Thermochemistry. Questions to ponder. Because 4/20/14. an ice-cube? an ice-cube? Part 2: Calorimetry. But I KNOW. Q=mc T, but T=0
|
|
|
- Clinton Walsh
- 5 years ago
- Views:
Transcription
1 Thermochemistry Part 2: Calorimetry p p If you leave your keys and your chemistry book sitting in the sun on a hot summer day, which one is hotter? Why is there a difference in temperature between the two objects? Because p Different substances have different specific heats (amount of energy needed to raise the temperature of 1 g of a substance by 1 degree Celsius). Q=mc T, but T=0 But I KNOW 0 1
2 How can I solve this??? Calorimetry!!! Heat reuired to melt ice (a.k.a. latent heat of fusion) cannot be measured directly, but calorimetry provides an experimental method allowing this heat transfer to be measured indirectly. Calorimetry!!! Calorimetry: measurement of the amount of heat evolved or absorbed in a chemical reaction, change of state or formation of a solution. CALORIMETRY p The enthalpy change associated with a chemical reaction or process can be determined experimentally. n Measure the heat gained or lost during a reaction at CONSTANT pressure p A calorimeter is a device used to measure the heat absorbed or released during a chemical or physical process Coffee Cup Calorimeter The cup is filled with water, which absorbs the heat evolved by the reaction, so: Coffee Cup Calorimeter p A more high tech drawing Styrofoam cup ice = - water OR rxn = - cal 2
3 What happens in a calorimeter? p One object will LOSE heat, and the other will ABSORB the heat n System loses heat to surroundings = EXO = - n System absorbs heat from surroundings = ENDO = + When a hot chunk of metal is dropped in a cool glass of water, the metal cools off. Where did the heat from the metal go? Did the metal lose more heat then the water gained? Magnitude of HEAT GAINED = HEAT LOST (ALWAYS!) To do calorimetry problems p Make a chart: measurement Heat () Mass (m) Specific Heat (c) Final Temp ( ) Initial Temp( ) The numbers in these two boxes are always the same, but with different signs (+/-). What heat one lost, the other gained. Cal (often water) Object/ Rxn EXAMPLE 1: A small pebble is heated and placed in a foam cup calorimeter containing 25.0 g of water at 25.0 C. The water reaches a maximum temperature of 26.4 C. How many joules of heat were released by the pebble? The specific heat of water is J/g C. Heat Water (cal) Mass 25.0 g Specific Heat Final Temp Initial Temp 26.4 o C 25.0 o C Pebble (rxn) The numbers in these two boxes are always the same, but with different signs (+/-). What heat one lost, the other gained. Hints: p The pebble lost heat because the water heated up from 25.0 C to 26.4 C. p Pebble loses heat (-, exothermic) while water gains heat (+, endothermic) p Do you calculation based on water (since the problem gave all the water s information) Heat Water (cal) Mass 25.0 g Specific Heat Final Temp Initial Temp 26.4 o C 25.0 o C cal = m water c water ΔT water Pebble (rxn) cal = (25.0g)(4.184J/g o C)(26.4 o C-25.0 o C) cal = 146 J rxn = J If the water ABSORBED 146 J of heat, then the pebble RELEASED 146 J of heat. Example 2: When 1.00 g of ammonium nitrate, NH 4 NO 3, is added to 50.0 g of water in a coffee cup calorimeter, it dissolves, NH 4 NO 3 (s) NH 4+ (a) + NO 3 - (a), and the temperature of the water drops from C to C. Calculate for the reaction system. m cal c g o C o C rxn 1.00 g cal = m w c w Δt cal = (50.0g)(4.18 J/g C)(-1.68 C) cal = J rxn = - cal rxn = 351 J (endothermic) When one substance absorbs heat, the other substance releases heat (energy) 3
4 Example 3 (similar to what you will do in tomorrow s lab) p Suppose that g of water at 22.4 C is placed in a calorimeter. A g sample of Al is removed from boiling water at a temperature of 99.3 C and uickly placed in a calorimeter. The substances reach a final temperature of 32.9 C. Determine the SPECIFIC HEAT of the metal. The specific heat of water is J/g C. è MAKE YOUR CHART Example 3: Suppose that g of water at 22.4 C is placed in a calorimeter. A g sample of Al is removed from boiling water at a temperature of 99.3 C and uickly placed in a calorimeter. The substances reach a final temperature of 32.9 C. Determine the SPECIFIC HEAT of the metal. The specific heat of water is J/g C. m cal (H 2 O) c g 32.9 o C 22.4 o C rxn (Al) g 32.9 o C 99.3 o C 1. Make chart 2. Calculate for water 3. Q for Al is the same (but with different sign) as for metal. 4. Using metal, calculate c metal Example 3 cont d: specific heat of aluminum cal (H 2 O) rxn (Al) 4,393.2 J -4,393.2 J m g g c J/g C 32.9 C 32.9 C 22.4 C 99.3 C cal = m w c w ΔT cal = (100.00g)(4.184J/g C)(10.5 C) cal = 4,393.2 J rxn = - cal rxn = -4,393.2 J rxn = m Al c Al ΔT -4,393.2 J = (75.25 g)(c Al )(-66.4 C) c Al = J/g C p What if you wanted to measure the heat of a reaction or process that couldn t be measured in a simple coffee cup calorimeter (e.g., heat of combustion of Mg)? You would need something like this Bomb Calorimeter 4
5 Another type of calorimeter is a Bomb Calorimeter If Heat Capacity (C) is known It is possible to calculate the amount of heat absorbed or evolved by the reaction if you know the heat capacity, C cal, and the temp. change, ΔT, of the calorimeter: cal = C cal ΔT NOTE: In a bomb calorimeter, heat is transferred from the sample to the oxygen-enriched chamber, to the metal that makes up the chamber, to the water thus we cannot just use the specific heat of water; instead heat capacity of the calorimeter, C cal, can be used or calculated. Everything else is the same (remember, the heat lost from the reaction goes into the calorimeter) EXAMPLE 4: The reaction between hydrogen and chlorine, H 2 + Cl 2 2HCl, can be studied in a bomb calorimeter. It is found that when a 1.00 g sample of H 2 reacts completely, the temp. rises from C to C. Taking the heat capacity of the calorimeter to be 9.33 kj/ C, calculate the amount of heat evolved in the reaction. EXAMPLE 5: When 1.00 mol of caffeine (C 8 H 10 N 4 O 2 ) is burned in air, 4.96 x 10 3 kj of heat is evolved. Five grams of caffeine is burned in a bomb calorimeter. The temperature is observed to increase by C. What is the heat capacity of the calorimeter in J/ C? c Cal 9.33 kj/ o C o C o C Rxn cal = C cal ΔT cal = (9.33 kj/ C)(9.82 C) cal = 91.6 kj rxn = kj *** must have a negative sign if heat evolved in the reaction = released 4.96 x 10 3 kj is for 1.00 mol of caffeine. We are burning only 5.00 g of caffeine to increase the temp by o C. We FIRST need to figure out how much heat energy is given off by just 5 grams. c kj = 194.0gC H N O Cal kj? ΔT o C 4 2 Rxn x 5.00 g kj x = kj KJ l = (C cal )(11.37 C) kj J C cal = 11.2! or11, 200! C C EXAMPLE 6: When twenty milliliters of ethyl ether, C 4 H 10 O. (d=0.714 g/ml) is burned in a bomb calorimeter, the temperature rises from 24.7 C to 88.9 C. The calorimeter heat capacity is kj/ C. (a) What is for the calorimeter? (b) What is when 20.0 ml of ether is burned? (c) What is for the combustion of one mole of ethyl ether? c Cal kj/ o C 88.9 o C 24.7 o C Rxn cal = C cal Δt cal = (10.34 kj/ C)(64.2 C) cal = 664 kj EXAMPLE 6: When twenty milliliters of ethyl ether, C 4 H 10 O. (d=0.714 g/ml) is burned in a bomb calorimeter, the temperature rises from 24.7 C to 88.9 C. The calorimeter heat capacity is kj/ C. (a) What is for the calorimeter? (b) What is when 20.0 ml of ether is burned? (c) What is for the combustion of one mole of ethyl ether? rxn = - cal rxn = -664 kj 5
6 EXAMPLE 6: When twenty milliliters of ethyl ether, C 4 H 10 O. (d=0.714 g/ ml) is burned in a bomb calorimeter, the temperature rises from 24.7 C to 88.9 C. The calorimeter heat capacity is kj/ C. (a) What is for the calorimeter? (b) What is when 20.0 ml of ether is burned? (c) What is for the combustion of one mole of ethyl ether? 664kJ 20.0mL 1mL g 74.0 g = mol 3 kj mol 6
Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes
 Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
THERMODYNAMICS. Energy changes in reactions Text chapter 3, 4, 5, 6 & 7
 1 THERMODYNAMICS Energy changes in reactions Text chapter 3, 4, 5, 6 & 7 TERMINOLOGY: Thermodynamics: study of heat changes that occur during chemical reactions. Energy (J): Cannot be seen, touched, smelled,
1 THERMODYNAMICS Energy changes in reactions Text chapter 3, 4, 5, 6 & 7 TERMINOLOGY: Thermodynamics: study of heat changes that occur during chemical reactions. Energy (J): Cannot be seen, touched, smelled,
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change
 Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Thermochemistry. The study of the ENERGY CHANGES that accompany changes in matter. 3 Ways: Monday, February 3, 2014
 Thermochemistry The study of the ENERGY CHANGES that accompany changes in matter 3 Ways: 1 Thermodynamics FIRST LAW OF THERMODYNAMICS the total amount of energy in the universe is constant (conservation
Thermochemistry The study of the ENERGY CHANGES that accompany changes in matter 3 Ways: 1 Thermodynamics FIRST LAW OF THERMODYNAMICS the total amount of energy in the universe is constant (conservation
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
Activity Calorimetry
 Activity 201 5 Calorimetry Directions: This GLA worksheet goes over the concepts of heat and calorimetry. Part A introduces the concepts of heat and specific heat capacity. Part B introduces calorimetry
Activity 201 5 Calorimetry Directions: This GLA worksheet goes over the concepts of heat and calorimetry. Part A introduces the concepts of heat and specific heat capacity. Part B introduces calorimetry
Chapter 8. Thermochemistry 강의개요. 8.1 Principles of Heat Flow. 2) Magnitude of Heat Flow. 1) State Properties. Basic concepts : study of heat flow
 강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 5 THERMO. THERMO chemistry. 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation
 Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Heat. Heat Terminology 04/12/2017. System Definitions. System Definitions
 System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
Chapter 6: Thermochemistry
 Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chapter 11 Thermochemistry Heat and Chemical Change
 Chemistry/ PEP Name: Date: Chapter 11 Thermochemistry Heat and Chemical Change Chapter 11:1 35, 57, 60, 61, 71 Section 11.1 The Flow of Energy - Heat 1. When 435 of heat is added to 3.4 g of olive oil
Chemistry/ PEP Name: Date: Chapter 11 Thermochemistry Heat and Chemical Change Chapter 11:1 35, 57, 60, 61, 71 Section 11.1 The Flow of Energy - Heat 1. When 435 of heat is added to 3.4 g of olive oil
Chapter 6. Thermochemistry
 Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Thermodynamics - Energy Relationships in Chemical Reactions:
 Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Chemistry 30: Thermochemistry. Practice Problems
 Name: Period: Chemistry 30: Thermochemistry Practice Problems Date: Heat and Temperature 1. Pretend you are doing a scientific study on the planet Earth. a. Name three things in the system you are studying.
Name: Period: Chemistry 30: Thermochemistry Practice Problems Date: Heat and Temperature 1. Pretend you are doing a scientific study on the planet Earth. a. Name three things in the system you are studying.
THERMOCHEMISTRY CHAPTER 11
 THERMOCHEMISTRY CHAPTER 11 ENERGY AND HEAT nthermochemistry: The study of the energy changes that accompany chemical reactions and changes in the physical states of matter. ENERGY AND HEAT nwork: Energy
THERMOCHEMISTRY CHAPTER 11 ENERGY AND HEAT nthermochemistry: The study of the energy changes that accompany chemical reactions and changes in the physical states of matter. ENERGY AND HEAT nwork: Energy
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
CRHS Academic Chemistry Unit 15 Thermochemistry HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 15 Thermochemistry HOMEWORK Due Date Assignment On-Time (100) Late (70) 15.1 15.2 15.3 15.4 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS
Name KEY Period CRHS Academic Chemistry Unit 15 Thermochemistry HOMEWORK Due Date Assignment On-Time (100) Late (70) 15.1 15.2 15.3 15.4 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS
Chemistry. Friday, March 30 th Monday, April 9 th, 2018
 Chemistry Friday, March 30 th Monday, April 9 th, 2018 Do-Now: BrainPOP: Heat 1. Write down today s FLT 2. Distinguish between exothermic and endothermic processes. 3. What is the specific heat of water?
Chemistry Friday, March 30 th Monday, April 9 th, 2018 Do-Now: BrainPOP: Heat 1. Write down today s FLT 2. Distinguish between exothermic and endothermic processes. 3. What is the specific heat of water?
The Nature of Energy Energy is the ability to do work or produce Heat, q or Q, is ; flows due to temperature differences (always to )
 CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Chapter 6. Heat Flow
 Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Chapter 5: Thermochemistry
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
I. Chemical Reactions that Involve Heat
 Unit 12 Energy I. Chemical Reactions that Involve Heat Thermochemistry: study of changes in heat in chemical reactions. Endothermic: absorbs heat; temp. goes down Exothermic: releases heat; temp. goes
Unit 12 Energy I. Chemical Reactions that Involve Heat Thermochemistry: study of changes in heat in chemical reactions. Endothermic: absorbs heat; temp. goes down Exothermic: releases heat; temp. goes
33. a. Heat is absorbed from the water (it gets colder) as KBr dissolves, so this is an endothermic process.
 31. This is an endothermic reaction so heat must be absorbed in order to convert reactants into products. The high temperature environment of internal combustion engines provides the heat. 33. a. Heat
31. This is an endothermic reaction so heat must be absorbed in order to convert reactants into products. The high temperature environment of internal combustion engines provides the heat. 33. a. Heat
Calculate the energy required to melt a 2.9 kg block of ice. Melting is a phase change - there is no change in temperature
 Calculation of Heat During a Phase Change or Reaction During a phase change, a physical process or chemical reaction, the temperature of the system does not change. Therefore the formula q = mc T would
Calculation of Heat During a Phase Change or Reaction During a phase change, a physical process or chemical reaction, the temperature of the system does not change. Therefore the formula q = mc T would
Chapter 8. Thermochemistry
 Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Chapter 11. Thermochemistry: Heat & Chemical Change
 Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Energetics. Topic
 Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE LEARNING OBJECTIVES
 Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Chapter 5: Thermochemistry. Molecular Kinetic Energy -Translational energy E k, translational = 1/2mv 2 -Rotational energy 5.
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Activity Calorimetry
 Activity 201 5 Calorimetry Directions: This GLA worksheet goes over the concepts of heat and calorimetry. Part A introduces the concepts of heat and specific heat capacity. Part B introduces calorimetry
Activity 201 5 Calorimetry Directions: This GLA worksheet goes over the concepts of heat and calorimetry. Part A introduces the concepts of heat and specific heat capacity. Part B introduces calorimetry
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
17.2 Thermochemical Equations
 17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
8.6 The Thermodynamic Standard State
 8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Measuring Energy Changes. Introducing Heat Capacity and Specific Heat
 Measuring Energy Changes Introducing Heat Capacity and Specific Heat Before Today s Discussion Begins Remember the differences between temperature, thermal energy, and heat Temperature is the average kinetic
Measuring Energy Changes Introducing Heat Capacity and Specific Heat Before Today s Discussion Begins Remember the differences between temperature, thermal energy, and heat Temperature is the average kinetic
Study Guide Chapter 5
 Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Energy Changes in Reactions p
 Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
I. Energy A. Terms and Definitions B. Energy Transfer as Heat C. Energy Transfer as Work D. Internal Energy
 Chapter 7 1 Thermochemistry is HOT! I. Energy A. Terms and Definitions B. Energy Transfer as Heat C. Energy Transfer as Work D. Internal Energy II. Chemistry and Energy A. Enthalpy and Enthalpies of Reaction
Chapter 7 1 Thermochemistry is HOT! I. Energy A. Terms and Definitions B. Energy Transfer as Heat C. Energy Transfer as Work D. Internal Energy II. Chemistry and Energy A. Enthalpy and Enthalpies of Reaction
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Calorimetry. Chapter 5. Week 2 Unit 1. Calorimetry. Since we cannot know the of the reactants and products, we measure H through, the of.
 Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
2. What is a measure of the average kinetic energy of particles? (A) heat capacity (B) molar enthalpy (C) specific heat (D) temperature
 Thermochemistry #1 Chemistry 3202 Name: 1. Classify the following systems as open or closed a) glass of cold water b) a gel filled freezer pack c) a burning candle d) a fluorescent lightbulb e) hot water
Thermochemistry #1 Chemistry 3202 Name: 1. Classify the following systems as open or closed a) glass of cold water b) a gel filled freezer pack c) a burning candle d) a fluorescent lightbulb e) hot water
Practice Test: Energy and Rates of Reactions
 Practice Test: Energy and Rates of Reactions NAME: /65 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. (20 marks) 1. What is the symbol for
Practice Test: Energy and Rates of Reactions NAME: /65 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. (20 marks) 1. What is the symbol for
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Enthalpy and Internal Energy
 Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Changes and Properties of Matter
 Changes and Properties of Matter Physical Properties of Matter Physical Changes: Changes that change only the appearance of a substance, not its chemical identity. Physical Properties: Properties that
Changes and Properties of Matter Physical Properties of Matter Physical Changes: Changes that change only the appearance of a substance, not its chemical identity. Physical Properties: Properties that
DETERMINING AND USING H
 DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
Energetics. These processes involve energy exchanges between the reacting system and its surroundings.
 Energetics Chemical reactions involve: the breaking of bonds between atoms the making of new bonds between atoms These processes involve energy exchanges between the reacting system and its surroundings.
Energetics Chemical reactions involve: the breaking of bonds between atoms the making of new bonds between atoms These processes involve energy exchanges between the reacting system and its surroundings.
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
ENERGY AND ENERGETICS PART ONE Keeping Track of Energy During a Chemical Reaction
 ENERGY AND ENERGETICS PART ONE Keeping Track of Energy During a Chemical Reaction ADEng. PROGRAMME Chemistry for Engineers Prepared by M. J. McNeil, MPhil. Department of Pure and Applied Sciences Portmore
ENERGY AND ENERGETICS PART ONE Keeping Track of Energy During a Chemical Reaction ADEng. PROGRAMME Chemistry for Engineers Prepared by M. J. McNeil, MPhil. Department of Pure and Applied Sciences Portmore
Additional Calculations: 10. How many joules are required to change the temperature of 80.0 g of water from 23.3 C to 38.8 C?
 Additional Calculations: 10. How many joules are required to change the temperature of 80.0 g of water from 23.3 C to 38.8 C? q = m C T 80 g (4.18 J/gC)(38.8-23.3C) = 5183 J 11. A piece of metal weighing
Additional Calculations: 10. How many joules are required to change the temperature of 80.0 g of water from 23.3 C to 38.8 C? q = m C T 80 g (4.18 J/gC)(38.8-23.3C) = 5183 J 11. A piece of metal weighing
3.2 Calorimetry and Enthalpy
 3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
Experiment 12 Determination of an Enthalpy of Reaction, Using Hess s Law
 Experiment 12 Determination of an Enthalpy of Reaction, Using Hess s Law Object: To measure the standard heat of formation, f, of MgO (s), and to become familiar with calorimetry as a toll for measuring
Experiment 12 Determination of an Enthalpy of Reaction, Using Hess s Law Object: To measure the standard heat of formation, f, of MgO (s), and to become familiar with calorimetry as a toll for measuring
THE ENERGY OF THE UNIVERSE IS CONSTANT.
 Chapter 6 Thermochemistry.notebook Chapter 6: Thermochemistry Jan 29 1:37 PM 6.1 The Nature of Energy Thermodynamics: The study of energy and its interconversions Energy: the capacity to do work or to
Chapter 6 Thermochemistry.notebook Chapter 6: Thermochemistry Jan 29 1:37 PM 6.1 The Nature of Energy Thermodynamics: The study of energy and its interconversions Energy: the capacity to do work or to
Unit 15 Energy and Thermochemistry Notes
 Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Measuring and Expressing Enthalpy Changes. Copyright Pearson Prentice Hall. Measuring and Expressing Enthalpy Changes. Calorimetry
 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
June Which is a closed system? (A) burning candle (B) halogen lightbulb (C) hot water in a sink (D) ripening banana
 June 2005 28. Which is a closed system? burning candle halogen lightbulb hot water in a sink ripening banana 29. Which involves the greatest energy change? chemical reaction nuclear reaction phase change
June 2005 28. Which is a closed system? burning candle halogen lightbulb hot water in a sink ripening banana 29. Which involves the greatest energy change? chemical reaction nuclear reaction phase change
Thermodynamics- Chapter 19 Schedule and Notes
 Thermodynamics- Chapter 19 Schedule and Notes Date Topics Video cast DUE Assignment during class time One Review of thermodynamics ONE and TWO Review of thermo Wksheet Two 19.1-4; state function THREE
Thermodynamics- Chapter 19 Schedule and Notes Date Topics Video cast DUE Assignment during class time One Review of thermodynamics ONE and TWO Review of thermo Wksheet Two 19.1-4; state function THREE
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
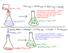 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
