SCHOLASTIC APTITUDE TEST (SAT) HINTS & SOLUTIONS
|
|
|
- Brett Oliver
- 5 years ago
- Views:
Transcription
1 NATIONAL TALENT SEARCH EXAMINATION-08-9, UTTAR PRADESH NATIONAL TALENT SEARCH EXAMINATION-08-9, UP SCHOLASTIC APTITUDE TEST (SAT) HINTS & SOLUTIONS Solution PHYSICS 0. [] When object at C image at C Q 5 0. []= = = A T 5 0. [ ] Image is formed at retina 0. [ ] kwh = J 05. [ ] Generator converts mechanical energy to Electrical energy. 06. [] = V C = C = 0 8 m/sec V rated 07. [ ] R rated = P rated = 8 Now V = 0 V ; R = 8 P = R V = 5 Watt [ ] P = = = 5D f [ ] 5 = + = V f u 50 V = 6 cm 0. [] minimum distance to hear echo = 7 m. [] Solar cells are used in satellite.. [] At ºC density is maimum hence volume for same mass is minimum V > V SOL. SAT NTSE STAGE-I-09 PAGE-
2 . [] each resistance after cutting 5 R R When connected in parallel 5 R = 5 R' CHEMISTRY. Ans.() MO formula of amide M M Valency of element M is + So the phosphate of element is + M PO M (PO ) 5. Ans.() Solid CO is also called dry Ice. 6. Ans.() F-have maimum electro negativity 7. Ans.() HgO Hg + O 8. Ans. () HgS is called cinnabar NTSE STAGE-I-07-8 PAGE-
3 9. Ans. () CHO is functional group of ethanal 0. Ans. () ph of water is = 7. Ans. () C H 5 OH ethanol. Ans. () Oalic acid present in tomato. Ans. () 5 ml NaOH neutralize to = 0 ml HCl them 0 ml NaOH neutralize to 0 ml HCl. Ans. () Baking soda = NaHCO 5. Ans. () Displacement reaction 6. Ans. () If aluminium carbide react with water them aluminium hydroide and methane will produce. Al C + H O Al (OH) + CH NTSE STAGE-I-07-8 PAGE-
4 Mathematics 8. P() = =...() = ( 8) = 9 = 9 = + + = 5 + = 5 = =... () Using () & () = + + = = 0 = 8 = 7 Which not in option, so it is bonus 8. y = + y = = 6 = y = a =, b = Which not in option, so it is bonus NTSE STAGE-I-07-8 PAGE-
5 8. A (, ), B (5, ) and C (, y) 5 0 y + = y = 9 = y = 5 (, 5) 8. (i) tan + sin = m tan sin = n (tan sin ) = m. n...() m + n = tan m n = sin (m + n) (m n) = tansin from equ. () sin cos sin = m. n sin cos cosec = n. n tan sin = mn tansin = mn tansin = n.m put tansin = m n m n = n.m = 75 ( ) = 75 5= () ( ) = () ( ) = () equation () + () ( ) + ( ) = 8 ( ) ( ) + 8 = = = = 75. NTSE STAGE-I-07-8 PAGE-5
6 86. = = y = y = = y = + y = = Option (a) 88. Let number of sides = n n 8 = 60 n = 60 8 = 0 n = ( )( )( )( 8) = 6 = P( 6, 8), O(0, 6) OP = ( 6 0)(s 0) = = A B Income 9 Epenses y y Save = Income Epenses 000 = 9 y...() 000 = y... () () () 000 = 9 y 000 = y = NTSE STAGE-I-07-8 PAGE-6
7 = 000 Income of B = = 000 = 8000 Answer not in option. So it is Bonus. 9. Let side of st square = a Let side of nd square = a Area of first square = a Area of second square = a a + a = 68 perimeter p = a p = a (a + a ) = 0 a + a = 0 (a + a ) = 900 a + a + a a = (0 a ) (a ) = 900 a (0 a ) = = a + 0a = 6 a 0a + 6 = 0 a 8a a + 6 = 0 a (a 8) (a 8) = 0 (a ) (a 8) = 0 a = a = 8 Difference 8 = (A ) area of ABC = 8 (A ) area of AEF = NTSE STAGE-I-07-8 PAGE-7
8 A A = BC EF Given EF = 8 = BC = BC BC = = D 6 C 8 A 6 B Area ABC s = 6 8 = 9 = 9 5 = 5 Area of parallelogram = area triangle = Cone radius = height = y Volume = ( ) (y) Cylinder radius = height = y V = () (y) (6)(y) V V 9 y = 8 NTSE STAGE-I-07-8 PAGE-8
9 96. 5 sin = 5 cos = 5 sin = sin cos = 5 5 = Let = y = = y = = y = = 6 9y = 7 = 6 9 y = y = 6 7 = =.. are + + = = Let 5 = y 5 5 = 6 5y + 5 y = 6 5y 6y + 5 = 0 5y 5y y + 5 = 0 5y(y 5) (y 5) = 0 (5y ) (y 5) = 0 y = 5, 5 =, = NTSE STAGE-I-07-8 PAGE-9
HINTS & SOLUTIONS NATIONAL STANDARD EXAMINATION IN JUNIOR SCIENCE (NSEJS)
 NATIONAL STANDARD EXAMINATION IN JUNIOR SCIENCE (NSEJS) DATE : 9--07. (a) 008, 09,.., 9997 a 008 d 0 a n 9997 a n a + (n )d 9997 008 + (n ) 0 8989 (n ) 0 89 n n 90. HINTS & SOLUTIONS CODE : JS-5. (c)...
NATIONAL STANDARD EXAMINATION IN JUNIOR SCIENCE (NSEJS) DATE : 9--07. (a) 008, 09,.., 9997 a 008 d 0 a n 9997 a n a + (n )d 9997 008 + (n ) 0 8989 (n ) 0 89 n n 90. HINTS & SOLUTIONS CODE : JS-5. (c)...
NATIONAL TALENT SEARCH EXAMINATION-2017, NTSE STAGE-II SCHOLASTIC APTITUDE TEST (SAT)_HINTS & SOLUTIONS
 NATIONAL TALENT SEARCH EXAMINATION-07, NTSE STAGE-II -05-07 SCHOLASTIC APTITUDE TEST (SAT)_HINTS & SOLUTIONS 5. In M O 3 valency of metal M +3 So the formula of metal nitride will be M +3 N 3 M 3 N 3 MN
NATIONAL TALENT SEARCH EXAMINATION-07, NTSE STAGE-II -05-07 SCHOLASTIC APTITUDE TEST (SAT)_HINTS & SOLUTIONS 5. In M O 3 valency of metal M +3 So the formula of metal nitride will be M +3 N 3 M 3 N 3 MN
SCHOLASTIC APTITUDE TEST (SAT) HINTS & SOLUTIONS
 NATIONAL TALENT SEARCH EXAMINATION-2018-19, ODISHA 04-11-2018 NATIONAL TALENT SEARCH EXAMINATION-2018-19, ODISHA SCHOLASTIC APTITUDE TEST (SAT) HINTS & SOLUTIONS 1. R. I of medium is more so nature change.
NATIONAL TALENT SEARCH EXAMINATION-2018-19, ODISHA 04-11-2018 NATIONAL TALENT SEARCH EXAMINATION-2018-19, ODISHA SCHOLASTIC APTITUDE TEST (SAT) HINTS & SOLUTIONS 1. R. I of medium is more so nature change.
MENTAL ABILITY TEST (MAT) HINTS & SOLUTIONS 2. 2+(2-1) =3
 NATIONAL TALENT SEARCH EXAMINATION-2018-19, JHARKHAND 04-11-2018 NATIONAL TALENT SEARCH EXAMINATION-2018-19, JHARKHAND MENTAL ABILITY TEST (MAT) HINTS & SOLUTIONS 2. 2+(2-1) =3 7+(7-6) = 8 14+ (14-12)
NATIONAL TALENT SEARCH EXAMINATION-2018-19, JHARKHAND 04-11-2018 NATIONAL TALENT SEARCH EXAMINATION-2018-19, JHARKHAND MENTAL ABILITY TEST (MAT) HINTS & SOLUTIONS 2. 2+(2-1) =3 7+(7-6) = 8 14+ (14-12)
Question Bank Organic Chemistry II
 Question Bank Organic Chemistry II 1. What are saturated and unsaturated hydrocarbons. Classify the following as saturated and unsaturated hydrocarbons. CH 4, C 2 H 2, C 2 H 6, C 3 H 6, C 3 H 4 Ans. Compounds
Question Bank Organic Chemistry II 1. What are saturated and unsaturated hydrocarbons. Classify the following as saturated and unsaturated hydrocarbons. CH 4, C 2 H 2, C 2 H 6, C 3 H 6, C 3 H 4 Ans. Compounds
1) Write the Brønsted-Lowry reaction for weak acid HCN reacting with H 2 O.
 1) Write the Brønsted-Lowry reaction for weak acid HCN reacting with H O. HCN + H O º H O + + CN ) Write the Brønsted-Lowry reaction for weak base NH reacting with H O NH + H O º OH + NH + ) Using the
1) Write the Brønsted-Lowry reaction for weak acid HCN reacting with H O. HCN + H O º H O + + CN ) Write the Brønsted-Lowry reaction for weak base NH reacting with H O NH + H O º OH + NH + ) Using the
SCHOLASTIC APTITUDE TEST (SAT) HINTS & SOLUTIONS
 NATIONAL TALENT SEARH EXAMINATION-08, RAJASTHAN 05--0 NATIONAL TALENT SEARH EXAMINATION-08, RAJASTHAN SHOLASTI APTITUE TEST (SAT) HINTS & SOLUTIONS. onstant speed means equal distance in equal interval
NATIONAL TALENT SEARH EXAMINATION-08, RAJASTHAN 05--0 NATIONAL TALENT SEARH EXAMINATION-08, RAJASTHAN SHOLASTI APTITUE TEST (SAT) HINTS & SOLUTIONS. onstant speed means equal distance in equal interval
PROGRESS TEST-2 RB , RBK-1806 RBS-1803 (JEE ADVANCED PATTERN)
 PROGRESS TEST- RB-83-84, RBK-806 RBS-803 (JEE ADVANCED PATTERN) Test Date: 6-09-07 [ ] PT-II (ADV) RB-83-84, RBK-806 & RBS-803_6.09.07. (A). (C) v 3v 3 m / s in downward direction B 3. (A) g 5 A PHYSICS
PROGRESS TEST- RB-83-84, RBK-806 RBS-803 (JEE ADVANCED PATTERN) Test Date: 6-09-07 [ ] PT-II (ADV) RB-83-84, RBK-806 & RBS-803_6.09.07. (A). (C) v 3v 3 m / s in downward direction B 3. (A) g 5 A PHYSICS
Q A. A B C A C D A,C,D A,B,C B,C B,C Q A. C C B SECTION-I. , not possible
 PAPER CODE SCORE-I LEADER & ENTHUSIAST COURSE TARGET : JEE (Main + Advanced) 04 PART- : MATHEMATICS SECTION-I SECTION-II SECTION-IV. Ans. (A) 0 C T 0 7 SECTION-I For x > & >, [x] - [] - + is not possible
PAPER CODE SCORE-I LEADER & ENTHUSIAST COURSE TARGET : JEE (Main + Advanced) 04 PART- : MATHEMATICS SECTION-I SECTION-II SECTION-IV. Ans. (A) 0 C T 0 7 SECTION-I For x > & >, [x] - [] - + is not possible
c. K 2 CO 3 d. (NH 4 ) 2 SO 4 Answer c
 Chem 130 Name Exam 2, Ch 4-6 July 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and
Chem 130 Name Exam 2, Ch 4-6 July 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and
Culinary Chemistry: Stoichiometry Made Simple
 Culinary Chemistry: Stoichiometry Made Simple Background Stoichiometry is based on the law of conservation of mass which states that the mass of the reactants in a chemical reaction equals the mass of
Culinary Chemistry: Stoichiometry Made Simple Background Stoichiometry is based on the law of conservation of mass which states that the mass of the reactants in a chemical reaction equals the mass of
CHEMICAL REACTIONS. Types of Reactions. Steps to Writing Reactions
 Types of Reactions CHEMICAL REACTIONS There are five types of chemical reactions we will talk about: 1. Synthesis reactions 2. reactions 3. Single displacement reactions 4. reactions 5. Combustion reactions
Types of Reactions CHEMICAL REACTIONS There are five types of chemical reactions we will talk about: 1. Synthesis reactions 2. reactions 3. Single displacement reactions 4. reactions 5. Combustion reactions
* Terms & Conditions apply. Name of the Student :... Reg. No. :... Duration : 1.30 hours Max. Marks : 114
 Vision Infinity Scholarship Award IIT JEE 03 Students of Vision Infinity who secure All India Rank in IIT JEE within top00,will be Awarded scholarship for four years during B.Tech in IIT * Terms & Conditions
Vision Infinity Scholarship Award IIT JEE 03 Students of Vision Infinity who secure All India Rank in IIT JEE within top00,will be Awarded scholarship for four years during B.Tech in IIT * Terms & Conditions
CHM 115 EXAM #3 Practice
 Name CHM 115 EXAM #3 Practice Complete the following showing all of your work. When calculations are required, show the set-up, units, and report answers with the correct number of significant figures.
Name CHM 115 EXAM #3 Practice Complete the following showing all of your work. When calculations are required, show the set-up, units, and report answers with the correct number of significant figures.
SCIENCE JSUNIL TUTORIAL CLASS 9. Activity 1
 Activity Objective To understand, that there is a change in mass when a chemical change takes place. (To understand law of conservation of mass experimentally). Procedure. Take one of the following sets,
Activity Objective To understand, that there is a change in mass when a chemical change takes place. (To understand law of conservation of mass experimentally). Procedure. Take one of the following sets,
Final Exam Review Questions You will be given a Periodic Table, Activity Series, and a Common Ions Chart CP CHEMISTRY
 Final Exam Review Questions You will be given a Periodic Table, Activity Series, and a Common Ions Chart CP CHEMISTRY Part A True-False State whether each statement is true or false. If false, correct
Final Exam Review Questions You will be given a Periodic Table, Activity Series, and a Common Ions Chart CP CHEMISTRY Part A True-False State whether each statement is true or false. If false, correct
RAMANUJAN MATHEMATICS CLUB,
 A. B Q R C A=, BQ= AC=11 BC=... In the adjacent figure, If A= cm, BQ= cm and AC=11 cm, then BC=...cm. 1 ) 1 ) ) 1 8. ; Match the following. a) r h ) b) ) c) ) d) 1 r r r h 1 a, b, c, d ) 1 d, c, b, a )
A. B Q R C A=, BQ= AC=11 BC=... In the adjacent figure, If A= cm, BQ= cm and AC=11 cm, then BC=...cm. 1 ) 1 ) ) 1 8. ; Match the following. a) r h ) b) ) c) ) d) 1 r r r h 1 a, b, c, d ) 1 d, c, b, a )
Copyright 2018 Dan Dill 1
 TP The expression for the equilibrium constant for the solubility equilibrium M 2 X 2 M X 2 is 1. sp 2 M X 2 / M 2 X 2. sp 2 M 2 X 2 / M 2 X 3. sp 2 M 2 X 2 4. sp M 2 X 2 Lecture 21 CH102 A1 (MWF 9:05
TP The expression for the equilibrium constant for the solubility equilibrium M 2 X 2 M X 2 is 1. sp 2 M X 2 / M 2 X 2. sp 2 M 2 X 2 / M 2 X 3. sp 2 M 2 X 2 4. sp M 2 X 2 Lecture 21 CH102 A1 (MWF 9:05
Titration region & buffer calculations. An acid base region is a circumstance
 CH102 Summer 1 2012 Boston University An acid base region is a circumstance not a sequence of operations Region 1: Only weak acid (base) Region 2: Both weak acid (base) and its conjugate base (acid) Region
CH102 Summer 1 2012 Boston University An acid base region is a circumstance not a sequence of operations Region 1: Only weak acid (base) Region 2: Both weak acid (base) and its conjugate base (acid) Region
SSC (Tier-II) (Mock Test Paper - 2) [SOLUTION]
![SSC (Tier-II) (Mock Test Paper - 2) [SOLUTION] SSC (Tier-II) (Mock Test Paper - 2) [SOLUTION]](/thumbs/96/127498144.jpg) SS (Tier-II) - 0 (Mock Test Paper - ) [SOLUTION]. () 7 7 7 7 7 8 8 7 8 7 7 9 R R 8 8 8. (D) Let the first odd integer the second odd integer + Difference between the squares of these two consecutive odd
SS (Tier-II) - 0 (Mock Test Paper - ) [SOLUTION]. () 7 7 7 7 7 8 8 7 8 7 7 9 R R 8 8 8. (D) Let the first odd integer the second odd integer + Difference between the squares of these two consecutive odd
Goal: During this lab students will gain a quantitative understanding of limiting reagents.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
UNIT 6338: SAMPLE ACTIVITY 28 SAMPLE ASSESSMENT ACTIVITIES AND SCHEDULES PROTON TRANSFERS
 UNIT 6338: SAMPLE ACTIVITY 28 SAMPLE ASSESSMENT ACTIVITIES AND SCHEDULES 6.129 6338 CHEMISTRY LEVEL 2 PROTON TRANSFERS This activity assesses: Unit: 6338 Characterise the behaviour of weak and strong acids
UNIT 6338: SAMPLE ACTIVITY 28 SAMPLE ASSESSMENT ACTIVITIES AND SCHEDULES 6.129 6338 CHEMISTRY LEVEL 2 PROTON TRANSFERS This activity assesses: Unit: 6338 Characterise the behaviour of weak and strong acids
NTSE TEST FULL TEST-2. Test Date :
 NTSE TEST FULL TEST- Test Date : 0..07 Corporate Office : Paruslok, Boring Road Crossing, Patna-0 Kankarbagh Office : A-0, st Floor, Patrakar Nagar, Patna-0 Bazar Samiti Office : Rainbow Tower, Sai Complex,
NTSE TEST FULL TEST- Test Date : 0..07 Corporate Office : Paruslok, Boring Road Crossing, Patna-0 Kankarbagh Office : A-0, st Floor, Patrakar Nagar, Patna-0 Bazar Samiti Office : Rainbow Tower, Sai Complex,
5/14/14. How can you measure the amount of heat released when a match burns?
 CHEMISTRY & YOU Chapter 7 Thermochemistry How can you measure the amount of heat released when a match burns? 7. The Flow of Energy 7.3 Heat in Changes of State 7.4 Calculating Heats of Reaction Remember:
CHEMISTRY & YOU Chapter 7 Thermochemistry How can you measure the amount of heat released when a match burns? 7. The Flow of Energy 7.3 Heat in Changes of State 7.4 Calculating Heats of Reaction Remember:
Pre RMO Exam Paper Solution:
 Paper Solution:. How many positive integers less than 000 have the property that the sum of the digits of each such number is divisible by 7 and the number itself is divisible by 3? Sum of Digits Drivable
Paper Solution:. How many positive integers less than 000 have the property that the sum of the digits of each such number is divisible by 7 and the number itself is divisible by 3? Sum of Digits Drivable
Cambridge International Examinations CambridgeOrdinaryLevel
 Cambridge International Examinations CambridgeOrdinaryLevel * 2 5 4 0 0 0 9 5 8 5 * ADDITIONAL MATHEMATICS 4037/12 Paper1 May/June 2015 2 hours CandidatesanswerontheQuestionPaper. NoAdditionalMaterialsarerequired.
Cambridge International Examinations CambridgeOrdinaryLevel * 2 5 4 0 0 0 9 5 8 5 * ADDITIONAL MATHEMATICS 4037/12 Paper1 May/June 2015 2 hours CandidatesanswerontheQuestionPaper. NoAdditionalMaterialsarerequired.
Example Exercise 10.1 Interpreting Chemical Equation Calculations
 Example Exercise 10.1 Interpreting Chemical Equation Calculations Given the chemical equation for the combustion of methane, CH 4, balance the equation and interpret the coefficients in terms of (a) moles
Example Exercise 10.1 Interpreting Chemical Equation Calculations Given the chemical equation for the combustion of methane, CH 4, balance the equation and interpret the coefficients in terms of (a) moles
Balancing Equations. Reactants: Zn + I 2 Product: Zn I 2
 Balancing Equations 1 Reactants: Zn + I 2 Product: Zn I 2 2 Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O 2 (g) ---> 2
Balancing Equations 1 Reactants: Zn + I 2 Product: Zn I 2 2 Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O 2 (g) ---> 2
Student Worksheet for Kinetics
 Student Worksheet for Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations Average Reaction
Student Worksheet for Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations Average Reaction
Chemistry 3202 Unit Test : Thermochemistry
 Chemistry 3202 Unit Test : Thermochemistry Name:_ Part A: Multiple Choice. Circle the best answer for each question. Answer all questions in this section. (24 pts) 1. A heated 250 g sample of aluminium
Chemistry 3202 Unit Test : Thermochemistry Name:_ Part A: Multiple Choice. Circle the best answer for each question. Answer all questions in this section. (24 pts) 1. A heated 250 g sample of aluminium
C hapter ATOMS. (c) (ii) and (iii) (d) (ii) and (iv)
 C hapter 3 ATOMS AND MOLECULES 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv) 1.2044 10 25 molecules
C hapter 3 ATOMS AND MOLECULES 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv) 1.2044 10 25 molecules
Chem 128, Final Exam May 5, 2004
 I. (70 points) This part of the final corresponds to Exam I. It covers the material in Chapters 10, 11, and 12. For parts A, C, D, E show all your work no matter how trivial. A. (20 points) Consider chloroform,
I. (70 points) This part of the final corresponds to Exam I. It covers the material in Chapters 10, 11, and 12. For parts A, C, D, E show all your work no matter how trivial. A. (20 points) Consider chloroform,
Patna Centre. Hint & Solution , 90, , 60, 2. 4 a b c d a b d Mean
 Patna Centre Hint & Solution Part I MT 1. Hints:- 10 40 3 10, 60, 180 60 3 180, 90, 90 3 70. Hints:- 3, 6, 9,1, 15, 18 3. Hints:- a b c d 100 4 a b c d 4 100 400 a b d 400 70 330 330 Mean 110 3 4. Hints:-
Patna Centre Hint & Solution Part I MT 1. Hints:- 10 40 3 10, 60, 180 60 3 180, 90, 90 3 70. Hints:- 3, 6, 9,1, 15, 18 3. Hints:- a b c d 100 4 a b c d 4 100 400 a b d 400 70 330 330 Mean 110 3 4. Hints:-
Sec 4 Maths SET D PAPER 2
 S4MA Set D Paper Sec 4 Maths Exam papers with worked solutions SET D PAPER Compiled by THE MATHS CAFE P a g e Answer all questions. Write your answers and working on the separate Answer Paper provided.
S4MA Set D Paper Sec 4 Maths Exam papers with worked solutions SET D PAPER Compiled by THE MATHS CAFE P a g e Answer all questions. Write your answers and working on the separate Answer Paper provided.
q 3 x 1.6 x 10 C, V 10V, W?
 Sat Sol: 1. 19 q 3 x 1.6 x 10 C, V 10V, W? W qv 19 W 3 x 1.6 x 10 x 10 18 W 4.8 x 10 J In moving one Lithium Nucleas, work done is 10 J So in moving 10 nucleus W ' 10W 17 4.8 x 10 J. Direction of Magnetic
Sat Sol: 1. 19 q 3 x 1.6 x 10 C, V 10V, W? W qv 19 W 3 x 1.6 x 10 x 10 18 W 4.8 x 10 J In moving one Lithium Nucleas, work done is 10 J So in moving 10 nucleus W ' 10W 17 4.8 x 10 J. Direction of Magnetic
Fill in as many empirical properties for acids and bases as you can...
 Fill in as many empirical properties for acids and bases as you can... Tastes sour Turn blue litmus red ph less than 7 Neutralize bases React w/ active metals to produce H 2(g) React w/ carbonates to produce
Fill in as many empirical properties for acids and bases as you can... Tastes sour Turn blue litmus red ph less than 7 Neutralize bases React w/ active metals to produce H 2(g) React w/ carbonates to produce
Reporting Category 1: Matter and Energy
 Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms 8.5A Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the
Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms 8.5A Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the
CHEM J-4 June 2014
 CHEM1102 2014-J-4 June 2014 Calculate the ph of a 0.010 M solution of aspirin at 25 C. The pk a of aspirin is 3.5 at this temperature. 7 Ammonia, NH 3, is a weak base in water. Write the equation for the
CHEM1102 2014-J-4 June 2014 Calculate the ph of a 0.010 M solution of aspirin at 25 C. The pk a of aspirin is 3.5 at this temperature. 7 Ammonia, NH 3, is a weak base in water. Write the equation for the
Chapter 5 Goals. Section Valence Electrons & Electron-Dot Symbols. Record into your notes
 Major Goals of Chapter 5: 1. Finding the exact location for valence electrons (outermost electrons) 2. Discuss the octet rule and why 8 is a magic number when Draw Lewis Dots 3. Define what an ionic substance
Major Goals of Chapter 5: 1. Finding the exact location for valence electrons (outermost electrons) 2. Discuss the octet rule and why 8 is a magic number when Draw Lewis Dots 3. Define what an ionic substance
1) What is the volume of a tank that can hold Kg of methanol whose density is 0.788g/cm 3?
 1) Convert the following 1) 125 g to Kg 6) 26.9 dm 3 to cm 3 11) 1.8µL to cm 3 16) 4.8 lb to Kg 21) 23 F to K 2) 21.3 Km to cm 7) 18.2 ml to cm 3 12) 2.45 L to µm 3 17) 1.2 m to inches 22) 180 ºC to K
1) Convert the following 1) 125 g to Kg 6) 26.9 dm 3 to cm 3 11) 1.8µL to cm 3 16) 4.8 lb to Kg 21) 23 F to K 2) 21.3 Km to cm 7) 18.2 ml to cm 3 12) 2.45 L to µm 3 17) 1.2 m to inches 22) 180 ºC to K
CHEMISTRY 101 EXAM 1 FORM 1J
 CHEMISTRY 101 EXAM 1 SECTIONS 540-550 Dr. Joy Heising FORM 1J September 26, 2003 Directions: 1. This examination consists of two parts: 17 multiple choice questions (4 points each) in Part 1 and 3 free
CHEMISTRY 101 EXAM 1 SECTIONS 540-550 Dr. Joy Heising FORM 1J September 26, 2003 Directions: 1. This examination consists of two parts: 17 multiple choice questions (4 points each) in Part 1 and 3 free
4) If ax 2 + bx + c = 0 has equal roots, then c is equal. b) b 2. a) b 2
 Karapettai Nadar Boys Hr. Sec. School One Word Test No 1 Standard X Time: 20 Minutes Marks: (15 1 = 15) Answer all the 15 questions. Choose the orrect answer from the given four alternatives and write
Karapettai Nadar Boys Hr. Sec. School One Word Test No 1 Standard X Time: 20 Minutes Marks: (15 1 = 15) Answer all the 15 questions. Choose the orrect answer from the given four alternatives and write
Acid Base Equilibria
 Acid Base Equilibria Acid Ionization, also known as acid dissociation, is the process in where an acid reacts with water to produce a hydrogen ion and the conjugate base ion. HC 2 H 3 O 2(aq) H + (aq)
Acid Base Equilibria Acid Ionization, also known as acid dissociation, is the process in where an acid reacts with water to produce a hydrogen ion and the conjugate base ion. HC 2 H 3 O 2(aq) H + (aq)
Part (1) Second : Trigonometry. Tan
 Part (1) Second : Trigonometry (1) Complete the following table : The angle Ratio 42 12 \ Sin 0.3214 Cas 0.5321 Tan 2.0625 (2) Complete the following : 1) 46 36 \ 24 \\ =. In degrees. 2) 44.125 = in degrees,
Part (1) Second : Trigonometry (1) Complete the following table : The angle Ratio 42 12 \ Sin 0.3214 Cas 0.5321 Tan 2.0625 (2) Complete the following : 1) 46 36 \ 24 \\ =. In degrees. 2) 44.125 = in degrees,
Reactant A (g) Reactant B (ml) Product (ml)
 Pre-Test Key Name: Chemical Reactions Date: 1. The chart below shows the amount of gas that is produced when two reactants (a solid and a liquid) are combined. However, one of the boxes is missing information.
Pre-Test Key Name: Chemical Reactions Date: 1. The chart below shows the amount of gas that is produced when two reactants (a solid and a liquid) are combined. However, one of the boxes is missing information.
Chapter 10 Worksheet 1 Name: Honors Accelerated Geometry Hour:
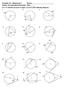 hapter 10 Worksheet 1 Name: Honors ccelerated Geometry Hour: For 1-15, find the measure of angle in each of the following diagrams. 1. 2.. 258 84 140 40 4. 5. 6. 2 y 80 y 72 7. 8. 9. 50 X 40 140 4 y 10.
hapter 10 Worksheet 1 Name: Honors ccelerated Geometry Hour: For 1-15, find the measure of angle in each of the following diagrams. 1. 2.. 258 84 140 40 4. 5. 6. 2 y 80 y 72 7. 8. 9. 50 X 40 140 4 y 10.
KEY Chem 1A First Midterm Examination February 7, 2005 Professor David Chandler
 KEY Chem 1A First Midterm Examination February 7, 2005 Professor David Chandler 1 2 3 4 5 6 7 8 9 10 Extra Name: Signature: Section: GSI: Instructions As indicated, either fill in blank space with appropriate
KEY Chem 1A First Midterm Examination February 7, 2005 Professor David Chandler 1 2 3 4 5 6 7 8 9 10 Extra Name: Signature: Section: GSI: Instructions As indicated, either fill in blank space with appropriate
IB ANS. -30 = -10 k k = 3
 IB ANS.. Find the value of k, if the straight lines 6 0y + 3 0 and k 5y + 8 0 are parallel. Sol. Given lines are 6 0y + 3 0 and k 5y + 8 0 a b lines are parallel a b -30-0 k k 3. Find the condition for
IB ANS.. Find the value of k, if the straight lines 6 0y + 3 0 and k 5y + 8 0 are parallel. Sol. Given lines are 6 0y + 3 0 and k 5y + 8 0 a b lines are parallel a b -30-0 k k 3. Find the condition for
Do Now April 24, 2017
 Do Now April 24, 2017 Obj: Observe and describe neutralization reactions. Copy: Neutralization is when an acid and base react to product a salt and water. e.g. HCl + NaOH NaCl + H 2 O acid base salt water
Do Now April 24, 2017 Obj: Observe and describe neutralization reactions. Copy: Neutralization is when an acid and base react to product a salt and water. e.g. HCl + NaOH NaCl + H 2 O acid base salt water
Name: Index Number: Class: CATHOLIC HIGH SCHOOL Preliminary Examination 3 Secondary 4
 Name: Inde Number: Class: CATHOLIC HIGH SCHOOL Preliminary Eamination 3 Secondary 4 ADDITIONAL MATHEMATICS 4047/1 READ THESE INSTRUCTIONS FIRST Write your name, register number and class on all the work
Name: Inde Number: Class: CATHOLIC HIGH SCHOOL Preliminary Eamination 3 Secondary 4 ADDITIONAL MATHEMATICS 4047/1 READ THESE INSTRUCTIONS FIRST Write your name, register number and class on all the work
Pre-Regional Mathematical Olympiad Solution 2017
 Pre-Regional Mathematical Olympiad Solution 07 Time:.5 hours. Maximum Marks: 50 [Each Question carries 5 marks]. How many positive integers less than 000 have the property that the sum of the digits of
Pre-Regional Mathematical Olympiad Solution 07 Time:.5 hours. Maximum Marks: 50 [Each Question carries 5 marks]. How many positive integers less than 000 have the property that the sum of the digits of
7.01 Chemical Reactions
 7.01 Chemical Reactions The Law of Conservation of Mass Dr. Fred Omega Garces Chemistry 100 Miramar College 1 Chemical Reactions Making Substances Chemical Reactions; the heart of chemistry is the chemical
7.01 Chemical Reactions The Law of Conservation of Mass Dr. Fred Omega Garces Chemistry 100 Miramar College 1 Chemical Reactions Making Substances Chemical Reactions; the heart of chemistry is the chemical
Solutions. S1. Ans.(c) Sol. Try Using option and divide With given option
 Solutions S1. Ans.(c) Try Using option and divide 64329 With given option S2. Ans.(a) Let the fraction be x/y. According to the questions, x y of 4 7 + 4 7 = 15 14 x y 4 7 + 4 7 = 15 14 x y 4 7 = 1 2 x
Solutions S1. Ans.(c) Try Using option and divide 64329 With given option S2. Ans.(a) Let the fraction be x/y. According to the questions, x y of 4 7 + 4 7 = 15 14 x y 4 7 + 4 7 = 15 14 x y 4 7 = 1 2 x
Test Corrections for Unit 1 Test
 MUST READ DIRECTIONS: Read the directions located on www.koltymath.weebly.com to understand how to properly do test corrections. Ask for clarification from your teacher if there are parts that you are
MUST READ DIRECTIONS: Read the directions located on www.koltymath.weebly.com to understand how to properly do test corrections. Ask for clarification from your teacher if there are parts that you are
Rao IIT Academy/ SSC - Board Exam 2018 / Mathematics Code-A / QP + Solutions JEE MEDICAL-UG BOARDS KVPY NTSE OLYMPIADS SSC - BOARD
 Rao IIT cademy/ SSC - oard Exam 018 / Mathematics Code- / QP + Solutions JEE MEDICL-UG ORDS KVPY NTSE OLYMPIDS SSC - ORD - 018 Date: 1.0.018 MTHEMTICS - PPER- - SOLUTIONS Q.1 ttempt any FIVE of the following
Rao IIT cademy/ SSC - oard Exam 018 / Mathematics Code- / QP + Solutions JEE MEDICL-UG ORDS KVPY NTSE OLYMPIDS SSC - ORD - 018 Date: 1.0.018 MTHEMTICS - PPER- - SOLUTIONS Q.1 ttempt any FIVE of the following
x n+1 = ( x n + ) converges, then it converges to α. [2]
![x n+1 = ( x n + ) converges, then it converges to α. [2] x n+1 = ( x n + ) converges, then it converges to α. [2]](/thumbs/92/110299841.jpg) 1 A Level - Mathematics P 3 ITERATION ( With references and answers) [ Numerical Solution of Equation] Q1. The equation x 3 - x 2 6 = 0 has one real root, denoted by α. i) Find by calculation the pair
1 A Level - Mathematics P 3 ITERATION ( With references and answers) [ Numerical Solution of Equation] Q1. The equation x 3 - x 2 6 = 0 has one real root, denoted by α. i) Find by calculation the pair
 APPLICATIONS OF DERIVATIVES OBJECTIVES. The approimate increase in the area of a square plane when each side epands from c m to.0 cm is () 0.00 sq. cm () 0.006 sq. cm () 0.06 sq. cm () None. If y log then
APPLICATIONS OF DERIVATIVES OBJECTIVES. The approimate increase in the area of a square plane when each side epands from c m to.0 cm is () 0.00 sq. cm () 0.006 sq. cm () 0.06 sq. cm () None. If y log then
CHEMISTRY 101 EXAM 1 FORM 1N
 CHEMISTRY 101 EXAM 1 SECTIONS 572-580 Dr. Joy Heising FORM 1N September 20, 2001 Directions: 1. Fill out your scantron sheet. a. Do not forget to include your SIGNATURE and ID number. b. Dept = CHEM, Course
CHEMISTRY 101 EXAM 1 SECTIONS 572-580 Dr. Joy Heising FORM 1N September 20, 2001 Directions: 1. Fill out your scantron sheet. a. Do not forget to include your SIGNATURE and ID number. b. Dept = CHEM, Course
Environmental Science and Technology Theory Examination. Combined Question and Answer Booklet
 Secondary Cycle 2, Year Two Theory Examination ( In plain English, grade 10) January 2013 EST-400.A01 (Sec. 4) Combined Question and Answer Booklet (We lower your eco-foot print by putting it all into
Secondary Cycle 2, Year Two Theory Examination ( In plain English, grade 10) January 2013 EST-400.A01 (Sec. 4) Combined Question and Answer Booklet (We lower your eco-foot print by putting it all into
7.01 Chemical Reactions
 7.01 Chemical Reactions The Law of Conservation of Mass Dr. Fred Omega Garces Chemistry 152 Miramar College 1 Chemical Reactions Making Substances Chemical Reactions; the heart of chemistry is the chemical
7.01 Chemical Reactions The Law of Conservation of Mass Dr. Fred Omega Garces Chemistry 152 Miramar College 1 Chemical Reactions Making Substances Chemical Reactions; the heart of chemistry is the chemical
HALF YEARLY EXAMINATIONS 2015/2016
 FORM 4 SECONDARY SCHOOLS HALF YEARLY EXAMINATIONS 2015/2016 MATHS NON-CALCULATOR PAPER Track 3 Time: 20 min Name: Non-Calculator Paper Class: Answer all questions. Each question carries 1 mark. Question
FORM 4 SECONDARY SCHOOLS HALF YEARLY EXAMINATIONS 2015/2016 MATHS NON-CALCULATOR PAPER Track 3 Time: 20 min Name: Non-Calculator Paper Class: Answer all questions. Each question carries 1 mark. Question
Final Review Packet. When 100% correct, you will receive a
 Final Review Packet When 100% correct, you will receive a 15-point bonus sticker to place on the final exam. Deadline: Wednesday, Feb. 8. NO EXCEPTIONS!!!!!! Note! The Final Exam will be worth two tests
Final Review Packet When 100% correct, you will receive a 15-point bonus sticker to place on the final exam. Deadline: Wednesday, Feb. 8. NO EXCEPTIONS!!!!!! Note! The Final Exam will be worth two tests
Geometry. A. Right Triangle. Legs of a right triangle : a, b. Hypotenuse : c. Altitude : h. Medians : m a, m b, m c. Angles :,
 Geometry A. Right Triangle Legs of a right triangle : a, b Hypotenuse : c Altitude : h Medians : m a, m b, m c Angles :, Radius of circumscribed circle : R Radius of inscribed circle : r Area : S 1. +
Geometry A. Right Triangle Legs of a right triangle : a, b Hypotenuse : c Altitude : h Medians : m a, m b, m c Angles :, Radius of circumscribed circle : R Radius of inscribed circle : r Area : S 1. +
Safety: Safety goggles should be worn at all times. Students should hold the balloons on the test tubes tightly while the reaction takes place.
 LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
LIMITING REAGENT LAB: THE REACTION BETWEEN VINEGAR AND BAKING SODA Goal: During this lab students will gain a quantitative understanding of limiting reagents. Safety: Safety goggles should be worn at all
CLASS X FORMULAE MATHS
 Real numbers: Euclid s division lemma Given positive integers a and b, there exist whole numbers q and r satisfying a = bq + r, 0 r < b. Euclid s division algorithm: This is based on Euclid s division
Real numbers: Euclid s division lemma Given positive integers a and b, there exist whole numbers q and r satisfying a = bq + r, 0 r < b. Euclid s division algorithm: This is based on Euclid s division
Note Taking Guide: Episode 701. Lab results: 1 doz grains of rice = g (Use this fact as a conversion factor.) Avogadro s Number - the = the number
 Note Taking Guide: Episode 701 Name Lab results: 1 doz grains of rice = g (Use this fact as a conversion factor.)? grains of rice = 1.94 g Avogadro s Number - the = the number Molar Mass the of one of
Note Taking Guide: Episode 701 Name Lab results: 1 doz grains of rice = g (Use this fact as a conversion factor.)? grains of rice = 1.94 g Avogadro s Number - the = the number Molar Mass the of one of
THE MOLE - PART 2. Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 THE MOLE - PART 2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which one of the following statements is a quantitative observation? a.
THE MOLE - PART 2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which one of the following statements is a quantitative observation? a.
CHEMISTRY. concentration of salt at equivalence point = C = 0.1 M. K = M b
 CHEMISTRY SECTION- I STRAIGHT OBJECTIVE TYPE This section contains 6 multiple choice questions. Each question has 4 choice (A), (B), (C) and (D), out of which ONLY-ONE is correct 47. 2.5 ml of 5 2 M weak
CHEMISTRY SECTION- I STRAIGHT OBJECTIVE TYPE This section contains 6 multiple choice questions. Each question has 4 choice (A), (B), (C) and (D), out of which ONLY-ONE is correct 47. 2.5 ml of 5 2 M weak
Math Day at the Beach 2018
 Multiple Choice Write your name and school and mark your answers on the answer sheet. You have 30 minutes to work on these problems. No calculator is allowed. 1. A bag has some white balls and some red
Multiple Choice Write your name and school and mark your answers on the answer sheet. You have 30 minutes to work on these problems. No calculator is allowed. 1. A bag has some white balls and some red
CHEMISTRY 102 Fall 2010 Hour Exam III. 1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with:
 1. My answers for this Chemistry 10 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E Consider the titration of 30.0 ml of 0.30 M HCN by 0.10
1. My answers for this Chemistry 10 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E Consider the titration of 30.0 ml of 0.30 M HCN by 0.10
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Practice Test 1-0308- Chapter 8 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Tell whether the angle is acute, right, obtuse, or straight. 1) 1)
Practice Test 1-0308- Chapter 8 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Tell whether the angle is acute, right, obtuse, or straight. 1) 1)
GCSE 4370/05 MATHEMATICS LINEAR PAPER 1 HIGHER TIER
 Surname Centre Number Candidate Number Other Names 0 GCSE 4370/05 MATHEMATICS LINEAR PAPER 1 HIGHER TIER A.M. WEDNESDAY, 6 November 2013 2 hours CALCULATORS ARE NOT TO BE USED FOR THIS PAPER For s use
Surname Centre Number Candidate Number Other Names 0 GCSE 4370/05 MATHEMATICS LINEAR PAPER 1 HIGHER TIER A.M. WEDNESDAY, 6 November 2013 2 hours CALCULATORS ARE NOT TO BE USED FOR THIS PAPER For s use
Chem 130 Name Exam 2 October 11, Points Part I: Complete all of problems 1-9
 Chem 130 Name Exam October 11, 017 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and significant
Chem 130 Name Exam October 11, 017 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and significant
O %lo ÆË v ÃO y Æ fio» flms ÿ,» fl Ê«fiÀ M, ÊMV fl KARNATAKA SECONDARY EDUCATION EXAMINATION BOARD, MALLESWARAM, BANGALORE
 CCE RF CCE RR O %lo ÆË v ÃO y Æ fio» flms ÿ,» fl Ê«fiÀ M, ÊMV fl 50 00 KARNATAKA SECONDARY EDUCATION EXAMINATION BOARD, MALLESWARAM, BANGALE 50 00 G È.G È.G È.. Æ fioê,» ^È% / HØ È 0 S. S. L. C. EXAMINATION,
CCE RF CCE RR O %lo ÆË v ÃO y Æ fio» flms ÿ,» fl Ê«fiÀ M, ÊMV fl 50 00 KARNATAKA SECONDARY EDUCATION EXAMINATION BOARD, MALLESWARAM, BANGALE 50 00 G È.G È.G È.. Æ fioê,» ^È% / HØ È 0 S. S. L. C. EXAMINATION,
Titration of a Weak Acid with a Strong Base
 Titration of a Weak Acid with a Strong Base Weak Acid w/ Strong Base Overall: INITIAL ph: Weak acids do not fully dissociate we need to do an ICE table to determine initial ph. We expect it to be weakly
Titration of a Weak Acid with a Strong Base Weak Acid w/ Strong Base Overall: INITIAL ph: Weak acids do not fully dissociate we need to do an ICE table to determine initial ph. We expect it to be weakly
PROBLEMS 13 - APPLICATIONS OF DERIVATIVES Page 1
 PROBLEMS - APPLICATIONS OF DERIVATIVES Page ( ) Water seeps out of a conical filter at the constant rate of 5 cc / sec. When the height of water level in the cone is 5 cm, find the rate at which the height
PROBLEMS - APPLICATIONS OF DERIVATIVES Page ( ) Water seeps out of a conical filter at the constant rate of 5 cc / sec. When the height of water level in the cone is 5 cm, find the rate at which the height
Titration a solution of known concentration, called a standard solution
 Acid-Base Titrations Titration is a form of analysis in which we measure the volume of material of known concentration sufficient to react with the substance being analyzed. Titration a solution of known
Acid-Base Titrations Titration is a form of analysis in which we measure the volume of material of known concentration sufficient to react with the substance being analyzed. Titration a solution of known
Lesson-3 TRIGONOMETRIC RATIOS AND IDENTITIES
 Lesson- TRIGONOMETRIC RATIOS AND IDENTITIES Angle in trigonometry In trigonometry, the measure of an angle is the amount of rotation from B the direction of one ray of the angle to the other ray. Angle
Lesson- TRIGONOMETRIC RATIOS AND IDENTITIES Angle in trigonometry In trigonometry, the measure of an angle is the amount of rotation from B the direction of one ray of the angle to the other ray. Angle
Stoichiometry SUPPLEMENTAL PROBLEMS CHAPTER 12. 3Si(s) 2N 2 N 4. (g) 0 Si 3. (s) PO 4. the reaction. Cr(s) H 3. (aq) 0.
 CHAPTER 12 Stoichiometry 1. Silicon nitride is used in the manufacturing of high-temperature thermal insulation for heat engines and turbines. It is produced by the following 3Si(s) 2N 2 (g) 0 Si 3 N 4
CHAPTER 12 Stoichiometry 1. Silicon nitride is used in the manufacturing of high-temperature thermal insulation for heat engines and turbines. It is produced by the following 3Si(s) 2N 2 (g) 0 Si 3 N 4
CHEMISTRY. SCIENCE Paper 2
 CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
Lab 2. Go Their Separate Ways: Separation of an Acid, Base, and Neutral Substance by Acid-Base Extraction
 Lab 2. Go Their Separate Ways: Separation of an Acid, Base, and Neutral Substance by Acid-Base Extraction How can I use an acid-base reaction to separate an acid-base-neutral mixture? Objectives 1. use
Lab 2. Go Their Separate Ways: Separation of an Acid, Base, and Neutral Substance by Acid-Base Extraction How can I use an acid-base reaction to separate an acid-base-neutral mixture? Objectives 1. use
1. Glyoxal consists of 41.4% C, 3.5% H, and 55.1% O by mass. What is the empirical formula of glyoxal? (A) CHO (B) CH 2 O (C) CH 2 O 2 (D) C 12 HO 16
 1 ACS Final Review **Questions are taken from actual past ACS USNCO exams. It is an overview of the topics that will be covered on the exam based on `materials covered. Please continue to study and review
1 ACS Final Review **Questions are taken from actual past ACS USNCO exams. It is an overview of the topics that will be covered on the exam based on `materials covered. Please continue to study and review
Example 1: Finding angle measures: I ll do one: We ll do one together: You try one: ML and MN are tangent to circle O. Find the value of x
 Ch 1: Circles 1 1 Tangent Lines 1 Chords and Arcs 1 3 Inscribed Angles 1 4 Angle Measures and Segment Lengths 1 5 Circles in the coordinate plane 1 1 Tangent Lines Focused Learning Target: I will be able
Ch 1: Circles 1 1 Tangent Lines 1 Chords and Arcs 1 3 Inscribed Angles 1 4 Angle Measures and Segment Lengths 1 5 Circles in the coordinate plane 1 1 Tangent Lines Focused Learning Target: I will be able
CHEMICAL REACTIONS WORDS, SYMBOLS AND ABBREVIATIONS
 CHEMICAL REACTIONS All chemical reactions have two parts: (1) A substance that undergoes a reaction is called a. In other words, reactants are the substances you start with. (2) When reactants undergo
CHEMICAL REACTIONS All chemical reactions have two parts: (1) A substance that undergoes a reaction is called a. In other words, reactants are the substances you start with. (2) When reactants undergo
 COORDINATE GEOMETRY LOCUS EXERCISE 1. The locus of P(x,y) such that its distance from A(0,0) is less than 5 units is x y 5 ) x y 10 x y 5 4) x y 0. The equation of the locus of the point whose distance
COORDINATE GEOMETRY LOCUS EXERCISE 1. The locus of P(x,y) such that its distance from A(0,0) is less than 5 units is x y 5 ) x y 10 x y 5 4) x y 0. The equation of the locus of the point whose distance
3Fe +2 (aq) + 2PO 4. NaHCO 3 + H 2 O. Chem 121 Quiz 3 Practice Fall 2017
 Chem 121 Quiz 3 Practice Fall 2017 The following quiz contains 22 questions (and bonuses!) valued at 1 point/question Name KEY G = H T S PV = nrt P 1 V 1 = P 2 V 2 P 1 /T 1 = P 2 /T 2 V 1 /T 1 = V 2 /T
Chem 121 Quiz 3 Practice Fall 2017 The following quiz contains 22 questions (and bonuses!) valued at 1 point/question Name KEY G = H T S PV = nrt P 1 V 1 = P 2 V 2 P 1 /T 1 = P 2 /T 2 V 1 /T 1 = V 2 /T
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON
 Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Chemistry 30: Thermochemistry. Practice Problems
 Name: Period: Chemistry 30: Thermochemistry Practice Problems Date: Heat and Temperature 1. Pretend you are doing a scientific study on the planet Earth. a. Name three things in the system you are studying.
Name: Period: Chemistry 30: Thermochemistry Practice Problems Date: Heat and Temperature 1. Pretend you are doing a scientific study on the planet Earth. a. Name three things in the system you are studying.
Narayana IIT Academy
 --7_Sr.T_Z_JEE-ADV_(_P)_CTA-4_Key &Sol s Narayana T Academy NDA Sec: Sr.T_Z JEE ADVANCE Date: --7 Time: : PM to 5: PM -P-Model Max Marks: 4 KEY & SOLUTONS KEY SHEET CHEMSTRY C C C 4 D 5 A 6 A 7 B 8 C 9
--7_Sr.T_Z_JEE-ADV_(_P)_CTA-4_Key &Sol s Narayana T Academy NDA Sec: Sr.T_Z JEE ADVANCE Date: --7 Time: : PM to 5: PM -P-Model Max Marks: 4 KEY & SOLUTONS KEY SHEET CHEMSTRY C C C 4 D 5 A 6 A 7 B 8 C 9
Buffer Calculations. The Standard Equilibrium Approach to Calculating a Buffer s ph
 Buffer Calculations A buffer is a solution that has the ability to resist a change in ph upon the addition of a strong acid or a strong base. For a buffer to exist it must satisfy two conditions: (1) the
Buffer Calculations A buffer is a solution that has the ability to resist a change in ph upon the addition of a strong acid or a strong base. For a buffer to exist it must satisfy two conditions: (1) the
Chapter 04 Molarity-Dilution-Stoichiometry with Solutions Worksheet (KEY)
 Chapter 04 Molarity-Dilution-Stoichiometry with Solutions Worksheet (KEY) 1. A person sufferin from hyponatremia has a sodium ion concentration in the blood of 0.118 M and a total blood volume of 4.6.
Chapter 04 Molarity-Dilution-Stoichiometry with Solutions Worksheet (KEY) 1. A person sufferin from hyponatremia has a sodium ion concentration in the blood of 0.118 M and a total blood volume of 4.6.
Pre-REGIONAL MATHEMATICAL OLYMPIAD, 2017
 P-RMO 017 NATIONAL BOARD FOR HIGHER MATHEMATICS AND HOMI BHABHA CENTRE FOR SCIENCE EDUCATION TATA INSTITUTE OF FUNDAMENTAL RESEARCH Pre-REGIONAL MATHEMATICAL OLYMPIAD, 017 TEST PAPER WITH SOLUTION & ANSWER
P-RMO 017 NATIONAL BOARD FOR HIGHER MATHEMATICS AND HOMI BHABHA CENTRE FOR SCIENCE EDUCATION TATA INSTITUTE OF FUNDAMENTAL RESEARCH Pre-REGIONAL MATHEMATICAL OLYMPIAD, 017 TEST PAPER WITH SOLUTION & ANSWER
Stoichiometry study of the relationships in a
 Note Taking Guide: Episode 801 Name Stoichiometry study of the relationships in a based on equations 2 Mg + O 2 2 MgO The in a give the for the involved in the. Ex. Problem: When elemental aluminum reacts
Note Taking Guide: Episode 801 Name Stoichiometry study of the relationships in a based on equations 2 Mg + O 2 2 MgO The in a give the for the involved in the. Ex. Problem: When elemental aluminum reacts
Complete this study guide to receive 5 bonus points on your test. Only study guides that are complete will receive the bonus.
 CHEMISTRY AND PERIODIC TABLE STUDY GUIDE Assigned: Thursday, 09 10 14 Due: Thursday, 09 18 14 Test Day: Friday, 09 19 14 Complete this study guide to receive 5 bonus points on your test. Only study guides
CHEMISTRY AND PERIODIC TABLE STUDY GUIDE Assigned: Thursday, 09 10 14 Due: Thursday, 09 18 14 Test Day: Friday, 09 19 14 Complete this study guide to receive 5 bonus points on your test. Only study guides
11 is the same as their sum, find the value of S.
 Answers: (998-99 HKMO Final Events) Created by: Mr. Francis Hung Last updated: July 08 Individual Events I P 4 I a 8 I a 6 I4 a I5 a IS a Q 8 b 0 b 7 b b spare b 770 R c c c c 0 c 57 S 0 d 000 d 990 d
Answers: (998-99 HKMO Final Events) Created by: Mr. Francis Hung Last updated: July 08 Individual Events I P 4 I a 8 I a 6 I4 a I5 a IS a Q 8 b 0 b 7 b b spare b 770 R c c c c 0 c 57 S 0 d 000 d 990 d
1983 FG8.1, 1991 HG9, 1996 HG9
 nswers: (1- HKMO Heat Events) reated by: Mr. Francis Hung Last updated: 6 February 017 - Individual 1 11 70 6 1160 7 11 8 80 1 10 1 km 6 11-1 Group 6 7 7 6 8 70 10 Individual Events I1 X is a point on
nswers: (1- HKMO Heat Events) reated by: Mr. Francis Hung Last updated: 6 February 017 - Individual 1 11 70 6 1160 7 11 8 80 1 10 1 km 6 11-1 Group 6 7 7 6 8 70 10 Individual Events I1 X is a point on
Chemical Reactions. Chemical Reactions Chemical reactions have a standard format when written:
 0.3.notebook A chemical property is a behaviour that occurs when substances change to create a new substance. When a new substance is created, a chemical change has occurred. New colour Evidence of chemical
0.3.notebook A chemical property is a behaviour that occurs when substances change to create a new substance. When a new substance is created, a chemical change has occurred. New colour Evidence of chemical
SSC [PRE+MAINS] Mock Test 230 [Answer with Solution]
![SSC [PRE+MAINS] Mock Test 230 [Answer with Solution] SSC [PRE+MAINS] Mock Test 230 [Answer with Solution]](/thumbs/91/106461951.jpg) SS [PRE+MAINS] Mock Test 0 [Answer with Solution]. () (x ) (x + ) (x ) (x + ) (x + x ) (x + x ) x + x x + x + x x x x + x + x x x + So, cofficient of x. () HF of fraction. () HF of,, LM of,8, t t x v x
SS [PRE+MAINS] Mock Test 0 [Answer with Solution]. () (x ) (x + ) (x ) (x + ) (x + x ) (x + x ) x + x x + x + x x x x + x + x x x + So, cofficient of x. () HF of fraction. () HF of,, LM of,8, t t x v x
Narayana IIT Academy
 INDIA Sec: Jr IIT_IZ CUT-18 Date: 18-1-17 Time: 07:30 AM to 10:30 AM 013_P MaxMarks:180 KEY SHEET PHYSICS 1 ABCD ACD 3 AC 4 BD 5 AC 6 ABC 7 ACD 8 ABC 9 A 10 A 11 A 1 C 13 B 14 C 15 B 16 C 17 A 18 B 19
INDIA Sec: Jr IIT_IZ CUT-18 Date: 18-1-17 Time: 07:30 AM to 10:30 AM 013_P MaxMarks:180 KEY SHEET PHYSICS 1 ABCD ACD 3 AC 4 BD 5 AC 6 ABC 7 ACD 8 ABC 9 A 10 A 11 A 1 C 13 B 14 C 15 B 16 C 17 A 18 B 19
2. An aldehyde can be obtained by the dehydrogenation of an alcohol. The catalyst used in the reaction is
 Class: 12 Subject: Chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Rectified spirit is converted to absolute alcohol taking advantage of the
Class: 12 Subject: Chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Rectified spirit is converted to absolute alcohol taking advantage of the
