Dynamic extraction of lipid tethers from cell membranes
|
|
|
- Douglas Alexander
- 6 years ago
- Views:
Transcription
1 Dynamic extraction of lipid tethers from cell membranes Sarah A. Nowak RAND Corporation, Los Angeles, CA Tom Chou Dept. of Biomathematics, UCLA, Los Angeles, CA Dept. of Mathematics, UCLA, Los Angeles, CA Abstract. When a ligand that is bound to an integral membrane receptor is pulled, the membrane and the underlying cytoskeleton can deform before either the membrane delaminates from the cytoskeleton or the ligand detaches from the receptor. If the membrane delaminates from the cytoskeleton, it may be further extruded and form a membrane tether. We develop a phenomenological model for these processes by assuming that deformations obey Hooke s law up to a critical force at which the cell membrane locally detaches from the cytoskeleton and a membrane tether forms. We compute the probability of tether formation and show that they can be extruded only within an intermediate range of force load rates and pulling velocities. The mean tether length that arises at the moment of ligand detachment is also computed and the force loading rates and pulling velocities that yield the longest tether found. 1. INTRODUCTION Adhesion between cells plays an important role in a number of biological processes involving cell motility and cell-cell communication. Cell-cell adhesion is mediated by integral membrane proteins on the surfaces of interacting cells. Cadherins, which mediate binding between cells of the same type within a tissue, bind to themselves, while integrins, which mediate binding between different cell types bind to Inter- Cellular Adhesion Molecules (ICAMs) or Vascular Cell Adhesion Molecules (VCAMs) [1]. Understanding the physics of this adhesive interaction requires an understanding of both the protein-protein bond as well as the cell s mechanical response when this bond becomes stressed. If forces act to pull the two cells apart, either of the cells cytoskeletons and plasma membranes may deform. Under certain conditions, the lipid membranes can delaminate from the underlying cytoskeleton and be pulled into long tethers. At any time during this process, the bonds holding the two cells together may also break, arresting tether extraction. A specific biological process in which bond dissociation and membrane deformation must be considered simultaneously is leukocyte extravasation, which is part of the process by which leukocytes are recruited to inflamed or infected tissue. Endothelial cells that make up blood vessels preferentially express cellular adhesion molecules, including selectins, near wounded tissue. Leukocytes circulating in the
2 Pulling Membrane Tethers 2 blood can then bind to the endothelial cells via their own cell surface proteins. Bonds between the leukocytes and the endothelial tissue are transiently made and broken as a shear flow in the blood vessel pushes the leukocyte, rolling it across the endothelial layer [2]. The rolling leukocytes contain microvilli that are enriched in adhesion molecules that preferentially attach to endothelial cells. During rolling, these microvilli tethers can extend under the hydrodynamic shear force of blood flow in the vessel [3]. At the same time, forces imposed on the endothelial membrane via the adhesion molecule can cause the endothelial membrane to form a tether [4]. Tether formation and extension of microvilli can decrease the force that the adhesive bond feels from the shear blood flow. Both the physics of cell membrane deformation and the mechanics of ligandreceptor bonds have been studied extensively. Micropipette and AFM techniques have been used to pull membrane tethers from cells and probe the properties of the cell membrane. The diameter of the extracted tether depends on the membrane surface tension and bending rigidity and, if the tether is being extended at a constant velocity, the membrane viscosity [5, 6]. Therefore, these quantities can be inferred from tether pulling or poking experiments [7]. Although theoretical models show only a 10% overshoot in the force-extension curve for pulling on an asymptotically flat membrane [8], some experiments show a significant force barrier to tether formation [9, 10]. When tethers are pulled from giant vesicles, the size of the force barrier increases when the area on which the pulling force is exerted is increased [9]. For smaller vesicles, area and volume constraints may also influence the tether force-extension relationship [11]. In living cells, large force barriers to membrane tether formation may be partly attributable to the actin cytoskeleton [12]. More sophisticated theoretical approaches have used Euler-Lagrange methods to compute equilibrium tether shapes and the force-extension curve for a tether pulled quasi-statically from a lipid vesicle [8]. The relative importance of nonequilibrium forces arising from the viscosity of both the membrane lipids and surrounding solution have also been estimated [13]. In many tether pulling experiments, as well as in their associated theoretical models, the molecular bond attaching the pulling device to the membrane remains intact. However, a typical ligand-receptor bond used to connect the pulling device to the lipid membrane (and possibly the underlying cytoskeleton) can rupture upon pulling. Although the details of a bond s energy landscape can be probed using dynamic force spectroscopy [14, 15, 16, 17], one can usually assume that bond rupturing is dominated by a single activation barrier that can be lowered by an externally applied pulling force. Most AFM studies of molecular strength are performed on proteins that have been isolated from cells. However, recent studies have probed bond strengths between proteins still embedded in a live cell membrane [18, 19]. While using live cells has the advantage that the post-translational modifications of membrane proteins are preserved, the mechanical deformation of the cell s cytoskeleton and membrane must also be taken into account. To model this system, a viscoelastic Kelvin model was used to fit experimental measurements of the force-extension relationship to determine effective cellular adhesion [19]. In this paper, we develop a dynamic model that can be directly used to analyze a wide class of experiments in which cell or lipid vesicle membranes are pulled. In contrast to an equilibrium model determining tether extraction and detachment from an adhered vesicle [11], we find relationships that define when tether extraction is likely, and the typical length of the tether pulled before the ligand-receptor bond ruptures. In the next section, we motivate a simple mechanical model using
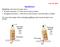
3 Pulling Membrane Tethers 3 phenomenological forms for the force-extension relationship of the membrane. The ligand-receptor bond dissociation kinetics and the elastic compliance of the pulling device are also incorporated within our simple mathematical treatment. Dynamical equations are written for two commonly employed experimental protocols, a linear force loading rate, and a constant bond pulling speed. In the Results and Discussion, we compute the probability of tether formation and plot universal curves that delineate regimes where tethers are likely to form. Mean tether lengths are also plotted for both pulling protocols. 2. Mathematical Model Consider the system depicted in Figure 1. A ligand is bound to an integral membrane protein, which may also be directly associated with the cytoskeleton. The ligand is attached to a pulling device via a spring and is pulled with either a fixed speed, or a force that increases linearly in time. (a) cell membrane F p or V cantilever x z cytoskeleton (b) x z Figure 1. (a) A cell membrane can be pulled by a device such as a cantilever. The device can be moved at fixed velocity V, or, at a specified force F p(t). Under specified force conditions, the transduction of force through the device is assumed instantaneous such that the F p(t) is always acting across the ligand-receptor bond. Before membrane delamination, the pulling results in small membrane and cytoskeletal deformations. (b) After membrane delamination, the pulling device can extrude long lipid tethers without deforming the cytoskeleton. The total system length z is the sum of the displacement x of the membrane protein from its initial position and l, the increase in the length of the cantilever spring from its unstretched length. As the ligand is pulled, the cytoskeleton first deforms, and eventually can detach from the membrane. At this point, the lipid membrane may flow into a tether. At any point during the cytoskeletal or membrane deformation, before or after membranecytoskeleton delamination and tether formation, the ligand affixed to the pulling device may detach from the membrane receptor protein.
4 Pulling Membrane Tethers Membrane Mechanics We first consider the response of the membrane-cytoskeleton system to an externally applied pulling force F p (t). The rate at which the receptor-ligand complex moves will be described by dx(t) dt = ξ [ U(x) x ] F p (t) x=x(t) where ξ is a mobility that is related to the viscosity of the membrane lipid [13]. In general, although ξ depends on the configuration of the system defined predominantly by x(t), we neglect the details of this dependence and assume constant ξ. The term U(x) represents the energetic cost associated with deforming the cell membrane and underlying cytoskeleton when the receptor is displaced a distance x normal to the flat membrane. Experiments in which tethers were pulled from live cells found a significant force barrier to tether formation [12, 20]. While a smaller force barrier can also arise in pure lipid membranes [8], we will assume that the plasma membrane is attached to an underlying cytoskeleton (with anchoring molecules), which we model as a linear elastic material provided the deformation is small. The receptor that binds the ligand that is attached to the pulling device can also be a transmembrane receptor that is directly attached to the cytoskeleton. As the membrane is initially pulled, the cytoskeleton will elastically deform as a Hookean spring. The receptor or anchoring molecules will break at a deformation x 0, and the lipid membrane will be drawn into a tether. This occurs at a critical delamination force F c. Thus, for displacements x < x 0, where the Hookean approximation for the membrane-cytoskeleton assembly is valid, the membrane-cytoskeleton carries an effective spring constant F c /x 0, where F c is the critical delamination force. The linear force extension relationship is thus U x = x x 0 F c, x < x 0. (2) At a displacement x = x 0, the membrane delaminates from the cytoskeleton and a lipid membrane tether forms. The tether can then elongate indefinitely under a constant force U/ x = F 0 that is intrinsic to the lipid tether and is determined by the membrane bending rigidity κ and surface tension σ [8]: F 0 = 2π 2κσ. The phenomenological membrane force-displacement relationship for both attached (solid curve) and free (dashed curve) membranes are shown in Figure 2. The barrier F c to tether formation is larger for receptors or membranes that are attached to the underlying cytoskeleton, than for the free lipid membrance case. In order to close the equations of our basic model, we must specify F p (t). Henceforth, we will consider two cases typically realized in experiments: a linearly increasing (in time) pulling force, and a fixed pulling speed Linear Force Ramp For a force linearly increasing in time, we set F p (t) = Γt, where Γ is the rate with which the force increases. Eq. 1 becomes [ ] dx(t) U(x) = ξ dt x Γt. (3) (1)
5 Pulling Membrane Tethers 5 Figure 2. We assume that when the receptor is initially associated with the cytoskeleton, xu(x) increases linearly until some maximum force F c in the barrier to tether formation is reached, after which the tether extends with constant force F 0 (solid curve). The qualitative features of this force-extension curve is observed in numerous systems [10, 20, 6, 12, 9]. For reference, we show xu(x) as a function of x when the only contributions to U(x) come from membrane surface tension and bending rigidity (dashed curve). This curve was calculated using the method described in [8] assuming a membrane bending rigidity of 20k B T and a surface tension of dynes/cm. Upon defining the time t 0 at which the membrane delaminates from the cytoskeleton provided the ligand-receptor bond has not yet ruptured by x(t = t 0 ) x 0, we have U x = (x/x 0)F c for t t 0, and U x = F 0 for t > t 0. Thus, Eq. 3 is solved by and x(t < t 0 ) = x 0Γ t Γx2 0 (1 e ξfct/x0 ), (4) F c ξf 2 c x(t > t 0 ) = x 0 ξf 0 (t t 0 ) + ξγ 2 (t2 t 2 0), where t 0 is determined from the solution to Γ t 0 Γx 0 (1 e ξfct0/x0 ) = 1. (6) F c ξf 2 c 2.3. Constant Pulling Speed In the case of constant pulling speed, we must include the dynamics of the device deformation l(t). Since the velocity of the pulling device is fixed, we note that the total displacement z(t) = x(t) + l(t) obeys dz dt = dx dt + dl = V. (7) dt This equation holds only when the ligand is attached to the membrane-bound receptor. Let us assume that the pulling device has an internal response that is modeled by a simple spring so that the force F p (t) that the spring exerts on the ligand is F p (t) = Kl(t), (8) (5)
6 Pulling Membrane Tethers 6 where K is the spring constant of the pulling device. Since the pulling force is proportional to l(t), it will ultimately depend on the pulling rate V and the physical properties of the pulling device (represented by an elastic cantilever in Figure 1) and cell membrane through Eq. 7. Upon integrating Eq. 7 and using the initial conditions x(t = 0) = l(t = 0) = 0, we find x(t) = V t l(t). (9) Substituting Eq. 8 into Eq. 1, and expressing the dynamics in terms of the device deformation, we find a closed equation for l(t): [ ] dl dt = V + ξ U(x) x Kl(t). (10) x=v t l(t) This equation is solved by for t < t 0, and l(t t 0 ) = V Kx 2 0 ξ(f c + Kx 0 ) 2 (1 e ξ(k+fc/x0)t ) + F cv t F c + Kx 0 (11) l(t > t 0 ) = V + ξf 0 ξk (1 e ξk(t t0) ) +l(t 0 )e ξk(t t0) (12) for t > t 0 (when U/ x = F 0 ). Here, the time t 0 at which tether formation occurs is found by evaluating Eq. 9 at time t = t 0, x(t 0 ) = x 0 = V t 0 l(t 0 ), yielding an implicit equation for t 0 : V t 0 x 0 = F cv t 0 F c + Kx 0 V Kx ξ(f c + Kx 0 ) 2 (1 ). e ξ(k+fc/x0)t0 After evaluating t 0 numerically, l(t) is found in terms of the K, V, ξ, F c, F 0, and x 0, and the membrane displacement can be found using Eq Ligand-Receptor Dissociation The dynamics described above for the membrane and pulling device deformations assume that the pulling device remains attached to the membrane through an intact ligand-receptor bond. Since all external forces are transduced through the ligandreceptor bond, the pulling force F p (t) in our fundamental equation 1 vanishes once the ligand-receptor bond ruptures. The probability of ligand-receptor bond dissociation itself depends on the applied force F p. We model the breaking of the ligand-receptor bond by a Poisson process and define a ligand-receptor bond survival probability Q(t) that obeys dq(t) dt (13) = k r (t)q(t), (14)
7 Pulling Membrane Tethers 7 where k r (t) is the force-dependent rupture (or dissociation) rate of the ligand from the receptor. We assume that k r takes a simple Arrenhius form: k r (t) = k 0 e Fp(t)d/kBT, (15) where d is the length of the ligand-receptor bond and k B T is the thermal energy. The solution to Eq. 14 is explicitly t ] Q(t) = exp [ k 0 e Fp(t )d/k BT dt. (16) 0 Molecular details such as the thermally-induced bond-breaking attempt frequency and the instrinsic free energy of the unstressed ligand-receptor bond are subsumed in the rate parameter k 0. Since dq(t)/dt = k r Q(t) is the bond rupture time distribution, the mean membrane displacement at the time of ligand-receptor rupture (the mean maximum displacement) is given by x = 0 k r (t)q(t)x(t)dt. (17) In our subsequent analysis, we explore, as a function of the physical parameters, the bond rupturing statistics, the probability of tether formation, and the length of pulled tethers should they form. To be concrete, we will use typical parameter values (listed in Table I) found from the relevant literature to guide our analysis. parameter range of values reference d nm [21] (streptaviden-haba) ξ 1µm/(pNs) [13] x 0 1 4µm [12] F pN calculated F c pN [22] (red blood cells) 1-100pN [23] (epithelial cells) K 8-11pN/µm [12] k /s [24] V 3 µm/s [12] Γ 10pN/s [12] 3. Results Here, we compute the dynamics of tether formation and ligand-receptor bond rupturing under both linear force loading and constant pulling velocity protocols Linear Force Ramp When F p (t) = Γt, the ligand-receptor survival probability defined by Eq. 16 is explicitly [ Q(t) = exp k ] 0k B T Γd (eγtd/kbt 1), (18) F 0 = 2 2π κσ where κ is the membrane bending rigidity and σ is the effective membrane surface tension[8]. We assumed σ = pN/µm[25] and κ = 10 20k B T [26]
8 Pulling Membrane Tethers 8 The bond survival probability, evaluated at the time of tether formation t 0 (found numerically from Eq. 6, Q(t 0 ) P T, defines the probability that a tether forms, and is plotted in Fig. 3(a) as a function of force loading rate Γ. Note that for our chosen parameter values, P T first increases with the force loading rate Γ, before decreasing again at very high loading rates. Membrane-cytoskeleton combinations that have weaker delamination forces F c yield a larger range of force loading rates that lead to tether formation. The limits of the force loading rates, Γ + and Γ, are defined by P T (Γ ± ) = 1/2. Figure 3. (a) Tether formation probability P T Q(t 0 ) as a function of force ramp rate Γ. This result is independent of the free tether restoring force F 0. Γ ± denote the linear loading rates at which the tether formation probability P T = 1/2. Applied loading rates Γ < Γ < Γ + are likely to lead to tether formation. For F c = 20pN, Γ 7.2pN/s and Γ + 435pN/s. Other parameters used were: k 0 = 0.01/s, d = 1nm, x 0 = 1µm and ξ = 1µm/(pNs). (b) Dimensionless parameter regimes in which P T 1/2. The regions of parameter space below each curve are associated with P T > 1/2, where tether formation is likely. The smaller the dimensionless bond dissociation rate α = k 0 x 0 d/(ξk B T), the wider the range of dimensionless loading rates γ = ξγ/(k0 2x 0) leading to tether formation. The maximum and minimum pulling rates γ min γ (f c = 0) and γ+ max γ + (f c = 0) are indicated for the α = curve, while the maximal dimensionless delamination force fc max is shown for α = (c) The minimum and maximum force ramps, and the maxima delamination force as functions of α. For γ < γ min, γ > γmax +, or fc > fmax c, tethers always have less than 50% chance of forming. For a more accurate determination of Γ ±, and their dependence on the other system parameters (F max, d, k B T, x 0, and ξ), it is convenient to define dimensionless
9 Pulling Membrane Tethers 9 parameters according to γ ξγ k0 2x, α k 0x 0 d 0 ξk B T, f c = ξf c, (19) k 0 x 0 and find parameter regimes within which P T 1/2. Upon using Eq. 18, the phase boundaries for tether formation are determined from the implicit solution to [ exp 1 ] αγ (eαγτ0(γ,fc) 1) = 1 2, (20) where τ 0 = k 0 t 0 is determined from the solution to the dimensionless form of Eq. 6: γτ 0 γ f c fc 2 (1 e fcτ0 ) = 1. (21) Figure 3(b) shows curves in dimensionless parameter space (f c and γ) below which P T > 1/2. Asymptotic analysis of the condition P T = 1/2 shows that for sufficiently large γ tether formation will always be suppressed. Additionally, as the dimensionless ligand-receptor dissociation rate α increases, the regime for tether formation shrinks. Conditions for tether formation can be further refined by computing universal parameter curves that define regimes for which P T can never be greater than 1/2. As a function of the intrinsic ligand-receptor dissociation rate, there is a band of pulling rates outside of which P T is always less than one half, even when tether extraction is barrierless (f c = 0). Fig. 3(c) shows γ min and γ+ max, the minimum and maximum dimensionless ramp rates at which P T = 1/2 when f c = 0. These delimiting loading rates are roots of Eq. 20. For small α, the lower root γ max 2/ ln O(α). Also plotted in Fig. 3(c) is fc max (α), the maximum membrane-cytoskeleton delamination force that can give rise to P T 1/2, for any ramp rate. One is unlikely to pull tethers from membranes that require more force than fc max to delaminate from the cytoskeleton. Moreover, there is a critical α max above which P T = 1/2 cannot be reached, independent of f c or γ. Finally, we plot in Fig. 4 the expected dimensionless maximum tether length X x /x 0 found from Eq. 17, as a function of the dimensionless force loading rate γ. Not only does the mean tether length that can be drawn decreases with increasing critical delamination force f c, as shown in Fig. 4(a), but the value of γ at which X is maximized decreases as f c is raised. Since tether extrusion is a competition between the rate of climb against the restoring force F c x/x 0 and ligand-receptor dissociation, as f c is increased, higher ramp rates are required to cross the delamination barrier faster, relative to the bond rupturing. Although the free membrane restoring force f 0 = ξf 0 /(k 0 x 0 ) does not come into play in the tether formation probability, it does influence the mean length of lipid tether that is extruded before the ligand detaches from the membrane-bound receptor. However, since f 0 does not affect the probability of tether nucleation, it does not influence the load rate γ at which the maximal X arises Constant Pulling Speed Now consider a constant pulling speed protocol. In this case, the bond survival probability is computed from t ] Q(t) = exp [ k 0 e Kl(t )d/k BT dt, (22) 0
10 Pulling Membrane Tethers 10 Figure 4. (a) Mean tether length X as a function of load rate γ for different critical delamination forces f c. (b) X at fixed f c and various f 0. Even though the cytoskeleton-free membrane restoring force does not affect tether formation probability P T, it does influence the length of tether possible. where l(t) is given by Eqs. 11 and 12. Initially, while the membrane and cytoskeleton are attached to each other, and constant speed pulling is applied, the ligand-receptor bond survival probability Q(t) first decreases rapidly. After delamination, the force on the ligand-receptor are fixed, arising only from F 0 and the viscous drag ξ 1. The subsequent decay of Q(t) arises from a slower, single exponential. Fig. 5(a) shows the corresponding P T as a function of pulling speed V, and as in the force ramp case, reveals an optimal pulling speed that maximizes the likelihood of pulling a tether. In the V 0 limit x(t) 0 (from Eqs. 9 and 12), and we expect P T 0 because when the ligand-receptor bond detaches, the membrane has not been sufficiently deformed. In the fast pulling limit, the detachment rate increases quickly and ligands detach at very short times t such that tether formation cannot occur. Upon defining P T (V ± ) = 1/2, pulling speeds V < V < V + are likely to result in tethers. To explore how V ± depends on other system parameters, we employ the same dimensionless parameters defined in Eqs. 19, along with a dimensionless pulling speed and pulling device stiffness, v V k 0 x 0 and µ Kξ k 0. (23) Figure 5(b) shows, for µ = 1000 and various values of α, the boundaries below which tether formation is likely. Fig. 5(c) shows the phase boundaries for fixed α = and various pulling device stiffnesses µ. Note that softer pulling devices suppress tether formation at low speeds since the forces are not immediately felt by the membrane, allowing the ligand more time to detach. However, softer pulling devices greatly enhance tether formation at large pull speeds because the accelerated delamination more than compensates for the drag-mediated acceleration of ligandreceptor dissociation. Note that analogous to the linear force ramp protocol, for particular α and µ, there is a maximum and minimum pulling speed (v min and vmax + ) beyond which tether formation is unlikely, even when the delamination force vanishes (Fig. 6(a)).
11 Pulling Membrane Tethers 11 Figure 5. (a) The probability P T that tether formation occurs before the ligand detaches from the receptor plotted as a function of pulling speed V. Analogous to the force ramp protocol, there is a band of pulling speeds V < V < V + within which the tether formation probability is greatest. Parameters used were: k 0 = 0.01/s, d = 1nm, ξ = 1µm/(pN s), F c = 20pN, K = 10pN/µm. (b) Phase diagram in the f c = ξf c/(k 0 x 0 ) and v = V/(k 0 x 0 ) parameter space for various α = k 0 x 0 d/(k B T) and fixed pulling device stiffness µ = ξk/k 0 = (c) Phase diagram for fixed α = and various pulling device stiffnesses µ. Conversely, there is a maximum delamination force fc max above which no pulling speed will result in likely tether formation (Fig. 6(b)). Finally, consider the mean receptor displacement at the moment of ligand detachment, X x /x 0, found from Eq. 17 with X(t) = vτ l(t)/x 0, and the appropriate Q(t) and k r (t). The same arguments that explain the non-monotonic behavior of P T as a function of V apply here, and the mean dimensionless receptor displacement at the moment of ligand detachment, X, is a non-monotonic function of V (Fig. 7). When the pulling velocity is small, the receptor moves slowly and the term x(t) in Eq. 17 will be small, rendering integral in Eq. 17 small. On the other hand, when V is sufficiently large, the viscosity ξ 1 allows a large force to be reached, accelerating ligand-receptor bond rupturing. Thus, Q(t) quickly decreases and the mean receptor displacement when the ligand detaches, x, will be small. Both constant force load rate and constant pulling speed protocols give qualitatively similar tether extraction probabilities and maximum delamination forces fc max. This is not surprising since in the two protocols are physically equivalent in both the infinitely stiff and infinitely soft pulling device limits, prior to delamination. In the large µ K limit, a constant pulling speed forces the ligand to have the
12 Pulling Membrane Tethers 12 Figure 6. (a) Universal curves for the critical values v min and vmax + as a function of dimensionless unstressed ligand-receptor dissociation rate α. Curves for three difference values of device stiffness µ are shown. For α 1 and µ, the minimum pull speed is asymptotically v min 1/(ln 2 α). In this limit, the critical α max beyond which v min and vmax + merge (and tether formation is unlikely, even for f c = 0) occurs at α max 0.24, slightly larger than the α max in the constant load rate protocol (not shown). (b) The maximal delamination force fc max as a function of α for two different stiffnesses µ. Figure 7. (a) The mean system length at ligand detachment, X, is a nonmonotonic function of the dimensionless pulling velocity, v, and increases with decreasing f c. The qualitative dependence of X on f 0 is similar to that shown in Fig. 4. (b) The mean maximum tether length as a function of v for various dimensionless pulling device stiffnesses µ. trajectory x(t) = V t. Because we assume a linear force-extension relationship before delamination, this protocol is equivalent to a high constant force loading rate of Γ KV (or γ µv). In the extremely soft pulling device limit, the elastic pulling device absorbs most of the extension and the force at which it acts on the ligand also increases linearly in time: Γ F c V/x 0 (or γ vf c ). However, we do find a qualitative difference in the mean tether length extracted, due to difference in the post-delamination forces between the two protocols. As functions of load rate and pulling speed, the maximum mean tether lengths attainable via linear force ramp are typically less than half of those achieved through constant
13 Pulling Membrane Tethers 13 pulling speed, all else being equal. This feature can be understood by considering how the receptor displacement, x(t) and the ligand dissociation rate, k r (t) depend on time in each case. When the pulling speed is constant, after the tether forms, x(t) increases linearly in time, and k r (t) is constant. When we apply a force ramp to the system, x(t) increases quadratically in time once tether formation occurs. This would seem to imply longer tethers under the force ramp protocol; however, in this case, the dissociation rate k r (t) also increases exponentially in time. Thus, ligand detachment is much faster in the force ramp case, resulting in shorter observed mean tether lengths. 4. Summary and Conclusions We modeled membrane-cytoskeleton delamination in series with a ligand-receptor bond and a deformable pulling device and determined the parameter regimes within which lipid tether extrusion is likely. For both linear force ramp and constant pulling speed protocols, we find a wide window of ramp rates and pulling speeds that likely lead to tether extraction. However, we also find critical values of a dimensionless membrane-cytoskeleton delamination force, and a dimensionless spontaneous ligandreceptor dissociation rate beyond which tether formation is unlikely, regardless of all other parameters. Both linear force ramp and constant pulling speed protocols yield intermediate tether formation regimes, with a specific pulling speed v and specific linear ramp rate γ that maximizes the mean tether length X in the respective protocol. However, they present different tether dynamics after delamination leading to different expected tether lengths x. Using both protocols, and our results, it may be possible to characterize membrane-cytoskeleton properties, provided sufficent information about the ligand-receptor binding energy and pulling device response are known. In general, such inverse problems are very ill-posed, but restricting the force-extension relationship to simple forms as we have done, one may be able to use the onset of tether formation as a way to estimate force parameters (such as F 0, F c and x 0 ). While we have framed our analysis in terms of AFM experiments in which the strength of a ligand-receptor bond is probed while the receptor is in the membrane of a live cell, our basic model is relevant to leukocyte rolling as well. In leukocyte rolling, a bond between protein on a leukocyte microvilli and a protein in the membrane of an endothelial cell becomes stressed. Because the microvilli act like Hookean springs when pulled [3], and simultaneous extension of microvilli and tether extraction from the endothelial cells has been observed [4], our analysis is directly applicable to this system, with the cantilever replaced with a microvilli. Finally, we note that we have treated the ligand-receptor bond rupturing as a stochastic Poisson process, while the deformation of membrane and cytoskeleton was considered deterministic. This approximation is good as long as x 0 d. However, if the experiment is repeated, each region of membrane may have highly variable attachments to the cytoskeleton. In this case, a distribution of delamination forces F c should be considered. Another source of stochasticity may arise when multiple adhesion points are being pulled, possibly leading to multiple tethers [27]. If the entire system is treated as a single, effective tether, the force-extension of this super-tether will rely on the statistics of how many individual tethers are still attached during the dynamics.
14 Pulling Membrane Tethers 14 Acknowledgments This work was supported by the NSF through Grant no. DMS and by the NIH through Grant no. K25AI [1] H. Lodish, A. Berk, P. Matsudaira, C. A. Kaiser, M. Krieger, M. P. Scott, S. L. Zipursky, and J. Darnell. Molecular Cell Biology. W. H. Freeman and Company, [2] C. B. Korn and U. S. Schwarz. Dynamic states of cells adhering in shear flow: from slipping to rolling. Phys. Rev. E, 77:041904, [3] J. Y. Shao, H. P. Ting-Beall, and R. M. Hochmuth. Static and dynamic lengths of neutrophil microvilli. Proceedings of the National Academy of Sciences of the United States of America, 95(12): , [4] G. Girdhar and J. Y. Shao. Simultaneous tether extraction from endothelial cells and leukocytes: Observation, mechanics, and significance. Biophys. J., 93(11): , [5] R. M. Hochmuth and E. Evans. Extensional flow of erythrocyte membrane from cell body to elastic tether. Biophys J., 39:71 81, [6] J. Dai and M. P. Sheetz. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys. J., 68(3): , [7] D. Fygenson, J. F. Marko, and A. Libchaber. Mechanics of microtubule-based membrane extension. Phys. Rev. Lett., 79: , [8] T. R. Powers, G. Huber, and R. E. Goldstein. Fluid-membrane tethers: Minimal surfaces and elastic boundary layers. Phys. Rev. E, 65(4):041901, [9] G. Koster, A. Cacciuto, I. Derenyi, D. Frenkel, and M. Dogterom. Force barriers for membrane tube formation. Phys Rev. Lett., 94:068101, [10] D. Cuvelier, N. Chiaruttini, P. Bassereau, and P. Nassoy. Pulling long tubes from firmly adhered vesicles. Europhys. Lett., 71: , [11] Ana-Suncana Smith, E. Sackmann, and U. Seifert. Pulling tethers from adhered vesicles. Phys. Rev. Lett., 92:208101, [12] M. Sun, J. S. Graham, B. Hegedus, F. Marga, Y. Zhang, G. Forgacs, and M. Grandbois. Multiple membrane tethers probed by atomic force microscopy. Biophys. J., 89(6): , [13] F. M. Hochmuth, J. Y. Shao, J. Dai, and M. P. Sheetz. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J., 70: , [14] R. Merkel, P. Nassoy, A. Leung, K. Richie, and E. Evans. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature, 397:50 53, [15] E. Evans. Probing the relation between force-lifetime and chemistry in single molecular bonds. Ann. Rev. Biophys Biomol. Struct, 30: , [16] B. Heymann and H. Grubmüller. Dynamic force spectroscopy of molecular adhesion bonds. Phys Rev. Lett., 84: , [17] O. K. Dudko, A. E. Filippov, J. Klafter, and M. Urbakh. Beyond the conventional description of dynamic force spectroscopy of adhesion bonds. Proceedings of the National Academy of Sciences of the United States of America, 100(20): , [18] W. Hanley, O. McCarty, S. Jadhav, Y. Tseng, D. Wirtz, and K. Konstantopoulos. Single molecule characterization of p-selectin/ligand binding. J. Biol. Chem., 278: , [19] J. Schmitz, M. Benoit, and K. E. Gottschalk. The viscoelasticity of membrane tethers and its importance for cell adhesion. Biophys J., 95: , [20] M. Benoit, D. Gabriel, G. Grerisch, and H. E. Gaub. Discrete interactions in cell adhsion measured by single-molecule force spectroscopy. Nat. Cell Biol., 2: , [21] D. Leckband, W. Müller, F.-J. Schmitt, and H. Ringsdorf. Molecular mechanisms determining the strength of receptor-mediated intermembrane adhesion. Biophys J., 69: , [22] N. Borghi and F. Brochard-Wyart. Tether extrusion from red blood cell: Integral proteins unbinding from cytoskeleton. Biophys J., 93: , [23] Y. Sako, A. Nagafuchi, S. Tsukita, M. Takeichi, and A. Kusumi. Cytoplasmic regulation of the movement of e-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: Coralling and tethering by the membrane cytoskeleton. J. Cell Biol., 140: , [24] M. D. Ward, M. Dembo, and D. A. Hammer. Kinetics of cell detachment: Effect of ligand density. Annals of Biomedical Engineering, 23: , [25] C. E. Morris and U. Homann. Cell surface area regulation and membrane tension. J. Membrane Biol., 179:79 102, 2001.
15 Pulling Membrane Tethers 15 [26] E. Evans and W. Rawicz. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett., 64: , [27] O. Bjrnham and O. Axner. Multipili attachment of bacteria with helix-like pili exposed to stress. J. Chem. Phys., 130:235102, 2009.
Lecture 17: Cell Mechanics
 Lecture 17: Cell Mechanics We will focus on how the cell functions as a mechanical unit, with all of the membrane and cytoskeletal components acting as an integrated whole to accomplish a mechanical function.
Lecture 17: Cell Mechanics We will focus on how the cell functions as a mechanical unit, with all of the membrane and cytoskeletal components acting as an integrated whole to accomplish a mechanical function.
APPENDIX. A.1. Sensitivity analysis of the non-dimensional equations for stretch growth
 335 336 337 338 339 340 34 342 343 344 APPENDIX A.. Sensitivity analysis of the non-dimensional equations for stretch growth Our goal in this section of the appendix is to show how the solutions of the
335 336 337 338 339 340 34 342 343 344 APPENDIX A.. Sensitivity analysis of the non-dimensional equations for stretch growth Our goal in this section of the appendix is to show how the solutions of the
Formation and breakage of noncovalent protein protein interactions
 Dynamics of unbinding of cell adhesion molecules: Transition from catch to slip bonds V. Barsegov and D. Thirumalai Institute for Physical Science and Technology and Department of Chemistry and Biochemistry,
Dynamics of unbinding of cell adhesion molecules: Transition from catch to slip bonds V. Barsegov and D. Thirumalai Institute for Physical Science and Technology and Department of Chemistry and Biochemistry,
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Nano-to-Micro Scale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. III. Numerical Simulation of Tethering under Flow
 1676 Biophysical Journal Volume 88 March 2005 1676 1683 Nano-to-Micro Scale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. III. Numerical Simulation of Tethering under Flow Michael R. King,*
1676 Biophysical Journal Volume 88 March 2005 1676 1683 Nano-to-Micro Scale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. III. Numerical Simulation of Tethering under Flow Michael R. King,*
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Anatoly B. Kolomeisky. Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS?
 Anatoly B. Kolomeisky Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS? Motor Proteins Enzymes that convert the chemical energy into mechanical
Anatoly B. Kolomeisky Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS? Motor Proteins Enzymes that convert the chemical energy into mechanical
Amneh Auben. Abdulrahman Jabr. Diala Abu-Hassan
 21 Amneh Auben Abdulrahman Jabr Diala Abu-Hassan Matrix polysaccharides Extracellular matrix (ECM): It s a collection of components that fills the spaces outside the cell or between the cells. ---------
21 Amneh Auben Abdulrahman Jabr Diala Abu-Hassan Matrix polysaccharides Extracellular matrix (ECM): It s a collection of components that fills the spaces outside the cell or between the cells. ---------
Untangling the Mechanics of Entangled Biopolymers
 Untangling the Mechanics of Entangled Biopolymers Rae M. Robertson-Anderson Physics Department University of San Diego students/postdocs: Cole Chapman, PhD Tobias Falzone, PhD Stephanie Gorczyca, USD 16
Untangling the Mechanics of Entangled Biopolymers Rae M. Robertson-Anderson Physics Department University of San Diego students/postdocs: Cole Chapman, PhD Tobias Falzone, PhD Stephanie Gorczyca, USD 16
Website: Selected readings Topics Introduction to Cell Biology Analysis of Cell Mechanics Cell
 Session 1 Website: http://faculty.washington.edu/nsniadec/me599/w13/ Selected readings Topics Introduction to Cell Biology Analysis of Cell Mechanics Cell Mechanics Modeling Measuring Cell Forces Mechanotransduction
Session 1 Website: http://faculty.washington.edu/nsniadec/me599/w13/ Selected readings Topics Introduction to Cell Biology Analysis of Cell Mechanics Cell Mechanics Modeling Measuring Cell Forces Mechanotransduction
Elasticity of the human red blood cell skeleton
 Biorheology 40 (2003) 247 251 247 IOS Press Elasticity of the human red blood cell skeleton G. Lenormand, S. Hénon, A. Richert, J. Siméon and F. Gallet Laboratoire de Biorhéologie et d Hydrodynamique Physico-Chimique,
Biorheology 40 (2003) 247 251 247 IOS Press Elasticity of the human red blood cell skeleton G. Lenormand, S. Hénon, A. Richert, J. Siméon and F. Gallet Laboratoire de Biorhéologie et d Hydrodynamique Physico-Chimique,
Nano-to-Microscale Mechanical Switches and Fuses Mediate Adhesive Contacts between Leukocytes and the Endothelium
 1482 J. Chem. Inf. Model. 2005, 45, 1482-1490 PERSPECTIVES Nano-to-Microscale Mechanical Switches and Fuses Mediate Adhesive Contacts between Leukocytes and the Endothelium Volkmar Heinrich,*,,# Andrew
1482 J. Chem. Inf. Model. 2005, 45, 1482-1490 PERSPECTIVES Nano-to-Microscale Mechanical Switches and Fuses Mediate Adhesive Contacts between Leukocytes and the Endothelium Volkmar Heinrich,*,,# Andrew
Measurements of interaction forces in (biological) model systems
 Measurements of interaction forces in (biological) model systems Marina Ruths Department of Chemistry, UMass Lowell What can force measurements tell us about a system? Depending on the technique, we might
Measurements of interaction forces in (biological) model systems Marina Ruths Department of Chemistry, UMass Lowell What can force measurements tell us about a system? Depending on the technique, we might
Physics of Cellular materials: Filaments
 Physics of Cellular materials: Filaments Tom Chou Dept. of Biomathematics, UCLA, Los Angeles, CA 995-766 (Dated: December 6, ) The basic filamentary structures in a cell are reviewed. Their basic structures
Physics of Cellular materials: Filaments Tom Chou Dept. of Biomathematics, UCLA, Los Angeles, CA 995-766 (Dated: December 6, ) The basic filamentary structures in a cell are reviewed. Their basic structures
SUPPLEMENTARY FIGURE 1. Force dependence of the unbinding rate: (a) Force-dependence
 (b) BSA-coated beads double exponential low force exponential high force exponential 1 unbinding time tb [sec] (a) unbinding time tb [sec] SUPPLEMENTARY FIGURES BSA-coated beads without BSA.2.1 5 1 load
(b) BSA-coated beads double exponential low force exponential high force exponential 1 unbinding time tb [sec] (a) unbinding time tb [sec] SUPPLEMENTARY FIGURES BSA-coated beads without BSA.2.1 5 1 load
Journal of Biomechanical Science and Engineering
 Science and Engineering Vol. 7, No., 1 Numerical Study on Platelet Adhesion to Vessel Walls using the Kinetic Monte Carlo Method* Seiji SHIOZAKI**, Kenichi L. ISHIKAWA**, *** and Shu TAKAGI**, *** **Computational
Science and Engineering Vol. 7, No., 1 Numerical Study on Platelet Adhesion to Vessel Walls using the Kinetic Monte Carlo Method* Seiji SHIOZAKI**, Kenichi L. ISHIKAWA**, *** and Shu TAKAGI**, *** **Computational
Modeling cell interactions under flow
 Modeling cell interactions under flow Claude Verdier, Cécile Couzon, Alain Duperray, Pushpendra Singh To cite this version: Claude Verdier, Cécile Couzon, Alain Duperray, Pushpendra Singh. Modeling cell
Modeling cell interactions under flow Claude Verdier, Cécile Couzon, Alain Duperray, Pushpendra Singh To cite this version: Claude Verdier, Cécile Couzon, Alain Duperray, Pushpendra Singh. Modeling cell
Lecture 4: viscoelasticity and cell mechanics
 Teaser movie: flexible robots! R. Shepherd, Whitesides group, Harvard 1 Lecture 4: viscoelasticity and cell mechanics S-RSI Physics Lectures: Soft Condensed Matter Physics Jacinta C. Conrad University
Teaser movie: flexible robots! R. Shepherd, Whitesides group, Harvard 1 Lecture 4: viscoelasticity and cell mechanics S-RSI Physics Lectures: Soft Condensed Matter Physics Jacinta C. Conrad University
Degenerate ground-state lattices of membrane inclusions
 PHYSICAL REVIEW E 78, 011401 008 Degenerate ground-state lattices of membrane inclusions K. S. Kim, 1 Tom Chou,, * and Joseph Rudnick 3 1 Lawrence Livermore National Laboratory, Livermore, California 94550,
PHYSICAL REVIEW E 78, 011401 008 Degenerate ground-state lattices of membrane inclusions K. S. Kim, 1 Tom Chou,, * and Joseph Rudnick 3 1 Lawrence Livermore National Laboratory, Livermore, California 94550,
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Letters to the Editor
 2318 Biophysical Journal Volume 83 October 2002 2318 2323 Letters to the Editor Measurement of Selectin Tether Bond Lifetimes Smith and others (Smith et al., 1999) estimated selectin bond lifetimes by
2318 Biophysical Journal Volume 83 October 2002 2318 2323 Letters to the Editor Measurement of Selectin Tether Bond Lifetimes Smith and others (Smith et al., 1999) estimated selectin bond lifetimes by
Supplementary Methods
 Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
3 Biopolymers Uncorrelated chains - Freely jointed chain model
 3.4 Entropy When we talk about biopolymers, it is important to realize that the free energy of a biopolymer in thermal equilibrium is not constant. Unlike solids, biopolymers are characterized through
3.4 Entropy When we talk about biopolymers, it is important to realize that the free energy of a biopolymer in thermal equilibrium is not constant. Unlike solids, biopolymers are characterized through
Biophysics of Macromolecules
 Biophysics of Macromolecules Lecture 11: Dynamic Force Spectroscopy Rädler/Lipfert SS 2014 - Forced Ligand-Receptor Unbinding - Bell-Evans Theory 22. Mai. 2014 AFM experiments with single molecules custom-built
Biophysics of Macromolecules Lecture 11: Dynamic Force Spectroscopy Rädler/Lipfert SS 2014 - Forced Ligand-Receptor Unbinding - Bell-Evans Theory 22. Mai. 2014 AFM experiments with single molecules custom-built
6 Mechanotransduction
 6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
Polymerization/depolymerization motors
 Polymerization/depolymerization motors Movement formation Kuo Lab, J.H.U. http://www.nature.com/nature/journal/v407/n6807/extref/40 71026a0_S3.mov http://www.bme.jhu.edu/~skuo/movies/macrophchase.mov http://www.bme.jhu.edu/~skuo/movies/gc_filo.mov
Polymerization/depolymerization motors Movement formation Kuo Lab, J.H.U. http://www.nature.com/nature/journal/v407/n6807/extref/40 71026a0_S3.mov http://www.bme.jhu.edu/~skuo/movies/macrophchase.mov http://www.bme.jhu.edu/~skuo/movies/gc_filo.mov
Sec. 2.1 Filaments in the cell 21 PART I - RODS AND ROPES
 Sec. 2.1 Filaments in the cell 21 PART I - RODS AND ROPES Sec. 2.1 Filaments in the cell 22 CHAPTER 2 - POLYMERS The structural elements of the cell can be broadly classified as filaments or sheets, where
Sec. 2.1 Filaments in the cell 21 PART I - RODS AND ROPES Sec. 2.1 Filaments in the cell 22 CHAPTER 2 - POLYMERS The structural elements of the cell can be broadly classified as filaments or sheets, where
CHAPTER V. Brownian motion. V.1 Langevin dynamics
 CHAPTER V Brownian motion In this chapter, we study the very general paradigm provided by Brownian motion. Originally, this motion is that a heavy particle, called Brownian particle, immersed in a fluid
CHAPTER V Brownian motion In this chapter, we study the very general paradigm provided by Brownian motion. Originally, this motion is that a heavy particle, called Brownian particle, immersed in a fluid
Figure 1.1: Flaccid (a) and swollen (b) red blood cells being drawn into a micropipette. The scale bars represent 5 µm. Figure adapted from [2].
![Figure 1.1: Flaccid (a) and swollen (b) red blood cells being drawn into a micropipette. The scale bars represent 5 µm. Figure adapted from [2]. Figure 1.1: Flaccid (a) and swollen (b) red blood cells being drawn into a micropipette. The scale bars represent 5 µm. Figure adapted from [2].](/thumbs/77/75728743.jpg) 1 Biomembranes 1.1 Micropipette aspiration 1.1.1 Experimental setup Figure 1.1: Flaccid (a) and swollen (b) red blood cells being drawn into a micropipette. The scale bars represent 5 µm. Figure adapted
1 Biomembranes 1.1 Micropipette aspiration 1.1.1 Experimental setup Figure 1.1: Flaccid (a) and swollen (b) red blood cells being drawn into a micropipette. The scale bars represent 5 µm. Figure adapted
Nano- to Microscale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. II. Tether Flow Terminated by P-Selectin Dissociation from PSGL-1
 Biophysical Journal Volume 88 March 2005 2299 2308 2299 Nano- to Microscale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. II. Tether Flow Terminated by P-Selectin Dissociation from PSGL-1
Biophysical Journal Volume 88 March 2005 2299 2308 2299 Nano- to Microscale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. II. Tether Flow Terminated by P-Selectin Dissociation from PSGL-1
The Polymers Tug Back
 Tugging at Polymers in Turbulent Flow The Polymers Tug Back Jean-Luc Thiffeault http://plasma.ap.columbia.edu/ jeanluc Department of Applied Physics and Applied Mathematics Columbia University Tugging
Tugging at Polymers in Turbulent Flow The Polymers Tug Back Jean-Luc Thiffeault http://plasma.ap.columbia.edu/ jeanluc Department of Applied Physics and Applied Mathematics Columbia University Tugging
Finite Element Method Analysis of the Deformation of Human Red Blood Cells
 331 Finite Element Method Analysis of the Deformation of Human Red Blood Cells Rie HIGUCHI and Yoshinori KANNO An erythrocyte and a spherocyte are subjected to aspiration pressure with a micropipette and
331 Finite Element Method Analysis of the Deformation of Human Red Blood Cells Rie HIGUCHI and Yoshinori KANNO An erythrocyte and a spherocyte are subjected to aspiration pressure with a micropipette and
arxiv:cond-mat/ v2 [cond-mat.soft] 29 Nov 2004
![arxiv:cond-mat/ v2 [cond-mat.soft] 29 Nov 2004 arxiv:cond-mat/ v2 [cond-mat.soft] 29 Nov 2004](/thumbs/80/82235863.jpg) arxiv:cond-mat/411654v2 [cond-mat.soft] 29 Nov 24 ork probability distribution in single molecule experiments Alberto Imparato ( ) and Luca Peliti ( ) Dipartimento di Scienze Fisiche and Unità INFM, Università
arxiv:cond-mat/411654v2 [cond-mat.soft] 29 Nov 24 ork probability distribution in single molecule experiments Alberto Imparato ( ) and Luca Peliti ( ) Dipartimento di Scienze Fisiche and Unità INFM, Università
4. Complex Oscillations
 4. Complex Oscillations The most common use of complex numbers in physics is for analyzing oscillations and waves. We will illustrate this with a simple but crucially important model, the damped harmonic
4. Complex Oscillations The most common use of complex numbers in physics is for analyzing oscillations and waves. We will illustrate this with a simple but crucially important model, the damped harmonic
BMB November 17, Single Molecule Biophysics (I)
 BMB 178 2017 November 17, 2017 14. Single Molecule Biophysics (I) Goals 1. Understand the information SM experiments can provide 2. Be acquainted with different SM approaches 3. Learn to interpret SM results
BMB 178 2017 November 17, 2017 14. Single Molecule Biophysics (I) Goals 1. Understand the information SM experiments can provide 2. Be acquainted with different SM approaches 3. Learn to interpret SM results
Lecture Note October 1, 2009 Nanostructure characterization techniques
 Lecture Note October 1, 29 Nanostructure characterization techniques UT-Austin PHYS 392 T, unique # 5977 ME 397 unique # 1979 CHE 384, unique # 151 Instructor: Professor C.K. Shih Subjects: Applications
Lecture Note October 1, 29 Nanostructure characterization techniques UT-Austin PHYS 392 T, unique # 5977 ME 397 unique # 1979 CHE 384, unique # 151 Instructor: Professor C.K. Shih Subjects: Applications
Micropipette aspiration of living cells
 Journal of Biomechanics 33 (2000) 15}22 Review Micropipette aspiration of living cells Robert M. Hochmuth* Department of Mechanical Engineering and Materials Science, Duke University, Durham, NC 27708-0300,
Journal of Biomechanics 33 (2000) 15}22 Review Micropipette aspiration of living cells Robert M. Hochmuth* Department of Mechanical Engineering and Materials Science, Duke University, Durham, NC 27708-0300,
Quantitative Analysis of Forces in Cells
 Quantitative Analysis of Forces in Cells Anders Carlsson Washington University in St Louis Basic properties of forces in cells Measurement methods and magnitudes of particular types of forces: Polymerization
Quantitative Analysis of Forces in Cells Anders Carlsson Washington University in St Louis Basic properties of forces in cells Measurement methods and magnitudes of particular types of forces: Polymerization
Intracellular transport
 Transport in cells Intracellular transport introduction: transport in cells, molecular players etc. cooperation of motors, forces good and bad transport regulation, traffic issues, Stefan Klumpp image
Transport in cells Intracellular transport introduction: transport in cells, molecular players etc. cooperation of motors, forces good and bad transport regulation, traffic issues, Stefan Klumpp image
Dynamic Competition between Catch and Slip Bonds in Selectins Bound to Ligands
 J. Phys. Chem. B 2006, 110, 26403-26412 26403 Dynamic Competition between Catch and Slip Bonds in Selectins Bound to Ligands V. Barsegov*, and D. Thirumalai*, Department of Chemistry, UniVersity of Massachusetts
J. Phys. Chem. B 2006, 110, 26403-26412 26403 Dynamic Competition between Catch and Slip Bonds in Selectins Bound to Ligands V. Barsegov*, and D. Thirumalai*, Department of Chemistry, UniVersity of Massachusetts
Monte Carlo simulations of rigid biopolymer growth processes
 THE JOURNAL OF CHEMICAL PHYSICS 123, 124902 2005 Monte Carlo simulations of rigid biopolymer growth processes Jenny Son and G. Orkoulas a Department of Chemical and Biomolecular Engineering, University
THE JOURNAL OF CHEMICAL PHYSICS 123, 124902 2005 Monte Carlo simulations of rigid biopolymer growth processes Jenny Son and G. Orkoulas a Department of Chemical and Biomolecular Engineering, University
Vesicle micro-hydrodynamics
 Vesicle micro-hydrodynamics Petia M. Vlahovska Max-Planck Institute of Colloids and Interfaces, Theory Division CM06 workshop I Membrane Protein Science and Engineering IPAM, UCLA, 27 march 2006 future
Vesicle micro-hydrodynamics Petia M. Vlahovska Max-Planck Institute of Colloids and Interfaces, Theory Division CM06 workshop I Membrane Protein Science and Engineering IPAM, UCLA, 27 march 2006 future
Polymerization and force generation
 Polymerization and force generation by Eric Cytrynbaum April 8, 2008 Equilibrium polymer in a box An equilibrium polymer is a polymer has no source of extraneous energy available to it. This does not mean
Polymerization and force generation by Eric Cytrynbaum April 8, 2008 Equilibrium polymer in a box An equilibrium polymer is a polymer has no source of extraneous energy available to it. This does not mean
Friction of Extensible Strips: an Extended Shear Lag Model with Experimental Evaluation
 Friction of Extensible Strips: an Extended Shear Lag Model with Experimental Evaluation Ahmad R. Mojdehi 1, Douglas P. Holmes 2, Christopher B. Williams 3, Timothy E. Long 4, David A. Dillard 1 1 Department
Friction of Extensible Strips: an Extended Shear Lag Model with Experimental Evaluation Ahmad R. Mojdehi 1, Douglas P. Holmes 2, Christopher B. Williams 3, Timothy E. Long 4, David A. Dillard 1 1 Department
Mechanics IV: Oscillations
 Mechanics IV: Oscillations Chapter 4 of Morin covers oscillations, including damped and driven oscillators in detail. Also see chapter 10 of Kleppner and Kolenkow. For more on normal modes, see any book
Mechanics IV: Oscillations Chapter 4 of Morin covers oscillations, including damped and driven oscillators in detail. Also see chapter 10 of Kleppner and Kolenkow. For more on normal modes, see any book
20.GEM GEM4 Summer School: Cell and Molecular Biomechanics in Medicine: Cancer Summer 2007
 MIT OpenCourseWare http://ocw.mit.edu 20.GEM GEM4 Summer School: Cell and Molecular Biomechanics in Medicine: Cancer Summer 2007 For information about citing these materials or our Terms of Use, visit:
MIT OpenCourseWare http://ocw.mit.edu 20.GEM GEM4 Summer School: Cell and Molecular Biomechanics in Medicine: Cancer Summer 2007 For information about citing these materials or our Terms of Use, visit:
MULTI-SCALE COMPUTATION IN HEMODYNAMICS NARCISSE ABOU N DRI
 MULTI-SCALE COMPUTATION IN HEMODYNAMICS By NARCISSE ABOU N DRI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
MULTI-SCALE COMPUTATION IN HEMODYNAMICS By NARCISSE ABOU N DRI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
Equilibrium. the linear momentum,, of the center of mass is constant
 Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
Diffusion in Reduced Dimensions. Clemens Bechinger 2. Physikalisches Institut, Universität Stuttgart
 Diffusion in Reduced Dimensions Clemens Bechinger 2. Physikalisches Institut, Universität Stuttgart Diffusion in narrow Channels t = 0 t = t 1 3D, 2D: mixing 1D: sequence unchanged 1D diffusion entirely
Diffusion in Reduced Dimensions Clemens Bechinger 2. Physikalisches Institut, Universität Stuttgart Diffusion in narrow Channels t = 0 t = t 1 3D, 2D: mixing 1D: sequence unchanged 1D diffusion entirely
QUANTIFYING CELL-ADHESION STRENGTH WITH MICROPIPETTE MANIPULATION: PRINCIPLE AND APPLICATION
 [Frontiers in Bioscience 9, 2183-2191, September 1, 2004] QUANTIFYING CELL-ADHESION STRENGTH WITH MICROPIPETTE MANIPULATION: PRINCIPLE AND APPLICATION Jin-Yu Shao, Gang Xu and Peng Guo Department of Biomedical
[Frontiers in Bioscience 9, 2183-2191, September 1, 2004] QUANTIFYING CELL-ADHESION STRENGTH WITH MICROPIPETTE MANIPULATION: PRINCIPLE AND APPLICATION Jin-Yu Shao, Gang Xu and Peng Guo Department of Biomedical
How DLS Works: Interference of Light
 Static light scattering vs. Dynamic light scattering Static light scattering measures time-average intensities (mean square fluctuations) molecular weight radius of gyration second virial coefficient Dynamic
Static light scattering vs. Dynamic light scattering Static light scattering measures time-average intensities (mean square fluctuations) molecular weight radius of gyration second virial coefficient Dynamic
Xiaoyi Li. Advisor: Dr. Kausik Sarkar
 iaoyi Li United Technologies Research Center Advisor: Dr. Kausik Sarkar Mechanical Engineering, University of Delaware Andreas Acrivos Dissertation Award Presentation 62 nd APS DFD meeting, Minneapolis,
iaoyi Li United Technologies Research Center Advisor: Dr. Kausik Sarkar Mechanical Engineering, University of Delaware Andreas Acrivos Dissertation Award Presentation 62 nd APS DFD meeting, Minneapolis,
Minimal encounter time and separation determine ligand-receptor binding in cell adhesion SUPPLEMENTARY INFORMATION
 Minimal encounter time and separation determine ligand-receptor binding in cell adhesion SUPPLEMENTARY INFORMATION Philippe Robert Alice Nicolas Said Aranda-Espinoza Pierre Bongrand Laurent Limozin Molecular
Minimal encounter time and separation determine ligand-receptor binding in cell adhesion SUPPLEMENTARY INFORMATION Philippe Robert Alice Nicolas Said Aranda-Espinoza Pierre Bongrand Laurent Limozin Molecular
Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling
 Nature Methods Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling Prithu Sundd, Edgar Gutierrez, Maria K Pospieszalska, Hong Zhang, Alexander Groisman & Klaus Ley Supplementary
Nature Methods Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling Prithu Sundd, Edgar Gutierrez, Maria K Pospieszalska, Hong Zhang, Alexander Groisman & Klaus Ley Supplementary
Intensity (a.u.) Intensity (a.u.) Raman Shift (cm -1 ) Oxygen plasma. 6 cm. 9 cm. 1mm. Single-layer graphene sheet. 10mm. 14 cm
 Intensity (a.u.) Intensity (a.u.) a Oxygen plasma b 6 cm 1mm 10mm Single-layer graphene sheet 14 cm 9 cm Flipped Si/SiO 2 Patterned chip Plasma-cleaned glass slides c d After 1 sec normal Oxygen plasma
Intensity (a.u.) Intensity (a.u.) a Oxygen plasma b 6 cm 1mm 10mm Single-layer graphene sheet 14 cm 9 cm Flipped Si/SiO 2 Patterned chip Plasma-cleaned glass slides c d After 1 sec normal Oxygen plasma
Supplemental Information - Glassy Dynamics in Composite Biopolymer Networks
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2018 Supplemental Information - Glassy Dynamics in Composite Biopolymer Networks Tom Golde, 1 Constantin
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2018 Supplemental Information - Glassy Dynamics in Composite Biopolymer Networks Tom Golde, 1 Constantin
BME 419/519 Hernandez 2002
 Vascular Biology 2 - Hemodynamics A. Flow relationships : some basic definitions Q v = A v = velocity, Q = flow rate A = cross sectional area Ohm s Law for fluids: Flow is driven by a pressure gradient
Vascular Biology 2 - Hemodynamics A. Flow relationships : some basic definitions Q v = A v = velocity, Q = flow rate A = cross sectional area Ohm s Law for fluids: Flow is driven by a pressure gradient
Dissipative Particle Dynamics: Foundation, Evolution and Applications
 Dissipative Particle Dynamics: Foundation, Evolution and Applications Lecture 4: DPD in soft matter and polymeric applications George Em Karniadakis Division of Applied Mathematics, Brown University &
Dissipative Particle Dynamics: Foundation, Evolution and Applications Lecture 4: DPD in soft matter and polymeric applications George Em Karniadakis Division of Applied Mathematics, Brown University &
Physics 141 Rotational Motion 2 Page 1. Rotational Motion 2
 Physics 141 Rotational Motion 2 Page 1 Rotational Motion 2 Right handers, go over there, left handers over here. The rest of you, come with me.! Yogi Berra Torque Motion of a rigid body, like motion of
Physics 141 Rotational Motion 2 Page 1 Rotational Motion 2 Right handers, go over there, left handers over here. The rest of you, come with me.! Yogi Berra Torque Motion of a rigid body, like motion of
Supplementary Figures:
 Supplementary Figures: Supplementary Figure 1: Simulations with t(r) 1. (a) Snapshots of a quasi- 2D actomyosin droplet crawling along the treadmilling direction (to the right in the picture). There is
Supplementary Figures: Supplementary Figure 1: Simulations with t(r) 1. (a) Snapshots of a quasi- 2D actomyosin droplet crawling along the treadmilling direction (to the right in the picture). There is
Polymer dynamics. Course M6 Lecture 5 26/1/2004 (JAE) 5.1 Introduction. Diffusion of polymers in melts and dilute solution.
 Course M6 Lecture 5 6//004 Polymer dynamics Diffusion of polymers in melts and dilute solution Dr James Elliott 5. Introduction So far, we have considered the static configurations and morphologies of
Course M6 Lecture 5 6//004 Polymer dynamics Diffusion of polymers in melts and dilute solution Dr James Elliott 5. Introduction So far, we have considered the static configurations and morphologies of
Chapter 1. Basic Concepts. 1.1 Trajectories
 Chapter 1 Basic Concepts 1.1 Trajectories We shall be concerned in this course with the motion of particles. Larger bodies will (with a few exceptions) be made up of collections of particles. We will find
Chapter 1 Basic Concepts 1.1 Trajectories We shall be concerned in this course with the motion of particles. Larger bodies will (with a few exceptions) be made up of collections of particles. We will find
A Model for Integrin Binding in Cells
 A Model for Integrin Binding in Cells By: Kara Huyett Advisor: Doctor Stolarska 31 st of August, 2015 Cell movement, and cell crawling in particular, has implications in various biological phenomena. For
A Model for Integrin Binding in Cells By: Kara Huyett Advisor: Doctor Stolarska 31 st of August, 2015 Cell movement, and cell crawling in particular, has implications in various biological phenomena. For
Anatoly B. Kolomeisky Department of Chemistry Center for Theoretical Biological Physics How to Understand Molecular Transport through Channels: The
 Anatoly B. Kolomeisy Department of Chemistry Center for Theoretical Biological Physics How to Understand Molecular Transport through Channels: The Role of Interactions Transport Through Channels Oil pumping
Anatoly B. Kolomeisy Department of Chemistry Center for Theoretical Biological Physics How to Understand Molecular Transport through Channels: The Role of Interactions Transport Through Channels Oil pumping
COMPLEX FLOW OF NANOCONFINED POLYMERS
 COMPLEX FLOW OF NANOCONFINED POLYMERS Connie B. Roth, Chris A. Murray and John R. Dutcher Department of Physics University of Guelph Guelph, Ontario, Canada N1G 2W1 OUTLINE instabilities in freely-standing
COMPLEX FLOW OF NANOCONFINED POLYMERS Connie B. Roth, Chris A. Murray and John R. Dutcher Department of Physics University of Guelph Guelph, Ontario, Canada N1G 2W1 OUTLINE instabilities in freely-standing
Scanning Force Microscopy. i.e. Atomic Force Microscopy. Josef A. Käs Institute for Soft Matter Physics Physics Department. Atomic Force Microscopy
 Scanning Force Microscopy i.e. Atomic Force Microscopy Josef A. Käs Institute for Soft Matter Physics Physics Department Atomic Force Microscopy Single Molecule Force Spectroscopy Zanjan 4 Hermann E. Gaub
Scanning Force Microscopy i.e. Atomic Force Microscopy Josef A. Käs Institute for Soft Matter Physics Physics Department Atomic Force Microscopy Single Molecule Force Spectroscopy Zanjan 4 Hermann E. Gaub
Chapter 4 Bidirectional membrane tubes driven by nonprocessive motors
 Chapter 4 Bidirectional membrane tubes driven by nonprocessive motors In cells, membrane tubes are extracted by molecular motors. Although individual motors cannot provide enough force to pull a tube,
Chapter 4 Bidirectional membrane tubes driven by nonprocessive motors In cells, membrane tubes are extracted by molecular motors. Although individual motors cannot provide enough force to pull a tube,
Supplementary Information
 Supplementary Information Switching of myosin-v motion between the lever-arm swing and Brownian search-and-catch Keisuke Fujita 1*, Mitsuhiro Iwaki 2,3*, Atsuko H. Iwane 1, Lorenzo Marcucci 1 & Toshio
Supplementary Information Switching of myosin-v motion between the lever-arm swing and Brownian search-and-catch Keisuke Fujita 1*, Mitsuhiro Iwaki 2,3*, Atsuko H. Iwane 1, Lorenzo Marcucci 1 & Toshio
Chapter 26 Elastic Properties of Materials
 Chapter 26 Elastic Properties of Materials 26.1 Introduction... 1 26.2 Stress and Strain in Tension and Compression... 2 26.3 Shear Stress and Strain... 4 Example 26.1: Stretched wire... 5 26.4 Elastic
Chapter 26 Elastic Properties of Materials 26.1 Introduction... 1 26.2 Stress and Strain in Tension and Compression... 2 26.3 Shear Stress and Strain... 4 Example 26.1: Stretched wire... 5 26.4 Elastic
Medical Biophysics II. Final exam theoretical questions 2013.
 Medical Biophysics II. Final exam theoretical questions 2013. 1. Early atomic models. Rutherford-experiment. Franck-Hertz experiment. Bohr model of atom. 2. Quantum mechanical atomic model. Quantum numbers.
Medical Biophysics II. Final exam theoretical questions 2013. 1. Early atomic models. Rutherford-experiment. Franck-Hertz experiment. Bohr model of atom. 2. Quantum mechanical atomic model. Quantum numbers.
For an imposed stress history consisting of a rapidly applied step-function jump in
 Problem 2 (20 points) MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING CAMBRIDGE, MASSACHUSETTS 0239 2.002 MECHANICS AND MATERIALS II SOLUTION for QUIZ NO. October 5, 2003 For
Problem 2 (20 points) MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING CAMBRIDGE, MASSACHUSETTS 0239 2.002 MECHANICS AND MATERIALS II SOLUTION for QUIZ NO. October 5, 2003 For
2.3 Damping, phases and all that
 2.3. DAMPING, PHASES AND ALL THAT 107 2.3 Damping, phases and all that If we imagine taking our idealized mass on a spring and dunking it in water or, more dramatically, in molasses), then there will be
2.3. DAMPING, PHASES AND ALL THAT 107 2.3 Damping, phases and all that If we imagine taking our idealized mass on a spring and dunking it in water or, more dramatically, in molasses), then there will be
Randomly Triangulated Surfaces as Models for Fluid and Crystalline Membranes. G. Gompper Institut für Festkörperforschung, Forschungszentrum Jülich
 Randomly Triangulated Surfaces as Models for Fluid and Crystalline Membranes G. Gompper Institut für Festkörperforschung, Forschungszentrum Jülich Motivation: Endo- and Exocytosis Membrane transport of
Randomly Triangulated Surfaces as Models for Fluid and Crystalline Membranes G. Gompper Institut für Festkörperforschung, Forschungszentrum Jülich Motivation: Endo- and Exocytosis Membrane transport of
CPGAN # 006. The Basics of Filament Stretching Rheometry
 Introduction Measurement of the elongational behavior of fluids is important both for basic research purposes and in industrial applications, since many complex flows contain strong extensional components,
Introduction Measurement of the elongational behavior of fluids is important both for basic research purposes and in industrial applications, since many complex flows contain strong extensional components,
Macromolecular Crowding
 Macromolecular Crowding Keng-Hwee Chiam Mathematical and Theoretical Biology Group Goodsell (1994) Macromolecular Crowding, Oct. 15, 2003 p.1/33 Outline What: introduction, definition Why: implications
Macromolecular Crowding Keng-Hwee Chiam Mathematical and Theoretical Biology Group Goodsell (1994) Macromolecular Crowding, Oct. 15, 2003 p.1/33 Outline What: introduction, definition Why: implications
Structural investigation of single biomolecules
 Structural investigation of single biomolecules NMR spectroscopy and X-ray crystallography are currently the most common techniques capable of determining the structures of biological macromolecules like
Structural investigation of single biomolecules NMR spectroscopy and X-ray crystallography are currently the most common techniques capable of determining the structures of biological macromolecules like
Graduate Institute t of fanatomy and Cell Biology
 Cell Adhesion 黃敏銓 mchuang@ntu.edu.tw Graduate Institute t of fanatomy and Cell Biology 1 Cell-Cell Adhesion and Cell-Matrix Adhesion actin filaments adhesion belt (cadherins) cadherin Ig CAMs integrin
Cell Adhesion 黃敏銓 mchuang@ntu.edu.tw Graduate Institute t of fanatomy and Cell Biology 1 Cell-Cell Adhesion and Cell-Matrix Adhesion actin filaments adhesion belt (cadherins) cadherin Ig CAMs integrin
Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding
 Supplementary information Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding Marcus Braun* 1,2,3, Zdenek Lansky* 1,2,3, Agata Szuba 1,2, Friedrich W. Schwarz 1,2, Aniruddha
Supplementary information Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding Marcus Braun* 1,2,3, Zdenek Lansky* 1,2,3, Agata Szuba 1,2, Friedrich W. Schwarz 1,2, Aniruddha
Stability Analysis of Micropipette Aspiration of Neutrophils
 Biophysical Journal Volume 79 July 2000 153 162 153 Stability Analysis of Micropipette Aspiration of Neutrophils Jure Derganc,* Bojan Božič,* Saša Svetina,* and Boštjan Žekš* *Institute of Biophysics,
Biophysical Journal Volume 79 July 2000 153 162 153 Stability Analysis of Micropipette Aspiration of Neutrophils Jure Derganc,* Bojan Božič,* Saša Svetina,* and Boštjan Žekš* *Institute of Biophysics,
Cytoskeleton dynamics simulation of the red blood cell
 1 Cytoskeleton dynamics simulation of the red blood cell Ju Li Collaborators: Subra Suresh, Ming Dao, George Lykotrafitis, Chwee-Teck Lim Optical tweezers stretching of healthy human red blood cell 2 Malaria
1 Cytoskeleton dynamics simulation of the red blood cell Ju Li Collaborators: Subra Suresh, Ming Dao, George Lykotrafitis, Chwee-Teck Lim Optical tweezers stretching of healthy human red blood cell 2 Malaria
Micro-Rheology Measurements with the NanoTracker
 Micro-Rheology Measurements with the NanoTracker JPK s NanoTracker optical tweezers system is a versatile high resolution force measurement tool. It is based on the principle of optical trapping and uses
Micro-Rheology Measurements with the NanoTracker JPK s NanoTracker optical tweezers system is a versatile high resolution force measurement tool. It is based on the principle of optical trapping and uses
Adaptive Response of Actin Bundles under Mechanical Stress
 Biophysical Journal, Volume 113 Supplemental Information Adaptive Response of Actin Bundles under Mechanical Stress Florian Rückerl, Martin Lenz, Timo Betz, John Manzi, Jean-Louis Martiel, Mahassine Safouane,
Biophysical Journal, Volume 113 Supplemental Information Adaptive Response of Actin Bundles under Mechanical Stress Florian Rückerl, Martin Lenz, Timo Betz, John Manzi, Jean-Louis Martiel, Mahassine Safouane,
Introduction to Continuous Systems. Continuous Systems. Strings, Torsional Rods and Beams.
 Outline of Continuous Systems. Introduction to Continuous Systems. Continuous Systems. Strings, Torsional Rods and Beams. Vibrations of Flexible Strings. Torsional Vibration of Rods. Bernoulli-Euler Beams.
Outline of Continuous Systems. Introduction to Continuous Systems. Continuous Systems. Strings, Torsional Rods and Beams. Vibrations of Flexible Strings. Torsional Vibration of Rods. Bernoulli-Euler Beams.
Dynamics of Solitary Waves Induced by Shock Impulses in a Linear Atomic Chain*
 Dynamics of Solitary Waves Induced by Shock Impulses in a Linear Atomic Chain* PHUOC X. TRAN, DONALD W. BRENNER, and C. T. WHITE Naval Research Laboratory, Washington, DC 20375-5000 Abstract The propagation
Dynamics of Solitary Waves Induced by Shock Impulses in a Linear Atomic Chain* PHUOC X. TRAN, DONALD W. BRENNER, and C. T. WHITE Naval Research Laboratory, Washington, DC 20375-5000 Abstract The propagation
PHYSICS 1 Simple Harmonic Motion
 Advanced Placement PHYSICS 1 Simple Harmonic Motion Student 014-015 What I Absolutely Have to Know to Survive the AP* Exam Whenever the acceleration of an object is proportional to its displacement and
Advanced Placement PHYSICS 1 Simple Harmonic Motion Student 014-015 What I Absolutely Have to Know to Survive the AP* Exam Whenever the acceleration of an object is proportional to its displacement and
On the stall force for growing microtubules
 Eur Biophys J (2000) 29: 2±6 Ó Springer-Verlag 2000 ARTICLE G. Sander van Doorn á Catalin Tanase á Bela M. Mulder Marileen Dogterom On the stall force for growing microtubules Received: 27 September 1999
Eur Biophys J (2000) 29: 2±6 Ó Springer-Verlag 2000 ARTICLE G. Sander van Doorn á Catalin Tanase á Bela M. Mulder Marileen Dogterom On the stall force for growing microtubules Received: 27 September 1999
MATERIALS. Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle?
 MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
The dynamics of small particles whose size is roughly 1 µmt or. smaller, in a fluid at room temperature, is extremely erratic, and is
 1 I. BROWNIAN MOTION The dynamics of small particles whose size is roughly 1 µmt or smaller, in a fluid at room temperature, is extremely erratic, and is called Brownian motion. The velocity of such particles
1 I. BROWNIAN MOTION The dynamics of small particles whose size is roughly 1 µmt or smaller, in a fluid at room temperature, is extremely erratic, and is called Brownian motion. The velocity of such particles
Available online at ScienceDirect. Procedia IUTAM 13 (2015 ) 82 89
 Available online at www.sciencedirect.com ScienceDirect Procedia IUTAM 13 (215 ) 82 89 IUTAM Symposium on Dynamical Analysis of Multibody Systems with Design Uncertainties The importance of imperfections
Available online at www.sciencedirect.com ScienceDirect Procedia IUTAM 13 (215 ) 82 89 IUTAM Symposium on Dynamical Analysis of Multibody Systems with Design Uncertainties The importance of imperfections
Magnetic tweezers and its application to DNA mechanics
 Biotechnological Center Research group DNA motors (Seidel group) Handout for Practical Course Magnetic tweezers and its application to DNA mechanics When: 9.00 am Where: Biotec, 3 rd Level, Room 317 Tutors:
Biotechnological Center Research group DNA motors (Seidel group) Handout for Practical Course Magnetic tweezers and its application to DNA mechanics When: 9.00 am Where: Biotec, 3 rd Level, Room 317 Tutors:
On the shape memory of red blood cells
 On the shape memory of red blood cells DANIEL CORDASCO AND PROSENJIT BAGCHI # Mechanical and Aerospace Engineering Department Rutgers, The State University of New Jersey Piscataway, NJ 08854, USA # Corresponding
On the shape memory of red blood cells DANIEL CORDASCO AND PROSENJIT BAGCHI # Mechanical and Aerospace Engineering Department Rutgers, The State University of New Jersey Piscataway, NJ 08854, USA # Corresponding
PROTEIN FLEXIBILITY AND THE CORRELATION RATCHET
 SIAM J. APPL. MATH. Vol. 61, No. 3, pp. 776 791 c 2 Society for Industrial and Applied Mathematics PROTEIN FLEXIBILITY AND THE CORRELATION RATCHET TIMOTHY C. ELSTON, DWIGHT YOU, AND CHARLES S. PESKIN Abstract.
SIAM J. APPL. MATH. Vol. 61, No. 3, pp. 776 791 c 2 Society for Industrial and Applied Mathematics PROTEIN FLEXIBILITY AND THE CORRELATION RATCHET TIMOTHY C. ELSTON, DWIGHT YOU, AND CHARLES S. PESKIN Abstract.
Johns Hopkins University What is Engineering? M. Karweit MATERIALS
 Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
Instabilities in Thin Polymer Films: From Pattern Formation to Rupture
 Instabilities in Thin Polymer Films: From Pattern Formation to Rupture John R. Dutcher*, Kari Dalnoki-Veress Η, Bernie G. Nickel and Connie B. Roth Department of Physics, University of Guelph, Guelph,
Instabilities in Thin Polymer Films: From Pattern Formation to Rupture John R. Dutcher*, Kari Dalnoki-Veress Η, Bernie G. Nickel and Connie B. Roth Department of Physics, University of Guelph, Guelph,
BRIEF COMMUNICATION TO SURFACES ANALYSIS OF ADHESION OF LARGE VESICLES
 BRIEF COMMUNICATION ANALYSIS OF ADHESION OF LARGE VESICLES TO SURFACES EVAN A. EVANS, Department ofbiomedical Engineering, Duke University, Durham, North Carolina 27706 U.S.A. ABSTRACT An experimental
BRIEF COMMUNICATION ANALYSIS OF ADHESION OF LARGE VESICLES TO SURFACES EVAN A. EVANS, Department ofbiomedical Engineering, Duke University, Durham, North Carolina 27706 U.S.A. ABSTRACT An experimental
Contents. Dynamics and control of mechanical systems. Focus on
 Dynamics and control of mechanical systems Date Day 1 (01/08) Day 2 (03/08) Day 3 (05/08) Day 4 (07/08) Day 5 (09/08) Day 6 (11/08) Content Review of the basics of mechanics. Kinematics of rigid bodies
Dynamics and control of mechanical systems Date Day 1 (01/08) Day 2 (03/08) Day 3 (05/08) Day 4 (07/08) Day 5 (09/08) Day 6 (11/08) Content Review of the basics of mechanics. Kinematics of rigid bodies
Bond tilting and sliding friction in a model of cell adhesion
 Bond tilting and sliding friction in a model of cell adhesion By S. Reboux, G. Richardson and O. E. Jensen School of Mathematical Sciences, University of Nottingham, University Park, Nottingham, NG7 2RD,
Bond tilting and sliding friction in a model of cell adhesion By S. Reboux, G. Richardson and O. E. Jensen School of Mathematical Sciences, University of Nottingham, University Park, Nottingham, NG7 2RD,
MOLECULAR, CELLULAR, & TISSUE BIOMECHANICS
 MOLECULAR, CELLULAR, & TISSUE BIOMECHANICS Spring 2015 Problem Set #6 - Cytoskeleton mechanics Distributed: Wednesday, April 15, 2015 Due: Thursday, April 23, 2015 Problem 1: Transmigration A critical
MOLECULAR, CELLULAR, & TISSUE BIOMECHANICS Spring 2015 Problem Set #6 - Cytoskeleton mechanics Distributed: Wednesday, April 15, 2015 Due: Thursday, April 23, 2015 Problem 1: Transmigration A critical
Effect of protein shape on multibody interactions between membrane inclusions
 PHYSICAL REVIEW E VOLUME 61, NUMBER 4 APRIL 000 Effect of protein shape on multibody interactions between membrane inclusions K. S. Kim, 1, * John Neu, and George Oster 3, 1 Department of Physics, Graduate
PHYSICAL REVIEW E VOLUME 61, NUMBER 4 APRIL 000 Effect of protein shape on multibody interactions between membrane inclusions K. S. Kim, 1, * John Neu, and George Oster 3, 1 Department of Physics, Graduate
PHYSICAL REVIEW LETTERS
 PHYSICAL REVIEW LETTERS VOLUME 80 1 JUNE 1998 NUMBER 22 Field-Induced Stabilization of Activation Processes N. G. Stocks* and R. Mannella Dipartimento di Fisica, Università di Pisa, and Istituto Nazionale
PHYSICAL REVIEW LETTERS VOLUME 80 1 JUNE 1998 NUMBER 22 Field-Induced Stabilization of Activation Processes N. G. Stocks* and R. Mannella Dipartimento di Fisica, Università di Pisa, and Istituto Nazionale
