A catalogue record for this book is available from the British Library
|
|
|
- Ezra Osborne
- 5 years ago
- Views:
Transcription
1
2
3 TUTORIAL CHEMISTRY TEXTS 9 Atomic Structure and Periodicity JACK BARRETT Imperial College of Science, Technology and Medicine, University of London RSC ROYAL SOCIETY OF CHEMISTRY
4 Cover images Murray Robertsonlvisual elements , taken from the 19 Visual Elements Periodic Table, available at glviselements ISBN A catalogue record for this book is available from the British Library The Royal Society of Chemistry 22 Published by The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 OWF, UK Registered Charity No For further information see our web site at Typeset in Great Britain by Wyvern 2 I, Bristol Printed and bound by Polestar Wheatons Ltd, Exeter
5 Preface This book deals with the fundamental basis of the modern periodic classification of the elements and includes a discussion of the periodicities of some atomic properties and the nature of the fluorides and oxides of the elements. An introductory chapter deals with the chemically important fundamental particles, the nature of electromagnetic radiation and the restrictions on our knowledge of atomic particles imposed by Heisenberg s uncertainty principle. Atomic orbitals are described with the minimum of mathematics, and then used to describe the electronic configurations of the elements and the construction of the Periodic Table. A chapter is devoted to the periodicities of the ionization energies, electron attachment energies, sizes and electronegativity coefficients of the elements. There is also a section on relativistic effects on atomic properties. A brief overview of chemical bonding is included as the basis of the remaining chapters, which describe the nature and stoichiometries of the fluorides and oxides of the elements. The book represents an attempt to present the periodicities of properties of the elements in a manner that is understandable from a knowledge of the electronic patterns on which the Periodic Table is based. It should be suitable for an introductory course on the subject and should give the reader a general idea of how the properties of atoms and some of their compounds vary across the periods and down the groups of the classification. This knowledge and understanding is essential for chemists who might very well find exceptions to the general rules described; such events being a great attraction in the continuing development of the subject. Apart from the underlying theoretical content, the general trends in periodicity of the elements may be appreciated by the simple statement that size matters, and so does charge. I thank Ellis Horwood for permission to use some material from my previous books, and Martyn Berry for helpful comments on the manuscript. I am very grateful to Pekka Pyykko for his comments on the section about relativistic effects. Jack Barrett London iii
6 TUTORIAL CHEMISTRY TEXTS EDITOR-IN-CHIEF ~ Professor E W A he1 EXEC I1 T I V E ED I TO R S ~ ~ Profissor A G Duvies Mr M Bmy Professor D Phillips Profissor J D Woollins EDlJCAT ONAL CONSULTANT This series of books consists of short, single-topic or modular texts, conceni rating on the fundamental areas of chemistry taught in undergraduate science courses. Each book provides a concise account of the basic principles underlying a given subject, embodying an independentlearning philosophy and including worked examples. The one topic, one book approach ensures that the series is adaptable to chemistry cpurses across a variety of institutions. TITLES IN THE SERIES FORTHCOM I NG TITLES Stereochemistry D C Morris Reactions and Characterization of Solids S E Dann Main Group Chemistry W HcJnderson d- and f-block Chemistry C J Joncs Structure and Bonding J Burrett Functional Group Chemistry J R Hunson Organotransition Metal Chemistry A F Hill Heterocyclic Chemistry M Sainshury Atomic Structure and Periodicity J Burrett Thermodynamics and Statistical Mechanics J M Setldon and J D Gule Basic Atomic and Moleciilar Spectroscopy Aromatic Chemistry Organic Synthetic Methods Quantum Mechanics for Chemists Mechanisms in Organic Reactions Molecular Interactions Reaction Kinetics X-ray Crystallography Lanthanide and Actinide Elements Maths for Chemists Bioinorganic Chemistry Chemistry of Solid Surfa8:es Biology for Chemists Mu1 ti-elemen t N MR Further infortnution about this series is availuble ut NWW. chenisoc. orgltct Orders und enquiries should be sent to: Sales and Customer Care, Royal Society of Chemistry, Thomas Graham Hcuse, Science Park, Milton Road, Cambridge CB4 OWF, UK Tel: +44 I ; Fax: ; sales@rsc.org
7 Contents I Atomic Particles, Photons and the Quantization of Electron Energies; Heisenberg s Uncertainty Principle 1,l Fundamental Particles Electromagnetic Radiation The Photoelectric Effect Wave-Particle Duality The Bohr Frequency Condition The Hydrogen Atom The Observation of Electrons; the Heisenberg Uncertainty Principle 17 2 Atomic Orbitals The Hydrogen Atom 21 3 The Electronic Configurations of Atoms; the Periodic Classification of the Elements Polyelectronic Atoms The Electronic Configurations and Periodic Classification of the Elements The Electronic Configurations of Elements Beyond Neon The Periodic Table Summarized 56 I V
8 vi Contents 4 Periodicity I: Some Atomic Properties; Relativistic Effects Periodicity of Ionization Energies Variations in Electron Attachment Energies Variations in Atomic Size Electronegativi ty Relativistic Effects Periodicity II: Valencies and Oxidation States An Overview of Chemical Bonding Valency and Oxidation State: Differences of Terminology Valency and the Octet and 18-Electron Rules Periodicity of Valency and Oxidation States in the s- and p-block Elements Oxidation States of the Transition Elements Oxidation States of the f-block Elements Periodicity 111: Standard Enthalpies of Atomization of the Elements Periodicity of the Standard Enthalpies of Atomization of the Elements Periodicity I V Fluorides and Oxides The Fluorides and Oxides of the Elements Fluorides of the Elements Oxides of the Elements Answers to Problems Subject Index
9 Atomic Particles, Photons and the Quantization of Electron Energies; Heisenberg s Uncertainty Principle As an introduction to the main topics of this book - atomic structure and the periodicity of atomic properties - the foundations of the subject, which lie in quantum mechanics, and the nature of atomic particles and electromagnetic radiation are described. By the end of this chapter you should understand: Which fundamental particles are important in chemistry The nature of electromagnetic radiation The photoelectric effect Wave-particle duality The relationship of electromagnetic radiation to changes of energy in nuclei, atoms, molecules and metals: the Bohr frequency condition The main features of the emission spectrum of the hydrogen atom and the quantization of the energies permitted for electrons in atoms Heisenberg s Uncertainty Principle and the necessity for quantum mechanics in the study of atomic structure l m i Fundamental Particles To describe adequately the chemical properties of atoms and molecules it is necessary only to consider three fundamental particles: protons and neutrons, which are contained by atomic nuclei, and electrons which surround the nuclei. Protons and neutrons are composite particles, each consisting of three quarks, and are therefore not fundamental particles in the true sense of that term. They may, however, be regarded as being 1
10 ~~~ 2 Atomic Structure and Periodicity The positive electron or positron has a mass identical to the has an opposite charge. Positrons are emitted by some radioactive nuclei and perish when they meet a negative electron, the two particles disappearing completely to form two y-ray photons. Such radiation is known as annihilation radiation: 2e- -+ 2hv fundamental particles for all chemical purposes. The physical properties of electrons, protons and neutrons are given in Table 1.1, together with Table 1.1 Properties of some fundamental particles and the hydrogen atom (e is the elementary unit of electronic charge = x 1-19 coulombs) Particle Symbol M~SS~/?@~~ kg RAM Charge Spin Proton P e tz Neutron n zero t? Electron e e t? H atom H zero - a The atomic unit of mass is given by mu = m(12c)/12 kg. The relative atomic mass (RAM) of an element is given by melement/mu, where melement is the mass of one atom of the element in kg. The absolute masses are given, together with their values on the relative atomic mass (RAM) scale, which is based on the unit of mass being equal to that of one twelfth of the mass of the I2C isotope, i.e. the RAM of 12C = 12. exactly. The spin values of the particles are important in determining the behaviour of nuclei in compounds when subjected to nuclear magnetic resonance spectroscopy (NMR) and electron spin resonance spectroscopy (ESR). The conventional way of representing an atom of an element and its nuclear properties is by placing the mass number, A [the whole number closest to the accurate relative mass (equal to the sum of the numbers of protons and neutrons in the nucleus)], as a lefthand superscript to the element symbol, with the nuclear charge, Ze+, expressed as the number of protons in the nucleus (the atomic number, 2) placed as a left-hand subscript, $X, where X is the chemical symbol for the element. The value of A - 2 is the number of neutrons in the nucleus. Mass numbers are very close to being whole numbers because the relative masses of nuclei are composed of numbers of protons and neutrons whose relative masses are very close to 1 on the RAM scale (see Table 1.1). The actual mass of an atom, M, can be expressed by the equation:
ATOMIC THEORY, PERIODICITY, and NUCLEAR CHEMISTRY
 ATOMIC THEORY, PERIODICITY, and NUCLEAR CHEMISTRY Note: For all questions referring to solutions, assume that the solvent is water unless otherwise stated. 1. The nuclide is radioactive and decays by the
ATOMIC THEORY, PERIODICITY, and NUCLEAR CHEMISTRY Note: For all questions referring to solutions, assume that the solvent is water unless otherwise stated. 1. The nuclide is radioactive and decays by the
Chemistry 111 Syllabus
 Chemistry 111 Syllabus Chapter 1: Chemistry: The Science of Change The Study of Chemistry Chemistry You May Already Know The Scientific Method Classification of Matter Pure Substances States of Matter
Chemistry 111 Syllabus Chapter 1: Chemistry: The Science of Change The Study of Chemistry Chemistry You May Already Know The Scientific Method Classification of Matter Pure Substances States of Matter
i. This is the best evidence for the fact that electrons in an atom surround the nucleus in certain allowed energy levels or orbitals ii.
 Atomic Structure I. The Atom A. Atomic theory: Devised in 1807 by John Dalton, states that all matter is made up of a small number of different kinds of atoms that are indivisible and indestructible but
Atomic Structure I. The Atom A. Atomic theory: Devised in 1807 by John Dalton, states that all matter is made up of a small number of different kinds of atoms that are indivisible and indestructible but
Electrons in Atoms. Section 5.1 Light and Quantized Energy
 Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
MODERN PHYSICS Frank J. Blatt Professor of Physics, University of Vermont
 MODERN PHYSICS Frank J. Blatt Professor of Physics, University of Vermont McGRAW-HILL, INC. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London Madrid Mexico Milan Montreal New Delhi
MODERN PHYSICS Frank J. Blatt Professor of Physics, University of Vermont McGRAW-HILL, INC. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London Madrid Mexico Milan Montreal New Delhi
Chemistry 121: Atomic and Molecular Chemistry Topic 3: Atomic Structure and Periodicity
 Text Chapter 2, 8 & 9 3.1 Nature of light, elementary spectroscopy. 3.2 The quantum theory and the Bohr atom. 3.3 Quantum mechanics; the orbital concept. 3.4 Electron configurations of atoms 3.5 The periodic
Text Chapter 2, 8 & 9 3.1 Nature of light, elementary spectroscopy. 3.2 The quantum theory and the Bohr atom. 3.3 Quantum mechanics; the orbital concept. 3.4 Electron configurations of atoms 3.5 The periodic
PhET Interactive Chemistry Simulations Aligned to an Example General Chemistry Curriculum
 PhET Interactive Chemistry Simulations Aligned to an Example General Chemistry Curriculum Alignment is based on the topics and subtopics addressed by each sim. Sims that directly address the topic area
PhET Interactive Chemistry Simulations Aligned to an Example General Chemistry Curriculum Alignment is based on the topics and subtopics addressed by each sim. Sims that directly address the topic area
Lecture 21 Fundamentals of Physics Phys 120, Fall 2015 Nuclear Physics
 Lecture 21 Fundamentals of Physics Phys 120, Fall 2015 Nuclear Physics A. J. Wagner North Dakota State University, Fargo, ND 58102 Fargo, November 13, 2015 Overview Why care about nuclei? How do nuclei
Lecture 21 Fundamentals of Physics Phys 120, Fall 2015 Nuclear Physics A. J. Wagner North Dakota State University, Fargo, ND 58102 Fargo, November 13, 2015 Overview Why care about nuclei? How do nuclei
FOUNDATIONS OF NUCLEAR CHEMISTRY
 FOUNDATIONS OF NUCLEAR CHEMISTRY Michele Laino January 8, 2016 Abstract In this brief tutorial, some of basics of nuclear chemistry are shown. Such tutorial it is mainly focused on binding energy of nuclei
FOUNDATIONS OF NUCLEAR CHEMISTRY Michele Laino January 8, 2016 Abstract In this brief tutorial, some of basics of nuclear chemistry are shown. Such tutorial it is mainly focused on binding energy of nuclei
Worksheet 2.1. Chapter 2: Atomic structure glossary
 Worksheet 2.1 Chapter 2: Atomic structure glossary Acceleration (in a mass spectrometer) The stage where the positive ions are attracted to negatively charged plates. Alpha decay The emission of an alpha
Worksheet 2.1 Chapter 2: Atomic structure glossary Acceleration (in a mass spectrometer) The stage where the positive ions are attracted to negatively charged plates. Alpha decay The emission of an alpha
Where are we? Check-In
 Where are we? Check-In ü Building Blocks of Matter ü Moles, molecules, grams, gases, ü The Bohr Model solutions, and percent composition Coulomb s Law ü Empirical and Molecular formulas Photoelectron Spectroscopy
Where are we? Check-In ü Building Blocks of Matter ü Moles, molecules, grams, gases, ü The Bohr Model solutions, and percent composition Coulomb s Law ü Empirical and Molecular formulas Photoelectron Spectroscopy
Chapter 44. Nuclear Structure
 Chapter 44 Nuclear Structure Milestones in the Development of Nuclear Physics 1896: the birth of nuclear physics Becquerel discovered radioactivity in uranium compounds Rutherford showed the radiation
Chapter 44 Nuclear Structure Milestones in the Development of Nuclear Physics 1896: the birth of nuclear physics Becquerel discovered radioactivity in uranium compounds Rutherford showed the radiation
Saint Lucie County Science Scope and Sequence
 Course: Honors Physics 1 Course Code: 2003390 UNIT 9 TOPIC of STUDY: Electricity STANDARDS: 10: Energy ~The electric force between two charged particles depends upon the size of the charge and the distance
Course: Honors Physics 1 Course Code: 2003390 UNIT 9 TOPIC of STUDY: Electricity STANDARDS: 10: Energy ~The electric force between two charged particles depends upon the size of the charge and the distance
THE NUCLEUS OF AN ATOM
 VISUAL PHYSICS ONLINE THE NUCLEUS OF AN ATOM Models of the atom positive charge uniformly distributed over a sphere J. J. Thomson model of the atom (1907) ~2x10-10 m plum-pudding model: positive charge
VISUAL PHYSICS ONLINE THE NUCLEUS OF AN ATOM Models of the atom positive charge uniformly distributed over a sphere J. J. Thomson model of the atom (1907) ~2x10-10 m plum-pudding model: positive charge
Materials Science. Atomic Structures and Bonding
 Materials Science Atomic Structures and Bonding 1 Atomic Structure Fundamental concepts Each atom consists of a nucleus composed of protons and neutrons which are encircled by electrons. Protons and electrons
Materials Science Atomic Structures and Bonding 1 Atomic Structure Fundamental concepts Each atom consists of a nucleus composed of protons and neutrons which are encircled by electrons. Protons and electrons
MARLBORO CENTRAL SCHOOL DISTRICT-CURRICULUM MAP. Subject: AP Chemistry 2015/16
 MARLBORO CENTRAL SCHOOL DISTRICT-CURRICULUM MAP Subject: AP Chemistry 205/6 Title or Topics (Unit organizing idea) Concepts (understandings) Tasks (What students actually do) Major Assessments (Tests,
MARLBORO CENTRAL SCHOOL DISTRICT-CURRICULUM MAP Subject: AP Chemistry 205/6 Title or Topics (Unit organizing idea) Concepts (understandings) Tasks (What students actually do) Major Assessments (Tests,
: the smallest particle that has the properties of an element. In, this Greek philosopher suggested that the universe was made of.
 Notes: ATOMS AND THE PERIODIC TABLE Atomic Structure: : the smallest particle that has the properties of an element. From the early concept of the atom to the modern atomic theory, scientists have built
Notes: ATOMS AND THE PERIODIC TABLE Atomic Structure: : the smallest particle that has the properties of an element. From the early concept of the atom to the modern atomic theory, scientists have built
Instead, the probability to find an electron is given by a 3D standing wave.
 Lecture 24-1 The Hydrogen Atom According to the Uncertainty Principle, we cannot know both the position and momentum of any particle precisely at the same time. The electron in a hydrogen atom cannot orbit
Lecture 24-1 The Hydrogen Atom According to the Uncertainty Principle, we cannot know both the position and momentum of any particle precisely at the same time. The electron in a hydrogen atom cannot orbit
CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester.
 Name Period CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester. Chapter 2 Measurement & Calculations Describe the
Name Period CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester. Chapter 2 Measurement & Calculations Describe the
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Evaluate Scientific Models for Atomic Structure
 Evaluate Scientific Models for Atomic Structure Directions: Answer all parts of each question below. Make sure your answers are in complete sentences and are concise, including ONLY necessary details.
Evaluate Scientific Models for Atomic Structure Directions: Answer all parts of each question below. Make sure your answers are in complete sentences and are concise, including ONLY necessary details.
2 Electons Electrons: Quantum Numbers, Energy Levels and Electron Configurations
 Electrons: Quantum Numbers, Energy Levels and Electron Configurations For chemical reactions to occur a collision between atoms or molecules must happen. These collisions typically result in an exchange
Electrons: Quantum Numbers, Energy Levels and Electron Configurations For chemical reactions to occur a collision between atoms or molecules must happen. These collisions typically result in an exchange
Chapter 6 - Electronic Structure of Atoms
 Chapter 6 - Electronic Structure of Atoms 6.1 The Wave Nature of Light To understand the electronic structure of atoms, one must understand the nature of electromagnetic radiation Visible light is an example
Chapter 6 - Electronic Structure of Atoms 6.1 The Wave Nature of Light To understand the electronic structure of atoms, one must understand the nature of electromagnetic radiation Visible light is an example
The Electronic Theory of Chemistry
 JF Chemistry CH1101 The Electronic Theory of Chemistry Dr. Baker bakerrj@tcd.ie Module Aims: To provide an introduction to the fundamental concepts of theoretical and practical chemistry, including concepts
JF Chemistry CH1101 The Electronic Theory of Chemistry Dr. Baker bakerrj@tcd.ie Module Aims: To provide an introduction to the fundamental concepts of theoretical and practical chemistry, including concepts
Unit title: Atomic and Nuclear Physics for Spectroscopic Applications
 Unit title: Atomic and Nuclear Physics for Spectroscopic Applications Unit code: Y/601/0417 QCF level: 4 Credit value: 15 Aim This unit provides an understanding of the underlying atomic and nuclear physics
Unit title: Atomic and Nuclear Physics for Spectroscopic Applications Unit code: Y/601/0417 QCF level: 4 Credit value: 15 Aim This unit provides an understanding of the underlying atomic and nuclear physics
Objective #1 (80 topics, due on 09/05 (11:59PM))
 Course Name: Chem 110 FA 2014 Course Code: N/A ALEKS Course: General Chemistry (First Semester) Instructor: Master Templates Course Dates: Begin: 06/27/2014 End: 06/27/2015 Course Content: 190 topics Textbook:
Course Name: Chem 110 FA 2014 Course Code: N/A ALEKS Course: General Chemistry (First Semester) Instructor: Master Templates Course Dates: Begin: 06/27/2014 End: 06/27/2015 Course Content: 190 topics Textbook:
Syllabus: General and Inorganic Chemistry. Code number: NP-01. Cycle: undergraduate. Semester: 1st. Course type
 Syllabus: General and Inorganic Chemistry Code number: NP-01 Cycle: undergraduate Semester: 1st Course type X Background/General knowledge Scientific area (pharmacy) Credit Units (ECTS): 4 Lectures (hours/week):
Syllabus: General and Inorganic Chemistry Code number: NP-01 Cycle: undergraduate Semester: 1st Course type X Background/General knowledge Scientific area (pharmacy) Credit Units (ECTS): 4 Lectures (hours/week):
Modern Atomic Theory and the Periodic Table
 Modern Atomic Theory and the Periodic Table Chapter 10 the exam would have to be given earlier Hein and Arena Version 1.1 Eugene Passer Chemistry Department Bronx Community 1 College John Wiley and Sons,
Modern Atomic Theory and the Periodic Table Chapter 10 the exam would have to be given earlier Hein and Arena Version 1.1 Eugene Passer Chemistry Department Bronx Community 1 College John Wiley and Sons,
Radiation Physics PHYS /251. Prof. Gocha Khelashvili
 Radiation Physics PHYS 571-051/251 Prof. Gocha Khelashvili Interaction of Radiation with Matter: Heavy Charged Particles Directly and Indirectly Ionizing Radiation Classification of Indirectly Ionizing
Radiation Physics PHYS 571-051/251 Prof. Gocha Khelashvili Interaction of Radiation with Matter: Heavy Charged Particles Directly and Indirectly Ionizing Radiation Classification of Indirectly Ionizing
Quick Review. 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior.
 Quick Review 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior. Emission spectra Every element has a unique emission spectrum
Quick Review 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior. Emission spectra Every element has a unique emission spectrum
Chapter 4 Arrangement of Electrons in Atoms. 4.1 The Development of a New Atomic Model
 Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Chemistry 2 nd 6 Weeks
 NAME OF UNIT UNIT II ESTIMATED # OF DAYS 2 nd 6 Weeks_ Weeks 1 Weeks 2-3 Weeks 4-5 Components Unit Name IIA: Nuclear Chemistry IIB: Light, Energy, and Periodic Trends IIC: Bonding Short Descriptive Overview
NAME OF UNIT UNIT II ESTIMATED # OF DAYS 2 nd 6 Weeks_ Weeks 1 Weeks 2-3 Weeks 4-5 Components Unit Name IIA: Nuclear Chemistry IIB: Light, Energy, and Periodic Trends IIC: Bonding Short Descriptive Overview
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
CHAPTER STRUCTURE OF ATOM
 12 CHAPTER STRUCTURE OF ATOM 1. The spectrum of He is expected to be similar to that [1988] H Li + Na He + 2. The number of spherical nodes in 3p orbitals are [1988] one three none two 3. If r is the radius
12 CHAPTER STRUCTURE OF ATOM 1. The spectrum of He is expected to be similar to that [1988] H Li + Na He + 2. The number of spherical nodes in 3p orbitals are [1988] one three none two 3. If r is the radius
Unit 2 - Electrons and Periodic Behavior
 Unit 2 - Electrons and Periodic Behavior I. The Bohr Model of the Atom A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The energy of the electron
Unit 2 - Electrons and Periodic Behavior I. The Bohr Model of the Atom A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The energy of the electron
CHEMISTRY 113 EXAM 3(A)
 Summer 2003 CHEMISTRY 113 EXAM 3(A) 1. Specify radiation with the greatest energy from the following list: A. ultraviolet B. gamma C. infrared D. radio waves 2. The photoelectric effect is: A. reflection
Summer 2003 CHEMISTRY 113 EXAM 3(A) 1. Specify radiation with the greatest energy from the following list: A. ultraviolet B. gamma C. infrared D. radio waves 2. The photoelectric effect is: A. reflection
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Bohr s Correspondence Principle Bohr s Correspondence Principle states that quantum mechanics is in agreement with classical physics when the energy differences between quantized
Chapter 28 Atomic Physics Bohr s Correspondence Principle Bohr s Correspondence Principle states that quantum mechanics is in agreement with classical physics when the energy differences between quantized
Chapter 13 Spectroscopy
 hapter 13 Spectroscopy Infrared spectroscopy Ultraviolet-Visible spectroscopy Nuclear magnetic resonance spectroscopy Mass Spectrometry 13.1 Principles of Molecular Spectroscopy: Electromagnetic Radiation
hapter 13 Spectroscopy Infrared spectroscopy Ultraviolet-Visible spectroscopy Nuclear magnetic resonance spectroscopy Mass Spectrometry 13.1 Principles of Molecular Spectroscopy: Electromagnetic Radiation
Chapter 4 The Structure of the Atom
 Chapter 4 The Structure of the Atom Read pg. 86-97 4.1 Early Theories of Matter The Philosophers Democritus Artistotle - Artistotle s influence so great and the science so primitive (lacking!) his denial
Chapter 4 The Structure of the Atom Read pg. 86-97 4.1 Early Theories of Matter The Philosophers Democritus Artistotle - Artistotle s influence so great and the science so primitive (lacking!) his denial
Arrangement of Electrons. Chapter 4
 Arrangement of Electrons Chapter 4 Properties of Light -Light s interaction with matter helps to understand how electrons behave in atoms -Light travels through space & is a form of electromagnetic radiation
Arrangement of Electrons Chapter 4 Properties of Light -Light s interaction with matter helps to understand how electrons behave in atoms -Light travels through space & is a form of electromagnetic radiation
Learning Objectives for Chemistry 173
 Learning Objectives for Chemistry 173 Glenbrook North High School Academic Year, 2017-2018 This outline provides a comprehensive list of the topics and concepts you will learn in this course. For each
Learning Objectives for Chemistry 173 Glenbrook North High School Academic Year, 2017-2018 This outline provides a comprehensive list of the topics and concepts you will learn in this course. For each
Modern Atomic Theory and Electron Configurations
 Chem 101 Modern Atomic Theory and Electron Configurations Lectures 8 and 9 Types of Electromagnetic Radiation Electromagnetic radiation is given off by atoms when they have been excited by any form of
Chem 101 Modern Atomic Theory and Electron Configurations Lectures 8 and 9 Types of Electromagnetic Radiation Electromagnetic radiation is given off by atoms when they have been excited by any form of
Chapter Test B. Chapter: Arrangement of Electrons in Atoms. possible angular momentum quantum numbers? energy level? a. 4 b. 8 c. 16 d.
 Assessment Chapter Test B Chapter: Arrangement of Electrons in Atoms PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question
Assessment Chapter Test B Chapter: Arrangement of Electrons in Atoms PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question
Atomic Quantum number summary. From last time. Na Optical spectrum. Another possibility: Stimulated emission. How do atomic transitions occur?
 From last time Hydrogen atom Multi-electron atoms This week s honors lecture: Prof. Brad Christian, Positron Emission Tomography Course evaluations next week Tues. Prof Montaruli Thurs. Prof. Rzchowski
From last time Hydrogen atom Multi-electron atoms This week s honors lecture: Prof. Brad Christian, Positron Emission Tomography Course evaluations next week Tues. Prof Montaruli Thurs. Prof. Rzchowski
Physics 100 PIXE F06
 Introduction: Ion Target Interaction Elastic Atomic Collisions Very low energies, typically below a few kev Surface composition and structure Ion Scattering spectrometry (ISS) Inelastic Atomic Collisions
Introduction: Ion Target Interaction Elastic Atomic Collisions Very low energies, typically below a few kev Surface composition and structure Ion Scattering spectrometry (ISS) Inelastic Atomic Collisions
Atoms, Electrons and Light MS. MOORE CHEMISTRY
 Atoms, Electrons and Light MS. MOORE CHEMISTRY Atoms Remember Rutherford??? What did he discover with his gold foil experiment. A: Atoms contain a dense nucleus where the protons and neutrons reside. ATOMS
Atoms, Electrons and Light MS. MOORE CHEMISTRY Atoms Remember Rutherford??? What did he discover with his gold foil experiment. A: Atoms contain a dense nucleus where the protons and neutrons reside. ATOMS
Modern Atomic Theory. (a.k.a. the electron chapter!) Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11
 Modern Atomic Theory (a.k.a. the electron chapter!) 1 Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11 ELECTROMAGNETIC RADIATION 2 Electromagnetic radiation. 3 4 Electromagnetic Radiation
Modern Atomic Theory (a.k.a. the electron chapter!) 1 Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11 ELECTROMAGNETIC RADIATION 2 Electromagnetic radiation. 3 4 Electromagnetic Radiation
General Chemistry, in broad strokes. I. Introduction to chemistry, matter, measurements, and naming -- The Language of Chemistry
 General Chemistry, in broad strokes. I. Introduction to chemistry, matter, measurements, and naming -- The Language of Chemistry II. Stoichiometry -- The Numerical Logic of Chemistry III. A survey of chemical
General Chemistry, in broad strokes. I. Introduction to chemistry, matter, measurements, and naming -- The Language of Chemistry II. Stoichiometry -- The Numerical Logic of Chemistry III. A survey of chemical
Physical Science Curriculum Guide Scranton School District Scranton, PA
 Scranton School District Scranton, PA Prerequisite: Successful completion of general science and biology courses. Students should also possess solid math skills. provides a basic understanding of physics
Scranton School District Scranton, PA Prerequisite: Successful completion of general science and biology courses. Students should also possess solid math skills. provides a basic understanding of physics
3. Write ground-state electron configurations for any atom or ion using only the Periodic Table. (Sections 8.3 & 9.2)
 Lecture 2: learning objectives, readings, topics, and resources: 1. Understand the significance of the quantum numbers, understand how they can be used to code for the electron energy levels within atoms
Lecture 2: learning objectives, readings, topics, and resources: 1. Understand the significance of the quantum numbers, understand how they can be used to code for the electron energy levels within atoms
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A
 CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
Chapter 30 Nuclear Physics and Radioactivity
 Chapter 30 Nuclear Physics and Radioactivity 30.1 Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: 30.1 Structure
Chapter 30 Nuclear Physics and Radioactivity 30.1 Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: 30.1 Structure
Lesson 1. Introduction to Nuclear Science
 Lesson 1 Introduction to Nuclear Science Introduction to Nuclear Chemistry What is nuclear chemistry? What is the relation of nuclear chemistry to other parts of chemistry? Nuclear chemistry vs nuclear
Lesson 1 Introduction to Nuclear Science Introduction to Nuclear Chemistry What is nuclear chemistry? What is the relation of nuclear chemistry to other parts of chemistry? Nuclear chemistry vs nuclear
Honors Ch3 and Ch4. Atomic History and the Atom
 Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
MIDTERM 3 REVIEW SESSION. Dr. Flera Rizatdinova
 MIDTERM 3 REVIEW SESSION Dr. Flera Rizatdinova Summary of Chapter 23 Index of refraction: Angle of reflection equals angle of incidence Plane mirror: image is virtual, upright, and the same size as the
MIDTERM 3 REVIEW SESSION Dr. Flera Rizatdinova Summary of Chapter 23 Index of refraction: Angle of reflection equals angle of incidence Plane mirror: image is virtual, upright, and the same size as the
Big Idea #1 : Atomic Structure
 The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements of atoms. These atoms retain their identity in chemical reactions. Big Idea
The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements of atoms. These atoms retain their identity in chemical reactions. Big Idea
A Macmillan Physics Text
 WAVES A Macmillan Physics Text Consulting Editor: Professor P. A. Matthews, F.R.S. Other titles MODERN ATOMIC PHYSICS: FUNDAMENTAL PRINCIPLES: B. Cagnacand J. -C. Pebay-Peyroula MODERN ATOMIC PHYSICS:
WAVES A Macmillan Physics Text Consulting Editor: Professor P. A. Matthews, F.R.S. Other titles MODERN ATOMIC PHYSICS: FUNDAMENTAL PRINCIPLES: B. Cagnacand J. -C. Pebay-Peyroula MODERN ATOMIC PHYSICS:
Chemistry. Atomic and Molecular Structure
 Chemistry Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates
Chemistry Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates
ADVANCED CHEMISTRY CURRICULUM. Unit 1: Mathematical Representation in Chemistry
 Chariho Regional School District - Science Curriculum September, 2016 ADVANCED CHEMISTRY CURRICULUM Unit 1: Mathematical Representation in Chemistry OVERVIEW Summary Measurements are fundamental to the
Chariho Regional School District - Science Curriculum September, 2016 ADVANCED CHEMISTRY CURRICULUM Unit 1: Mathematical Representation in Chemistry OVERVIEW Summary Measurements are fundamental to the
CHEMISTRY Matter and Change
 CHEMISTRY Matter and Change Chapter 5: Electrons in Atoms 5 Section 5.1 Section Section 5.3 Table Of Contents Light and Quantized Energy Electron Configuration Compare the wave and particle natures of
CHEMISTRY Matter and Change Chapter 5: Electrons in Atoms 5 Section 5.1 Section Section 5.3 Table Of Contents Light and Quantized Energy Electron Configuration Compare the wave and particle natures of
Warm-up For sulfur: 1. How many valence electrons does it have? 2. What ion does this typically form? 3. Write the electron configuration for the ion.
 Warm-up For sulfur: 1. How many valence electrons does it have? 2. What ion does this typically form? 3. Write the electron configuration for the ion. Nucleus Contains 99.9% of the mass of an atom Found
Warm-up For sulfur: 1. How many valence electrons does it have? 2. What ion does this typically form? 3. Write the electron configuration for the ion. Nucleus Contains 99.9% of the mass of an atom Found
AP Chemistry A. Allan Chapter 7 Notes - Atomic Structure and Periodicity
 AP Chemistry A. Allan Chapter 7 Notes - Atomic Structure and Periodicity 7.1 Electromagnetic Radiation A. Types of EM Radiation (wavelengths in meters) 10-1 10-10 10-8 4 to 7x10-7 10-4 10-1 10 10 4 gamma
AP Chemistry A. Allan Chapter 7 Notes - Atomic Structure and Periodicity 7.1 Electromagnetic Radiation A. Types of EM Radiation (wavelengths in meters) 10-1 10-10 10-8 4 to 7x10-7 10-4 10-1 10 10 4 gamma
The structure of atoms.
 The structure of atoms. What will be covered? 1. The nucleus 2. Atomic weight 3. Electronic structure 4. Electronic configuration of the elements 5. Valence 6. Hybridization 7. Periodic table Why do we
The structure of atoms. What will be covered? 1. The nucleus 2. Atomic weight 3. Electronic structure 4. Electronic configuration of the elements 5. Valence 6. Hybridization 7. Periodic table Why do we
Atomic and nuclear physics
 Chapter 4 Atomic and nuclear physics INTRODUCTION: The technologies used in nuclear medicine for diagnostic imaging have evolved over the last century, starting with Röntgen s discovery of X rays and Becquerel
Chapter 4 Atomic and nuclear physics INTRODUCTION: The technologies used in nuclear medicine for diagnostic imaging have evolved over the last century, starting with Röntgen s discovery of X rays and Becquerel
Physics for Scientists & Engineers with Modern Physics Douglas C. Giancoli Fourth Edition
 Physics for Scientists & Engineers with Modern Physics Douglas C. Giancoli Fourth Edition Pearson Education Limited Edinburgh Gate Harlow Essex CM20 2JE England and Associated Companies throughout the
Physics for Scientists & Engineers with Modern Physics Douglas C. Giancoli Fourth Edition Pearson Education Limited Edinburgh Gate Harlow Essex CM20 2JE England and Associated Companies throughout the
14-Feb-18 INSIDE STARS. Matter and Energy. Matter and Energy. Matter and Energy. What is matter?
 What is matter? What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements,
What is matter? What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements,
Introductory College Chemistry
 Introductory College Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to
Introductory College Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to
1 amu 1 amu 0 amu. Chapter 2 part 1.notebook September 16, Modern Atomic Theory
 Chapter 2 The Atom Elements are the basic substances that make up all matter. An atom is the smallest particle of an element. Average atoms are 10 10 m in diameter. If you could put 6.02 x 10 23 p + and
Chapter 2 The Atom Elements are the basic substances that make up all matter. An atom is the smallest particle of an element. Average atoms are 10 10 m in diameter. If you could put 6.02 x 10 23 p + and
Chemistry 6 12 Section 03
 Chemistry 6 12 Section 03 1 Knowledge of the nature of matter 1. Differentiate between pure substances, homogeneous mixtures, and heterogeneous mixtures. 2. Determine the effects of changes in temperature,
Chemistry 6 12 Section 03 1 Knowledge of the nature of matter 1. Differentiate between pure substances, homogeneous mixtures, and heterogeneous mixtures. 2. Determine the effects of changes in temperature,
ATOMS. 1. DALTON'S ATOMIC THEORY. Modelos atómicos
 ATOMS An atom is the smallest particle of any element that still retains the characteristics of that element. However, atoms consist of even smaller particles. 1. DALTON'S ATOMIC THEORY. Modelos atómicos
ATOMS An atom is the smallest particle of any element that still retains the characteristics of that element. However, atoms consist of even smaller particles. 1. DALTON'S ATOMIC THEORY. Modelos atómicos
Contents. 1 Matter: Its Properties and Measurement 1. 2 Atoms and the Atomic Theory Chemical Compounds Chemical Reactions 111
 Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
CHAPTER 28 Quantum Mechanics of Atoms Units
 CHAPTER 28 Quantum Mechanics of Atoms Units Quantum Mechanics A New Theory The Wave Function and Its Interpretation; the Double-Slit Experiment The Heisenberg Uncertainty Principle Philosophic Implications;
CHAPTER 28 Quantum Mechanics of Atoms Units Quantum Mechanics A New Theory The Wave Function and Its Interpretation; the Double-Slit Experiment The Heisenberg Uncertainty Principle Philosophic Implications;
Key Terms and Definitions
 Topic: Atomic Structure Duration: Traditional (50 minute periods) : 8-13 days (adjust to student needs using professional discretion) Block Schedule (90 minute periods) : 3-5 days (adjust to student needs
Topic: Atomic Structure Duration: Traditional (50 minute periods) : 8-13 days (adjust to student needs using professional discretion) Block Schedule (90 minute periods) : 3-5 days (adjust to student needs
UNDERLYING STRUCTURE OF MATTER
 1 UNDERLYING STRUCTURE OF MATTER Chapter 4 Atomic Structure DEFINING THE ATOM Earlier theories of matter: A. Even though his hypothesis lacked evidence at the time, the Greek philosopher Democritus (460
1 UNDERLYING STRUCTURE OF MATTER Chapter 4 Atomic Structure DEFINING THE ATOM Earlier theories of matter: A. Even though his hypothesis lacked evidence at the time, the Greek philosopher Democritus (460
Chemistry Vocabulary. These vocabulary words appear on the Chemistry CBA in addition to being tested on the Chemistry Vocabulary Test.
 Chemistry Vocabulary These vocabulary words appear on the Chemistry CBA in addition to being tested on the Chemistry Vocabulary Test. atom the smallest unit of an element that still represents that element.
Chemistry Vocabulary These vocabulary words appear on the Chemistry CBA in addition to being tested on the Chemistry Vocabulary Test. atom the smallest unit of an element that still represents that element.
General Chemistry Notes Name
 Bio Honors General Chemistry Notes Name Directions: Carefully read the following information. Look for the ** directions in italics** for prompts where you can do some work. Use the information you have
Bio Honors General Chemistry Notes Name Directions: Carefully read the following information. Look for the ** directions in italics** for prompts where you can do some work. Use the information you have
Chapter 6 Part 1 Structure of the atom
 Chapter 6 Part 1 Structure of the atom What IS the structure of an atom? What are the properties of atoms? REMEMBER: structure affects function! Important questions: Where are the electrons? What is the
Chapter 6 Part 1 Structure of the atom What IS the structure of an atom? What are the properties of atoms? REMEMBER: structure affects function! Important questions: Where are the electrons? What is the
U N I T T E S T P R A C T I C E
 South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
Chemistry 101 Chapter 11 Modern Atomic Theory
 Chemistry 101 Chapter 11 Modern Atomic Theory Electromagnetic radiation: energy can be transmitted from one place to another by lightmore properly called electromagnetic radiation. Many kinds of electromagnetic
Chemistry 101 Chapter 11 Modern Atomic Theory Electromagnetic radiation: energy can be transmitted from one place to another by lightmore properly called electromagnetic radiation. Many kinds of electromagnetic
CHEM GENERAL CEMISTRY
 CHEM 100-12 GENERAL CEMISTRY Course Synopsis: The fundamental principles of chemistry, including atomic and molecular structure, bonding, elementary thermochemistry and thermodynamics, oxidation-reduction
CHEM 100-12 GENERAL CEMISTRY Course Synopsis: The fundamental principles of chemistry, including atomic and molecular structure, bonding, elementary thermochemistry and thermodynamics, oxidation-reduction
Characteristics and Structure of Matter Perry Sprawls, Ph.D.
 Online Textbook Perry Sprawls, Ph.D. Table of Contents INTRODUCTION AND OVERVIEW NUCLEAR STRUCTURE Composition Nuclides Isotopes Isobars Isomers Isotones NUCLEAR STABILITY Radioactivity NUCLEAR ENERGY
Online Textbook Perry Sprawls, Ph.D. Table of Contents INTRODUCTION AND OVERVIEW NUCLEAR STRUCTURE Composition Nuclides Isotopes Isobars Isomers Isotones NUCLEAR STABILITY Radioactivity NUCLEAR ENERGY
"What Do I Remember From Introductory Chemistry?" - A Problem Set
 1 "What Do I Remember From Introductory Chemistry?" - A Problem Set These problems review a portion of the material from introductory chemistry that you should be very familiar with. 1. The electron configuration
1 "What Do I Remember From Introductory Chemistry?" - A Problem Set These problems review a portion of the material from introductory chemistry that you should be very familiar with. 1. The electron configuration
Subatomic Particles. proton. neutron. electron. positron. particle. 1 H or 1 p. 4 α or 4 He. 0 e or 0 β
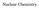 Nuclear Chemistry Subatomic Particles proton neutron 1n 0 1 H or 1 p 1 1 positron electron 0 e or 0 β +1 +1 0 e or 0 β 1 1 particle 4 α or 4 He 2 2 Nuclear Reactions A balanced nuclear equation has the
Nuclear Chemistry Subatomic Particles proton neutron 1n 0 1 H or 1 p 1 1 positron electron 0 e or 0 β +1 +1 0 e or 0 β 1 1 particle 4 α or 4 He 2 2 Nuclear Reactions A balanced nuclear equation has the
WDHS Curriculum Map: Created by Erin Pence September 2010
 WDHS Curriculum Map: Created by Erin Pence September 2010 Course: Chemistry CP Text: Modern Chemistry (Holt) Text: Chemistry ( Lab Book: Chemistry The Study of Matter () Course Units Covered MP1 Units
WDHS Curriculum Map: Created by Erin Pence September 2010 Course: Chemistry CP Text: Modern Chemistry (Holt) Text: Chemistry ( Lab Book: Chemistry The Study of Matter () Course Units Covered MP1 Units
General Physics (PHY 2140)
 General Physics (PHY 140) Lecture 18 Modern Physics Nuclear Physics Nuclear properties Binding energy Radioactivity The Decay Process Natural Radioactivity Last lecture: 1. Quantum physics Electron Clouds
General Physics (PHY 140) Lecture 18 Modern Physics Nuclear Physics Nuclear properties Binding energy Radioactivity The Decay Process Natural Radioactivity Last lecture: 1. Quantum physics Electron Clouds
Introduction to Nuclear Reactor Physics
 Introduction to Nuclear Reactor Physics J. Frýbort, L. Heraltová Department of Nuclear Reactors 19 th October 2017 J. Frýbort, L. Heraltová (CTU in Prague) Introduction to Nuclear Reactor Physics 19 th
Introduction to Nuclear Reactor Physics J. Frýbort, L. Heraltová Department of Nuclear Reactors 19 th October 2017 J. Frýbort, L. Heraltová (CTU in Prague) Introduction to Nuclear Reactor Physics 19 th
RFSS: Lecture 6 Gamma Decay
 RFSS: Lecture 6 Gamma Decay Readings: Modern Nuclear Chemistry, Chap. 9; Nuclear and Radiochemistry, Chapter 3 Energetics Decay Types Transition Probabilities Internal Conversion Angular Correlations Moessbauer
RFSS: Lecture 6 Gamma Decay Readings: Modern Nuclear Chemistry, Chap. 9; Nuclear and Radiochemistry, Chapter 3 Energetics Decay Types Transition Probabilities Internal Conversion Angular Correlations Moessbauer
Basic Chemistry 2014 Timberlake
 A Correlation of 2014 Timberlake A Correlation of, INTRODUCTION This document demonstrates how, Timberlake 2014 meets the, grades 9-12. Correlation page references are to the Student Edition. Maintaining
A Correlation of 2014 Timberlake A Correlation of, INTRODUCTION This document demonstrates how, Timberlake 2014 meets the, grades 9-12. Correlation page references are to the Student Edition. Maintaining
Principles of Alchemy (Chemistry) by Dr Jamie Love from Merlin Science Syllabus and correlation/alignment with Standards
 Principles of Alchemy (Chemistry) by Dr Jamie Love from Merlin Science www.synapse.co.uk/alchemy Syllabus and correlation/alignment with Standards Here you will find 1. a simple syllabus of the course
Principles of Alchemy (Chemistry) by Dr Jamie Love from Merlin Science www.synapse.co.uk/alchemy Syllabus and correlation/alignment with Standards Here you will find 1. a simple syllabus of the course
1, 2, 3, 4, 6, 14, 17 PS1.B
 Correlations to Next Generation Science Standards Physical Science Disciplinary Core Ideas PS-1 Matter and Its Interactions PS1.A Structure and Properties of Matter Each atom has a charged substructure
Correlations to Next Generation Science Standards Physical Science Disciplinary Core Ideas PS-1 Matter and Its Interactions PS1.A Structure and Properties of Matter Each atom has a charged substructure
Photoelectron Spectroscopy Evidence for Electronic Structure Guided-Inquiry Learning Activity for AP* Chemistry
 Introduction Photoelectron Spectroscopy Evidence for Electronic Structure Guided-Inquiry Learning Activity for AP* Chemistry Catalog No. AP7710 Publication No. 7710AS The chemical properties of elements
Introduction Photoelectron Spectroscopy Evidence for Electronic Structure Guided-Inquiry Learning Activity for AP* Chemistry Catalog No. AP7710 Publication No. 7710AS The chemical properties of elements
2 Atomic Theory Development of Theory
 Atomic Theory Development of Theory Historical Atomic Models Democritus Greek philosopher who postulated that matter is comprised of atoms as the smallest part (ca 400 BC) John Dalton Max Planck J.J. Thompson
Atomic Theory Development of Theory Historical Atomic Models Democritus Greek philosopher who postulated that matter is comprised of atoms as the smallest part (ca 400 BC) John Dalton Max Planck J.J. Thompson
Chapter 2: The Structure of the Atom and the Periodic Table
 Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Nuclear Medicine Treatments and Clinical Applications
 INAYA MEDICAL COLLEGE (IMC) RAD 243- LECTURE 4 Nuclear Medicine Treatments and Clinical Applications DR. MOHAMMED MOSTAFA EMAM References "Advancing Nuclear Medicine Through Innovation". Committee on State
INAYA MEDICAL COLLEGE (IMC) RAD 243- LECTURE 4 Nuclear Medicine Treatments and Clinical Applications DR. MOHAMMED MOSTAFA EMAM References "Advancing Nuclear Medicine Through Innovation". Committee on State
CHEM 1364 Detailed Learning Outcomes Fall 2011 Buckley
 CHEM 1364 Introduction: Matter and Measurement (Chapter 1) Textbook references to Brown, LeMay, Bursten, Murphy, and Woodward 12 th Edition Classification of matter Given sufficient information be able
CHEM 1364 Introduction: Matter and Measurement (Chapter 1) Textbook references to Brown, LeMay, Bursten, Murphy, and Woodward 12 th Edition Classification of matter Given sufficient information be able
16.5 Coulomb s Law Types of Forces in Nature. 6.1 Newton s Law of Gravitation Coulomb s Law
 5-10 Types of Forces in Nature Modern physics now recognizes four fundamental forces: 1. Gravity 2. Electromagnetism 3. Weak nuclear force (responsible for some types of radioactive decay) 4. Strong nuclear
5-10 Types of Forces in Nature Modern physics now recognizes four fundamental forces: 1. Gravity 2. Electromagnetism 3. Weak nuclear force (responsible for some types of radioactive decay) 4. Strong nuclear
Updated: Page 1 of 5
 A. Academic Division: Health Sciences B. Discipline: Science MASTER SYLLABUS 2018-2019 C. Course Number and Title: CHEM1210 Chemistry I D. Course Coordinator: Assistant Dean: Melinda S. Roepke, MSN, RN
A. Academic Division: Health Sciences B. Discipline: Science MASTER SYLLABUS 2018-2019 C. Course Number and Title: CHEM1210 Chemistry I D. Course Coordinator: Assistant Dean: Melinda S. Roepke, MSN, RN
Lecture 1. Introduction to Nuclear Science
 Lecture 1 Introduction to Nuclear Science Composition of atoms Atoms are composed of electrons and nuclei. The electrons are held in the atom by a Coulomb attraction between the positively charged nucleus
Lecture 1 Introduction to Nuclear Science Composition of atoms Atoms are composed of electrons and nuclei. The electrons are held in the atom by a Coulomb attraction between the positively charged nucleus
Unit Two Test Review. Click to get a new slide. Choose your answer, then click to see if you were correct.
 Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Radiation and the Atom
 Radiation and the Atom PHYS Lecture Departamento de Física Instituto Superior de Engenharia do Porto cav@isep.ipp.pt Overview SI Units and Prefixes Radiation Electromagnetic Radiation Electromagnetic Spectrum
Radiation and the Atom PHYS Lecture Departamento de Física Instituto Superior de Engenharia do Porto cav@isep.ipp.pt Overview SI Units and Prefixes Radiation Electromagnetic Radiation Electromagnetic Spectrum
