About how long ago did it form? 2. What are the two most common gases in the atmosphere? What percentage of the atmosphere s molecules are made of each gas? 3. About what fraction
1. Why photons? 2. Photons in a vacuum
 Photons and Other Messengers 1. Why photons? Ask class: most of our information about the universe comes from photons. What are the reasons for this? Let s compare them with other possible messengers,
Photons and Other Messengers 1. Why photons? Ask class: most of our information about the universe comes from photons. What are the reasons for this? Let s compare them with other possible messengers,
Chapter 5 Light and Matter: Reading Messages from the Cosmos. 5.1 Light in Everyday Life. How do we experience light?
 Chapter 5 Light and Matter: Reading Messages from the Cosmos 5.1 Light in Everyday Life Our goals for learning: How do we experience light? How do light and matter interact? How do we experience light?
Chapter 5 Light and Matter: Reading Messages from the Cosmos 5.1 Light in Everyday Life Our goals for learning: How do we experience light? How do light and matter interact? How do we experience light?
Absorption and scattering
 Absorption and scattering When a beam of radiation goes through the atmosphere, it encounters gas molecules, aerosols, cloud droplets, and ice crystals. These objects perturb the radiation field. Part
Absorption and scattering When a beam of radiation goes through the atmosphere, it encounters gas molecules, aerosols, cloud droplets, and ice crystals. These objects perturb the radiation field. Part
Lecture 5: Greenhouse Effect
 Lecture 5: Greenhouse Effect S/4 * (1-A) T A 4 T S 4 T A 4 Wien s Law Shortwave and Longwave Radiation Selected Absorption Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation
Lecture 5: Greenhouse Effect S/4 * (1-A) T A 4 T S 4 T A 4 Wien s Law Shortwave and Longwave Radiation Selected Absorption Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation
LIFE CYCLE OF A STAR
 LIFE CYCLE OF A STAR First stage = Protostar PROTOSTAR Cloud of gas and dust many light-years across Gravity tries to pull the materials together Eventually, at the center of the ball of dust and gas,
LIFE CYCLE OF A STAR First stage = Protostar PROTOSTAR Cloud of gas and dust many light-years across Gravity tries to pull the materials together Eventually, at the center of the ball of dust and gas,
Kinds of Energy. Defining Energy is Hard! EXPLAIN: 1. Energy and Radiation. Conservation of Energy. Sco; Denning CSU ESMEI ATS 1
 Defining Energy is Hard! EXPLAIN: 1. Energy and Radiation Energy is the capacity to perform work (but physicists have a special definition for work, too!) Part of the trouble is that scientists have appropriated
Defining Energy is Hard! EXPLAIN: 1. Energy and Radiation Energy is the capacity to perform work (but physicists have a special definition for work, too!) Part of the trouble is that scientists have appropriated
Appendix A. The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System
 Appendix A The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System Real quantum mechanical systems have the tendency to become mathematically
Appendix A The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System Real quantum mechanical systems have the tendency to become mathematically
Newton s Laws of Motion
 Newton s Laws of Motion #1: A body continues at rest or in uniform motion in a straight line unless acted upon by a force. Why doesn t the soccer ball move on its own? What causes a soccer ball to roll
Newton s Laws of Motion #1: A body continues at rest or in uniform motion in a straight line unless acted upon by a force. Why doesn t the soccer ball move on its own? What causes a soccer ball to roll
Lecture 2 Solutions to the Transport Equation
 Lecture 2 Solutions to the Transport Equation Equation along a ray I In general we can solve the static transfer equation along a ray in some particular direction. Since photons move in straight lines
Lecture 2 Solutions to the Transport Equation Equation along a ray I In general we can solve the static transfer equation along a ray in some particular direction. Since photons move in straight lines
Introduction to Electromagnetic Radiation and Radiative Transfer
 Introduction to Electromagnetic Radiation and Radiative Transfer Temperature Dice Results Visible light, infrared (IR), ultraviolet (UV), X-rays, γ-rays, microwaves, and radio are all forms of electromagnetic
Introduction to Electromagnetic Radiation and Radiative Transfer Temperature Dice Results Visible light, infrared (IR), ultraviolet (UV), X-rays, γ-rays, microwaves, and radio are all forms of electromagnetic
Planetary Atmospheres: Earth and the Other Terrestrial Worlds Pearson Education, Inc.
 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning: What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric properties
Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning: What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric properties
Chapter 5 Light: The Cosmic Messenger. Copyright 2012 Pearson Education, Inc.
 Chapter 5 Light: The Cosmic Messenger 5.1 Basic Properties of Light and Matter Our goals for learning: What is light? What is matter? How do light and matter interact? What is light? Light is an electromagnetic
Chapter 5 Light: The Cosmic Messenger 5.1 Basic Properties of Light and Matter Our goals for learning: What is light? What is matter? How do light and matter interact? What is light? Light is an electromagnetic
1. Estimate the lifetime of an excited state of hydrogen. Give your answer in terms of fundamental constants.
 Sample final questions.. Estimate the lifetime of an excited state of hydrogen. Give your answer in terms of fundamental constants. 2. A one-dimensional harmonic oscillator, originally in the ground state,
Sample final questions.. Estimate the lifetime of an excited state of hydrogen. Give your answer in terms of fundamental constants. 2. A one-dimensional harmonic oscillator, originally in the ground state,
14 Time-dependent perturbation theory
 TFY4250/FY2045 Lecture notes 14 - Time-dependent perturbation theory 1 Lecture notes 14 14 Time-dependent perturbation theory (Sections 11.1 2 in Hemmer, 9.1 3 in B&J, 9.1 in Griffiths) 14.1 Introduction
TFY4250/FY2045 Lecture notes 14 - Time-dependent perturbation theory 1 Lecture notes 14 14 Time-dependent perturbation theory (Sections 11.1 2 in Hemmer, 9.1 3 in B&J, 9.1 in Griffiths) 14.1 Introduction
( r) = 1 Z. e Zr/a 0. + n +1δ n', n+1 ). dt ' e i ( ε n ε i )t'/! a n ( t) = n ψ t = 1 i! e iε n t/! n' x n = Physics 624, Quantum II -- Exam 1
 Physics 624, Quantum II -- Exam 1 Please show all your work on the separate sheets provided (and be sure to include your name) You are graded on your work on those pages, with partial credit where it is
Physics 624, Quantum II -- Exam 1 Please show all your work on the separate sheets provided (and be sure to include your name) You are graded on your work on those pages, with partial credit where it is
Open quantum systems
 Open quantum systems Wikipedia: An open quantum system is a quantum system which is found to be in interaction with an external quantum system, the environment. The open quantum system can be viewed as
Open quantum systems Wikipedia: An open quantum system is a quantum system which is found to be in interaction with an external quantum system, the environment. The open quantum system can be viewed as
Radiation in the Earth's Atmosphere. Part 1: Absorption and Emission by Atmospheric Gases
 Radiation in the Earth's Atmosphere Part 1: Absorption and Emission by Atmospheric Gases Electromagnetic Waves Electromagnetic waves are transversal. Electric and magnetic fields are perpendicular. In
Radiation in the Earth's Atmosphere Part 1: Absorption and Emission by Atmospheric Gases Electromagnetic Waves Electromagnetic waves are transversal. Electric and magnetic fields are perpendicular. In
Rotational Raman Spectroscopy
 Rotational Raman Spectroscopy If EM radiation falls upon an atom or molecule, it may be absorbed if the energy of the radiation corresponds to the separation of two energy levels of the atoms or molecules.
Rotational Raman Spectroscopy If EM radiation falls upon an atom or molecule, it may be absorbed if the energy of the radiation corresponds to the separation of two energy levels of the atoms or molecules.
Lecture 10. Transition probabilities and photoelectric cross sections
 Lecture 10 Transition probabilities and photoelectric cross sections TRANSITION PROBABILITIES AND PHOTOELECTRIC CROSS SECTIONS Cross section = = Transition probability per unit time of exciting a single
Lecture 10 Transition probabilities and photoelectric cross sections TRANSITION PROBABILITIES AND PHOTOELECTRIC CROSS SECTIONS Cross section = = Transition probability per unit time of exciting a single
Lecture 6: The Physics of Light, Part 1. Astronomy 111 Wednesday September 13, 2017
 Lecture 6: The Physics of Light, Part 1 Astronomy 111 Wednesday September 13, 2017 Reminders Star party tonight! Homework #3 due Monday Exam #1 Monday, September 25 The nature of light Look, but don t
Lecture 6: The Physics of Light, Part 1 Astronomy 111 Wednesday September 13, 2017 Reminders Star party tonight! Homework #3 due Monday Exam #1 Monday, September 25 The nature of light Look, but don t
Physics 221A Fall 1996 Notes 13 Spins in Magnetic Fields
 Physics 221A Fall 1996 Notes 13 Spins in Magnetic Fields A nice illustration of rotation operator methods which is also important physically is the problem of spins in magnetic fields. The earliest experiments
Physics 221A Fall 1996 Notes 13 Spins in Magnetic Fields A nice illustration of rotation operator methods which is also important physically is the problem of spins in magnetic fields. The earliest experiments
RAYLEIGH AND RAMAN SCATTERING
 CHAPTER 9 RAYLEIGH AND RAMAN SCATTERING 9.1. Introduction The statistical multi-level approach of the preceding chapter that is based on the density matrix formalism provides a foundation for physical
CHAPTER 9 RAYLEIGH AND RAMAN SCATTERING 9.1. Introduction The statistical multi-level approach of the preceding chapter that is based on the density matrix formalism provides a foundation for physical
Many-Body Problems and Quantum Field Theory
 Philippe A. Martin Francois Rothen Many-Body Problems and Quantum Field Theory An Introduction Translated by Steven Goldfarb, Andrew Jordan and Samuel Leach Second Edition With 102 Figures, 7 Tables and
Philippe A. Martin Francois Rothen Many-Body Problems and Quantum Field Theory An Introduction Translated by Steven Goldfarb, Andrew Jordan and Samuel Leach Second Edition With 102 Figures, 7 Tables and
Temperature Scales
 TEMPERATURE is a measure of the internal heat energy of a substance. The molecules that make up all matter are in constant motion. By internal heat energy, we really mean this random molecular motion.
TEMPERATURE is a measure of the internal heat energy of a substance. The molecules that make up all matter are in constant motion. By internal heat energy, we really mean this random molecular motion.
Ψ t = ih Ψ t t. Time Dependent Wave Equation Quantum Mechanical Description. Hamiltonian Static/Time-dependent. Time-dependent Energy operator
 Time Dependent Wave Equation Quantum Mechanical Description Hamiltonian Static/Time-dependent Time-dependent Energy operator H 0 + H t Ψ t = ih Ψ t t The Hamiltonian and wavefunction are time-dependent
Time Dependent Wave Equation Quantum Mechanical Description Hamiltonian Static/Time-dependent Time-dependent Energy operator H 0 + H t Ψ t = ih Ψ t t The Hamiltonian and wavefunction are time-dependent
Infrared Spectroscopy
 Infrared Spectroscopy The Interaction of Light with Matter Electric fields apply forces to charges, according to F = qe In an electric field, a positive charge will experience a force, but a negative charge
Infrared Spectroscopy The Interaction of Light with Matter Electric fields apply forces to charges, according to F = qe In an electric field, a positive charge will experience a force, but a negative charge
221B Lecture Notes Many-Body Problems I (Quantum Statistics)
 221B Lecture Notes Many-Body Problems I (Quantum Statistics) 1 Quantum Statistics of Identical Particles If two particles are identical, their exchange must not change physical quantities. Therefore, a
221B Lecture Notes Many-Body Problems I (Quantum Statistics) 1 Quantum Statistics of Identical Particles If two particles are identical, their exchange must not change physical quantities. Therefore, a
Phonons I - Crystal Vibrations (Kittel Ch. 4)
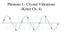 Phonons I - Crystal Vibrations (Kittel Ch. 4) Displacements of Atoms Positions of atoms in their perfect lattice positions are given by: R 0 (n 1, n 2, n 3 ) = n 10 x + n 20 y + n 30 z For simplicity here
Phonons I - Crystal Vibrations (Kittel Ch. 4) Displacements of Atoms Positions of atoms in their perfect lattice positions are given by: R 0 (n 1, n 2, n 3 ) = n 10 x + n 20 y + n 30 z For simplicity here
12 주 /15 주 작은구멍이나장애물을만나면넘어가거나돌아간다. 원거리에돌이 ( 프라운호퍼에돌이 ) 에돌이 ( 회절 )- 불확정성의원리 근거리에돌이 ( 프레스넬에돌이 )
 12 주 /15 주 작은구멍이나장애물을만나면넘어가거나돌아간다. 원거리에돌이 ( 프라운호퍼에돌이 ) 에돌이 ( 회절 )- 불확정성의원리 근거리에돌이 ( 프레스넬에돌이 ) 2014-12-17 1 현대물리 : 광학 5 장 Diffraction 목포해양대학교기관공학부 김상훈 2014-12-17 2 2014-12-17 3 2014-12-17 4 2014-12-17 5
12 주 /15 주 작은구멍이나장애물을만나면넘어가거나돌아간다. 원거리에돌이 ( 프라운호퍼에돌이 ) 에돌이 ( 회절 )- 불확정성의원리 근거리에돌이 ( 프레스넬에돌이 ) 2014-12-17 1 현대물리 : 광학 5 장 Diffraction 목포해양대학교기관공학부 김상훈 2014-12-17 2 2014-12-17 3 2014-12-17 4 2014-12-17 5
Atmospheric "greenhouse effect" - How the presence of an atmosphere makes Earth's surface warmer
 Atmospheric "greenhouse effect" - How the presence of an atmosphere makes Earth's surface warmer Some relevant parameters and facts (see previous slide sets) (So/) 32 W m -2 is the average incoming solar
Atmospheric "greenhouse effect" - How the presence of an atmosphere makes Earth's surface warmer Some relevant parameters and facts (see previous slide sets) (So/) 32 W m -2 is the average incoming solar
Discussion Review Test #2. Units 12-19: (1) (2) (3) (4) (5) (6)
 Discussion Review Test #2 Units 12-19: (1) (2) (3) (4) (5) (6) (7) (8) (9) Galileo used his observations of the changing phases of Venus to demonstrate that a. the sun moves around the Earth b. the universe
Discussion Review Test #2 Units 12-19: (1) (2) (3) (4) (5) (6) (7) (8) (9) Galileo used his observations of the changing phases of Venus to demonstrate that a. the sun moves around the Earth b. the universe
LECTURES ON QUANTUM MECHANICS
 LECTURES ON QUANTUM MECHANICS GORDON BAYM Unitsersity of Illinois A II I' Advanced Bock Progrant A Member of the Perseus Books Group CONTENTS Preface v Chapter 1 Photon Polarization 1 Transformation of
LECTURES ON QUANTUM MECHANICS GORDON BAYM Unitsersity of Illinois A II I' Advanced Bock Progrant A Member of the Perseus Books Group CONTENTS Preface v Chapter 1 Photon Polarization 1 Transformation of
Wave Motion and Sound
 Wave Motion and Sound 1. A back and forth motion that repeats itself is a a. Spring b. Vibration c. Wave d. Pulse 2. The number of vibrations that occur in 1 second is called a. A Period b. Frequency c.
Wave Motion and Sound 1. A back and forth motion that repeats itself is a a. Spring b. Vibration c. Wave d. Pulse 2. The number of vibrations that occur in 1 second is called a. A Period b. Frequency c.
