Chemistry 424 Organometallic Chemistry
|
|
|
- Scott Harvey
- 5 years ago
- Views:
Transcription
1 hemistry 424 rganometallic hemistry 424 chem ourse Dr. Waed Z Al-Kayali First term, Department of hemistry King Saud University lass eeting: theory (Sun, Tues)(10-11) and (sun 8-9) Semester credit hours: 3.0 credits, second term Total ontact Hours: 39 hr. E.mail: wwaed1956@yahoo.com oom No:242-third floor ffice Hours: Sun.11-1 First id Term: Sunday 2/1 (10-11) Second id Term: Sunday 7/2 (10-11) hemistry rganometallic hemistry Syllabus This course covers the organometallic chemistry of the transition metals with emphasis on basic reaction types and the natural extensions to the very relevant area of homogeneous (and heterogeneous) catalysis. I. igand Systems and Electron ounting 1. xidation States, d electron configurations, 18-electron "rule 2. arbonyls, hosphines & Hydrides 3. σ bound carbon ligands: alkyls, aryls 4. σ/π-bonded carbon ligands: carbenes, carbynes 5. π -bonded carbon ligands: alkene, allyl, cyclobutadiene, arenes, cyclopentadienyl 6. etal-etal bonding II. eaction chemistry of complexes 1. eactions involving the gain and loss of ligands 2. eactions involving modifications of the ligand 3. atalytic processes by the complexes 1
2 ecommended Book: "The rganometallic hemistry of the Transition etals" by obert rabtree (4th Edition, Wiley). eference Book: The rinciples and Applications of Transition etal hemistry, by ollman, Hegedus, Norton and Finke Study Groups: The class will form study groups of 3 students to work together on the homework (each student hands in their own copy of the HW) and to answer questions in class (work alone on quizzes & exams). ourse onstruction: Two 50 min Exams: 2/1, 7/9 40% Final Exam (2 hrs): 40% Homeworks:3-5 homeworks 10% Quizzes: Two quizess 10% rganometallic hemistry Definition: Definition of an organometallic compound Anything with bond =, H (hydride) etal (of course) eriodic Table down & left electropositive element (easily loses electrons) NT: omplex which binds ligands via, N,, S, other - carboxylates, ethylenediamine, water X where complex has organometallic behavior, reactivity patterns e.g., low-valent oxidation State d n for compounds of transition elements nd < (n+1) s or (n+1) p in compounds e.g., 3d < 4s or 4p I. igand Systems and Electron ounting Fundamentals You Need to Know: - Electronegative/Electropositive concepts Where do the partial positive and negative charges in a molecule reside? This is important for determining how much electron (e-) density will be donated from a ligand to a metal and where a nucleophile or electrophile will likely attack for chemical reactions. 2
3 - ewis dot structures and valence electron counts - Important for determining the number of electrons on a ligand and what the charge of the ligand is. - We almost always deal with ligands with even # s of electrons. - If a ligand has an odd # of electrons we add additional electrons to get to an even #, usually to form a closed shell electron configuration with a formal negative charge(s). Exception = Boron. xidation States of the central atom (metal) rganic line notation for drawing structures Electron Density is the presence of higher energy valence electrons around an atom. Electrons are represented by a probability distribution spread out over a region of space defined by the orbital: s, p, d, f, and/or hybrid orbitals such sp 3, sp 2, sp, etc. Atoms with quite a few valence electrons such as t(0) d 10 and/or contracted orbitals have a high electron density. Atoms with fewer valence electrons (e.g., Na + ) and/or diffuse orbitals (electrons spread out over a larger region of space) can be considered to have low electron densities. ** Do not confuse electron density with electronegativity. Electron-rich: Atoms that are willing to readily donate electron pairs to other atoms are called electron rich. Ease of ionization is another property associated with electron-rich atoms. The willingness to share or donate electron pairs is related to lower electronegativity, larger numbers of valence electrons, good donor groups on the atom in question, negative charges, or some combination of these factors. Using organic terminology I would consider an electronrich atom to be a good nucleophile (electron pair donating). 3
4 Electron-deficient (poor): Atoms that are NT willing to donate or share electron pairs to other atoms are called electron deficient (poor). These atoms typically have lower lying empty orbitals that can accept electron pairs from other atoms. The un-willingness to donate or share electron pairs could be caused by: high electronegativity, cationic charge(s), lack of electron pairs, or some combination of these. I would consider many (but not all) electron-deficient atoms/molecules to be good electrophiles (electron-pair accepting) and certainly poor donors. Fluoride anion, F - : This anion has high electron density due to the negative charge, filled octet of electrons, and small size. But NT electron-rich, meaning not good electron donor. The extremely high electronegativity of a fluorine atom means that it desperately wants to pick up an extra electron to form the fluoride anion, which is extremely stable. The filled valence orbitals are fairly low in energy for F - and generally poor donors. It is certainly not electron-deficient as it doesn t have any low-lying empty orbitals and does not want to accept any more electrons. It is not electron-rich either since it is a very poor nucleophile and generally a poor ligand for most metals ethyl anion, H 3- : This anion is very electron-rich and a powerful nucleophile. The electron-richness comes from: the lower electronegativity of carbon, and the high energy of the anionic sp 3 -hybridized lone pair that makes it a strong donor group. 4
5 e 3 vs. (e) 3 : The methyl groups are considered to be electron donating making the center more electron-rich. The methoxy groups are electron-withdrawing due to the electronegative oxygen atoms, making the center more electron deficient. Note the higher energy of the lone pair (highest occupied molecular orbital, H), greater spatial extent (generally better overlap with metal d-orbitals), and lower positive charge on for e 3 relative to (e) 3. plot of the lone pair orbital (H) for e 3. Dashed outline indicates the spatial extent of the lone pair for (e) 3. e 3 H = ev harge on = (e) 3 H = ev harge on = Transition etal atalysis A + B [catalyst] A catalyst is a substance that increases the rate of rxn without itself being consumed (but it is involved!) in the reaction. A catalyst speeds up the rate at which a chemical reaction reaches equilibrium. The overall thermodynamics of the rxn is NT changed by the catalyst. Therefore, very endothermic (non-spontaneous) reactions are usually NT suitable for catalytic applications. G Ea eactants G eaction oordinate atalyzed rxn proceeding through an intermediate E a catalyzed roducts A catalyst provides an alternate mechanism (or pathway) for the reactants to be transformed into products. The catalyzed mechanism has an activation energy that is lower than the original uncatalyzed rxn. An excellent catalyst will lower the activation energy the most. 5
6 helate Effect: chelate is from the Greek meaning claw or to grab on to. Since most metal-ligand bonds are relatively weak compared to - bonds. - bonds can often be broken rather easily, leading to dissociation of the ligand from the metal. + From a kinetic viewpoint, if one of the ligands dissociates, it will remain close enough to the metal center to have a high probability of re-coordinating before another ligand can get in an bind. From a thermodynamic viewpoint, by tethering two donor ligands together, one removes most of the entropic driving force for dissociating a ligand and thus making more particles in solution (more disorder). h x eta-x was originally developed to indicate how many contiguous donor atoms of a p-system were coordinated to a metal center. Hapticity is another word used to describe the bonding mode of a ligand to a metal center. An h 5 - cyclopentadienyl ligand, for example, has all five carbons of the ring bonding to the transition metal center. h x values for all-carbon based ligands where the x value is odd usually indicate anionic carbon ligands (e.g., h 5 -p, h 1 -H 3, h 1 -allyl or h 3 -allyl, h 1 - H=H 2 ). The # of electrons donated (ionic method of electron counting) by the ligand is usually equal to x + 1. Even h x values usually indicate neutral carbon p- system ligands (e.g., h 6-6 H 6, h 2 -H 2 =H 2, h 4 -butadiene, h 4 -cyclooctadiene). The # of electrons donated by the ligand in the even (neutral) case is usually just equal to x. 6
7 k x kappa-x was developed to indicate how many non-contiguous donor atoms of a ligand system were coordinated to a metal center. This usually refers to non-carbon donor atoms, but can include carbons. A k 1 -dppe (h 2 H 2 H 2 h 2 ) ligand, for example, has only one of the two phosphorus donors bonded to the transition metal center. m x h 2 h 2 mu-x is the nomenclature used to indicate the presence of a bridging ligand between two or more metal centers. The x refers to the number of metal centers being bridged by the ligand. Usually most authors omit x = 2 and just use m to indicate that the ligand is bridging the simplest case of two metals. There are two different general classes of bridging ligands: 1) Single atom bridges 2) Two donor atoms separated by a bridging group (typically organic) rdering in Formula - Formulas with p (cyclopentadienyl) ligands, the p usually comes first, followed by the metal center: p 2 Til 2 - Formulas with hydride ligands, the hydride is sometimes listed first, Hh()(h 3 ) 2 and p 2 TiH 2 - Bridging ligands are usually placed next to the metals in question, then followed by the other ligands: o 2 (m-) 2 () 6, h 2 (m-l) 2 () 4, p 2 Fe 2 (m-) 2 () 2 - Anionic ligands are often listed before neutral ligands: hl(h 3 ) 3, pul(=h 2 Et)(h 3 ) (neutral carbene ligand), tie 2 ( )(bipy). ommon oordination Geometries 6-oordinate: ctahedral (90 & 180 angles) 5-oordinate: Trigonal Bypyramidal or Square yramidial axial equitorial apical (90 & 120 ) (~100 & 90 ) basal 7
8 4-oordinate: Square lanar or Tetrahedral (90 & 180 ) (109 ) Square planar geometry is generally limited to h, Ir, Ni, d, t, and Au in the d 8 electronic state when coordinated to 2e- donor ligands. roblem: Sketch structures for the following: a) pul(=h 2 Et)(h 3 ) b) o 2 (m-) 2 () 6 (o-o bond, several possible structures) c) trans-hh()(h 3 ) 2 [h(+1) = d 8 ] d) Ir 2 (m-l) 2 () 4 [Ir(+1) = d 8 ] e) p 2 Til 2 Bonding and rbitals y z x s z z z y x y x y x p z p x p y z z y x y x z d z 2 z d x 2 - y 2 z y x y x y x dyz d xz d xy 8
9 verlap Efficiency The strength of a chemical bond (covalent or dative) is related to the amount of overlap between two atomic (or hybrid) orbitals. The overlap efficiency can be thought of as the orbital overlap area divided by the nonoverlapping area. The smaller this ratio, the weaker the bonding. verlap efficiency verlap efficiency also applies to s-bonds between atoms. Dihalogen bond strengths increase F 2 < l 2 < Br 2 < I 2. But decrease as one goes from - > Si- > Ge- > Sn- > b-. For most transition metal - single bonds the trend is fairly consistent: first row < second row < third row. But for - quadruple bonds one has: r-r << o-o > W-W. Square lanar Square planar complexes typically have d 8 (sometimes d 9 ) electronic configurations and are usually limited to the following elements: h, Ir, Ni, d, t, u, & Au. 9
10 10
11 ctahedral rbital Diagram 11
12 18-Electron ule The vast majority of stable diamagnetic organometallic compounds have 16 or 18 valence electrons due to the presence of the five d orbitals which can hold 10 more electrons relative to,, N, etc. Electron counting is the process of determining the number of valence electrons about a metal center in a given transition metal complex. To figure out the electron count for a metal complex: 1) Determine the oxidation state of the transition metal center(s) and the metal centers resulting d-electron count. To do this one must: a) note any overall charge on the metal complex b) know the charges of the ligands bound to the metal center (ionic ligand method) c) know the number of electrons being donated to the metal center from each ligand (ionic ligand method) 2) Add up the electron counts for the metal center and ligands 18 e- counts are referred to as saturated, because there are no empty lowlying orbitals to which another incoming ligand can coordinate. Electron counts lower than 18e- are called unsaturated and can electronically bind additional ligands unless the coordination site is sterically blocked. 12
13 Exceptions to the 18-Electron ule Early Transition etals 16e- and sub-16econfigurations are common oordination geometries higher than 6 relatively common iddle Transition etals 18e- configurations are common oordination geometries of 6 are common d 6 ate Transition etals 16e- and sub-16econfigurations are common oordination geometries of 5 and lower are common: d 8 = square planar igands, Bonding Types, harges, and Donor # s igands, harges, and Donor # s ationic 2e- donor: Neutral 2e- donors: Anionic 2e- donors: Anionic 4e- donors: Anionic 6e- donors: Ionic ethod of electron-counting N + (nitrosyl) 3 (phosphines), (carbonyl), 2 = 2 (alkenes), (alkynes, can also donate 4 e-), N (nitriles) l - (chloride), Br - (bromide), I - (iodide), H 3 - (methyl), 3 - (alkyl), h - (phenyl), H - (hydride) The following can also donate 4 e- if needed, but initially count them as 2e- donors (unless they are acting as bridging ligands): - (alkoxide), S - (thiolate), N 2 - (inorganic amide), 2 - (phosphide) 3 H 5 - (allyl), 2- (oxide), S 2- (sulfide), N 2- (imido), 2 2- (alkylidene) and from the previous list: - (alkoxide), S - (thiolate), N 2 - (inorganic amide), 2 - p - (cyclopentadienyl), N 3- (nitride) 13
14 e-counting Examples: Simple 1) There is no overall charge on the complex 2) There is one anionic ligand (H 3 -, methyl group) 3) The e metal atom must have a +1 charge to compensate for the one negatively charged ligand. So the e is the in the +1 oxidation state. We denote this three different ways: e(+1), e(i), or e I. e(+1) d e- 2 4e- H - 3 2e- H 2 =H 2 2e- Total: 18e- e-counting Examples: Simple (but semi-unusual ligand) 1) There is a +2 charge on the complex 2) The NH 3 (methyl isocyanide) ligand is neutral, but lets check the ewis Dot structure to make sure that is correct: o(+2) d 4 7 NH 3 14e- Total: 18e- 3) Because there is a +2 charge on the complex and all neutral ligands present, the o has a +2 charge & oxidation state. e-counting Examples: igand Analysis 1) emove the metal atom(s) and examine the ligand by itself: 2) If the donor atoms have an odd # of e- s, add enough to get an even # and (usually) a filled octet. As you add e- s don t forget to add negative charges!! 14
15 e-counting Examples: Tricky System 1) There is no overall charge on the complex 2) There is one anionic ligand ( 3 H 5 -, allyl) 3) The top ligand is NT a ep -! It is a neutral diene that has a H attached to the methylsubstituted ring carbon. This is a neutral 4e- donor. 3) Because the complex is neutral and there is one anionic ligand present, the h atom must have a +1 charge to compensate for the one negatively charged ligand. So the h atom is in the +1 oxidation state. h(+1) d 8 3 2e- h 4-5 H 5 e 4eh 3-3 H - 5 4e- Total: 18e- e-counting Examples: - Bonded System 2 2 o l o l 2 o(+1) d e- 2 4e- 2m-l - 4e- Sub-total: 17e- o-o 1e- TTA: 18e- 2 1) Generally treat metal-metal (-) bonds to be simple covalent bonds with each metal contributing 1e- to the bond. If you have two metal atoms next to one another and each has an odd electron-count, pair the odd electrons to make a - bond. 2) Bridging ligands, like halides, with at least 2 lone pairs almost always donate 2e- to each metal center. 3) xidation state determination: Total of two anionic ligands for two metal centers (overall complex is neutral). Thus each metal center needs to have a +1 oxidation state to balance the anionic ligands. Very ommon istake: Students determining the oxidation state for complexes with 2 or more metal centers often add up all the anionic ligands and then figure out the oxidation state for only one of the metal centers based on this. 15
16 e-counting Examples: - Bonded System e e N N d H 2 d H 3 N N e e xidation state analysis: Total of 3 negative charges on the ligands (anionic methyl, dianionic alkylidene) and a positive charge on the complex. Therefore the two d centers must have a TTA of a +4 charge, or a +2 charge (oxidation state) on each. igand analysis: The chelating N ligand is a bis-imine, is neutral, with each N atom donating 2e-. Two different bridging ligands an anionic H 3 - (methyl group) and a dianionic H 2 2- (carbene or alkylidene). The H 3 - only has one lone pair of electrons, so it has to split these between the two metals (1e- to each). The H 2 2- alkylidene ligand, on the other hand, has 2 lone pairs & donates 2e- to each. d(+2) d 8 2 imines 4em-H - 3 1em-H 2-2 2e- Sub-total: 15e- d-d 1e- TTA: 16e- e-counting roblems: e e(+1) d 6 h 6 -benzene 6 h 5 -p 6 18 o (e) 3 (e) 3 l l Ta 3 3 Ne 2 Ni N e 2 N o Ne 2 Ne 2 N r N N e 3 Ti Br Br W e 3 d Ni N N o h 3 n e 16
17 Sc e o e 3 e 3 e 3 e 3 r e 3 H h 2 h 2 W H 2t-Bu H Fe? Fe Fe? Fe s s? s H H o? o Br Au? Au Au? Au H 3 o? o h? h roblem. Sketch out a structure showing the geometry about the metal center as accurately as possible and clearly show the electron counting for the complexes below. hosphine ligand abbreviations are defined in your notes (see the phosphine ligand section). 17
18 18
19 roblem. ropose an 18e- structure for the following metal/ligand combinations. Use at least one of each metal and ligand listed. omplexes should be neutral. Don t use more than 2 metal centers. Show your electron counting. igands are shown without charges, please indicate the proper ligand charge in your electron counting. Draw a reasonable structure showing the geometry about the metal center(s). 19
20 roblem. For each complex below, provide the oxidation state of the metal, the number of d electrons,and the total electron count for the complex. If there is more than one metal center, consider each separately. roblem. For each of the following reactions, indicate whether the metal is oxidized, reduced, or retains the same oxidation state? 20
21 ewis Base igands - Halides X Increasing polarizability F, l, Br, I rimary Halides Strongest nucleophile for low oxidation state metals centers X X X 2e- terminal 4e- m-bridging 6e- m 3 -bridging ommon isconception: The halides are anionic ligands, so they are NT electron-withdrawing ligands. In organic chemistry the halogens can be considered neutral ligands and do drain electron density from whatever they are attached to. But here they are anionic and are perfectly happy with that charge. Their electronegativity makes the halides poor donor ligands. As one moves from F - to I -, the donor ability increases as the electro negativity drops. xygen Donors Sulfur Donors 21
22 Nitrogen Donors N In general, alkylated amines are not particularly good ligands. This is mainly due to the relatively short N- bond distances and the stereoelectronic problems generated from this. helating amines have fewer steric problems and are better ligands for transition metal centers. erhaps the most famous neutral nitrogen donor ligand is bipyridine or bipyridyl, almost universally abbreviated bipy. N N N N Bipy = Bipyridine hen = phenanthroline Inorganic Amides N Strong Base & Nucelophile Terminal (2 or 4e-) donor 4e- Bridging donor The lone pairs in an amide are about 2eV higher in energy than in -. This makes an amide a considerably stronger donor. N 2e- donor pyramidal geometry N 4e- donor trigonal planar Alkyl-Imido (nitrene) igand 4e- or 6e- Terminal N N + - N 4e- Bridging 6e- Triply bridging - Imido (nitrene) Bent (nucleophillic nitrogen) inear (electrophillic nitrogen) N N N s 2 N Ta N 2 N 2 l h 3 e l e h 3 l As one moves to the right-hand side of the periodic table, one tends to get less - multiple bonding 22
23 roblem: onsider the complexes e(ne 2 )() 3 (dmpe) and W(Ne 2 )Br(dmpe) 2. In the e complex the Ne 2 ligand is not acting as a π-donating ligand, while in the W complex it is acting as a strong π- donating ligand. Discuss how the Ne 2 ligand is acting as a π-donating ligand for the W complex and why it is not acting as such for the e complex, even though the e atom is probably more electron deficient due to the three π-backbonding ligands. When the Ne 2 ligand acts as a 4e- σ- and π-donating ligand, its geometry is different from when it acts as a simple σ-donating 2e- donor. What is this difference in geometry? The e complex counts up to 18e- with the amide ligand acting as a simple 2e- donor. If it was a 4eπ-donating ligand, one would get a 20e- count, which would be bad. Thus, there is no reason for it to want to donate more than 2e-. The W complex, on the other hand, has a 16e- count with the amide acting as a simple 2e- donor. Thus, there is an empty metal orbital available that can interact with the filled lone pair on the amide, allowing it to act as a π-donating 4e- donor ligand. 2. arbonyls, hosphines & Hydrides arbonyl igands - Examples of neutral, binary metal carbonyls: Ti V() 6 r() 6 n 2 () 10 Fe 2 () 9 Fe() 5 Fe 3 () 12 o 2 () 8 o 4 () 12 Ni() 4 u Zr Nb o() 6 Tc 2 () 10 u() 5 u 3 () 12 h 4 () 12 h 6 () 16 d Ag Hf Ta W() 6 e 2 () 10 s() 5 s 3 () 12 Ir 4 () 12 t Au 23
24 olecular rbital () Diagram Experimental Data Supporting Nature of s in Species onfig - Å n cm -1 omment (5s) (5s) s is weakly antibonding * (5s) 1 (2p) 1 S p is strongly antibonding T Three types (two of which are important) of -etal bonding interactions: - bond: increases increases increases - bond: increases decreases decreases n freq: increases decreases decreases 24
25 arbonyl Infrared (I) Stretching Frequencies The position of the carbonyl bands in the I depends mainly on the bonding mode of the (terminal, bridging) and the amount of electron density on the metal being p-backbonded to the. The number (and intensity) of the carbonyl bands observed depends on the number of ligands present and the symmetry of the metal complex. There are also secondary effects such as Fermi resonance and overtone interactions that can complicate carbonyl I spectra. free terminal mode m 2 bridging m 3 bridging n I (cm ) (for neutral metal complexes) 2. As the metal center becomes increasingly electron rich the stretching frequency drops 25
26 Summary 1. As the bridges more metal centers its stretching frequency drops same for all p ligands ore back donation Electronic Effects on n As the electron density on a metal center increases, more p- back bonding to the ligand(s) takes place. This further weakens the - bond by pumping more electron density into the formally empty carbonyl p* orbital. This increases the - bond strength making it more double-bond-like, i.e., the resonance structure == assumes more importance. d x omplex n cm -1 free 2143 d 10 [Ag()] Ni() [o() 4 ] [Fe() 4 ] [n() 6 ] d 6 r() [V() 6 ]
27 Fe h2 h 2 Fe Fe h 2 Fe h Wavenumbers (cm-1) 1800 Ni 2( m-)() 2(dppm) 2 Ni Ni - + Ni 2() 4(dppm) 2 Ni Ni Ni() ( h 3 -dppm) Ni Wavenumbers (cm-1 ) 1700 igand Electronic Effects on n omplex n cm -1 o() 3 (F 3 ) , 2055 o() 3 (l 3 ) , 1991 o() 3 [(e) 3 ] , 1888 o() 3 (h 3 ) , 1835 o() 3 (NH 3 ) , 1783 o() 3 (triamine) , 1758 o() 3 (pyridine) , 1746 Based on I stretching frequencies, the following ligands can be ranked from best p-acceptor to worst: N + > > F 3 > N > l 3 > () 3 > 3 > N > NH 3 27
28 Semi-Bridging arbonyls ~ 150 Unsymmetrical bridging form. p* system accepts electron density from second metal center. Distortions away from a linear - (180 ) or a symmetrically bridging (120 ). Typical - angle around 150 (but with considerable variations). Fe Fe p* empty antibonding acceptor orbital filled Fe d orbital N N otton & Troup JAS, 1974, 96, 1233 s/p Bridging s acting as p-donor or p-acceptor? 2.22Å Nb p p 1.30Å Nb 2.25Å Nb 1.97Å p Herrman & coworkers JAS, 1981, 103, 1692 roblem: Which of the following metal carbonyl I spectra represents the compound with the least amount of electron density on the metal center? Briefly discuss the reasoning for your choice. Which compound will lose the easiest? 28
29 roblem: Which of the following metal carbonyl compounds will have the highest n stretching frequency in the I? Why? Will this be the most electron-rich or deficient compound? a) b) F Ir F F Br Br Ir Br c) e 2 N e 2 N Ir Ne 2 roblem. For each of the following pairs of metal complexes, circle the one that will have the highest stretching frequency. Briefly and clearly discuss your reasoning for each case. 29
30 30
31 roblem: The nitrosyl ligand usually coordinates as a cationic ligand, N +. It can, however, occasionally act as an anionic N - ligand. When it is behaving as an anionic ligand it adopts a bent coordination geometry. Discuss (using ewis dot-like figures) the distribution of electrons in both kinds of -N complexes and how these affect the structures (linear vs. bent). Assume in both cases that you are dealing with a [-N] + unit (positive charge on the overall complex) where the metal has 2 or more d electrons. learly show the relative oxidation states of the metal and the relative d electron count for each bonding case (linear vs. bent). It is mentioned that in some ways N - is the extreme case of N + acting as a hyper π backbonding ligand. Explain what is meant by that statement. N + is isoelectronic with, that is, it has the same bonding and electronic structure. The difference is that the more electronegative nitrogen atom combined with the net positive charge work together to make N + the strongest π - backbonding ligand known. In fact, it can backbond enough to formally oxidize the metal center by two electrons to transform the N + ligand into a N - ligand. This is shown below in the transfer of a pair of electrons from the metal to the N + ligand to produce the bent N - ligand. The lone pair that used to be on the metal center is now on the nitrogen of the N - ligand. This is what I was referring to as hyper-π- backbonding. When the N + ligand turns into a N - ligand it has formally oxidized the metal center by 2 electrons. That represents the ultimate in π-backbonding. hosphine igands 3 31
32 Tolman s one Angle and Electronic arameter The electron-donating ability of a phosphine ligand was determined by measuring the n of a Ni() 3 ( 3 ) complex: owest stretching frequency: most donating phosphine The size or steric bulk of a phosphine ligand was determined from simple 3-D space-filling models of the phosphine ligand coordinated to a Ni atom: Highest stretching frequency: least donating phosphine (best p-acceptor) cone angle one Angle (Tolman) Steric hindrance: A cone angle of 180 degrees -effectively protects (or covers) one half of the coordination sphere of the metal complex hosphine igand H 3 F 3 (e) 3 e 3 e 2 h Et 3 h 3 y 3 (t-bu) 3 (mesityl) 3 one Angle 87 o 104 o 107 o 118 o 122 o 132 o 145 o 170 o 182 o 212 o ommonly Used olydentate hosphines h 2 h 2 dppm (121 ) diphenylphosphinomethane bis(diphenyl)phosphinomethane bridging ligand h 2 h 2 h h S h 2 h 2 A-Frame bimetallic h 2(m-S)() 2(dppm) 2 Kubiak & Eisenberg JAS, 1977, 99, 6129 h 2 h 2 dppe (125 ) diphenylphosphinoethane bis(diphenyl)phosphinoethane chelating ligand 86.9 h 2 h 2 Ni l l 95.5 typical -- angle for a 5-membered chelate ring Nil 2(dppe) van Koten, et al Acta rys., 1987, 43, 1878 e 2 dmpe (107 ) dimethylphosphinoethane bis(dimethyl)phosphinoethane chelating ligand electron-rich, strong donor e 2 h 2 h 2 dppp (127 ) diphenylphosphinopropane bis(diphenyl)phosphinopropane chelating ligand forms 6-membered rings typical -- angle for a 6-membered chelate ring
33 Some Structural Information hosphines have only been characterized as simple 2 e- donating, terminal-only ligands. No true m-bridging monophosphines are known (although bridging phosphides, 2 -, are very common). hosphines generally tend to orient trans to one another in order to minimize steric interactions (especially true for bulky 3 ). helating bisphosphine ligands are used to enforce cisoidal coordination geometries when needed. Some typical first row - 3 average bond distances: Ti- 2.6 Å V- 2.5 Å r- 2.4 Å Ni- 2.1 Å - bonds are the strongest for alkylated phosphine ligands bonding to a neutral or monocationic middle to later transition metal center that is electron-deficient. High oxidation state early transition metals are too hard to have very effective bonding to most phosphines, although more and more early transition metal phosphine complexes are being characterized and found to be reasonably stable. Bond ength vs. Bond Strength steric effect r() ( ) 5 3 l 3 e 3 r- = 2.24Å r- = 2.37Å r- = 1.90Å r- = 1.85Å - = 1.14Å - = 1.15Å onclusions For most systems a shorter bond usually indicates a stronger bond when comparing similar atoms and bonds. For metal-ligand complexes there can be exceptions to this when the ligands in question have fairly different donor/acceptor properties.in r() 5 (l 3 ) the shorter bond distance relative to r() 5 (e 3 ) arises due to the combination of a contracted lower energy orbitals and moderate to significant π -backbonding. The DFT calculations indicate that the e 3 complex has stronger - bonding despite the significantly longer r- distance (2.37Å vs. 2.24Å) 33
34 onsider the following equilibrium: obr2()2 obr22 + The equilibrium constants for the reaction with various ligands are given in the table below. Explain the trends in Kd. Hydride igands H - H anionic 2e donor { H Hydride nomenclature comes from the N behavior: bridging mode H 1e to each -H ~ -5 to -25 ppm for d 1 d 9 metals!! Ho() 4 upfield shift indicates hydridic chemical nature 1H N = ppm BUT: Ho() 4 H + + [o()4] - strong acid in H 2, eh similar to Hl!! d 0 p* 2 ZrH 2 = ppm d 10 [Hu{(p-tolyl) 3 }] 6 = ppm I Spectra: -H cm -1 } can be very weak or absent 2 (m-h) cm -1 } broader (weak or absent) 34
35 roblem: For each of the following pairs of metal hydride complexes, circle the one that should have the lowest pka value. Briefly and clearly discuss your reasoning for each case. 35
36 36
37 3. s bound carbon ligands: alkyls and aryls Alkyls and Aryls H 3 Anionic 2 e- donors Alkyls are typically very strong mono-anionic s- donors, second only to hydrides. They have virtually no p-acceptor ability. Increasing the carbon substitution (replacing hydrogens with hydrocarbon groups such as methyl, ethyl, isopropyl) usually increases the donor strength, but steric factors can come into play and weaken the metal-alkyl bond (e.g., t-butyl groups are often too sterically hindered to bind well). Alkyls and Aryls eplacing the hydrogens with fluorine atoms (very electron withdrawing) dramatically reduces the donor ability of the alkyl (aryl). For example, F 3 - and 6 F 5 - are not very strong donors. etal alkyls are also typically quite to extremely reactive to molecular 2, water, and a variety of other ligands and reagents. As with hydrides, they play a very important and active role in catalysis. b-hydride Elimination Note that in order to have a b-hydride elimination you UST have a empty orbital on the metal cisoidal (next) to the alkyl ligand. You also must have b-hydrogens present on the alkyl. 37
38 b-hydride Elimination In order to prepare stable -alkyl complexes one generally needs to stay away from alkyls with b- hydrogens (or avoid metals with empty coordination sites). Some common ligands used to avoid b-hydride elimination reactions are shown below. e H 3 Si e e methyl neopentyl benzyl trimethylsilylmethyl e e e 38
39 roblems: a) Why doesn t a 16e- -phenyl do a b-hydride elimination? H H H H H b) Would a 16 e- -(t-butyl) complex be stable or not? Why? e e e Aryl igands Aryl ligands are relatively strong anionic two electron donors, like alkyls. Since they cannot easily b-hydride eliminate metal-aryls are relatively stable. Aryls do have the potential for both p-donation and p-backbonding through the filled aryl p-orbitals and empty p* antibonding orbitals. 39
40 4. s/p-bonded carbon ligands: carbenes, carbynes Fischer arbenes In 1964 Fischer s group prepared the first transition metal carbon double bond, which he called a carbene, after the very reactive neutral organic 2 fragment. () 5W W() 6 + H 3i H 3 (H3) 3 + () 5W H 3 H 3 () 5W H 3 Ernst. Fischer Technical University of unich, Germany Structure on () 5 r=(et)[n(i-r) 2 ] Et 2.13 Å (r- single bond distances are Å) r N(ir) Å (normal distance should be 1.41 Å, a 0.06 Å shortening) 1.33 Å (normal distance should be 1.45 Å, a 0.12 Å shortening) 40
41 r Et r Et N(ir) 2 N(ir) 2 Fischer arbenes are usually treated as neutral 2e- donor ligands that typically only makes a single bond to the metal (BUT, we often draw it as a double bond!!). etal Weak = Electron-deficient (electron withdrawing ligands like, N, 1 st row metal, electronegative metal) Strong = Electron-rich (electron donating ligands, 3 rd row metal) Good donating functional groups that Simple sigma donors can p-bond to the like H or H3 that can t arbene groups carbene (like N2, S, p-donate to the arbene, h); more than one carbon atom. donating group really weakens the - bond!! ost Fischer arbenes favor the weak bonding situation, where the metal has a d 6 configuration (counting the carbene as neutral ligand), ligands, and the carbene has p-donating groups. The d 6 configuration naturally favors the middle to late transition metals. The strong carbene bonding situation is actually considerably more reactive, much like the reactivity of a = double bond vs. a - single bond. roblem: ircle the correct ordering of the following group of Fisher carbenes from the strongest = 2 bond to weakest. Explain your reasoning. 41
42 Schrock Alkylidenes In 1973 ichard Schrock, while working at Duont central research, prepared the first early transition metal complex with a = double bond: (t-butyl-h 2 ) 3 Tal 2 + 2i(H 2 -t-butyl) ichard Schrock IT Nobel rize in elimination Ta(H 2 -t-butyl) 5 (t-butyl-h 2 ) 3 Ta H unstable intermediate + neopentane e e e 138 Ta H 3 H Å The Ta=H 2 bond is distinctly shorter than the Ta-H 3 single bond! 2.03 Å Fischer arbenes Nucleophillic attacks at carbon atom of carbene (carbon is electron deficient) Electrophillic attacks on metal center (metal is more electron-rich, often d 6 18 e- system) arbene is stabilized by heteroatom groups that can p-bond to it. ikes N 2, S,, or h groups. ater transition metals favored, especially with d6 counts (carbene as neutral 2e- donor ligand) Schrock Alkylidenes Electrophillic attacks at carbon atom of alkylidene (carbon is electron-rich) Nucleophillic attacks on metal center (metal is electron-deficient, usually d 2 or d 0 16 or 14 e- count) Alkylidene is destabilized by heteroatom groups that can p-bond to it. Strongly prefers H or simple alkyl groups. Early transition metals favored, especially with d0 centers (alkylidene as dianionic 4e- donor) 42
43 The bonding description commonly used to describe Schrock Alkylidenes is to treat the alkylidene as a dianionic 4e- donor ligand, which is what the electron counting and valence rules from the first chapter would indicate. So How Should I Electron ount?? The various methods of electron-counting carbenes and alkylidenes are: 1) both as neutral 2 e- donor ligands (but still draw a = double bond) 2) both as dianionic 4 e- donor ligands 3) Fischer carbenes as neutral 2 e- donor ligands. Typically group 6 or higher metals with a d 6 or d 8 electron count (sometimes d 4 ). 4) Schrock alkylidenes as dianionic 4 e- donor ligands. Typically group 4 or 5 metals with d 0 electron counts. Also later transition metals in high oxidation states (d 0, d 2, or d 4 ). f course, in order to do method 3 or 4, you have to realize whether you have a Fischer or Schrock system. As far as the overall electron-count is concerned, it DESN T matter which electron-counting method you use, since both give you the same overall electron-count!! It can be important to tell them apart since Schrock alkylidenes almost always have stronger (but often still very reactive) = bonds compared to Fischer carbenes. So on a question asking you to order a series of carbene and/or alkylidene complexes, it is generally important to figure out which is which. roblem: Identify the following complexes as a Fischer carbene or Schrock alkylidene. 43
44 roblem: rder the following = complexes from the one with the highest = 2 rotational barrier to the lowest. What factors affect the = rotational barrier? Identify each complex as either a Fisher carbene or a Schrock alkylidene. a) H H 3 H 3 H 3 s H 3 e b) h e 3 e 3 Fe e 3 Br Br e c) e 2 N Ne 2 u l l d) h e (e) 3 Fe (e) 3 l l arbynes/alkylidynes E.. Fischer accidentally prepared the first - triple bonded compound in 1973: e + BX 3 = r, o, W X = l, Br, I = e, Et, h planned rxn didn't work X X Thus, one can simply treat carbynes and alkylidynes as trianionic (-3) 6e- donating ligands. They are very strong donors as might be expected from the relatively low electronegativity of carbon and the -3 formal charge. h n = 1938 cm -1 l W e 3 e 3 l 1.78Å H h W h l W e 3 e 3 y e 3 H 2 e 3 H 2.25Å 1.94Å n = 1870 cm -1 44
45 e e 2.69Å e e 1.94Å Schrock, J, 1996 roblem: Which of the following ligands will coordinate the strongest to the empty coordination site on the metal complexes shown below., e 3, (e) 3, H 3-, F -, F 3 - a) [n() 5 ] + b) ebr(e 3 ) 4 c) [Ni(H 3 )() 2 ] + 5. π-bonded carbon ligands: alkene, alkynes, allyl, cyclobutadiene, arenes, cyclopentadienyl Alkenes/Alkynes Alkenes are typically relatively weakly coordinating ligands. They are also extremely important substrates for catalytic reactions. The strongest alkene-metal bonds occur with third row metals (as with almost all ligands) and when one can get more p-backbonding to occur. The amount of p-backbonding depends strongly on how electron-rich the metal center is and whether or not there are electron-withdrawing groups on the alkene to make it a better acceptor ligand. 45
46 Alkenes/Alkynes Dewar-hatt- Duncanson bonding model (1953) s-donation via the filled alkene p-system p-back donation via the empty alkene p -system 46
47 H H H H l t l l t(2+) = = 1.37Å Zeiss's Salt H H H H t 3 3 t(0) = = 1.43Å N N N N t 3 3 t(+2) -- = 1.49Å metallocyclopropane If the metal is electron-rich enough and/or if there are electron-withdrawing groups on the alkene, one can actually get a formal oxidation of the metal via the transfer of 2e- to the alkene to form a dianionic metallocyclopropane ligand that is now coordinated via two anionic alkyl s-bonds (thus the assignment of t(+2)). F = = 1.40Å F h- = 2.02Å h H H F F H H = = 1.35Å h- = 2.16Å The electronwithdrawing fluorine groups on the F 2 =F 2 alkene makes it a better p-acceptor ligand. This weakens the = bond, but strengthens the alkene-metal bond. 1.45Å 1.46Å 1.46Å 1.40Å 1.36Å Fe Zr 1.45Å Zr Zr is in a very low oxidation state (+2, but really wants to be +4) and is, therefore, extremely electron-rich. So electron-rich that it transfers two electrons to the butadiene via the p-backdonation and generates a metallo cyclopentene resonance structure 47
48 Electronic Effects Ethylene omplex n= (cm -1 ) Free Ethylene 1623 [Ag(H 2 =H 2 ) 2 ] Fe() 4 (H 2 =H 2 ) 1551 [e() 4 (H 2 =H 2 ) 2 ] [pfe() 2 (H 2 =H 2 )] d 2 l 4 (H 2 =H 2 ) [tl 3 (H 2 =H 2 )] pn() 2 (H 2 =H 2 ) 1508 t 2 l 4 (H 2 =H 2 ) ph(h 2 =H 2 ) The thermodynamic stability of metal-alkene complexes is strongly affected by the nature of the alkene (and metal): 1) Electron-withdrawing groups on the alkene generally increase the strength of the metalalkene bonding, while electron-donating groups generally decrease the stability. Exception (backdonation) 2) In cases where cis-trans isomerism is possible, the more stable complex is almost always formed by the cis-alkene (steric factors). 3) etal complexes of ring-strained cycloalkenes (e.g., cyclopropene) display higher than expected stability. The ring strain raises the energy of the cycloalkene ring system making it a better donor to the metal center (better orbital energy matching). 4) helating dienes show the expected stabilization from the chelate effect. The most common examples are norbornadiene and cyclooctadiene as shown. norbornadiene complex cyclooctadiene complex 5) Third-row metals form the strongest bonds and most stable complexes (as with most ligands). 48
49 yclobutadiene A triumph of the early days of organometallic chemistry was the successful synthesis of (h 4-4 H 4 ) 2 Ni 2 (m-l) 2 l 2, a stable metalcoordinated cyclobutadiene molecule, by riegee in This was actually predicted theoretically by onguet-higgins and rgel in 1956 using an early form of molecular orbital theory. e e e e l e l e + Ni() l 4 Ni Ni l l e e e l e e e A simpler route was discovered shortly after involving the cyclodimerization of diphenyl acetylene by Fe()5: 2 h h + Fe() 5 h Fe h h h Fe Fe s p Fe metal d orbitals Fe Fe Fe p non-bonding cyclobutadiene p, non-bonding, and p* orbitals Fe s Fe Fe Alkynes Alkynes are essentially like alkenes, only with another perpendicular pair of p-electrons. Thus they can act as neutral 2 or 4 e- donors, depending on the needs of the metal center. They are also much better bridging ligands because of this second set of p-electrons. Note how the bridging alkyne is drawn. This indicates a perpendicular bridging mode and that both carbons are interacting equally with both metals (the alkyne is donating 2e- to each metal). It dos NT indicate that each carbon has 6 bonds to it!! 49
50 When alkynes bridge, they almost always do so perpendicular to the - axis, the parallel bridging mode is known, but is quite rare: onsider p 2 h 2 [μ-(f 3 F 3 )]()(N). The h-h bond distance is 2.67 Å strongly indicating the presence of a covalent bond between the two rhodium atoms. (a) show the electron-counting for this complex including h oxidation state, ligand charges, # of e- donated, etc. nly one h center needs to be counted since both the and N ligands are neutral 2e- donors making the complex electronically symmetrical from an electron counting viewpoint. The electron-withdrawing groups on the alkyne allow it to oxidize each h center by 1e- to put each into the +2 oxidation state (d 7 ) and convert the alkyne into a dianionic bridging alkene ligand. This is analogous to the alkene example on the first page of the alkene chapter where the electron withdrawing cyano groups allow it to formally oxidize the t center and make a σ-coodinated metallocyclopropane complex. (b) Why does the alkyne ligand orient parallel to the h- h bond? From an organic hybridization and bonding viewpoint how should the alkyne be considered? Draw a simple orbital picture showing how the filled alkyne orbitals are overlapping with the empty h orbitals (use the diagram below as a starting point, ignore all other ligands). The 2e- reduction of the alkyne changes the carbon hybridization from sp to sp 2 (double bond like). Each carbon center now has a sp 2 hybrid orbital in the plane of the double bond with a lone-pair to bond to each h center. By using these stronger σ-donating orbitals the alkyne ligand now must orient parallel to the h- h bond axis. emember that ligands with π-systems and σ-lone pairs generally prefer bonding to the metal via the σ-lone pairs. 50
51 Broblem: Aside from, what other ligands mentioned in the lectures can act like π-backbonding (or π-acceptor) ligands and would have easily monitored I stretching frequencies (in the cm 1 region) that might prove useful as sensors for measuring the amount of electron density (or lack there of) on a transition metal center? [Hint: there are 3 or 4 reasonable choices] Discuss which of these would be the best choice for this and why. We are looking for ligands that have X=Y or X Y (X, Y =, N, ) with double or triple bonding between the two atoms. nly these will have characteristic I stretching frequencies in the range indicated. The best, of course, is N +, followed by N- (isocyanide), - - (alkynes), and 2 = 2 (alkenes). Nitriles (N - ) are another possibility, but it was mentioned in the notes and lecture that these are not particularly good π-acceptors. Anionic ligands like N, -, and H= 2 are not good π-acceptors due to their anionic charges. Arenes Arenes (benzene being the simplest member of this family) typically coordinate in an h6 fashion and as such are neutral 6 e- donors, although they can adopt lower coordination modes (h 4 and h 2 ). h 6 h 4 51
52 p-backbonding p-backdonation plays a relatively important role in arene bonding and chemistry. Arenes tend to favor metals in low oxidation states and often generate surprisingly stable complexes. r( 6 H 6 ) 2, for example, is kinetically inert to most substitution reactions, no doubt due to its 18 e- configuration, but also due to the mix of p- bonding and backbonding. emember that and N + are far, far stronger p-backbonding ligands. A dramatic example of the power of the 18e- electronic configuration is seen for [u( 6 e 6 ) 2 ] 2+. This can be reduced to neutral u( 6 e 6 ) 2, but electroncounting with two h 6-6 e 6 ligands gives you a 20e- complex. e e e e e u e e e e e e h 6 h 4 yclopentadienyl ligands p s H 3 H 3 6estrong donor H 3 H 3 6e- stronger donor bulky ligand H 3 p p* H h 5 h 3 h 1 52
53 Structural Features - p p - Fe [Fe] p p u s o [o] Ni The changes in the neutral Fe, o, Ni metallocenes are a direct result of going from 18e- (Fe) to 19e- (o) to 20e- (Ni) counts. The extra electrons for the o and Ni complexes are going into - p antibonding orbitals, which are delocalized and progressively weaken the -p bonding, leading to the increase in bond distances. This in spite of the fact that the metal s covalent radius is decreasing as one goes from Fe to Ni. omparison of p - vs. Arene igands Benzene-etal omplex p yclopentadienyl-etal omplex p p metal d orbitals p metal d orbitals p p p 6- etal-etal Bonding ovalent: Electron precise bonds. - bond counts as one e- from each metal center. ost common type of - bonding. Dative: Where one metal uses a filled d orbital lone pair to coordinate to an empty orbital on a second, more unsaturated metal. ost dative bonding situations can also be electron-counted as covalent bonds. Symmetry: Weak metal-metal interactions caused by molecular orbital symmetry interactions of filled & empty - bonding and/or antibonding orbitals. Typically seen for d8 metals. Not at all common. 53
54 d z 2 s d yz d xz p d xy the dx2 - y2orbitals (not shown) are used for - bonding d z 2 d yz d xy d xy d yz d z 2 d xz the dx2- y2, s and px,y orbitals are not shown since they are used for -ligand bonding s p p s - antibonding orbitals d xz - bonding orbitals Electron ount esulting - Bond d 1 - d 1 Single bond d 2 - d 2 Double bond d 3 - d 3 Triple bond d 4 - d 4 Quadruple bond optimum d 5 - d 5 Triple bond d 6 - d 6 Double bond (- bonding usually dominates) d 7 - d 7 Single bond d 8 - d 8 No bond (symmetry interaction) 54
55 Some ovalent ultiple Bonded Examples: Double Bonds t-bu t-bu l l l l Ta Ta l l l s s l Ta=Ta = 2.68 Å Triple Bonds hisholm d 3 -d 3 Triple Bonds hh2 H2h hh 2 o o H 2h hh2 H2h o-o = 2.17 Å s=s = 2.30 Å d 5 -d 5 Triple Bond r r r-r = 2.27 Å Quadruple Bonds (otton) d 4 -d 4 electronic configurations often lead to the formation of quadruple - bonds. rof. F. Albert otton at Texas A& was famous for his discovery and extensive studies of - quadruple bonds (and other - bonded systems). H 3 H 3 H 3 e H 3 2- H 3 e H 3 H 3 H 3 e-e = 2.18 Å e r r e 4 F. Albert otton Texas A& University r-r = 1.85 Å Dative - Bonds (unsymmetrical - bonded complexes) planar coordination like Ni(+2) Ni- = 2.16 Å Ni- = 1.70 Å t-bu t-bu Ni Ni t-bu t-bu Ni-Ni = 2.41 Å Ni- = 2.24 Å Ni- = 1.78 Å tetrahedral coordination like Ni(0) ovalent - Bonding eft Ni ight Ni Ni(+1) d9 Ni(+1) d9 [m-2]- 2e- [m-2]- 2em-2 2e- m-2 2e- 2e- 2 4e- - 1e- - 1e- eft Ni Ni(+2) d8 2[m-2]- 4e- 2e- Ni Ni(0) 2e- Dative ight Ni Ni(0) d10 2m-2 4e- 2 4e- Total 16e- Total 18e- Total 16e- Total 18e- 55
56 Weak - Interactions by Symmetry Based on the diagram at the beginning of this section, d 8 -d 8 systems shouldn t have any - bonding due to the filling of all the - antibonding orbitals, which cancels out the - bonding orbitals. But Harry Gray and others noted that more than a few bior polymetallic d 8 complexes do show the presence of weak - bonding interactions, both in solution and the solid-state. N N N 3 N N Ir Ir Ir N N l N N N p z d z 2 s s s s Harry Gray altech pz d z 2 56
Course 201N 1 st Semester Inorganic Chemistry Instructor: Jitendra K. Bera
 andout-8 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. Organometallic hemistry yclopentadienyl, Alkyl and Alkene yclopentadienyl p The cyclopentadienyl ligand
andout-8 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. Organometallic hemistry yclopentadienyl, Alkyl and Alkene yclopentadienyl p The cyclopentadienyl ligand
Oxidative Addition/Reductive Elimination 1. Oxidative Addition
 Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Reaction Mechanisms - Ligand Substitutions. ML n-x P x + xl
 Reaction chanisms - igand Substitutions igand Substitutions 1 A substitution reaction is one in which an existing ligand on a metal center is replaced by another ligand. Exactly how this occurs depends
Reaction chanisms - igand Substitutions igand Substitutions 1 A substitution reaction is one in which an existing ligand on a metal center is replaced by another ligand. Exactly how this occurs depends
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N2 Kashiwa ampus, October 16, 2009 Properties I. Nature of II. Nature of L II.a. Ligands with -atom attached to II.b. Ligands
Organometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N2 Kashiwa ampus, October 16, 2009 Properties I. Nature of II. Nature of L II.a. Ligands with -atom attached to II.b. Ligands
O CH 3. Mn CH 3 OC C. 16eelimination
 igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
Course 201N 1 st Semester Inorganic Chemistry Instructor: Jitendra K. Bera
 andout-9 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. rganometallic hemistry xidative Addition, Reductive Elimination, Migratory Insertion, Elimination
andout-9 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. rganometallic hemistry xidative Addition, Reductive Elimination, Migratory Insertion, Elimination
Introduction to Organometallic Chemistry. Stability of organometallic reagents
 Introduction to rganometallic hemistry. rganometallic chemistry is the chemistry of compounds that contain M- bonds, where M is either a main group metal or a transition metal.. ne common feature of all
Introduction to rganometallic hemistry. rganometallic chemistry is the chemistry of compounds that contain M- bonds, where M is either a main group metal or a transition metal.. ne common feature of all
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
Chem 634. Introduction to Transition Metal Catalysis. Reading: Heg Ch 1 2 CS-B 7.1, , 11.3 Grossman Ch 6
 Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Metal Hydrides, Alkyls, Aryls, and their Reactions
 Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
Organometallic Chemistry and Homogeneous Catalysis
 rganometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N8 Kashiwa ampus, December 11, 2009 Types of reactions in the coordination sphere of T 3. Reductive elimination X-L n -Y L n +
rganometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N8 Kashiwa ampus, December 11, 2009 Types of reactions in the coordination sphere of T 3. Reductive elimination X-L n -Y L n +
PART 2. -BONDED ORGANOMETALLICS
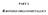 PART 2. -BODED ORGAOETALLIS V() 6 r() 6 o() 6 W() 6 Fe() 5 Ru() 5 Os() 5 i() 4 Figure 1. Binary, ononuclear etal arbonyls n n O Fe O O Fe O o O o n 2 () 10 Fe 2 () 9 o 2 () 8 Fe Fe Fe Ir() 3 Os Os Os o
PART 2. -BODED ORGAOETALLIS V() 6 r() 6 o() 6 W() 6 Fe() 5 Ru() 5 Os() 5 i() 4 Figure 1. Binary, ononuclear etal arbonyls n n O Fe O O Fe O o O o n 2 () 10 Fe 2 () 9 o 2 () 8 Fe Fe Fe Ir() 3 Os Os Os o
Paper 3: INORGANIC CHEMISTRY I (Stereochemistry, Metal-Ligand Equilibria and Reaction Mechanism of Transition Metal Complexes) Module 3: dπ-pπ bonding
 Subject Paper No and Title Module No and Title Module Tag 3, INORGANIC CHEMISTRY I (Stereochemistry, Metal- Ligand Equilibria and Reaction Mechanism of Transition Metal Complexes) 3, dπ-pπ bonding CHE_P3_M3
Subject Paper No and Title Module No and Title Module Tag 3, INORGANIC CHEMISTRY I (Stereochemistry, Metal- Ligand Equilibria and Reaction Mechanism of Transition Metal Complexes) 3, dπ-pπ bonding CHE_P3_M3
σ Bonded ligands: Transition Metal Alkyls and Hydrides
 σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Problem 1 (4 points) D2h. C2v. Part A.
 Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Insertion Reactions. 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (1,1) atom
 Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Chapter 8. Acidity, Basicity and pk a
 Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Acids and Bases: Molecular Structure and Acidity
 Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Introduction to Organic Chemistry
 Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
π bonded ligands alkene complexes alkyne complexes allyl complexes diene complexes cyclopentadienyl complexes arene complexes metallacycles
 π bonded ligands alkene complexes alkyne complexes allyl complexes diene complexes cyclopentadienyl complexes arene complexes metallacycles M Transition metal alkene complexes The report in 1825 by William
π bonded ligands alkene complexes alkyne complexes allyl complexes diene complexes cyclopentadienyl complexes arene complexes metallacycles M Transition metal alkene complexes The report in 1825 by William
Review questions CHAPTER 5. Practice exercises 5.1 F F 5.3
 CHAPTER 5 Practice exercises 5.1 S 5.3 5.5 Ethane is symmetrical, so does not have a dipole moment. However, ethanol has a polar H group at one end and so has a dipole moment. 5.7 xygen has the valence
CHAPTER 5 Practice exercises 5.1 S 5.3 5.5 Ethane is symmetrical, so does not have a dipole moment. However, ethanol has a polar H group at one end and so has a dipole moment. 5.7 xygen has the valence
Chapter 20: Aldehydes and Ketones
 hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
Chapter 2 Acids and Bases. Arrhenius Acid and Base Theory. Brønsted-Lowry Acid and Base Theory
 hapter 2 Acids and Bases A significant amount of chemistry can be described using different theories of acids and bases. We ll consider three different acid-base theories (listed in order of increasing
hapter 2 Acids and Bases A significant amount of chemistry can be described using different theories of acids and bases. We ll consider three different acid-base theories (listed in order of increasing
Chapter 6 Molecular Structure
 hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 9: Chemical Bonding I: Lewis Theory. Lewis Theory: An Overview
 Chapter 9: Chemical Bonding I: Lewis Theory Dr. Chris Kozak Memorial University of ewfoundland, Canada Lewis Theory: An verview Valence e - play a fundamental role in chemical bonding. e - transfer leads
Chapter 9: Chemical Bonding I: Lewis Theory Dr. Chris Kozak Memorial University of ewfoundland, Canada Lewis Theory: An verview Valence e - play a fundamental role in chemical bonding. e - transfer leads
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Organometallics Study Meeting
 rganometallics Study eeting 04/21/2011.itsunuma 1. rystal field theory(ft) and ligand field theory(lft) FT: interaction between positively charged metal cation and negative charge on the non-bonding electrons
rganometallics Study eeting 04/21/2011.itsunuma 1. rystal field theory(ft) and ligand field theory(lft) FT: interaction between positively charged metal cation and negative charge on the non-bonding electrons
Reaction mechanisms offer us insights into how reactions work / how molecules react with one another.
 Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Survey of Elements. 1s - very small atom All noble gases have very high first I.E. s which is in line with their chemical inertness
 Survey of Elements 354 Hydrogen 1s 1 Naturally, loss of one e - dominates 1s Helium 1s 2 - closed shell 1s - very small atom All noble gases have very high first I.E. s which is in line with their chemical
Survey of Elements 354 Hydrogen 1s 1 Naturally, loss of one e - dominates 1s Helium 1s 2 - closed shell 1s - very small atom All noble gases have very high first I.E. s which is in line with their chemical
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
Introduction to Organometallic Compounds. Metal. R MgX. Wilkinson s catalyst is used for hydrogenation of alkene and alkyne. Wilkinson's catalyst
 Introduction to rganometallic mpounds 1 Chapter 1 Introduction to rganometallic mpounds Introduction : Edward Frankland was father of organometallic chemistry for a complex to be organometallic compound,
Introduction to rganometallic mpounds 1 Chapter 1 Introduction to rganometallic mpounds Introduction : Edward Frankland was father of organometallic chemistry for a complex to be organometallic compound,
Ch.2 Polar Bonds and Their Consequences. 2.1 Polar Covalent Bonds and Electronegativity. polar covalent bonds: electron distribution is unsymmetrical
 2.1 Polar ovalent Bonds and Electronegativity polar covalent bonds: electron distribution is unsymmetrical Ionic haracter δ+ δ- + - X Y X Y X Y symmetrical covalent bond polar covalent bond ionic bond
2.1 Polar ovalent Bonds and Electronegativity polar covalent bonds: electron distribution is unsymmetrical Ionic haracter δ+ δ- + - X Y X Y X Y symmetrical covalent bond polar covalent bond ionic bond
5 Polyatomic molecules
 s manual for Burrows et.al. Chemistry 3 Third edition 5 Polyatomic molecules Answers to worked examples WE 5.1 Formal charges in N 2 (on p. 221 in Chemistry 3 ) Use formal charges to decide whether oxygen
s manual for Burrows et.al. Chemistry 3 Third edition 5 Polyatomic molecules Answers to worked examples WE 5.1 Formal charges in N 2 (on p. 221 in Chemistry 3 ) Use formal charges to decide whether oxygen
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
: Bond Order = 1.5 CHAPTER 5. Practice Questions
 CAPTER 5 Practice Questions 5.1 5.3 S 5.5 Ethane is symmetrical, so does not have a dipole moment. owever, ethanol has a polar group at one end and so has a dipole moment. 5.7 xygen has the valence electron
CAPTER 5 Practice Questions 5.1 5.3 S 5.5 Ethane is symmetrical, so does not have a dipole moment. owever, ethanol has a polar group at one end and so has a dipole moment. 5.7 xygen has the valence electron
Organometallics Study Meeting Part 4. Reactions of Organometallic Complexes
 rganometallics Study eeting art 4. eactions of rganometallic Complexes 1. asics 2011/4/28 oshino(d1).10 1-1. igand Exchange Dissosiative echanism ' n n-1 ' n-1 Fe(C) 5 (18e):hoto-promoteddissociationofigand
rganometallics Study eeting art 4. eactions of rganometallic Complexes 1. asics 2011/4/28 oshino(d1).10 1-1. igand Exchange Dissosiative echanism ' n n-1 ' n-1 Fe(C) 5 (18e):hoto-promoteddissociationofigand
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
CONTENTS PART I STRUCTURES OF THE TRANSITION-METAL COMPLEXES
 CONTENTS Introduction... 1 1. Organization of the text... 1 2. Frontiers of organometallic chemistry... 2 3. Situation of the book with respect to teaching... 2 4. Reference books and other selected references...
CONTENTS Introduction... 1 1. Organization of the text... 1 2. Frontiers of organometallic chemistry... 2 3. Situation of the book with respect to teaching... 2 4. Reference books and other selected references...
5.03 In-Class Exam 3
 5.03 In-Class Exam 3 Christopher C. Cummins April 9, 2010 Instructions Clearly write your name at the top of this front page, but otherwise do not write on this front page as it will be used for scoring.
5.03 In-Class Exam 3 Christopher C. Cummins April 9, 2010 Instructions Clearly write your name at the top of this front page, but otherwise do not write on this front page as it will be used for scoring.
Orbital Shapes Carbon: Electron configuration Carbon: Full. Short form. Orbital energy diagram. Orbital energy levels diagram
 rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
1. Lewis Structures. (Smith Chap 1 sections 1 7)
 Grossman, E 230 1. Lewis Structures. (Smith hap 1 sections 1 7) 1.1 Ways of drawing structures Even before we begin taking about Lewis structures, I would like to discuss one of the ways in which organic
Grossman, E 230 1. Lewis Structures. (Smith hap 1 sections 1 7) 1.1 Ways of drawing structures Even before we begin taking about Lewis structures, I would like to discuss one of the ways in which organic
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry. Zachary Chin, Alex Li, Alex Liu
 A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
Chapter 2 Polar Covalent Bonds; Acids and Bases SAMPLE. Chapter Outline
 Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Practice Hour Examination # 1-1
 CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
Chapter 8 Basic Concepts of Chemical Bonding
 hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
EPIC LIGAND SURVEY: CARBON MONOXIDE
 EPIC LIGAND SURVEY: CARBON MONOXIDE As a young, growing field, organometallic chemistry may be taught in many ways. Some professors (e.g., Shaugnessy) spend a significant chunk of time discussing ligands,
EPIC LIGAND SURVEY: CARBON MONOXIDE As a young, growing field, organometallic chemistry may be taught in many ways. Some professors (e.g., Shaugnessy) spend a significant chunk of time discussing ligands,
Loudon Chapter 18 Review: Vinyl/Aryl Reactivity Jacquie Richardson, CU Boulder Last updated 2/21/2016
 Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Recommended Reading: 20, , 13 (3rd edition); 19, , 13 (4th edition)
 Recommended Reading: 20, 21.2-21.4, 13 (3rd edition); 19, 20.2-20.4, 13 (4th edition) h 102 Problem Set 5 Due: Thursday, May 17 Before lass Problem 1 (2 points) Part A Hard and soft acids and bases (HSAB)
Recommended Reading: 20, 21.2-21.4, 13 (3rd edition); 19, 20.2-20.4, 13 (4th edition) h 102 Problem Set 5 Due: Thursday, May 17 Before lass Problem 1 (2 points) Part A Hard and soft acids and bases (HSAB)
Chapter 2 Polar Covalent Bonds; Acids and Bases. Chapter Outline
 rganic Chemistry 9th Edition McMurry SLUTINS MANUAL Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurrysolutions-manual/ rganic Chemistry 9th Edition McMurry
rganic Chemistry 9th Edition McMurry SLUTINS MANUAL Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurrysolutions-manual/ rganic Chemistry 9th Edition McMurry
CHAPTER 2. Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules
 CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
Classes of Organic Compounds
 Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Covalent Bonding. Chapter 8. Diatomic elements. Covalent bonding. Molecular compounds. 1 and 7
 hapter 8 ovalent bonding ovalent Bonding A metal and a nonmetal transfer An ionic bond Two metals just mix and don t react An alloy What do two nonmetals do? Neither one will give away an electron So they
hapter 8 ovalent bonding ovalent Bonding A metal and a nonmetal transfer An ionic bond Two metals just mix and don t react An alloy What do two nonmetals do? Neither one will give away an electron So they
Name Date Class STUDY GUIDE FOR CONTENT MASTERY. covalent bond molecule sigma bond exothermic pi bond
 Covalent Bonding Section 9.1 The Covalent Bond In your textbook, read about the nature of covalent bonds. Use each of the terms below just once to complete the passage. covalent bond molecule sigma bond
Covalent Bonding Section 9.1 The Covalent Bond In your textbook, read about the nature of covalent bonds. Use each of the terms below just once to complete the passage. covalent bond molecule sigma bond
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Helpful Hints Lewis Structures Octet Rule For Lewis structures of covalent compounds least electronegative
 Helpful Hints Lewis Structures Octet Rule Lewis structures are a basic representation of how atoms are arranged in compounds based on bond formation by the valence electrons. A Lewis dot symbol of an atom
Helpful Hints Lewis Structures Octet Rule Lewis structures are a basic representation of how atoms are arranged in compounds based on bond formation by the valence electrons. A Lewis dot symbol of an atom
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
1. methyl 2. methylene 3. methine 4. primary 5. secondary 6. tertiary 7. quarternary 8. isopropyl
 hem 201 Sample Midterm Beauchamp Exams are designed so that no one question will make or break you. The best strategy is to work steadily, starting with those problems you understand best. Make sure you
hem 201 Sample Midterm Beauchamp Exams are designed so that no one question will make or break you. The best strategy is to work steadily, starting with those problems you understand best. Make sure you
Hour Examination # 1
 CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
Carbenes and Olefin Metathesis
 arbenes and Olefin etathesis Peter H.. Budzelaar etal-carbon multiple bonds any transition metals form not only - single bonds but also = and (more rare) even bonds. omplexes containing an = bond are called
arbenes and Olefin etathesis Peter H.. Budzelaar etal-carbon multiple bonds any transition metals form not only - single bonds but also = and (more rare) even bonds. omplexes containing an = bond are called
Chapter 10 Practice Problems
 Chapter 10 Practice Problems Q 10.1 0-1 -1-1 S +2 +2 S S +2 0-1 -1-1 0 C in S - 6 6 1 2 1 2 C in S = 6 4 1 4 0 2 C S 6 0 1 8 2 2 Q 10.2 Correct Answer: B Two oxygen atoms will have a formal charge of 1
Chapter 10 Practice Problems Q 10.1 0-1 -1-1 S +2 +2 S S +2 0-1 -1-1 0 C in S - 6 6 1 2 1 2 C in S = 6 4 1 4 0 2 C S 6 0 1 8 2 2 Q 10.2 Correct Answer: B Two oxygen atoms will have a formal charge of 1
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
Carbon and Its Compounds
 Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
2. Acids and Bases (text )
 2009, Department of hemistry, The University of Western ntario 2.1 2. Acids and Bases (text 2.1 2.6) Acid-base reactions are one of the most important reaction types in organic chemistry and biology, e.g.:
2009, Department of hemistry, The University of Western ntario 2.1 2. Acids and Bases (text 2.1 2.6) Acid-base reactions are one of the most important reaction types in organic chemistry and biology, e.g.:
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Chapters 9&10 Structure and Bonding Theories
 Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Ch 2 Polar Covalent Bonds
 h 2 Polar ovalent Bonds Two primary bond types: ovalent (shared e -1 s) and Ionic (transferred e -1 s) Ionic bonds can have covalent character, such as with Na:l. An e -1 pair on l -1 can fill the 3s orbital
h 2 Polar ovalent Bonds Two primary bond types: ovalent (shared e -1 s) and Ionic (transferred e -1 s) Ionic bonds can have covalent character, such as with Na:l. An e -1 pair on l -1 can fill the 3s orbital
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
antidisestablishmenttarianism an-ti-dis-es-tab-lish-ment-ta-ri-an-ism
 What do you do when you encounter a very long, difficult word? 1 antidisestablishmenttarianism break it up into syllables: an-ti-dis-es-tab-lish-ment-ta-ri-an-ism meaning: antidisestablishmenttarianism
What do you do when you encounter a very long, difficult word? 1 antidisestablishmenttarianism break it up into syllables: an-ti-dis-es-tab-lish-ment-ta-ri-an-ism meaning: antidisestablishmenttarianism
CHEMISTRY Topic #1: Functional Groups and Drawing Organic Molecules Fall 2014 Dr. Susan Findlay
 EMISTRY 2500 Topic #1: Functional Groups and Drawing rganic Molecules Fall 2014 Dr. Susan Findlay Drawing rganic Molecules (Basics) Recall the steps for drawing Lewis structures in EM 1000: 1. Determine
EMISTRY 2500 Topic #1: Functional Groups and Drawing rganic Molecules Fall 2014 Dr. Susan Findlay Drawing rganic Molecules (Basics) Recall the steps for drawing Lewis structures in EM 1000: 1. Determine
The Organic Acids. Carboxylic Acids * *
 arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. cleophiles and Electrophiles V. Acids and Bases What a chemical
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. cleophiles and Electrophiles V. Acids and Bases What a chemical
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Exam Analysis: Organic Chemistry, Midterm 1
 Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Basic Organic Chemistry
 Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Module 10 : Reaction mechanism. Lecture 1 : Oxidative addition and Reductive elimination. Objectives. In this lecture you will learn the following
 Module 10 : Reaction mechanism Lecture 1 : Oxidative addition and Reductive elimination Objectives In this lecture you will learn the following The oxidative addition reactions. The reductive elimination
Module 10 : Reaction mechanism Lecture 1 : Oxidative addition and Reductive elimination Objectives In this lecture you will learn the following The oxidative addition reactions. The reductive elimination
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Chapter 8 Covalent Boding
 Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Learning Guide for Chapter 1 - Atoms and Molecules
 Learning Guide for hapter 1 - Atoms and Molecules I. Introduction to organic chemistry - p 1 II. Review of atomic structure - p 3 Elementary particles Periodic Table of Elements Electronegativity Atomic
Learning Guide for hapter 1 - Atoms and Molecules I. Introduction to organic chemistry - p 1 II. Review of atomic structure - p 3 Elementary particles Periodic Table of Elements Electronegativity Atomic
CHAPTER 8 BONDING: GENERAL CONCEPTS Ionic solids are held together by strong electrostatic forces that are omnidirectional.
 CAPTER 8 BDIG: GEERAL CCEPTS 1 CAPTER 8 BDIG: GEERAL CCEPTS Questions 15. a. This diagram represents a polar covalent bond as in. In a polar covalent bond, there is an electron rich region (indicated by
CAPTER 8 BDIG: GEERAL CCEPTS 1 CAPTER 8 BDIG: GEERAL CCEPTS Questions 15. a. This diagram represents a polar covalent bond as in. In a polar covalent bond, there is an electron rich region (indicated by
4 Molecules and Compounds
 4 Molecules and ompounds 4 Molecules and ompounds Atoms that have assembled into substances (new or old) are bonded (glued) together. The way the atoms are bonded together will create different properties
4 Molecules and ompounds 4 Molecules and ompounds Atoms that have assembled into substances (new or old) are bonded (glued) together. The way the atoms are bonded together will create different properties
Chem 232. Representation of Reaction Mechanisms. A Simple Guide to "Arrow Pushing"
 Chem 232 D. J. Wardrop wardropd@uic.edu Representation of Reaction Mechanisms A Simple Guide to "Arrow Pushing" 1. For a given reaction, draw out the structure of the reactants and reagents. Check that
Chem 232 D. J. Wardrop wardropd@uic.edu Representation of Reaction Mechanisms A Simple Guide to "Arrow Pushing" 1. For a given reaction, draw out the structure of the reactants and reagents. Check that
