STRUCTURAT ASPECTS OF TFIE MARCASITE-PYRITE TRANSFORTVTATION
|
|
|
- Evan Miles
- 5 years ago
- Views:
Transcription
1 STRUCTURAT ASPECTS OF TFIE MARCASITE-PYRITE TRANSFORTVTATION M. E. FLEET. Department oj Geology, (Jn'ivers'ity of Western On'tario, London, Ontar'io AesrRAcr Oriented single crystals of marcasite have been inverted to pyrite by heating under vacuum in the temperature range 425 to 475"C. X-ray diffraclion studies reveal two preferred orientations of pyrite, with az of pyrite parallel to b of marcasite and or and os sub-parallel to [101] of marcasite, agreeing with the orientation of some natural intergronths of the two minerals. It is suggested that the inversion occurs by rotation of the Fe-S chains in alternate layers of the {101} planes of marcasite. The shape of the r-ray reflections recorded in the plane normal to the common b axis suggests that the crystallites have been rotated about this axis: the nature and extent of the movement and the displacement between the two pyrite orientations within this plane tend to agree with the proposed transformation model. hvrnooucrton Pyrite and marcasite are regarded commonly as polymorphic {orms of FeS2: pyrite is cubic, a : A (Swanson, Gilfrich & Ugrinic 1955) ' Z : imarcasiteisorthorhombic,a : A,b : A,c :3.381A (Buerger L937a), Z : 2. Their stability relationship has been the subject of much discussion over the years. Pure natural marcasite readily inverts to pyrite at temperatures greater than 400'C. However, the reaction is irreversible and it is not known if marcasite has a field of stability at stoichiometric FeSz at temperatures less than this. It has been suggested that the two are not polymorphic, since marcasite appears to be S deficient* (Buerger 1934), However, marcasite readily precipitates from acid aqueous solutions (Allen, Crenshaw, & Merwin 1914) but has not been synthesized under dry conditions and this prompted Kullerud (1967) to speculate that H-S bonds might be significant in stabilizing it. The present study is devoted to the structural aspects of the transformation; the stability relation is not treated although the results might throw some light on this problem The structure of pyrite (Bragg 1914; Parker ct Whitehouse 1932) and of marcasite (Buerger 1931 ; 1937b) are well known, and only certain aspects pertinent to the present discussion will be presented. The FeSz molecule contains a disulphide group. The basic structural unit in each modification is a chain of alternating Fe atoms and disulphide groups, in which the *The deviation from the theoretical composition is probabiy within the limits of analytical errors (Dana, System oj M'ineral,ogy, 1944,
2 226 TIIE CANADIAN MINERALOGIsT disulphide groups lie at an angle to the directions of the chain, giving it a zig-zag appearance. In the marcasite structure the chains o<tend in the b direction, arranged in layers parallel to the basal plane, and in directions normal to the b axis within the {101} planes (Fig. 1). Each S atom is in four-fold co-ordination, three Fe atoms in a triangular group on the opposite side to the other S in the disulphide group, and each Fe atom is in a slightly distorted octahedral co-ordination (Table 1). In tjre pyrite structure the Fe-S chains extend in three mutually perpendicular directions (a-axes), so that the disulphide groups are centred on the midpoints of the cube edges and cube body-centre, and lie on four non-intersecting triad axes (Fig. 2). The co-ordinations of the Fe and S atoms are similar to those in marcasite. A comparison of some of the interatomic distances in the two structures is given in Table L. In marcasite, the disulphide groups of adjacent chains within the same basal layer are relatively close to each otjrer (2.97 A). In contrast, the S-S distance between equivalent basal layers is relatively large (3.38 A) ; in fact, there is a slight gap in the structure between the disulphide groups of these layers. There is a good correlation between <\ -{- +./ a./ --a-- b a c I o_ Frc. 1. Representation of the crystal structure of marcasite, Full circles, Fe at 0: open circles, Fe at l/2; arrows indicate the direction of inclination of disulphide groups.
3 MARCAS 227 / Qo a Frc. 2. The crystal structure of pyrite. Tl,sr-s 1 Bond Length (A) Fe-S $-S (S2 group) S_S Pyrite L Marcasite ( 2.23 \'2.2L z.zs r 3.38 I Within same basal layer Between adjacent basal layers Between equivalent basal layers Between adjacent basal layer Within same basal layer the b parameter and d(101) of marcasite and the cell edge of pyrite. The fact that all are related to directions of Fe-S chains emphasizes the importance of these chains in tjrese two structures. A related phenomenon is that natural intergrowths with {101} of marcasite parallel to {001} of pyrite are quite common when the two minerals occur together. ExpBnrMsr.rrAl INvEsrrcATroN Natural marcasite, from Bird Dog Mine, Cardin, Oklahoma, was used in the investigation. A prismatic piece from the edge of a 'cockscomb'
4 228 TIIE CANADIAN MINIqRALoGIST crystal aggregate, bounded in part by a pair of {1or} faces and elongated in the & direction, was placed ina 0.3 mm. diameter quartzglass capillary. The marcasite fragment was held in place at the end of the capillaiy bya flush-fitting, glass fibre and the capillary was evacuated and sealed. to a length of t to g inches. inversion -The experiments were carried out in an Jc-ray heating camera, ;arhich' essentially, consisted of a water-cooled, cylindrically heating element, wound, suspended coaxially inside the cassette of a unican rotation camera. The heating process was controiled manually with a variable transformer, and achromel-alumel thermocouple, placed near the specimen was used to record the temperature at trrat point. The temperature.of the sample was inferred by calibrating the furnace remperature against the known thermal expansion of gold (Merryman 1964). The calibration was done by recording powder diffraction patterns, at various temperatures, of a small piece of gold supported in a capillary tube. The recorded sample temperatures are believed accurate to within +10oC, although the precision is much better. The sealed capillary tube, supporled at the end of a small ceramic tube, was mounted ona goniometer head so that, during a run witfi the furnace in place, all of the vapour space surrounding the specimen was within the furnace and heated by it, albeit with a thermal gradient along the length. Although the marcasite had previously been oriented and prlcession and rotation exposures taken, it was not possible to record an fi-ray pattern at high temperature since, when oriented, the capillary tube was invariably inclined at a greater angle than was allowed bv the internal diameter of the furnace. The normal procedure, then, rvas to heat the specimen, remove the furnace and reset the goniometer arcs to the values obtained previously and obtain a rotation pattern to estimate the extent, if. any, of the inversion of the marcasite to pyrite. Experience with several samples indicated that the inversion was partially complete after heating for 12 hours at 425oC and essentially complete at 425"C with less than 4 hours heating..precession photographs were taken at the conclusion of each experiment with tfre spe_gime1 mounted in the capilrary tube (,i..e. with rhe originar marcasite b direction parallel to the dial axis) and u'ith the specimen lor. fragment of it) removed and remounted to allow for pr"""."i,or about this direction. DrscussroN - Rotation and precession photographs showed that pyrite had developed from the marcasite with a preferred orientation,."ith orr" cube axis (arbitrarily designated a2) parallel to the marcasite & axis. The other cube
5 MARCASITE-PYRITE TI'ANSFORIIATION 229 axes (o1 and os) are subparallel to the [101] directions of marcasite. On precession photographs taken with b parallel to the dial axis the reflections of the pyrite are relatively sharp. It is evident from the apparent four-fold symmetry that two pyrite orientations had developed. On precession photographs precessed about the marcasite b axis the diffraction spots are spread about this axis (Fig. S): the two pyrite orientations are made more evident because of the extinction rule for hul, reflections, I, * 2n. Each orientation is related to a [101] marcasite direction and has been rotated (or spread) toward the original marcasite c direction. :;i ii ilr i.aiiil!ilrriq ii,i.!,4!9,!,8; ffi ft E h.'' '; :::;: i:: iair il: : ::.:.aa!!.::l: ; iitiir ilr!.]::::l ;i i: iii-14, il,t.'' Frc, 3. Zero level, precession photograph ol partially inverted marcasite crystal, precessed about the mircasite b axis, The diffraction streaks of the remnant marcasite lre identified by the spots within them. The original a and c axes of marcasite are indicated. Mo Ka radiation, 35 kv, 20 ma, 16 hour exposure.
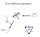
6 230 THE CANADIAN MINERALOGIST The preferred orientation of the pyrite and the rapidity of the reaction suggest that the structural reorganization involved in the transformation is minimal. A model for the transformation, based on the structural elements common to both minerals, is outlined below. The diagonal layer of Fe-s chains in the marcasite structure (A-A in Fig. 1) is similar to the layer c-c in pyrite (Fig. 2). A distorted pyrite structure can be produced from the marcasite structure, simply by rotating those Fe-s chains in the adjacent layers (of type R-B in Fig. l) approximately g0 degrees clockwise about the chain axes (Fig. 4), and translating the whole structure L/2 along b. The disulphide groups within these layers, then, are directed toward the adjacent 'basal' layers, fitting into the gap, noted above, in the marcasite structure. In the transformation, only one in six of the Fe-s bonds are broken: these are the chain-to-chain bonds witrrin the B-B layers. Because of the new positions taken by tlre disulphide.rt utjr' I rf.,\p.,t -z." o+ I -'.- '.., 't.. -'.. {,. FIc..4. Development of the pyrite structure from that of marcasite, illustrating the redistribution of the Fe-S bonds.
7 MARCASITE-PYRITE TRANSFORMATION groups in these diagonal layers, the strucfure is contracted in the marcasite a direction and expanded in the marcasite c direction. The original marcasite crystal will be stressed in these directions, and those crystallites in it which invert to pyrite in the manner outlined ('i.e. based on layers parallel to (101)) will tend to be rotated counter-clockwise. It is possible to produce a second pyrite orientation by counter-clockwise rotation of the Fe-S chains in the layers of the type A'-A' (Fig. L)' a.a. based on layers parallel to (10T). Crystallites formed with this orientation will tend to be rotated clockwise. The {101} planes in marcasite intersect at 74"36'. Ideally, the two pyrite orientations should each be rotated 7o42' so that they are superimposed (ar of the one parallel to as of the other alc.). However, each crystallite is subject to interference from neighbouring crystallites in the host crystal and there is an overall reduction involume of 2.1 per cent. The angular separation of tl1e two orientations in several separate crystals varied f.rom 2 to 7 degrees. In the specimen illustrated (Fig. 3) the separation is 3 degrees, and the spread of the (002) and (400) reflections of the remnant marcasite crystallites is in the range 15 to 20 degrees, suggesting that they have been displaced up to 10 degrees from their mean position in the original marcasite crystal. These observations agree quite well with the proposed mode of the transformation. 23L RBrBnBltcns Ar,lon, E. T., CnoNsuew' J. L. & Mnnwrx, H. E. (1914): Efiect of temperature and.cidity in the formation of marcasite (FeSz) and wurtzite (ZtS), Am. J. Sc'i'ence,38, 393. Bnecc, W. L. (1914): The analysis of crystals by the r-ray spectrometer, Proc, Roy' Soc', A, 89, 468. Buonoon, M. J. (1931): The crystal structure of marcasite, Am. M'ineral'.,16' 361' (1934): The pyrite-marcasite relation, Am. M'ineral,, 19,,37. (193?a): Inteiatomic distances in marcasite and notes on the bonding in crystals of ldllingite, arsonopyrite, and marcasite types, Ze'i't. Kr'ist,,97, 504., (1g37b): A common orientation and a classification for crystals based upon a marcasite-like packing, Am. M'ineral,, 22, 48. Kur,r-enuo, G. (19dDr Sulfide studies, in Researckes 'in geocham'istry,2, Abelson, P'H', Edit., John Wiley, New York. MonnruAs-, R. G. (1-964): A study of temperature measurement precision in Debye- Scherrer specimens during high temperature r-ray diffraction measurement of thermal expansion, Los Al,amos Scicntifn Laboratory Report,.Lil Penrsn, H. M. & Wuireuouso, W. J. (19i2): An r-ray analysis of iron pyrites by the method of Fourier Series, Ph'i'l'. Mag., Tth ser., 14,939. SweNsor.r, H. E., Gn-rnrcs, \i. T. & Ucnrwrc, G. M. (1955): Standard r-ray diffraction patterns, Nat, Bur. Stand'arils, C'irc. 539, no. 6,29, Manuscr'i'pt received' August 21, 1969
The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures.
 657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
Fro. 2. Fibrous brucite in vein of chrysotile asbestos, Asbestos, Quebec. Pr,ern I. Frc. 1. Photograph of fibrous brucite from Asbestos, Quebec.
 Pr,ern I Frc. 1. Photograph of fibrous brucite from Asbestos, Quebec. sbe stos Fro. 2. Fibrous brucite in vein of chrysotile asbestos, Asbestos, Quebec. Frc. 3. Sketch of photograph in Fig. 2, to show
Pr,ern I Frc. 1. Photograph of fibrous brucite from Asbestos, Quebec. sbe stos Fro. 2. Fibrous brucite in vein of chrysotile asbestos, Asbestos, Quebec. Frc. 3. Sketch of photograph in Fig. 2, to show
INVESTIGATION OF THE DEGREE OF PERFECTION OF A CRYSTAL BY MEANS OF POLARIZED X-RAYS BY S. RAMASESHAN AND G. N. RAMACHANDRAN, F.A.Sc.
 INVESTIGATION OF THE DEGREE OF PERFECTION OF A CRYSTAL BY MEANS OF POLARIZED X-RAYS BY S. RAMASESHAN AND G. N. RAMACHANDRAN, F.A.Sc.* (Department of Physics, Indian Institute of Science, Bangalore 3) Received
INVESTIGATION OF THE DEGREE OF PERFECTION OF A CRYSTAL BY MEANS OF POLARIZED X-RAYS BY S. RAMASESHAN AND G. N. RAMACHANDRAN, F.A.Sc.* (Department of Physics, Indian Institute of Science, Bangalore 3) Received
The distinction of pyrite from marcasite in nodular growths.
 179 The distinction of pyrite from marcasite in nodular growths. By F. A. BANNISTER, M.A. Assistant-Keeper in the Mineral Department of the British Museum of Natural History. [Read March 15, 1932.] A RECENT
179 The distinction of pyrite from marcasite in nodular growths. By F. A. BANNISTER, M.A. Assistant-Keeper in the Mineral Department of the British Museum of Natural History. [Read March 15, 1932.] A RECENT
X-ray Diffraction. Diffraction. X-ray Generation. X-ray Generation. X-ray Generation. X-ray Spectrum from Tube
 X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
The Solid State. Phase diagrams Crystals and symmetry Unit cells and packing Types of solid
 The Solid State Phase diagrams Crystals and symmetry Unit cells and packing Types of solid Learning objectives Apply phase diagrams to prediction of phase behaviour Describe distinguishing features of
The Solid State Phase diagrams Crystals and symmetry Unit cells and packing Types of solid Learning objectives Apply phase diagrams to prediction of phase behaviour Describe distinguishing features of
Chem 728 Introduction to Solid Surfaces
 Chem 728 Introduction to Solid Surfaces Solids: hard; fracture; not compressible; molecules close to each other Liquids: molecules mobile, but quite close to each other Gases: molecules very mobile; compressible
Chem 728 Introduction to Solid Surfaces Solids: hard; fracture; not compressible; molecules close to each other Liquids: molecules mobile, but quite close to each other Gases: molecules very mobile; compressible
SOLID STATE 18. Reciprocal Space
 SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
X-ray determination of centrosymmetry in three felspars. 1
 759 X-ray determination of centrosymmetry in three felspars. 1 By S. W. BAILEY, R. B. FERUUSO~, and W. It. TAYLOR. Crystallographic Laboratory, Cavendish Laboratory, Cambridge. [Read May 16, 1951.] T Introduction.
759 X-ray determination of centrosymmetry in three felspars. 1 By S. W. BAILEY, R. B. FERUUSO~, and W. It. TAYLOR. Crystallographic Laboratory, Cavendish Laboratory, Cambridge. [Read May 16, 1951.] T Introduction.
Electron Diffraction
 Exp-3-Electron Diffraction.doc (TJR) Physics Department, University of Windsor Introduction 64-311 Laboratory Experiment 3 Electron Diffraction In 1924 de Broglie predicted that the wavelength of matter
Exp-3-Electron Diffraction.doc (TJR) Physics Department, University of Windsor Introduction 64-311 Laboratory Experiment 3 Electron Diffraction In 1924 de Broglie predicted that the wavelength of matter
X-ray practical: Crystallography
 X-ray practical: Crystallography Aim: To familiarise oneself with the operation of Tex-X-Ometer spectrometer and to use it to determine the lattice spacing in NaCl and LiF single crystals. Background:
X-ray practical: Crystallography Aim: To familiarise oneself with the operation of Tex-X-Ometer spectrometer and to use it to determine the lattice spacing in NaCl and LiF single crystals. Background:
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 11 Crystallography and Crystal Structures, Part 3 Suggested Reading Chapter 6 in Waseda Chapter 1 in F.D. Bloss, Crystallography and Crystal Chemistry: An Introduction,
Analytical Methods for Materials Lesson 11 Crystallography and Crystal Structures, Part 3 Suggested Reading Chapter 6 in Waseda Chapter 1 in F.D. Bloss, Crystallography and Crystal Chemistry: An Introduction,
(si1tm)2 - sin O)n 2
 Vo., 8, 1922 PHYSICS: DA VIS AND TERRILL 357 tory explanation has been found for the profound effect of such a small amount of gas or the behavior of the tube. The investigation is being continued and
Vo., 8, 1922 PHYSICS: DA VIS AND TERRILL 357 tory explanation has been found for the profound effect of such a small amount of gas or the behavior of the tube. The investigation is being continued and
The structure of liquids and glasses. The lattice and unit cell in 1D. The structure of crystalline materials. Describing condensed phase structures
 Describing condensed phase structures Describing the structure of an isolated small molecule is easy to do Just specify the bond distances and angles How do we describe the structure of a condensed phase?
Describing condensed phase structures Describing the structure of an isolated small molecule is easy to do Just specify the bond distances and angles How do we describe the structure of a condensed phase?
Chapter 2. X-ray X. Diffraction and Reciprocal Lattice. Scattering from Lattices
 Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
4. Interpenetrating simple cubic
 2 1. The correct structure t of CsClCl crystal is 1. Simple cubic 2. Body centered cubic 3. Face centered cubic 4. Interpenetrating simple cubic If corner as well as the particle at the center are same
2 1. The correct structure t of CsClCl crystal is 1. Simple cubic 2. Body centered cubic 3. Face centered cubic 4. Interpenetrating simple cubic If corner as well as the particle at the center are same
Igneous petrology EOSC 321 Laboratory 8: Intermediate and Felsic Volcanic Rocks. Pyroclastic Rocks
 321 Lab 8 Instructor: L. Porritt - 1 - Igneous petrology EOSC 321 Laboratory 8: Intermediate and Felsic Volcanic Rocks. Pyroclastic Rocks Learning Goals. After this Lab, you should be able: Identify fine-grained
321 Lab 8 Instructor: L. Porritt - 1 - Igneous petrology EOSC 321 Laboratory 8: Intermediate and Felsic Volcanic Rocks. Pyroclastic Rocks Learning Goals. After this Lab, you should be able: Identify fine-grained
ANALYSIS OF STRAINS CONCEPT OF STRAIN
 ANALYSIS OF STRAINS CONCEPT OF STRAIN Concept of strain : if a bar is subjected to a direct load, and hence a stress the bar will change in length. If the bar has an original length L and changes by an
ANALYSIS OF STRAINS CONCEPT OF STRAIN Concept of strain : if a bar is subjected to a direct load, and hence a stress the bar will change in length. If the bar has an original length L and changes by an
CHEM Principles of Chemistry II Chapter 10 - Liquids and Solids
 CHEM 1212 - Principles of Chemistry II Chapter 10 - Liquids and Solids 10.1 Intermolecular Forces recall intramolecular (within the molecule) bonding whereby atoms can form stable units called molecules
CHEM 1212 - Principles of Chemistry II Chapter 10 - Liquids and Solids 10.1 Intermolecular Forces recall intramolecular (within the molecule) bonding whereby atoms can form stable units called molecules
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION
 THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
Comparison of the Wehner Spots With Angle Distribution Sputtered Atoms Materials
 Comparison of the Wehner Spots With Angle Distribution Sputtered Atoms Materials Dj. Boubetra and L.Selmani LMSE, Centre Universitaire de Bordj Bou Arreridj El Anasser 34265, Algeria boubetra@gmail.com
Comparison of the Wehner Spots With Angle Distribution Sputtered Atoms Materials Dj. Boubetra and L.Selmani LMSE, Centre Universitaire de Bordj Bou Arreridj El Anasser 34265, Algeria boubetra@gmail.com
THERMAL EXPANSION OF FLUORSPAR AND IRON PYRITE BY D. C. PRESS
 THERMAL EXPANSION OF FLUORSPAR AND IRON PYRITE BY D. C. PRESS (From the Department of Physics, Indian Institute of Science, Bangalore) Received October 26, 1949 (Communicated by Prof. R. S. Krishnan, F.A.SC.)
THERMAL EXPANSION OF FLUORSPAR AND IRON PYRITE BY D. C. PRESS (From the Department of Physics, Indian Institute of Science, Bangalore) Received October 26, 1949 (Communicated by Prof. R. S. Krishnan, F.A.SC.)
THE CONTRIBUTIONS WHICH HAVE
 Reprinted from SCIENCE, July 23, 1948, Vol. 108, No. 2795, pages 69-75. X-Ray, Electron, and Neutron Diffraction C. G. Shull and E. O. Wollan Oak Ridge National Laboratory, Oak Ridge, Tennessee THE CONTRIBUTIONS
Reprinted from SCIENCE, July 23, 1948, Vol. 108, No. 2795, pages 69-75. X-Ray, Electron, and Neutron Diffraction C. G. Shull and E. O. Wollan Oak Ridge National Laboratory, Oak Ridge, Tennessee THE CONTRIBUTIONS
Structure Report for J. Reibenspies
 X-ray Diffraction Laboratory Center for Chemical Characterization and Analysis Department of Chemistry Texas A & M University Structure Report for J. Reibenspies Project Name: Sucrose Date: January 29,
X-ray Diffraction Laboratory Center for Chemical Characterization and Analysis Department of Chemistry Texas A & M University Structure Report for J. Reibenspies Project Name: Sucrose Date: January 29,
Review of Last Class 1
 Review of Last Class 1 X-Ray diffraction of crystals: the Bragg formulation Condition of diffraction peak: 2dd sin θθ = nnλλ Review of Last Class 2 X-Ray diffraction of crystals: the Von Laue formulation
Review of Last Class 1 X-Ray diffraction of crystals: the Bragg formulation Condition of diffraction peak: 2dd sin θθ = nnλλ Review of Last Class 2 X-Ray diffraction of crystals: the Von Laue formulation
Physics Department, Sardar Vallabhbhai Vidyapeeth, Vallabh Vidyanagar, India
 860 Acta Cryst. (1962). 15, 860 Etching of Mica Cleavages :BY A. R. PATEL AND S. I{AMANATttAN Physics Department, Sardar Vallabhbhai Vidyapeeth, Vallabh Vidyanagar, India (Received 29 April 1961 and in
860 Acta Cryst. (1962). 15, 860 Etching of Mica Cleavages :BY A. R. PATEL AND S. I{AMANATttAN Physics Department, Sardar Vallabhbhai Vidyapeeth, Vallabh Vidyanagar, India (Received 29 April 1961 and in
The Crystal Structure of Sodium Pentafluorodistannate(II), NaSn2Fs*
 1104 Acta Cryst. (1964). 17, 1104 The Crystal Structure of Sodium Pentafluorodistannate(II), NaSn2Fs* :BY ROBERT R. MCDONALD,'~ ALLEN C. I~RSON, AND Do~ T. CROMER University of California, Los Alamos Scientific
1104 Acta Cryst. (1964). 17, 1104 The Crystal Structure of Sodium Pentafluorodistannate(II), NaSn2Fs* :BY ROBERT R. MCDONALD,'~ ALLEN C. I~RSON, AND Do~ T. CROMER University of California, Los Alamos Scientific
Roger Johnson Structure and Dynamics: Displacive phase transition Lecture 9
 9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
Symmetry. 2-D Symmetry. 2-D Symmetry. Symmetry. EESC 2100: Mineralogy 1. Symmetry Elements 1. Rotation. Symmetry Elements 1. Rotation.
 Symmetry a. Two-fold rotation = 30 o /2 rotation a. Two-fold rotation = 30 o /2 rotation Operation Motif = the symbol for a two-fold rotation EESC 2100: Mineralogy 1 a. Two-fold rotation = 30 o /2 rotation
Symmetry a. Two-fold rotation = 30 o /2 rotation a. Two-fold rotation = 30 o /2 rotation Operation Motif = the symbol for a two-fold rotation EESC 2100: Mineralogy 1 a. Two-fold rotation = 30 o /2 rotation
THE DIFFERENTIAL THERMAL ANALYZER AS A MICRO-CALORIMETER* M.q,nr Wtttnr.s, M as s achus ett s I n stitute of T echnolo gy, C ombriil ge, Mass.
 THE DIFFERENTIAL THERMAL ANALYZER AS A MICRO-CALORIMETER* M.q,nr Wtttnr.s, M as s achus ett s I n stitute of T echnolo gy, C ombriil ge, Mass. ABSTRACT Calorimetric measurements of very small magnitude
THE DIFFERENTIAL THERMAL ANALYZER AS A MICRO-CALORIMETER* M.q,nr Wtttnr.s, M as s achus ett s I n stitute of T echnolo gy, C ombriil ge, Mass. ABSTRACT Calorimetric measurements of very small magnitude
9 Crystal Structures
 9 Crystal Structures Supporting interactive 3D images of crystal structures and more advanced material may be found at:http://www-teach.ch.cam.ac.uk/links/3dindex.html www.xtremepapers.com A Introduction
9 Crystal Structures Supporting interactive 3D images of crystal structures and more advanced material may be found at:http://www-teach.ch.cam.ac.uk/links/3dindex.html www.xtremepapers.com A Introduction
Solid-state physics. Laue diagrams: investigating the lattice structure of monocrystals. LEYBOLD Physics Leaflets P
 Solid-state physics Properties of crystals X-ray structural analysis LEYBOLD Physics Leaflets P7.1.2.2 Laue diagrams: investigating the lattice structure of monocrystals Objects of the experiment Evaluating
Solid-state physics Properties of crystals X-ray structural analysis LEYBOLD Physics Leaflets P7.1.2.2 Laue diagrams: investigating the lattice structure of monocrystals Objects of the experiment Evaluating
Observation of a robust zero-energy bound state in iron-based superconductor Fe(Te,Se)
 Materials and Methods: SUPPLEMENTARY INFORMATION Observation of a robust zero-energy bound state in iron-based superconductor Fe(Te,Se) All the crystals, with nominal composition FeTe0.5Se0.5, used in
Materials and Methods: SUPPLEMENTARY INFORMATION Observation of a robust zero-energy bound state in iron-based superconductor Fe(Te,Se) All the crystals, with nominal composition FeTe0.5Se0.5, used in
Beamline Practice at BL02B2 (Powder diffraction)
 Beamline Practice at BL02B2 (Powder diffraction) Rietveld Refinement using Synchrotron X-ray Powder Diffraction Data Shogo Kawaguchi, Bagautdin Bagautdinov, and Kunihisa Sugimoto (JASRI) 1. Introduction
Beamline Practice at BL02B2 (Powder diffraction) Rietveld Refinement using Synchrotron X-ray Powder Diffraction Data Shogo Kawaguchi, Bagautdin Bagautdinov, and Kunihisa Sugimoto (JASRI) 1. Introduction
Condensed Matter Physics Prof. G. Rangarajan Department of Physics Indian Institute of Technology, Madras
 (Refer Slide Time: 00:11) Condensed Matter Physics Prof. G. Rangarajan Department of Physics Indian Institute of Technology, Madras Diffraction Methods for Crystal Structure Worked Examples Next, we have
(Refer Slide Time: 00:11) Condensed Matter Physics Prof. G. Rangarajan Department of Physics Indian Institute of Technology, Madras Diffraction Methods for Crystal Structure Worked Examples Next, we have
Structure and therm al properties associated with some hydrogen bonds in crystals IV. Isotope effects in some acid phosphates
 Interaction between adsorbed substances. I I 399 R eferen ces Adam, N. K. 1938 The physics and chemistry of surfaces. 2n Adam, N. K., Askew, F. A. and Pankhurst, K. G. A. 1939 Proc. Roy. A, 170, 485. Guastalla,
Interaction between adsorbed substances. I I 399 R eferen ces Adam, N. K. 1938 The physics and chemistry of surfaces. 2n Adam, N. K., Askew, F. A. and Pankhurst, K. G. A. 1939 Proc. Roy. A, 170, 485. Guastalla,
3.012 Structure An Introduction to X-ray Diffraction
 3.012 Structure An Introduction to X-ray Diffraction This handout summarizes some topics that are important for understanding x-ray diffraction. The following references provide a thorough explanation
3.012 Structure An Introduction to X-ray Diffraction This handout summarizes some topics that are important for understanding x-ray diffraction. The following references provide a thorough explanation
Lecture course on crystallography, 2015 Lecture 5: Symmetry in crystallography
 Dr Semën Gorfman Department of Physics, University of SIegen Lecture course on crystallography, 2015 Lecture 5: Symmetry in crystallography What is symmetry? Symmetry is a property of an object to stay
Dr Semën Gorfman Department of Physics, University of SIegen Lecture course on crystallography, 2015 Lecture 5: Symmetry in crystallography What is symmetry? Symmetry is a property of an object to stay
Matter and Minerals. Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more).
 1 2 Matter and Minerals Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... 3 4 5 6 7 8 9 10 11 12 13 14 Also crystalline,
1 2 Matter and Minerals Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... 3 4 5 6 7 8 9 10 11 12 13 14 Also crystalline,
Chapter 10. Liquids and Solids
 Chapter 10 Liquids and Solids Chapter 10 Table of Contents 10.1 Intermolecular Forces 10.2 The Liquid State 10.3 An Introduction to Structures and Types of Solids 10.4 Structure and Bonding in Metals 10.5
Chapter 10 Liquids and Solids Chapter 10 Table of Contents 10.1 Intermolecular Forces 10.2 The Liquid State 10.3 An Introduction to Structures and Types of Solids 10.4 Structure and Bonding in Metals 10.5
complexity they have been described as the most complex known for the
 256 C,HE.AIISTR Y: PA CJLING 4 ND CORE PROC. N. A. S. This investigation was aided by grants from The Rockefeller Foundationi, The National Foundation for Infantile Paralysis, and The United States Public
256 C,HE.AIISTR Y: PA CJLING 4 ND CORE PROC. N. A. S. This investigation was aided by grants from The Rockefeller Foundationi, The National Foundation for Infantile Paralysis, and The United States Public
Physical Structure of Matter. K a doublet splitting of molybdenum X-rays / fine structure Physics of the Electron.
 Physics of the Electron Physical Structure of Matter K a doublet splitting of molybdenum X-rays / fine structure What you can learn about Characteristic X-ray radiation Energy levels Selection rules The
Physics of the Electron Physical Structure of Matter K a doublet splitting of molybdenum X-rays / fine structure What you can learn about Characteristic X-ray radiation Energy levels Selection rules The
THE CRYSTAL STRUCTURE OF STILPNOMELANE PART I. THE SUBCELL
 THE CRYSTAL STRUCTURE OF STILPNOMELANE PART I. THE SUBCELL by R. A. EGGLETON and S. W. BAILEY University of Wisconsin, Madison, Wisconsin ABSTRACT FERRIC-IRON stilpnomelane is triclinic, space group PI,
THE CRYSTAL STRUCTURE OF STILPNOMELANE PART I. THE SUBCELL by R. A. EGGLETON and S. W. BAILEY University of Wisconsin, Madison, Wisconsin ABSTRACT FERRIC-IRON stilpnomelane is triclinic, space group PI,
Introductory Nanotechnology ~ Basic Condensed Matter Physics ~
 Introductory Nanotechnology ~ Basic Condensed Matter Physics ~ Atsufumi Hirohata Department of Electronics Go into Nano-Scale Lateral Size [m] 10-3 10-6 Micron-scale Sub-Micron-scale Nano-scale Human hair
Introductory Nanotechnology ~ Basic Condensed Matter Physics ~ Atsufumi Hirohata Department of Electronics Go into Nano-Scale Lateral Size [m] 10-3 10-6 Micron-scale Sub-Micron-scale Nano-scale Human hair
THE CRYSTAT STRUCTURE OF DAWSONITE NaAl(CoJ(oHb
 THE CRYSTAT STRUCTURE OF DAWSONTE NaAl(CoJ(oHb A. J. FRUEH, JR. AND J. P. GOLGHTLY Cry stal,l,ograph'i,c Lab oratory, McGill, Un&ter sity, M ontreal,, euebec Ansrn-tcr The structure of dawsonite v/as determined
THE CRYSTAT STRUCTURE OF DAWSONTE NaAl(CoJ(oHb A. J. FRUEH, JR. AND J. P. GOLGHTLY Cry stal,l,ograph'i,c Lab oratory, McGill, Un&ter sity, M ontreal,, euebec Ansrn-tcr The structure of dawsonite v/as determined
Chapter 10. Liquids and Solids
 Chapter 10 Liquids and Solids Section 10.1 Intermolecular Forces Section 10.1 Intermolecular Forces Section 10.1 Intermolecular Forces Section 10.1 Intermolecular Forces Metallic bonds Covalent bonds Ionic
Chapter 10 Liquids and Solids Section 10.1 Intermolecular Forces Section 10.1 Intermolecular Forces Section 10.1 Intermolecular Forces Section 10.1 Intermolecular Forces Metallic bonds Covalent bonds Ionic
GEOL. 40 ELEMENTARY MINERALOGY
 CRYSTAL DESCRIPTION AND CALCULATION A. INTRODUCTION This exercise develops the framework necessary for describing a crystal. In essence we shall discuss how we fix the position of any crystallographic
CRYSTAL DESCRIPTION AND CALCULATION A. INTRODUCTION This exercise develops the framework necessary for describing a crystal. In essence we shall discuss how we fix the position of any crystallographic
CRYSTAL MEASUREMENT AND AXIAL RATIO LABORATORY
 CRYSTAL MEASUREMENT AND AXIAL RATIO LABORATORY George R. McCormick Department of Geology The University of Iowa Iowa City, Iowa 52242 george_mccormick@uiowa.edu Goals of the Exercise This exercise is designed
CRYSTAL MEASUREMENT AND AXIAL RATIO LABORATORY George R. McCormick Department of Geology The University of Iowa Iowa City, Iowa 52242 george_mccormick@uiowa.edu Goals of the Exercise This exercise is designed
Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering
 Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
THE UNIT CELL AND SPACE GROUP OF LINDGRENITE Wrr-uau H. Bnnxrs, National' Research Council', Ottawa, Ontar'io, Canada'
 THE UNIT CELL AND SPACE GROUP OF LINDGRENITE Wrr-uau H. Bnnxrs, National' Research Council', Ottawa, Ontar'io, Canada' AsstRAcr The unit cell constants and the space group of lindgrenite, 2CuMoOr'Cu(OH]'ll:1"
THE UNIT CELL AND SPACE GROUP OF LINDGRENITE Wrr-uau H. Bnnxrs, National' Research Council', Ottawa, Ontar'io, Canada' AsstRAcr The unit cell constants and the space group of lindgrenite, 2CuMoOr'Cu(OH]'ll:1"
for the darker autunite and the formula
 MINERALOGICAL NOTES TIIE AMERICAN MINERALOGIST, VOL. 48, NOVEMBER-DECEMBER, 1963 THE CRYSTALLOGRAPHY OF META-AUTUNITE (I)1 M.lr.cor.rt Ross, [/. S. Geological Suraey, Washington 25, D. C. INrnolucrroN
MINERALOGICAL NOTES TIIE AMERICAN MINERALOGIST, VOL. 48, NOVEMBER-DECEMBER, 1963 THE CRYSTALLOGRAPHY OF META-AUTUNITE (I)1 M.lr.cor.rt Ross, [/. S. Geological Suraey, Washington 25, D. C. INrnolucrroN
NAME GEOL FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY
 NAME GEOL.2150 - FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY I. Introduction Minerals are crystalline solids. Individual minerals are distinguished on the basis of chemistry and the way in
NAME GEOL.2150 - FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY I. Introduction Minerals are crystalline solids. Individual minerals are distinguished on the basis of chemistry and the way in
S = k log W CHEM Thermodynamics. Change in Entropy, S. Entropy, S. Entropy, S S = S 2 -S 1. Entropy is the measure of dispersal.
 , S is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints on
, S is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints on
THE UNMIXING OF CHALCOPYRITE FROM SPHALERITE N. W. Buoncen, Massachuselts Institute oj Technology.
 THE UNMIXING OF CHALCOPYRITE FROM SPHALERITE N. W. Buoncen, Massachuselts Institute oj Technology. PnBvrous Wonr Schneiderhcihn and Ramdohrl suggest that certain intergrowths of sphalerite and chalcopyrite
THE UNMIXING OF CHALCOPYRITE FROM SPHALERITE N. W. Buoncen, Massachuselts Institute oj Technology. PnBvrous Wonr Schneiderhcihn and Ramdohrl suggest that certain intergrowths of sphalerite and chalcopyrite
STRUCTURES OF MERCURY MERCAPTIDES
 STRUCTURES OF MERCURY MERCAPTIDES PART 11. X-RAY STRUCTURAL ANALYSIS OF MERCURY ETHYLMERCAPTIDE D. C. BRADLEY~ AND N. R. KUNCHUR~ Department of Chemistry, University of Western Ontario, London, Ontario
STRUCTURES OF MERCURY MERCAPTIDES PART 11. X-RAY STRUCTURAL ANALYSIS OF MERCURY ETHYLMERCAPTIDE D. C. BRADLEY~ AND N. R. KUNCHUR~ Department of Chemistry, University of Western Ontario, London, Ontario
AP* Chapter 10. Liquids and Solids. Friday, November 22, 13
 AP* Chapter 10 Liquids and Solids AP Learning Objectives LO 1.11 The student can analyze data, based on periodicity and the properties of binary compounds, to identify patterns and generate hypotheses
AP* Chapter 10 Liquids and Solids AP Learning Objectives LO 1.11 The student can analyze data, based on periodicity and the properties of binary compounds, to identify patterns and generate hypotheses
Probing Atomic Crystals: Bragg Diffraction
 1 Probing Atomic Crystals: Bragg Diffraction OBJECTIVE: To learn how scientists probe the structure of solids, using a scaled-up version of X-ray Diffraction. APPARATUS: Steel ball "crystal", microwave
1 Probing Atomic Crystals: Bragg Diffraction OBJECTIVE: To learn how scientists probe the structure of solids, using a scaled-up version of X-ray Diffraction. APPARATUS: Steel ball "crystal", microwave
Applications of X-ray and Neutron Scattering in Biological Sciences: Symmetry in direct and reciprocal space 2012
 Department of Drug Design and Pharmacology Applications of X-ray and Neutron Scattering in Biological Sciences: Symmetry in direct and reciprocal space 2012 Michael Gajhede Biostructural Research Copenhagen
Department of Drug Design and Pharmacology Applications of X-ray and Neutron Scattering in Biological Sciences: Symmetry in direct and reciprocal space 2012 Michael Gajhede Biostructural Research Copenhagen
THERMAL TRANSFORMATIONS OF PYROPHYLLITE AND TALC AS REVEALED BY X-RAY AND ELECTRON DIFFRACTION STUDIES*
 THERMAL TRANSFORMATIONS OF PYROPHYLLITE AND TALC AS REVEALED BY X-RAY AND ELECTRON DIFFRACTION STUDIES* by M. NAKAHIRA AND T. KATO Pennsylvania State University, University Park, Pa., U.S.A. and University
THERMAL TRANSFORMATIONS OF PYROPHYLLITE AND TALC AS REVEALED BY X-RAY AND ELECTRON DIFFRACTION STUDIES* by M. NAKAHIRA AND T. KATO Pennsylvania State University, University Park, Pa., U.S.A. and University
DEVELOPMENT OF MEASURING SYSTEM FOR STRESS BY MEANS OF IMAGE PLATE FOR LABORATORY X-RAY EXPERIMENT
 Copyright JCPDS - International Centre for Diffraction Data 003, Advances in X-ray Analysis, Volume 46. 6 DEVELOPMENT OF MEASURING SYSTEM FOR STRESS BY MEANS OF IMAGE PLATE FOR LABORATORY X-RAY EXPERIMENT
Copyright JCPDS - International Centre for Diffraction Data 003, Advances in X-ray Analysis, Volume 46. 6 DEVELOPMENT OF MEASURING SYSTEM FOR STRESS BY MEANS OF IMAGE PLATE FOR LABORATORY X-RAY EXPERIMENT
ON THE GEOMETRY OF THE QUANTUM REFLECTION OF X-RAYS IN DIAMOND By P. RAMA PISHAROTY
 ON THE GEOMETRY OF THE QUANTUM REFLECTION OF X-RAYS IN DIAMOND By P. RAMA PISHAROTY (From the Department of Physics, Indian Institute of Science, Bangalore) Received June 27, 1941 (Communicated by Sir
ON THE GEOMETRY OF THE QUANTUM REFLECTION OF X-RAYS IN DIAMOND By P. RAMA PISHAROTY (From the Department of Physics, Indian Institute of Science, Bangalore) Received June 27, 1941 (Communicated by Sir
CRYSTAL CHEMISTRY OF THE BASIC MANGANESE ARSENATES: III. The CRYSTAL STRUCTURE OF EVEITE, Mn 2 (OH) (AsO 4 )
 THE AMERICAN MINERALOGIST, VOL. 53, NOVEMBER-DECEMBER, 1968 CRYSTAL CHEMISTRY OF THE BASIC MANGANESE ARSENATES: III. The CRYSTAL STRUCTURE OF EVEITE, Mn 2 (OH) (AsO 4 ) PAUL B. MOORE and JOSEPH R. SMYTH,
THE AMERICAN MINERALOGIST, VOL. 53, NOVEMBER-DECEMBER, 1968 CRYSTAL CHEMISTRY OF THE BASIC MANGANESE ARSENATES: III. The CRYSTAL STRUCTURE OF EVEITE, Mn 2 (OH) (AsO 4 ) PAUL B. MOORE and JOSEPH R. SMYTH,
The thermal expansion of the sodalite group of minerals
 761 The thermal expansion of the sodalite group of minerals By D. TAYLOR, B.Sc., Ph.D. Department of Geology, University of Manchester 1 [Read 14 March 1968] Summary. Thermal expansion data up to 920 ~
761 The thermal expansion of the sodalite group of minerals By D. TAYLOR, B.Sc., Ph.D. Department of Geology, University of Manchester 1 [Read 14 March 1968] Summary. Thermal expansion data up to 920 ~
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/2/3/e1501725/dc1 Supplementary Materials for Discovery of natural MgSiO3 tetragonal garnet in a shocked chondritic meteorite The PDF file includes: Naotaka Tomioka,
advances.sciencemag.org/cgi/content/full/2/3/e1501725/dc1 Supplementary Materials for Discovery of natural MgSiO3 tetragonal garnet in a shocked chondritic meteorite The PDF file includes: Naotaka Tomioka,
PSD '17 -- Xray Lecture 5, 6. Patterson Space, Molecular Replacement and Heavy Atom Isomorphous Replacement
 PSD '17 -- Xray Lecture 5, 6 Patterson Space, Molecular Replacement and Heavy Atom Isomorphous Replacement The Phase Problem We can t measure the phases! X-ray detectors (film, photomultiplier tubes, CCDs,
PSD '17 -- Xray Lecture 5, 6 Patterson Space, Molecular Replacement and Heavy Atom Isomorphous Replacement The Phase Problem We can t measure the phases! X-ray detectors (film, photomultiplier tubes, CCDs,
Chapter 1 X-ray Absorption Fine Structure (EXAFS)
 1 Chapter 1 X-ray Absorption Fine Structure (EXAFS) 1.1 What is EXAFS? X-ray absorption fine structure (EXAFS, XAFS) is an oscillatory modulation in the X-ray absorption coefficient on the high-energy
1 Chapter 1 X-ray Absorption Fine Structure (EXAFS) 1.1 What is EXAFS? X-ray absorption fine structure (EXAFS, XAFS) is an oscillatory modulation in the X-ray absorption coefficient on the high-energy
The atomic structure of fluor-apatite and its relation to that of tooth and bone material.
 254 I The atomic structure of fluor-apatite and its relation to that of tooth and bone material. (With Plates XVI--XVIII.) ]~y C. A. BEEVERS, D.Sc., F.Inst.P., F.R.S.E., and D. B. MCINTYRE, B.Sc. Dewar
254 I The atomic structure of fluor-apatite and its relation to that of tooth and bone material. (With Plates XVI--XVIII.) ]~y C. A. BEEVERS, D.Sc., F.Inst.P., F.R.S.E., and D. B. MCINTYRE, B.Sc. Dewar
S = k log W 11/8/2016 CHEM Thermodynamics. Change in Entropy, S. Entropy, S. Entropy, S S = S 2 -S 1. Entropy is the measure of dispersal.
 Entropy is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints
Entropy is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints
Determination of the Rydberg constant, Moseley s law, and screening constant (Item No.: P )
 Determination of the Rydberg constant, Moseley s law, and screening constant (Item No.: P2541001) Curricular Relevance Area of Expertise: ILIAS Education Level: Physik Topic: Hochschule Subtopic: Moderne
Determination of the Rydberg constant, Moseley s law, and screening constant (Item No.: P2541001) Curricular Relevance Area of Expertise: ILIAS Education Level: Physik Topic: Hochschule Subtopic: Moderne
the crystal structure of aravaipaite.
 American Mineralogist, Volume 86, pages 927 931, 2001 The crystal structure of aravaipaite ANTHONY R. KAMPF* Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California
American Mineralogist, Volume 86, pages 927 931, 2001 The crystal structure of aravaipaite ANTHONY R. KAMPF* Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California
X-ray diffraction geometry
 X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
Chapter 3. The structure of crystalline solids 3.1. Crystal structures
 Chapter 3. The structure of crystalline solids 3.1. Crystal structures 3.1.1. Fundamental concepts 3.1.2. Unit cells 3.1.3. Metallic crystal structures 3.1.4. Ceramic crystal structures 3.1.5. Silicate
Chapter 3. The structure of crystalline solids 3.1. Crystal structures 3.1.1. Fundamental concepts 3.1.2. Unit cells 3.1.3. Metallic crystal structures 3.1.4. Ceramic crystal structures 3.1.5. Silicate
Overview - Macromolecular Crystallography
 Overview - Macromolecular Crystallography 1. Overexpression and crystallization 2. Crystal characterization and data collection 3. The diffraction experiment 4. Phase problem 1. MIR (Multiple Isomorphous
Overview - Macromolecular Crystallography 1. Overexpression and crystallization 2. Crystal characterization and data collection 3. The diffraction experiment 4. Phase problem 1. MIR (Multiple Isomorphous
Introduction to X-ray and neutron scattering
 UNESCO/IUPAC Postgraduate Course in Polymer Science Lecture: Introduction to X-ray and neutron scattering Zhigunov Alexander Institute of Macromolecular Chemistry ASCR, Heyrovsky sq., Prague -16 06 http://www.imc.cas.cz/unesco/index.html
UNESCO/IUPAC Postgraduate Course in Polymer Science Lecture: Introduction to X-ray and neutron scattering Zhigunov Alexander Institute of Macromolecular Chemistry ASCR, Heyrovsky sq., Prague -16 06 http://www.imc.cas.cz/unesco/index.html
Wigner function for nonparaxial wave fields
 486 J. Opt. Soc. Am. A/ Vol. 18, No. 10/ October 001 C. J. R. Sheppard and K. G. Larin Wigner function for nonparaxial wave fields Colin J. R. Sheppard* and Kieran G. Larin Department of Physical Optics,
486 J. Opt. Soc. Am. A/ Vol. 18, No. 10/ October 001 C. J. R. Sheppard and K. G. Larin Wigner function for nonparaxial wave fields Colin J. R. Sheppard* and Kieran G. Larin Department of Physical Optics,
Determining Protein Structure BIBC 100
 Determining Protein Structure BIBC 100 Determining Protein Structure X-Ray Diffraction Interactions of x-rays with electrons in molecules in a crystal NMR- Nuclear Magnetic Resonance Interactions of magnetic
Determining Protein Structure BIBC 100 Determining Protein Structure X-Ray Diffraction Interactions of x-rays with electrons in molecules in a crystal NMR- Nuclear Magnetic Resonance Interactions of magnetic
Geometry of Crystal Lattice
 0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
The nature of special points on unit cell parameters temperature dependences for crystal substances
 Z. Kristallogr. Suppl. 26 (2007) 447-452 by Oldenbourg Wissenschaftsverlag, München 447 The nature of special points on unit cell parameters temperature dependences for crystal substances Stanislav K.
Z. Kristallogr. Suppl. 26 (2007) 447-452 by Oldenbourg Wissenschaftsverlag, München 447 The nature of special points on unit cell parameters temperature dependences for crystal substances Stanislav K.
Lecture 05 Structure of Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch03, McGraw-Hill, 2000.
 MME 467 Ceramics for Advanced Applications Lecture 05 Structure of Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch03, McGraw-Hill, 2000. Prof. A. K. M. Bazlur Rashid Department of MME, BUET, Dhaka
MME 467 Ceramics for Advanced Applications Lecture 05 Structure of Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch03, McGraw-Hill, 2000. Prof. A. K. M. Bazlur Rashid Department of MME, BUET, Dhaka
ANOMALIES IN TILE ETHYLENE GLYCOL SOLVA- TION TECHNIQUE USED IN X-RAY DIFFRACTION * ABSTRACT
 ANOMALIES IN TILE ETHYLENE GLYCOL SOLVA- TION TECHNIQUE USED IN X-RAY DIFFRACTION * G. W. KUNZE Agricultural and lv[echanical College of Texas ABSTRACT X-ray diffraction results are presented to show that
ANOMALIES IN TILE ETHYLENE GLYCOL SOLVA- TION TECHNIQUE USED IN X-RAY DIFFRACTION * G. W. KUNZE Agricultural and lv[echanical College of Texas ABSTRACT X-ray diffraction results are presented to show that
Minerals: Minerals: Building blocks of rocks. Atomic Structure of Matter. Building Blocks of Rocks Chapter 3 Outline
 Minerals: Building Blocks of Rocks Chapter 3 Outline Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Minerals: Building blocks of rocks Definition
Minerals: Building Blocks of Rocks Chapter 3 Outline Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Minerals: Building blocks of rocks Definition
Chapter 12 Solids and Modern Materials
 Sec$on 10.3 An Introduc+on to Structures and Types of Solids Chapter 12 Solids and Modern Materials Sec$on 10.3 An Introduc+on to Structures and Types of Solids Solids Amorphous Solids: Disorder in the
Sec$on 10.3 An Introduc+on to Structures and Types of Solids Chapter 12 Solids and Modern Materials Sec$on 10.3 An Introduc+on to Structures and Types of Solids Solids Amorphous Solids: Disorder in the
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
Stratigraphical etch patterns on the rhombohedral cleavages of natural quartz crystals
 MINERALOGICAL MAGAZINE, DECEMBER 1973, VOL. 39, PP- 482-7 Stratigraphical etch patterns on the rhombohedral cleavages of natural quartz crystals M. S. JOSHI AND BABY K. PAUL Department of Physics, Sardar
MINERALOGICAL MAGAZINE, DECEMBER 1973, VOL. 39, PP- 482-7 Stratigraphical etch patterns on the rhombohedral cleavages of natural quartz crystals M. S. JOSHI AND BABY K. PAUL Department of Physics, Sardar
Jordan M. Rhodes, Caleb A. Jones, Lucas B. Thal, Janet E. Macdonald*
 Supporting Information for: Jordan M. Rhodes, Caleb A. Jones, Lucas B. Thal, Janet E. Macdonald* Department of Chemistry and Vanderbilt Institute for Nanoscale Science and Engineering, Vanderbilt University,
Supporting Information for: Jordan M. Rhodes, Caleb A. Jones, Lucas B. Thal, Janet E. Macdonald* Department of Chemistry and Vanderbilt Institute for Nanoscale Science and Engineering, Vanderbilt University,
Bonding and Packing: building crystalline solids
 Bonding and Packing: building crystalline solids The major forces of BONDING Gravitational forces: F = G m m 1 2 F = attractive forces between 2 bodies G = universal graviational constant (6.6767 * 10
Bonding and Packing: building crystalline solids The major forces of BONDING Gravitational forces: F = G m m 1 2 F = attractive forces between 2 bodies G = universal graviational constant (6.6767 * 10
Further refinement of the goyazite structure
 MNERALOGCAL JOURNAL, VOL. 13, NO.6, PP. 390-396, APRL 1987 Short Communication Further refinement of the goyazite structure Toshio KATO nstitute of Earth Sciences, Faculty of Liberal Arts, Yamaguchi University,
MNERALOGCAL JOURNAL, VOL. 13, NO.6, PP. 390-396, APRL 1987 Short Communication Further refinement of the goyazite structure Toshio KATO nstitute of Earth Sciences, Faculty of Liberal Arts, Yamaguchi University,
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Protein Crystallography
 Protein Crystallography Part II Tim Grüne Dept. of Structural Chemistry Prof. G. Sheldrick University of Göttingen http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview The Reciprocal Lattice The
Protein Crystallography Part II Tim Grüne Dept. of Structural Chemistry Prof. G. Sheldrick University of Göttingen http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview The Reciprocal Lattice The
We need to be able to describe planes and directions.
 We need to be able to describe planes and directions. Miller Indices & XRD 1 2 Determining crystal structure and identifying materials (B) Plastic deformation Plastic deformation and mechanical properties
We need to be able to describe planes and directions. Miller Indices & XRD 1 2 Determining crystal structure and identifying materials (B) Plastic deformation Plastic deformation and mechanical properties
Homework 4 Due 25 October 2018 The numbers following each question give the approximate percentage of marks allocated to that question.
 Name: Homework 4 Due 25 October 218 The numbers following each question give the approximate percentage of marks allocated to that question. 1. Use the reciprocal metric tensor again to calculate the angle
Name: Homework 4 Due 25 October 218 The numbers following each question give the approximate percentage of marks allocated to that question. 1. Use the reciprocal metric tensor again to calculate the angle
Efficient sorting of orbital angular momentum states of light
 CHAPTER 6 Efficient sorting of orbital angular momentum states of light We present a method to efficiently sort orbital angular momentum (OAM) states of light using two static optical elements. The optical
CHAPTER 6 Efficient sorting of orbital angular momentum states of light We present a method to efficiently sort orbital angular momentum (OAM) states of light using two static optical elements. The optical
The densest and the least dense packings of equal spheres.
 479 The densest and the least dense packings of equal spheres. By SIDNEY MELMORE, B.Sc. [Read June 24, 1948.J The densest packings.--of the results which have so far been achieved by study of the packing
479 The densest and the least dense packings of equal spheres. By SIDNEY MELMORE, B.Sc. [Read June 24, 1948.J The densest packings.--of the results which have so far been achieved by study of the packing
Reversible uptake of HgCl 2 in a porous coordination polymer based on the dual functions of carboxylate and thioether
 Supplementary Information Reversible uptake of HgCl 2 in a porous coordination polymer based on the dual functions of carboxylate and thioether Xiao-Ping Zhou, a Zhengtao Xu,*,a Matthias Zeller, b Allen
Supplementary Information Reversible uptake of HgCl 2 in a porous coordination polymer based on the dual functions of carboxylate and thioether Xiao-Ping Zhou, a Zhengtao Xu,*,a Matthias Zeller, b Allen
Crystal Structure. Dr Bindu Krishnan
 Solid State Physics-1 Crystal Structure Dr Bindu Krishnan CRYSTAL LATTICE What is crystal (space) lattice? In crystallography, only the geometrical properties of the crystal are of interest, therefore
Solid State Physics-1 Crystal Structure Dr Bindu Krishnan CRYSTAL LATTICE What is crystal (space) lattice? In crystallography, only the geometrical properties of the crystal are of interest, therefore
The luminescence of diamond-i
 Curr. Sci. 19 357-363 (1950) The luminescence of diamond-i SIR C V RAMAN 1. Introduction' No less than seventy-five distinct papers which concerned themselves with the structure and properties of diamond
Curr. Sci. 19 357-363 (1950) The luminescence of diamond-i SIR C V RAMAN 1. Introduction' No less than seventy-five distinct papers which concerned themselves with the structure and properties of diamond
GEOLOGIC OCCURRENCE OF GRATONITE AT CERRO DE PASCO, PERU* Gnonco W. Rusr, Cerro tle Pasco Copper Corporation'.
 GEOLOGIC OCCURRENCE OF GRATONITE AT CERRO DE PASCO, PERU* Gnonco W. Rusr, Cerro tle Pasco Copper Corporation'. GrNBn.a,r, Gnorocv The copper-silver-gold and lead-zinc mineralizations at Cerro de Pasco
GEOLOGIC OCCURRENCE OF GRATONITE AT CERRO DE PASCO, PERU* Gnonco W. Rusr, Cerro tle Pasco Copper Corporation'. GrNBn.a,r, Gnorocv The copper-silver-gold and lead-zinc mineralizations at Cerro de Pasco
The University of Hong Kong Department of Physics
 The University of Hong Kong Department of Physics Physics Laboratory PHYS3551 Introductory Solid State Physics Experiment No. 3551-2: Electron and Optical Diffraction Name: University No: This experiment
The University of Hong Kong Department of Physics Physics Laboratory PHYS3551 Introductory Solid State Physics Experiment No. 3551-2: Electron and Optical Diffraction Name: University No: This experiment
Atomic and Nuclear Physics
 Atomic and Nuclear Physics Introductory experiments ualism of wave and particle L Physics Leaflets P6.1.5.1 iffraction of electrons in a polycrystalline lattice (ebye-scherrer diffraction) Objects of the
Atomic and Nuclear Physics Introductory experiments ualism of wave and particle L Physics Leaflets P6.1.5.1 iffraction of electrons in a polycrystalline lattice (ebye-scherrer diffraction) Objects of the
