Biochemistry 3300 Problems (and Solutions) Metabolism I
|
|
|
- Junior Maxwell
- 5 years ago
- Views:
Transcription
1 (1) Provide a reasonable systematic name for an enzyme that catalyzes the following reaction: fructose + ATP > fructose-1 phosphate + ADP (2) The IUBMB has a developed a set of rules for classifying enzymes based upon the type of reaction they catalyze. The classification scheme assigns an Enzyme Commission (EC) number to each enzyme. Answer the following questions regarding EC numbers and the class of reaction catalyzed: (a) What name is associated with class 4 enzymes and what type of reaction do they catalyze? (b) Oxidoreductases catalyze redox reactions. What class of enzymes are oxidoreductases? (c) What types of reactions are catalyzed by ligases? (3) List four characteristics that define a metabolic pathway. (4) Identify two amino acids that commonly act as nucleophiles in enzyme catalyzed reactions. (5) What is the difference between an (1) isomerization reaction and (2) a rearrangement? (6) Enzyme catalyzed reactions that make or break C-C bonds typically involve carbanion intermediates. One means of stabilizing a carbanion intermediate involves the formation of a Schiff's base. Draw a strcuture of : (a) a Schiff's base carbanion (b) the resonance stabilized Schiff's base in the eneamine form (7) How can metabolic inhibitors be used to determine the sequence of reactions in a metabolic pathway? (8) Isotopes of C, N, S, P and H have been extensively used to investigate metabolic pathways. Why? (9) The standard transformed free energy change for the following bimolecular reaction: Glucose + ATP ----> Glucose-6-phosphate + ADP is kj/mol. Consequently, this reaction is spontaneous under standard conditions. Under what conditions would this reaction NOT be spontaneous at 298K? (10) If an enzyme catalyzed the direct transfer of a phosphate to glucose Glucose + P i ---> Glucose-6-phosphate the standard, transformed free energy change would be kj/mol. Under what conditions would this reaction be favourable at 298K?
2 (11) Place an 'x' on each of the high energy bonds shown in the structures below. (12) Consider the oxidation-reduction reaction between cytochrome b and ubiquinol. (a) What is the G'º for the reduction of cytochrome b by ubiquinol? (Note: you will need to use the tables of standard reduction potentials) (b) Under what conditions will the reduction of cytochrome b by ubiquinol be favourable at 298K? (13) Indicate whether the following statements are true or false. If false, change a single word or number to make the statement true. (a) (6-13 C)Glucose (ie. C6 label) is converted to (1-13 C)Glyceraldehyde-3-phosphate during the preparatory phase of glycolysis. (b) Phosphoglycerate kinase requires catalytic amounts of 2,3-bisphosphoglycerate for activity. (c) Glyceraldehyde-3-phosphate dehydrogenase couples the addition of phosphate to glyceraldehyde-3-phosphate and the reduction of NAD +. (14) Identify the glycolytic enzyme with the following properties: (a) Forms a Schiff's base intermediate during its catalytic cycle. (b) Synthesized in an inactive form. (c) Is inhibited by alkylating agents such as iodoacetate. (d) Is a lyase. (15) Answer the following questions regarding glycolysis: (a) What is the overall chemical reaction for the 'preparatory' phase of glycolysis? (b) What is substrate-level phosphorylation? (c) Under cellular conditions, which glycolytic enzymes are likely to be regulatory targets? (d) Which glycolytic intermediates are 'high-energy' compounds?
3
4 Answers 1- Fructose:ATP phosphotransferase or Fructose:ATP kinase 2- (a) Lyases catalyze group eliminations that form double bonds (b) Class 1 (c) Bond formation coupled to ATP hydrolysis. 3- Metabolic pathways are (1) irreversible, (2) have a committed step, (3) are regulated and (4) catabolic and anabolic pathways differ. 4- Serine, threonine and cysteine are nucleophilic in their ionized form, while histidine and lysine are nucleophiles in their neutral-charged form. 5- Isomerization reactions inevitably involve the movement of an H atom without changing the carbon backbone while rearrangements involve changes to the carbon backbone. 6-(a) Schiff's base carbanion (b) Schiff's base eneamine form 7- Metabolic inhibitors specifically block a metabolic pathway and result in the accumulation of metabolic pathway intermediates. By identifying the metabolic pathway intermediate(s) that accumulate, one can identify the step in a metabolic pathway that has been inhibited. The use of several metabolic inhibitors that act at different points in the pathway (together with 'chemical intuition') is sufficient to propose and verify the sequence of reactions associated with a metabolic pathway. 8- Greatest difficulty in metabolism research is identifying the intermediates of a metabolic pathway. The large number of related compounds in an organism make the identification of metabolic intermediates difficult. Further, many metabolic intermediate are only present at very low levels making them difficult to detect. The use of isotopes (especially radioisotopes) can overcome this problem as they can be readily detected at very low concentrations and serve to 'tag' or 'label' each of the metabolic intermediates of a pathway. 9 - Reactions are only spontaneous when the G' < 0. Given our equation for calculating G': G' = G' + RT ln ([Products]/[Reactants]) 0 > G' + RT ln ([Products]/[Reactants]) for a spontaneous reaction 0 > kj mol J mol -1 K K ln ([Products]/[Reactants]) 16.7 kj mol -1 / 2.48 kj mol -1 > ln ([Products]/[Reactants])
5 6.73 > ln ([Products]/[Reactants]) e 6.73 > ([Products]/[Reactants]) > ([Products]/[Reactants]) When the ratio of Products to Reactants is greater than or equal to 837.1, the reaction will no longer be spontaneous. (Note, at exactly fold excess of products to reactants the reaction will be at equilibrium) 10 - This reaction will only be favourable when G' is negative. G' = G' + RT ln ([Products]/[Reactants]) 0 > G' + RT ln ([Products]/[Reactants]) for a spontaneous reaction 0 > 13.8 kj mol J mol -1 K K ln ([Products]/[Reactants]) kj mol -1 / 2.48 kj mol -1 > ln ([Products]/[Reactants]) > ln ([Products]/[Reactants]) e > ([Products]/[Reactants]) > ([Products]/[Reactants]) or < ([Reactants]/[Products]) While the reactant concentration is fold higher (or more) than the product concentration, the reaction will be favourable Please note that in the case of PEP (left) and 1,3-bisphosphoglycerate (top-middle) you could have placed the 'x' on the adjacent anhydride-like bond. 12- (a) Cyto b (ox) + e - cyto b (red) 0.077V ubiquinol + H 2 ubiquinone + 2e - + 2H V 2 Cyto b (ox) + ubiquinol + H 2 2 cyto b (red) + 2H V G'º = -nf E'º = -2 (96.5 kj/v mol) (0.032 V mol) = -6.2 kj/mol (b) There are two ways to solve this problem; using standard reduction potentials or using free energy changes.
6 G' = G' + RT ln ([Products]/[Reactants]) 0 > G' + RT ln ([Products]/[Reactants]) for a spontaneous reaction 0 > -6.2 kj mol J mol -1 K K ln ([Products]/[Reactants]) -6.2 kj mol -1 /2.48 kj mol -1 > ln ([Products]/[Reactants]) > ([Products]/[Reactants]) or 12.1[Reactants] > [Products] The reaction will no longer be favourable when the products are 12.1 times more concentrated than reactants. OR E' = E ' - (RT/nF) ln ([Products]/[Reactants]) 0 < E ' - (RT/nF) ln ([Products]/[Reactants]) for a spontaneous reaction 0 < V - (8.31 J mol -1 K K / (2) 96,480 J V -1 mol -1 ) ln ([Products]/[Reactants]) 0 < V ( V) ln ([Products]/[Reactants]) V/ V > ln ([Products]/[Reactants]) 12.1 > ([Products]/[Reactants]) or 12.1[Reactants] > [Products] The reaction will no longer be favourable when the products are 12.1 times more concentrated than reactants. 13- (a) False. Change 1 to 3. (b) False. Change kinase to mutase. (c) True. 14- (a) Aldolase catalyzes the cleavage of a C-C bond via a Schiff's base. (b) Phosphoglycerate Mutase must be activated by 2,3-bisphosphoglycerate. (c) Glyceraldehyde-3-phosphate dehydrogenase has an active site thiol. (d) Enolase forms a double bond while eliminating a H 2 O molecule Aldolase forms a ketone while eliminating glyceraldehyde-3-phosphate. 15- (a) Glucose + 2ATP 2 Glyceraldehyde-3-phosphate + 2ADP (b) P i is a substrate in the formation of a 'high-energy' phosphodiester. In glycolysis, glyceraldehyde-3-phosphate, P i and NAD + are converted to 1,3-bisphosphoglycerate and NADH + H +. Phosphate addition to the aldehyde of glyceraldehyde-3-phosphate generates a 'high-energy' phosphodiester bond in 1,3-bisphosphoglycerate. (c) Regulatory targets are typically enzymes that catalyze reactions with large G'. In the particular case of glycolysis, only hexokinase, phosphofructokinase and pyruvate kinase have large G's and are regulatory targets. Aldolase (unfavourable under standard conditions) and phosphoglycerate kinase (Energy coupled with glyceraldehyde-3-phosphate Dehydrogenase) have much smaller G's under cellular conditions and are not regulatory targets. (d) 1,3-bisphosphoglycerate, phosphoenolpyruvate
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Energy in Chemical and Biochemical Reactions
 Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
CHAPTER 15 Metabolism: Basic Concepts and Design
 CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Enzymes I. Dr. Mamoun Ahram Summer semester,
 Enzymes I Dr. Mamoun Ahram Summer semester, 2017-2018 Resources Mark's Basic Medical Biochemistry Other resources NCBI Bookshelf: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=books The Medical Biochemistry
Enzymes I Dr. Mamoun Ahram Summer semester, 2017-2018 Resources Mark's Basic Medical Biochemistry Other resources NCBI Bookshelf: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=books The Medical Biochemistry
Basic Concepts of Enzyme Action. Enzymes. Rate Enhancement 9/17/2015. Stryer Short Course Chapter 6
 Basic Concepts of Enzyme Action Stryer Short Course Chapter 6 Enzymes Biocatalysts Active site Substrate and product Catalyzed rate Uncatalyzed rate Rate Enhancement Which is a better catalyst, carbonic
Basic Concepts of Enzyme Action Stryer Short Course Chapter 6 Enzymes Biocatalysts Active site Substrate and product Catalyzed rate Uncatalyzed rate Rate Enhancement Which is a better catalyst, carbonic
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Department of Chemistry and Biochemistry University of Lethbridge. Biochemistry II. Bioenergetics
 Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Review Questions - Lecture 5: Metabolism, Part 1
 Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
Review Questions - Lecture 5: Metabolism, Part 1 Questions: 1. What is metabolism? 2. What does it mean to say that a cell has emergent properties? 3. Define metabolic pathway. 4. What is the difference
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Chapter 8 Notes. An Introduction to Metabolism
 Chapter 8 Notes An Introduction to Metabolism Describe how allosteric regulators may inhibit or stimulate the activity of an enzyme. Objectives Distinguish between the following pairs of terms: catabolic
Chapter 8 Notes An Introduction to Metabolism Describe how allosteric regulators may inhibit or stimulate the activity of an enzyme. Objectives Distinguish between the following pairs of terms: catabolic
Biologic catalysts 1. Shared properties with chemical catalysts a. Enzymes are neither consumed nor produced during the course of a reaction. b.
 Enzyme definition Enzymes are protein catalysts that increase the velocity of a chemical reaction and are not consumed during the reaction they catalyze. [Note: Some types of RNA can act like enzymes,
Enzyme definition Enzymes are protein catalysts that increase the velocity of a chemical reaction and are not consumed during the reaction they catalyze. [Note: Some types of RNA can act like enzymes,
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Key Concepts 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 8.2 The free-energy change of a reaction tells us
Chapter 8: An Introduction to Metabolism Key Concepts 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 8.2 The free-energy change of a reaction tells us
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
Exam 4 April 15, 2005 CHEM 3511 Print Name: KEY Signature
 1) (8 pts) General Properties of Enzymes. Give four properties of enzymaticallycatalyzed reactions. The answers should indicate how enzymatic reactions differ from non-enzymatic reactions. Write four only
1) (8 pts) General Properties of Enzymes. Give four properties of enzymaticallycatalyzed reactions. The answers should indicate how enzymatic reactions differ from non-enzymatic reactions. Write four only
I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI.
 Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Chapter 6 Energy & Metabolism I. Flow of Energy in Living Things II. Laws of Thermodynamics & Free Energy III. Activation Energy IV. Enzymes V. Reaction Coupling VI. Metabolism I. Flow of Energy in Living
Applications of Free Energy. NC State University
 Chemistry 433 Lecture 15 Applications of Free Energy NC State University Thermodynamics of glycolysis Reaction kj/mol D-glucose + ATP D-glucose-6-phosphate + ADP ΔG o = -16.7 D-glucose-6-phosphate p D-fructose-6-phosphate
Chemistry 433 Lecture 15 Applications of Free Energy NC State University Thermodynamics of glycolysis Reaction kj/mol D-glucose + ATP D-glucose-6-phosphate + ADP ΔG o = -16.7 D-glucose-6-phosphate p D-fructose-6-phosphate
An Introduction to Metabolism
 An Introduction to Metabolism Chapter 8 Objectives Distinguish between the following pairs of terms: catabolic and anabolic pathways; kinetic and potential energy; open and closed systems; exergonic and
An Introduction to Metabolism Chapter 8 Objectives Distinguish between the following pairs of terms: catabolic and anabolic pathways; kinetic and potential energy; open and closed systems; exergonic and
Chapter 8: An Introduction to Metabolism. 1. Energy & Chemical Reactions 2. ATP 3. Enzymes & Metabolic Pathways
 Chapter 8: An Introduction to Metabolism 1. Energy & Chemical Reactions 2. ATP 3. Enzymes & Metabolic Pathways 1. Energy & Chemical Reactions 2 Basic Forms of Energy Kinetic Energy (KE) energy in motion
Chapter 8: An Introduction to Metabolism 1. Energy & Chemical Reactions 2. ATP 3. Enzymes & Metabolic Pathways 1. Energy & Chemical Reactions 2 Basic Forms of Energy Kinetic Energy (KE) energy in motion
Chapter 8: An Introduction to Metabolism
 AP Biology Reading Guide Name Chapter 8: An Introduction to Metabolism Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. 2.
AP Biology Reading Guide Name Chapter 8: An Introduction to Metabolism Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. 2.
Oxidative Phosphorylation versus. Photophosphorylation
 Photosynthesis Oxidative Phosphorylation versus Photophosphorylation Oxidative Phosphorylation Electrons from the reduced cofactors NADH and FADH 2 are passed to proteins in the respiratory chain. In eukaryotes,
Photosynthesis Oxidative Phosphorylation versus Photophosphorylation Oxidative Phosphorylation Electrons from the reduced cofactors NADH and FADH 2 are passed to proteins in the respiratory chain. In eukaryotes,
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
An Introduction to Metabolism
 Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
Introduction to Metabolism (Or Energy Management) Chapter 8
 Introduction to Metabolism (Or Energy Management) Chapter 8 Metabolism of the chemical reactions in the organism Building up molecules Breaking down molecules Managing energy and materials Route to end-product
Introduction to Metabolism (Or Energy Management) Chapter 8 Metabolism of the chemical reactions in the organism Building up molecules Breaking down molecules Managing energy and materials Route to end-product
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. 2. There are two types
Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. 2. There are two types
Biochemical Pathways
 Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
BIOLOGICAL SCIENCE. Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge. FIFTH EDITION Freeman Quillin Allison
 BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
Chapter 8 Metabolism: Energy, Enzymes, and Regulation
 Chapter 8 Metabolism: Energy, Enzymes, and Regulation Energy: Capacity to do work or cause a particular change. Thus, all physical and chemical processes are the result of the application or movement of
Chapter 8 Metabolism: Energy, Enzymes, and Regulation Energy: Capacity to do work or cause a particular change. Thus, all physical and chemical processes are the result of the application or movement of
2) This is a learning problem. You will learn something. You will appreciate this information. If not soon, than someday. Ready?
 Problem Set 2 Hello Class. This is a Big Round problem set. Lots of word problems. I do this because science is a language. Much of it, and medicine too, is transacted as spoken word: seminars, grand rounds,
Problem Set 2 Hello Class. This is a Big Round problem set. Lots of word problems. I do this because science is a language. Much of it, and medicine too, is transacted as spoken word: seminars, grand rounds,
Part II => PROTEINS and ENZYMES. 2.5 Enzyme Properties 2.5a Enzyme Nomenclature 2.5b Transition State Theory
 Part II => PROTEINS and ENZYMES 2.5 Enzyme Properties 2.5a Enzyme Nomenclature 2.5b Transition State Theory Section 2.5a: Enzyme Nomenclature Synopsis 2.5a - Enzymes are biological catalysts they are almost
Part II => PROTEINS and ENZYMES 2.5 Enzyme Properties 2.5a Enzyme Nomenclature 2.5b Transition State Theory Section 2.5a: Enzyme Nomenclature Synopsis 2.5a - Enzymes are biological catalysts they are almost
What is an enzyme? Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics. Margaret A. Daugherty Fall General Properties
 Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2003 ENZYMES: Why, what, when, where, how? All but the who! What: proteins that exert kinetic control over
Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2003 ENZYMES: Why, what, when, where, how? All but the who! What: proteins that exert kinetic control over
An Introduction to Metabolism
 An Introduction to Metabolism I. All of an organism=s chemical reactions taken together is called metabolism. A. Metabolic pathways begin with a specific molecule, which is then altered in a series of
An Introduction to Metabolism I. All of an organism=s chemical reactions taken together is called metabolism. A. Metabolic pathways begin with a specific molecule, which is then altered in a series of
Microbiology II Microbial physiology I Energetics
 Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses
Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
What is an enzyme? Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics. Margaret A. Daugherty Fall 2004 KEY FEATURES OF ENZYMES
 Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2004 What is an enzyme? General Properties Mostly proteins, but some are actually RNAs Biological catalysts
Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2004 What is an enzyme? General Properties Mostly proteins, but some are actually RNAs Biological catalysts
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
An Introduction to Metabolism. Chapter 8
 An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
Chapter 6 Active Reading Guide An Introduction to Metabolism
 Name: AP Biology Mr. Croft Section 1 1. Define metabolism. Chapter 6 Active Reading Guide An Introduction to Metabolism 2. There are two types of reactions in metabolic pathways: anabolic and catabolic.
Name: AP Biology Mr. Croft Section 1 1. Define metabolism. Chapter 6 Active Reading Guide An Introduction to Metabolism 2. There are two types of reactions in metabolic pathways: anabolic and catabolic.
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Chapter 8: An Introduction to Metabolism
 Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. 2. There are two types of reactions in metabolic pathways: anabolic
Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. 2. There are two types of reactions in metabolic pathways: anabolic
Bioenergetics and high-energy compounds
 Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Chapter 6. Ground Rules Of Metabolism
 Chapter 6 Ground Rules Of Metabolism Alcohol Dehydrogenase An enzyme Breaks down ethanol and other toxic alcohols Allows humans to drink Metabolism Is the totality of an organism s chemical reactions Arises
Chapter 6 Ground Rules Of Metabolism Alcohol Dehydrogenase An enzyme Breaks down ethanol and other toxic alcohols Allows humans to drink Metabolism Is the totality of an organism s chemical reactions Arises
Chapter 6- An Introduction to Metabolism*
 Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Activity: Identifying forms of energy
 Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
9/25/2011. Outline. Overview: The Energy of Life. I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V.
 Chapter 8 Introduction to Metabolism Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes Overview: The Energy of Life Figure 8.1 The living cell is a miniature
Chapter 8 Introduction to Metabolism Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes Overview: The Energy of Life Figure 8.1 The living cell is a miniature
Welcome to Class 8! Introductory Biochemistry! Announcements / Reminders! Midterm TA led Review Sessions!
 Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Announcements / Reminders Midterm TA led Review Sessions Welcome to Class 8 Sunday, February 23 from 8-10pm Location: Science Center Main Room (315) Office Hours Prof Salomon: SFH 270 on Thursday Feb 20,
Unit 3: Cellular Energetics Guided Reading Questions (50 pts total)
 AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
AP Biology Biology, Campbell and Reece, 10th Edition Adapted from chapter reading guides originally created by Lynn Miriello Name: Chapter 8 An Introduction to Metabolism Unit 3: Cellular Energetics Guided
Lecture #14. Chapter 17 Free Energy and Equilibrium Constants
 Lecture #14 Chapter 17 Free Energy and Equilibrium Constants Josiah W. Gibbs 1839-1903 Gibbs Free Energy (G) The maximum energy released by a system occurring at constant temperature and pressure that
Lecture #14 Chapter 17 Free Energy and Equilibrium Constants Josiah W. Gibbs 1839-1903 Gibbs Free Energy (G) The maximum energy released by a system occurring at constant temperature and pressure that
Pyruvate is reduced to lactate in anaerobic metabolism in muscle cells
 Pyruvate is reduced to lactate in anaerobic metabolism in muscle cells Transferases and hydrolases catalyze group transfer reactions Acyl transfer: Hexokinase catalyzes a phosphoryl transfer from ATP to
Pyruvate is reduced to lactate in anaerobic metabolism in muscle cells Transferases and hydrolases catalyze group transfer reactions Acyl transfer: Hexokinase catalyzes a phosphoryl transfer from ATP to
ATP ATP. The energy needs of life. Living economy. Where do we get the energy from? 9/11/2015. Making energy! Organisms are endergonic systems
 Making energy! ATP The energy needs of life rganisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active transport temperature regulation 2007-2008
Making energy! ATP The energy needs of life rganisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active transport temperature regulation 2007-2008
Overview of Kinetics
 Overview of Kinetics [P] t = ν = k[s] Velocity of reaction Conc. of reactant(s) Rate of reaction M/sec Rate constant sec -1, M -1 sec -1 1 st order reaction-rate depends on concentration of one reactant
Overview of Kinetics [P] t = ν = k[s] Velocity of reaction Conc. of reactant(s) Rate of reaction M/sec Rate constant sec -1, M -1 sec -1 1 st order reaction-rate depends on concentration of one reactant
CHAPTER 8. An Introduction to Metabolism
 CHAPTER 8 An Introduction to Metabolism WHAT YOU NEED TO KNOW: Examples of endergonic and exergonic reactions. The key role of ATP in energy coupling. That enzymes work by lowering the energy of activation.
CHAPTER 8 An Introduction to Metabolism WHAT YOU NEED TO KNOW: Examples of endergonic and exergonic reactions. The key role of ATP in energy coupling. That enzymes work by lowering the energy of activation.
An Introduction to Metabolism
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism
Glycolysis and Fermentation. Chapter 8
 Glycolysis and Fermentation Chapter 8 Cellular Respiration and Photosynthesis 0 Things to know in these chapters 0 Names and order of the processes 0 Reactants and products of each process 0 How do they
Glycolysis and Fermentation Chapter 8 Cellular Respiration and Photosynthesis 0 Things to know in these chapters 0 Names and order of the processes 0 Reactants and products of each process 0 How do they
Energy Transformation and Metabolism (Outline)
 Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Energy and Cellular Metabolism
 1 Chapter 4 About This Chapter Energy and Cellular Metabolism 2 Energy in biological systems Chemical reactions Enzymes Metabolism Figure 4.1 Energy transfer in the environment Table 4.1 Properties of
1 Chapter 4 About This Chapter Energy and Cellular Metabolism 2 Energy in biological systems Chemical reactions Enzymes Metabolism Figure 4.1 Energy transfer in the environment Table 4.1 Properties of
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Chapter 6: Energy Flow in the Life of a Cell
 Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Energetics of metabolism
 Energetics of metabolism Dr. Bódis Emőke October 7, 2015 JJ9 Why do we study difficult thermodynamics? The laws and principles of the thermodynamics describe the characteristics of matter- and energy flow
Energetics of metabolism Dr. Bódis Emőke October 7, 2015 JJ9 Why do we study difficult thermodynamics? The laws and principles of the thermodynamics describe the characteristics of matter- and energy flow
Ground Rules of Metabolism CHAPTER 6
 Ground Rules of Metabolism CHAPTER 6 Antioxidants You ve heard the term. What s the big deal? Found naturally in many fruits and vegetables Added to many products What do they actually do? Antioxidants
Ground Rules of Metabolism CHAPTER 6 Antioxidants You ve heard the term. What s the big deal? Found naturally in many fruits and vegetables Added to many products What do they actually do? Antioxidants
What Is Energy? Energy is the capacity to do work. First Law of Thermodynamics. Types of energy
 What Is Energy? Energy is the capacity to do work. Synthesizing molecules Moving objects Generating heat and light Types of Kinetic: of movement otential: stored First Law of Thermodynamics Energy cannot
What Is Energy? Energy is the capacity to do work. Synthesizing molecules Moving objects Generating heat and light Types of Kinetic: of movement otential: stored First Law of Thermodynamics Energy cannot
AP Biology Cellular Respiration
 AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
AP Biology Cellular Respiration The bonds between H and C represents a shared pair of electrons These are high-energy electrons This represents chemical potential energy Hydro-carbons posses a lot of chemical
Exam 3 Review (4/12/2011) Lecture note excerpt covering lectures (Exam 3 topics: Chapters 8, 12, 14 & 15)
 Exam 3 Review (4/12/2011) Lecture note excerpt covering lectures 17-23 (Exam 3 topics: Chapters 8, 12, 14 & 15) Enzyme Kinetics, Inhibition, and Regulation Chapter 12 Enzyme Kinetics When the concentration
Exam 3 Review (4/12/2011) Lecture note excerpt covering lectures 17-23 (Exam 3 topics: Chapters 8, 12, 14 & 15) Enzyme Kinetics, Inhibition, and Regulation Chapter 12 Enzyme Kinetics When the concentration
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
BIOLOGY 10/11/2014. An Introduction to Metabolism. Outline. Overview: The Energy of Life
 8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
Objectives INTRODUCTION TO METABOLISM. Metabolism. Catabolic Pathways. Anabolic Pathways 3/6/2011. How to Read a Chemical Equation
 Objectives INTRODUCTION TO METABOLISM. Chapter 8 Metabolism, Energy, and Life Explain the role of catabolic and anabolic pathways in cell metabolism Distinguish between kinetic and potential energy Distinguish
Objectives INTRODUCTION TO METABOLISM. Chapter 8 Metabolism, Energy, and Life Explain the role of catabolic and anabolic pathways in cell metabolism Distinguish between kinetic and potential energy Distinguish
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
Biochemistry and Physiology ID #:
 BCHM 463 Your Name: Biochemistry and Physiology ID #: Exam II, November 4, 2002 Prof. Jason Kahn You have 50 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded under any
BCHM 463 Your Name: Biochemistry and Physiology ID #: Exam II, November 4, 2002 Prof. Jason Kahn You have 50 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded under any
Enzymes Enzyme Mechanism
 Mechanisms of Enzymes BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
Mechanisms of Enzymes BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
Mechanism of CO 2 Fixation: Rubisco Step 1
 dark means light-indendent! The Dark Reaction: Carbon Fixation Requires Rubisco: Ribulose-1,5-bisphosphate carboxylase RuBPi + C 6 x 3PG MW = 550, 000 g/mol Subunits eight small (14,000 g/mol) eight large
dark means light-indendent! The Dark Reaction: Carbon Fixation Requires Rubisco: Ribulose-1,5-bisphosphate carboxylase RuBPi + C 6 x 3PG MW = 550, 000 g/mol Subunits eight small (14,000 g/mol) eight large
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
Enzymes Enzyme Mechanism
 BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Mechanisms of Enzymes Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Mechanisms of Enzymes Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
Chapter 6: Outline-2. Chapter 6: Outline Properties of Enzymes. Introduction. Activation Energy, E act. Activation Energy-2
 Chapter 6: Outline- Properties of Enzymes Classification of Enzymes Enzyme inetics Michaelis-Menten inetics Lineweaver-Burke Plots Enzyme Inhibition Catalysis Catalytic Mechanisms Cofactors Chapter 6:
Chapter 6: Outline- Properties of Enzymes Classification of Enzymes Enzyme inetics Michaelis-Menten inetics Lineweaver-Burke Plots Enzyme Inhibition Catalysis Catalytic Mechanisms Cofactors Chapter 6:
Free Energy. because H is negative doesn't mean that G will be negative and just because S is positive doesn't mean that G will be negative.
 Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
An Introduction to Metabolism
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION The
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION The
An Introduction to Metabolism
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: The Energy of Life The
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: The Energy of Life The
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Metabolism and enzymes
 Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Chemistry 1506: Allied Health Chemistry 2. Section 10: Enzymes. Biochemical Catalysts. Outline
 Chemistry 1506 Dr. Hunter s Class Section 10 Notes - Page 1/14 Chemistry 1506: Allied Health Chemistry 2 Section 10: Enzymes Biochemical Catalysts. Outline SECTION 10.1 INTRODUCTION...2 SECTION SECTION
Chemistry 1506 Dr. Hunter s Class Section 10 Notes - Page 1/14 Chemistry 1506: Allied Health Chemistry 2 Section 10: Enzymes Biochemical Catalysts. Outline SECTION 10.1 INTRODUCTION...2 SECTION SECTION
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Enzymes are biological macromolecules that increase the rate of the reaction. Six major groups of enzymes (pgs. 94-95/98-99) Oxidoreductases:
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Enzymes are biological macromolecules that increase the rate of the reaction. Six major groups of enzymes (pgs. 94-95/98-99) Oxidoreductases:
This is an example of cellular respiration, which can be used to make beer and wine using different metabolic pathways For these reasons we call this
 Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6: Energy and Metabolism
 Chapter 6: Energy and Metabolism Student: 1. Oxidation and reduction reactions are chemical processes that result in a gain or loss in A) atoms. B) neutrons. C) electrons. D) molecules. E) protons. 2.
Chapter 6: Energy and Metabolism Student: 1. Oxidation and reduction reactions are chemical processes that result in a gain or loss in A) atoms. B) neutrons. C) electrons. D) molecules. E) protons. 2.
Lecture 7: Enzymes and Energetics
 Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
Enzyme Kinetics: The study of reaction rates. For each very short segment dt of the reaction: V k 1 [S]
![Enzyme Kinetics: The study of reaction rates. For each very short segment dt of the reaction: V k 1 [S] Enzyme Kinetics: The study of reaction rates. For each very short segment dt of the reaction: V k 1 [S]](/thumbs/71/66235891.jpg) Enzyme Kinetics: The study of reaction rates. For the one-way st -order reaction: S the rate of reaction (V) is: V P [ P] moles / L t sec For each very short segment dt of the reaction: d[ P] d[ S] V dt
Enzyme Kinetics: The study of reaction rates. For the one-way st -order reaction: S the rate of reaction (V) is: V P [ P] moles / L t sec For each very short segment dt of the reaction: d[ P] d[ S] V dt
An Introduction to Metabolism
 Chapter 8 An Introduction to Metabolism Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 8 1. An organism s metabolism transforms matter and energy, subject to the laws of
Chapter 8 An Introduction to Metabolism Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 8 1. An organism s metabolism transforms matter and energy, subject to the laws of
BIOLOGY. An Introduction to Metabolism CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 8 An Introduction to Metabolism Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick The Energy of Life The living
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 8 An Introduction to Metabolism Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick The Energy of Life The living
Lecture 21 - Introduction to Metabolism: Bioenergetics
 Lecture 21 - Introduction to Metabolism: Bioenergetics Key Concepts Energy conversion in biological systems Metabolic redox reactions Review of thermodynamic principles and coupled reactions The adenylate
Lecture 21 - Introduction to Metabolism: Bioenergetics Key Concepts Energy conversion in biological systems Metabolic redox reactions Review of thermodynamic principles and coupled reactions The adenylate
General Biology. The Energy of Life The living cell is a miniature factory where thousands of reactions occur; it converts energy in many ways
 Course No: BNG2003 Credits: 3.00 General Biology 5. An Introduction into Cell Metabolism The Energy of Life The living cell is a miniature factory where thousands of reactions occur; it converts energy
Course No: BNG2003 Credits: 3.00 General Biology 5. An Introduction into Cell Metabolism The Energy of Life The living cell is a miniature factory where thousands of reactions occur; it converts energy
*The entropy of a system may decrease, but the entropy of the system plus its surroundings must always increase
 AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
AP biology Notes: Metabolism Metabolism = totality of an organism's chemical process concerned with managing cellular resources. Metabolic reactions are organized into pathways that are orderly series
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Outer Glycolysis mitochondrial membrane Glucose ATP
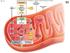 Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
