Spin-orbit coupling and chirality in carbon nanotubes Master Thesis. Frederik Treue Supervisor: Karten Flensberg
|
|
|
- Jennifer Bradley
- 5 years ago
- Views:
Transcription
1 Spin-orbit coupling and chirality in carbon nanotubes Master Thesis Frederik Treue Supervisor: Karten Flensberg April, 1
2 ii
3 Contents 1 Introduction Carbon nanotubes and quantum computers Organization of the thesis Graphene 5.1 Basic definitions Symmetry of graphene Bloch s theorem for graphene Folding up the nanotube 1.1 Chirality and unitcell of the CNT The helical unitcell The translational unitcell Comparing the unitcells Cyclic boundary conditions. Metallic and semiconducting nanotubes σ/π band mixing π π orbital coupling σ π orbital coupling σ σ orbital coupling Selfenergy of the σ orbitals Solving the full CNT hamiltonian Spin orbit interaction in CNTs Basics of the spin-orbit coupling The atomic hamiltonian The pertubative hamiltonian The basic problem The effective spin-orbit/curvature perturbation calculation of Ĥ The pertubative hamiltonian iii
4 CONTENTS 6 Izumidas derivation Their coordinatesystem The effective hamiltonian The ĤCV hamiltonian Ĥ SO The graphene hamiltonian Ĥ Ĥ () SOCV and Ĥ() CV CV Magnetic field Zeeman effect The Aharonov-Bohm effect Numerical results Nummerical Hückel calculations The extended Hückel method Semi-heuristic argument for EHT EHT for magnetic field and spin-orbit coupling A more efficient method Conclusion 91 A σ σ coupling elements 9 B Explicit H so matrix 95 B.1 Explicit H so matrix C Oddness of derivative operator 97 D Electromagnetic field in quantummechanics. Canonical and Kinematic impulse 99 iv
5 Chapter 1 Introduction In this first section, I aim to present my personal entry point to the study of carbon nanotubes (CNTs), ie. the reason why I think this particular systems are of any interests. I then outline the thesis organization, ie. present an overview of the chapters. 1.1 Carbon nanotubes and quantum computers In a standard classical computer, every piece of data is represented by a vector of bits which can each take the value of or 1. In the first part of the last century it was shown by Alan Turing and others, that any algorithm could be implemented by repeated application of the NAND gate to this vector. However, in 198, Richard Feynman sparked a new field inside physics, mathematics and computer science, when he pointed out that while classical computers can indeed carry out any algorithm, only a subset of the computational operations made available by the Schrodinger equation [] are described by the classical gates such as the NAND gate. In quantum physics we know that states need not be in either one state or another, but can be in a superposition of eg. state and state 1, and, more importantly, we know that different states may be entangled with each other, eg. the state Ψ = 1 ( ) where each state depends on the other state, so that if a measurement is made on the first state, and it is found to be in the position, the other one immediately shifts from being in a superposition of and 1 to being in position 1 with unit certainty. Now, we may imagine having a vector of N such states, or qubits 1, and preparing them in a superposition of all N possible (classical) states, ie. if we use n to denote the state where the qubits in some given ordering is the binary representation of the number n, we may 1 N prepare the state n n. Now, if we have an algorithm that we wish to evaluate, one way of formally characterizing the quantum computation (due to Kitaev[]), is to note we must have some physical system that implement a time development operator U, such that for a given input vector i, we have that o = U i is the desired output, for any input vector i - such an operation have been shown to be implementable with an array consisting of a number of quantum gates, 1 QUantum BITS 1
6 CHAPTER 1. INTRODUCTION each of which is one of basic 1 or qubit operations, reminiscent of the NAND gate mentioned above, shown in e.g. [4]. Combining these two things, U and Ψ, we find that we can generate a superposition of all possible output states of the system, since U Ψ = U 1 N n n = 1 N n o n where o n is defined as the algorithms output when given input n. Since the U operation was used only once, the time needed for this calculation is the same as the time needed to calculate the result for only one input state, and so this principle have been named quantum parallelism - however if we try to measure the result of the calculation, the wavefunction will collapse and we will receive only one of the answers, so it was not clear if this scheme provides any advantages over the classical computer, but through the work of e.g. Deutch[5],Grover[6] and especially Shor[7], quantum algorithms with significant speedup over classical computation was discovered, some of which also had significance for the society at large. The theory of quantum computation have developed much in its approx. year long existence, however the implementation of these ideas in actual physical systems have proven to be a formidable challenge in its own respect. There are some fundamental reasons for this, but it all comes down to 5 demands made of a quantum computer, some of which seems mutually exclusive. To qualify as a physical system for quantum computation, a system must have 1. Strong controllable interaction, providing a handle, so we can set the qubit and interact with it.. Weak interaction with surroundings, so that when we leave the system, the data contained in the qubit is preserved.. Readout: We need a way to measure the state of a qubit once calculation is done. 4. Scalability: One qubit is not (very) interesting - we need easy way to expand the number of qubits for larger calculations 5. A way to make two qubits interact in a controlled way. So far, probably the most successful implementation of this has been the iontrap quantum computer, first proposed by [1]. However, it is at its core an atomic physics implementation, meaning that all operations is carried out on individual ions in a single trap, making scalability the major problem of this implementation. As an alternative route to the quantum computer, solid state implementations have been considered, since it may be thought that the scalability will be easier here, as has been seen for classical computers. However, here one runs into the problem that most physical variables is strongly coupled to the surroundings in for instance crystals: The charge of a given area is eg. coupled by the strong Coulomb potentials of nearby charges - thus, what we need is a variable that is weakly coupled with the environment. The spin of an (unpaired) electron of a spin- atom (to avoid hyperfine interaction) is a potentially good candidate, since the spin is typically relatively weakly interacting with the environment compared to e.g. the charge, although there are also problems with speed: By design, the quantum computer will never get far above 1 4 quantum gates pr. second
7 1.. ORGANIZATION OF THE THESIS thus fulfilling condition above, but we then need a strong interaction to control the qubit: The magnetic field is an obvious candidate, but changing magnetic field is relatively slow compared to changing electric fields, and thus one could search for a system where one has electric control of the spin. One possible candidate for such a system is carbon nanotubes: They can, on one hand, be made using only C-1 atoms, which have nuclear spin, and since the spin-orbit coupling here gives a physical interaction between the motion around the circumference of the nanotube and the spin of the electrons[14], it could thus be hoped, that by electrically controlling the orbital motion of the electrons, one could, in a controlled way, alter their spin, thus fulfilling condition 1 above. Around the beginning of the work on this thesis, an article showing new, relevant experimental data was published [8] where several unexpected features due to spin-orbit coupling in carbon nanotubes were observed. This prompted us to attempt a more thorough examination of the effects of spin-orbit coupling in carbon nanotubes, which was found independently by us, has a highly nontrivial correlation with the so-called chirality of the nanotube, which is a measure of the specific geometry of a given carbon nanotube. 1. Organization of the thesis This thesis is roughly organized as a gradually more advanced description of the physical effects in a carbon nanotube, each chapter adding a new part to the hamiltonian of the system: The first chapter concerns graphene, which forms the basis of the description of the carbon nanotubes, since these may be thought of as rolled up sheets of graphene. Then follows a chapter describing the effects of the cylindrical like topology of the carbon nanotube. Then we describe the effect of spin-orbit coupling in a nanotube. In chapter 5 we describe the first of the results from a numerical simulation of the system, and explain it, using a pertubative approach. As an interlude, chapter 6 describes another derivation, reaching the same principal result as us, but with a slightly different, and perhaps more elegant, way of deriving it. Then follows a chapter on the effect of applying a magnetic field along the tube axis direction, where we explain some further numerical results. Finally, a chapter describes the numerical algorithm used to generate the numerical data in this thesis.
8 CHAPTER 1. INTRODUCTION 4
9 Chapter Graphene In order to solve the graphene system, we shall employ Bloch s theorem, so we assume that we may deal with an infinite sheet of graphene. We use a tight-binding approximation, ie. we shall assume that the electron wavefunctions can be described as linear combinations of atomic orbitals, also known as the LCAO approximation, so we shall ignore e.g. ionized basis functions - this follows the literature standard for solving graphene, which was first developed for graphite by []. First of all, however, we must define the unitcell to be used in these calculations, along with a few other important basics..1 Basic definitions In figure.1 we see the honeycomb lattice of graphene. The first thing one notices (or remembers from basic solid state physics) is that the honeycomb lattice is not a bravais lattice, but rather, composed by two sub(bravais)lattices or in other words, the primitive unitcell will contain two atoms, and thus the two vectors, a 1 and a in figure.1 are chosen to be our lattice vectors, and, defining the latticeconstant (the length of the lattice vectors), as a, we find through simple geometry that ( a 1 = a 1 ) ( a = a 1 From these, it is easy to see that the reciprocal lattice will be spanned by the two vectors: ) (.1) b 1 = π a ( 1 1 ) b = π a ( 1 1 ) (.) Notice also that from the real lattice, we may gather that the distance between neighboring atoms is a cc a, and since the lattice constant is approximately a =.46 Å[] we find that a cc = 1.4Å. 5
10 CHAPTER. GRAPHENE τ τ 1 τ a 1 y a x Figure.1: The crystal structure of graphene. The red(blue) dots denotes carbon atoms on sublattice A(B) and the lines denote the σ-orbital bonds between the atoms. Note, that with the coordinatesystem shown, the σ orbitals presented in the text denotes the orbitals on the A sublattice sites.. Symmetry of graphene In principle, one could now start out with the full hamiltonian for the graphene system, including all 6 electrons (pr. atom). However, a few assumptions vastly decreases the work required. First, we shall assume that the 1s electrons are so tightly bound to the carbon nuclei that they are too far down in the Fermi sea to play any role, reducing our problem to 4 electrons pr. atom. Now, in graphene, the symmetry helps reduce this even further 1 : From figure.1 it is obvious that there is a threefold rotation symmetry around each atom, and therefore the crystal field part of the full hamiltonian will also have this symmetry, and consequently it will be easier to solve the system if we can construct atomic orbitals that have this symmetry inbuilt and use them, instead of the usual continuously rotationally symmetric nlm (hydrogenic) functions. This is indeed possible: we define the z direction to be perpendicular to the graphene plane, and start out from the wellknown three, realvalued orbitals lying along the axis s, build from the standard nlm as follows: p x = 1 ( ) p y = i ( ) p z = 1 (.) and the remaining s shell. First we see that the p z shell, since it is invariant under any rotation around the z axis, immediately fulfills the crystal symmetry, and second we make the linear 1 note, however, that this relies on the planar geometry of the graphene sheet - things become somewhat more involved once we consider the cylindrical CNTs 6
11 .. BLOCH S THEOREM FOR GRAPHENE Figure.: The isoprobability curves of the first sigma orbitals. The nucleus is at (,) and the units are Ångstrøm, and the neighboring atom would thus be at (1.4,) combinations, known as the σ orbitals σ 1 = 1 s + p x σ = 1 1 s 6 p x + 1 p y σ = 1 1 s 6 p x 1 p y (.4) The isoprobability contours of the σ 1 orbital has been shown in figure. - We note that the orbitals evidently rotate onto one another under the fold rotation we are considering. In graphene, the crystal field clearly causes the sp orbitals to have a lower energy than the p z bands, since the sp orbitals get closer to the surrounding, positive nuclei. Therefore out of the 4 electrons in the outer shell of carbon will fall into these orbitals, and form bonds with the surrounding atoms, holding the crystal structure together. The fourth electron could in principle fall into these orbitals as well, but that would cost a large amount of coulomb energy, since they are all halffilled, and so we may assume that it goes into the energetically less favorable, but free, p z orbital, also known as the π orbital. Since these do not form any bonds (graphene is pr. definition a monolayer crystal), it is appropriate to use Bloch s theorem for these electrons, identifying the crystal lattice as the periodic potential.. Bloch s theorem for graphene From basic solid state physics, we know that the eigenfunctions of the electrons (Ψ kn (r)) in a periodic potential, U(r), can be chosen to be blochwaves, that is Ψ nk (r) = e ik r u nk (r) (.5) 7
12 CHAPTER. GRAPHENE where n refers to the band number and u nk (r) has the same periodicity as the crystal potential, U(r), so they fulfill the Bloch condition Ψ nk (r + R) = e ik r+r u nk (r + R) = e ik R e ik r u nk (r) = e ik R Ψ nk (r) (.6) where R is a lattice vector. We now wish to couple this to the atomic orbitals described above, in particular to the π orbitals at each atomic site, since this will put us in a position to use the standard LCAO (Linear Combination of Atomic Orbitals) machinery. This is done by first noting that we may make an inverse Fourier transform of any Bloch wavefunction, that is, any function fulfilling (.6), since it is periodic in the reciprocal lattice. So Ψ nk (r) = R e ik R φ n (r,r) (.7) Now, if we can establish that the φ n functions may be represented by atomic orbitals, we will be ready to solve the graphene problem. This will eventually rely on an approximation, but we may make some rigorous progress using the standard Wannier decomposition method, as in chapter 1 of [9], by noting that by virtue of the mechanics of the Fourier transform, we know that φ n (r,r) = 1 e ir k Ψ nk (r) (.8) A k where A is as usual, the area of the graphene unitcell. From this we may see that the φ n (r,r) may be viewed as molecular orbitals on each unitcell, since they may be expressed as a function solely of the difference between r and R, which is seen by recalling that Ψ nk fulfills (.6) and thus, φ n (r,r) = φ n (r + R l,r + R l ) where R l is a lattice vector, and thus the solution to the problem can be viewed as the φ n (r,r) functions placed at each unitcell of the crystal, with the appropriate Bloch phase. Now we make the LCAO assumption that these φ n functions may be expressed as linear combinations of the atomic orbitals present in the unitcell, thus solving two problems with one stroke, namely the problem of connecting the Bloch waves to the atomic orbitals and the complication of the diatomic unitcell, since the molecular orbitals φ n is now assumed to be linear combinations of atomic orbitals, so that, inserting into (.7), we may express the Bloch wavefunctions as Ψ nk (r) = 1 e ik R (aπ A (r R) + be ik l 1 π B (r R l 1 )) N R a ψ n,k A + be ik l 1 ψ n,k B (.9) ( 1 ) where l 1 = a is the vector between the two atoms in a unitcell, ie. between different sublatices, N is the number of unitcells, the A/B subscripts on the wavefunctions denotes the One could ask why we don t just use the Wannier functions instead of making this assumption. The main reason for this is, as stated above, that we may find the elements of the Hamiltonian and the overlap matrix,s, for the atomic orbitals, but it is important to remember that the choice of basisfunctions are not fixed by the theory and other sets of functions may serve us better in other circumstances, as we shall indeed see in section 8 8
13 .. BLOCH S THEOREM FOR GRAPHENE sublattice, and a and b are the coefficients to be determined from solving the Schrödinger equation: ĤΨ nk = E n Ψ nk (.1) Notice that in (.9), we explicitly write out the relative blochphase between the atoms in a given unitcell. This may seem slightly at odds with the molecular orbitals approach, since we might have made it a part of the coefficient, ie. used b = be il1 k instead of b - however, this explicit representation will be convenient later, and so it is left in. Now, we have to be slightly careful when we use π orbitals instead of the Wannier functions as in (.9): Whereas the Wannier functions were orthogonal by construction, the atomic orbitals at different sites are not. This requires a slight modification of the Schrödinger equation: let us define a non-orthonormal, but complete, set of ξ m. The fact that the set is complete means that the eigenstates of H may be expressed as Ψ nk = a c ank ξ a - then, if we define the overlap matrix S ab = ξ a ξ b, the usual schrödinger equation (.1) is Ĥc ank ξ a = E n c ank ξ a = a H ba c ank = E n c ank S ba = a a a ĤΨ nk = E n SΨ nk (.11) where the first implication follows from multiplying from the left with ξ b, and the second one follows from the fact that the second line in (.11) is in fact one row of the equation Ĥc nk = E n Sc nk, where c nk is now a column of the coefficients for the nth eigenvector. This equation is equivalent to the last line of (.11). Now we apply the schrödinger equation to the LCAO Bloch waves (.9) in order to find E n,a and b: ĤΨ nk (r) = 1 e ik R (aĥπ A(r R) + be il1 k Ĥπ B (r R l 1 )) = N R E nk SΨ nk (r) = 1 e ik R (asπ A (r R) + be il1 k Sπ B (r R l 1 )) (.1) N R Thus, we see that we need the matrix elements of Ĥ and S in order to proceed, and in order to make progress with this, we employ our next assumption, namely that only nearest neighbour atoms interact through the hamiltonian, and have a significant overlap. Notice from figure.1 that nearest neighbours are always from different sublattices. Clearly, since we have a diatomic unitcell, and since we consider only one atomic orbital pr. atom, the hamiltonian and overlap matrix may be written as x matrices in the A-B space in which the eigenfunctions (.9) is described by the vector of the coefficients on the sublattices ie. A ij ψ nk i A ψ nk j for A = these matrices one by one: ( a b ), and consequently, we need {Ĥ,S }, and i,j denoting sublatices. We consider the elements of 9
14 CHAPTER. GRAPHENE The H AB and S AB element: As observed above, the graphene crystal have a threefold rotational symmetry, which immediately yields that the transfer integrals between nearest neighbour are equal (since they are rotated onto one another by the symmetry operation), and we denote this transfer integral γ. From figure.1 one sees that an A sublattice orbital couples to orbitals in the B sublattice, at positions l 1,l a ( 1 1 the sum over l in the following derivation: R ),l a ( 1 1 ) relative to the A orbital, which leads to ψ nk A Ĥ ψ nk B = 1 e i((r+l 1) k R k ) π A (r R )Hπ B (r R l 1 ) = N R,R 1 e ir (k k ) e il k γ = δ k,k e ik l j γ Γ(k)γ (.1) N j j A completely analogous argument yields the S AB element, except that the transport integral γ between neighbours is of cause exchanged with s, the overlap integral between neighbours: Thus S AB = Γ(k)s. These calculations also immediately gives us the H BA element since the hamiltonian is hermitian, so H BA = HAB, in agreement with (.1), since the only change in the derivation comes from the fact, that while the A sublattice orbitals couple to the B sublattice orbitals at the unitcells at positions l 1,l,l relative to the original unitcell, the B sublattice couple to A orbitals at relative positions l 1, l, l, which we see is the same as interchanging Γ(k) with Γ(k) in (.1). Thus, choosing γ real, we have that H BA = Γ(k) γ = (Γ(k)γ ) = HAB. This also applies to S BA = SAB. Now we turn to the diagonal elements S AA and H AA. Since we know from eg. [1] that the overlap between nearest neighbours is small (s =.19) and the overlap decreases exponentially with distance (since the π orbitals have an exponential decaying dependency on distance), we are vindicated in the nearest neighbour assumption, and since the π orbitals are normalised, the derivation of S AA becomes easy by using the fact that none of the nearest neighbour to the atoms on the A sublattice are themselves part of the A sublatice: ψ nk A S ψ nk A = 1 e i(r k R k ) π A (R)Sπ A (R ) N br,r = 1 δ R,R e ir (k k ) = δ k,k (.14) N R,R Since this derivation only involves orbitals from the same unitcell, indeed the same atom, it is precisely the same for S BB, so S BB = S AA. Furthermore, an analogous derivation gives us H AA (and H BB ) except we need to exchange the overlap (1) with the energy of the π orbital, ie. ǫ π Ĥ π, thus giving H BB = H AA = δ k,k ǫ. Notice that all the elements have a delta Dirac function between k and k, proving that eigenfunctions at different points in the Brillouin zone does not couple to one other - this is a (standard) consequence of the assumption of an infinite sheet. The parameters γ and s are usually found by formally solving the problem, and then fitting the resulting dispersion relation either to DFT calculations or to experiments. In this thesis I use 1
15 .. BLOCH S THEOREM FOR GRAPHENE Figure.: The Brillouin zone of graphene. Note the 6 points where the valence and conduction band touch - two of them are in-equivalent, and is conventionally denoted K and K. The dispersion relation close to these points is linear in k, thus giving rise to the name Dirac points for these points. The units are Å 1 for the two horizontal axises, and ev for the vertical axis. the numerical estimates from [1] for the parameters, since they have all the parameters for the σ orbitals as well, which I will use later in the thesis. It should be noted however, that it is not clear how and to what the fit is made since the article cites private communication - however, from the plots in the article, it is clear that the parameters produce the wellknown dispersion relation for graphene (compare figure 6a in [1] with eg. figure in [11]). We then have to solve the following generalized eigenvalue problem: ( ) ) ( ) ǫ γ Γ(k) a γ Γ(k) ǫ ) ( a b = E n ( 1 s Γ(k) s Γ(k) 1 b (.15) This problem is easily solvable, by noting that ĤΨ n = E n SΨ n = (Ĥ E ns)ψ n =, and since this linear equation has nonzero solutions for Ψ n precisely when the determinant of Ĥ E n S is zero, we get the following expressions for the two energy bands: ǫ + (k) = ǫ γ Γ(k) 1 + s Γ(k) ǫ (k) = ǫ + γ Γ(k) 1 s Γ(k) (.16) The two energy bands have been depicted in figure.. Note that since γ =.ev we have that ǫ + (k) ǫ (k) for all k. By the standard ( ) ± a method we find that the eigenfunctions of the graphene hamiltonian may be described as = ) b ( Γ(k) 1 Γ(k). 1 We work in the socalled halffilling regime throughout the thesis, meaning simply that there is 1 π band electron pr. carbon atom (ie. the graphene sheet is electrically neutral), so since there are π orbitals pr. atom (due to spin) the lower half of the states in the Brillouin zone is filled, corresponding to the states with energies described by ǫ (k). Thus we see that the points 11
16 CHAPTER. GRAPHENE where ǫ + (k) = ǫ (k) are of special interest, since these are the points where the dispersion relation touches the fermisurface. From (.16) we see that this happens exactly when Γ(k) = - we now also see that if we define ǫ = we have set the Fermi energy ǫ F =. There are two inequivalent points in the Brillouin zone where Γ(k) =, namely the two socalled diracpoints K and K : K( ) = π a ( 1, ± 1 ) (.17) where the ± distinguishes between K and K. We are mainly interested in the bandstructure near the fermisurface, since these are the states that will be of interest in a quantum dot setup. Therefore, we expand the energy in a small deviation k around the K and K point in the Brillouin zone. We have seen that the crucial part is the Γ(K+k), and thus we expand this function around eg. K: Γ(K + k) = e il1 K e il1 k + e il K e il k + e il K e il k ( 1 i )(1 + il 1 k)+ ( 1 + i )(1 + il k) + (1 + il k) = ( 1 i )(1 ia k x )+ ( 1 + i )(1 + i(a k x + a k y )) + (1 + i(a k x a k y )) = a 4 ( k x + k y + i( k y k x )) a (kc + ik T) (.18) where we have defined a new coordinatesystem, {k C, k T } = { k x + 1 k y, 1 k x + k y} (.19) the naming convention of which will become more meaningful in the next chapter. An equivalent calculation for the K point gives yields Γ(K + k) a ( k C + ik T ). From this we see that the dispersion relation is linear around the K and K point, giving rise to the term Dirac points, since the gapless, linear dispersion is also found in relativistic quantum mechanics for massless particles. It is also of worth to note that this leads to the concept of an effective hamiltonian, ie. a hamiltonian that gives the physics of the interesting states near the Fermi surfaces, which is obtained by inserting the approximated expression of Γ(k) from (.18) into the hamiltonian part (or indeed into Ĥ E ks) from (.15) to obtain ( ǫ E n (γ E n s ) a (γ E n s ) a (τk C ik T ) ǫ E n where τ is 1(-1) for K(K ). This concludes the introduction to the physics of graphene. ) (a (τk C + ik T ) ) = b ( ) (.) note, that we can only choose ǫ to as long as we only wish to study states close to these Dirac points. If the size of Γ(k) becomes appreciable, we see from (.16) that it is no longer just an energy shift, and we will have to choose our energy scale to set ǫ F to explicitly, if we wish to do so 1
17 Chapter Folding up the nanotube We now turn to the task of rolling up the graphene sheet into a cylinder called a carbon nanotube or CNT. This gives rise to two effects, namely the imposition of cyclic boundary conditions and the breaking of the graphene plane mirror symmetry, most importantly leading to mixing of the π and σ bands, which were separate in graphene. We shall treat these two effects in turn, but first we need to make some basic definitions, especially concerning the crucial concept of chirality..1 Chirality and unitcell of the CNT In folding up the CNT we have a choice of how exactly to do so - Essentially we may specify everything about the folding of the nanotube by specifying two equivalent atoms in the graphene lattice that will be folded onto one another - this is done by specifying the chiral vector, C going from these two sites, and thus going around the circumference, and is illustrated in figure.1. We shall use two equivalent methods for specifying the chiral vector: by specifying the two numbers n and m, so that C = na 1 + ma. Because of the sixfold rotational symmetry and the mirror symmetry around a line parallel with a given nearest neighbour bond (see figure.1), we see that we may impose the condition n m. It is customary to denote a CNT by its chiral vector as (n, m) eg. a (4, ) CNT, and also to denote (n, ) CNTs as zigzag nanotubes, and (n, n) as armchair nanotubes. All other CNTs are denoted chiral nanotubes. by specifying the radius r of the nanotube (giving the length of C, since obviously C = πr) and the chiral angle, θ, which we define as the angle between C and a 1. From the above restriction on n and m, it is clear that θ π. We see that zigzag nanotubes have θ = 6 and armchair nanotubes have θ = π. Note that this implies that θ becomes larger if we turn 6 C in a clockwise direction. We also need to consider the possible unitcells of the CNT. First, we shall prove that we may still use the diatomic unitcell from graphene (although of cause curved) following the derivations of [1], and then we shall consider the larger socalled translational unitcell,which is easier to handle numerically than the primitive unitcell. 1
18 CHAPTER. FOLDING UP THE NANOTUBE k T k C a1 a θ C,1 Figure.1: The (,1) chiral vector in a graphene sheet. The two broken lines (perpendicular to C) are folded onto one another, so the chiral vector goes around the circumference. The coordinatesystem {k C,k T } is also shown..1.1 The helical unitcell Following [1], we first observe, that the two atoms that make up a unitcell of graphene may be mapped onto the CNT surface by arbitrarily placing the first atom onto the surface, and then, since the vector between two atoms in the same unitcell is d = a 1+a, and since, as we noted above, C is mapped onto the circumference of the CNT. We may therefore divide d into an rotation around the tube axis by πd C radians, and a translation along the tube axis direction, which is simply C d C, since Usually (for all but the armchair case) the direction of the d vector will not coincide with the direction of the C vector. Thus the unitcell may be uniquely mapped onto the surface of the nanotube - we now turn to the task of proving that this mapped unitcell is indeed a unitcell of the CNT: First observe, that there may be unitcells at the same coordinate along the tubule axis as the first unitcell placed, but at different angles around the circumference: This happens, if C can be expressed as a shorter chiral vector, C = ma 1 + ña, times some factor M, since this means that we may reach another unitcell from the first one, by translating C in the graphene plane, or correspondingly rotating C π C radians around the tubule axis. Since m and ñ must both be integers, we see that the shortest C possible in the direction of C, corresponding to the largest M possible, is the C for which M is the greatest common divisor of n and m, ie. M = gcd(n, m). This leads us to the first of our lattice vectors for the CNT primitive cell, namely the vector C/M corresponding to a rotation of π M around the tubule axis. With this lattice vector alone we can place M unitcells in a helical motif around the circumference of the CNT. We now desire a second operation, S(h, α) that, when applied successively to this motif, generates the nanotube, ie. we desire a unique pair of integers k and l, such that, given an origo on the CNT, the lattice point of any unitcell may be found by applying the rotational operation, ie. rotating π radians around the tube axis, k times, and then applying the helical operation S(h, α) l M 14
19 .1. CHIRALITY AND UNITCELL OF THE CNT times. First we notice that we may couple the S(h, α) operation to a corresponding lattice vector 1 in the graphene plane H = pa 1 + qa (.1) by noting that h = H C and α = C Hπ. We may insure that H and C are indeed lattice vectors, C C M by demanding that h = H C be as small as possible: since per definition all unitcells in graphene C are alike, all unitcells with the distance r h perpendicular to C from a given origo unitcell, can be found by adding rh (corresponding to r applications of S(h, α) on the CNT) to a unique number of C (corresponding to a given number of C π rotations around the CNT axis) - here r must be M an integer, since, if the number h is the minimal distance perpendicular to C from one unitcell to some other, it is the minimal distance perpendicular to C from any unitcell to some other. Conversely only one linear combination of H and C will yield the vector R connecting two given unitcells, since they clearly span the graphene plane. Thus, all we now need to do is to find the numbers h and α, or equivalently p and q. One way to do this, is to note (as in [1]) that the area covered by the helical motif is A motif = M a 1 a (.) since the area of a unitcell is a 1 a, but at the same time the nanotube can be thought of as a repetition of the helical motif, rotated by α and stacked at distance h along the tubule axis, so there is a new motif for each step of distance h along the axis, and thus, the area covered by one motif must be the area of a cylinder of height h and circumference C or, in terms of H, A motif = H C = (pa 1 + qa ) (na 1 + ma ) = (pm qn) a 1 a (.) so that, by comparing the two expressions for the area covered by a helical motif, we have the identity pm qn = ±M (.4) Clearly there are some ambiguities here, owing to the fact that S(h, α) = S(h, α + jπ) for any integer j, which leads to H being indistinguishable from H + jc, and thus to the pair (p, q) being indistinguishable from (p + jn, q + jm). Also, since the inverse operation of S(h, α), namely S( h, α) would work just as well, corresponding to H being as good as H, we also have that (p, q) is indistinguishable from ( p, q) as seen from the ± in (.4). Clearly we can reach unambiguity by selecting n > p, and thus, we may find p and q, and thus α and h from n and m, using (.4) together with the standard identity H = ( C H ) C H + ( ) (.5) C C Since the main purpose of this section is to prove the existence of the diatomic unitcell, we choose not to carry out this calculation: It is sufficient for our purposes to prove its existence. 1 ie. the vector connecting the two unitcells in graphene which, when mapped onto the CNT surface, will be separated by the length h along the axis, and a rotation of α radians around the axis, so that S(h, α) will translate one onto the other. 15
20 CHAPTER. FOLDING UP THE NANOTUBE.1. The translational unitcell We now turn to the other possible unitcell, namely the socalled translational unitcell, consisting of a cylinder segment of height T and circumference C which may be repeated along the tube axis to generate the CNT, where T is to be found in order to prove the existence of this unitcell. In order to show that such a unitcell exists, we only need to prove the existence of a lattice vector T which is perpendicular to C - this vector will be mapped onto the CNT along the tube axis, and will thus satisfy the condition of connecting two cylinder segments. However the proof of the existence of this vector { is rather trivial, since if we define t 1 and t so that T = t 1 a 1 + t a we 1 for i = j have, using a i a j = 1 for i j, that for C and T to be perpendicular, = T C = t 1 m + t n + t 1 m + t n = t 1 (m + n) = t (n + m) (.6) In order for T to be a lattice vector, t 1 and t must be integers, and we see, that such integers exists, and we may also find smallest possible translatory unitcell by setting m + n t 1 = gcd(m + n, n + m) t = n + m gcd(m + n, n + m) (.7) It is of interest to note that the area of the translatoric compared to the primitive unitcell is N = C T a 1 a = nt mt 1 a 1 a = (n + m + nm) a 1 a gcd(n + m, m + n) (.8) For example the translatoric unitcell of a (1,9) CNT is 54 times larger than the primitive unitcell! We may now divide the k vector of the blochwave into a part along reciprocal basis vector b T of T, and a part along the reciprocal basis vector b C of C, ie. b C b T k k t b T + k c b C = k ˆ tb T + k CbC ˆ (.9) Given the definition of the reciprocal basis vector, ie. A b B = πδ A,B, we find that b C = t b 1 + t 1 b N b T = mb 1 nb N (.1).1. Comparing the unitcells The two unitcells are of cause in principle equivalent, but whereas the translatory unitcell is well suited for the numerical experiments, and, as we shall see in the following section, to describe the effect of cyclic boundary conditions (since only k C will be effected), the larger size of the unitcell is an insurmountable drawback when doing analytical calculations, since the amount of basis functions in eg. a tight-binding model is of cause proportional to the number of atoms considered, and thus to the size of the unitcell. Therefore we shall use either unitcell when convenient throughout this thesis. 16
21 .. CYCLIC BOUNDARY CONDITIONS. METALLIC AND SEMICONDUCTING NANOTUBES. Cyclic boundary conditions. Metallic and semiconducting nanotubes Having established the basic structure of the CNT, we now turn to the first of the two effects of curling up the nanotube, namely the simple fact that the phase of the wavefunctions available to the electrons must be single valued, and so the phase change when r is changed by one circumference ( C ) must be an integer multiplum of π, or, if we choose to work with the translational unitcell from section.1, we have the constraint that, since the blochcondition (.6) for r = C now states Ψ nk (r + C) = e ik C Ψ nk (r) = Ψ nk (r), we must have that, if we recall (.9) l C π = k C = k C C b C b C = k C C = k C = l Cπ C (.11) where the third equality follows from the fact that b C is parallel to C which in turn follows from the fact that T and C are perpendicular, and where we have defined the integer l C, which must be restricted in order to insure that k cannot be written as some shorter vector plus a reciprocal lattice vector. Using (.9) and (.1) and the above definition of l C, we find k = k tˆbt + k CˆbC = k tˆbt + l Cπb C C b C = k tˆb T + l C N ( t b 1 + t 1 b ) (.1) Now, using that pr. construction of t 1 and t, gcd(t 1, t ) = 1, we see that if l C < N, t l C and t 1l c N N cannot both be integers so l C ( t b 1 + t 1 b ) is not a reciprocal lattice vector, and thus we have our constraint: l C N 1 (.1) Turning now to the effects of this constraint on the bandstructure, we see that this is the (main) reason why some CNTs are semiconducting and others are metallic: If the condition excludes the K and K points, there are no allowed k-values for which the bands touch the fermisurface, and thus the CNT will be semiconducting, and conversely, if the K and K points are allowed values of k C, the CNT will be metallic - in figure. this is illustrated for an example of either group. In order to understand the results from the Hückel code shown later in the thesis, it is important to note that we can now see the dispersion relation as being one dimensional (ie. along the k T direction) and then using a zone folding scheme to include all the N possible values of k C as different bands - this is of cause the natural Brillouin zone to use when we start out with the translational unitcell, rather than the (constrained) D unitcell from figure. which, as is readily seen from its size, corresponds to the diatomic, helical unitcell. In figure. we see the 1 dimensional bands corresponding to the two chiralities, one metallic, the other semiconducting. 17
22 CHAPTER. FOLDING UP THE NANOTUBE (7,) (9,) k y /[AA 1] k y /[AA 1] k x /[AA 1] k x /[AA 1] Figure.: The allowed k values are shown as black lines for a semiconducting (7,) CNT (left) and a metallic (9,) CNT (right), where we can see explicitly that the lines in the (9,) CNT touch the diracpoints, while they do not in the (7,) CNT - the hexagon denotes the first Brillouin zone of graphene. 15 Reduced zone band structure for (4,1) CNT 15 Reduced zone band structure for (6,) CNT E/[eV] E/[eV] k T /[AA 1] k T /[AA 1] Figure.: Reduced zone bandstructures for two different chiralities: left is the semiconducting (4,1) CNT, and right is the metallic (6,) CNT. Occupied bands are shown in red, unoccupied are shown in blue. Note that the small gap in the (6,) spectrum is due to imprecision in the numerical code, and not curvature effects. 18
23 .. CYCLIC BOUNDARY CONDITIONS. METALLIC AND SEMICONDUCTING NANOTUBES For completeness, we shall now derive the condition for metallic vs. semiconducting nanotubes from the numbers (n, m) specifying the chirality (see.1). Since K( ) = π ( 1 a, τ ), where τ = ±1 distinguishes K from K, we find that πl C = K( ) C = π(n + m + τ n m ) = { for τ = 1 : lc = n+m for τ = 1 : l C = m+n (.14) Observing that, since m is always an integer, m+n Z n m Z, and from that we know that m+n + n m = n+m Z, so if K C is an allowed value, then so is K C, and thus we have A CNT is metallic if and only if n+m is an integer. It is also instructive to derive the effective hamiltonian, and thus the energies close the the K( ) point, which is easily done by building on the effective hamiltonian description from last section (.), and for simplicity ignore overlap (ie. s = ) and furthermore set the fermilevel so that ǫ =. First of all, we now see the logic behind the naming convention in (.19): For the zigzag case, ie. θ =, we have precisely that k C = kc and k T = kt. From studying figure.1 we see that, in general, kc = cosθk C + sin θk T and kt = sin θk C + cos θk T, leading to τk C + ik T = τ cosθk C + τ sin θk T i sin θk C + i cosθk T = e iτθ (τk C + ik T ) (.15) where we use that τ = 1. This can then be inserted into (.), following the spirit of [1], [18], and others but I have used a slightly non-standard coordinatesystem and definition of the chiral angle, so care should be taken when comparing the results. We find ( ) a E n γ (a (τk C + ik T ) ) a γ (τk C ik T ) E = n b ( ) a E n γ (a ) ( ) (τk C + ik T )e iτθ a γ = (τk C ik T )e iτθ E n b (.16) Now we must take the constraint of k C into account. Here we need to remember that it is of cause the total vector that has to fulfill the constrain, ie. (k + K( )) C = πl c. Therefore, using (.14) and the fact that C = Rπ where R is the radius of the CNT, we have πl C = (k + K( )) C = k C Rπ + π(n + m + τ n m ) = k C = l c R n + m + τ n m R (.17) From this, we find the energy to be (recalling (.)) E ± = γ a a τkc + ik T = γ (Rk T ) R + (l c + 19 τ(m n) + n m ) 6 (.18)
24 CHAPTER. FOLDING UP THE NANOTUBE It is of cause important to remember, that this is the result of an expansion, so the formula is only accurate for the l C values closest to the Dirac-points, ie. { τ(m n) + n m for metallic tubes l c + = 6 ± 1 for semiconducting tubes (.19) Since we can choose k T freely (due to the infinitely long tube), it is now clear that the energy gap between the HOMO and the LUMO band in semiconducting CNTs should be inversely proportional to the radius, irrespective of the details of the chirality. When we alter (.) with our restriction on k we end up with the effective hamiltonian ( ) a E n γ (a R (ν + irk ) T))e iτθ a γ R (ν irk = T)e iτθ E n b ( ) (.) where ν is the family number of the CNT chirality, which is for metallic tubes, or ±1 for semiconducting tubes, depending on whether the smallest numeric value of k C is + or 1 in (.19) off, compared to the Dirac points - later we shall see that ν plays an important role for the understanding of spin-orbit coupling. One final manipulation of this hamiltonian is to realize that we may rotate it to get rid of the phases in the off-diagonal elements, by evaluating Ĥeff U ˆ Heff CNT U 1 with U = Ĥ eff CNT = ( ( e iτθ 1 ), so we finally have ) a E n γ (a R (ν + irk ) T)) a γ R (ν irk T) E n b = ( ) CNT = (.1) This hamiltonian finally yields the following expressions for the eigenenergies near the K( ) point: E n ± = γ a ( ν R ) + R kt (.). σ/π band mixing We now turn to the other major effect of curling up the nanotube, namely the breaking of the mirror symmetry that allowed us to ignore the σ bands in the case of graphene. The first important observation is now that the π bands on neighbouring sites now tilt towards one another - secondly we shall find that the matrix elements between the σ orbitals and the π orbitals are no longer zero. In order to parametrize these effects, we shall first study the geometry of the coupling between the n = shells on two neighbouring atoms, and due to symmetry reasons we shall find that in principle only 4 different parameters are necessary, when we make the assumption that the hamiltonian matrix element is zero if the orbital overlap is zero. In table.1 we see the fundamentally different pairs of orbitals around two neighbouring carbon atoms (denoted A and B) separated by a cc along the x axis (notice, that the matrix elements we seek are of cause the same if we exchange the A and B orbital, except for a complex conjugation,
25 .. σ/π BAND MIXING y,z x y,z x y,z x + + y,z x y,z x + + y,z x y,z x Table.1: The seven inequivalent orbit pairs at two neighbouring carbon atoms. The upper row is shown in the text to have no overlap, and then by assumption no hamiltonian, matrix elements. In. the overlap and hamiltonian elements for the remaining combinations is denoted, for the second row, left to right, V π pp /sπ pp,v σ ss /sσ ss,v σ sp /sσ sp V σ pp/s σ pp., and the coupling shown in the third row is denoted stemming from the hermiticity of the hamiltonian). From these figures, and from the wellknown property of the real p-orbitals that p i is an odd function along the i axis, and even along the two other axises, plus the fact that s-orbitals are even along all Cartesian coordinates, it is easy to see that overlap between the p x,a and p (y,z),b (the y and z axises are indistinguishable in this case) is, since the integrant p x,a p (y,z),b is odd along either the y or z axis respectively. Likewise, the overlap between p y,a and p z,b is zero (since the integrant is then odd along both the y and z axis), and finally the overlap between S A and p (y,z),b is zero, since again the integrant is odd along either the y or the z direction - as mentioned, we then assume that the hamiltonian matrix elements between these orbitals are also. This leaves four pairs of orbitals have non-zero overlap/hamiltonian matrix elements, and, using the numerical values from [1], we have drp (y,z),aĥp (y,z),b = Vpp π =.ev, drp (y,z),ap (y,z),b = s π pp =.19 drp x,aĥp x,b = Vpp σ = 5.7eV, drp x,a p x,b = s σ pp =.146 drs AĤp x,b = Vsp σ = 5.58eV, drsa p x,b = s σ pp =.1 drs AĤS B = Vss σ = 6.769eV, drsas B = s σ ss =.1 (.) We need one final parameter to describe the entire n = shell: In the previous sections, we have only dealt with one self-energy, ǫ, being the diagonal element of the hamiltonian of a p orbital. We now have to include an energy corresponding to the S orbital, ǫ S = 8.868eV. We choose, following [14] to define the π orbitals on the CNT as the ones perpendicular to the cylinder surface and we also choose to call the other three n = orbitals, formed in the three-clover 1
26 CHAPTER. FOLDING UP THE NANOTUBE π A ^ C R i α i π B α i Figure.4: The geometry of two neighbouring atoms, projected onto the endface of the cylinder, ie. T is perpendicular to the paper. Shown is the definition of α i and the directions of the π orbitals at the two atom positions, A and B, R i vector projected onto the plane of the paper, perpendicular to the tube axis. form as in graphene, and perpendicular to the radial, π direction, σ orbitals, although of cause the symmetry that lead us to employ the sp is now broken. Now we make some observations and definitions concerning the geometrical effects of curvature. In figure.4 we define the angle α to parametrise the curvature effect between two neighbouring atoms. We wish to couple this angle to the chirality of a CNT, and to this end, we know the radius as seen in section.1, either as one of the parameters that define the chirality, or as C, and thus π for a given pair of carbon atoms, we need the length of the vector between them ( R i, where the i subscript denotes the bond) projected onto the face perpendicular to the tube axis (which is of cause not just a cc, since the bond between neighbours will usually be partly along the tube axis direction as well) - ie. we need the length R i Ĉ. However, if one studies the honeycomb lattice, it is clear that only three different R i s exists, as we still restrict ourselves to nearest neighbour interaction and only the direction of the bonds (compared to C) plays any role in the calculation of α. Rigorously, if we define the angle between R i and C as β i, we find that β i = cos 1 ( R i Ĉ ) = sin(α i ) = R i Ĉ a cc R = cos(β i)a cc R ηcos(β i ) (.4) We should note here, that there is actually an assumption hidden in this calculation, since it is not clear whether R i Ĉ (which is well defined in flat graphene) is, after rolling up the CNT, the length along the straight line in figure.4 - we could also have chosen it to be the length along the curved line, ie. the surface of the cylinder. There are a few things to say about this The difference between the two is minute for anything but the smallest CNTs (of the order of femtometers). The bondlength (a cc ) should be the length of the vector connecting the two atoms, regardless of the cylindrical form we imagine for the tube. The procedure for including the σ orbitals into the hamiltonian can be divided it three steps: First, we shall derive the angle dependent hamiltonian between the π orbitals on neighbouring
structure of graphene and carbon nanotubes which forms the basis for many of their proposed applications in electronics.
 Chapter Basics of graphene and carbon nanotubes This chapter reviews the theoretical understanding of the geometrical and electronic structure of graphene and carbon nanotubes which forms the basis for
Chapter Basics of graphene and carbon nanotubes This chapter reviews the theoretical understanding of the geometrical and electronic structure of graphene and carbon nanotubes which forms the basis for
Refering to Fig. 1 the lattice vectors can be written as: ~a 2 = a 0. We start with the following Ansatz for the wavefunction:
 1 INTRODUCTION 1 Bandstructure of Graphene and Carbon Nanotubes: An Exercise in Condensed Matter Physics developed by Christian Schönenberger, April 1 Introduction This is an example for the application
1 INTRODUCTION 1 Bandstructure of Graphene and Carbon Nanotubes: An Exercise in Condensed Matter Physics developed by Christian Schönenberger, April 1 Introduction This is an example for the application
Electrons in a periodic potential
 Chapter 3 Electrons in a periodic potential 3.1 Bloch s theorem. We consider in this chapter electrons under the influence of a static, periodic potential V (x), i.e. such that it fulfills V (x) = V (x
Chapter 3 Electrons in a periodic potential 3.1 Bloch s theorem. We consider in this chapter electrons under the influence of a static, periodic potential V (x), i.e. such that it fulfills V (x) = V (x
PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR
 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR The aim of this project is to present the student with a perspective on the notion of electronic energy band structures and energy band gaps
PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR The aim of this project is to present the student with a perspective on the notion of electronic energy band structures and energy band gaps
From Graphene to Nanotubes
 From Graphene to Nanotubes Zone Folding and Quantum Confinement at the Example of the Electronic Band Structure Christian Krumnow christian.krumnow@fu-berlin.de Freie Universität Berlin June 6, Zone folding
From Graphene to Nanotubes Zone Folding and Quantum Confinement at the Example of the Electronic Band Structure Christian Krumnow christian.krumnow@fu-berlin.de Freie Universität Berlin June 6, Zone folding
Theoretical Concepts of Spin-Orbit Splitting
 Chapter 9 Theoretical Concepts of Spin-Orbit Splitting 9.1 Free-electron model In order to understand the basic origin of spin-orbit coupling at the surface of a crystal, it is a natural starting point
Chapter 9 Theoretical Concepts of Spin-Orbit Splitting 9.1 Free-electron model In order to understand the basic origin of spin-orbit coupling at the surface of a crystal, it is a natural starting point
Chapter 2. Linear Algebra. rather simple and learning them will eventually allow us to explain the strange results of
 Chapter 2 Linear Algebra In this chapter, we study the formal structure that provides the background for quantum mechanics. The basic ideas of the mathematical machinery, linear algebra, are rather simple
Chapter 2 Linear Algebra In this chapter, we study the formal structure that provides the background for quantum mechanics. The basic ideas of the mathematical machinery, linear algebra, are rather simple
Quantum Condensed Matter Physics Lecture 4
 Quantum Condensed Matter Physics Lecture 4 David Ritchie QCMP Lent/Easter 2019 http://www.sp.phy.cam.ac.uk/drp2/home 4.1 Quantum Condensed Matter Physics 1. Classical and Semi-classical models for electrons
Quantum Condensed Matter Physics Lecture 4 David Ritchie QCMP Lent/Easter 2019 http://www.sp.phy.cam.ac.uk/drp2/home 4.1 Quantum Condensed Matter Physics 1. Classical and Semi-classical models for electrons
Electrons in periodic potential
 Chapter 9 Electrons in periodic potential The computation of electronic states in a solid is a nontrivial problem. A great simplification can be achieved if the solid is a crystal, i.e. if it can be described
Chapter 9 Electrons in periodic potential The computation of electronic states in a solid is a nontrivial problem. A great simplification can be achieved if the solid is a crystal, i.e. if it can be described
Introduction to Electronic Structure Theory
 Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Introduction to DFTB. Marcus Elstner. July 28, 2006
 Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
ELECTRONIC ENERGY DISPERSION AND STRUCTURAL PROPERTIES ON GRAPHENE AND CARBON NANOTUBES
 ELECTRONIC ENERGY DISPERSION AND STRUCTURAL PROPERTIES ON GRAPHENE AND CARBON NANOTUBES D. RACOLTA, C. ANDRONACHE, D. TODORAN, R. TODORAN Technical University of Cluj Napoca, North University Center of
ELECTRONIC ENERGY DISPERSION AND STRUCTURAL PROPERTIES ON GRAPHENE AND CARBON NANOTUBES D. RACOLTA, C. ANDRONACHE, D. TODORAN, R. TODORAN Technical University of Cluj Napoca, North University Center of
Energy bands in two limits
 M. A. Gusmão IF-UFRGS 1 FIP10601 Text 4 Energy bands in two limits After presenting a general view of the independent-electron approximation, highlighting the importance and consequences of Bloch s theorem,
M. A. Gusmão IF-UFRGS 1 FIP10601 Text 4 Energy bands in two limits After presenting a general view of the independent-electron approximation, highlighting the importance and consequences of Bloch s theorem,
Lecture 4: Basic elements of band theory
 Phys 769 Selected Topics in Condensed Matter Physics Summer 010 Lecture 4: Basic elements of band theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 Introduction Most matter, in particular most insulating
Phys 769 Selected Topics in Condensed Matter Physics Summer 010 Lecture 4: Basic elements of band theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 Introduction Most matter, in particular most insulating
Chm 331 Fall 2015, Exercise Set 4 NMR Review Problems
 Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
Introduction to Condensed Matter Physics
 Introduction to Condensed Matter Physics The Reciprocal Lattice M.P. Vaughan Overview Overview of the reciprocal lattice Periodic functions Reciprocal lattice vectors Bloch functions k-space Dispersion
Introduction to Condensed Matter Physics The Reciprocal Lattice M.P. Vaughan Overview Overview of the reciprocal lattice Periodic functions Reciprocal lattice vectors Bloch functions k-space Dispersion
Brief review of Quantum Mechanics (QM)
 Brief review of Quantum Mechanics (QM) Note: This is a collection of several formulae and facts that we will use throughout the course. It is by no means a complete discussion of QM, nor will I attempt
Brief review of Quantum Mechanics (QM) Note: This is a collection of several formulae and facts that we will use throughout the course. It is by no means a complete discussion of QM, nor will I attempt
Quantum Condensed Matter Physics
 QCMP-2017/18 Problem sheet 2: Quantum Condensed Matter Physics Band structure 1. Optical absorption of simple metals Sketch the typical energy-wavevector dependence, or dispersion relation, of electrons
QCMP-2017/18 Problem sheet 2: Quantum Condensed Matter Physics Band structure 1. Optical absorption of simple metals Sketch the typical energy-wavevector dependence, or dispersion relation, of electrons
2 Symmetry. 2.1 Structure of carbon nanotubes
 2 Symmetry Carbon nanotubes are hollow cylinders of graphite sheets. They can be viewed as single molecules, regarding their small size ( nm in diameter and µm length), or as quasi-one dimensional crystals
2 Symmetry Carbon nanotubes are hollow cylinders of graphite sheets. They can be viewed as single molecules, regarding their small size ( nm in diameter and µm length), or as quasi-one dimensional crystals
1 Mathematical preliminaries
 1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
Carbon nanotubes and Graphene
 16 October, 2008 Solid State Physics Seminar Main points 1 History and discovery of Graphene and Carbon nanotubes 2 Tight-binding approximation Dynamics of electrons near the Dirac-points 3 Properties
16 October, 2008 Solid State Physics Seminar Main points 1 History and discovery of Graphene and Carbon nanotubes 2 Tight-binding approximation Dynamics of electrons near the Dirac-points 3 Properties
Graphene and Planar Dirac Equation
 Graphene and Planar Dirac Equation Marina de la Torre Mayado 2016 Marina de la Torre Mayado Graphene and Planar Dirac Equation June 2016 1 / 48 Outline 1 Introduction 2 The Dirac Model Tight-binding model
Graphene and Planar Dirac Equation Marina de la Torre Mayado 2016 Marina de la Torre Mayado Graphene and Planar Dirac Equation June 2016 1 / 48 Outline 1 Introduction 2 The Dirac Model Tight-binding model
Time-Independent Perturbation Theory
 4 Phys46.nb Time-Independent Perturbation Theory.. Overview... General question Assuming that we have a Hamiltonian, H = H + λ H (.) where λ is a very small real number. The eigenstates of the Hamiltonian
4 Phys46.nb Time-Independent Perturbation Theory.. Overview... General question Assuming that we have a Hamiltonian, H = H + λ H (.) where λ is a very small real number. The eigenstates of the Hamiltonian
Topological insulator with time-reversal symmetry
 Phys620.nb 101 7 Topological insulator with time-reversal symmetry Q: Can we get a topological insulator that preserves the time-reversal symmetry? A: Yes, with the help of the spin degree of freedom.
Phys620.nb 101 7 Topological insulator with time-reversal symmetry Q: Can we get a topological insulator that preserves the time-reversal symmetry? A: Yes, with the help of the spin degree of freedom.
Chapter 1. Crystal structure. 1.1 Crystal lattices
 Chapter 1 Crystal structure 1.1 Crystal lattices We will concentrate as stated in the introduction, on perfect crystals, i.e. on arrays of atoms, where a given arrangement is repeated forming a periodic
Chapter 1 Crystal structure 1.1 Crystal lattices We will concentrate as stated in the introduction, on perfect crystals, i.e. on arrays of atoms, where a given arrangement is repeated forming a periodic
CHAPTER 6 CHIRALITY AND SIZE EFFECT IN SINGLE WALLED CARBON NANOTUBES
 10 CHAPTER 6 CHIRALITY AND SIZE EFFECT IN SINGLE WALLED CARBON NANOTUBES 6.1 PREAMBLE Lot of research work is in progress to investigate the properties of CNTs for possible technological applications.
10 CHAPTER 6 CHIRALITY AND SIZE EFFECT IN SINGLE WALLED CARBON NANOTUBES 6.1 PREAMBLE Lot of research work is in progress to investigate the properties of CNTs for possible technological applications.
Phonons and lattice dynamics
 Chapter Phonons and lattice dynamics. Vibration modes of a cluster Consider a cluster or a molecule formed of an assembly of atoms bound due to a specific potential. First, the structure must be relaxed
Chapter Phonons and lattice dynamics. Vibration modes of a cluster Consider a cluster or a molecule formed of an assembly of atoms bound due to a specific potential. First, the structure must be relaxed
CHAPTER 10 Tight-Binding Model
 CHAPTER 0 Tight-Binding Model Linear Combination of Atomic Orbitals (LCAO) Application to Bands from s-levels General Features of Tight-Binding Levels Wannier Functions 6 a : S S P 3S Core FE Semicore
CHAPTER 0 Tight-Binding Model Linear Combination of Atomic Orbitals (LCAO) Application to Bands from s-levels General Features of Tight-Binding Levels Wannier Functions 6 a : S S P 3S Core FE Semicore
Linear Algebra March 16, 2019
 Linear Algebra March 16, 2019 2 Contents 0.1 Notation................................ 4 1 Systems of linear equations, and matrices 5 1.1 Systems of linear equations..................... 5 1.2 Augmented
Linear Algebra March 16, 2019 2 Contents 0.1 Notation................................ 4 1 Systems of linear equations, and matrices 5 1.1 Systems of linear equations..................... 5 1.2 Augmented
conventions and notation
 Ph95a lecture notes, //0 The Bloch Equations A quick review of spin- conventions and notation The quantum state of a spin- particle is represented by a vector in a two-dimensional complex Hilbert space
Ph95a lecture notes, //0 The Bloch Equations A quick review of spin- conventions and notation The quantum state of a spin- particle is represented by a vector in a two-dimensional complex Hilbert space
3: Many electrons. Orbital symmetries. l =2 1. m l
 3: Many electrons Orbital symmetries Atomic orbitals are labelled according to the principal quantum number, n, and the orbital angular momentum quantum number, l. Electrons in a diatomic molecule experience
3: Many electrons Orbital symmetries Atomic orbitals are labelled according to the principal quantum number, n, and the orbital angular momentum quantum number, l. Electrons in a diatomic molecule experience
Three Most Important Topics (MIT) Today
 Three Most Important Topics (MIT) Today Electrons in periodic potential Energy gap nearly free electron Bloch Theorem Energy gap tight binding Chapter 1 1 Electrons in Periodic Potential We now know the
Three Most Important Topics (MIT) Today Electrons in periodic potential Energy gap nearly free electron Bloch Theorem Energy gap tight binding Chapter 1 1 Electrons in Periodic Potential We now know the
Electrons in a weak periodic potential
 Electrons in a weak periodic potential Assumptions: 1. Static defect-free lattice perfectly periodic potential. 2. Weak potential perturbative effect on the free electron states. Perfect periodicity of
Electrons in a weak periodic potential Assumptions: 1. Static defect-free lattice perfectly periodic potential. 2. Weak potential perturbative effect on the free electron states. Perfect periodicity of
Spin Superfluidity and Graphene in a Strong Magnetic Field
 Spin Superfluidity and Graphene in a Strong Magnetic Field by B. I. Halperin Nano-QT 2016 Kyiv October 11, 2016 Based on work with So Takei (CUNY), Yaroslav Tserkovnyak (UCLA), and Amir Yacoby (Harvard)
Spin Superfluidity and Graphene in a Strong Magnetic Field by B. I. Halperin Nano-QT 2016 Kyiv October 11, 2016 Based on work with So Takei (CUNY), Yaroslav Tserkovnyak (UCLA), and Amir Yacoby (Harvard)
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
3 Symmetry Protected Topological Phase
 Physics 3b Lecture 16 Caltech, 05/30/18 3 Symmetry Protected Topological Phase 3.1 Breakdown of noninteracting SPT phases with interaction Building on our previous discussion of the Majorana chain and
Physics 3b Lecture 16 Caltech, 05/30/18 3 Symmetry Protected Topological Phase 3.1 Breakdown of noninteracting SPT phases with interaction Building on our previous discussion of the Majorana chain and
The Quantum Heisenberg Ferromagnet
 The Quantum Heisenberg Ferromagnet Soon after Schrödinger discovered the wave equation of quantum mechanics, Heisenberg and Dirac developed the first successful quantum theory of ferromagnetism W. Heisenberg,
The Quantum Heisenberg Ferromagnet Soon after Schrödinger discovered the wave equation of quantum mechanics, Heisenberg and Dirac developed the first successful quantum theory of ferromagnetism W. Heisenberg,
1 Measurement and expectation values
 C/CS/Phys 191 Measurement and expectation values, Intro to Spin 2/15/05 Spring 2005 Lecture 9 1 Measurement and expectation values Last time we discussed how useful it is to work in the basis of energy
C/CS/Phys 191 Measurement and expectation values, Intro to Spin 2/15/05 Spring 2005 Lecture 9 1 Measurement and expectation values Last time we discussed how useful it is to work in the basis of energy
I. CSFs Are Used to Express the Full N-Electron Wavefunction
 Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
3-month progress Report
 3-month progress Report Graphene Devices and Circuits Supervisor Dr. P.A Childs Table of Content Abstract... 1 1. Introduction... 1 1.1 Graphene gold rush... 1 1.2 Properties of graphene... 3 1.3 Semiconductor
3-month progress Report Graphene Devices and Circuits Supervisor Dr. P.A Childs Table of Content Abstract... 1 1. Introduction... 1 1.1 Graphene gold rush... 1 1.2 Properties of graphene... 3 1.3 Semiconductor
Lecture 10: A (Brief) Introduction to Group Theory (See Chapter 3.13 in Boas, 3rd Edition)
 Lecture 0: A (Brief) Introduction to Group heory (See Chapter 3.3 in Boas, 3rd Edition) Having gained some new experience with matrices, which provide us with representations of groups, and because symmetries
Lecture 0: A (Brief) Introduction to Group heory (See Chapter 3.3 in Boas, 3rd Edition) Having gained some new experience with matrices, which provide us with representations of groups, and because symmetries
5. Atoms and the periodic table of chemical elements. Definition of the geometrical structure of a molecule
 Historical introduction The Schrödinger equation for one-particle problems Mathematical tools for quantum chemistry 4 The postulates of quantum mechanics 5 Atoms and the periodic table of chemical elements
Historical introduction The Schrödinger equation for one-particle problems Mathematical tools for quantum chemistry 4 The postulates of quantum mechanics 5 Atoms and the periodic table of chemical elements
5 Symmetries and point group in a nut shell
 30 Phys520.nb 5 Symmetries and point group in a nut shell 5.1. Basic ideas: 5.1.1. Symmetry operations Symmetry: A system remains invariant under certain operation. These operations are called symmetry
30 Phys520.nb 5 Symmetries and point group in a nut shell 5.1. Basic ideas: 5.1.1. Symmetry operations Symmetry: A system remains invariant under certain operation. These operations are called symmetry
Topic 9-1: Bloch Theorem and the Central Equation Kittel Pages:
 Topic 9-1: Bloch Theorem and the Central Equation Kittel Pages: 167-174 Summary: We begin here by postulating Bloch s theorems which develop the form of the wavefunction in a periodic solid. We then show
Topic 9-1: Bloch Theorem and the Central Equation Kittel Pages: 167-174 Summary: We begin here by postulating Bloch s theorems which develop the form of the wavefunction in a periodic solid. We then show
Physics 541: Condensed Matter Physics
 Physics 541: Condensed Matter Physics Final Exam Monday, December 17, 2012 / 14:00 17:00 / CCIS 4-285 Student s Name: Instructions There are 24 questions. You should attempt all of them. Mark your response
Physics 541: Condensed Matter Physics Final Exam Monday, December 17, 2012 / 14:00 17:00 / CCIS 4-285 Student s Name: Instructions There are 24 questions. You should attempt all of them. Mark your response
ECE 659, PRACTICE EXAM II Actual Exam Friday, Feb.21, 2014, FNY B124, PM CLOSED BOOK. = H nm. Actual Exam will have five questions.
 1 ECE 659, PRACTICE EXAM II Actual Exam Friday, Feb.1, 014, FNY B14, 330-40PM CLOSED BOOK Useful relation h( k ) = H nm [ ] e +i k.( r m r n ) Actual Exam will have five questions. The following questions
1 ECE 659, PRACTICE EXAM II Actual Exam Friday, Feb.1, 014, FNY B14, 330-40PM CLOSED BOOK Useful relation h( k ) = H nm [ ] e +i k.( r m r n ) Actual Exam will have five questions. The following questions
The Postulates of Quantum Mechanics Common operators in QM: Potential Energy. Often depends on position operator: Kinetic Energy 1-D case: 3-D case
 The Postulates of Quantum Mechanics Common operators in QM: Potential Energy Often depends on position operator: Kinetic Energy 1-D case: 3-D case Time Total energy = Hamiltonian To find out about the
The Postulates of Quantum Mechanics Common operators in QM: Potential Energy Often depends on position operator: Kinetic Energy 1-D case: 3-D case Time Total energy = Hamiltonian To find out about the
3.15. Some symmetry properties of the Berry curvature and the Chern number.
 50 Phys620.nb z M 3 at the K point z M 3 3 t ' sin 3 t ' sin (3.36) (3.362) Therefore, as long as M 3 3 t ' sin, the system is an topological insulator ( z flips sign). If M 3 3 t ' sin, z is always positive
50 Phys620.nb z M 3 at the K point z M 3 3 t ' sin 3 t ' sin (3.36) (3.362) Therefore, as long as M 3 3 t ' sin, the system is an topological insulator ( z flips sign). If M 3 3 t ' sin, z is always positive
Angular Momentum Quantization: Physical Manifestations and Chemical Consequences
 Angular Momentum Quantization: Physical Manifestations and Chemical Consequences Michael Fowler, University of Virginia 7/7/07 The Stern-Gerlach Experiment We ve established that for the hydrogen atom,
Angular Momentum Quantization: Physical Manifestations and Chemical Consequences Michael Fowler, University of Virginia 7/7/07 The Stern-Gerlach Experiment We ve established that for the hydrogen atom,
The quantum state as a vector
 The quantum state as a vector February 6, 27 Wave mechanics In our review of the development of wave mechanics, we have established several basic properties of the quantum description of nature:. A particle
The quantum state as a vector February 6, 27 Wave mechanics In our review of the development of wave mechanics, we have established several basic properties of the quantum description of nature:. A particle
Quantum Mechanics Solutions
 Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
3.024 Electrical, Optical, and Magnetic Properties of Materials Spring 2012 Recitation 8 Notes
 Overview 1. Electronic Band Diagram Review 2. Spin Review 3. Density of States 4. Fermi-Dirac Distribution 1. Electronic Band Diagram Review Considering 1D crystals with periodic potentials of the form:
Overview 1. Electronic Band Diagram Review 2. Spin Review 3. Density of States 4. Fermi-Dirac Distribution 1. Electronic Band Diagram Review Considering 1D crystals with periodic potentials of the form:
Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory
 Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory 1. Introduction Bound state perturbation theory applies to the bound states of perturbed systems,
Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory 1. Introduction Bound state perturbation theory applies to the bound states of perturbed systems,
7. Numerical Solutions of the TISE
 7. Numerical Solutions of the TISE Copyright c 2015 2016, Daniel V. Schroeder Besides the infinite square well, there aren t many potentials V (x) for which you can find the energies and eigenfunctions
7. Numerical Solutions of the TISE Copyright c 2015 2016, Daniel V. Schroeder Besides the infinite square well, there aren t many potentials V (x) for which you can find the energies and eigenfunctions
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
PH575 Spring Lecture #28 Nanoscience: the case study of graphene and carbon nanotubes.
 PH575 Spring 2014 Lecture #28 Nanoscience: the case study of graphene and carbon nanotubes. Nanoscience scale 1-100 nm "Artificial atoms" Small size => discrete states Large surface to volume ratio Bottom-up
PH575 Spring 2014 Lecture #28 Nanoscience: the case study of graphene and carbon nanotubes. Nanoscience scale 1-100 nm "Artificial atoms" Small size => discrete states Large surface to volume ratio Bottom-up
Unitary Dynamics and Quantum Circuits
 qitd323 Unitary Dynamics and Quantum Circuits Robert B. Griffiths Version of 20 January 2014 Contents 1 Unitary Dynamics 1 1.1 Time development operator T.................................... 1 1.2 Particular
qitd323 Unitary Dynamics and Quantum Circuits Robert B. Griffiths Version of 20 January 2014 Contents 1 Unitary Dynamics 1 1.1 Time development operator T.................................... 1 1.2 Particular
Chemistry 120A 2nd Midterm. 1. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (1-electron):
 April 6th, 24 Chemistry 2A 2nd Midterm. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (-electron): E n = m e Z 2 e 4 /2 2 n 2 = E Z 2 /n 2, n =, 2, 3,... where Ze is
April 6th, 24 Chemistry 2A 2nd Midterm. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (-electron): E n = m e Z 2 e 4 /2 2 n 2 = E Z 2 /n 2, n =, 2, 3,... where Ze is
Summary of free theory: one particle state: vacuum state is annihilated by all a s: then, one particle state has normalization:
 The LSZ reduction formula based on S-5 In order to describe scattering experiments we need to construct appropriate initial and final states and calculate scattering amplitude. Summary of free theory:
The LSZ reduction formula based on S-5 In order to describe scattering experiments we need to construct appropriate initial and final states and calculate scattering amplitude. Summary of free theory:
Identical Particles in Quantum Mechanics
 Identical Particles in Quantum Mechanics Chapter 20 P. J. Grandinetti Chem. 4300 Nov 17, 2017 P. J. Grandinetti (Chem. 4300) Identical Particles in Quantum Mechanics Nov 17, 2017 1 / 20 Wolfgang Pauli
Identical Particles in Quantum Mechanics Chapter 20 P. J. Grandinetti Chem. 4300 Nov 17, 2017 P. J. Grandinetti (Chem. 4300) Identical Particles in Quantum Mechanics Nov 17, 2017 1 / 20 Wolfgang Pauli
1.1 A Scattering Experiment
 1 Transfer Matrix In this chapter we introduce and discuss a mathematical method for the analysis of the wave propagation in one-dimensional systems. The method uses the transfer matrix and is commonly
1 Transfer Matrix In this chapter we introduce and discuss a mathematical method for the analysis of the wave propagation in one-dimensional systems. The method uses the transfer matrix and is commonly
Lecture 26: Qualitative Molecular Orbital Theory: Hückel Theory
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.6 Physical Chemistry I Fall, 07 Professor Robert W. Field Lecture 6: Qualitative Molecular Orbital Theory: Hückel Theory Models in Physical Chemistry Our job is
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.6 Physical Chemistry I Fall, 07 Professor Robert W. Field Lecture 6: Qualitative Molecular Orbital Theory: Hückel Theory Models in Physical Chemistry Our job is
Multi-Electron Atoms II
 Multi-Electron Atoms II LS Coupling The basic idea of LS coupling or Russell-Saunders coupling is to assume that spin-orbit effects are small, and can be neglected to a first approximation. If there is
Multi-Electron Atoms II LS Coupling The basic idea of LS coupling or Russell-Saunders coupling is to assume that spin-orbit effects are small, and can be neglected to a first approximation. If there is
5.5. Representations. Phys520.nb Definition N is called the dimensions of the representations The trivial presentation
 Phys50.nb 37 The rhombohedral and hexagonal lattice systems are not fully compatible with point group symmetries. Knowing the point group doesn t uniquely determine the lattice systems. Sometimes we can
Phys50.nb 37 The rhombohedral and hexagonal lattice systems are not fully compatible with point group symmetries. Knowing the point group doesn t uniquely determine the lattice systems. Sometimes we can
[Disclaimer: This is not a complete list of everything you need to know, just some of the topics that gave people difficulty.]
![[Disclaimer: This is not a complete list of everything you need to know, just some of the topics that gave people difficulty.] [Disclaimer: This is not a complete list of everything you need to know, just some of the topics that gave people difficulty.]](/thumbs/82/86615903.jpg) Math 43 Review Notes [Disclaimer: This is not a complete list of everything you need to know, just some of the topics that gave people difficulty Dot Product If v (v, v, v 3 and w (w, w, w 3, then the
Math 43 Review Notes [Disclaimer: This is not a complete list of everything you need to know, just some of the topics that gave people difficulty Dot Product If v (v, v, v 3 and w (w, w, w 3, then the
Electrons in Crystals. Chris J. Pickard
 Electrons in Crystals Chris J. Pickard Electrons in Crystals The electrons in a crystal experience a potential with the periodicity of the Bravais lattice: U(r + R) = U(r) The scale of the periodicity
Electrons in Crystals Chris J. Pickard Electrons in Crystals The electrons in a crystal experience a potential with the periodicity of the Bravais lattice: U(r + R) = U(r) The scale of the periodicity
Reciprocal Lattice. Let A(r) be some function featuring discrete translation symmetry:
 Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Lecture 5. Hartree-Fock Theory. WS2010/11: Introduction to Nuclear and Particle Physics
 Lecture 5 Hartree-Fock Theory WS2010/11: Introduction to Nuclear and Particle Physics Particle-number representation: General formalism The simplest starting point for a many-body state is a system of
Lecture 5 Hartree-Fock Theory WS2010/11: Introduction to Nuclear and Particle Physics Particle-number representation: General formalism The simplest starting point for a many-body state is a system of
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
H ψ = E ψ. Introduction to Exact Diagonalization. Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden
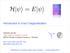 H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
Introduction to Group Theory
 Chapter 10 Introduction to Group Theory Since symmetries described by groups play such an important role in modern physics, we will take a little time to introduce the basic structure (as seen by a physicist)
Chapter 10 Introduction to Group Theory Since symmetries described by groups play such an important role in modern physics, we will take a little time to introduce the basic structure (as seen by a physicist)
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Phys 460 Describing and Classifying Crystal Lattices
 Phys 460 Describing and Classifying Crystal Lattices What is a material? ^ crystalline Regular lattice of atoms Each atom has a positively charged nucleus surrounded by negative electrons Electrons are
Phys 460 Describing and Classifying Crystal Lattices What is a material? ^ crystalline Regular lattice of atoms Each atom has a positively charged nucleus surrounded by negative electrons Electrons are
Tight Binding Method: Linear Combination of Atomic Orbitals (LCAO)
 Tight Binding Method: Linear Combination of Atomic Orbitals (LCAO) W. E. Pickett (Dated: April 14, 2014) This write-up is a homemade introduction to the tight binding representation of the electronic structure
Tight Binding Method: Linear Combination of Atomic Orbitals (LCAO) W. E. Pickett (Dated: April 14, 2014) This write-up is a homemade introduction to the tight binding representation of the electronic structure
Introduction to Quantum Computing
 Introduction to Quantum Computing Part I Emma Strubell http://cs.umaine.edu/~ema/quantum_tutorial.pdf April 12, 2011 Overview Outline What is quantum computing? Background Caveats Fundamental differences
Introduction to Quantum Computing Part I Emma Strubell http://cs.umaine.edu/~ema/quantum_tutorial.pdf April 12, 2011 Overview Outline What is quantum computing? Background Caveats Fundamental differences
C/CS/Phys C191 Particle-in-a-box, Spin 10/02/08 Fall 2008 Lecture 11
 C/CS/Phys C191 Particle-in-a-box, Spin 10/0/08 Fall 008 Lecture 11 Last time we saw that the time dependent Schr. eqn. can be decomposed into two equations, one in time (t) and one in space (x): space
C/CS/Phys C191 Particle-in-a-box, Spin 10/0/08 Fall 008 Lecture 11 Last time we saw that the time dependent Schr. eqn. can be decomposed into two equations, one in time (t) and one in space (x): space
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together.
 Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Calculating Electronic Structure of Different Carbon Nanotubes and its Affect on Band Gap
 Calculating Electronic Structure of Different Carbon Nanotubes and its Affect on Band Gap 1 Rashid Nizam, 2 S. Mahdi A. Rizvi, 3 Ameer Azam 1 Centre of Excellence in Material Science, Applied Physics AMU,
Calculating Electronic Structure of Different Carbon Nanotubes and its Affect on Band Gap 1 Rashid Nizam, 2 S. Mahdi A. Rizvi, 3 Ameer Azam 1 Centre of Excellence in Material Science, Applied Physics AMU,
Supplementary Figure S1. STM image of monolayer graphene grown on Rh (111). The lattice
 Supplementary Figure S1. STM image of monolayer graphene grown on Rh (111). The lattice mismatch between graphene (0.246 nm) and Rh (111) (0.269 nm) leads to hexagonal moiré superstructures with the expected
Supplementary Figure S1. STM image of monolayer graphene grown on Rh (111). The lattice mismatch between graphene (0.246 nm) and Rh (111) (0.269 nm) leads to hexagonal moiré superstructures with the expected
Physics 342 Lecture 26. Angular Momentum. Lecture 26. Physics 342 Quantum Mechanics I
 Physics 342 Lecture 26 Angular Momentum Lecture 26 Physics 342 Quantum Mechanics I Friday, April 2nd, 2010 We know how to obtain the energy of Hydrogen using the Hamiltonian operator but given a particular
Physics 342 Lecture 26 Angular Momentum Lecture 26 Physics 342 Quantum Mechanics I Friday, April 2nd, 2010 We know how to obtain the energy of Hydrogen using the Hamiltonian operator but given a particular
LS coupling. 2 2 n + H s o + H h f + H B. (1) 2m
 LS coupling 1 The big picture We start from the Hamiltonian of an atomic system: H = [ ] 2 2 n Ze2 1 + 1 e 2 1 + H s o + H h f + H B. (1) 2m n e 4πɛ 0 r n 2 4πɛ 0 r nm n,m Here n runs pver the electrons,
LS coupling 1 The big picture We start from the Hamiltonian of an atomic system: H = [ ] 2 2 n Ze2 1 + 1 e 2 1 + H s o + H h f + H B. (1) 2m n e 4πɛ 0 r n 2 4πɛ 0 r nm n,m Here n runs pver the electrons,
Floquet Topological Insulator:
 Floquet Topological Insulator: Understanding Floquet topological insulator in semiconductor quantum wells by Lindner et al. Condensed Matter Journal Club Caltech February 12 2014 Motivation Motivation
Floquet Topological Insulator: Understanding Floquet topological insulator in semiconductor quantum wells by Lindner et al. Condensed Matter Journal Club Caltech February 12 2014 Motivation Motivation
VALENCE Hilary Term 2018
 VALENCE Hilary Term 2018 8 Lectures Prof M. Brouard Valence is the theory of the chemical bond Outline plan 1. The Born-Oppenheimer approximation 2. Bonding in H + 2 the LCAO approximation 3. Many electron
VALENCE Hilary Term 2018 8 Lectures Prof M. Brouard Valence is the theory of the chemical bond Outline plan 1. The Born-Oppenheimer approximation 2. Bonding in H + 2 the LCAO approximation 3. Many electron
ECE 535 Theory of Semiconductors and Semiconductor Devices Fall 2015 Homework # 5 Due Date: 11/17/2015
 ECE 535 Theory of Semiconductors and Semiconductor Devices Fall 2015 Homework # 5 Due Date: 11/17/2015 Problem # 1 Two carbon atoms and four hydrogen atoms form and ethane molecule with the chemical formula
ECE 535 Theory of Semiconductors and Semiconductor Devices Fall 2015 Homework # 5 Due Date: 11/17/2015 Problem # 1 Two carbon atoms and four hydrogen atoms form and ethane molecule with the chemical formula
Physics 113!!!!! Spring 2009!! Quantum Theory Seminar #9
 Physics 113!!!!! Spring 2009!! Quantum Theory Seminar #9 Readings:!! Zettili - Chapter - 6!! Boccio - Chapter - 9 - Sections 9.6.1-9.6.13 Presentations: 3D Finite Well!!!!! _Sarah_ (Section 9.6.3)! 2D
Physics 113!!!!! Spring 2009!! Quantum Theory Seminar #9 Readings:!! Zettili - Chapter - 6!! Boccio - Chapter - 9 - Sections 9.6.1-9.6.13 Presentations: 3D Finite Well!!!!! _Sarah_ (Section 9.6.3)! 2D
Incompatibility Paradoxes
 Chapter 22 Incompatibility Paradoxes 22.1 Simultaneous Values There is never any difficulty in supposing that a classical mechanical system possesses, at a particular instant of time, precise values of
Chapter 22 Incompatibility Paradoxes 22.1 Simultaneous Values There is never any difficulty in supposing that a classical mechanical system possesses, at a particular instant of time, precise values of
2 The Density Operator
 In this chapter we introduce the density operator, which provides an alternative way to describe the state of a quantum mechanical system. So far we have only dealt with situations where the state of a
In this chapter we introduce the density operator, which provides an alternative way to describe the state of a quantum mechanical system. So far we have only dealt with situations where the state of a
Translation Groups; Introduction to Bands and Reciprocal Space. Timothy Hughbanks, Texas A& M University
 Translation Groups; Introduction to Bands and Reciprocal Space Timothy Hughbanks, Texas A& M University The One-dimensional Translation Group If chemists wish to understand the symmetry properties of a
Translation Groups; Introduction to Bands and Reciprocal Space Timothy Hughbanks, Texas A& M University The One-dimensional Translation Group If chemists wish to understand the symmetry properties of a
Vector Spaces in Quantum Mechanics
 Chapter 8 Vector Spaces in Quantum Mechanics We have seen in the previous Chapter that there is a sense in which the state of a quantum system can be thought of as being made up of other possible states.
Chapter 8 Vector Spaces in Quantum Mechanics We have seen in the previous Chapter that there is a sense in which the state of a quantum system can be thought of as being made up of other possible states.
Graphene and Carbon Nanotubes
 Graphene and Carbon Nanotubes 1 atom thick films of graphite atomic chicken wire Novoselov et al - Science 306, 666 (004) 100μm Geim s group at Manchester Novoselov et al - Nature 438, 197 (005) Kim-Stormer
Graphene and Carbon Nanotubes 1 atom thick films of graphite atomic chicken wire Novoselov et al - Science 306, 666 (004) 100μm Geim s group at Manchester Novoselov et al - Nature 438, 197 (005) Kim-Stormer
Electron States of Diatomic Molecules
 IISER Pune March 2018 Hamiltonian for a Diatomic Molecule The hamiltonian for a diatomic molecule can be considered to be made up of three terms Ĥ = ˆT N + ˆT el + ˆV where ˆT N is the kinetic energy operator
IISER Pune March 2018 Hamiltonian for a Diatomic Molecule The hamiltonian for a diatomic molecule can be considered to be made up of three terms Ĥ = ˆT N + ˆT el + ˆV where ˆT N is the kinetic energy operator
Physics 221A Fall 1996 Notes 19 The Stark Effect in Hydrogen and Alkali Atoms
 Physics 221A Fall 1996 Notes 19 The Stark Effect in Hydrogen and Alkali Atoms In these notes we will consider the Stark effect in hydrogen and alkali atoms as a physically interesting example of bound
Physics 221A Fall 1996 Notes 19 The Stark Effect in Hydrogen and Alkali Atoms In these notes we will consider the Stark effect in hydrogen and alkali atoms as a physically interesting example of bound
5 Problems Chapter 5: Electrons Subject to a Periodic Potential Band Theory of Solids
 E n = :75, so E cont = E E n = :75 = :479. Using E =!, :479 = m e k z =! j e j m e k z! k z = r :479 je j m e = :55 9 (44) (v g ) z = @! @k z = m e k z = m e :55 9 = :95 5 m/s. 4.. A ev electron is to
E n = :75, so E cont = E E n = :75 = :479. Using E =!, :479 = m e k z =! j e j m e k z! k z = r :479 je j m e = :55 9 (44) (v g ) z = @! @k z = m e k z = m e :55 9 = :95 5 m/s. 4.. A ev electron is to
14. Structure of Nuclei
 14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
2 Electronic structure theory
 Electronic structure theory. Generalities.. Born-Oppenheimer approximation revisited In Sec..3 (lecture 3) the Born-Oppenheimer approximation was introduced (see also, for instance, [Tannor.]). We are
Electronic structure theory. Generalities.. Born-Oppenheimer approximation revisited In Sec..3 (lecture 3) the Born-Oppenheimer approximation was introduced (see also, for instance, [Tannor.]). We are
Physics 221A Fall 2017 Notes 27 The Variational Method
 Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2017 Notes 27 The Variational Method 1. Introduction Very few realistic problems in quantum mechanics are exactly solvable, so approximation methods
Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2017 Notes 27 The Variational Method 1. Introduction Very few realistic problems in quantum mechanics are exactly solvable, so approximation methods
Chapter 9: Multi- Electron Atoms Ground States and X- ray Excitation
 Chapter 9: Multi- Electron Atoms Ground States and X- ray Excitation Up to now we have considered one-electron atoms. Almost all atoms are multiple-electron atoms and their description is more complicated
Chapter 9: Multi- Electron Atoms Ground States and X- ray Excitation Up to now we have considered one-electron atoms. Almost all atoms are multiple-electron atoms and their description is more complicated
Quantum Oscillations in Graphene in the Presence of Disorder
 WDS'9 Proceedings of Contributed Papers, Part III, 97, 9. ISBN 978-8-778-- MATFYZPRESS Quantum Oscillations in Graphene in the Presence of Disorder D. Iablonskyi Taras Shevchenko National University of
WDS'9 Proceedings of Contributed Papers, Part III, 97, 9. ISBN 978-8-778-- MATFYZPRESS Quantum Oscillations in Graphene in the Presence of Disorder D. Iablonskyi Taras Shevchenko National University of
Translation Symmetry, Space Groups, Bloch functions, Fermi energy
 Translation Symmetry, Space Groups, Bloch functions, Fermi energy Roberto Orlando and Silvia Casassa Università degli Studi di Torino July 20, 2015 School Ab initio Modelling of Solids (UniTo) Symmetry
Translation Symmetry, Space Groups, Bloch functions, Fermi energy Roberto Orlando and Silvia Casassa Università degli Studi di Torino July 20, 2015 School Ab initio Modelling of Solids (UniTo) Symmetry
Physics 202 Laboratory 5. Linear Algebra 1. Laboratory 5. Physics 202 Laboratory
 Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
