SOME ANALYSES OF SOIL MONTMORIN, VERMICULITE, MICA, CHLORITE, ANI) INTERSTRATIFIED LAYER SILICATES 1
|
|
|
- Jasmin Horton
- 5 years ago
- Views:
Transcription
1 SOME ANALYSES OF SOIL MONTMORIN, VERMICULITE, MICA, CHLORITE, ANI) INTERSTRATIFIED LAYER SILICATES 1 By M. L. JACKSON, L. D. WH[TTIG, R. C. VANDEN HEUVEL, A. KAUFMAN, and B. E. BROWN Department of Soils, University of Wisconsin, Madison ABSTRACT Analyses were made of 12 clay fractions and one silt fraction taken from 10 different soil horizons and clay deposits. The analyses presented were selected to represent a wide variely of analytical problems with materials containing 2:1 layer silicates, 2:2 (chlorite) layer silicates, varying amounts of 1:1 layer silicates, and in some cases with appreciable amounts of quartz, feldspars, gibbsite, hematite, magnetite, and (or) anatase. The analyses were performed by x-ray diffraction, specific surface, integral heating weight loss, and elemental analyses. The x-ray diffraction analyses included both wedge-powder (film recorded) and oriented (Geiger counter recorded) techniques. Vermiculites of soils were found to vary in the degree to which K-saturation would close the interlayer space, but vermiculite is established by: (a) a 14 A spacing when solvated (even with glycerol); (b) closure of the interlayer spacing on heating to 500 ~ C; and (c) an interlayer surface of about 800 m 2 per gin. In the absence of adequate criteria, vermiculites can be mistakenly identified as chlorites. The data obtained from the various techniques were combined by a system of allocation so that the minerals reported represented a unique fit of all the data. The specific surface measurement, including the measurement of interlayer surface, is a key criterion in the allocation. Interstratified ("mixed layer") layer silicates are determined as the individual component minerals. The beidellite of Iron River and Putnam soil clay finer tban 0.08 microns is extensively interstratified with chlorite, mica, and vermiculite. No discrete x-ray crystalline zones of any of the latter three minerals were observed, but a 19.6 to 19.2 A spacing may be indicative of a superlattice of these materials and beidellite with mica and chlorite at the electron density nodes. A titaniferous ceramic clay contains 43 percent mica (as determined by allocating the 5.03 percent K~O to muscovite), 20 percent kaolinite (water loss in ~ range), 21 percent TiO2 and 17 percent of other constituents (by elemental and surface analysis). This occurrence of the full complement of K20 in clay micas (both biotite and muscovite type) is general for the various clays examined, when the interlayer surface is calculated to halloysite, vermiculite, and montmorin. A general occurrence of interstratification of layer silicates is proposed. 1 This work was supported in part by the University of Wisconsin Research Committee through grant of funds from the Wisconsin Alumni Research Foundation. 218
2 ANALYSES OF SOIL SILICATES 219 INTRODUCTION A single type of analytical technique, such as x-ray diffraction or differential thermal analysis, often yields important qualitatiz'c information as to the more abundant mineral or minerals of polycomponent clays, but a combination of several analytical methods is essential for a quantitati've mineralogical analysis. While pcrforming the analyses presented herein the authors have been impressed by the fact that one or two criteria, which are indicative qualitatively, often give a completely distorted view of the quantitative mineral content. This is especially true of the x-ray diffraction technique which, while indispensable for qualitative identification of layer silicates, is virtually without quantitative reliability because of the general occurrence of interstratification ("mixed layer" structure) among the 2:1 and 2:2 (chlorite) layer silicates. This interstratification is of the type referred to by Gruner (1934) as "meta-crystalline". Use of specific surface, integral heating weight loss, and elemental analyses together with qualitative x-ray diffraction and differential thermal analyses permits a decisive quantitative analysis of the minerals present. The objectives of this paper are: a. To present sonle representative analyses of soil mineral colloids containing representatives of the main classes of layer silicates, namely montmorin series, vermiculite, micas (illite), and chlorite, and interstratifled mixtures of these, with and without kaolin. b. To illustrate the residue mechanism of chemical weathering in the system Hydrol Humic Latosol (mostly hydrous oxides) to titaniferous ceramic clay (abundant in micas). c. To present, through a system of ~tllocation of several types of analytical data, evidence of the occurrence of random interstratification of different layer silicate series in clays generally. The soils employed are identified within the respective sections in which data are presented. The literature is reviewed in the sections to which it pertains. EXPERIMENTAL PROCEDURES The mineral colloid fractions were separated by the procedure of Jackson, et al. (1950), with iron oxide extraction by the procedure of Aguilera and Jackson (1953). The criteria for the analyses reported herein include x-ray diffraction analysis with various exchange saturation, solvation, and heat treatments (Jackson, et al., 1955) ; internal and total surface measurement (Vanden Heuvel and Jackson, 1955); elemental analysis (Corey and Jackson, 1953); and integral heating weight loss analysis; followed by allocation to mineral components on the basis of the combined data. The systcm of allocation, details of which are to be published elsewhere, is based on allocation of water and hydroxyl (as a function of temperature) and of elements (particularly K, Ca, and Na) to feldspars and
3 220 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS micas, on the basis of the qualitative indication on the x-ray diffraction patterns, within the limits of vermiculite and montmorin set by the specific interlayer surface. The diffraction analyses were carried out on three specimens, two fiat (oriented) specimens and one wedge-powder specimen. Of the two fiat specimens, one was exchange saturated with Mg and dried from water at room temperature in the presence of glycerol (weight equal to the colloid) and a second was exchange saturated with K or NH4 and dried at room temperature from water in the absence of glycerol; they were x-rayed with the General Electric XRD-3 generator and Geiger counter recorder, with copper radiaticm filtered with Ni. Each fiat specimen was then heated at various temperatures up to 500~ and further x-radiated; changes in diffraction intensities of a specimen of a given cation saturation resulting from heat treatment are therefore attributable to the heating (use of the same mount eliminates any possibility of variation in particle orientation as might occur between different mountings). The wedge-powder x-ray diffraction patterns (General Electric XRD-1, with Fe radiation) we,'e made by the White, et al., procedurel in which the Mg colloid is solvated with glycerol dissolved in a benzene-ethanol solution. COLLOIDS CONTAINING MUCH OF THE MONTMORIN SERIES MINERALS Analyses of soil and clay colloids containing much montmorin (term after Correns ; if preferred, this may be read "montmorillonite isomorphous series" which consists of 2:1 layer silicates with an 18 A diffraction spacing when dried in the presence of glycerol) are illustrated with six samples. These analyses illustrate an effective technique for the evaluation of the several associated layer silicate species by allocation of the several types of data. The fact that soil mineral colloids are generally polycomponent has been emphasized earlier (Byers, et al., 1935; Jackson, et al., 1948). Ahmeek Loam, A 1 Horizon The Ahmeek loam soil is a Brown Forest soil from northern Wisconsin (location described by the U. S. Department of Agriculture, 1952). The micron size fraction from the A 1 horizon gave a relatively intense 18 A diffraction spacing (Fig. 1, A) when the sample was dried at room temperature in the presence of glycerol. Drying of this specimen at a steam hotplate temperature caused the volatilization of considerable glycerol and a shift of the basal spacing to 14 A, thus coinciding with that of the vermiculite present. This shift of spacing from 18 to 14 A has been noted commonly in our laboratory with soil beidellites from many localities (with the Hagerstown colloid, Fig. 3 below, for example), although the shift does not occur with many other soil montmorins and with most 1 Manuscript to be published (essentials reviewed by Jeffries and Jackson, 1949).
4 ANALYSES OF SOIL SILICATES 221 s/:.~ J.ISN3 j~ll, :...::... 9 ~,. ~_ ~... "'-2 ~,~ "".i'... *- o < ~ - ~ < ~, < ~. - ~ % ~'~-:::::'..-'~----_.-: ~ ~-~ o ~ ~r ~ ~ <, 9 i- ~ -~ "", i trl $/0,Ik.LISN~IJ. NI g 8 ~. - I m J - ~ FIGURE 1.--X-ray diffraction patterns of coarse clay from Ahmeek loam; (A) ttl horizon, showing the shift of basal beidellite 18 A spacing as a result of ll0~ drying even in the presence of glycerol; (B) (;z horizon, which stlow the retention of 7.2 and 14 A spacings of chlorite on heating to 500~ thus differentiating the spacings from those of vermiculite or kaolin. The zero intensity base lines are shifted vertically for the successive patterns as indicated with a bar at left.
5 222 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS..... ~.. ~ z 9 9 < ~ o o o o o 11 o o.< OQ ~0 oo o ~ ~ ~. II v~ o~ 0,< < 0 V V V V ~V~V~.~'~.< < 0 "~.~ I~ "~.u,< 0 I o.o o -~ =~=-<~-~".~- ~.~,.q,~ < ~o < ~-.~O~-o~.= i
6 ANALYSES OF ~OIL ~ILICATES 223 1nontmorins from bentonites. The controlling factor is believed to be related to the layer charge and this is proposed as a possible means of subdividing the montmorins into two classes. These data show the necessity for restricting the drying temperature to room temperature for the x-ray diffraction test for the tnontnlorin series (including beidellite). The presence of some chlorite in the Ahmeek A 1 colloid is indicated by the persistence of the 7.2 A spacing after heating to 500~ On the basis of allocation of surface and elenaental analyses (Tables 1 and 2) with consideration of the powder pattern which clearly reveals quartz and feldspars, the minerals present were calculated (Table 3). The disproportionality of the montmorin and vermiculite diffraction intensity (Fig. 1, A) to that of the other components is attributed to the occurrence of these two constituents (a) in large crystals and (b) with little interstratification. The chlorite formula derived by the Marshall (1935) type calculations is : (Si3.4aA10.~7) (A11.4tTi0.59)(A10.s6Mg0.93Fe02.~ 4 F(a+ )O10(OH)s 0-15 in which the total brucite cations = The mica formula is: 3+ Kl.oo (Si3.ooAll.00) (A10.98Mgo.5oFe2o.~9 Fe 0.0s TJ0.31) O10 (OH) 2 in which the octahedral cations = The 3:1 Si to A1 ratio is not inherent in the method of calculation. The vermiculite plus montmorin average formula is similar to that of the mica except for Xo.54(Si3.5o A10.5o ) in the first three terms. 1 Intermediate octahedral cation values are expected because of the heterogeneous sources of the colloidal mineral particle population. Iron River Loam, d 2 Horizon An x-ray diffraction maximum at 19.6 A was found (Fig. 2) with the colloid less than 0.08 microns separated from Iron River loam, A 2 horizon, a Podzol soil of northern Wisconsin (U.S.D.A., 1952). The 19.6 A spacing is noted in the sample which was Mg saturated and dried at room temperature; an inflection at 10 A spacing (arrow, Fig. 2) indicates some mica, the presence of which is confirmed by a K20 content of 1.67 percent. The diffraction background is high. It is well known that K or NH 4 saturation of any montmorin, followed by drying from water at room temperature in the absence of glycerol, allows the crystal layers to come together to 11.5 to 13.5 A as observed in this material. According to Walker (1951, p. 203), a vermiculite spacing may decrease to 10.5 A when mineral is NH4 saturated. Ammonium versus potassium saturation for the heated specimen.--when the sample was heated to 500~ a clearly defined diffraction maximum at 10 A was obtained when the sample had been previously saturated with ammonium, and a less distinct one was obtained when it was previously 1 X refers to equivalent of exchangeable cations.
7 224 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS IRON RIVER LOAM Az:O-Sin PODZOL (MODERATE} NO 331W 15 ;t FRACTION ~O08u SATURATION DRYING TEMP ~oea NH~ H-:... / io ea~]~ /- / ~ v,, -;/ K~/V 50~0~ r3.=.~ 200 / 1 25~ :~.aa J M~I /f " qo HI5 RiO /! O DIFFRACTION ANGLE (2e) FIGURE 2.--X-ray diffraction patterns of the fine clay of Iron River loam, A2 horizon. A high background occurs in association with the 19.6 A spacing associated with a superlattice. Drying at 500 ~ C x~,ith ammonium saturation gave a more clearly defined 10 A maximum than drying with K saturation. saturated with K. This is interpreted as an indication that the K ion holds the crystal layers apart somewhat (illustrated in Fig. 2) because the layers have rotated at random around the Z-axis, so as to lose the correspondence of opposing holes in the surfaces of the layers. The ammonium saturated material becomes hydrogen saturated during heating, and this ion would not hold the layers apart appreciably. The coming together of many layers to 10 A on heating disqualifies the material as a "swelling chlorite" (Stephen and MacEwan, 1951). Although the heating tests on the Iron River colloid
8 ANALYSES OF ~OIL ~ILICATES 225 indicate that ammonium saturation is preferable to potassium saturation, tests with other materials indicate that ammonium is not consistently superior. A quaternary interslratified systcm.--the low intensity 10 A diffraction maxinauna on heating the Iron River colloid (Fig. 2) proves the presence of some chlorite, the brucite layer of which holds some layers apart after heating. ]nterstratification is intimate enough to prevent either a 14 or a 7 A diffraction maximum to be formed. The mica content is 17 percent (from the K20 analysis) and the chlorite content is 15 percent (Table 3) based on the allocation of interlayer surface (Table 1) to montmorin (37 percent) and vermiculite (31 percent). An intense (hko) diffraction maximum at 4.45 A observed in the fihn-recorded pattern (not shown) proves the predominance of layer silicates rather than truly amorphous materials. The system is therefore appropriately termed a complexly interstratified mixture of layer silicates. It is probably in part quaternary interstratification. Essentially the same characteristics are found in the Putnam and Ahmeek C2 colloids (below) and in many other soil colloids that have weathered from micas. Possible structure. -- Although not a premise to the above conclusion on interstratification, one possible structural possibility to account for the increase in basal spacing to 19.6 A and the broadening and low intensity is the occurrence of a "superlattice" of which the 19.6 A spacing is a higher order. Nodes of electron density may be formed by mica and chlorite assemblages, while the electron density internodes would be formed by vermiculite and montmorin sequences. A sequence of 5Mt-F- TABLE 2.- ELEMENTAL ANALYSIS AND ]{EATING WEIGHT Loss DATA ON CLAY FRACTIONS STUDIED, PERCENTAGES AS OXIDES Soil Ahmeek loam Ti Ceram. clay Davidson Horizon (and No.) A~(1) C2(2) --(9) A(10) Size fraction, microns <' < 0.2 SiO A FeO [ FezO ~ TiO MgO CaO K Na H20, ~ " 2.42* ~ C [" 11.4t to 900~ ** ~c 3.50~ Total _400~ t ~ 9 * From temperature of previous loss entry, corrected for ferrous iron oxidation. :~ Uncorrected for ferrous iron oxidation, since iron content is low.
9 226 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS 2Vm-+-2Chl+lMi, for example, gives a 156 A spacing of nodes of which the eighth order is 19.5 A; also, a sequence of 3Mt+lChl+lMi gives a spacing of 78 A of which the fourth order is 19.5 A. These and related layer assemblages would be expected to follow a probability distribution around the mica nucleus rather than a regular sequence. Their complexly interstratified system is similar to but more complex than the Hendricks and Teller (1942) binary randomly interstratified systems. The occurrence of long (2 to 24-layer) unit cell sequences in micas was previously proposed by Hendricks (1939) and such structural units, accompanied by a periodicy of ionic substitution, might result in similarly spaced preferential weathering planes which grow to form the internodes. It is suggested that the nodes around a mica-chlorite nucleus reflect the mica-to-chlorite-tovermiculite-to-montmorin weathering sequence (Jackson, et al., 1952); Jackson and Sherman, 1953), wherein the weathering is more extensive (to montmorin) the further from the mica node. Chlorite formed adjacent to mica layers would be the "aluminous chlorite" of Jackson and Sherman (1953, p. 236). A distribution of metallic cation saturation, ph, and Mg activity would be expected to follow in phase with the electron density nodes on a "superlattice" unit cell scale, the weathering sequence thus following in consequence. Such a "superlattice" system would represent a fourth recapitulation of the weathering sequence of Jackson, et al. (1948). Another possible source of a 19.5 A spacing would be the presence of two loosely packed brucite lavers between some pairs of 2:1 layers. Either the "superlattice" or the double brucite layer mechanism suggested provides a correlation of the relatively low glycerol sorption (Table 1) with the basal spacing higher than that of pure montmorin (which might lead to expectation of more than aaormal glycerol sorption). Either allows for a high background and line broadening without requiring an excessively fine particle size (which is precluded by the low specific surface). TABLE MINERAL CONTENT OF SOIL AND CLAY FRACTIONS BASED ON ALLOCATION OF COMBINED DATA Minerals present ~expressed in percent * Soil or clay fraction FI Qr Mi Chl Vm Mt KI Gb Gt Hm Ma An 1. Ahmeek, A1, 2-0.2t~ I n.d. n.d Ahmeek, C2, 2-02# n.d. n.d Iron River, A2 <0.08# n.d. n.d Putnam, B <0.2# n.d. n.d Beidellite, Ark. <0.2# n.d. n.d Montmorin, Wyo. <0.2# n.d. n.d Ti Ceram. clay, 20-2# Ti Ceram. clay, (2# Davidson, 2-0.2# n.d. n.d. 0 0 Davidson, <0.2# n.d. n.d. 0 0 * Fl--feldspars; Qr--quartz; Mi--micas; Chl--chlorites ; VmIvermiculites ; Mt-- montmorin series; KlIkaolin family; GbIgibbsite; GtIgoethite; Hm--hematite; Ma--magnetite; An--anatase; n.d.--not determined--reported percentages are Oll samples from which free Fe20:~ was extracted.
10 ANALYSES of ~OIL ~ILICATES 227 Hagerstown Silt Loam, B Horizon The Hagerstown silt loam, B horizon, a Gray-Brown Podzolic soil, was sampled from the profile near Zion, Pa. (a few miles northwest of State College, displayed during the National Soil Science Society Meetings, in 1951). The Hagerstown soil colloid of less than 0.08 microns gave a distinct 18 A montnaorin diffraction spacing (Fig. 3). This fine clay fraction makes up 17.7 percent of the total clay and 6.7 percent of the soil and thus is a very important component of the soil. The surface measurement (Table 1) is also high and thus corroborates the interpretation of a high beidellite content. hnportance of size segregation.- X-ray diffraction patterns are shown also (Fig. 3) for the Hagerstown fine silt (5-2 micron), coarse clay ( HAGERSTOWN SILT LOAM >- z bj I-- Z >- I- z bt.i I-- z DIFFRACTION ANGLE (20) FIGURE.3.--X-ray diffraction patterns of fractions from Hagerstown, B horizon. The presence of beidellite (18 A spacing when Mg saturated and dried from water in the presence of glycerol) can only be shown when the less than 0.08 fraction is separated from the rest of the clay fraction. The other fractions contain mainly mica and quartz. Dotted lines lead to the appropriate intensity scale for the respective zero base lines.
11 228 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS micron), medium clay ( micron) and total clay (less than 2 micron) fractions to illustrate the advantage of separation of the fine clay fraction (less than 0.08 micron) in order to detect the beidetlite (18 A in the presence of glycerol) in this soil. Because of its low 18 A diffraction intensity, the beidellite cannot be detected in the diffraction pattern of the total clay, less than 2 microns (upper curve in Fig. 3). The need for segregation at a small size such as 0.06 or 0.08 micron to detect beidellite in some soils was pointed out by Bray (1937). The occurrence of otherwise undetected amorphous mineral colloid in the less than 0.08 micron fraction has been reported in the kaolinic Chester soil clay (Pennington and Jackson, 1948) and in the vermiculitic Omega soil clay (Jackson, et al., 1955). Separation of the mineral colloids at the 0.08 and 0.2 micron limits is included as one of the necessary criteria for soil mineral colloid identification and characterization (Jackson, et al., 1955). Mica diffraction enhancement.- The intensity of the 10 A diffraction maximum is greater in the sample of total clay of Hagerstown than in any of its clay subfractions (Fig. 3). This phenomenon has been noted in other soils (Jackson, et al., 1955) also, and is attributed to the enhancement of orientation of the coarse clay mica crystals by the presence of fine colloidal (non-mica) particles. These observations are being tested with other materials. Putnam Silt Loam, B Horizon The Putnam silt loam, B horizon (kindly furnished by Dr. C. E. Marshall) is a grassland Planosol developed on loess, the sample being obtained from the Sanborn field of the Missouri Agricultural Experiment Station. The results with Putnam colloid were compared with those from a beidellite (kindly furnished by C. S. Ross) from Nashville, Arkansas, and with the colloid of Wyoming bentonite (kindly furnished by the American Colloid Co., Chicago, Ill.) from Upton, Wyoming. In each case, the finer than 0.2 micron size fraction was studied. The basal spacing of the Putnam colloid (Fig. 4) was found to be 19.2 A as compared with 18 A for the usual montmorin. The intensity of diffraction of the Putnam colloid is less than one-tenth that of the Arkansas and Wyoming montmorins (Fig. 4) and shows a much broader first order peak. These characteristics of the Putnam colloid are similar to those of the Iron River colloid (above). The contents of mica and montmorin are similar, but more chlorite and less vermiculite are present (Table 3). The lower basal diffraction spacing of 19.2 A in Putnam colloid, compared with 19.6 A in that of Iron River may arise from the slightly different proportion of these layer silicate constituents in the complexly interstratified system. Randomness and heating changes.- No higher orders are apparent in either the Putnam or Arkansas sample, and this is taken as indicative of randomness in the basal spacing distribution in both samples. The beidellite
12 ANALYSES OF SOIL SILICATES 229 PUTNAM CLAY (Mo.) BEIDELLITE B: in. FRACTION < O.2u... MONT. <O.2u (Wyo. Bent.) NASHVILLE~ ARK. FRACTION < 0.2u DRYING TEMP: INTENSITY 1 C/S ~ ~, ~ 400,.,,-.3_ DRYING TEMP. l 5OO 4O0 _200 '200 II0 II0 / Jtl! ~ I. 5o i ' " ~ I I I0 20 DIFFRACTION SPACINGS - A FIGURE 4.---X-ray diffraction patterns of fine clay from Putnam, B horizon, in comparison with those of beidellite and a mommorin from V~ryoming bentonite. (randomness through distribution of only 7 percent mica) has nearly as strong an 18 A diffraction intensity as the Wyoming montmorin, but the latter shows six distinct orders (has no randomness). It will be noted that the Putnam colloid maintained its 19 A spacing through the ll0~ temperature (stabilized by vermiculite) whereas the 18 A spacing of Arkansas beidellite (not so stabilized) decreased appreciably at the same temperature (cf. the more extensive decrease with Ahmeek,41 and Hagerstown colloids wherein the montmorin is in large zones as shown by sharp 18 A peaks). The occurrence of only a low 10 A peak in the heated Putnam samples confirms the presence of chlorite and the other constituents shown in the analysis (Table 3).
13 230 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS A COLLOID CONTAINING MUCH MICA AND CHLORITE Ahmcek Loam, C2 Horizon The x-ray diffraction patterns of the coarse clay fraction of Ahmeek loam C2 horizon (42-48 inches, Fig, 1, B) clearly reveal the presence of mica (illite, 10 A spacing unchanged by heating to 500~ as well as chlorite (14 and 7.2 A spacings which remain unchanged on heating to 500~ The small maximum at 19.2 A shows that some montmorin is present, interstratified with chlorite and mica in the superlattice or other complexly interstratified system (as above), but the vermiculite content is lower (Table 3). This maximum disappears as a result of heating to ll0~ as does that of the montmorin in the surface of this soil (Fig. 1, A). The diffraction intensity of this soil chlorite is low compared with that of standard chlorite ground and fractionated at microns (Fig 1, B). In addition, the background in the region from 7 to 20 A is much lower for the standard chlorite than in the soil colloid of the same size fraction. These observations are taken to indicate the probable interstratification of some vermiculite and montmorin spacings in zones too small to be x-crystalline among the chlorite and mica x-crystalline zones. Because this is a coarse clay fraction from which the particles less than 0.2 microns in diameter had been removed after thorough dispersion, the high background cannot be attributed to particle size effect, except insofar as randomness along the Z-axis of single ultimate grains is truly a one-axis particle size effect. The chlorite formula for the material is, 2+ ~ 3+ (Si3.3sAlo.~2) (All.~sTio.32) (Alo.~4Mg1.20Feo.32Feo.44) O10 (OH)s in which brucite cations = The mica present has the formula: KI.oo (Si3.01Alo.99)(A11.00Mgo.67Feo.16FCo.23Tio.16), 2+ ~.3+ O10 (OH)2 in which octahedral cations = The vermiculite plus montmorin average formula is similar except for Xo.3z (Si3.5sAt0.42) in the first three terms. The departure from full dioctahedral or trioctahedral systems is expected because of the heterogeneous sources of the colloidal mineral particle population. Geochemical Relationships The depth function (Jackson, et al., 1948) of the weathering sequence is illustrated by the Ahmeek loam profile including the various horizons from//1 to C2, of which only the coarse clay fractions of two horizons are considered here. The weathering stages advance from much ordered chlorite and mica (stages 4 to 6) in the subsoil to much ordered vermiculite and montmorin (stages 8 and 9) in the surface. In the C2 horizon the mica and chlorite are largely in x-crystalline zones with vermiculite and montmorin split up in x-amorphous zones (Fig. 1, B); in the A 1 horizon the vermiculite and montmorin zones have grown large enough
14 ANALYSES OF ~OIL ~ILICATES 231 to be highly x-crystalline (Fig. 1, A). The percentage changes (Table 3) ark much less radical than the diffraction patterns indicate. Similarly, the percentage of feldspar species and quartz change systematically as follows :.,I 1 horizon C2 horizon Feldspars Anorthite 1 7 Albite 8 9 Orthoclase 10 5 Quartz The anorthite and albite decrease in the A 1 horizon in accord with the weathering sequence depth function (Jackson, et al., 1948; Jackson and Sherman, 1953) while quartz and the more resistant feldspar, orthoclase, increase. The occurrence of much feldspars in the clay fraction is not commou in fine-textured Wisconsin soils; it is attributed to the sandy texture and youthful weathering stage ill this soil. Increase of quartz in the A 1 horizon relative to the C 2 horizon (seemingly contrary to the weathering depth function) is common for the coarse clay fraction and is caused by (a) the size function and (b) the residue mechanism of weathering, the layer silicates having weathered out into the finer fraction. The weathering sequence "quartz to mica" (stage 6 to 7) applies best for the finer clay fractions, and applies better for size fractions "less than" a given diameter than to coarser fractions from which the layer silicates resulting from weathering have been removed. VERMICULITE AND MICA IN A ttydrol HUMIC LATOSOL IN RELATION TO MICA AND KAOLIN IN A TITANIFEROUS CERAMIC CLAY OF HAWAII Hilo, a Hydrol ttumic LatosoI The lnineral colloids of the Hilo and Akaka family of the Hydrol Humic Latosols of Hawaii contain (Tamura, et al., 1953) predominantly amorphous (allophane) materials, with some crystalline minerals including gibbsite, goethite, anatase, and a small percentage of mica. These analyses were made separately on the untreated and less than 0.2 micron fractions. The powder diffraction patterns of these colloids recorded on fihn show but faint evidence of vermiculite and mica. The B1 horizon of Hilo was further examined in the present study by means of the flat oriented sample technique applied to the mineral colloid after preconcentration of the 2:1 layer silicates by the iron oxide extraction procedure. The iron oxides extracted constituted 25.8 percent of the sample. Analyses of aluminmn in the extract solution showed that only 2.75 percent of AI(OH)a was removed, and thus the Aguilera and Jackson (1953) procedure is shown not to attack the aluminous components very much. (A
15 232 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS o 0 o t~l o o S/O AIISN':IIN I o o,i toa,~i ~ OI N 0 r o o,~,i J. o '-' ~ zl "u~ 4".n :~ ~._-- ~._.~ ~ r",r nr",,r,~.~ g i ~ o o o s/0 ),.LISN 3.LNI o o o N ~ 0d c) N~.~ o'-x o~,,d o, no N ~ g g, ~ a -J :D og~ - - I1: :l: :1: tu Fi6u~v: 5. I X-ray diffraction pallerns of lhe clay fractions from the Hilo family of tile Hydrol Humic Latosol group; (A) less than 0.2 micron fraction, containing much vermiculite and mica; (B) micron fraction containing much interstratified material.
16 ANALYSES OF SOIL SILICATES 233 control test of the procedure on a bauxite sample containing 81.1 percent gibbsite also showed that only 3.05 percent Al(OH)oo was dissolved.) The x-ray diffraction results for the Hilo fine clay (less than 0.2 microns, Fig. 5, A) show an abundance of vermiculite with some kaolin. The Mg saturated and glycerol solvated coarse clay fraction (Fig. 5, B) shows broad weak diffraction maxima over a range from 14 A to 10 A; its change to a strong 10 A maximum on heating establishes rather conclusively that the sample contains 2:1 layer silicates with various spacings randomly distributed and that the results cannot be explained by fineness of particle size. Potassium saturation of the specimen markedly enhanced the 10 A diffraction maximum without heating (the "Barshad effect"), indicating closure of some of the expanded interspaces. Barshad (1948) noted closure of 14 A spacings of Mg-treated biotite to 10 A on resaturation with K. Some quartz and kaolin are present, in agreement with Tamura, et al. (1953); further detailed study is being given to the vermiculite and other interstratified layer silicates of this clay on the basis of the present findings. A 14 A spacing (heat-shifted to 10 A, thus of vermiculite) was reported in an aluminous (gibbsite, boehmite) Latosot from Haiti by Aguilera and Jackson (1953), after the extraction of iron oxides. Titaniferous Ceramic Clay The titaniferous ceramic clay (kindly furnished by G. D. Sherman of the Department of Soils and Agricultural Chemistry, University of Hawaii) is a product of extreme tropical leaching under reducing conditions in the presence of acid surface organic matter. The annual rainfall is several hundred inches. The analyses.- The diffraction patterns of the clay fraction (Fig. 6) show that the material is very high in kaolin and illite with some interstratification (11.3 A spacing in lower curve). It was found that K saturation (as compared with Mg saturation) enhanced both the 10 A and 7.2 A diffraction maxima in the sample dried at room temperature, This enhancement may be attributable in part to increased basal orientation of the particles in the K saturated specimen, but the loss of the 11.3 A spacing proves that some "Barshad effect" closure occurred, enhancing the 10 A diffraction. The 7.2 A maximum remained constant in intensity in the K saturated specimen on heating from 25 to 350~ while the 10 A maximum increased progressively. Heating of the K-saturated st)ecimen to 400~ increased the diffraction intensity of both the 10 A and 7.2 A maximum as compared with the 350~ heating. It lowered the background under the 10 A maximum, indicating decrease in randonmess of basal spacings with dehydration of vermiculite and (or) montmorin interlayers interstratified in the mica. The increase of the 7.2 A maximum is attributed to expulsion of water from the small amount of hydrated halloysite present (confirmed by a few rods appearing in electron micrographs). 13rindley (1951, p. 55) described trapping of a portion of halloysite water
17 234 SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS TfTANIFEROUS CERAMIC CLAY O Io 5 z DIFFRACTrON AN6LE (2 e) FIGURE 6.--X-ray diffraction pattern of titaniferous ceramic clay fraction less than 2 microns. The marked increase in the 7.2 and 10 A spacings on heating to 400~ together with lowered background, indicate expanded interspaces in both the 1:1 and 2:1 components. The 5.05 percent K20 content must be allocated to mica containing 11.8 percent K20 when the kaolin, titanium (anatase) and interlayer surface components are weighed with the elemental data. during dehydration until a temperature of 350 to 400~ was reached. In the present case, the water expulsion from halloysite allows the halloysite diffraction to augment that of kaolinite (strong 7.19 A spacing before heating). Interstratification of a few hydrated layers in the kaolinite would of course also produce the diffraction intensity changes noted (Fig. 6). Calculation of the surface to vermiculite (5 percent), ~ water loss to kaolin (20 percent), TiO2 to anatase (21 percent), and suitable allocation of the other constituents gives 43 percent mica (Table 3). Since the total K20 content is 5.03 percent, the mica has ~20 or an amount equal to the full theoretical formula for muscovite. This is a
18 ANALYSES O1" SOIL SILICATES 235 significant finding when considered in relation to the firm qualitative and quantitative basis for the amounts of other constituents reported. A similar allocation shows 37 percent anatase, together with other constituents (Table 3), in the silt fraction. Related 9eochcmistry.--The question has been raised: What is the source of the mica in this extremely weathered titaniferous-illite-kaolin formation? The titanium content (Table 1) corresponds to the most advanced stage of chemical weathering (Jackson, et al., 1948; Jackson and Sherman, 1953), while mica in large amounts is not ordinarily associated with the anatase stage of weathering. The finding of a small but distinct residue of mica, kaolin, and vermiculite in the Hydrol Humic Latosol after removal of the iron oxides suggests that the mica of the titaniferous ceramic clay is a residue remaining after the free oxides and hydroxides have been leached out by the combined action of acidity and chelation by organic acids. The predominance of the residue mechanism in causing mica accumulation in soils has been advocated by Grim (1942). The kaolin and vermiculite contents of the two materials suggest that the vermiculite largely weathered to kaolin. The mica concentration process has already begun in the Hydrol Humic Latosol, since the iron oxide content is only half that in the Ferruginous ttumic Latosol (Tamura, et al., 1953). Titanium is less subject to leaching though it apparently leaches somewhat (Sherman, 1952). On the basis that good oxidation and open drainage have a positive sign (Jackson, et al., 1948) in the weathering function, leaching in the presence of an organic layer and reducing conditions has a negative functional sign. The weathering reactions, and therefore the weathering sequence, are reversed (advanced, oxide stages removed; early stage, mica left as a residue). COLLOIDS HIGH IN KAOLIN AND GIBBSITE Application of the method of allocation to colloids high in gibbsite and kaolin (both kaolinite and hydrated halloysite) provides a more severe test of its applicability than the above colloids, because of the increased variety of water loss energies, specific surface, and elemental sources. Pearson and Ensminger (1949) reported analyses of high kaolin with some gibbsite in the clay of Davidson soil of Alabama. They observed an unidentified diffraction maxinmm at 14.3 A, indicating the presence of some 2:1 and (or) 2:2 layer silicates, and a relatively high cation exchange capacity of 30 to 45 meq per 100 g. Dr. R. W. Pearson kindly furnished the authors a Davidson soil sample from Alabama similar to the one reported in 1949 for purposes of further analysis and test of our methods. The "free" iron oxides (2.9 percent FeeOa) were removed and excluded from the following analysis.
19 ~ SECOND NATIONAL CONFERENCE ON CLAYS AND CLAY MINERALS Davldson Soil, Fine Clay The diffraction patterns of the colloid finer than 0.2 microns (Fig. 7 A) show much kaolinite and some halloysite (increased 3.55 A diffraction intensity on heating), some gibbsite, and the 14 A diffraction maximum in accord with Pearson and Ensminger (1949). Saturation with K shifted the spacing to 13.8 A (a bit of "Barshad effect" in the interstratified vermiculite). Heating to 500~ decreased the spacing to 12.3 A, showing the presence of chlorite with interstratified vermiculite and (or) montmorin. The presence of chlorite is established by failure of the 10 A diffraction maximum to be resolved by heating to 500~ Allocation of the low temperature water loss to gibbsite, halloysite, and vermiculite, the K20 to mica, the interlayer surface to vermiculite and montmorin and the other elements and water loss accordingly gave the mineral content reported in Table 3. The distribution of elements is such that the percentage of each component is certain within narrow limits. The montmorin is definitely established by the surface allocation between that mineral and vermiculite. The presence of the vermiculite and montmorin explains the relatively high exchange capacities observed by Pearson and Ensminger (1949), although the presence of montmorin was not detected by diffraction methods in either their study or ours because the DAVII3SON CLAY / <O.2 FRACTION f SATUR- DRYING f IOO DAVIDSON CLAY FRACTION / / 13~2A~ SATUR- DRYING T_F.,Y_2.,1,o"" / I00 K 525 ~ ~ ~ K 525 ~ zjaa I fl.,',a~ ~.~st,~00 o,,.,;r ~. 4.80A ~ Z - ; \.oo, II,A)/ - Mg 25' ~' ' ' 3'(I DIFFRACTION ANGLE (2e) DIFFRACTION ANGLE ('20) FIGURI'; 7.--X-ray diflraction patterns of (A) fine clay fractions, and (B) coarse clay fractions, of Davidson clay soil showing abundant kaolin and presence of interstratified layer silicates including vermiculite and (or) montmorin with chlorite.
20 ANALYSES OF SOIL SILICATES 237 montmorin, like the mica present, is entirely interstratified in zones too limited to show their diffraction maxima. Davidson Soil, Coarse Clay The x-ray diffraction patterns of the coarse clay (2-0.2 microns, Fig. 7 B ) show nmch kaolin and the 14 A diffraction maximum, in accord with Pearson and Fnsminger (1949). Rise of the kaolin 7.13 A maximum on heating to 400~ establishes the presence of some halloysite, and the shift of the 14 A diffraction maxinmm shows that some vermiculite and (or) montmorin are interstratified with chlorite. Heating to 500~ shifted the 14 ~k spacings to a range of 10 to 14 A; this establishes the presence of some chlorite with the vermiculite and (or) montmorin. Allocation of all the low temperature water to halloysite was not permissible, from the elemental analyses. The presence of about 5 percent gibbsite was indicated although no gibbsite diffraction maximum was noted for this fraction. Again, as for the fine clay, the allocation of interlayer surface, water, and elements gave the mineral composition shown in Table 3, within surprisingly narrow confidence limits. The montmorin, as in the fine clay, is interstratified and thus does not show a discrete diffraction maximum. OCCURRENCE OF INTERSTRATIFICATION LAYER SILICATES GENERALLY A comparison of the intensities of the diffraction maxima of pure specimens with those of soils and many sediments shows a radically higher intensity of the diffraction maxima of the pure specimen material. The presence of fine particle size, crystal defects, and of amorphous materials could in part explain the lower basal diffraction intensity. However, the frequent rise in 10 to 7 A diffraction intensity on heating could scarcely occur except through decrease in randonmess of the Z-axis spacings through removal of water layers. Also, the composition and properties of the soil colloids and the general persistence of a fairly strong 4.45 A (hko) diffraction line in powder diffraction patterns (fihn recorded, not shown) suggest that they are layer silicates which have much x-amorphous (randomly spaced) material distributed along the Z-axis, i.e., that the materials are interstratified. The fact that the simpler binary (2-component) randomness (Gruner, 1934) occurs commonly to give the Hendricks and Teller (1942) intermediate spacings, for example the 11.3 A spacings in Figure 6, is also in accord with the expectation of not uncommon occurrence of ternary, quaternary (mica-montmorin-vermiculite-chlorite), and greater randomness (binary-binary systems, etc.) through interstratification along the Z-axis. In this way, much or all of the basal diffraction is reduced to a general low angle scatter consisting of a merging of the broadened maxima of virtually all spacings. The common IN
21 238 SECOND NATIONAL CONFERI,.'NCE ON CLAYS AND CLAY MINERALS occurrence of a definite though broad diffraction maxinmnl at approximately 3.5 A (corresponding to 005 of 18 A, 004 of 14 A, 003 of 10 A, and 002 of 7 A spacings) points to the occurrence of these Z-axis spacings in x-amorphous zones. Each x-amorphous zone has too few repetitions to show a distinct lnaximum, but the SUln total of their scattering is responsible for the high intensity of low angle diffraction background. The specific surface measurements do not allow fine enough particle size to permit the high background to be atlributed to ordinary particle size effects alone. (Randomness or interstratification along the Z-axis is a o~e dimv~sional fine-particle si,~e c.!tcct.) Excessively broadened diffractmn maxima and high backgrounds were in fact correlated with lower specific surface than that of some of the samples with high sharp diffraction maxima. The broadening has therefore been attributed to interstratification of low surface minerals, such as micas and chlorite with high surface minerals such as vermiculite and monhnorin. Even with the relatively intense 10 A diffraction maximum for illite in the titaniferous ceramic clay, the intensity was markedly increased by heating in increments up to 400~ This fact indicates closure of spacings greater than 10 A due to interstratified vermiculite or montmorin with consequent decrease in randonmess along the Z-axis in the assemblages which contain nmch relatively pure mica. Increases in the 7.2 A diffraction lnaximum of this sample on heating similarly indicate decrease in randomness of the halloysite spacings. Hendricks and Alexander (1941) showed the presence of up to 20 percent montmorin in illite (Grim, et al., 1937) and in the Ordovician bentonite from High Bridge, Kentucky, based on the exothermic peak at ~ obtained in differential thermal analysis (Hendricks, et al., 1940). This was confirmed through hydrogen-cerous competitive exchange studies. The cerous fraction was 0.06 for sericite (mica ratio), and on the order of 0.9 for montmorins. The fraction was 0.30 for a glauconite (greensand, New Jersey), anti 0.22 for the type illite referred to above. The fraction was about 0.9 for Ordovician bentonites (micaceous) and glimmerton (Maegdefrau and tlofmann, 1937) ; the similarity of the ratio for these micaceous materials to that of montmorin was explained by the presence of montmorin in them in appreciable amounts. The montmorin component would dominate the ratio because of the low exchange capacity of the true mica component. Interstratification of montmorin with mica spacings was shown in illite from Pennsylvanian underclay (Jackson, et al., 1952, Fig. 1, b). Complex intcrstratification with clearly defined diffraction maxima shown for mica and vermiculite and their interlnixtures in a single crystal were shown also (Jackson, ct al., 1952, Fig. 1, a) and the occurrence of open interspaces (montmorin and vermiculite) in m icaceous layer silicate clays was explained bv the preferential weathering plane principle. These findings are in accord with the present findings of the rather general occurrence of interstratification of different 2:1 anti 2:2 (chlorite) layer
22 ANALYSES OF ~OIL ~ILICATES 239 silicates in clays. Partially dehydrated halloysite accounts for Z-axis spacing randomness in the 1:1 layer silicates encountered in this study. SUMMARY Allocations of the necessary analytical data (including diffraction, thermal, surface, and elemental data) show the l)ol),component nature of clays generally, and also the general occurrence of intcrstratification in layer silicates. The interstratification may possibly involve a "supcrlattice" which reflects the mica-to-chlorite to-vermiculite to-montmorin weathering sequence. RFA:I:ICF.NCES Aguilera, N. H., and Jackson, M. I.. (1953) h'on oa'ide removal from soils and clays: Soil Sci. Soc. Am. Proc., v. 17, p Barshad, I. (1948) l'ermiculite and its relation to biotite as revealed by base exchange re~lctions,.r-ray analyses, differential thermal curves, and water content: Am. Min., v. 33, p Bray, R. H. (1937) The significance of particle si;e within the clay fraction: Jour. Am. Ceram. Soc., v. 20, p Brindley, G. \V. (195l) The kaolin minerals: In "X-ray identification and cryshd structures of clay minerals": Mineralogical Society of Great Britain Monograph, Chap. 2, p Byers, if. G., Alexander, L. T., and Holmes, R. S. (1935) The composition and constitution of colloids of certain of the great groups of soils: U. S. Dept. Agrie. Tech. Bull Corey, R. B., and Jackson, M. L. (1953) Silicate analysis by a rapid semi-microchemical system: Anal. Chem., v. 25, p Grim, R. E. (1942) Modern concepts of clay materials: Jour. Geol., v. 50, p , Bray, R. H., and Bradley, W. F. (1937) The mica in argillaceous sediments: Am. Min., v. 22, p Gruner, J. W. (1934) The structure of vermiculites and their collapse by dehydration: Am. Min., v. 19, p Hendrieks, S. B. (1939) Polymorphisn~ of the micas and diffuse x-ray scattering of layer silicate lattices: Nature, v. 143, p. 800., and Alexander, L. T. (1941) Nemiquqntitative estimation of montmorillonite in clay: Soil Sei. Soc. Am. Proe., v. 5, p , Nelson, R. A., and Alexander, L. T. (1940) Hydration mechanism of the clay mineral montmorillonite saturated with various cations: Jour. Am. Chem. Soc., v. 62, p , and Teller, E. (1942) Interference by disordered lattices: Jour. Chem. Phys., v. 10, p Jackson, M. L., Hseung, Y., Corey, R. B., Ewms, E. J., and Vanden Heuvel, R. C. (1952) l/veathering sequence of clay-sice minerals in soils and sediments: II. Chemical weathering of layer silicates: Soil Sci. Soc. Am. Proc., v. 16, p Jackson, M. L., and Sherman, G. D. (1953) Chemical weathering of minerals in soils: Adv. in Agron., v. 5, p. 21%318. Jackson, M. L., Tyler, S. A., Willis, A. L., Bourbeau, G. A., and Pennington, R. P. (1948) Weathering sequence of clay-site minerals in soils and sediments: 1. Fundamental 9enerali.vations: Jour. Plays. Coll. Chem., v. 52, p Jackson, M. L., Whittig, L. D., and I'ennington, R. P. (1950) Segregation procedure for the mineralogical analysis of soils: Soil Sci. Soc. Am. Proc., v. 14, p
23 240 SECOND NATIONAl. CONFERENCE ON CLAYS AND CLAY ~IINERALS Jackson, M. L., \Vhittig, L. D,, and Vanden Heuvel, R. C. (1955) Criteria for the idontificatian of callaidal layer silicate series and assacialed amorphous material in soils and sediments: Soil Sci. Soc. Am. Proc., v. 19 (in press). Jeffries, C. D., and Jackson, M. L. (1949) Minoralagical analysis of soils: Soil Sci., v. 67, p Maegdefrau, E., and l tofmann, U. (1937) U. 91immerali.qe Mincralien als Tonsubstanzen: Zlschr. Nrist., v. 98A, p Marshall, C. E. (1935) Mineralaaical methods for the study of sills and clays: Ztschr. Krist., v. 90, p Pearson, R. \V., and Ensminger, L. E. (1049) Types of clay minerals in Alabama sails: Soil Sci. Soc. Am. Proc., v. 13, p Pemfington, R. P., and Jackson, M. L. (1948).S'eqreaatian of the clay minerals of palycampanent sail clays: Soil Sci. Soc. Am. Proc., v. 12, p Sherman, G. D. (1952) The titanium cantent of Hawaiian soils and its sianificance: Soil Sci. Soc. Am. Proc., v. 16, p Stephen. I., and Macgwan, I). M. C. (1951) 5"ome chlaritic clay minerals of unusual type: Clay Min. Bult. 1, p Tamura, T., Jackson, M. L., and Sherman, G. D. (1953) Mineral content af Low tlumic, llumic and Hydrol Humic Latosols of Hawaii: Soil Sci. Soc. Am. t:'roc., v. 17, p U.S.D.A. (1952) Yield and laborato13, data on some Podzol, Brown Podzalic, Brown Farest, and Gray 14Zoadcd sails in narthern United Stales and southern Canada: Soil Survey Laboratory Memorandum No. 1. Vanden Heuvel, R. C., and Jackson, M. L. (1955).S'urface determination of mineral colloids by 9lyerol sorptian and its application to interstratified layer silicates: Soil Sci. Soc. Am. Proc., v. 19 (in press). \Valker, G. F. (195t) }'ermiculites and some related mixed-layer ~ninerals: In "X-ray identification and crystal structures of clay minerals": Mineralogical Society of Great Britain Monograph, Chap. 7, p
MINERAL CONTENT AND DISTRIBUTION AS INDEXES OF WEATHERING IN THE OMEGA AND AHMEEK SOILS OF NORTHERN WISCONSIN
 MINERAL CONTENT AND DISTRIBUTION AS INDEXES OF WEATHERING IN THE OMEGA AND AHMEEK SOILS OF NORTHERN WISCONSIN By L. D. WHITTIG 1 AND M. L. JACKSON University of Wisconsin, Madison, Wisconsin ABSTRACT Quantitative
MINERAL CONTENT AND DISTRIBUTION AS INDEXES OF WEATHERING IN THE OMEGA AND AHMEEK SOILS OF NORTHERN WISCONSIN By L. D. WHITTIG 1 AND M. L. JACKSON University of Wisconsin, Madison, Wisconsin ABSTRACT Quantitative
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
CLAY MINERAL DISTRIBUTION IN THE HIAWATHA SANDY SOILS OF NORTHERN WISCONSIN 1
 CLAY MINERAL DISTRIBUTION IN THE HIAWATHA SANDY SOILS OF NORTHERN WISCONSIN 1 By B. E. BROWN AND M. L. JACKSON Department of Soils, University of Wisconsin, Madison ABSTRACT Mineralogical analyses were
CLAY MINERAL DISTRIBUTION IN THE HIAWATHA SANDY SOILS OF NORTHERN WISCONSIN 1 By B. E. BROWN AND M. L. JACKSON Department of Soils, University of Wisconsin, Madison ABSTRACT Mineralogical analyses were
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION
 THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
Soil Colloidal Chemistry. Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India
 Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
CHEMICAL, PHYSICAL, AND MINERALOGICAL PROPERTIES OF CERTAIN SOIL PROFILES IN THE LOWER MISSISSIPPI DELTA B. N. DRISKELL ABSTRACT
 CHEMICAL, PHYSICAL, AND MINERALOGICAL PROPERTIES OF CERTAIN SOIL PROFILES IN THE LOWER MISSISSIPPI DELTA B. N. DRISKELL Louisiana State University ABSTRACT The soils of the lower Mississippi Delta are
CHEMICAL, PHYSICAL, AND MINERALOGICAL PROPERTIES OF CERTAIN SOIL PROFILES IN THE LOWER MISSISSIPPI DELTA B. N. DRISKELL Louisiana State University ABSTRACT The soils of the lower Mississippi Delta are
ANOMALIES IN TILE ETHYLENE GLYCOL SOLVA- TION TECHNIQUE USED IN X-RAY DIFFRACTION * ABSTRACT
 ANOMALIES IN TILE ETHYLENE GLYCOL SOLVA- TION TECHNIQUE USED IN X-RAY DIFFRACTION * G. W. KUNZE Agricultural and lv[echanical College of Texas ABSTRACT X-ray diffraction results are presented to show that
ANOMALIES IN TILE ETHYLENE GLYCOL SOLVA- TION TECHNIQUE USED IN X-RAY DIFFRACTION * G. W. KUNZE Agricultural and lv[echanical College of Texas ABSTRACT X-ray diffraction results are presented to show that
Adsorption of ions Ion exchange CEC& AEC Factors influencing ion
 Adsorption of ions Ion exchange CEC& AEC Factors influencing ion exchange- Significance. Adsorption of ions Ion adsorption and subsequent exchange are important processes that take place between soil colloidal
Adsorption of ions Ion exchange CEC& AEC Factors influencing ion exchange- Significance. Adsorption of ions Ion adsorption and subsequent exchange are important processes that take place between soil colloidal
Analysis of Clays and Soils by XRD
 Analysis of Clays and Soils by XRD I. Introduction Proper sample preparation is one of the most important requirements in the analysis of powder samples by X-ray diffraction (XRD). This statement is especially
Analysis of Clays and Soils by XRD I. Introduction Proper sample preparation is one of the most important requirements in the analysis of powder samples by X-ray diffraction (XRD). This statement is especially
HETEROGENEITY IN MONTMORILLONITE. JAMES L. MCATEE, JR. Baroid Division, National Lead Co., Houston, Texas
 HETEROGENEITY IN MONTMORILLONITE By JAMES L. MCATEE, JR. Baroid Division, National Lead Co., Houston, Texas ABSTRACT X-ray diffraction patterns and cation-exchange data are presented for centrifuged Wyoming
HETEROGENEITY IN MONTMORILLONITE By JAMES L. MCATEE, JR. Baroid Division, National Lead Co., Houston, Texas ABSTRACT X-ray diffraction patterns and cation-exchange data are presented for centrifuged Wyoming
Weathering and mineral equilibria. Seminar at NGU 23 May 2016 Håkon Rueslåtten
 Weathering and mineral equilibria Seminar at NGU 23 May 2016 Håkon Rueslåtten Weathering is the breakdown of rocks and minerals that are exposed to surface processes (climatically controlled). Water is
Weathering and mineral equilibria Seminar at NGU 23 May 2016 Håkon Rueslåtten Weathering is the breakdown of rocks and minerals that are exposed to surface processes (climatically controlled). Water is
The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures.
 657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
Particle size distribution of mineral species, a measure of chemical weathering and a control of chemical activity through specific surface
 Page 100 Chapter 3 MINERAL FRACTIONATION FOR SOILS Particle size distribution of mineral species, a measure of chemical weathering and a control of chemical activity through specific surface Contents:...
Page 100 Chapter 3 MINERAL FRACTIONATION FOR SOILS Particle size distribution of mineral species, a measure of chemical weathering and a control of chemical activity through specific surface Contents:...
CERAMIC MATERIALS I. Asst. Prof. Dr. Ayşe KALEMTAŞ
 CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
PRETREATMENT OF SOILS AND CLAYS FOR MEASUREMENT OF EXTERNAL SURFACE AREA BY GLYCEROL RETENTION
 PRETREATMENT OF SOILS AND CLAYS FOR MEASUREMENT OF EXTERNAL SURFACE AREA BY GLYCEROL RETENTION by EARL B. Kn~TEB AND SIDNEY D~_MO~D Division of Physical Research, Bureau of Public Roads, Washington, D.C.
PRETREATMENT OF SOILS AND CLAYS FOR MEASUREMENT OF EXTERNAL SURFACE AREA BY GLYCEROL RETENTION by EARL B. Kn~TEB AND SIDNEY D~_MO~D Division of Physical Research, Bureau of Public Roads, Washington, D.C.
 26. MIXED-LAYER ILLITE/MONTMORILLONITE CLAYS FROM SITES 146 AND 149 Herman E. Roberson, State University of New York, Binghamton, New York INTRODUCTION The purpose of this report is to describe the clay
26. MIXED-LAYER ILLITE/MONTMORILLONITE CLAYS FROM SITES 146 AND 149 Herman E. Roberson, State University of New York, Binghamton, New York INTRODUCTION The purpose of this report is to describe the clay
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
A few more details on clays, Soil Colloids and their properties. What expandable clays do to surface area. Smectite. Kaolinite.
 A few more details on clays, Soil Colloids and their properties What expandable clays do to surface area Kaolinite Smectite Size 0.5-5 µm External surface 10-30 m 2 /g Internal surface - Size 0.1-1 µm
A few more details on clays, Soil Colloids and their properties What expandable clays do to surface area Kaolinite Smectite Size 0.5-5 µm External surface 10-30 m 2 /g Internal surface - Size 0.1-1 µm
Clays and Clay Minerals
 Clays and Clay Minerals Fields of interest for clays Various definitions Acients: Earths in the earth-air-fire-water system Definition of clay depends on discipline: Geologist grain size
Clays and Clay Minerals Fields of interest for clays Various definitions Acients: Earths in the earth-air-fire-water system Definition of clay depends on discipline: Geologist grain size
Tikrit University. College of Engineering Civil engineering Department SOIL PROPERTES. Soil Mechanics. 3 rd Class Lecture notes Up Copyrights 2016
 Tikrit University SOIL PROPERTES College of Engineering Civil engineering Department Soil Mechanics 3 rd Class Lecture notes Up Copyrights 2016 1-Soil Composition -Solids -Water -Air 2-Soil Phases -Dry
Tikrit University SOIL PROPERTES College of Engineering Civil engineering Department Soil Mechanics 3 rd Class Lecture notes Up Copyrights 2016 1-Soil Composition -Solids -Water -Air 2-Soil Phases -Dry
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
ON THE DIFFERENTIATION OF VERMICULITES AND SMECTITES IN CLAYS
 ON THE DIFFERENTIATION OF VERMICULITES AND SMECTITES IN CLAYS By G. F. WALKER. C.S.I.R.O., Division of Industrial Chemistry, Melbourne, Australia. [MS. received 26th March, 1957.] ABSTRACT Certain of the
ON THE DIFFERENTIATION OF VERMICULITES AND SMECTITES IN CLAYS By G. F. WALKER. C.S.I.R.O., Division of Industrial Chemistry, Melbourne, Australia. [MS. received 26th March, 1957.] ABSTRACT Certain of the
Prof. B V S Viswanadham, Department of Civil Engineering, IIT Bombay
 05 Clay particle-water interaction & Index properties Electrical nature of clay particles a) Electrical charges i) The two faces of all platy particles have a negative charge. Resulting due to isomorphous
05 Clay particle-water interaction & Index properties Electrical nature of clay particles a) Electrical charges i) The two faces of all platy particles have a negative charge. Resulting due to isomorphous
CHLORITIZED WEATHERING PRODUCTS OF A NEW ENGLAND GLACIAL TILL
 CHLORITIZED WEATHERING PRODUCTS OF A NEW ENGLAND GLACIAL TILL R. M. QUIGLEY~ AND R. T. MARTIN Soil Engineering Division, Massachusetts Institute of Technology, Cambridge, Massachusetts ABSTRACT The clay
CHLORITIZED WEATHERING PRODUCTS OF A NEW ENGLAND GLACIAL TILL R. M. QUIGLEY~ AND R. T. MARTIN Soil Engineering Division, Massachusetts Institute of Technology, Cambridge, Massachusetts ABSTRACT The clay
Soil Mechanics Prof. B.V.S. Viswanadham Department of Civil Engineering Indian Institute of Technology, Bombay Lecture 3
 Soil Mechanics Prof. B.V.S. Viswanadham Department of Civil Engineering Indian Institute of Technology, Bombay Lecture 3 In the previous lecture we have studied about definitions of volumetric ratios and
Soil Mechanics Prof. B.V.S. Viswanadham Department of Civil Engineering Indian Institute of Technology, Bombay Lecture 3 In the previous lecture we have studied about definitions of volumetric ratios and
Be sure to show all calculations so that you can receive partial credit for your work!
 Agronomy 365T Exam 1 Spring 2004 Exam Score: Name TA Lab Hour Be sure to show all calculations so that you can receive partial credit for your work! 1) List 14 of the plant essential nutrient for plant
Agronomy 365T Exam 1 Spring 2004 Exam Score: Name TA Lab Hour Be sure to show all calculations so that you can receive partial credit for your work! 1) List 14 of the plant essential nutrient for plant
J. U. ANDERSON. Agronomy Department, New Mexico State University, University Park, New Mexico ABSTRACT
 Page - 380 - AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS OF SAMPLES CONTAINING ORGANIC MATTER by J. U. ANDERSON Agronomy Department, New Mexico
Page - 380 - AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS OF SAMPLES CONTAINING ORGANIC MATTER by J. U. ANDERSON Agronomy Department, New Mexico
NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE
 Clay Minerals (1986) 21, 225-230 225 NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE Albite, muscovite granite and greisens of the Montebras cupola, Creuse,
Clay Minerals (1986) 21, 225-230 225 NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE Albite, muscovite granite and greisens of the Montebras cupola, Creuse,
Lecture 13 More Surface Reactions on Mineral Surfaces. & Intro to Soil Formation and Chemistry
 Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
CESIUM SORPTION REACTIONS AS INDICATOR CLAY MINERAL STRUCTURES
 CESIUM SORPTION REACTIONS AS INDICATOR CLAY MINERAL STRUCTURES OF TsuNEo by TAMURA Health Physics Division, Oak Ridge National Laboratory, 1 Oak Ridge, Tennessee ABSTRACT At low cesium ion concentrations
CESIUM SORPTION REACTIONS AS INDICATOR CLAY MINERAL STRUCTURES OF TsuNEo by TAMURA Health Physics Division, Oak Ridge National Laboratory, 1 Oak Ridge, Tennessee ABSTRACT At low cesium ion concentrations
Soil Sequences in the Hawaiian Islands 1
 THE WIDE RANGE of conditions under which soils have developed in the Hawaiian Islands has produced a pattern of soil geography which reflects the differential influence of the intensity and capacity factors
THE WIDE RANGE of conditions under which soils have developed in the Hawaiian Islands has produced a pattern of soil geography which reflects the differential influence of the intensity and capacity factors
AN EXPANSIBLE MINERAL HAVING
 Clays and Clay Minerals, Vol. 21, pp. 185-190. Pergamon Press 1973. Printed in Greal Britain AN EXPANSIBLE MINERAL HAVING REHYDRATION ABILITY HIGH KATSUTOSHI TOMITA and MITSUHIKO DOZONO Institute of Earth
Clays and Clay Minerals, Vol. 21, pp. 185-190. Pergamon Press 1973. Printed in Greal Britain AN EXPANSIBLE MINERAL HAVING REHYDRATION ABILITY HIGH KATSUTOSHI TOMITA and MITSUHIKO DOZONO Institute of Earth
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
CATION EXCHANGE BETWEEN MIXTURES OF CLAY MINERALS AND BETWEEN A ZEOLITE AND A CLAY MINERAL*
 CATION EXCHANGE BETWEEN MIXTURES OF CLAY MINERALS AND BETWEEN A ZEOLITE AND A CLAY MINERAL* by PATRICK J. DENNY and RUSTUM ROY Pennsylvania State University, University Park, Pa. ABSTRACT The electron
CATION EXCHANGE BETWEEN MIXTURES OF CLAY MINERALS AND BETWEEN A ZEOLITE AND A CLAY MINERAL* by PATRICK J. DENNY and RUSTUM ROY Pennsylvania State University, University Park, Pa. ABSTRACT The electron
CLAY MINERALS AT A PENNSYLVANIAN DISCONFORMITY 1
 CLAY MINERALS AT A PENNSYLVANIAN DISCONFORMITY 1 By JANE A. DALTON z, ADA SWINEFORD, AND J. M. JEWETT State Geological Survey, University of Kansas, Lawrence ABSTRACT At the Dcsmoinesian-Missourian disconformity
CLAY MINERALS AT A PENNSYLVANIAN DISCONFORMITY 1 By JANE A. DALTON z, ADA SWINEFORD, AND J. M. JEWETT State Geological Survey, University of Kansas, Lawrence ABSTRACT At the Dcsmoinesian-Missourian disconformity
Particles in aqueous environments
 Lecture 11 Particle-Aqueous Solute Interactions Today 1. Particle types and sizes 2. Particle charges 3. Particle-solute Interactions Next time Please continue to read Manahan Chapter 4. 1. Fresh-salt
Lecture 11 Particle-Aqueous Solute Interactions Today 1. Particle types and sizes 2. Particle charges 3. Particle-solute Interactions Next time Please continue to read Manahan Chapter 4. 1. Fresh-salt
WEATHERING ACCORDING TO THE CATIONIC BONDING ENERGIES OF COLLOIDS I ABSTRACT
 WEATHERING ACCORDING TO THE CATIONIC BONDING ENERGIES OF COLLOIDS I By E. R. GRAHAM University of Missouri ABSTRACT A study was made of the energy changes of several colloidal systems in relation to weathering.
WEATHERING ACCORDING TO THE CATIONIC BONDING ENERGIES OF COLLOIDS I By E. R. GRAHAM University of Missouri ABSTRACT A study was made of the energy changes of several colloidal systems in relation to weathering.
A horizon. Clay Basics. Clays: A horizon. Phyllosilicates phyllon = leaves. Clay formation. Zone were parent materials weather
 A horizon lay Basics Start focus on A horizon Zone were parent materials weather Original rocks and minerals break down into smaller and smaller pieces Eventually dissolve A horizon Zone were new materials
A horizon lay Basics Start focus on A horizon Zone were parent materials weather Original rocks and minerals break down into smaller and smaller pieces Eventually dissolve A horizon Zone were new materials
Lecture 14: Cation Exchange and Surface Charging
 Lecture 14: Cation Exchange and Surface Charging Cation Exchange Cation Exchange Reactions Swapping of cations between hydrated clay interlayers and the soil solution Also occurs on organic matter functional
Lecture 14: Cation Exchange and Surface Charging Cation Exchange Cation Exchange Reactions Swapping of cations between hydrated clay interlayers and the soil solution Also occurs on organic matter functional
Chapter I Basic Characteristics of Soils
 Chapter I Basic Characteristics of Soils Outline 1. The Nature of Soils (section 1.1 Craig) 2. Soil Texture (section 1.1 Craig) 3. Grain Size and Grain Size Distribution (section 1.2 Craig) 4. Particle
Chapter I Basic Characteristics of Soils Outline 1. The Nature of Soils (section 1.1 Craig) 2. Soil Texture (section 1.1 Craig) 3. Grain Size and Grain Size Distribution (section 1.2 Craig) 4. Particle
A Method, tor Determining the Slope. or Neutron Moisture Meter Calibration Curves. James E. Douglass
 Station Paper No. 154 December 1962 A Method, tor Determining the Slope or Neutron Moisture Meter Calibration Curves James E. Douglass U.S. Department of Agriculture-Forest Service Southeastern Forest
Station Paper No. 154 December 1962 A Method, tor Determining the Slope or Neutron Moisture Meter Calibration Curves James E. Douglass U.S. Department of Agriculture-Forest Service Southeastern Forest
CLAY MINERALS BULLETIN
 CLAY MINERALS BULLETIN JULY, 196 Vol. 4, No. 23 CHANGES EFFECTED IN LAYER SILICATES BY HEATING BELOW 55~ * By C. M. WARSHAW, P. E. ROSENBERG and R. RoY. The Pennsylvania State University, University Park,
CLAY MINERALS BULLETIN JULY, 196 Vol. 4, No. 23 CHANGES EFFECTED IN LAYER SILICATES BY HEATING BELOW 55~ * By C. M. WARSHAW, P. E. ROSENBERG and R. RoY. The Pennsylvania State University, University Park,
A QUANTITATIVE METHOD FOR THE DETERMINATION OF MONTMORILLONITE IN SOILS*
 Cta)'.s am/ Clay Mim'rals. Vol, 23. pp. 85 89. Pergamon Press 1975. Printed in Great Britain A QUANTITATIVE METHOD FOR THE DETERMINATION OF MONTMORILLONITE IN SOILS* RACHEL LEVY and C. W. FRANCIS t Agricultural
Cta)'.s am/ Clay Mim'rals. Vol, 23. pp. 85 89. Pergamon Press 1975. Printed in Great Britain A QUANTITATIVE METHOD FOR THE DETERMINATION OF MONTMORILLONITE IN SOILS* RACHEL LEVY and C. W. FRANCIS t Agricultural
Lecture 6: Soil Profiles: Diagnostic Horizons
 Lecture 6: Soil Profiles: Diagnostic Horizons Complexity in Soil Profiles Soil Horizons Soils display distinct layering O Horizon: Partially decomposed organic matter (OM) A Horizon: Near surface, mineral
Lecture 6: Soil Profiles: Diagnostic Horizons Complexity in Soil Profiles Soil Horizons Soils display distinct layering O Horizon: Partially decomposed organic matter (OM) A Horizon: Near surface, mineral
Types of Occurrence of Nontronite and Nontronite-like Minerals in Soils l
 Types of Occurrence of Nontronite and Nontronite-like Minerals in Soils l G. DONALD SHERMAN, HARUYOSHI IKAWA, GORO UEHARA, AND ERNEST OKAZAKI 2 NONTRONITE, the iron-rich dioctahedral mineral of the montmorillonite
Types of Occurrence of Nontronite and Nontronite-like Minerals in Soils l G. DONALD SHERMAN, HARUYOSHI IKAWA, GORO UEHARA, AND ERNEST OKAZAKI 2 NONTRONITE, the iron-rich dioctahedral mineral of the montmorillonite
Soil physical and chemical properties the analogy lecture. Beth Guertal Auburn University, AL
 Soil physical and chemical properties the analogy lecture. Beth Guertal Auburn University, AL Soil Physical Properties Porosity Pore size and pore size distribution Water holding capacity Bulk density
Soil physical and chemical properties the analogy lecture. Beth Guertal Auburn University, AL Soil Physical Properties Porosity Pore size and pore size distribution Water holding capacity Bulk density
STABILITIES OF THREE-LAYER PHYLLOSILICATES RELATED TO THEIR IONIC-COVALENT BONDING by
 STABILITIES OF THREE-LAYER PHYLLOSILICATES RELATED TO THEIR IONIC-COVALENT BONDING by JOHN W. TLAPEK The California Company, Jackson, Miasissippi and W. D. KELLER University of Missouri, Columbia, Missouri
STABILITIES OF THREE-LAYER PHYLLOSILICATES RELATED TO THEIR IONIC-COVALENT BONDING by JOHN W. TLAPEK The California Company, Jackson, Miasissippi and W. D. KELLER University of Missouri, Columbia, Missouri
X-RAY STUDIES OF THE ALTERATION OF SODA FELDSPAW ABSTRACT
 X-RAY STUDIES OF THE ALTERATION OF SODA FELDSPAW By G. W. BRINDLEY AND E. W. RADOSLOVICH Department of Ceramic Technology, The Pennsylvania State University, University Park, Pennsylvania ABSTRACT Studies
X-RAY STUDIES OF THE ALTERATION OF SODA FELDSPAW By G. W. BRINDLEY AND E. W. RADOSLOVICH Department of Ceramic Technology, The Pennsylvania State University, University Park, Pennsylvania ABSTRACT Studies
EXPANSION OF POTASSIUM-DEPLETED MUSCOVITE*
 EXPANSION OF POTASSIUM-DEPLETED MUSCOVITE* by A. D. SCOTT and M. G. REEDf Iowa State University, Ames, Iowa ABSTRACT MUSCOVITE samples were K-depleted with NaCl-NaTPB solutions. By varying the extraction
EXPANSION OF POTASSIUM-DEPLETED MUSCOVITE* by A. D. SCOTT and M. G. REEDf Iowa State University, Ames, Iowa ABSTRACT MUSCOVITE samples were K-depleted with NaCl-NaTPB solutions. By varying the extraction
Clay Science 9, (1996)
 Clay Science 9, 335-345 (1996) ALTERATION OF MICA AND CHLORITE IN PADDY SOILS DERIVED FROM TRIASSIC AND JURASSIC SEDIMENTS YASUO KITAGAWA and KATSUHIKO ITAMI Fukui Prefectural University, Matsuoka, Fukui
Clay Science 9, 335-345 (1996) ALTERATION OF MICA AND CHLORITE IN PADDY SOILS DERIVED FROM TRIASSIC AND JURASSIC SEDIMENTS YASUO KITAGAWA and KATSUHIKO ITAMI Fukui Prefectural University, Matsuoka, Fukui
X-RAY DIFFRACTION STUDIES OF ORGANIC CATION-STABILIZED BENTONITE I
 X-RAY DIFFRACTION STUDIES OF ORGANIC CATION-STABILIZED BENTONITE I by E. A. I:~OSAUER, 1~. L. HANDY AND TURGUT D~MmEL ~ Iowa State University, Ames, Iowa ABSTRACT Studies of quaternary ammonium chlorides
X-RAY DIFFRACTION STUDIES OF ORGANIC CATION-STABILIZED BENTONITE I by E. A. I:~OSAUER, 1~. L. HANDY AND TURGUT D~MmEL ~ Iowa State University, Ames, Iowa ABSTRACT Studies of quaternary ammonium chlorides
CHARACTERIZATION OF SMECTITES SYNTHESISED FROM ZEOLITES AND MECHANISM OF SMECTITE SYNTHESIS
 Clay Minerals (1985) 20, 181-188 CHARACTERZATON OF SMECTTES SYNTHESSED FROM ZEOLTES AND MECHANSM OF SMECTTE SYNTHESS S. KOMARNEN AND E. BREVAL Materials Research Laboratory, The Pennsylvania State University,
Clay Minerals (1985) 20, 181-188 CHARACTERZATON OF SMECTTES SYNTHESSED FROM ZEOLTES AND MECHANSM OF SMECTTE SYNTHESS S. KOMARNEN AND E. BREVAL Materials Research Laboratory, The Pennsylvania State University,
How Does Redox Status Influence Exchangeable Potassium In Soil?
 How Does Redox Status Influence Exchangeable Potassium In Soil? Michael L. Thompson Taslima Stephen Iowa State University 1 Why do we care about exchangeable K? K is required by all plants. A soil may
How Does Redox Status Influence Exchangeable Potassium In Soil? Michael L. Thompson Taslima Stephen Iowa State University 1 Why do we care about exchangeable K? K is required by all plants. A soil may
SURFACE AREAS OF CLAY MINERALS AS DERIVED FROM MEASUREMENTS OF GLYCEROL RETENTION
 SURFACE AREAS OF CLAY MINERALS AS DERIVED FROM MEASUREMENTS OF GLYCEROL RETENTION By SIDNEY DIAMOND AND EARL B. KII~TER Bureau of Public Roads, Physical Research Division, Washington, D.C. ABSTRACT The
SURFACE AREAS OF CLAY MINERALS AS DERIVED FROM MEASUREMENTS OF GLYCEROL RETENTION By SIDNEY DIAMOND AND EARL B. KII~TER Bureau of Public Roads, Physical Research Division, Washington, D.C. ABSTRACT The
Clays and Clay Minerals
 Clays and Clay Minerals Fields of interest for clays Various definitions Acients: Earths in the earth-air-fire-water system Definition of clay depends on discipline: Geologist grain size
Clays and Clay Minerals Fields of interest for clays Various definitions Acients: Earths in the earth-air-fire-water system Definition of clay depends on discipline: Geologist grain size
ELECTRON SPIN RESONANCE STUDIES OF MONTMORILLONITES
 Clay Minerals (1985) 20, 281-290 ELECTRON SPIN RESONANCE STUDIES OF MONTMORILLONITES C. CRACIUN AND AURELIA MEGHEA* Institutul de Cercethri pentru Pedologie ~i A grochimie and *Institutul Politeehnie Bueure~ti,
Clay Minerals (1985) 20, 281-290 ELECTRON SPIN RESONANCE STUDIES OF MONTMORILLONITES C. CRACIUN AND AURELIA MEGHEA* Institutul de Cercethri pentru Pedologie ~i A grochimie and *Institutul Politeehnie Bueure~ti,
THE NATURE OF ILLITE
 THE NATURE OF ILLITE by H. E. GAUDETTE, J. L. EADES, and R. E. GRIM University of Illinois, Urbana, Illinois ABSTRACT CHEMICAL composition. X-ray diffraction and other analytical data of a series of samples
THE NATURE OF ILLITE by H. E. GAUDETTE, J. L. EADES, and R. E. GRIM University of Illinois, Urbana, Illinois ABSTRACT CHEMICAL composition. X-ray diffraction and other analytical data of a series of samples
THE DECOMPOSITION PRODUCTS OF ANORTHITE ATTACKED BY PURE WATER AT ELEVATED TEMPERA- TURES AND PRESSURE
 THE DECOMPOSITION PRODUCTS OF ANORTHITE ATTACKED BY PURE WATER AT ELEVATED TEMPERA- TURES AND PRESSURE By A. F. FREDERICKSON AND J. E. Cox, JR., Washington University, St. Louis, Mo. ABSTRACT The behavior
THE DECOMPOSITION PRODUCTS OF ANORTHITE ATTACKED BY PURE WATER AT ELEVATED TEMPERA- TURES AND PRESSURE By A. F. FREDERICKSON AND J. E. Cox, JR., Washington University, St. Louis, Mo. ABSTRACT The behavior
Determination of the composition of montmorillonite in the presence of natural admixtures
 Determination of the composition of montmorillonite in the presence of natural admixtures Ľ. NOVÁKOVÁ Institute of Inorganic Chemistry, Slovak Academy of Sciences, 89 34 Bratislava Received 9 February
Determination of the composition of montmorillonite in the presence of natural admixtures Ľ. NOVÁKOVÁ Institute of Inorganic Chemistry, Slovak Academy of Sciences, 89 34 Bratislava Received 9 February
IRREVERSIBLE DEHYDRATION IN MONTMORILLONITE
 IRREVERSIBLE DEHYDRATION IN MONTMORILLONITE PART II BY R. GREENE-KELLY Introduction.--Most of the important properties of montmorillonite arise.because of the high specific surface of this mineral. The
IRREVERSIBLE DEHYDRATION IN MONTMORILLONITE PART II BY R. GREENE-KELLY Introduction.--Most of the important properties of montmorillonite arise.because of the high specific surface of this mineral. The
T6 soil base cation weathering rates
 T6 soil base cation weathering rates julian aherne :: trent university FORFLUX :: biogeochemistry of irish forests [RSF 07510] Advisory Group Meeting [5 6 December 2011] objective (a) to determine the
T6 soil base cation weathering rates julian aherne :: trent university FORFLUX :: biogeochemistry of irish forests [RSF 07510] Advisory Group Meeting [5 6 December 2011] objective (a) to determine the
It is important to recognize two distinct but overlapping uses of the term "clay":
 Soil Texture (Particle Size Analysis or Mechanical Analysis) Introduction Texture, or size distribution of mineral particles (or its associated pore volume), is one of the most important measures of a
Soil Texture (Particle Size Analysis or Mechanical Analysis) Introduction Texture, or size distribution of mineral particles (or its associated pore volume), is one of the most important measures of a
Soil Fertility. Fundamentals of Nutrient Management June 1, Patricia Steinhilber
 Soil Fertility Fundamentals of Nutrient Management June 1, 2010 Patricia Steinhilber Ag Nutrient Management Program University of Maryland College Park Main Topics plant nutrition functional soil model
Soil Fertility Fundamentals of Nutrient Management June 1, 2010 Patricia Steinhilber Ag Nutrient Management Program University of Maryland College Park Main Topics plant nutrition functional soil model
WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS?
 WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS? David Bish and David Vaniman Indiana University Los Alamos National Laboratory Products of Mineralogical Studies Mars surface mineralogy
WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS? David Bish and David Vaniman Indiana University Los Alamos National Laboratory Products of Mineralogical Studies Mars surface mineralogy
NATURE AND ORIGIN OF AN ALUMINOUS VERMICULITIC WEATHERING PRODUCT IN ACID SOILS FROM UPLAND CATCHMENTS IN SCOTLAND
 Clay Minerals (1990) 25, 467-475 NATURE AND ORIGIN OF AN ALUMINOUS VERMICULITIC WEATHERING PRODUCT IN ACID SOILS FROM UPLAND CATCHMENTS IN SCOTLAND D. C. BAIN, A. MELLOR* AND M. J. WILSON The Macaulay
Clay Minerals (1990) 25, 467-475 NATURE AND ORIGIN OF AN ALUMINOUS VERMICULITIC WEATHERING PRODUCT IN ACID SOILS FROM UPLAND CATCHMENTS IN SCOTLAND D. C. BAIN, A. MELLOR* AND M. J. WILSON The Macaulay
PERTURBATION OF STRUCTURAL Fe 3+ IN SMECTITES BY EXCHANGE IONS
 Cla~':, and Clay Minerals. Vol 23. pp. 103 107 Pergamon Press 1975. Printed in Great Britain PERTURBATON OF STRUCTURAL Fe 3+ N SMECTTES BY EXCHANGE ONS M. B. MCBRO!. T. J. PNrqAVAA and M. M. MORTLAND Contribution
Cla~':, and Clay Minerals. Vol 23. pp. 103 107 Pergamon Press 1975. Printed in Great Britain PERTURBATON OF STRUCTURAL Fe 3+ N SMECTTES BY EXCHANGE ONS M. B. MCBRO!. T. J. PNrqAVAA and M. M. MORTLAND Contribution
SOIL FORMATION AND CHARACTERIZATION
 CHAPTER 2 SOIL FORMATION AND CHARACTERIZATION 2.1 INTRODUCTION The word 'soil' has different meanings for different professions. To the agriculturist, soil is the top thin layer of earth within which organic
CHAPTER 2 SOIL FORMATION AND CHARACTERIZATION 2.1 INTRODUCTION The word 'soil' has different meanings for different professions. To the agriculturist, soil is the top thin layer of earth within which organic
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
The Iron and Titanium Minerals in the Titaniferous Ferruginous latosols of Hawaii!
 The Iron and Titanium Minerals in the Titaniferous Ferruginous latosols of Hawaii! JAMES L. WALKER,2 G. DONALD SHERMAN,2 AND TAKASHI KATSURA3 ABSTRACT: Titaniferous Ferruginous latosols are an important
The Iron and Titanium Minerals in the Titaniferous Ferruginous latosols of Hawaii! JAMES L. WALKER,2 G. DONALD SHERMAN,2 AND TAKASHI KATSURA3 ABSTRACT: Titaniferous Ferruginous latosols are an important
Ch. 4 - Clay Minerals, Rock Classification Page 1. Learning Objectives. Wednesday, January 26, 2011
 Ch. 4 - Clay Minerals, Rock Classification Page 1 Learning Objectives Ch. 4 - Clay Minerals, Rock Classification Page 2 Symbols Ch. 4 - Clay Minerals, Rock Classification Page 3 Clay Minerals and Structure
Ch. 4 - Clay Minerals, Rock Classification Page 1 Learning Objectives Ch. 4 - Clay Minerals, Rock Classification Page 2 Symbols Ch. 4 - Clay Minerals, Rock Classification Page 3 Clay Minerals and Structure
CLAY MINERALOGY OF PALEOZOIC K-BENTONITES OF THE EASTERN UNITED STATES (PART 1)
 CLAY MINERALOGY OF PALEOZOIC K-BENTONITES OF THE EASTERN UNITED STATES (PART 1) by RICHARD W. LOUNSBURY and WILTON N. MELHORN Purdue University, Lafayette, Indiana ABSTRACT Samples of altered volcanic
CLAY MINERALOGY OF PALEOZOIC K-BENTONITES OF THE EASTERN UNITED STATES (PART 1) by RICHARD W. LOUNSBURY and WILTON N. MELHORN Purdue University, Lafayette, Indiana ABSTRACT Samples of altered volcanic
Studying the Effect of Crystal Size on Adsorption Properties of Clay
 Studying the Effect of Crystal Size on Adsorption Properties of Clay M. M. Abdellatif Nuclear and Radiological Regulatory Authority, 3 Ahmed El Zomer st. Nasr City, 11762 Egypt. Email: magdadel200@hotmail.com
Studying the Effect of Crystal Size on Adsorption Properties of Clay M. M. Abdellatif Nuclear and Radiological Regulatory Authority, 3 Ahmed El Zomer st. Nasr City, 11762 Egypt. Email: magdadel200@hotmail.com
Circle the correct (best) terms inside the brackets:
 1 Circle the correct (best) terms inside the brackets: 1) Soils are [consolidated / unconsolidated] [natural / artificial] bodies at the earth s surface. Soils contain mineral and organic material, which
1 Circle the correct (best) terms inside the brackets: 1) Soils are [consolidated / unconsolidated] [natural / artificial] bodies at the earth s surface. Soils contain mineral and organic material, which
INVESTIGATION ON GEOTHERMAL DRILLING MUDS WITH HIGH TEMPERATURE STABILITY
 INVESTIGATION ON GEOTHERMAL DRILLING MUDS WITH HIGH TEMPERATURE STABILITY Umran Serpen ITÜ, Petroleum and Natural Gas Eng. Dept., Maslak, 80626, Istanbul, Turkey Key Words: Drilling-mud, geothermal, sepiolite,
INVESTIGATION ON GEOTHERMAL DRILLING MUDS WITH HIGH TEMPERATURE STABILITY Umran Serpen ITÜ, Petroleum and Natural Gas Eng. Dept., Maslak, 80626, Istanbul, Turkey Key Words: Drilling-mud, geothermal, sepiolite,
Chunmei Chen A,B and Donald L Sparks A. Delaware, Newark, DE 19711, USA.
 Environ. Chem. 2015, 12, 64 CSIRO 2015 Supplementary material Multi-elemental scanning transmission X-ray microscopy near edge X-ray absorption fine structure spectroscopy assessment of organo mineral
Environ. Chem. 2015, 12, 64 CSIRO 2015 Supplementary material Multi-elemental scanning transmission X-ray microscopy near edge X-ray absorption fine structure spectroscopy assessment of organo mineral
INFLUENCE OF EXCHANGE IONS ON THE b-dimensions OF DIOCTAHEDRAL VERMICULITE*
 INFLUENCE OF EXCHANGE IONS ON THE b-dimensions OF DIOCTAHEDRAL VERMICULITE* by R. A. LEONARD t and S. B. WEED North Carolina State University, Raleigh, North Carolina ABSTRACT THE 1--5 /~ size fractions
INFLUENCE OF EXCHANGE IONS ON THE b-dimensions OF DIOCTAHEDRAL VERMICULITE* by R. A. LEONARD t and S. B. WEED North Carolina State University, Raleigh, North Carolina ABSTRACT THE 1--5 /~ size fractions
Trioctahedral minerals in the soil-clays of north-east Scotland.
 72 Trioctahedral minerals in the soil-clays of north-east Scotland. By G. F. WALKER, B.Sc., Ph.D. Macaulay Institute for Soil Research, Aberdeen. [Read at the meeting of the Clay Minerals Group, November
72 Trioctahedral minerals in the soil-clays of north-east Scotland. By G. F. WALKER, B.Sc., Ph.D. Macaulay Institute for Soil Research, Aberdeen. [Read at the meeting of the Clay Minerals Group, November
The structural formula of a hydrous amphibole.
 717 The structural formula of a hydrous amphibole. By G. D. NIC~OLLS, B.A., Ph.D., F.G.S. and J. ZVSSMAN, M.A., Ph.D. Department of Geology, University of Manchester. T [Read January 27, 1955.] HE majority
717 The structural formula of a hydrous amphibole. By G. D. NIC~OLLS, B.A., Ph.D., F.G.S. and J. ZVSSMAN, M.A., Ph.D. Department of Geology, University of Manchester. T [Read January 27, 1955.] HE majority
EXPERIMENTAL TRANSFORMATION OF 2M SERICITE INTO A RECTORITE-TYPE MIXED-LAYER MINERAL BY TREATMENT WITH VARIOUS SALTS
 Clays and Clay Minerals, Vol. 25, pp. 302-308. Pergamon Press 1977. Printed in Great Britain EXPERIMENTAL TRANSFORMATION OF 2M SERICITE INTO A RECTORITE-TYPE MIXED-LAYER MINERAL BY TREATMENT WITH VARIOUS
Clays and Clay Minerals, Vol. 25, pp. 302-308. Pergamon Press 1977. Printed in Great Britain EXPERIMENTAL TRANSFORMATION OF 2M SERICITE INTO A RECTORITE-TYPE MIXED-LAYER MINERAL BY TREATMENT WITH VARIOUS
EXPERT SYSTEM FOR STRUCTURAL CHARACTERIZATION OF PHYLLOSILICATES: II. APPLICATION TO MIXED-LAYER MINERALS
 Clay Minerals (1994) 29, 39-45 EXPERT SYSTEM FOR STRUCTURAL CHARACTERIZATION OF PHYLLOSILICATES: II. APPLICATION TO MIXED-LAYER MINERALS V.A. DRITS AND A. PLAN~ON* Geological Institute, Academy of Sciences,
Clay Minerals (1994) 29, 39-45 EXPERT SYSTEM FOR STRUCTURAL CHARACTERIZATION OF PHYLLOSILICATES: II. APPLICATION TO MIXED-LAYER MINERALS V.A. DRITS AND A. PLAN~ON* Geological Institute, Academy of Sciences,
Trinitite the Atomic Rock
 Trinitite the Atomic Rock Nelson Eby, EEAS, University of Massachusetts, Lowell, MA Norman Charnley, Earth Sciences, University of Oxford, Oxford, UK John Smoliga, Roxbury, CT Special thanks to Robert
Trinitite the Atomic Rock Nelson Eby, EEAS, University of Massachusetts, Lowell, MA Norman Charnley, Earth Sciences, University of Oxford, Oxford, UK John Smoliga, Roxbury, CT Special thanks to Robert
A mineralogical study of soil formation in four rhyolite-derived soils from New Zealand
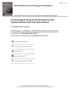 New Zealand Journal of Geology and Geophysics ISSN: 0028-8306 (Print) 1175-8791 (Online) Journal homepage: http://www.tandfonline.com/loi/tnzg20 A mineralogical study of soil formation in four rhyolite-derived
New Zealand Journal of Geology and Geophysics ISSN: 0028-8306 (Print) 1175-8791 (Online) Journal homepage: http://www.tandfonline.com/loi/tnzg20 A mineralogical study of soil formation in four rhyolite-derived
SOIL and WATER CHEMISTRY
 SOIL and WATER CHEMISTRY An Integrative Approach MICHAEL E. ESSINGTON CRC PRESS Boca Raton London New York Washington, D.C. Table of Contents Chapter 1 The Soil Chemical Environment: An Overview 1 1.1
SOIL and WATER CHEMISTRY An Integrative Approach MICHAEL E. ESSINGTON CRC PRESS Boca Raton London New York Washington, D.C. Table of Contents Chapter 1 The Soil Chemical Environment: An Overview 1 1.1
Solapur University, Solapur. Syllabus for B.Sc. II- Geochemistry - (IDS) Semester System - CGPA To be implemented from Academic Year
 Solapur University, Solapur Syllabus for B.Sc. II- Geochemistry - (IDS) Semester System - CGPA To be implemented from Academic Year- 2015-16 Course Structure Total Credit 16 - (Theory (4 x 3) = 12+Practical
Solapur University, Solapur Syllabus for B.Sc. II- Geochemistry - (IDS) Semester System - CGPA To be implemented from Academic Year- 2015-16 Course Structure Total Credit 16 - (Theory (4 x 3) = 12+Practical
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Effect of EcSS 3000 on Expansive Clays
 Effect of EcSS 3000 on Expansive Clays R. Malek Director, Particle Characterization Laboratory, Materials Research Institute, Pennsylvania State University, University Park, PA 16802. RQM@PSU.EDU (814)
Effect of EcSS 3000 on Expansive Clays R. Malek Director, Particle Characterization Laboratory, Materials Research Institute, Pennsylvania State University, University Park, PA 16802. RQM@PSU.EDU (814)
GROUNDWATER GEOCHEMISTRY AND ASSOCIATED HARDPANS IN SOUTHWESTERN AUSTRALIA
 254 GROUNDWATER GEOCHEMISTRY AND ASSOCIATED HARDPANS IN SOUTHWESTERN AUSTRALIA Sam Lee CRC LEME, Department of Applied Geology, Curtin University of Technology, Bentley, Western Australia, 6485 INTRODUCTION
254 GROUNDWATER GEOCHEMISTRY AND ASSOCIATED HARDPANS IN SOUTHWESTERN AUSTRALIA Sam Lee CRC LEME, Department of Applied Geology, Curtin University of Technology, Bentley, Western Australia, 6485 INTRODUCTION
LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1
 EESC 2100: Mineralogy LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1 Learning Objectives: Students will be able to identify minerals that occur commonly in sandstones (quartz and feldspars), both
EESC 2100: Mineralogy LAB 3: COMMON MINERALS IN SEDIMENTARY ROCKS, Part 1 Learning Objectives: Students will be able to identify minerals that occur commonly in sandstones (quartz and feldspars), both
APPENDIX F PHOTOGRAPHS OF CORE SAMPLES
 EKATI DIAMOND MINE LONG LAKE CONTAINMENT FACILITY INVESTIGATION 2013 EBA FILE: E14103013-01.002 MAY 2014 ISSUED FOR USE APPENDIX F PHOTOGRAPHS OF CORE SAMPLES LLCF Geotechnical Site Investigation Report_IFU.docx
EKATI DIAMOND MINE LONG LAKE CONTAINMENT FACILITY INVESTIGATION 2013 EBA FILE: E14103013-01.002 MAY 2014 ISSUED FOR USE APPENDIX F PHOTOGRAPHS OF CORE SAMPLES LLCF Geotechnical Site Investigation Report_IFU.docx
Objectives of this Lab. Introduction. The Petrographic Microscope
 Geological Sciences 101 Lab #9 Introduction to Petrology Objectives of this Lab 1. Understand how the minerals and textures of rocks reflect the processes by which they were formed. 2. Understand how rocks
Geological Sciences 101 Lab #9 Introduction to Petrology Objectives of this Lab 1. Understand how the minerals and textures of rocks reflect the processes by which they were formed. 2. Understand how rocks
COLLAPSE OF POTASSIUM MONTMORILLONITE CLAYS UPON HEATING--"POTASSIUM FIXATION"
 COLLAPSE OF POTASSIUM MONTMORILLONITE CLAYS UPON HEATING--"POTASSIUM FIXATION" by H. VA~ OLPHEN Shell Development Company (A Division of Shell Oil Company), Exploration and Production Research Division,
COLLAPSE OF POTASSIUM MONTMORILLONITE CLAYS UPON HEATING--"POTASSIUM FIXATION" by H. VA~ OLPHEN Shell Development Company (A Division of Shell Oil Company), Exploration and Production Research Division,
Maine Agricultural and Forest Experiment Station
 The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 5-1-1979 TB91: The Effect of Acidity, Organic Matter, and Sesquioxide Polymers on the
The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 5-1-1979 TB91: The Effect of Acidity, Organic Matter, and Sesquioxide Polymers on the
Mechanism of formation of Jbrsterite and enstatite from serpentine
 189 Mechanism of formation of Jbrsterite and enstatite from serpentine By G. W. BRNDLEY, M.Sc., Ph.D., and RYozo HAYAM, M.S. Materials Research Laboratory, The Pennsylvania State University, University
189 Mechanism of formation of Jbrsterite and enstatite from serpentine By G. W. BRNDLEY, M.Sc., Ph.D., and RYozo HAYAM, M.S. Materials Research Laboratory, The Pennsylvania State University, University
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Chapter 4 Implications of paleoceanography and paleoclimate
 Age ka / Chapter 4 Implications of paleoceanography and paleoclimate 4.1 Paleoclimate expression 4.2 Implications of paleocirculation and tectonics 4.3 Paleoenvironmental reconstruction MD05-2901 (Liu
Age ka / Chapter 4 Implications of paleoceanography and paleoclimate 4.1 Paleoclimate expression 4.2 Implications of paleocirculation and tectonics 4.3 Paleoenvironmental reconstruction MD05-2901 (Liu
Influence of soil type and land use on the nature of mobile colloids: implications for the metal transfer in soils
 Symposium no. 01 Paper no. 1289 Presentation: poster Influence of soil type and land use on the nature of mobile colloids: implications for the metal transfer in soils CITEAU Laëtitia, LAMY Isabelle, van
Symposium no. 01 Paper no. 1289 Presentation: poster Influence of soil type and land use on the nature of mobile colloids: implications for the metal transfer in soils CITEAU Laëtitia, LAMY Isabelle, van
High Temperature Materials. By Docent. N. Menad. Luleå University of Technology ( Sweden )
 Course KGP003 Ch. 12 High Temperature Materials By Docent. N. Menad Dept. of Chemical Engineering and Geosciences Div. Of process metallurgy Luleå University of Technology ( Sweden ) Ceramic materials
Course KGP003 Ch. 12 High Temperature Materials By Docent. N. Menad Dept. of Chemical Engineering and Geosciences Div. Of process metallurgy Luleå University of Technology ( Sweden ) Ceramic materials
NAME GEOL FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY
 NAME GEOL.2150 - FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY I. Introduction Minerals are crystalline solids. Individual minerals are distinguished on the basis of chemistry and the way in
NAME GEOL.2150 - FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY I. Introduction Minerals are crystalline solids. Individual minerals are distinguished on the basis of chemistry and the way in
THE ROLE OF WATER VAPOUR IN THE DEHYDROXYLATION OF CLAY MINERALS
 THE ROLE OF WATER VAPOUR IN THE DEHYDROXYLATION OF CLAY MINERALS By G. W. BRI~qDLE~C and M. NAtOa~RA Department of Ceramic Technology, The Pennsylvania State University, University Park, Pa., U.S.A. [MS.
THE ROLE OF WATER VAPOUR IN THE DEHYDROXYLATION OF CLAY MINERALS By G. W. BRI~qDLE~C and M. NAtOa~RA Department of Ceramic Technology, The Pennsylvania State University, University Park, Pa., U.S.A. [MS.
