A Gene Discovery Lab Manual For Undergraduates:
|
|
|
- Chester Lawrence
- 5 years ago
- Views:
Transcription
1 A Gene Discovery Lab Manual For Undergraduates: Searching For Genes Required To Make A Seed Honors Collegium 70AL Summer 2014 By Kelli Henry Anhthu Q. Bui Brandon Le Bob Goldberg Department of Molecular, Cell & Developmental Biology UCLA Sponsored by NSF
2 HC70AL Protocols Summer 2014 Professor Bob Goldberg Table of Contents Experiments: Experiment 1 Introduction to General Molecular Biology Techniques Experiment 2 Screening Salk T-DNA Mutagenesis Lines (Gene ONE) Experiment 3 Identifying Features of Mutant Seeds Using Nomarski Microscopy (Gene ONE) Experiment 4 Screening Salk T-DNA Mutagenesis Lines (Gene TWO) Experiment 5 Identifying Features of Mutant Seeds Using Nomarski Microscopy (Gene TWO) Appendicies: Appendix 1A Preparation of an Agarose Gel A1A.1 A1A.3 Appendix 1B NanoDrop Spectrophotometer A1B.1 A1B.5 Appendix 1C 100 bp DNA Ladder A1C.1 Appendix 1D 1 Kb Plus DNA Ladder A1D.1 Appendix 1E Ex Taq DNA Polymerase A1E.1 Appendix 1F QIAquick Spin Handbook A1F.1 A1F.23 Appendix 2 Bioinformatics Presentations Parts I and II A2.1 A2.16 Table of Contents
3 EXPERIMENT 1 INTRODUCTION TO GENERAL MOLECULAR BIOLOGY TECHNIQUES STRATEGY I. PIPETTING EXERCISE II. SERIAL DILUTION EXPERIMENT Introduction to General Molecular Biology Techniques 1.1
4 I. PIPETTING EXERCISE Purpose: To learn how to use pipettes Taken From: DNA Science: A First Course, Second Edition Laboratory 1: Measurements, Micropipetting, and Sterile Techniques p (ISBN ) Solutions Needed:! Four Dye Solutions Labeled I-IV Solution I: Blue Solution II: Red Solution III: Yellow Solution IV: Green Materials Needed:! Set of pipettes (P-10, P-20, P-200 & P-1000)! Pipet tips (regular, non-filter tips)! 1.5 ml microcentrifuge tubes! Microcentrifuge tube rack PROCEDURE A. Small Volume Pipette Exercise This exercise simulates setting up a reaction, using a pipette with a range of 1-10 µl or 2-20 µl. 1. Use a permanent marker (sharpie) to label THREE 1.5 ml tubes A, B and C and your initials. 2. Use the table below as a checklist while adding solutions to each reaction tube. Introduction to General Molecular Biology Techniques 1.2
5 Tube Sol. I (Blue) Sol. II (Red) Sol. III (Yellow) Sol. IV (Green) Total Volume A 4 µl 5 µl 1 µl - 10 µl B 4 µl 5 µl - 1 µl 10 µl C 4 µl 4 µl 1 µl 1 µl 10 µl 3. Set the pipette to 4 µl and add Solution I to each reaction tube. 4. Use a fresh tip to add the appropriate volume of Solution II to a clean spot inside reaction tubes A, B and C. 5. Use a fresh tip to add 1 µl of Solution III to tubes A and C. 6. Use a fresh tip to add 1 µl of Solution IV to tubes B and C. 7. Close lids. Pool and mix reagents by using one of the following methods: a. Sharply tap the tube bottom on the bench top. Make sure that the drops have pooled into one drop at the bottom of the tube. Or b. Place the tubes in a microcentrifuge and apply a short, few-second pulse. Make sure that the reaction tubes are placed in a balanced configuration in the microcentrifuge rotor. Caution: Spinning tubes in an unbalanced position will damage the microcentrifuge. 8. A total of 10 µl of reagents were added to each reaction tube. To check that the previous pipetting measurements were accurate, set the pipette to 10 µl and very carefully withdraw the solution from each tube. a. Is the tip just filled? What does this suggest? Or b. Is a small volume of fluid left in tube? What does this suggest? Or c. After extracting all the fluid, is an air space left in the tip end? What does this suggest? (The air can be displaced and the actual volume determined simply Introduction to General Molecular Biology Techniques 1.3
6 by rotating the volume adjustment to push the fluid to the very end of the tip. Then, read the volume directly.) 9. If several measurements were inaccurate, repeat this exercise to obtain near-perfect results. B. Large Volume Pipette Exercise This exercise simulates a bacterial transformation or plasmid preparation, for which a P-1000 pipette is used. It is far easier to measure incorrectly when using a largevolume pipette. If the plunger is not released slowly, an air bubble may form or solution may be drawn into the piston. 1. Use a permanent marker to label TWO 1.5 ml microcentrifuge tubes D and E and your initials. 2. Use the matrix below as a checklist while adding solutions to each reaction tube. Tube Sol. I (Blue) Sol. II (Red) Sol. III (Yellow) Sol. IV (Green) Total Volume D 100 µl 200 µl 150 µl 550 µl 1000 µl E 150 µl 250 µl 350 µl 250 µl 1000 µl 3. Set the pipette to add the appropriate volume of Solutions I-IV to reaction tubes D and E. Follow the same procedure as for the Small Volume Pipette Exercise to add Solutions I-IV to each reaction tube. 4. Close lids. Pool and mix reagents by using one of the following methods: a. Sharply tap the tube bottom on the bench top. Make sure that the drops have pooled into one drop at the bottom of the tube. Or b. Place the tubes in a microcentrifuge and apply a short, few-second pulse. Make sure that the reaction tubes are placed in a balanced configuration in Introduction to General Molecular Biology Techniques 1.4
7 the microcentrifuge rotor. Caution: Spinning tubes in an unbalanced position will damage the microcentrifuge. 5. A total of 1000 µl of reagents were added to each tube. To check that the measurements were accurate, set the pipette to 1000 µl and very carefully withdraw the solution from each tube. a. Is the tip just filled? What does this suggest? Or b. Is a small volume of fluid left in tube? What does this suggest? Or c. After extracting all the fluid, is an air space left in the tip end? (The air can be displaced and the actual volume determined simply by rotating the volume adjustment to push the fluid to the very end of the tip. Then, read the volume directly.) 6. If several measurements were inaccurate, repeat this exercise to obtain near-perfect results. Introduction to General Molecular Biology Techniques 1.5
8 II. SERIAL DILUTION EXPERIMENT Purpose: To test the accuracy and precision of pipetting Reference: Anhthu Bui Introduction: Diluting is simply the addition of a solution (or plain solvent) to a substance in order to decrease the concentration of the latter substance. In this exercise, the substance is DNA and the solution is TE Buffer. By the end of this exercise, you will learn how to calculate the dilution factor and determine the accuracy of your pipetting technique as determined by gel electrophoresis and spectrophotometer readings. Solutions Needed:! DNA stock (known concentration)! TE Buffer! Agarose! 1x TAE buffer! 10,000x SYBR Safe DNA gel stain (Invitrogen)! 50 ng/µl 1 Kb Plus DNA ladder (Invitrogen)! 6x Loading Dye containing xylene cyanol and bromophenol blue dyes Materials Needed:! Pipettes (P-10 & P-20)! Pipet tips (regular, non-filter tips)! 1.5 ml microcentrifuge tubes! Microcentrifuge tube rack! NanoDrop spectrophotometer! Kimwipes! 250 ml Erlenmeyer flask! 25 ml Erlenmeyer flask! Saran wrap! Scale! Microwave! 55 C water bath! Hot hand protector! Gel cast! Gel comb Introduction to General Molecular Biology Techniques 1.6
9 ! Round bubble level! Gel box! Cables! Electrophoresis power supply! Plastic container for carrying the gel! Gel document system (Bio-Rad) PROCEDURE A. Serial Dilution of a DNA Stock 1. Label THREE 1.5 ml microcentrifuge tubes as: Dil #1 for dilution #1 Dil #2 for dilution #2 Dil #3 for dilution #3 2. Pipet 15 µl of TE buffer solution into each microcentrifuge tube in step 1. (Use the P-20 pipette) 3. Vortex the DNA stock solution for 5 seconds. Then, spin the tube for 10 seconds to ensure that all of the solution is at the bottom of the tube. 4. Pipet 5 µl of your DNA stock solution into the Dil #1 microcentrifuge tube. (Use the P-10 or P-20 pipette) 5. Vortex the contents of the tube for 5 seconds. Then, spin the tube for 10 seconds to ensure that all of the solution is at the bottom of the tube. 6. Pipet 5 µl of DNA solution from the Dil #1 tube into the Dil #2 tube. 7. Vortex the contents of the Dil #2 tube for 5 seconds. Then, spin the tube for 10 seconds to ensure that all of the solution is at the bottom of the tube. 8. Pipet 5 µl of DNA solution from the Dil #2 tube into the Dil #3 tube. 9. Vortex the contents of the Dil #3 tube for 5 seconds. Then, spin the tube for 10 seconds to ensure that all of the solution is at the bottom of the tube. Introduction to General Molecular Biology Techniques 1.7
10 B. Determination of Pipetting Accuracy by Gel Electrophoresis (See Appendix 1A) 1. Label THREE microcentrifuge tubes with the letters A, B, C and D. 2. Pipet 10 µl of DNA solution to tubes A, B, C and D: from DNA Stock Dil #1 Dil #2 Dil #3 to Tube A Tube B Tube C Tube D 3. Pipet 2 µl of 6x loading dye into tubes A, B, C and D. Mix by pipetting up and down 5 times. The total volume for each solution is 12 µl. 4. Load the contents of tubes A, B, C and D into lanes 1, 2, 3 and Add 10 µl of 10,000x SYBR Safe DNA gel stain to the running buffer at the anode (positively charged) side of the gel box. (The anode is on the side opposite the wells.) Note: Similar to ethidium bromide, SYBR Safe DNA Gel Stain is positively charged. Therefore, it migrates towards the negative side of the gel box, from anode to cathode. (Opposite the direction of DNA migration). Remember that DNA is negatively charged; so, it migrates to the positive end of the gel box. (DNA migrates from cathode to anode). 6. Put the lid on the gel box and connect the electrodes to the power supply (RED to RED and BLACK to BLACK). Note: SYBR Safe gel stain is unstable in UV or bright room light. If possible, run the gel in the dark by either turning off the lights, covering the gel with a cardboard box or aluminum foil, or run the gel inside of a drawer. Realistically, hours of constant UV or bright room light exposure are required to cause any significant loss of signal. 7. Record the identity of samples loaded on the gel. Lane Sample 1 DNA Stock 2 Dilution #1 3 Dilution #2 4 Dilution #3 Introduction to General Molecular Biology Techniques 1.8
11 8. Run the gel at 105 volts for 1-2 hours or until the lower dye (bromophenol blue) has migrated one-half or two-thirds of the gel length. Time power supply turned ON: Time power supply turned OFF: How long was the gel run? hour(s) and minutes 10. After 1-2 hours of running the gel, turn off the power supply. 11. Remove the lid of the gel box. Put the gel in its gel cast into a small plastic container and bring the container to room 4128A2. Caution: It is a MUST to put the gel into a plastic container so that the gel cannot slide off the gel cast, fall on the floor and be broken into pieces while walking. 12. Take a picture of the gel using the Bio-Rad Gel Document System. Label the picture using the text program of the Gel Document System. (Your TA will show you how.) Alternatively: Print out the picture. Tape it to a piece of paper by putting a piece of white tape at a position immediately above the wells. Label the wells with the sample names. 13. Print out the picture. Store the labeled picture in your lab notebook. C. Determination of Pipetting Accuracy Using a Spectrophotometer While running the gel, determine the concentration of DNA solutions in the tubes labeled DNA Stock, Dil #1, Dil #2 and Dil #3 by using the NanoDrop Spectrophotometer (Your instructor will demonstrate how to use the instrument). What is a spectrophotometer? (See Appendix 1B) 1. For each tube, read the concentration at least TWICE, using a fresh drop each time. 2. Record the DNA concentration (in ng/µl) from each tube. Sample DNA Stock Dil #1 Dil #2 Concentration (ng/!l) Introduction to General Molecular Biology Techniques 1.9
12 Dil #3 D. Questions and Summary 1. What did you expect to see on your gel? 2. How is your pipetting accuracy as determined by gel electrophoresis? 3. Does the gel result show what you expected? If not, what might be the problem? 4. What is the dilution factor in this exercise? 5. Given the stock DNA concentration is 1!g/!L, what is the expected DNA concentration in tubes Dil #1, Dil #2 and Dil #3? Hint: Use the equation V i x C i = V f x C f where, V i = initial volume (the volume of original DNA solution is 5 µl) C i = initial concentration (reading from the spectrophotometer; example: 1000 ng/µl) V f = final volume (the volume of Dil #1 is 20 µl) C f = final concentration (the concentration of Dil #1) 6. Make a plot on log graph paper or Excel of the logarithm with base 2 of the expected DNA concentration (this will be your standard curve) as shown in the graph below: The x-axis: Tubes (DNA stock, Dil #1, Dil #2 and Dil #3) The y-axis: The logarithm with base 2 of the expected DNA concentration Dilution & Pipetting Accuracy DNA Concentration (log scale) DNA Stock Dil #1 Dil #2 Dil #3 Tubes 7. Plot the logarithm with base 2 of the DNA concentration readings you obtained from the spectrophotometer. Introduction to General Molecular Biology Techniques 1.10
13 8. How does your DNA concentration reading deviate from the expected DNA concentration? Introduction to General Molecular Biology Techniques 1.11
Materials: Micropipettes (2-20 µl range pipette, µl range, µl range), tips, test tubes with color dye, well plates
 Virtually every chemical reaction in a lab or manufacturing facility, as in cells, occurs in a watery environment or solution. A lab technician, therefore, must be able to quickly prepare any volume of
Virtually every chemical reaction in a lab or manufacturing facility, as in cells, occurs in a watery environment or solution. A lab technician, therefore, must be able to quickly prepare any volume of
THE DYE/INDICATOR LAB SEPARATION OF MOLECULES USING AGAROSE GEL ELECTROPHORESIS
 THE DYE/INDICATOR LAB SEPARATION OF MOLECULES USING AGAROSE GEL ELECTROPHORESIS OBJECTIVES: YOU WILL BE ABLE TO PERFORM THE FOLLOWING ACTIVITIES. Make and load the wells of an agarose gel. Electrophorese
THE DYE/INDICATOR LAB SEPARATION OF MOLECULES USING AGAROSE GEL ELECTROPHORESIS OBJECTIVES: YOU WILL BE ABLE TO PERFORM THE FOLLOWING ACTIVITIES. Make and load the wells of an agarose gel. Electrophorese
Molecular Rainbow Dye Electrophoresis Student Materials
 Dye Electrophoresis Student Materials Introduction. 2 Pre-Lab Questions 4 Lab Protocol. 5 Data Collection Worksheet 6 Post-Lab Questions and Analysis.. 7 Last updated: 10/23/2017 Introduction Take just
Dye Electrophoresis Student Materials Introduction. 2 Pre-Lab Questions 4 Lab Protocol. 5 Data Collection Worksheet 6 Post-Lab Questions and Analysis.. 7 Last updated: 10/23/2017 Introduction Take just
Student Guide. bluegel TM Electrophoresis Rainbow Lab. 1. Synopsis
 bluegel TM Electrophoresis Rainbow Lab Student Guide Contents 1. Synopsis p. 1 2. Background p. 2 3. Student laboratory guide p. 7 4. Student data table p. 13 5. Study questions p. 14 1. Synopsis In this
bluegel TM Electrophoresis Rainbow Lab Student Guide Contents 1. Synopsis p. 1 2. Background p. 2 3. Student laboratory guide p. 7 4. Student data table p. 13 5. Study questions p. 14 1. Synopsis In this
Micropipetting Basics
 S44 EdvoKit #S44 Micropipetting Basics Experiment Objective: The objective of this experiment is to learn how to accurately pipet different microliter volumes using a micropipet and to practice micropipetting
S44 EdvoKit #S44 Micropipetting Basics Experiment Objective: The objective of this experiment is to learn how to accurately pipet different microliter volumes using a micropipet and to practice micropipetting
Molecular Rainbow Dye Electrophoresis Teacher Materials
 Molecular Rainbow Dye Electrophoresis Teacher Materials Students will perform an introductory electrophoresis experiment. Five standard dye samples will be used to identify the dyes found in three unknown
Molecular Rainbow Dye Electrophoresis Teacher Materials Students will perform an introductory electrophoresis experiment. Five standard dye samples will be used to identify the dyes found in three unknown
Principles of Thin Layer Chromatography
 REVISED & UPDATED Edvo-Kit #113 Principles of Thin Layer Chromatography Experiment Objective: The objective of this experiment is to gain an understanding of the theory and methods of thin layer chromatography.
REVISED & UPDATED Edvo-Kit #113 Principles of Thin Layer Chromatography Experiment Objective: The objective of this experiment is to gain an understanding of the theory and methods of thin layer chromatography.
PROTOCOL: GCP Protein Quantification Assay and QC (QUANT)
 PROTOCOL: GCP Protein Quantification Assay and QC (QUANT) Purpose To determine overall protein yield and histone purity after histone extraction. SDS PAGE gels are not required, only when changing variables
PROTOCOL: GCP Protein Quantification Assay and QC (QUANT) Purpose To determine overall protein yield and histone purity after histone extraction. SDS PAGE gels are not required, only when changing variables
SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis):
 SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis): Aim: SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) is one of the common methods used in the molecular biology
SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis): Aim: SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) is one of the common methods used in the molecular biology
Sampling and DNA Extraction from Wastewater Activated Sludge Standard Protocol
 Sampling and DNA Extraction from Wastewater Activated Sludge Standard Protocol Version 7.0 Skill Prerequisites: DNA handling Introduction This protocol explains sampling and DNA extraction from activated
Sampling and DNA Extraction from Wastewater Activated Sludge Standard Protocol Version 7.0 Skill Prerequisites: DNA handling Introduction This protocol explains sampling and DNA extraction from activated
Experimental Procedure. Lab 406
 Experimental Procedure Lab 406 Overview This experiment is to be complete in cooperation with other chemists/chemist groups in the laboratory. In PART A, a standardized solution of hydrochloric acid is
Experimental Procedure Lab 406 Overview This experiment is to be complete in cooperation with other chemists/chemist groups in the laboratory. In PART A, a standardized solution of hydrochloric acid is
Spectrophotometry Materials
 Spectrophotometry Materials Item per Class per Bench Genesys 10UV Spectrophotometer 6 1 13 ml test tubes box 7 Test tube racks 6 1 1% Albumin solution 25 ml/one flask 2 ml 0.7% Albumin solution (unknown
Spectrophotometry Materials Item per Class per Bench Genesys 10UV Spectrophotometer 6 1 13 ml test tubes box 7 Test tube racks 6 1 1% Albumin solution 25 ml/one flask 2 ml 0.7% Albumin solution (unknown
Instructor s Advance Preparation
 INSTRUCTOR'S MANUAL Instructor s Advance Preparation This protocol is designed for 80 workstations of 4 students. Each group will prepare a set of standards, a blank, and 2 milk samples (can be a blind
INSTRUCTOR'S MANUAL Instructor s Advance Preparation This protocol is designed for 80 workstations of 4 students. Each group will prepare a set of standards, a blank, and 2 milk samples (can be a blind
Density of an Unknown
 Experiment 3 Density of an Unknown Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The density of an
Experiment 3 Density of an Unknown Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The density of an
Preparing Extension Products for Electrophoresis
 Preparing Extension Products for Electrophoresis Overview Preparation of extension products for electrophoresis will vary depending on the cycle sequencing chemistry used. Dye Terminator Chemistries Unincorporated
Preparing Extension Products for Electrophoresis Overview Preparation of extension products for electrophoresis will vary depending on the cycle sequencing chemistry used. Dye Terminator Chemistries Unincorporated
Bio 6 Photosynthesis Lab
 Bio 6 Photosynthesis Lab Introduction In order to survive, organisms require a source of energy and molecular building blocks to construct all of their biological molecules. The ultimate source of energy
Bio 6 Photosynthesis Lab Introduction In order to survive, organisms require a source of energy and molecular building blocks to construct all of their biological molecules. The ultimate source of energy
E.Z.N.A. MicroElute Clean-up Kits Table of Contents
 E.Z.N.A. MicroElute Clean-up Kits Table of Contents Introduction... 2 Kit Contents... 3 Preparing Reagents/Storage and Stability... 4 Guideline for Vacuum Manifold... 5 MicroElute Cycle-Pure - Spin Protocol...
E.Z.N.A. MicroElute Clean-up Kits Table of Contents Introduction... 2 Kit Contents... 3 Preparing Reagents/Storage and Stability... 4 Guideline for Vacuum Manifold... 5 MicroElute Cycle-Pure - Spin Protocol...
Inserting grids into capsule Removing capsules from grid box Immunolabeling using template guide Preparing for TEM insertion
 Protocol Immunolabeling protocols are easily adapted to mprep/g processing. This document illustrates a typical protocol using a primary antibody and a secondary antibody conjugated to colloidal gold,
Protocol Immunolabeling protocols are easily adapted to mprep/g processing. This document illustrates a typical protocol using a primary antibody and a secondary antibody conjugated to colloidal gold,
Determining Evolutionary Relationships Among Ungulates!
 Determining Evolutionary Relationships Among Ungulates! Today s Lab: 1)! Gel electrophoresis of lactate dehydrogenase isozymes 2)! Morphological examination of representative ungulates a)! Hoof morphology
Determining Evolutionary Relationships Among Ungulates! Today s Lab: 1)! Gel electrophoresis of lactate dehydrogenase isozymes 2)! Morphological examination of representative ungulates a)! Hoof morphology
Working with Solutions. (and why that s not always ideal)
 Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
MOHAWK COLLEGE OF APPLIED ARTS AND TECHNOLOGY CHEMICAL AND ENVIRONMENTAL TECHNOLOGY DEPARTMENT. Lab Report ROOM NO: FE E309
 MOHAWK COLLEGE OF APPLIED ARTS AND TECHNOLOGY CHEMICAL AND ENVIRONMENTAL TECHNOLOGY DEPARTMENT Lab Report ROOM NO: FE E309 EXPERIMENT NO : 4 TITLE : UV Spectrophotometric Analysis of DNA Submitted by Class
MOHAWK COLLEGE OF APPLIED ARTS AND TECHNOLOGY CHEMICAL AND ENVIRONMENTAL TECHNOLOGY DEPARTMENT Lab Report ROOM NO: FE E309 EXPERIMENT NO : 4 TITLE : UV Spectrophotometric Analysis of DNA Submitted by Class
Introduction to Spectroscopy: Analysis of Copper Ore
 Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution delivered 2.47 ml of solution delivered 50.00 ml Volumetric Flask Reading a buret: Burets are
Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution delivered 2.47 ml of solution delivered 50.00 ml Volumetric Flask Reading a buret: Burets are
Buffers for Biological Systems Laboratory Instructor s Manual
 Buffers for Biological Systems Laboratory Instructor s Manual 1. Purpose and Concepts Covered...1 2. Effect of Temperature and Concentration on ph...1 A. Preparing Buffers...2 B. Analysis and Discussion...3
Buffers for Biological Systems Laboratory Instructor s Manual 1. Purpose and Concepts Covered...1 2. Effect of Temperature and Concentration on ph...1 A. Preparing Buffers...2 B. Analysis and Discussion...3
Protein extraction and quantification by Teresa Fan, University of Kentucky BUFFER PREPARATION AND STORAGE
 Protein extraction and quantification by Teresa Fan, University of Kentucky Note: This procedure follows [Fan_Extract_Polar_Lipid_Prot_SOP] Step 11 (middle part, in 1.5 ml tube). BUFFER PREPARATION AND
Protein extraction and quantification by Teresa Fan, University of Kentucky Note: This procedure follows [Fan_Extract_Polar_Lipid_Prot_SOP] Step 11 (middle part, in 1.5 ml tube). BUFFER PREPARATION AND
Module 3. Establishing Standard Curve (Bradford Assay)
 Module 3. Establishing Standard Curve (Bradford Assay) 1. Melissa Myoglobin and the Missing Molecules Melissa Myoglobin had a little tube filled with a clear protein solution. To do more research with
Module 3. Establishing Standard Curve (Bradford Assay) 1. Melissa Myoglobin and the Missing Molecules Melissa Myoglobin had a little tube filled with a clear protein solution. To do more research with
Please use only the valid version of the package insert provided with the kit. This kit is intended for Research Use Only.
 Please use only the valid version of the package insert provided with the kit This kit is intended for Research Use Only. Not for use in diagnostic procedures. INTENDED USE The Vitamin Niacin test is a
Please use only the valid version of the package insert provided with the kit This kit is intended for Research Use Only. Not for use in diagnostic procedures. INTENDED USE The Vitamin Niacin test is a
Functional Genomics Research Stream. Lecture: February 17, 2009 Masses, Volumes, Solutions & Dilutions
 Functional Genomics Research Stream Lecture: February 17, 2009 Masses, Volumes, Solutions & Dilutions Agenda Lab Work: Last Week New Equipment Solution Preparation: Fundamentals Solution Preparation: How
Functional Genomics Research Stream Lecture: February 17, 2009 Masses, Volumes, Solutions & Dilutions Agenda Lab Work: Last Week New Equipment Solution Preparation: Fundamentals Solution Preparation: How
Instructor s Guide. bluegel TM Electrophoresis Rainbow Lab
 bluegel TM Electrophoresis Rainbow Lab Instructor s Guide Contents Page 1. Synopsis 1 2. Learning goals and skills developed 2 3. Standards alignment 3 4. Background 4 5. Laboratory set-up manual 10 6.
bluegel TM Electrophoresis Rainbow Lab Instructor s Guide Contents Page 1. Synopsis 1 2. Learning goals and skills developed 2 3. Standards alignment 3 4. Background 4 5. Laboratory set-up manual 10 6.
user manual blotting Hoefer PR648 Slot blot manifold um PR648-IM/Rev.F0/08-12
 user manual blotting Hoefer PR648 Slot blot manifold um PR648-IM/Rev.F0/08-12 Page finder Introduction...1 Specifications...2 Procedure for standard use of the Hoefer PR648...3 Setting up the Hoefer PR648...3
user manual blotting Hoefer PR648 Slot blot manifold um PR648-IM/Rev.F0/08-12 Page finder Introduction...1 Specifications...2 Procedure for standard use of the Hoefer PR648...3 Setting up the Hoefer PR648...3
Sero: p30 Crossover Electrophoresis for Semen
 Safety Principle Equipment and supplies SAFETY WARNING Electrophoretic procedures have electrical hazards associated with them. Therefore, at minimum, the presence of two analysts is required during these
Safety Principle Equipment and supplies SAFETY WARNING Electrophoretic procedures have electrical hazards associated with them. Therefore, at minimum, the presence of two analysts is required during these
Chem 2115 Experiment #7. Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets
 Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution, analysis of vinegar & antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Globin-Zero Gold Kit
 Cat. No. GZG1206 (Contains 1 box of Cat. No. GZRR1306 and 1 box of Cat. No. MRZ116C) Cat. No. GZG1224 (Contains 1 box of Cat. No. GZRR1324 and 1 box of Cat. No. MRZ11124C) Connect with Epicentre on our
Cat. No. GZG1206 (Contains 1 box of Cat. No. GZRR1306 and 1 box of Cat. No. MRZ116C) Cat. No. GZG1224 (Contains 1 box of Cat. No. GZRR1324 and 1 box of Cat. No. MRZ11124C) Connect with Epicentre on our
Preparing the sample for determination of Viability
 Preparing the sample for determination of Viability I. For preparing one sample for analysis you will need: - 1 pcs CELLCHIP - 1 piece of colored Eppendorf with SOFIA GREEN lyophilized dye - 1 piece of
Preparing the sample for determination of Viability I. For preparing one sample for analysis you will need: - 1 pcs CELLCHIP - 1 piece of colored Eppendorf with SOFIA GREEN lyophilized dye - 1 piece of
OESTREICH LAB CHIP PROTOCOL
 OESTREICH LAB CHIP PROTOCOL Buffers (all starred (*) buffers must be autoclaved before use) 10X Cell Lysis Buffer*: 100mL 0.214g Potassium Acetate (KOAc) = 10mM 0.147g Magnesium Acetate (MgAc) = 15mM 10mL
OESTREICH LAB CHIP PROTOCOL Buffers (all starred (*) buffers must be autoclaved before use) 10X Cell Lysis Buffer*: 100mL 0.214g Potassium Acetate (KOAc) = 10mM 0.147g Magnesium Acetate (MgAc) = 15mM 10mL
CH 112 Special Assignment #4 Chemistry to Dye for: Part C
 CH 112 Special Assignment #4 Chemistry to Dye for: Part C PRE-LAB ASSIGNMENT: Make sure that you read this handout and bring the essentials to lab with you. Review Light, energy and color (pp 17-18), Measuring
CH 112 Special Assignment #4 Chemistry to Dye for: Part C PRE-LAB ASSIGNMENT: Make sure that you read this handout and bring the essentials to lab with you. Review Light, energy and color (pp 17-18), Measuring
Introduction to Binding Equilibrium Module
 IntroductionToBindingEquilibrium.docx Page 1 Introduction to Binding Equilibrium Module Specific information about the spectrophotometer below will change depending on the make and model used. Avidin and
IntroductionToBindingEquilibrium.docx Page 1 Introduction to Binding Equilibrium Module Specific information about the spectrophotometer below will change depending on the make and model used. Avidin and
Mag-Bind Soil DNA Kit. M preps M preps M preps
 Mag-Bind Soil DNA Kit M5635-00 5 preps M5635-01 50 preps M5635-02 200 preps January 2013 Mag-Bind Soil DNA Kit Table of Contents Introduction and Overview...2 Kit Contents/Storage and Stability...3 Preparing
Mag-Bind Soil DNA Kit M5635-00 5 preps M5635-01 50 preps M5635-02 200 preps January 2013 Mag-Bind Soil DNA Kit Table of Contents Introduction and Overview...2 Kit Contents/Storage and Stability...3 Preparing
The Basic Skills of the. Biotechnology Workplace
 The Basic Skills of the Biotechnology Workplace Learning Outcomes Determine the most appropriate tool for measuring specific volumes of masses Describe how to select, set, and use a variety of micropipets
The Basic Skills of the Biotechnology Workplace Learning Outcomes Determine the most appropriate tool for measuring specific volumes of masses Describe how to select, set, and use a variety of micropipets
In-gel digestion of immunoprecipitated proteins separated by SDS-PAGE
 In-gel digestion of immunoprecipitated proteins separated by SDS-PAGE (Lamond Lab / April 2008)! Perform all the pipetting steps in a laminar flow hood. We routinely do our digestions in our TC room hoods.
In-gel digestion of immunoprecipitated proteins separated by SDS-PAGE (Lamond Lab / April 2008)! Perform all the pipetting steps in a laminar flow hood. We routinely do our digestions in our TC room hoods.
VDL ENDOTOXIN ASSAY: ASSAY FOR LIMULUS AMEBOCYTE LYSATE
 1. Purpose 1.1. The purpose of this protocol is test purified viral vectors for endotoxin contamination. 1.2. This procedure is routinely performed in the Vector Development Laboratory (VDL) following
1. Purpose 1.1. The purpose of this protocol is test purified viral vectors for endotoxin contamination. 1.2. This procedure is routinely performed in the Vector Development Laboratory (VDL) following
Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006
 Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006 Introduction: Capillary electrophoresis (CE) is a relatively new, but rapidly growing separation
Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006 Introduction: Capillary electrophoresis (CE) is a relatively new, but rapidly growing separation
Introduction to Spectroscopy: Analysis of Copper Ore
 Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution 2.47 ml of solution 50.00 ml delivered delivered Volumetric Flask Reading a buret: Burets are
Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution 2.47 ml of solution 50.00 ml delivered delivered Volumetric Flask Reading a buret: Burets are
Substances and Mixtures:Separating a Mixture into Its Components
 MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
DIFFUSION THROUGH MEMBRANES STANDARDS B C.4 INTRODUCTION
 DIFFUSION THROUGH MEMBRANES STANDARDS 3.2.12.B.1 3.2.12.C.4 INTRODUCTION Westminster College Many aspects of the life of a cell depend on the fact that atoms and molecules have kinetic energy and are constantly
DIFFUSION THROUGH MEMBRANES STANDARDS 3.2.12.B.1 3.2.12.C.4 INTRODUCTION Westminster College Many aspects of the life of a cell depend on the fact that atoms and molecules have kinetic energy and are constantly
Protein Quantification Kit (Bradford Assay)
 Protein Quantification Kit (Bradford Assay) Booklet Item NO. KTD3002 Product Name Protein Quantification Kit (Bradford Assay) ATTENTION For laboratory research use only. Not for clinical or diagnostic
Protein Quantification Kit (Bradford Assay) Booklet Item NO. KTD3002 Product Name Protein Quantification Kit (Bradford Assay) ATTENTION For laboratory research use only. Not for clinical or diagnostic
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Safety Rules Reminders
 Safety Rules Reminders 1. Take your time doing your experiment. 2. Wash your hands when the class ends. 3. No horseplay, but it s ok to have fun. 4. No cell phones, put away phone and ear buds. 5. Know
Safety Rules Reminders 1. Take your time doing your experiment. 2. Wash your hands when the class ends. 3. No horseplay, but it s ok to have fun. 4. No cell phones, put away phone and ear buds. 5. Know
Activity 2: Determine the Effect of Temperature on the Reaction Rate
 STUDENT MANUAL Activity 2: Determine the Effect of Temperature on the Reaction Rate Temperature can affect the speed of the reaction. Heat can speed up the movement of the substrate and enzyme molecules,
STUDENT MANUAL Activity 2: Determine the Effect of Temperature on the Reaction Rate Temperature can affect the speed of the reaction. Heat can speed up the movement of the substrate and enzyme molecules,
GLUTEN RAPID KIT. Product: GLTN Lateral Flow Devices for Qualitative Analysis of Gluten Proteins. Storage at 4-8 C (35-45 F)
 GLUTEN RAPID KIT Lateral Flow Devices for Qualitative Analysis of Gluten Proteins Product: GLTN-1005 Storage at 4-8 C (35-45 F) UPDATED FEBRUARY 2013 Gluten Rapid LFD Analysis Note: This kit format has
GLUTEN RAPID KIT Lateral Flow Devices for Qualitative Analysis of Gluten Proteins Product: GLTN-1005 Storage at 4-8 C (35-45 F) UPDATED FEBRUARY 2013 Gluten Rapid LFD Analysis Note: This kit format has
Experiment 13. Dilutions and Data Handling in a Spreadsheet rev 1/2013
 Absorbance Experiment 13 Dilutions and Data Handling in a Spreadsheet rev 1/2013 GOAL: This lab experiment will provide practice in making dilutions using pipets and introduce basic spreadsheet skills
Absorbance Experiment 13 Dilutions and Data Handling in a Spreadsheet rev 1/2013 GOAL: This lab experiment will provide practice in making dilutions using pipets and introduce basic spreadsheet skills
Prelab vocabulary (complete before turning in beginning of Lab 2) Name: Date: Gen Bio 1 Lab #1: Microscopes and Measurements
 Name: Date: Gen Bio 1 Lab #1: Microscopes and Measurements Prelab Reading Assignment: Pages 94-97 in Campbell 10 th edition. Appendix C and D at the back of your text. Please look at the following websites
Name: Date: Gen Bio 1 Lab #1: Microscopes and Measurements Prelab Reading Assignment: Pages 94-97 in Campbell 10 th edition. Appendix C and D at the back of your text. Please look at the following websites
Chem 2115 Experiment #7. Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution and the analysis of antacid tablets
 Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution and the analysis of antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
Chem 2115 Experiment #7 Volumetric Analysis & Consumer Chemistry Standardization of an unknown solution and the analysis of antacid tablets OBJECTIVE: The goals of this experiment are to learn titration
The Determination of an Equilibrium Constant
 The Determination of an Equilibrium Constant Chemistry 102 10 Chemical reactions occur to reach a state of equilibrium. The equilibrium state can be characterized by quantitatively defining its equilibrium
The Determination of an Equilibrium Constant Chemistry 102 10 Chemical reactions occur to reach a state of equilibrium. The equilibrium state can be characterized by quantitatively defining its equilibrium
Lab #3 Reduction of 3-Nitroacetophenone
 Lab #3 Reduction of 3-Nitroacetophenone Introduction: Extraction: This method uses a different technique in which the two chemical compounds being separated are in immiscible solvents, also known as phases.
Lab #3 Reduction of 3-Nitroacetophenone Introduction: Extraction: This method uses a different technique in which the two chemical compounds being separated are in immiscible solvents, also known as phases.
Biology Unit 2, Structure of Life, Lab Activity 2-2
 Biology Unit 2, Structure of Life, Lab Activity 2-2 Photosynthesis is the process by which energy used by living systems is converted from electromagnetic radiation from the sun to chemical energy. This
Biology Unit 2, Structure of Life, Lab Activity 2-2 Photosynthesis is the process by which energy used by living systems is converted from electromagnetic radiation from the sun to chemical energy. This
When a solution of thiosulfate is acidified, the following reaction takes place: S2O3 2 - (aq) + 2H + (aq) H2O + SO2 (g) + S (s) (1)
 EXPERIMENT 1 The Kinetics of a Thiosulfate Solution INTRODUCTION: Various approaches are used to study the kinetics of reactions. A usual procedure is to monitor some property, such as intensity of color
EXPERIMENT 1 The Kinetics of a Thiosulfate Solution INTRODUCTION: Various approaches are used to study the kinetics of reactions. A usual procedure is to monitor some property, such as intensity of color
DETERMINATION OF K c FOR AN EQUILIBRIUM SYSTEM
 DETERMINATION OF K c FOR AN EQUILIBRIUM SYSTEM 1 Purpose: To determine the equilibrium constant K c for an equilibrium system using spectrophotometry to measure the concentration of a colored complex ion.
DETERMINATION OF K c FOR AN EQUILIBRIUM SYSTEM 1 Purpose: To determine the equilibrium constant K c for an equilibrium system using spectrophotometry to measure the concentration of a colored complex ion.
MODULO DIAGRAMMA-FOTO Instructions for the correct disposal of waste
 SGI_00_01_POG MDF Pagina Page 1 of 12 SGI_00_01_POG MDF Pagina Page 2 of 12 CER 150110* CER 150202* CER 070704* Material contaminated Absorbing material Chemical liquid waste with dangerous contaminated
SGI_00_01_POG MDF Pagina Page 1 of 12 SGI_00_01_POG MDF Pagina Page 2 of 12 CER 150110* CER 150202* CER 070704* Material contaminated Absorbing material Chemical liquid waste with dangerous contaminated
TPH (Total Petroleum Hydrocarbons)
 TPH (Total Petroleum Hydrocarbons) DOC316.53.01475 Immunoassay 1 Method 10050 Scope and application: For water. 1 This test is semi-quantitative. Results are shown as more or less than the threshold value
TPH (Total Petroleum Hydrocarbons) DOC316.53.01475 Immunoassay 1 Method 10050 Scope and application: For water. 1 This test is semi-quantitative. Results are shown as more or less than the threshold value
Learn to do quantitative titration reactions. Observe the mole ratios of several simple chemical reactions.
 CHAPTER 6 Stoichiometry of Reactions in Solution Objectives The objectives of this laboratory are to: Learn to do quantitative titration reactions. Observe the mole ratios of several simple chemical reactions.
CHAPTER 6 Stoichiometry of Reactions in Solution Objectives The objectives of this laboratory are to: Learn to do quantitative titration reactions. Observe the mole ratios of several simple chemical reactions.
Chemistry Calibration of a Pipet and Acid Titration
 Chemistry 3200 Today you are given a chance to brush up on some of the techniques that you will be using during the remainder of the semester. Lab grades will be based on obtaining the correct answer in
Chemistry 3200 Today you are given a chance to brush up on some of the techniques that you will be using during the remainder of the semester. Lab grades will be based on obtaining the correct answer in
Photosynthesis Investigation 1
 Photosynthesis Investigation 1 Part 1. Measuring the Rate of Photosynthesis You will use the "floating leaf disk" method to measure the rate of photosynthesis. To begin, cut several disks from a spinach
Photosynthesis Investigation 1 Part 1. Measuring the Rate of Photosynthesis You will use the "floating leaf disk" method to measure the rate of photosynthesis. To begin, cut several disks from a spinach
LAB 7 Photosynthesis
 LAB 7 Photosynthesis Introduction In order to survive, organisms require a source of energy and molecular building blocks to construct all of their biological molecules. The ultimate source of energy for
LAB 7 Photosynthesis Introduction In order to survive, organisms require a source of energy and molecular building blocks to construct all of their biological molecules. The ultimate source of energy for
GENERAL INSTRUCTIONS
 Read these before doing any work in laboratory Safety: GENERAL INSTRUCTIONS 1) Eye protection must be worn at all times in the laboratory. Minimum eye protection is eye glasses with side shields. Safety
Read these before doing any work in laboratory Safety: GENERAL INSTRUCTIONS 1) Eye protection must be worn at all times in the laboratory. Minimum eye protection is eye glasses with side shields. Safety
Supernatant: The liquid layer lying above the solid layer after a precipitation reaction occurs.
 Limiting Reagent Introduction The quantities of substances involved in a chemical reaction represented by a balanced equation are often referred to as stoichiometric amounts. Solution stoichiometry is
Limiting Reagent Introduction The quantities of substances involved in a chemical reaction represented by a balanced equation are often referred to as stoichiometric amounts. Solution stoichiometry is
Protocol for 2D-E. Protein Extraction
 Protocol for 2D-E Protein Extraction Reagent 1 inside the ReadyPrep TM Sequential Extraction kit (in powder form) 50ml of deionized water is used to dissolve all the Reagent 1. The solution is known as
Protocol for 2D-E Protein Extraction Reagent 1 inside the ReadyPrep TM Sequential Extraction kit (in powder form) 50ml of deionized water is used to dissolve all the Reagent 1. The solution is known as
Genetic Material Uptake in E. Coli
 Genetic Material Uptake in E. Coli Christine Watkins 31 March 2015 Lab Group Number: 7 Taylor BIOL 1111: General Biology I Lab Spring 2015 Lab Section: 103 Lab Instructor: Alex Aitken Genetic Material
Genetic Material Uptake in E. Coli Christine Watkins 31 March 2015 Lab Group Number: 7 Taylor BIOL 1111: General Biology I Lab Spring 2015 Lab Section: 103 Lab Instructor: Alex Aitken Genetic Material
INSTITUT FÜR ANGEWANDTE LABORANALYSEN GMBH. First-Beer Magnetic DNA Kit. Extraktion von Hefe- und Bakterien-DNA aus Bier und anderen Getränken
 First-Beer INSTITUT FÜR ANGEWANDTE LABORANALYSEN GMBH First-Beer Magnetic DNA Kit Extraktion von Hefe- und Bakterien-DNA aus Bier und anderen Getränken Extraction of bacteria and yeast DNA from beer and
First-Beer INSTITUT FÜR ANGEWANDTE LABORANALYSEN GMBH First-Beer Magnetic DNA Kit Extraktion von Hefe- und Bakterien-DNA aus Bier und anderen Getränken Extraction of bacteria and yeast DNA from beer and
Aspirin Lab By Maya Parks Partner: Ben Seufert 6/5/15, 6/8/15
 Aspirin Lab By Maya Parks Partner: Ben Seufert 6/5/15, 6/8/15 Abstract: This lab was performed to synthesize acetyl salicylic acid or aspirin from a carboxylic acid and an alcohol. We had learned in class
Aspirin Lab By Maya Parks Partner: Ben Seufert 6/5/15, 6/8/15 Abstract: This lab was performed to synthesize acetyl salicylic acid or aspirin from a carboxylic acid and an alcohol. We had learned in class
Standard Operating Procedure
 Standard Operating Procedure Procedure Radioimmunoassay with I Department Location SOP Prepared By: Section 1: Purpose Radioimmunoassays are used for detecting the concentration of a specific antigen or
Standard Operating Procedure Procedure Radioimmunoassay with I Department Location SOP Prepared By: Section 1: Purpose Radioimmunoassays are used for detecting the concentration of a specific antigen or
THE TYPICAL HOME KITCHEN has bowls,
 Operating Laboratory Equipment and Measurement Devices THE TYPICAL HOME KITCHEN has bowls, spoons, whisks, knives, mixers, rollers, measuring cups, and many other tools designed for specific uses. Similarly,
Operating Laboratory Equipment and Measurement Devices THE TYPICAL HOME KITCHEN has bowls, spoons, whisks, knives, mixers, rollers, measuring cups, and many other tools designed for specific uses. Similarly,
TruSight Cancer Workflow on the MiniSeq System
 TruSight Cancer Workflow on the MiniSeq System Prepare Library Sequence Analyze Data TruSight Cancer 1.5 days ~ 24 hours < 2 hours TruSight Cancer Library Prep MiniSeq System Local Run Manager Enrichment
TruSight Cancer Workflow on the MiniSeq System Prepare Library Sequence Analyze Data TruSight Cancer 1.5 days ~ 24 hours < 2 hours TruSight Cancer Library Prep MiniSeq System Local Run Manager Enrichment
The Synthesis and Analysis of Aspirin
 The Synthesis and Analysis of Aspirin Computer 22 Aspirin, the ubiquitous pain reliever, goes by the chemical name acetylsalicylic acid. One of the compounds used in the synthesis of aspirin is salicylic
The Synthesis and Analysis of Aspirin Computer 22 Aspirin, the ubiquitous pain reliever, goes by the chemical name acetylsalicylic acid. One of the compounds used in the synthesis of aspirin is salicylic
QuickReferenceCard. OncoScan CNV FFPE Assay Kit 25 Samples. Starting Afternoon Day 1. Finished ~ Noon Day 3
 QuickReferenceard OncoScan NV P ssay Kit Workflow Overview The ffymetrix OncoScan NV P ssay Kit protocol is optimized for processing 1 to 23 samples at a time to obtain whole genome copy number information
QuickReferenceard OncoScan NV P ssay Kit Workflow Overview The ffymetrix OncoScan NV P ssay Kit protocol is optimized for processing 1 to 23 samples at a time to obtain whole genome copy number information
THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE
 THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE INTRODUCTION FACTORS INFLUENCING REACTION RATE: The study of chemical reactions is not complete without a consideration of the rates at which
THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE INTRODUCTION FACTORS INFLUENCING REACTION RATE: The study of chemical reactions is not complete without a consideration of the rates at which
Protease Inhibitor Cocktail A (1 tablet / 7 10 ml, Roche Cat# ) Protease inhibitor Cocktail B (0.5ml per 250ml, Calbiochem Cat# )
 Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
Protocol for Western Blotting Tissue/Cell Sample Preparation Lysis Buffer 1 (ph8.0) o 50mM Tris-Cl o 150mM NaCl o 1% v/v NP40 o protease inhibitor cocktail A/B Lysis Buffer 2 (RIPA) (ph 8.0) o 50mM Tris-Cl
RNA Transport. R preps R preps
 RNA Transport R0527-00 5 preps R0527-01 50 preps July 2014 RNA Transport Table of Contents Introduction...2 Kit Contents/Storage and Stability...3 Protocol...4 Storage Procedure...4 Recovery Procedure...5
RNA Transport R0527-00 5 preps R0527-01 50 preps July 2014 RNA Transport Table of Contents Introduction...2 Kit Contents/Storage and Stability...3 Protocol...4 Storage Procedure...4 Recovery Procedure...5
FerroZine Method 1 Method to 100 µg/l Fe (10-cm cell) Reagent Solution. Instrument Adapter Sample cell DR 6000 LZV
 Iron, Total DOC316.53.01338 FerroZine Method 1 Method 10264 1 to 100 µg/l Fe (10-cm cell) Reagent Solution Scope and application: For ultrapure water. 1 Adapted from Stookey, L.L., Anal. Chem., 42(7),
Iron, Total DOC316.53.01338 FerroZine Method 1 Method 10264 1 to 100 µg/l Fe (10-cm cell) Reagent Solution Scope and application: For ultrapure water. 1 Adapted from Stookey, L.L., Anal. Chem., 42(7),
Experiment 1: Preparation of Vanillyl Alcohol
 Experiment 1: Preparation of Vanillyl Alcohol INTRDUCTIN A common method for preparing alcohols is the reduction of aldehydes to form primary alcohols [equation (1)] or of ketones to produce secondary
Experiment 1: Preparation of Vanillyl Alcohol INTRDUCTIN A common method for preparing alcohols is the reduction of aldehydes to form primary alcohols [equation (1)] or of ketones to produce secondary
Experiment 1: The Borohydride Reduction of 9-Fluorenone to 9-Fluorenol
 Experiment 1: The Borohydride Reduction of 9-Fluorenone to 9-Fluorenol Background: In this week s experiment, a metal hydride will be used as a reducing agent. Metal hydrides can be quite reactive, and
Experiment 1: The Borohydride Reduction of 9-Fluorenone to 9-Fluorenol Background: In this week s experiment, a metal hydride will be used as a reducing agent. Metal hydrides can be quite reactive, and
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB5009.
 Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
AP Biology Lab 4 PLANT PIGMENTS AND PHOTOSYNTHESIS
 AP Biology Laboratory Date: Name and Period: AP Biology Lab 4 PLANT PIGMENTS AND PHOTOSYNTHESIS OVERVIEW In this lab you will: 1. separate plant pigments using chromatography, and 2. measure the rate of
AP Biology Laboratory Date: Name and Period: AP Biology Lab 4 PLANT PIGMENTS AND PHOTOSYNTHESIS OVERVIEW In this lab you will: 1. separate plant pigments using chromatography, and 2. measure the rate of
AP BIOLOGY Photosynthesis
 AP BIOLOGY Photosynthesis The process of photosynthesis involves the use of light energy to convert carbon dioxide and water into sugar, oxygen, and other organic compounds. This process is often summarized
AP BIOLOGY Photosynthesis The process of photosynthesis involves the use of light energy to convert carbon dioxide and water into sugar, oxygen, and other organic compounds. This process is often summarized
Modified Adams Assay for Phenolics in Wine
 Modified Adams Assay for Phenolics in Wine 1. Total Iron-Reactive Phenolics THIS VALUE WILL DETERMINE DILUTIONS FOR TANNIN & POLYMERIC PIGMENT ANALYSES 1.1 Into a reduced volume cuvette, pipette in the
Modified Adams Assay for Phenolics in Wine 1. Total Iron-Reactive Phenolics THIS VALUE WILL DETERMINE DILUTIONS FOR TANNIN & POLYMERIC PIGMENT ANALYSES 1.1 Into a reduced volume cuvette, pipette in the
RayBio Nickel Magnetic Particles
 RayBio Nickel Magnetic Particles Catalog #: 801-108 User Manual Last revised January 4 th, 2017 Caution: Extraordinarily useful information enclosed ISO 1348 Certified 3607 Parkway Lane, Suite 100 Norcross,
RayBio Nickel Magnetic Particles Catalog #: 801-108 User Manual Last revised January 4 th, 2017 Caution: Extraordinarily useful information enclosed ISO 1348 Certified 3607 Parkway Lane, Suite 100 Norcross,
FOR RESEARCH USE ONLY.
 MAN-10039-04 Vantage 3D DNA SNV Qualification Kit Vantage 3D DNA SNV Qualification Kit The ncounter Vantage 3D DNA SNV Qualification Kit is designed to assess whether the ncounter MAX, FLEX, or SPRINT
MAN-10039-04 Vantage 3D DNA SNV Qualification Kit Vantage 3D DNA SNV Qualification Kit The ncounter Vantage 3D DNA SNV Qualification Kit is designed to assess whether the ncounter MAX, FLEX, or SPRINT
DEVELOP YOUR LAB, YOUR WAY
 Technical brochure DEVELOP YOUR LAB, YOUR WAY The FLOW Solution: Expand your potential today Redesign your lab with the FLOW Solution The FLOW Solution is a highly flexible modular, semi-automated data
Technical brochure DEVELOP YOUR LAB, YOUR WAY The FLOW Solution: Expand your potential today Redesign your lab with the FLOW Solution The FLOW Solution is a highly flexible modular, semi-automated data
Functional Genomics Research Stream
 Functional Genomics Research Stream http://fc09.deviantart.net/fs70/i/2010/214/2/f/dna_heart_by_micche.jpg http://www.ryersondesigns.com/skanndelus/dnaheart.jpg Research Meeting: February 14, 2012 Nucleic
Functional Genomics Research Stream http://fc09.deviantart.net/fs70/i/2010/214/2/f/dna_heart_by_micche.jpg http://www.ryersondesigns.com/skanndelus/dnaheart.jpg Research Meeting: February 14, 2012 Nucleic
Rate law Determination of the Crystal Violet Reaction Using the Isolation Method
 Rate law Determination of the Crystal Violet Reaction Using the Isolation Method Introduction A common challenge in chemical kinetics is to determine the rate law for a reaction with multiple reactants.
Rate law Determination of the Crystal Violet Reaction Using the Isolation Method Introduction A common challenge in chemical kinetics is to determine the rate law for a reaction with multiple reactants.
Determining the Concentration of a Solution: Beer s Law
 Determining the Concentration of a Solution: Beer s Law LabQuest 11 The primary objective of this experiment is to determine the concentration of an unknown nickel (II) sulfate solution. You will be using
Determining the Concentration of a Solution: Beer s Law LabQuest 11 The primary objective of this experiment is to determine the concentration of an unknown nickel (II) sulfate solution. You will be using
NAD + /NADH Assay [Colorimetric]
![NAD + /NADH Assay [Colorimetric] NAD + /NADH Assay [Colorimetric]](/thumbs/91/105496846.jpg) G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name NAD + /NADH Assay [Colorimetric] (Cat. #786 1539, 786 1540) think proteins! think G-Biosciences
G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name NAD + /NADH Assay [Colorimetric] (Cat. #786 1539, 786 1540) think proteins! think G-Biosciences
CHEMICAL SEPARATION EXPERIMENT 2
 CHEMICAL SEPARATION EXPERIMENT 2 INTRODUCTION The term analysis in chemistry usually refer to the quantitative and qualitative determination of the components of a sample. Qualitative refering to the identity
CHEMICAL SEPARATION EXPERIMENT 2 INTRODUCTION The term analysis in chemistry usually refer to the quantitative and qualitative determination of the components of a sample. Qualitative refering to the identity
often display a deep green color due to where the SPR occurs (i.e., the wavelength of light that interacts with this specific morphology).
 Synthesis-Dependent Catalytic Properties of Gold Nanoparticles Nanoscience is the study of materials that have dimensions, intuitively, on the nanoscale, typically between 1 100 nm. This field has received
Synthesis-Dependent Catalytic Properties of Gold Nanoparticles Nanoscience is the study of materials that have dimensions, intuitively, on the nanoscale, typically between 1 100 nm. This field has received
Lesson Plan: Diffusion
 Lesson Plan: Diffusion Background Particles in cells show rapid back and forth movement, or Brownian motion, which is also known as diffusion. The back and forth motion consists of random steps from a
Lesson Plan: Diffusion Background Particles in cells show rapid back and forth movement, or Brownian motion, which is also known as diffusion. The back and forth motion consists of random steps from a
[C] [D] K eq = [A] [B]
![[C] [D] K eq = [A] [B] [C] [D] K eq = [A] [B]](/thumbs/76/73734780.jpg) Enzyme Kinetics: Properties of -Galactosidase Preparation for Laboratory: Web Tutorial 4, Beta Galactosidase - submit answers to questions Additonal background: Freeman, Proteins pp 51-54 and Box 3.3 pp56-57,
Enzyme Kinetics: Properties of -Galactosidase Preparation for Laboratory: Web Tutorial 4, Beta Galactosidase - submit answers to questions Additonal background: Freeman, Proteins pp 51-54 and Box 3.3 pp56-57,
AP CHEMISTRY LAB RATES OF CHEMICAL REACTIONS (II)
 PURPOSE: Observe a redox reaction. AP CHEMISTRY LAB RATES OF CHEMICAL REACTIONS (II) Apply graphing techniques to analyze data. Practice computer skills to develop a data table. Determine the order of
PURPOSE: Observe a redox reaction. AP CHEMISTRY LAB RATES OF CHEMICAL REACTIONS (II) Apply graphing techniques to analyze data. Practice computer skills to develop a data table. Determine the order of
Ribo-Zero Magnetic Gold Kit*
 Ribo-Zero Magnetic Gold Kit* (Epidemiology) Cat. No. MRZE706 (Contains 1 box of Cat. No. RZE1206 and 1 box of Cat. No. MRZ116C) Cat. No. MRZE724 (Contains 1 box of Cat. No. RZE1224 and 1 box of Cat. No.
Ribo-Zero Magnetic Gold Kit* (Epidemiology) Cat. No. MRZE706 (Contains 1 box of Cat. No. RZE1206 and 1 box of Cat. No. MRZ116C) Cat. No. MRZE724 (Contains 1 box of Cat. No. RZE1224 and 1 box of Cat. No.
Experiment 7: ACID-BASE TITRATION: STANDARDIZATION OF A SOLUTION
 Experiment 7: ACID-BASE TITRATION: STANDARDIZATION OF A SOLUTION Purpose: Determine molarity of a solution of unknown concentration by performing acid-base titrations Performance Goals: Apply the concepts
Experiment 7: ACID-BASE TITRATION: STANDARDIZATION OF A SOLUTION Purpose: Determine molarity of a solution of unknown concentration by performing acid-base titrations Performance Goals: Apply the concepts
E lectrophores is with S DS buffer kit ph 8.0 INS T R UC T IONS
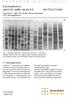 A M E R S H A M B I O S C I E N C E S E lectrophores is with S DS buffer kit ph 8.0 INS T R UC T IONS C leang elª with S DS B uffer K it for horizontal S DS electrophores is Ð Fig. 1. SD S electrophoresis
A M E R S H A M B I O S C I E N C E S E lectrophores is with S DS buffer kit ph 8.0 INS T R UC T IONS C leang elª with S DS B uffer K it for horizontal S DS electrophores is Ð Fig. 1. SD S electrophoresis
Experiment 1. Chemical Equilibria and Le Châtelier s Principle
 Experiment 1 Chemical Equilibria and Le Châtelier s Principle A local theatre company is interested in preparing solutions that look like blood for their upcoming production of Lizzie Borden. They have
Experiment 1 Chemical Equilibria and Le Châtelier s Principle A local theatre company is interested in preparing solutions that look like blood for their upcoming production of Lizzie Borden. They have
