Fission Reactors. Alternatives Inappropriate. Fission Reactors
|
|
|
- Silvester Gaines
- 5 years ago
- Views:
Transcription
1 Page 1 of 5 Fission Reactors The Polywell Reactor Nuclear Reactions Alternatives Inappropriate Hidden Costs of Carbon Web Site Home Page Fission Reactors There are about 438 Neutron Fission Power Reactors (NFPRs) producing commercial electricity in the world today. They use uranium fuel to produce heat, which boils water to make steam, which spins a turbine, which spins a generator to make the electricity. Neutron Fission Power Reactors (NFPRs) are more often called Nuclear Power Reactors, but this commonly accepted terminology is ambiguous because it does not properly distinguish between fusion and fission; and the difference is important. NFPRs (Neutron Fission Power Reactors) get their energy from uranium fission:
2 Page 2 of 5 When elements with very large mass numbers such as U236 split apart to make smaller more stable nuclei closer to 56 in mass number, we say that fission has occurred. In the reaction above left, a neutron combines with a U235 nucleus to produce an unstable U236 nucleus. The unstable U236 nucleus fissions to produce 3 neutrons, a Barium nucleus, and a Krypton nucleus. The multiple neutrons produced are very important in sustaining the chain reaction, which characterizes NFPRs. In the chain reaction above right, neutrons split uranium nuclei which release more neutrons which split more uranium nuclei which release more neutrons which split more uranium nuclei and so on. A neutron fission chain reaction is the primary distinguishing trait of NFPRs; the chain reaction sets NFPRs apart from other nuclear power devices such as the polywell. People who insist on saying, Nuclear Power Reactor, when they mean "Neutron Fission Power Reactor" are putting all nuclear reactors in the same category, which is a very big mistake. NFPRs have very serious problems, which are not shared by the polywell. It is extremely important that people who are worried about these problems, distinguish between devices that truly have those problems, and other devices that do not. At this time, the polywell is the only device which can promise to safely provide all the energy needs of our civilization. To confuse it with other nuclear devices, is to jeopardize the very future of humankind. Nomenclature Control Rods absorb neutrons. When the control rods are inserted into a Neutron Fission Power Reactor, the chain reaction stops. As the control rods are gradually removed, the chain reaction (and thus, the energy produced) increases. As the reaction rate increases, the temperature of the reactor increases. The control rods are usually made out of Cadmium, Hafnium, or Boron10 a neutron absorbing isotope of Boron. The Coolant is a liquid that circulates through the reactor, absorbs the heat, and carries it away to make the steam to drive the turbine. The coolant is usually water or heavy water. (Heavy water is made from deuterium and oxygen.) The neutrons produced during a chain reaction are moving too fast to split Uranium nucleii. It is necessary to slow the neutrons down enough for them to react. Nuclear Cross-Section in Millibarns (a measure of reaction probability) varies with the speed of neutrons, just as it varies with the speeds of the hydrogen and barium nucleii in the polywell and the tokamak. Moderators slow down the neutrons so the chain reaction can
3 Page 3 of 5 continue. In addition to acting as a coolant, water or heavy water can also act as a moderator. Graphite is also used as a moderator in some reactors. There are 264 Pressurized Water Reactors (PWR) in use today in the United States, France, and Japan. PWRs have two separate water circulation systems. The coolant water reaches a temperature of about 325C in the reactor core, and it is pressurized to 150 atmospheres. This very hot pressurized water passes through a boiler where it heats water from the turbine loop, and boils it to make steam for the turbine. Types of NFPRs There are 94 Boiling Water Reactors (BWR) in use today in the United States, Japan, and Sweden. In a BWR (right), the steam for the turbine is produced by boiling the water in the reactor. Since there is only one loop, a break in the steam line to the turbine could release reactor contaminants; so the turbine is often placed inside the reactor containment. Unfortunately, water is the moderator in both the PWR and BWR reactors. Because water does not moderate the neutrons to optimum speed (crosssection), it is necessary to enrich the uranium fuel used in these reactors. Natural Uranium, as it comes out of the ground, is only about 0.7% (seven tenths of one percent) U235. It is necessary to increase that percentage to about 3.5 to 5.0%, through enrichment, in order to sustain a chain reaction using water as a moderator. There are 43 CANDU reactors in use today in Canada. The CANDU uses heavy water as a coolant and moderator but, except as noted below, it is other-wise essentially the same as the PWR. There are 12 Реактор Ьольшой Мошности Канальный (RBMKs) in use today in Russia. Like the BWR, the RBMK has only one water circulation system, and the steam for the turbine is produced by boiling the water in the reactor. The RBMK, however, uses graphite as a moderator. Both the CANDU and the RBMK are able to use natural Uranium as a fuel, rather than enriched Uranium. Using heavy water or graphite as a moderator to better optimize the speeds (cross-sections) of the neutrons makes this possible. With only two notable exceptions, most of these reactors have functioned for many years without incident. Nevertheless, many people have serious concerns about them.
4 Page 4 of 5 Concerns 1. Meltdowns: NFPRs depend upon chain reactions to make neutrons. The chain reaction is controlled by the control rods, which can be gradually inserted into and removed from the reactor. If a malfunction causes the control rods to be removed and they cannot be reinserted, the chain reaction can go out of control and release too much energy, causing critical parts of the reactor to melt down or otherwise selrf-destruct. Also, in some reactors, failure of the reactor coolant system can cause a meltdown. Such events have already happened three times: in 2011 at the Fukushima Daiichi Plant in Japan; 1986 in Chernobyl, Ukraine; and 1979 at Three Mile Island, United States. In both Japan and the Ukraine, these meltdowns breached the reactor containment, releasing substantial amounts of radioactive substances into the biosphere. 2. Nuclear Weapons: Some NFPRs can be used to produce fuels such as plutonium for nuclear weapons. Plutonium can also be extracted from the nuclear waste of some reactors. There is concern that unscrupulous nations may take advantage of their NFPRs to produce nuclear weapons. Indeed, such threats have already been implied by North Korea and Iran. Also, there is the concern that as plutonium becomes more readily available, it will somehow be acquired by a terrorist group and used to make nuclear weapons. 3. Limited Fuel: NFPRs use uranium as their fuel. Uranium may be a limited resource. It has been estimated that the world supply of uranium will be depleted in about 50 years at the current rate of consumption and the present price of uranium. 4. Nuclear Waste: An operating NFPR produces some radioactive isotopes, which cannot conveniently be recycled. Many of these isotopes are hazardous because of their emissions, and some are toxic as well. At present, there is no safe, cost-effective way to dispose of them. Such dangerous nuclear waste has been accumulating for more than 30 years at various locations world-wide. So, while it is true that NFPRs do not produce greenhouse gases, the above concerns, when taken together with the following information, would seem to suggest the need for a safer source of energy such as the polywell device. For Future Cost Comparison How many 1,000,000,000 watt (1000 mw) Boiling Water NFPRs would be required to replace just half of the 12,900,000,000,000 watts (12.9 Terawatts), presently produced from carbon-based fuels every hour here on Earth, assuming that the Boiling Water NFPRs are operating at 90% (0.9) efficiency? And how much would it cost for that many Boiling Water NFPRs? 6,450,000,000,000 watts/(1,000,000,000 watts per NFPR x 0.9) = 7,167 NFPRs Each 1000 mw NFPR costs $4 billion to $8 billion installed. (Call it $6 billion.) $6,000,000,000/NFPR x 7,167 NFPRs = $43,002,000,000,000 Excuse me? More than $43 TRILLION dollars????
5 Page 5 of 5 It's a pretty scary number, but just for future comparison purposes, to eventually put this number into perspective, let's calculate the cost per Terawatt: $43 Trillion/6.45 Terawatts = $6.66 Trillion/Terawatt (remember this number!) Next
turbine (a) (i) Which part of the power station provides thermal (heat) energy from a chain reaction?
 Nuclear fission and radiation 1 The diagram shows parts of a nuclear power station. control rods boiler steam generator electricity out turbine condenser nuclear reactor (a) (i) Which part of the power
Nuclear fission and radiation 1 The diagram shows parts of a nuclear power station. control rods boiler steam generator electricity out turbine condenser nuclear reactor (a) (i) Which part of the power
WELCOME TO PERIOD 18: CONSEQUENCES OF NUCLEAR ENERGY
 WELCOME TO PERIOD 18: CONSEQUENCES OF NUCLEAR ENERGY Homework #17 is due today. Midterm 2: Weds, Mar 27, 7:45 8:55 pm (Same room as your midterm 1 exam.) Covers periods 10 19 and videos 3 & 4 Review: Tues,
WELCOME TO PERIOD 18: CONSEQUENCES OF NUCLEAR ENERGY Homework #17 is due today. Midterm 2: Weds, Mar 27, 7:45 8:55 pm (Same room as your midterm 1 exam.) Covers periods 10 19 and videos 3 & 4 Review: Tues,
Term 3 Week 2 Nuclear Fusion & Nuclear Fission
 Term 3 Week 2 Nuclear Fusion & Nuclear Fission Tuesday, November 04, 2014 Nuclear Fusion To understand nuclear fusion & fission Nuclear Fusion Why do stars shine? Stars release energy as a result of fusing
Term 3 Week 2 Nuclear Fusion & Nuclear Fission Tuesday, November 04, 2014 Nuclear Fusion To understand nuclear fusion & fission Nuclear Fusion Why do stars shine? Stars release energy as a result of fusing
Physics 30 Modern Physics Unit: Fission and Fusion
 Physics 30 Modern Physics Unit: Fission and Fusion Nuclear Energy For years and years scientists struggled to describe where energy came from. They could see the uses of energy and the results of energy
Physics 30 Modern Physics Unit: Fission and Fusion Nuclear Energy For years and years scientists struggled to describe where energy came from. They could see the uses of energy and the results of energy
Introducing nuclear fission The Fizzics Organization
 Nuclear Fission is the splitting of the nucleus of an atom into two or more parts by hitting it with a small particle, almost always a neutron (a proton would be repelled from the positive nucleus and
Nuclear Fission is the splitting of the nucleus of an atom into two or more parts by hitting it with a small particle, almost always a neutron (a proton would be repelled from the positive nucleus and
The discovery of nuclear reactions need not bring about the destruction of mankind any more than the discovery of matches - Albert Einstein
 The world has achieved brilliance without wisdom, power without conscience. Ours is a world of nuclear giants and ethical infants. - Omar Bradley (US general) The discovery of nuclear reactions need not
The world has achieved brilliance without wisdom, power without conscience. Ours is a world of nuclear giants and ethical infants. - Omar Bradley (US general) The discovery of nuclear reactions need not
Nuclear Energy Learning Outcomes
 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons. Describe the structure and operation of a nuclear reactor.
1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons. Describe the structure and operation of a nuclear reactor.
Nuclear Energy Learning Outcomes. Nuclear Fission. Chain Reaction
 by fastfission public domain by fastfission public domain 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons.
by fastfission public domain by fastfission public domain 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons.
2 Energy from the Nucleus
 CHAPTER 4 2 Energy from the Nucleus SECTION Atomic Energy BEFORE YOU READ After you read this section, you should be able to answer these questions: What is nuclear fission? What is nuclear fusion? What
CHAPTER 4 2 Energy from the Nucleus SECTION Atomic Energy BEFORE YOU READ After you read this section, you should be able to answer these questions: What is nuclear fission? What is nuclear fusion? What
AN OVERVIEW OF NUCLEAR ENERGY. Prof. Mushtaq Ahmad, MS, PhD, MIT, USA
 AN OVERVIEW OF NUCLEAR ENERGY Prof. Mushtaq Ahmad, MS, PhD, MIT, USA Outline of the Seminar 2 Motivation and Importance of Nuclear Energy Future Energy Planning in the Kingdom Current Status of Nuclear
AN OVERVIEW OF NUCLEAR ENERGY Prof. Mushtaq Ahmad, MS, PhD, MIT, USA Outline of the Seminar 2 Motivation and Importance of Nuclear Energy Future Energy Planning in the Kingdom Current Status of Nuclear
Carbon Dating. Principles of Radiometric Dating. 03 nuclear decay and the standard model June 05, 2013
 Principles of Radiometric Dating http://facstaff.gpc.edu/~pgore/geology/geo102/radio.htm Naturally occurring radioactive materials break down into other materials at known rates. This is known as radioactive
Principles of Radiometric Dating http://facstaff.gpc.edu/~pgore/geology/geo102/radio.htm Naturally occurring radioactive materials break down into other materials at known rates. This is known as radioactive
Announcements. Projected Energy Consumption. Fossil fuel issues. By the end of class today
 Announcements Projected Energy Consumption Ecological Footprint assignment starts this afternoon to be completed by 10 AM Thursday Today: Alternatives to fossil fuels? Nuclear power Energy efficiency Thursday:
Announcements Projected Energy Consumption Ecological Footprint assignment starts this afternoon to be completed by 10 AM Thursday Today: Alternatives to fossil fuels? Nuclear power Energy efficiency Thursday:
c) O-16 d) Pu An unstable nucleus emits. a) Atoms b) Electricity c) Plasma d) Radiation 3. Many of uranium are radioactive. a) Ions b) Isomers
 Physical Science Domain 1 Nuclear Decay Review 1. Which nucleus would be MOST likely to be radioactive? a) C-12 b) Ca-40 c) O-16 d) Pu-241 2. An unstable nucleus emits. a) Atoms b) Electricity 3. Many
Physical Science Domain 1 Nuclear Decay Review 1. Which nucleus would be MOST likely to be radioactive? a) C-12 b) Ca-40 c) O-16 d) Pu-241 2. An unstable nucleus emits. a) Atoms b) Electricity 3. Many
The Physics of Nuclear Reactors. Heather King Physics 420
 The Physics of Nuclear Reactors Heather King Physics 420 Nuclear Reactions A nuclear reaction is a reaction that involves atomic nuclei, or nuclear particles (protons, neutrons), producing products different
The Physics of Nuclear Reactors Heather King Physics 420 Nuclear Reactions A nuclear reaction is a reaction that involves atomic nuclei, or nuclear particles (protons, neutrons), producing products different
Nuclear Chemistry. Chapter 24
 Nuclear Chemistry Chapter 24 Radioactivity Radioisotopes are isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic configurations in a process called radioactive decay.
Nuclear Chemistry Chapter 24 Radioactivity Radioisotopes are isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic configurations in a process called radioactive decay.
The Electromagnetic Spectrum. 7.1 Atomic Theory and Radioactive Decay. Isotopes. 19K, 19K, 19K Representing Isotopes
 7.1 Atomic Theory and Radioactive Decay Natural background radiation exists all around us. Radioactivity is the release of high energy particles or waves When atoms lose high energy particles and waves,
7.1 Atomic Theory and Radioactive Decay Natural background radiation exists all around us. Radioactivity is the release of high energy particles or waves When atoms lose high energy particles and waves,
nuclear fission nucleus slightly mass
 Nuclear Fuel A nuclear fuel pellet contains about 4 grams of fuel It produces the same amount of energy as a ton of coal or 150 gallons of gasoline It s fairly cheap - $3 per pellet (compare to 150 gallons
Nuclear Fuel A nuclear fuel pellet contains about 4 grams of fuel It produces the same amount of energy as a ton of coal or 150 gallons of gasoline It s fairly cheap - $3 per pellet (compare to 150 gallons
Lecture 14, 8/9/2017. Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion
 Lecture 14, 8/9/2017 Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place
Lecture 14, 8/9/2017 Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place
Nuclear Energy ECEG-4405
 Nuclear Energy ECEG-4405 Today s Discussion Technical History and Developments Atom Nuclear Energy concepts and Terms Features Fission Critical Mass Uranium Fission Nuclear Fusion and Fission Fusion Fission
Nuclear Energy ECEG-4405 Today s Discussion Technical History and Developments Atom Nuclear Energy concepts and Terms Features Fission Critical Mass Uranium Fission Nuclear Fusion and Fission Fusion Fission
R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada
 NUCLEAR REACTOR CONFIGURATION R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Nuclear Reactors, Reactor Types, Reactor Arrangement, Technical Data Contents
NUCLEAR REACTOR CONFIGURATION R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Nuclear Reactors, Reactor Types, Reactor Arrangement, Technical Data Contents
L 36 Atomic and Nuclear Physics-4. Radioactivity. Nuclear reactions: E = mc 2. Hazards of radiation. Biological effects of nuclear radiation
 L 36 Atomic and Nuclear Physics- Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, half-life carbon dating Nuclear energy nuclear fission nuclear fusion nuclear
L 36 Atomic and Nuclear Physics- Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, half-life carbon dating Nuclear energy nuclear fission nuclear fusion nuclear
Energy. on this world and elsewhere. Visiting today: Prof. Paschke
 Energy on this world and elsewhere Visiting today: Prof. Paschke Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu,
Energy on this world and elsewhere Visiting today: Prof. Paschke Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu,
Nuclear power plants can generate large amounts of electricity.
 7.3 Nuclear Reactions Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of nuclei Fusion = the joining of nuclei Nuclear power plants can
7.3 Nuclear Reactions Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of nuclei Fusion = the joining of nuclei Nuclear power plants can
Nuclear fission and fusion are processes that involve extremely large amounts of energy.
 Nuclear Reactions & Energy Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of a large nucleus into two smaller nuclei, subatomic particles
Nuclear Reactions & Energy Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of a large nucleus into two smaller nuclei, subatomic particles
PHYS:1200 LECTURE 36 ATOMIC AND NUCLEAR PHYSICS (4)
 1 PHYS:1200 LECTURE 36 ATOMIC AND NUCLEAR PHYSICS (4) This last lecture of the course will focus on nuclear energy. There is an enormous reservoir of energy in the nucleus and it can be released either
1 PHYS:1200 LECTURE 36 ATOMIC AND NUCLEAR PHYSICS (4) This last lecture of the course will focus on nuclear energy. There is an enormous reservoir of energy in the nucleus and it can be released either
Radioactivity. L 38 Modern Physics [4] Hazards of radiation. Nuclear Reactions and E = mc 2 Einstein: a little mass goes a long way
![Radioactivity. L 38 Modern Physics [4] Hazards of radiation. Nuclear Reactions and E = mc 2 Einstein: a little mass goes a long way Radioactivity. L 38 Modern Physics [4] Hazards of radiation. Nuclear Reactions and E = mc 2 Einstein: a little mass goes a long way](/thumbs/80/81251381.jpg) L 38 Modern Physics [4] Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
L 38 Modern Physics [4] Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
nuclear chemical change CH4 + 2O2 CO2 + 2H2O carbon dating
 Nuclear Chemistry I. What is nuclear chemistry? a. Nuclear changes vs. chemical changes i. A nuclear change is a change in which the nucleons (things in the nucleus) change. For instance, if the number
Nuclear Chemistry I. What is nuclear chemistry? a. Nuclear changes vs. chemical changes i. A nuclear change is a change in which the nucleons (things in the nucleus) change. For instance, if the number
Science 10: Radioactivity! Comparing Fission and Fusion Notes (Ch 11)
 http://www.atomicarchive.com/movies/index.shtml Science 10: Radioactivity! Comparing Fission and Fusion Notes (Ch 11) Nuclear Reactions: an atom s nucleus changes by gaining or releasing particles or energy.
http://www.atomicarchive.com/movies/index.shtml Science 10: Radioactivity! Comparing Fission and Fusion Notes (Ch 11) Nuclear Reactions: an atom s nucleus changes by gaining or releasing particles or energy.
NUCLEAR ENERGY! DAY 1: (RADIATION, FISSION, FUSION)
 NUCLEAR ENERGY! DAY 1: (RADIATION, FISSION, FUSION) Nucleus Stability Stability of the nucleus depends on the nuclear forces that act between protons and neutrons Protons repel each other Protons attract
NUCLEAR ENERGY! DAY 1: (RADIATION, FISSION, FUSION) Nucleus Stability Stability of the nucleus depends on the nuclear forces that act between protons and neutrons Protons repel each other Protons attract
Chemistry 500: Chemistry in Modern Living. Topic 5: The Fires of Nuclear Fission. Atomic Structure, Nuclear Fission and Fusion, and Nuclear.
 Chemistry 500: Chemistry in Modern Living 1 Topic 5: The Fires of Nuclear Fission Atomic Structure, Nuclear Fission and Fusion, and Nuclear Weapons Chemistry in Context, 2 nd Edition: Chapter 8, Pages
Chemistry 500: Chemistry in Modern Living 1 Topic 5: The Fires of Nuclear Fission Atomic Structure, Nuclear Fission and Fusion, and Nuclear Weapons Chemistry in Context, 2 nd Edition: Chapter 8, Pages
Name: New Document 1. Class: Date: 54 minutes. Time: 54 marks. Marks: Comments: Page 1 of 22
 New Document Name: Class: Date: Time: 54 minutes Marks: 54 marks Comments: Page of 22 (a) Uranium has two natural isotopes, uranium-235 and uranium-238. Use the correct answer from the box to complete
New Document Name: Class: Date: Time: 54 minutes Marks: 54 marks Comments: Page of 22 (a) Uranium has two natural isotopes, uranium-235 and uranium-238. Use the correct answer from the box to complete
Students will distinguish the characteristics and components of radioactivity.
 Students will distinguish the characteristics and components of radioactivity. A. Differentiate among alpha, beta, and gamma radiation. B. Differentiate between fission and fusion. C. Explain the process
Students will distinguish the characteristics and components of radioactivity. A. Differentiate among alpha, beta, and gamma radiation. B. Differentiate between fission and fusion. C. Explain the process
L 36 Modern Physics :006 FINAL EXAM. Nuclear reactions: E = mc 2. Radioactivity. Hazards of radiation. Biological effects of nuclear radiation
 9:006 FINAL EXAM The final exam is on Monday MAY 7:30 AM - 9:30 AM in W90 CB The FE is not cumulative, and will cover lectures 3 through 36. (50 questions) The last regular lecture (Lec. 36) will be given
9:006 FINAL EXAM The final exam is on Monday MAY 7:30 AM - 9:30 AM in W90 CB The FE is not cumulative, and will cover lectures 3 through 36. (50 questions) The last regular lecture (Lec. 36) will be given
Nuclear fission is used in nuclear power stations to generate electricity. Nuclear fusion happens naturally in stars.
 1 (a) Nuclear fission is used in nuclear power stations to generate electricity. Nuclear fusion happens naturally in stars. (i) Explain briefly the difference between nuclear fission and nuclear fusion.
1 (a) Nuclear fission is used in nuclear power stations to generate electricity. Nuclear fusion happens naturally in stars. (i) Explain briefly the difference between nuclear fission and nuclear fusion.
Fundamentals of Nuclear Power. Original slides provided by Dr. Daniel Holland
 Fundamentals of Nuclear Power Original slides provided by Dr. Daniel Holland Nuclear Fission We convert mass into energy by breaking large atoms (usually Uranium) into smaller atoms. Note the increases
Fundamentals of Nuclear Power Original slides provided by Dr. Daniel Holland Nuclear Fission We convert mass into energy by breaking large atoms (usually Uranium) into smaller atoms. Note the increases
Chapter 7 Review. Block: Date:
 Science 10 Chapter 7 Review Name: KEY Block: Date: 1. Radioactivity is the release of high-energy particles and rays from a substance as a result of changes in the nuclei of its atoms.. _Natural background
Science 10 Chapter 7 Review Name: KEY Block: Date: 1. Radioactivity is the release of high-energy particles and rays from a substance as a result of changes in the nuclei of its atoms.. _Natural background
Chapter 18: Consequences of Nuclear Energy Use
 Chapter 18: Consequences of Nuclear Energy Use Goals of Period 18 Section 18.1: To discuss the generation of electricity by nuclear reactors Section 18.2: To describe the design of nuclear fission reactors
Chapter 18: Consequences of Nuclear Energy Use Goals of Period 18 Section 18.1: To discuss the generation of electricity by nuclear reactors Section 18.2: To describe the design of nuclear fission reactors
RADIOACTIVITY & HALF-LIFE Part 3
 RADIOACTIVITY & HALF-LIFE Part 3 Half-Life Half-life: is the rate of decay for a radioactive isotope. is the time required for half of an original quantity of an element to decay. is constant and independent
RADIOACTIVITY & HALF-LIFE Part 3 Half-Life Half-life: is the rate of decay for a radioactive isotope. is the time required for half of an original quantity of an element to decay. is constant and independent
u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig [1]
![u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig [1] u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig [1]](/thumbs/79/80122691.jpg) 1 (a) Fig. 6.1 shows the quark composition of some particles. proton neutron A B u u d u d d u d u u u u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig. 6.1. (ii) State
1 (a) Fig. 6.1 shows the quark composition of some particles. proton neutron A B u u d u d d u d u u u u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig. 6.1. (ii) State
NUCLEAR ENGINEERING. 6. Amongst the following, the fissionable materials are (a) U233andPu239 (b) U23iandPu233 (c) U235andPu235 (d) U238andPu239
 NUCLEAR ENGINEERING 1. The efficiency of a nuclear power plant in comparsion to a conventional thermal power plant is (a) same (b) more (c) less (d) may be less or mote depending on size (e) unpredictable.
NUCLEAR ENGINEERING 1. The efficiency of a nuclear power plant in comparsion to a conventional thermal power plant is (a) same (b) more (c) less (d) may be less or mote depending on size (e) unpredictable.
Nuclear Fusion 1 of 24 Boardworks Ltd 2011
 Nuclear Fusion 1 of 24 Boardworks Ltd 2011 2 of 24 Boardworks Ltd 2011 How do we get energy from atoms? 3 of 24 Boardworks Ltd 2011 Energy is produced from atoms in power stations using the process of
Nuclear Fusion 1 of 24 Boardworks Ltd 2011 2 of 24 Boardworks Ltd 2011 How do we get energy from atoms? 3 of 24 Boardworks Ltd 2011 Energy is produced from atoms in power stations using the process of
Nuclear Reactions and E = mc 2. L 38 Modern Physics [4] Hazards of radiation. Radiation sickness. Biological effects of nuclear radiation
![Nuclear Reactions and E = mc 2. L 38 Modern Physics [4] Hazards of radiation. Radiation sickness. Biological effects of nuclear radiation Nuclear Reactions and E = mc 2. L 38 Modern Physics [4] Hazards of radiation. Radiation sickness. Biological effects of nuclear radiation](/thumbs/77/76435546.jpg) L 38 Modern Physics [4] Nuclear physics what s s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
L 38 Modern Physics [4] Nuclear physics what s s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
Radioactivity One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of Marie Curie
 1 Nuclear Chemistry Radioactivity 2 One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of Marie Curie (1876-1934). She discovered radioactivity or radioactive
1 Nuclear Chemistry Radioactivity 2 One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of Marie Curie (1876-1934). She discovered radioactivity or radioactive
Special!Area!of!Study!1! Energy!from!the!nucleus!
 Outcome Year11PhysicsUnit1 SpecialAreaofStudy1 Energyfromthenucleus Chapter12 Oncompletionofthischapter,youshouldbeabletodescribeandexplaintypical fission and fusion reactions, energy transfer and transformation
Outcome Year11PhysicsUnit1 SpecialAreaofStudy1 Energyfromthenucleus Chapter12 Oncompletionofthischapter,youshouldbeabletodescribeandexplaintypical fission and fusion reactions, energy transfer and transformation
SRI VIDYA COLLEGE OF ENGINEERING & TECHNOLOGY QUESTION BANK UNIT II -TWOMARKS. UNIT-II NUCLEAR POWER PLANTS:
 -TWOMARKS. UNIT-II NUCLEAR POWER PLANTS: 1.What is meant by radioactivity? It refers to the german name of Radio-Activitat. Radioactivity is the spontaneous disintegration of atomic nuclei. The nucleus
-TWOMARKS. UNIT-II NUCLEAR POWER PLANTS: 1.What is meant by radioactivity? It refers to the german name of Radio-Activitat. Radioactivity is the spontaneous disintegration of atomic nuclei. The nucleus
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
Nuclear Fission. Conceptual Physics 11 th Edition. Nuclear Fission. Nuclear Fission. Nuclear Fission. This lecture will help you understand:
 Conceptual Physics 11 th Edition A typical uranium fission reaction: Chapter 34: NUCLEAR FISSION AND FUSION Note the mass number as well as atomic numbers balance. This lecture will help you understand:
Conceptual Physics 11 th Edition A typical uranium fission reaction: Chapter 34: NUCLEAR FISSION AND FUSION Note the mass number as well as atomic numbers balance. This lecture will help you understand:
Science 10 Radioactivity Review v3
 Class: Date: Science 10 Radioactivity Review v3 Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 1. An atom
Class: Date: Science 10 Radioactivity Review v3 Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 1. An atom
Step 2: Calculate the total amount of U-238 present at time=0. Step 4: Calculate the rate constant for the decay process.
 LP#9. A meteor contains 0.556 g of Pb-206 to every 1.00g U-238. Determine the age of the meteor. Step 1: Calculate the moles of each nuclide present. 0.566g Pb-206 x 1.00g U-238 x Step 2: Calculate the
LP#9. A meteor contains 0.556 g of Pb-206 to every 1.00g U-238. Determine the age of the meteor. Step 1: Calculate the moles of each nuclide present. 0.566g Pb-206 x 1.00g U-238 x Step 2: Calculate the
Isotopes 1. Carbon-12 and Carbon-14 have a different number of. A. Protons B. Neutrons C. Both D. Neither
 Isotopes 1. Carbon-12 and Carbon-14 have a different number of A. Protons B. Neutrons C. Both D. Neither 2. Which statement is true about an isotope s half life? Radioactive Isotopes A. Isotopes of the
Isotopes 1. Carbon-12 and Carbon-14 have a different number of A. Protons B. Neutrons C. Both D. Neither 2. Which statement is true about an isotope s half life? Radioactive Isotopes A. Isotopes of the
Power Installations based on Activated Nuclear Reactions of Fission and Synthesis
 Yu.V. Grigoriev 1,2, A.V. Novikov-Borodin 1 1 Institute for Nuclear Research RAS, Moscow, Russia 2 Joint Institute for Nuclear Research, Dubna, Russia Power Installations based on Activated Nuclear Reactions
Yu.V. Grigoriev 1,2, A.V. Novikov-Borodin 1 1 Institute for Nuclear Research RAS, Moscow, Russia 2 Joint Institute for Nuclear Research, Dubna, Russia Power Installations based on Activated Nuclear Reactions
Nuclear Reactions A Z. Radioactivity, Spontaneous Decay: Nuclear Reaction, Induced Process: x + X Y + y + Q Q > 0. Exothermic Endothermic
 Radioactivity, Spontaneous Decay: Nuclear Reactions A Z 4 P D+ He + Q A 4 Z 2 Q > 0 Nuclear Reaction, Induced Process: x + X Y + y + Q Q = ( m + m m m ) c 2 x X Y y Q > 0 Q < 0 Exothermic Endothermic 2
Radioactivity, Spontaneous Decay: Nuclear Reactions A Z 4 P D+ He + Q A 4 Z 2 Q > 0 Nuclear Reaction, Induced Process: x + X Y + y + Q Q = ( m + m m m ) c 2 x X Y y Q > 0 Q < 0 Exothermic Endothermic 2
Chapter 10 Section 4 Notes
 Chapter 10 Section 4 Notes This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The
Chapter 10 Section 4 Notes This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The
Physics 11 Nuclear Process. Nuclear Fusion Reactors Terminology Waste Storage Radiation and living things Nuclear Fission
 Physics 11 Nuclear Process Nuclear Fusion Reactors Terminology Waste Storage Radiation and living things Nuclear Fission Nuclear Reactors Terminology Fission Control Rods, moderator, chain reaction half-life
Physics 11 Nuclear Process Nuclear Fusion Reactors Terminology Waste Storage Radiation and living things Nuclear Fission Nuclear Reactors Terminology Fission Control Rods, moderator, chain reaction half-life
10.4 Fission and Fusion
 This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The alchemists failed in their
This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The alchemists failed in their
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
Nuclear Power MORE CHAPTER 11, #6. Nuclear Fission Reactors
 MORE CHAPTER 11, #6 Nuclear Power Nuclear Fission Reactors The discovery that several neutrons are emitted in the fission process led to speculation concerning the possibility of using these neutrons to
MORE CHAPTER 11, #6 Nuclear Power Nuclear Fission Reactors The discovery that several neutrons are emitted in the fission process led to speculation concerning the possibility of using these neutrons to
NUCLEI. Atomic mass unit
 13 NUCLEI Atomic mass unit It is a unit used to express the mass of atoms and particles inside it. One atomic mass unit is the mass of atom. 1u = 1.660539 10. Chadwick discovered neutron. The sum of number
13 NUCLEI Atomic mass unit It is a unit used to express the mass of atoms and particles inside it. One atomic mass unit is the mass of atom. 1u = 1.660539 10. Chadwick discovered neutron. The sum of number
Neutron reproduction. factor ε. k eff = Neutron Life Cycle. x η
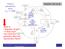 Neutron reproduction factor k eff = 1.000 What is: Migration length? Critical size? How does the geometry affect the reproduction factor? x 0.9 Thermal utilization factor f x 0.9 Resonance escape probability
Neutron reproduction factor k eff = 1.000 What is: Migration length? Critical size? How does the geometry affect the reproduction factor? x 0.9 Thermal utilization factor f x 0.9 Resonance escape probability
Science 30 Unit D Energy and the Environment
 Science 30 Unit D Energy and the Environment Outcome 2: Students will describe the sun as Earth s main source of energy and explain the functioning of some conventional and alternative technologies that
Science 30 Unit D Energy and the Environment Outcome 2: Students will describe the sun as Earth s main source of energy and explain the functioning of some conventional and alternative technologies that
Question to the class: What are the pros, cons, and uncertainties of using nuclear power?
 Energy and Society Week 11 Section Handout Section Outline: 1. Rough sketch of nuclear power (15 minutes) 2. Radioactive decay (10 minutes) 3. Nuclear practice problems or a discussion of the appropriate
Energy and Society Week 11 Section Handout Section Outline: 1. Rough sketch of nuclear power (15 minutes) 2. Radioactive decay (10 minutes) 3. Nuclear practice problems or a discussion of the appropriate
Isotopes. An isotope is an atom of the same element (same number of protons) that varies in the number of neutrons.
 Nuclear Chemistry Isotopes An isotope is an atom of the same element (same number of protons) that varies in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Nuclear Chemistry Isotopes An isotope is an atom of the same element (same number of protons) that varies in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Isotopes. An isotope is an atoms of the same element (same number of protons) that vary in the number of neutrons.
 Nuclear Chemistry Isotopes An isotope is an atoms of the same element (same number of protons) that vary in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Nuclear Chemistry Isotopes An isotope is an atoms of the same element (same number of protons) that vary in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
11.5 Nuclear Reactions: Fusion
 11.5 Nuclear Reactions: Fusion Nuclear fusion reactions occur in the Sun and supply the energy needed to sustain life on Earth (Figure 1). Nuclear fusion is the fusing or joining of two small nuclei to
11.5 Nuclear Reactions: Fusion Nuclear fusion reactions occur in the Sun and supply the energy needed to sustain life on Earth (Figure 1). Nuclear fusion is the fusing or joining of two small nuclei to
Production. David Nusbaum Project on Managing the Atom, Belfer Center October 4, 2011
 Production David Nusbaum Project on Managing the Atom, Belfer Center October 4, 2011 Where are we? Nuclear Fuel Cycle Background Pu- Radioactive, chemical element, of the actinoid series of the periodic
Production David Nusbaum Project on Managing the Atom, Belfer Center October 4, 2011 Where are we? Nuclear Fuel Cycle Background Pu- Radioactive, chemical element, of the actinoid series of the periodic
1. Which is the most commonly used molten metal for cooling of nuclear reactors? A. Zinc B. Sodium C. Calcium D. Mercury
 1. Which is the most commonly used molten metal for cooling of nuclear reactors? A. Zinc B. Sodium C. Calcium D. Mercury 2. Commercial power generation from fusion reactor is not yet possible, because
1. Which is the most commonly used molten metal for cooling of nuclear reactors? A. Zinc B. Sodium C. Calcium D. Mercury 2. Commercial power generation from fusion reactor is not yet possible, because
Write down the nuclear equation that represents the decay of neptunium 239 into plutonium 239.
 Q1.A rod made from uranium 238 ( U) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium 238 absorbs a neutron it becomes unstable and decays to neptunium
Q1.A rod made from uranium 238 ( U) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium 238 absorbs a neutron it becomes unstable and decays to neptunium
The outermost container into which vitrified high level waste or spent fuel rods are to be placed. Made of stainless steel or inert alloy.
 Glossary of Nuclear Waste Terms Atom The basic component of all matter; it is the smallest part of an element having all the chemical properties of that element. Atoms are made up of protons and neutrons
Glossary of Nuclear Waste Terms Atom The basic component of all matter; it is the smallest part of an element having all the chemical properties of that element. Atoms are made up of protons and neutrons
Episode 528: Controlling fission
 Episode 528: Controlling fission In this episode, you can look at the different features of the core of a nuclear reactor, and explain its operation using your students knowledge of nuclear physics. Summary
Episode 528: Controlling fission In this episode, you can look at the different features of the core of a nuclear reactor, and explain its operation using your students knowledge of nuclear physics. Summary
Radioactivity & Nuclear. Chemistry. Mr. Matthew Totaro Legacy High School. Chemistry
 Radioactivity & Nuclear Chemistry Mr. Matthew Totaro Legacy High School Chemistry The Discovery of Radioactivity Antoine-Henri Becquerel designed an experiment to determine if phosphorescent minerals also
Radioactivity & Nuclear Chemistry Mr. Matthew Totaro Legacy High School Chemistry The Discovery of Radioactivity Antoine-Henri Becquerel designed an experiment to determine if phosphorescent minerals also
Thinking Like a Chemist About Nuclear Change!
 Thinking Like a Chemist About Nuclear Change! What are we going to learn today? Nuclear Changes REACTIONS ENERGY RELEASED DECAY Poll: Clicker Question There was a nuclear emergency in Japan. The emergency
Thinking Like a Chemist About Nuclear Change! What are we going to learn today? Nuclear Changes REACTIONS ENERGY RELEASED DECAY Poll: Clicker Question There was a nuclear emergency in Japan. The emergency
Chapter 12: Nuclear Reaction
 Chapter 12: Nuclear Reaction A nuclear reaction occurs when a nucleus is unstable or is being bombarded by a nuclear particle. The product of a nuclear reaction is a new nuclide with an emission of a nuclear
Chapter 12: Nuclear Reaction A nuclear reaction occurs when a nucleus is unstable or is being bombarded by a nuclear particle. The product of a nuclear reaction is a new nuclide with an emission of a nuclear
Chapter 10. Answers to examination-style questions. Answers Marks Examiner s tips. 1 (a) (i) 238. (ii) β particle(s) 1 Electron antineutrinos 1
 (a) (i) 238 92 U + 0 n 239 92 U (ii) β particle(s) Electron antineutrinos (b) For: Natural uranium is 98% uranium-238 which would be otherwise unused. Plutonium-239 would not need to be stored long-term
(a) (i) 238 92 U + 0 n 239 92 U (ii) β particle(s) Electron antineutrinos (b) For: Natural uranium is 98% uranium-238 which would be otherwise unused. Plutonium-239 would not need to be stored long-term
Nuclear Chemistry. Transmutations and the Creation of Elements
 Nuclear Chemistry Transmutations and the Creation of Elements Nuclear Fusion When two smaller elements are fused together to form a larger element. Fusion is Hard! There are two competing forces in an
Nuclear Chemistry Transmutations and the Creation of Elements Nuclear Fusion When two smaller elements are fused together to form a larger element. Fusion is Hard! There are two competing forces in an
Radioactive Materials
 Radioactive Materials (OCR) The structure of the atom ELECTRON negative, mass nearly nothing NEUTRON neutral, same mass as proton ( 1 ) PROTON positive, same mass as neutron ( 1 ) Isotopes An isotope is
Radioactive Materials (OCR) The structure of the atom ELECTRON negative, mass nearly nothing NEUTRON neutral, same mass as proton ( 1 ) PROTON positive, same mass as neutron ( 1 ) Isotopes An isotope is
Aim: What are the two types of Nuclear. Reactions? Do Now: 1. Get into your groups and compare your answers to your homework.
 Aim: What are the two types of Nuclear Reactions? Do Now: 1. Get into your groups and compare your answers to your homework. Nuclear Energy In nuclear reaction, mass is converted into energy; there is
Aim: What are the two types of Nuclear Reactions? Do Now: 1. Get into your groups and compare your answers to your homework. Nuclear Energy In nuclear reaction, mass is converted into energy; there is
1ST SEM MT CHAP 22 REVIEW
 1ST SEM MT CHAP 22 REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. (CAPITAL LETTERS ONLY PLEASE) 1. Mass defect is the difference between the mass
1ST SEM MT CHAP 22 REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. (CAPITAL LETTERS ONLY PLEASE) 1. Mass defect is the difference between the mass
B C G H I J. In which section(s) would you find: a) the metals? b) the nonmetals? c) the halogens? d) the actinides? e) the alkaline earth metals?
 Pretest: Nuclear Technology (PSC 4010) 1. A B C D E F G H I J In which section(s) would you find: a) the metals? b) the nonmetals? c) the halogens? d) the actinides? e) the alkaline earth metals? f) the
Pretest: Nuclear Technology (PSC 4010) 1. A B C D E F G H I J In which section(s) would you find: a) the metals? b) the nonmetals? c) the halogens? d) the actinides? e) the alkaline earth metals? f) the
Unit 1 Test A Atomic Theory & Nuclear Decay 1. Which of these BEST describes any two atoms of the same element? a. same number of protons
 1. Which of these BEST describes any two atoms of the same element? same number of protons same number of chemical bonds same number of neutrons same number of particles in the nucleus Self Assessment
1. Which of these BEST describes any two atoms of the same element? same number of protons same number of chemical bonds same number of neutrons same number of particles in the nucleus Self Assessment
YEAR 11 Physics Unit 1
 Hampton Park Secondary College Student s Name: Senior School Examinations Semester 1 2011 Home Group: Student Number Figures Words YEAR 11 Physics Unit 1 Written Examination QUESTION AND ANSWER BOOK Structure
Hampton Park Secondary College Student s Name: Senior School Examinations Semester 1 2011 Home Group: Student Number Figures Words YEAR 11 Physics Unit 1 Written Examination QUESTION AND ANSWER BOOK Structure
Name Date Class NUCLEAR CHEMISTRY
 25 NUCLEAR CHEMISTRY SECTION 25.1 NUCLEAR RADIATION (pages 799 802) This section describes the nature of radioactivity and the process of radioactive decay. It characterizes alpha, beta, and gamma radiation
25 NUCLEAR CHEMISTRY SECTION 25.1 NUCLEAR RADIATION (pages 799 802) This section describes the nature of radioactivity and the process of radioactive decay. It characterizes alpha, beta, and gamma radiation
Ch Radioactivity. Henry Becquerel, using U-238, discovered the radioactive nature of elements in 1896.
 Ch. 10 - Radioactivity Henry Becquerel, using U-238, discovered the radioactive nature of elements in 1896. Radioactivity the process in which an unstable atomic nucleus emits charged particles and energy
Ch. 10 - Radioactivity Henry Becquerel, using U-238, discovered the radioactive nature of elements in 1896. Radioactivity the process in which an unstable atomic nucleus emits charged particles and energy
 Nuclear Fission and Fusion A. Nuclear Fission. The process of splitting up of the nucleus of a heavy atom into two nuclei more or less of equal fragments when bombarded with neutron simultaneously releasing
Nuclear Fission and Fusion A. Nuclear Fission. The process of splitting up of the nucleus of a heavy atom into two nuclei more or less of equal fragments when bombarded with neutron simultaneously releasing
Nuclear Chemistry. Technology Strategies for Success PO Box 1485 East Northport, NY (631) NYS-PREP
 Nuclear Chemistry Technology Strategies for Success PO Box 1485 East Northport, NY 11725 (631)734-0115 1-888-NYS-PREP techstrategies@gmail.com Nuclear Chemistry Table of Contents 1.0 Nuclear Chemistry...3
Nuclear Chemistry Technology Strategies for Success PO Box 1485 East Northport, NY 11725 (631)734-0115 1-888-NYS-PREP techstrategies@gmail.com Nuclear Chemistry Table of Contents 1.0 Nuclear Chemistry...3
This Week. Production, Usage and Distribution of Energy. 8/4/2017 Physics 214 Fall
 This Week Fission and fusion What made our world Atomic and Hydrogen bombs Big bangs in small packages Nuclear power plants The solution to the energy problem? Enrichment What does it mean and why is it
This Week Fission and fusion What made our world Atomic and Hydrogen bombs Big bangs in small packages Nuclear power plants The solution to the energy problem? Enrichment What does it mean and why is it
Cambridge University Press An Introduction to the Engineering of Fast Nuclear Reactors Anthony M. Judd Excerpt More information
 INTRODUCTION WHAT FAST REACTORS CAN DO Chain Reactions Early in 1939 Meitner and Frisch suggested that the correct interpretation of the results observed when uranium is bombarded with neutrons is that
INTRODUCTION WHAT FAST REACTORS CAN DO Chain Reactions Early in 1939 Meitner and Frisch suggested that the correct interpretation of the results observed when uranium is bombarded with neutrons is that
This Week. 3/23/2017 Physics 214 Summer
 This Week Fission and fusion What made our world Atomic and Hydrogen bombs Big bangs in small packages Nuclear power plants The solution to the energy problem? Enrichment What does it mean and why is it
This Week Fission and fusion What made our world Atomic and Hydrogen bombs Big bangs in small packages Nuclear power plants The solution to the energy problem? Enrichment What does it mean and why is it
Physics Lecture 25
 Physics 3313 - Lecture 25 Monday May 3, 2010 Dr. Andrew Brandt CH 12: The Nucleus 4/26/2010 1 3313 Andrew Brandt CHAPTER 12 The Atomic Nucleus 12.6 Radioactive Decay 12.7 Alpha+ Beta Decay 4/26/2010 3313
Physics 3313 - Lecture 25 Monday May 3, 2010 Dr. Andrew Brandt CH 12: The Nucleus 4/26/2010 1 3313 Andrew Brandt CHAPTER 12 The Atomic Nucleus 12.6 Radioactive Decay 12.7 Alpha+ Beta Decay 4/26/2010 3313
Fission and Chain Reactions
 The Harnessed Atom Lesson Five Fission and Chain Reactions What you need to know about Fission and Chain Reactions: Fission Chain reaction Uranium fuel Mining Milling Enrichment Fuel fabrication 2 Nuclear
The Harnessed Atom Lesson Five Fission and Chain Reactions What you need to know about Fission and Chain Reactions: Fission Chain reaction Uranium fuel Mining Milling Enrichment Fuel fabrication 2 Nuclear
NUCLEAR PHYSICS: solutions to higher level questions
 NUCLEAR PHYSICS: solutions to higher level questions 2015 Question 12 (d) (i) What is meant by the term radioactive? (Spontaneous) disintegration of a nucleus with the emission of radiation (ii) Name a
NUCLEAR PHYSICS: solutions to higher level questions 2015 Question 12 (d) (i) What is meant by the term radioactive? (Spontaneous) disintegration of a nucleus with the emission of radiation (ii) Name a
1. What is the phenomenon that best explains why greenhouse gases absorb infrared radiation? D. Diffraction (Total 1 mark)
 1. What is the phenomenon that best explains why greenhouse gases absorb infrared radiation? A. Resonance B. Interference C. Refraction D. Diffraction 2. In which of the following places will the albedo
1. What is the phenomenon that best explains why greenhouse gases absorb infrared radiation? A. Resonance B. Interference C. Refraction D. Diffraction 2. In which of the following places will the albedo
UNIT 13: NUCLEAR CHEMISTRY
 UNIT 13: NUCLEAR CHEMISTRY REVIEW: ISOTOPE NOTATION An isotope notation is written as Z A X, where X is the element, A is the mass number (sum of protons and neutrons), and Z is the atomic number. For
UNIT 13: NUCLEAR CHEMISTRY REVIEW: ISOTOPE NOTATION An isotope notation is written as Z A X, where X is the element, A is the mass number (sum of protons and neutrons), and Z is the atomic number. For
Nuclear Energy; Effects and Uses of Radiation
 Nuclear Energy; Effects and Uses of Radiation Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical
Nuclear Energy; Effects and Uses of Radiation Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical
Unpressurized steam reactor. Controlled Fission Reactors. The Moderator. Global energy production 2000
 From last time Fission of heavy elements produces energy Only works with 235 U, 239 Pu Fission initiated by neutron absorption. Fission products are two lighter nuclei, plus individual neutrons. These
From last time Fission of heavy elements produces energy Only works with 235 U, 239 Pu Fission initiated by neutron absorption. Fission products are two lighter nuclei, plus individual neutrons. These
Lecture Presentation. Chapter 21. Nuclear Chemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 21, Inc. James F. Kirby Quinnipiac University Hamden, CT Energy: Chemical vs. Chemical energy is associated with making and breaking chemical bonds. energy is enormous in comparison.
Lecture Presentation Chapter 21, Inc. James F. Kirby Quinnipiac University Hamden, CT Energy: Chemical vs. Chemical energy is associated with making and breaking chemical bonds. energy is enormous in comparison.
25.3 Fission and Fusion of Atomic Nuclei 25.3 FOCUS. Guide for Reading INSTRUCT. Nuclear Fission. Section Resources.
 25.3 25.3 Fission and Fusion of Atomic Nuclei 1 FOCUS Objectives 25.3.1 Describe what happens in a nuclear chain reaction. 25.3.2 Explain the role of water in the storage of spent fuel rods. 25.3.3 Distinguish
25.3 25.3 Fission and Fusion of Atomic Nuclei 1 FOCUS Objectives 25.3.1 Describe what happens in a nuclear chain reaction. 25.3.2 Explain the role of water in the storage of spent fuel rods. 25.3.3 Distinguish
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 21 REVIEW Nuclear Chemistry SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Based on the information about the three elementary particles in the text, which has
CHAPTER 21 REVIEW Nuclear Chemistry SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Based on the information about the three elementary particles in the text, which has
Nuclear processes: Vocabulary: Radioactive decay Isotope Alpha particle Beta particle Transmutation Strong Nuclear Force Fusion Fission
 Nuclear processes: Students will develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive
Nuclear processes: Students will develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive
Nuclear Chemistry Unit
 Nuclear Chemistry Unit January 28th HW Due Thurs. 1/30 Read pages 284 291 Define: Radioactivity Nuclear Radiation Alpha Particle Beta Particle Gamma Ray Half-Life Answer: -Questions 1-3 -Write the symbols
Nuclear Chemistry Unit January 28th HW Due Thurs. 1/30 Read pages 284 291 Define: Radioactivity Nuclear Radiation Alpha Particle Beta Particle Gamma Ray Half-Life Answer: -Questions 1-3 -Write the symbols
Nuclear processes: Vocabulary: Radioactive decay Isotope Alpha particle Beta particle Transmutation Strong Nuclear Force Fusion fission
 Nuclear processes: Students will develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive
Nuclear processes: Students will develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive
