The chemical bond The topological approach
|
|
|
- Hugh Hall
- 5 years ago
- Views:
Transcription
1 The chemical bond The topological approach Julia Contreras-García 1. Why and how studying chemical bonds? 2. Topology a) critical points b) topological partitions 3. Chemical functions a) electron density b) ELF c) 4. Applications Why studying chemical bonds? 1
2 Motivation Why studying the chemical bond in solids? Many solid chemists have isolated themselves from their organic or even inorganic colleagues by choosing not to see bonds in their materials. One is unlikely to understand new materials with novel properties if one is wearing purely chemical or physical blinkers. We should aim at a coupled approach a chemical understanding of bonding merged with a deep physical description. Nobel Prize Winner Prof. Roald Hoffmann How studying chemical bonds? Is reconciliation between classical and quantum chemistry possible? Quantum Chem System:Global Concepts: rigurous, QUANTITATIVE Classical Chem System: Atoms, Bonds Concepts: empirical, QUALITATIVE We will see one of these way-outs: the topological approach 6 2
3 The topological approach reconciles quantum and classical chemistry through a real space description of the system and its partition into chemically meaninful (a la classical) parts Quantum Chem System:Global Concepts: rigurous, QUANTITATIVE Real space Classical Chem System: Atoms, Bonds Concepts: empirical, QUALITATIVE It provides a mathematical definition of atoms in a molecule, bonds, etc. 7 QCT in a nutshell Quantum topology Classical Chemistry 3D Quantum chemistry QM Ψ Chemical function 3D f [Ψ](r) Topology Shape of f max, min, V, q Why studying chemical bonds? 2. Topology a) critical points b) topological partitions 3. Chemical functions a) electron density b) ELF c) 4. Application to solid state 3
4 Study of a function: 1D Example: f is a cubic function given by f (x) = x 3 Analyse f 10 Study of a function: 1D Example: f is a cubic function given by f (x) = 3x 2 f (x) = 0 -> x=0 There is a critical point at x=0 f (x) = 6x f (0)=0 -> saddle point 11 Study of a function: 1D 12 4
5 There are 4 types of CPs in 3D Maximum Saddle point of order 1 13 There are 4 types of CPs in 3D Saddle point of order 2 Minimum 14 There are 4 types of CPs in 3D (3,-3) 1st order saddle (3,-1) 2nd order saddle (3,+1) (3,+3) 15 5
6 Topology Critical points Topological partition 16 Topological partitions are intuitive 17 Topological partitions are intuitive We automatically Identify cusps Divide through the valleys Identify the lowest point in the valley 6
7 Topological partitions are intuitive We automatically Identify cusps Divide through the valleys Identify the lowest point in the valley Use this information to see three regions Meaning is inherited These regions contain orography information How is it done mathematically? We follow the zero gradient path 20 IMPORTANT PROPERTIES Each maximum has an associated region of space (basin) Non overlapping They fill up the volume R 3 R 3 7
8 QCT in a nutshell Topology Chemically sound functions Quantum chemical Topology (QTC) Chemical functions Electron density ELF 23 The density ρ(r) is a fundamental property of any electronic system ρ r r r r r r ) = 2 N... ( x, x,..., x ) dsdx... Ψ ( 1 2 N 2 x N It condenses all the information of the system (Hohenberg-Kohn theorem) 24 8
9 Electron density is maximal at the nuclei H H 25 The electron density (3,-1) occur at chemical bonds Plane that contains the nuclei Plane perpendicular to the internuclear line at the critical point that contains the nuclei 26 The density 3D Density Density isosurfaces 27 9
10 The density 3D Density C x H x C Density isosurfaces Saddle point=bond 28 We can predict the presence of strong bonds thanks to the topology of the density QTAIM C-H bcp C-C bcp Ring cp C H Density ρ topology Chemical structure Maxima = nuclei Bond = 1 st order saddle point (bcp) 29 Old concepts Li I Contact Polyhedra Electron density basins 30 10
11 Controversial cases It recovers the molecular graph: atoms and bonds (3,+1) 31 Quantification Each region has its own properties, which can be obtained by integration of density properties within each region E.g. if we integrate the density within a volume defined by ELF as a bond, we will know the bond charge q bond ρdv = Ω bond These properties are additive recover the molecular value N = allspace ρdv = i Ω ρdv i 32 Electron density Each maximum has an associated region of space (basin) Non overlapping They fill up the volume Li H R 3 R
12 Electron density Each maximum has an associated region of space (basin) Non overlapping They fill up the volume Have a chemical meaning Li Li H H R 3 R 3 34 Electron density Each maximum has an associated region of space (basin) Non overlapping We can integrate properties! They fill up the volume Have a chemical meaning Li 2e Li H H 2e R 3 R 3 35 Chemical functions Electron density ELF 36 12
13 The Electron Localization Function (ELF) Defined by Becke and Edgecombe, 1990 It can be interpreted as an excess of local kinetic energy density due to Pauli repulsion. r r 1 ρ( r ) t ( ) = t( ) P r 8 ( ) t ( r ρ r ) P χ( ) = r ELF = 5 3 c ρ( ) F 2 1 r 2 ( 1+ χ ( r )) 37 The Electron Localization Function (ELF) It recovers the Lewis picture of a system ELF is close to one in: Atomic shells 38 The Electron Localization Function (ELF) It recovers the Lewis picture of a system H 3 C-CH 3 ELF is close to one in: Atomic shells Bonds 39 13
14 The Electron Localization Function (ELF) It recovers the Lewis picture of a system HCΞCH ELF is close to one in: Atomic shells Bonds H 2 C=CH 2 H 3 C-CH 3 40 The Electron Localization Function (ELF) It recovers the Lewis picture of a system ELF is close to one in: Atomic shells Bonds Lone pairs H 2 O
15 ELF critical points 43 ELF and Lewis Example CH 3 OH H H C H O H Lewis structure 44 ELF and Lewis 45 15
16 ELF and Lewis Basin population N i = ρ (r ) dr Ω ELF N C core 2.12 O core 2.22 Bond C-H 2.04 Bond O-H 1.66 LP O 2.34 Bond C-O 1.22 V(C, H) 2.04 C(C) 2.12 V(C, O) 1.22 V(O, H) 1.66 V(O) 2.34 C(O) ELF and VSEPR Basin size V = i dr Ω ELF V C core 0.13 O core 0.05 Bond C-H Bond O-H 6.8 LP O 8.6 Bond C-O 0.9 Core < Bond < Lone pair V(C, H) 11.7 C(C) 0.13 V(C, O) 0.9 V(O, H) 6.8 V(O) 8.6 C(O) Chemical functions Electron density ELF 48 16
17 The electron density alone is not able to provide a satisfactory picture of weak bonds Density Critical points Unsolved issue Pair interactions are not well described by critical points 49 s = c F ρ 4 / 3 ρ is a method for the visualization of non covalent interactions based on the analysis of reduced density gradient at low densities J. Am. Chem. Soc. 132, 6498 (2010) Free densities s = 1 2 1/ 3 2(3π ) ρ ρ 4 / 3 s( ρ) ρ 1/ 3 ρ 0 Atomic tails J. Am. Chem. Soc. 132, 6498 (2010) 17
18 Interacting densities s = 1 2 1/ 3 2(3π ) ρ ρ 4 / 3 r 0 / 3 3 ( ) R s ρ e r + O[ r] 0 Free tails Non covalent region J. Am. Chem. Soc. 132, 6498 (2010) attractive repulsive J. Am. Chem. Soc. 132, 6498 (2010) Distinguish interaction type 1 Density is proportional to the strength of the interaction Hydrogen bond Very weak interactions ρ ~0 Weak interactions low ρ vdw Steric clash 18
19 Distinguish interaction type 2 Sign of the second eigenvalue Bonding interactions charge accumulation (λ H < 0) Antibonding interactions charge depletion (λ H > 0) λ H < 0 λ H = 0 s λ H < 0 Density accumulation λ H > 0 Density depletion λ H > 0 λ H ~ 0 s sign(λ 2 ) ρ ρ J. Am. Chem. Soc. 132, 6498 (2010) 1) We distinghish 3 ranges of interaction 2) We colour the isosurfaces in a continuum 3-range scheme ρ > 0 λ H < 0 ρ increasing ρ 0 λ H 0 ρ decreasing ρ > 0 λ H > 0 H- bonds Van der Waals Steric clashes Strongly attractive J. Am. Chem. Soc. 132, 6498 (2010) Very weakly attractive Strongly repulsive H- bonds Van der Waals Steric clashes J. Am. Chem. Soc. 132, 6498 (2010) 19
20 H- bonds Van der Waals Steric clashes J. Am. Chem. Soc. 132, 6498 (2010) Summary We have complementary functions to describe all the chemical entities of our systems The density, which usually has a one-to-one correspondence with the atoms in the system However, the charge density alone does not describe bonding in its entirety, especially the mechanism of electron pairing: we use ELF However, the density and ELF do not show non covalent interactions, foundamental in molecular crystals: 59 Method AIM ELF Function density Pauli kinetic energy density Chemical meaning Critical points Reduced density gradient Atoms Lewis pairs Non covalent interactions Maxima=atoms Maxima=Lewis pairs Minima=s Regions Atoms Lewis pairs Non covalent interactions ELF = 0.9 s = 0.6, ρ <
21 Applications Reactivity Protein structure New materials 61 Reactivity Following organic reactions Proteins Polipeptide: α helix with 15 alanine residues Hydrogen bonds stabilize the helix Big region of van der Waals interaction inside the helix and between methyle lateral chains one step away 63 21
22 Proteins HB between C=O and N-H groups in parallel chains Van der Waals interactions between CH 2 groups 64 DNA C-G H-bonds between bases A-T Crystallographic coordinates π-stacking 19/01/2017 J. Chem. Theor. Comp. 7, 625 (2011) 65 New materials : Electronic structure of high pressure metals Under pressure, solids exhibit increasingly shorter interatomic distances. Intuitively, this response is expected to be accompanied by an increase in the widths of the valence and conduction bands and hence a more pronounced free-electronlike behavior. However, recent experiments have shown a pressure-induced transformation of Na into an optically transparent and insulating phase at 200 GPa (5.0-fold compression) What is the electronic structure behind this new state of matter? Phys. Rev. Lett. 103, (2009) 66 22
23 Na Localization of valence electrons is again observed under pressure ci16 Op8 Hp4 pressure Phys. Rev. B 83, (2011) 67 K Same structure as A 2 X and AX 2 compounds! Electrons in same position as anions! COUMPOUND IONIC-LIKE ELECTRONS PSEUDOANIONS ELF=0.3 ELF= GPa Phys. Rev. Lett. 103, (2009) 68 Thanks to the topological approach we have been able to Understand the electronic structure of a new class of materials beyond historical classifications of materials types and bonding types. We have understood the microscopic nature of a new electronic property (insulating state of metals) We have connected strong and weak chemical bonding with reactivity We have shown that weak interactions enable us to foresee the outcome of a reaction mechanism We have visualized the interactions that stabilize protein structure and provide them with different properties and functionalities 69 23
Atom in Molecules a Quantum Theory (AIM)
 Atom in Molecules a Quantum Theory (AIM) Richard F. W. Bader* The primary purpose in postulating the existence of atoms in molecules is a consequence of the observation that atoms or groupings of atoms
Atom in Molecules a Quantum Theory (AIM) Richard F. W. Bader* The primary purpose in postulating the existence of atoms in molecules is a consequence of the observation that atoms or groupings of atoms
On the topology of the reduced density gradient. Roberto A. Boto, Julia Contreras García, Julien Tierny and Jean-Philip Piquemal
 On the topology of the reduced density gradient Roberto A. Boto, Julia Contreras García, Julien Tierny and Jean-Philip Piquemal ICS-UPMC, Paris, France LCT, UMR 7616, UPMC Univ July 10, 2015 Roberto A.
On the topology of the reduced density gradient Roberto A. Boto, Julia Contreras García, Julien Tierny and Jean-Philip Piquemal ICS-UPMC, Paris, France LCT, UMR 7616, UPMC Univ July 10, 2015 Roberto A.
Lecture 4: Band theory
 Lecture 4: Band theory Very short introduction to modern computational solid state chemistry Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonding in Reciprocal space
Lecture 4: Band theory Very short introduction to modern computational solid state chemistry Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonding in Reciprocal space
Lecture 2: Bonding in solids
 Lecture 2: Bonding in solids Electronegativity Van Arkel-Ketalaar Triangles Atomic and ionic radii Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonds in Reciprocal space
Lecture 2: Bonding in solids Electronegativity Van Arkel-Ketalaar Triangles Atomic and ionic radii Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonds in Reciprocal space
Topic 2. Structure and Bonding Models of Covalent Compounds of p-block Elements
 Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Downloaded from
 Remedial action plan :- Chemical bonding # focus on important topic 1. Octet rule 2. Lewis structure Q.7. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly.
Remedial action plan :- Chemical bonding # focus on important topic 1. Octet rule 2. Lewis structure Q.7. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly.
Big Idea #5: The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter.
 KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
Chemical bonding in solids from ab-initio Calculations
 Chemical bonding in solids from ab-initio Calculations 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India & Center for Materials Science and Nanotechnology, University
Chemical bonding in solids from ab-initio Calculations 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India & Center for Materials Science and Nanotechnology, University
QUANTUM MECHANICS AND MOLECULAR STRUCTURE
 6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
1. What is the formula for the compound formed by calcium and nitrogen?
 IB Chem 1 Name Topic 4 Bonding - Sample Test Problems 1. What is the formula for the compound formed by calcium and nitrogen? A. CaN B. Ca 2 N C. Ca 2 N 3 D. Ca 3 N 2 2. Element X is in group 2, and element
IB Chem 1 Name Topic 4 Bonding - Sample Test Problems 1. What is the formula for the compound formed by calcium and nitrogen? A. CaN B. Ca 2 N C. Ca 2 N 3 D. Ca 3 N 2 2. Element X is in group 2, and element
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester Christopher J. Cramer. Lecture 30, April 10, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
The wavefunction that describes a bonding pair of electrons:
 4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
Chapter 3. Crystal Binding
 Chapter 3. Crystal Binding Energy of a crystal and crystal binding Cohesive energy of Molecular crystals Ionic crystals Metallic crystals Elasticity What causes matter to exist in three different forms?
Chapter 3. Crystal Binding Energy of a crystal and crystal binding Cohesive energy of Molecular crystals Ionic crystals Metallic crystals Elasticity What causes matter to exist in three different forms?
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Chapter 10 Chemical Bonding II
 Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Lone Pairs: An Electrostatic Viewpoint
 S1 Supporting Information Lone Pairs: An Electrostatic Viewpoint Anmol Kumar, Shridhar R. Gadre, * Neetha Mohan and Cherumuttathu H. Suresh * Department of Chemistry, Indian Institute of Technology Kanpur,
S1 Supporting Information Lone Pairs: An Electrostatic Viewpoint Anmol Kumar, Shridhar R. Gadre, * Neetha Mohan and Cherumuttathu H. Suresh * Department of Chemistry, Indian Institute of Technology Kanpur,
Hybridisation of Atomic Orbitals
 Lecture 7 CHEM101 Hybridisation of Atomic Orbitals Dr. Noha Osman Learning Outcomes Understand the valence bond theory Understand the concept of hybridization. Understand the different types of orbital
Lecture 7 CHEM101 Hybridisation of Atomic Orbitals Dr. Noha Osman Learning Outcomes Understand the valence bond theory Understand the concept of hybridization. Understand the different types of orbital
Can atomic orbitals explain these shapes or angles? What s in Chapter 9: Shapes of molecules affect: reactivity physical properties
 What s in Chapter 9: Can atomic orbitals explain these shapes or angles? Shapes of molecules affect: reactivity physical properties Shapes of molecules explained by: VSEPR Valence bond theory Why molecules
What s in Chapter 9: Can atomic orbitals explain these shapes or angles? Shapes of molecules affect: reactivity physical properties Shapes of molecules explained by: VSEPR Valence bond theory Why molecules
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Lecture B6 Molecular Orbital Theory. Sometimes it's good to be alone.
 Lecture B6 Molecular Orbital Theory Sometimes it's good to be alone. Covalent Bond Theories 1. VSEPR (valence shell electron pair repulsion model). A set of empirical rules for predicting a molecular geometry
Lecture B6 Molecular Orbital Theory Sometimes it's good to be alone. Covalent Bond Theories 1. VSEPR (valence shell electron pair repulsion model). A set of empirical rules for predicting a molecular geometry
Carbon and Its Compounds
 Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
***Occurs when atoms of elements combine together to form compounds.*****
 CHEMICAL BONDING ***Occurs when atoms of elements combine together to form compounds.***** Formation of compounds involve adjustments in the position of one or more valence electrons. PE is lower in bonded
CHEMICAL BONDING ***Occurs when atoms of elements combine together to form compounds.***** Formation of compounds involve adjustments in the position of one or more valence electrons. PE is lower in bonded
Electrons and Molecular Forces
 Electrons and Molecular Forces Chemistry 30 Ms. Hayduk Electron Configuration Atomic Structure Atomic Number Number of protons in the nucleus Defines the element Used to organize the periodic table 1 Bohr
Electrons and Molecular Forces Chemistry 30 Ms. Hayduk Electron Configuration Atomic Structure Atomic Number Number of protons in the nucleus Defines the element Used to organize the periodic table 1 Bohr
Atomic structure & interatomic bonding. Chapter two
 Atomic structure & interatomic bonding Chapter two 1 Atomic Structure Mass Charge Proton 1.67 х 10-27 kg + 1.60 х 10-19 C Neutron 1.67 х 10-27 kg Neutral Electron 9.11 х 10-31 kg - 1.60 х 10-19 C Electron
Atomic structure & interatomic bonding Chapter two 1 Atomic Structure Mass Charge Proton 1.67 х 10-27 kg + 1.60 х 10-19 C Neutron 1.67 х 10-27 kg Neutral Electron 9.11 х 10-31 kg - 1.60 х 10-19 C Electron
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes. Reference: Chapter 9 10 in textbook
 PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes Reference: Chapter 9 10 in textbook 1 Valence Electrons Valence ae Electron Define: the outer shell electrons Important for determination
PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes Reference: Chapter 9 10 in textbook 1 Valence Electrons Valence ae Electron Define: the outer shell electrons Important for determination
The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals.
 Physical Metallurgy The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals. Crystal Binding In our discussions
Physical Metallurgy The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals. Crystal Binding In our discussions
VSEPR Model. Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds
 Molecular Shapes VSEPR Model Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds They repel each other and stay as far away from each other as
Molecular Shapes VSEPR Model Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds They repel each other and stay as far away from each other as
AIM Bond Concepts. U A bond is defined along the bond line between two nuclei, called a bond path, along which electron density is concentrated.
 AIM Bond Concepts U A bond is defined along the bond line between two nuclei, called a bond path, along which electron density is concentrated. U The bond critical point, ρ b, is a point along the bond
AIM Bond Concepts U A bond is defined along the bond line between two nuclei, called a bond path, along which electron density is concentrated. U The bond critical point, ρ b, is a point along the bond
Molecular Geometries. Molecular Geometries. Remember that covalent bonds are formed when electrons in atomic orbitals are shared between two nuclei.
 Molecular Geometries Lewis dot structures are very useful in determining the types of bonds in a molecule, but they may not provide the best insight into the spatial geometry of a molecule, i.e., how the
Molecular Geometries Lewis dot structures are very useful in determining the types of bonds in a molecule, but they may not provide the best insight into the spatial geometry of a molecule, i.e., how the
Carbon Compounds. Electronegativity. Chemical Bonding Part 1c. Bond Polarity. Bond Polarity
 Electronegativity Carbon Compounds Electronegativity is a relative measure on the pull of electrons by an atom in a bond. Most bonds fall somewhere in between and these bonds are considered polar. Chemical
Electronegativity Carbon Compounds Electronegativity is a relative measure on the pull of electrons by an atom in a bond. Most bonds fall somewhere in between and these bonds are considered polar. Chemical
Chapter 7. Chemical Bonding I: Basic Concepts
 Chapter 7. Chemical Bonding I: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 7. Chemical Bonding I: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
of its physical and chemical properties.
 8.4 Molecular Shapes VSEPR Model The shape of a molecule determines many of its physical and chemical properties. Molecular l geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion
8.4 Molecular Shapes VSEPR Model The shape of a molecule determines many of its physical and chemical properties. Molecular l geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Organic Chemistry. Review Information for Unit 1. Atomic Structure MO Theory Chemical Bonds
 Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
Chapter 8. Molecular Shapes. Valence Shell Electron Pair Repulsion Theory (VSEPR) What Determines the Shape of a Molecule?
 PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
ELEMENTARY BAND THEORY
 ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Can you see atoms? M
 Can you see atoms? http://www.youtube.com/watch?v=s_okfvbzd9 M 2. Life requires about 25 chemical elements About 25 of the 92 natural elements are known to be essential for life. Four elements - carbon
Can you see atoms? http://www.youtube.com/watch?v=s_okfvbzd9 M 2. Life requires about 25 chemical elements About 25 of the 92 natural elements are known to be essential for life. Four elements - carbon
Andrew Rosen *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same
 *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
*Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
Unit 3 - Chemical Bonding and Molecular Structure
 Unit 3 - Chemical Bonding and Molecular Structure Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction
Unit 3 - Chemical Bonding and Molecular Structure Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction
Ligand Close-Packing Model
 Ligand Close-Packing Model! VSEPR is an electronic model for explaining molecular geometry.! The Ligand Close-Packing (LCP) model is a steric model. L The geometry of an AX n molecule is that which allows
Ligand Close-Packing Model! VSEPR is an electronic model for explaining molecular geometry.! The Ligand Close-Packing (LCP) model is a steric model. L The geometry of an AX n molecule is that which allows
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Valence Bond Theory. Localized Electron Model. Hybridize the Orbitals! Overlap and Bonding. Atomic Orbitals are. mmmkay. Overlap and Bonding
 Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
like carbon, has fewer than an octet. It is simply less likely but still imperative to draw.
 Andrew Rosen Chapter 1: The Basics - Bonding and Molecular Structure 1.1 - We Are Stardust - Organic chemistry is simply the study of carbon-based compounds, hydrocarbons, and their derivatives, which
Andrew Rosen Chapter 1: The Basics - Bonding and Molecular Structure 1.1 - We Are Stardust - Organic chemistry is simply the study of carbon-based compounds, hydrocarbons, and their derivatives, which
c. Ionic bonding d. Covalent bonding i. nonpolar covalent bonding
 Chapter 11: Chemical Bonding I. Introduction to Chemical Bonding a. Types of chemical bonding i. A chemical bond is a mutual attraction between nuclei and the valence electrons of different atoms that
Chapter 11: Chemical Bonding I. Introduction to Chemical Bonding a. Types of chemical bonding i. A chemical bond is a mutual attraction between nuclei and the valence electrons of different atoms that
Paper No. 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry)
 Subject Chemistry Paper No and Title Paper 1: ORGANIC - I (Nature of Bonding Module No and Title Module Tag CHE_P1_M10 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Non-Covalent Interactions
Subject Chemistry Paper No and Title Paper 1: ORGANIC - I (Nature of Bonding Module No and Title Module Tag CHE_P1_M10 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Non-Covalent Interactions
AP Chemistry Chapter 7: Bonding
 AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
Chapter 8. Bonding : General Concepts Chemical Bondings
 Chapter 8. onding : General Concepts Chemical ondings create Diversity in the Universe Why and how do they make chemical bonds? and what do they make? Types of Chemical onds Chemical bonds: orces that
Chapter 8. onding : General Concepts Chemical ondings create Diversity in the Universe Why and how do they make chemical bonds? and what do they make? Types of Chemical onds Chemical bonds: orces that
Constitutional isomers. Two bracelets with the same structural parts assembled differently
 Constitutional isomers 1 6 6 Two bracelets with the same structural parts assembled differently Constitutional isomers C C N C C N C 2 7 N Two molecules with the same constituent atoms assembled differently
Constitutional isomers 1 6 6 Two bracelets with the same structural parts assembled differently Constitutional isomers C C N C C N C 2 7 N Two molecules with the same constituent atoms assembled differently
Lecture 32: The Periodic Table
 Lecture 32: The Periodic Table (source: What If by Randall Munroe) PHYS 2130: Modern Physics Prof. Ethan Neil (ethan.neil@colorado.edu) Announcements Homework #9 assigned, due next Wed. at 5:00 PM as usual.
Lecture 32: The Periodic Table (source: What If by Randall Munroe) PHYS 2130: Modern Physics Prof. Ethan Neil (ethan.neil@colorado.edu) Announcements Homework #9 assigned, due next Wed. at 5:00 PM as usual.
Atoms & Their Interactions
 Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 8 Covalent Boding
 Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Ionic and Covalent Bonding
 1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
Big Idea: Ionic Bonds: Ionic Bonds: Metals: Nonmetals: Covalent Bonds: Ionic Solids: What ions do atoms form? Electron Electron
 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS
 1 CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS PERIODIC TRENDS: See pages 214-216, 221 Table 11.3, and 227 + 228 of text. Lewis Structures of Atoms: The Lewis Dot Diagram
1 CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS PERIODIC TRENDS: See pages 214-216, 221 Table 11.3, and 227 + 228 of text. Lewis Structures of Atoms: The Lewis Dot Diagram
General Physical Chemistry II
 General Physical Chemistry II Lecture 10 Aleksey Kocherzhenko October 7, 2014" Last time " promotion" Promotion and hybridization" [He] 2s 2 2p x 1 2p y 1 2p z0 " 2 unpaired electrons" [He] 2s 1 2p x 1
General Physical Chemistry II Lecture 10 Aleksey Kocherzhenko October 7, 2014" Last time " promotion" Promotion and hybridization" [He] 2s 2 2p x 1 2p y 1 2p z0 " 2 unpaired electrons" [He] 2s 1 2p x 1
Shapes of Molecules VSEPR
 Shapes of Molecules In this section we will use Lewis structures as an introduction to the shapes of molecules. The key concepts are: Electron pairs repel each other. Electron pairs assume orientations
Shapes of Molecules In this section we will use Lewis structures as an introduction to the shapes of molecules. The key concepts are: Electron pairs repel each other. Electron pairs assume orientations
Lecture B1 Lewis Dot Structures and Covalent Bonding
 Lecture B1 Lewis Dot Structures and Covalent Bonding G.N. Lewis & Linus Pauling Two American Chemists G. N. Lewis 1875-1946 Linus Pauling 1901-1994 The Covalent Bond 1. First proposed by G.N. Lewis in
Lecture B1 Lewis Dot Structures and Covalent Bonding G.N. Lewis & Linus Pauling Two American Chemists G. N. Lewis 1875-1946 Linus Pauling 1901-1994 The Covalent Bond 1. First proposed by G.N. Lewis in
CHEMISTRY Topic #1: Bonding What Holds Atoms Together? Spring 2012 Dr. Susan Lait
 CHEMISTRY 2000 Topic #1: Bonding What Holds Atoms Together? Spring 2012 Dr. Susan Lait Why Do Bonds Form? An energy diagram shows that a bond forms between two atoms if the overall energy of the system
CHEMISTRY 2000 Topic #1: Bonding What Holds Atoms Together? Spring 2012 Dr. Susan Lait Why Do Bonds Form? An energy diagram shows that a bond forms between two atoms if the overall energy of the system
Molecular Structure. Valence Bond Theory Overlap of atomic orbitals is a covalent bond that joins atoms together to form a molecule
 Molecular Structure Topics 3-D structure shape (location of atoms in space) Molecular Geometry Valence Bond Theory Hybrid Orbitals Multiple Bonds VSEPR (Valence Shell Electron Pair Repulsion) Valence Bond
Molecular Structure Topics 3-D structure shape (location of atoms in space) Molecular Geometry Valence Bond Theory Hybrid Orbitals Multiple Bonds VSEPR (Valence Shell Electron Pair Repulsion) Valence Bond
Chapter 13: Phenomena
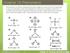 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
UNIT TWO BOOKLET 1. Molecular Orbitals and Hybridisation
 DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT TWO BOOKLET 1 Molecular Orbitals and Hybridisation In the inorganic unit we learned about atomic orbitals and how they could be used to write the electron
DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT TWO BOOKLET 1 Molecular Orbitals and Hybridisation In the inorganic unit we learned about atomic orbitals and how they could be used to write the electron
Chapter 14: Phenomena
 Chapter 14: Phenomena p p Phenomena: Scientists knew that in order to form a bond, orbitals on two atoms must overlap. However, p x, p y, and p z orbitals are located 90 from each other and compounds like
Chapter 14: Phenomena p p Phenomena: Scientists knew that in order to form a bond, orbitals on two atoms must overlap. However, p x, p y, and p z orbitals are located 90 from each other and compounds like
Molecular Geometry and Bonding Theories. Molecular Shapes. Molecular Shapes. Chapter 9 Part 2 November 16 th, 2004
 Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Quantum Topology of the Charge density of Chemical Bonds. QTAIM analysis of the C-Br and O-Br bonds.
 Procedia Computer Science Volume 51, 2015, Pages 1872 1877 ICCS 2015 International Conference On Computational Science Quantum Topology of the Charge density of Chemical Bonds. QTAIM analysis of the C-Br
Procedia Computer Science Volume 51, 2015, Pages 1872 1877 ICCS 2015 International Conference On Computational Science Quantum Topology of the Charge density of Chemical Bonds. QTAIM analysis of the C-Br
Atomic and Molecular Dimensions
 1 Atomic and Molecular Dimensions Equilibrium Interatomic Distances When two atoms approach each other, their positively charged nuclei and negatively charged electronic clouds interact. The total interaction
1 Atomic and Molecular Dimensions Equilibrium Interatomic Distances When two atoms approach each other, their positively charged nuclei and negatively charged electronic clouds interact. The total interaction
The solid state. Ga Ge As Se Br d 10 4s 2. Sn Xe 1.49 I Sb Te In d 10 5s 2. Pb 0.
 Molecular shape The shapes of molecules: van t Hoff (1874): CH 4 tetrahedron Werner (1893): Pt(NH 3 ) 2 Cl 2 planar Lewis (1915): Electron pairs and octets Sidgwick and Powell (1940): Foundations of Valence
Molecular shape The shapes of molecules: van t Hoff (1874): CH 4 tetrahedron Werner (1893): Pt(NH 3 ) 2 Cl 2 planar Lewis (1915): Electron pairs and octets Sidgwick and Powell (1940): Foundations of Valence
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Earth Solid Earth Rocks Minerals Atoms. How to make a mineral from the start of atoms?
 Earth Solid Earth Rocks Minerals Atoms How to make a mineral from the start of atoms? Formation of ions Ions excess or deficit of electrons relative to protons Anions net negative charge Cations net
Earth Solid Earth Rocks Minerals Atoms How to make a mineral from the start of atoms? Formation of ions Ions excess or deficit of electrons relative to protons Anions net negative charge Cations net
CHEMICAL BONDING IN MATTER Text p ) Chemical Bonds are attractive electrostatic forces that hold atoms or ions together in a substance.
 CHEMICAL BONDING IN MATTER Text p. 162 164) Chemical Bonds are attractive electrostatic forces that hold atoms or ions together in a substance. Bohr's Model of the Atom protons and neutrons are located
CHEMICAL BONDING IN MATTER Text p. 162 164) Chemical Bonds are attractive electrostatic forces that hold atoms or ions together in a substance. Bohr's Model of the Atom protons and neutrons are located
Lab: Model Building with Covalent Compounds - Introduction
 Name Date Period # Lab: Model Building with Covalent Compounds - Introduction Most of our learning is in two dimensions. We see pictures in books and on walls and chalkboards. We often draw representations
Name Date Period # Lab: Model Building with Covalent Compounds - Introduction Most of our learning is in two dimensions. We see pictures in books and on walls and chalkboards. We often draw representations
Structural Bioinformatics (C3210) Molecular Mechanics
 Structural Bioinformatics (C3210) Molecular Mechanics How to Calculate Energies Calculation of molecular energies is of key importance in protein folding, molecular modelling etc. There are two main computational
Structural Bioinformatics (C3210) Molecular Mechanics How to Calculate Energies Calculation of molecular energies is of key importance in protein folding, molecular modelling etc. There are two main computational
Bonding in Chemistry. Chemical Bonds All chemical reactions involve breaking of some bonds and formation of new ones where new products are formed.
 CHEMICAL BONDS Atoms or ions are held together in molecules or compounds by chemical bonds. The type and number of electrons in the outer electronic shells of atoms or ions are instrumental in how atoms
CHEMICAL BONDS Atoms or ions are held together in molecules or compounds by chemical bonds. The type and number of electrons in the outer electronic shells of atoms or ions are instrumental in how atoms
Unit Six --- Ionic and Covalent Bonds
 Unit Six --- Ionic and Covalent Bonds Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples
Unit Six --- Ionic and Covalent Bonds Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples
Intramolecular Bonding. Chapters 4, 12 Chemistry Mr. McKenzie
 Intramolecular Bonding Chapters 4, 12 Chemistry Mr. McKenzie What determines the type of intramolecular bond? An intramolecular bond is any force that holds two atoms together to form a compound; 3 types
Intramolecular Bonding Chapters 4, 12 Chemistry Mr. McKenzie What determines the type of intramolecular bond? An intramolecular bond is any force that holds two atoms together to form a compound; 3 types
Question 1.1. Electron Configurations Noble Gases and The Rule of Eight. Chapter 1
 ~ 0.1 nm Chapter 1 Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Acids and Bases Nucleus = 1/10,000 of the atom 1 nm = 10
~ 0.1 nm Chapter 1 Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Acids and Bases Nucleus = 1/10,000 of the atom 1 nm = 10
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed.
 Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Critical Temperature - the temperature above which the liquid state of a substance no longer exists regardless of the pressure.
 Critical Temperature - the temperature above which the liquid state of a substance no longer exists regardless of the pressure. Critical Pressure - the vapor pressure at the critical temperature. Properties
Critical Temperature - the temperature above which the liquid state of a substance no longer exists regardless of the pressure. Critical Pressure - the vapor pressure at the critical temperature. Properties
VALENCE Hilary Term 2018
 VALENCE Hilary Term 2018 8 Lectures Prof M. Brouard Valence is the theory of the chemical bond Outline plan 1. The Born-Oppenheimer approximation 2. Bonding in H + 2 the LCAO approximation 3. Many electron
VALENCE Hilary Term 2018 8 Lectures Prof M. Brouard Valence is the theory of the chemical bond Outline plan 1. The Born-Oppenheimer approximation 2. Bonding in H + 2 the LCAO approximation 3. Many electron
Lesmahagow High School AHChemistry Inorganic and Physical Chemistry Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Shapes of Molecules and Polyatomic Ions 1 Shapes of molecules Bonding and Electronegativity Revision A Covalent
Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Shapes of Molecules and Polyatomic Ions 1 Shapes of molecules Bonding and Electronegativity Revision A Covalent
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Its Bonding Time. Chemical Bonds CH 12
 Its Bonding Time Chemical Bonds CH 12 What is a chemical bond? Octet Rule: Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its
Its Bonding Time Chemical Bonds CH 12 What is a chemical bond? Octet Rule: Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Bonding Test pg 1 of 4 Name: Pd. Date:
 Bonding Test pg 1 of 4 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How many electrons are shared in a single covalent bond? 1. A) 2 B) 3 C)
Bonding Test pg 1 of 4 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How many electrons are shared in a single covalent bond? 1. A) 2 B) 3 C)
Name: Class: Date: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Name: Class: Date: SCH4U Chapter 4 Formative Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following statements about
Name: Class: Date: SCH4U Chapter 4 Formative Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following statements about
Chapter 7: Chemical Bonding and Molecular Structure
 Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
