Group Members: Your Name In Class Exercise #6. Photon A. Energy B
|
|
|
- Hortense Thompson
- 6 years ago
- Views:
Transcription
1 Group Members: Your Name In Class Exercise #6 Shell Structure of Atoms Part II Photoelectron Spectroscopy Photoelectron spectroscopy is closely related to the photoelectric effect. When high energy photons hit an atom, molecule, or solid surface an electron can be emitted if the energy of the photon is greater than the ionization energy of the matter. By conservation of energy Ephoton = KE + IE Where KE is the kinetic energy of the electron and IE is the ionization energy (this would be the work function in the photoelectric effect). There are several modes for running the photoelectron spectrum; throughout this exercise we will consider the photoelectron experiment in which a high energy X-ray photon is used as the source and both the kinetic energy and the number of electrons emitted are measured. Because we know the energy of the photon and measure the the KEs of emitted electrons we can determine the IE. IE = Ephoton KE 1. Let s say that a hypothetical atom with one electron in its ground state is irradiated with photons that have energy of MJ/mole. On the drawing below, the large arrow represents the photon energy and the circle represents the electron in the lowest energy level of the atom. Which energy (A or B) represents the KE of the electron emitted and which represents the IE? Photon A Energy B
2 Upon ionization the emitted electrons all have energy of 40.8 MJ/mole. What is the ionization energy for atom in the ground state? What is the energy of the electron in the n=1 level (be sure to use the proper sign)? If the atom were in an excited state, would the kinetic energy of the emitted electron be greater or less? Explain. Consider the hypothetical atom to be in the first excited state (i.e. the electron is in the n=2 level). Knowing that the energy of a level is proportional to 1/n 2 and knowing the energy for n=1, what is the energy for the n=2 level? What will be the IE and KE for the atom in the excited state if a photon with 144.0MJ/mole energy is used? Photon Energy The axes shown below represent the photoelectron experiment results: the number of electrons emitted is the vertical scale and the horizontal axis represents the KE of the electron. Now imagine that the photoelectron spectrum of a sample in which 50% of the atoms were in the ground state and 50% were in the first excited state was measured using 144.0MJ/mole photons. Place labeled peaks on the graph below at appropriate places. No. of Electrons Typical peak shape Kinetic Energy of Electron (MJ/mole) 2
3 More commonly, the results of photoelectron spectrum are plotted with the horizontal axis as the Ionization Energy. Using this axis, plot the photoelectron spectrum of the sample with 50% atoms in the ground state and 50% of the atoms in the first excited state. No. of Electrons Ionization Energy (MJ/mole) 50 In most measurements of photoelectron spectra, the atoms are in their ground state. However multiple peaks may be seen in the spectra if there are multiple shells of electrons. For example, Li has two shells of electrons and exhibits the photoelectron spectrum shown below. Li Z=3 q core 1 No. of Electrons Ionization Energy (MJ/mole) Note that there are two peaks one at low energy that is about ½ the size of the peak at high energy. This spectrum illustrates important general features of the photoelectron spectrum: electrons are emitted from each shell and the size of the peak is proportional to the number of electrons in the shell. 2. For the photoelectron spectrum of Li, from what shell are electrons being ionized to give the the peak at 0.5 MJ/mole? Why is the peak at 6.3 MJ/mole twice as large as the peak at 0.5MJ/mole? 3
4 Low values of the ionization energy indicate that the electron is very easy to remove from the atom. Which electrons are closer to the nucleus, those in the inner shell or the outer shell? Which peaks correspond to ionization from the inner shell and outer shell? Orbitals and the Shell Model The shell structure and the experimental photoelectron spectrum of Ne are shown below. No. of Electrons Ne Z=10 q core Ionization Energy (MJ/mole) Note that three peaks are observed with IEs of 84.0 MJ/mole, 4.68 MJ/mole, and 2.08 MJ/mole. Also note that the peak at 2.08 MJ/mole is abut three times larger than either of the other two peaks. We must give up the notion that only the shell number (that is, the principal quantum number, n) determines the IE. We know from the mathematical description of the hydrogen atom that the n=2 shell has two different kinds of orbitals: s- (l=0) and p-orbitals (l=1). The p-orbitals comprise three types: p x, p y, and p z (m l =-1,0,1). Each orbital can hold two electrons so a more detailed description of the Ne atom is given by the electron configuration: 4
5 1s 2 2s 2 2p 6. The photoelectron spectrum of Ne exhibits three peaks, suggesting that the ionization of electrons may come from either the 1s, 2s, or 2p orbitals and that the energies of these ionization processes are different. 3. Which peak corresponds to ionization from the 1s orbital? Explain your reasoning, paying close attention to the ionization energy. Which peak corresponds to ionization from the 2s shell? Explain. Why are the peaks for ionization from the 2s and 2p shells of different size? Label the spectrum shown above with the names of the orbitals from which ionization occurred. Use the radial distribution curves shown below to rationalize why the IE for the 2s orbital is greater than the IE for the 2p orbital. Your rationalization must use the terms shielding and effective nuclear charge. (Hint: for which orbital can the electrons best get between the 1s shell and the nucleus). Ionization Energies and Electron Configurations The table below gives the orbital ionization energies for the first 18 elements. Table 1. Orbital Ionization Energies (MJ/mole) and Some Electron Configurations Element IE 1s IE 2s IE 2p IE 3s IE 3p Configuration 5
6 H s 1 He s 2 Li s 2 2s 1 Be s 2 2s 2 B s 2 2s 2 2p 1 C N O F Ne s 2 2s 2 2p 6 Na Mg s 2 2s 2 2p 6 3s 2 Al Si P S Cl Ar Compare the photoelectron spectrum of Ne with the energies listed above to verify the table. Another way of illustrating the electron configuration and orbital energies of Ne is given to the right. 2p Neon What is the electron configuration for N? For P? Fill in the rest of the table. 1s Draw the photoelectron spectra that you would expect for Na and Ar. 6
7 The first ionization energy is the energy to remove an electron from the highest energy filled orbital. Based on the shell model why do you think that the first ionization energy of Li is less than that of H? If the 19 th electron of K is found in the n=4 shell, would the ionization energy be closest to 0.42, 1.4, or 2.0 MJ/mole? Explain. 7
Photoelectron Spectroscopy
 32 ChemActivity 8 Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?) From our previous examination of the ionization energies of the atoms, we proposed a shell model of the atom, and noted
32 ChemActivity 8 Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?) From our previous examination of the ionization energies of the atoms, we proposed a shell model of the atom, and noted
Photoelectron Spectroscopy
 Photoelectron Spectroscopy Why? There are many data that have lead to the development of the shell model of the atom such as ionization energy. In this activity we will examine another one of these data
Photoelectron Spectroscopy Why? There are many data that have lead to the development of the shell model of the atom such as ionization energy. In this activity we will examine another one of these data
Photoelectron Spectroscopy Evidence for Electronic Structure Guided-Inquiry Learning Activity for AP* Chemistry
 Introduction Photoelectron Spectroscopy Evidence for Electronic Structure Guided-Inquiry Learning Activity for AP* Chemistry Catalog No. AP7710 Publication No. 7710AS The chemical properties of elements
Introduction Photoelectron Spectroscopy Evidence for Electronic Structure Guided-Inquiry Learning Activity for AP* Chemistry Catalog No. AP7710 Publication No. 7710AS The chemical properties of elements
The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006.
 The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006. The first ionization energy (IE 1 ) is the minimum energy required to remove
The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006. The first ionization energy (IE 1 ) is the minimum energy required to remove
Chemistry: A Guided Inquiry 5e
 Chemistry: A Guided Inquiry 5e ISBN 9781119919629 Copyright 2013 John Wiley & Sons, Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted
Chemistry: A Guided Inquiry 5e ISBN 9781119919629 Copyright 2013 John Wiley & Sons, Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted
Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?)
 32 I Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?) From our previous examination of the ionization energies of the atoms, we proposed a shell model of the atom, and noted that the number
32 I Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?) From our previous examination of the ionization energies of the atoms, we proposed a shell model of the atom, and noted that the number
a) how many electrons do you see in the picture? How many protons? d) compare the energy from 3b with the energy in 2a and then in 2c.
 During Class Invention Question: How are electrons arranged in an atom? 1. Describe the nature of the interaction between protons and electrons in an atom? Consider using some or all of the following terms
During Class Invention Question: How are electrons arranged in an atom? 1. Describe the nature of the interaction between protons and electrons in an atom? Consider using some or all of the following terms
2. Why do all elements want to obtain a noble gas electron configuration?
 AP Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Electron Configuration Metal, Nonmetal or Semi-metal Metalloid)? Group 1, Period 1 Group 11,
AP Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Electron Configuration Metal, Nonmetal or Semi-metal Metalloid)? Group 1, Period 1 Group 11,
Question: How are electrons arranged in an atom?
 Honors Chemistry: Coulomb s Law and periodic trends Question: How are electrons arranged in an atom? Coulomb s Law equation: 1. A) Define what each of the following variables in the equation represents.
Honors Chemistry: Coulomb s Law and periodic trends Question: How are electrons arranged in an atom? Coulomb s Law equation: 1. A) Define what each of the following variables in the equation represents.
Suggested time minutes (22 points) minutes (16 points) minutes (38 points) 4. 9 minutes (24 points) Total (100 points) Name
 First Hour Exam 5.111 Write your name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read each part of each problem carefully and thoroughly.
First Hour Exam 5.111 Write your name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read each part of each problem carefully and thoroughly.
(3 pts) 2. In which gas sample do the molecules have a lower average kinetic energy? (A) Gas A (B) Gas B (C) Neither
 Consider two samples of gas, A and B, as shown in the figure below. Both containers are at the same temperature and pressure. Gas A 1.0 L 0.32 g Gas B 1.0 L 0.48 g (3 pts) 1. Which gas sample contains
Consider two samples of gas, A and B, as shown in the figure below. Both containers are at the same temperature and pressure. Gas A 1.0 L 0.32 g Gas B 1.0 L 0.48 g (3 pts) 1. Which gas sample contains
Name: Block: Date: Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom.
 Name: Block: Date: Chemistry 11 Trends Activity Assignment Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom. Ionic Radius: the distance from the center
Name: Block: Date: Chemistry 11 Trends Activity Assignment Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom. Ionic Radius: the distance from the center
U N I T T E S T P R A C T I C E
 South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
Periodic Trends. Atomic Radius: The distance from the center of the nucleus to the outer most electrons in an atom.
 Periodic Trends Study and learn the definitions listed below. Then use the definitions and the periodic table provided to help you answer the questions in the activity. By the end of the activity you should
Periodic Trends Study and learn the definitions listed below. Then use the definitions and the periodic table provided to help you answer the questions in the activity. By the end of the activity you should
Chemistry (
 Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
To review Rutherford s model of the atom To explore the nature of electromagnetic radiation To see how atoms emit light
 Objectives To review Rutherford s model of the atom To explore the nature of electromagnetic radiation To see how atoms emit light 1 A. Rutherford s Atom.but there is a problem here!! 2 Using Rutherford
Objectives To review Rutherford s model of the atom To explore the nature of electromagnetic radiation To see how atoms emit light 1 A. Rutherford s Atom.but there is a problem here!! 2 Using Rutherford
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE The Electromagnetic Spectrum The Wave
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE The Electromagnetic Spectrum The Wave
LIGHT AND THE QUANTUM MODEL
 LIGHT AND THE QUANTUM MODEL WAVES Wavelength ( ) - length of one complete wave Frequency ( ) - # of waves that pass a point during a certain time period hertz (Hz) = 1/s Amplitude (A) - distance from the
LIGHT AND THE QUANTUM MODEL WAVES Wavelength ( ) - length of one complete wave Frequency ( ) - # of waves that pass a point during a certain time period hertz (Hz) = 1/s Amplitude (A) - distance from the
Electrons in Atoms. So why does potassium explode in water? Quantum Mechanics Periodic Trends Chemical Bonding
 Electrons in Atoms So why does potassium explode in water? Quantum Mechanics Periodic Trends Chemical Bonding 12.1 Development of Atomic Models Dalton s Thompson s Rutherford s Bohr s carbon Quantum Model
Electrons in Atoms So why does potassium explode in water? Quantum Mechanics Periodic Trends Chemical Bonding 12.1 Development of Atomic Models Dalton s Thompson s Rutherford s Bohr s carbon Quantum Model
Unit 7. Atomic Structure
 Unit 7. Atomic Structure Upon successful completion of this unit, the students should be able to: 7.1 List the eight regions of the electromagnetic spectrum in the designated order and perform calculations
Unit 7. Atomic Structure Upon successful completion of this unit, the students should be able to: 7.1 List the eight regions of the electromagnetic spectrum in the designated order and perform calculations
Quick Review. 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior.
 Quick Review 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior. Emission spectra Every element has a unique emission spectrum
Quick Review 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior. Emission spectra Every element has a unique emission spectrum
PART 2 Electronic Structure and the Periodic Table. Reference: Chapter 7 8 in textbook
 PART 2 Electronic Structure and the Periodic Table Reference: Chapter 7 8 in textbook 1 Experiment to Discover Atom Structure -particle: He 2+ mass number = 4 Nucleus and Electron Model 2 Atomic Structure
PART 2 Electronic Structure and the Periodic Table Reference: Chapter 7 8 in textbook 1 Experiment to Discover Atom Structure -particle: He 2+ mass number = 4 Nucleus and Electron Model 2 Atomic Structure
Atomic Structure. 1. For a hydrogen atom which electron transition requires the largest amount of energy?
 Atomic Structure 1. For a hydrogen atom which electron transition requires the largest amount of energy? A. n = 4 to n = 10 B. n = 3 to n = 2 C. n = 3 to n = 4 D. n = 1 to n = 3 E. n = 2 to n = 4 2. Which
Atomic Structure 1. For a hydrogen atom which electron transition requires the largest amount of energy? A. n = 4 to n = 10 B. n = 3 to n = 2 C. n = 3 to n = 4 D. n = 1 to n = 3 E. n = 2 to n = 4 2. Which
3.9 The First Ionization Energy
 3.9 THE FIRST IONIZATION ENERGY 83 The characteristic color of the light emitted by various elements can be demonstrated by filling a series of salt shakers with a variety of powdered metals (such as Mg
3.9 THE FIRST IONIZATION ENERGY 83 The characteristic color of the light emitted by various elements can be demonstrated by filling a series of salt shakers with a variety of powdered metals (such as Mg
Modern Atomic Theory CHAPTER OUTLINE
 Chapter 3B Modern Atomic Theory 1 CHAPTER OUTLINE Waves Electromagnetic Radiation Dual Nature of Light Bohr Model of Atom Quantum Mechanical Model of Atom Electron Configuration Electron Configuration
Chapter 3B Modern Atomic Theory 1 CHAPTER OUTLINE Waves Electromagnetic Radiation Dual Nature of Light Bohr Model of Atom Quantum Mechanical Model of Atom Electron Configuration Electron Configuration
How we describe bonding is affected strongly by how we describe where/how electrons are held by atoms in molecules.
 CHEM 2060 Lecture 8: Atomic Configurations L8-1 Electronic Configurations How we describe bonding is affected strongly by how we describe where/how electrons are held by atoms in molecules. We know that
CHEM 2060 Lecture 8: Atomic Configurations L8-1 Electronic Configurations How we describe bonding is affected strongly by how we describe where/how electrons are held by atoms in molecules. We know that
CLEP Chemistry - Problem Drill 10: Atomic Structure and Electron Configuration
 CLEP Chemistry - Problem Drill 10: Atomic Structure and Electron Configuration No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully (2) Work the problems on paper as 1.
CLEP Chemistry - Problem Drill 10: Atomic Structure and Electron Configuration No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully (2) Work the problems on paper as 1.
The Shell Model (II)
 22 ChemActivity 5 The Shell Model (II) Model 1: Valence Electrons, Inner-Shell Electrons, and Core Charge. The electrons in the outermost shell of an atom are referred to as valence electrons. Electrons
22 ChemActivity 5 The Shell Model (II) Model 1: Valence Electrons, Inner-Shell Electrons, and Core Charge. The electrons in the outermost shell of an atom are referred to as valence electrons. Electrons
CH 101Fall 2018 Discussion #12 Chapter 8, Mahaffy, 2e sections Your name: TF s name: Discussion Day/Time:
 CH 11Fall 218 Discussion #12 Chapter 8, Mahaff, 2e sections 8.3-8.7 Your name: TF s name: Discussion Da/Time: Things ou should know when ou leave Discussion toda for one-electron atoms: ΔE matter=e n-e
CH 11Fall 218 Discussion #12 Chapter 8, Mahaff, 2e sections 8.3-8.7 Your name: TF s name: Discussion Da/Time: Things ou should know when ou leave Discussion toda for one-electron atoms: ΔE matter=e n-e
Gilbert Kirss Foster. Chapter3. Atomic Structure. Explaining the Properties of Elements
 Gilbert Kirss Foster Chapter3 Atomic Structure Explaining the Properties of Elements Chapter Outline 3.1 Waves of Light 3.2 Atomic Spectra 3.3 Particles of Light: Quantum Theory 3.4 The Hydrogen Spectrum
Gilbert Kirss Foster Chapter3 Atomic Structure Explaining the Properties of Elements Chapter Outline 3.1 Waves of Light 3.2 Atomic Spectra 3.3 Particles of Light: Quantum Theory 3.4 The Hydrogen Spectrum
Particle Behavior of Light 1. Calculate the energy of a photon, mole of photons 2. Find binding energy of an electron (know KE) 3. What is a quanta?
 Properties of Electromagnetic Radiation 1. What is spectroscopy, a continuous spectrum, a line spectrum, differences and similarities 2. Relationship of wavelength to frequency, relationship of E to λ
Properties of Electromagnetic Radiation 1. What is spectroscopy, a continuous spectrum, a line spectrum, differences and similarities 2. Relationship of wavelength to frequency, relationship of E to λ
Trends in the Periodic Table
 Trends in the Periodic Table OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret group and period trends in atomic radii, ionization energies and electronegativity The Periodic Table
Trends in the Periodic Table OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret group and period trends in atomic radii, ionization energies and electronegativity The Periodic Table
Periodic Relationships
 Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
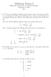 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Edexcel Chemistry A-level
 Edexcel Chemistry A-level Topic 1 - Atomic Structure and Periodic Table Flashcards What was stated in Dalton s atomic theory? (4) What was stated in Dalton s atomic theory? Atoms are tiny particles made
Edexcel Chemistry A-level Topic 1 - Atomic Structure and Periodic Table Flashcards What was stated in Dalton s atomic theory? (4) What was stated in Dalton s atomic theory? Atoms are tiny particles made
The Quantum Mechanical Model
 Recall The Quantum Mechanical Model Quantum Numbers Four numbers, called quantum numbers, describe the characteristics of electrons and their orbitals Quantum Numbers Quantum Numbers The Case of Hydrogen
Recall The Quantum Mechanical Model Quantum Numbers Four numbers, called quantum numbers, describe the characteristics of electrons and their orbitals Quantum Numbers Quantum Numbers The Case of Hydrogen
Chemistry 1210, Section 3, Fall semester 2012 Second Hour Exam October 24, 2012 Dr. Scott Ensign ExamVersion 0001
 Chemistry 1210, Section 3, Fall semester 2012 Second Hour Exam October 24, 2012 Dr. Scott Ensign ExamVersion 0001 Instructions: Be sure to mark the exam version number (0001) on your scantron Do not begin
Chemistry 1210, Section 3, Fall semester 2012 Second Hour Exam October 24, 2012 Dr. Scott Ensign ExamVersion 0001 Instructions: Be sure to mark the exam version number (0001) on your scantron Do not begin
Problems with the Wave Theory of Light (Photoelectric Effect)
 CHEM101 NOTES Properties of Light Found that the wave theory could not work for some experiments e.g. the photovoltaic effect This is because the classic EM view of light could not account for some of
CHEM101 NOTES Properties of Light Found that the wave theory could not work for some experiments e.g. the photovoltaic effect This is because the classic EM view of light could not account for some of
Chapter 2 Atoms and Elements. Electromagnetic Radiation. Electromagnetic Spectrum. Electron Energy Levels. 2.6 Electron Energy Levels
 Chapter 2 Atoms and Elements Electromagnetic Radiation 2.6 Electron Energy Levels Electromagnetic radiation Consists of energy particles called photons that travel as waves. Includes low energy particles
Chapter 2 Atoms and Elements Electromagnetic Radiation 2.6 Electron Energy Levels Electromagnetic radiation Consists of energy particles called photons that travel as waves. Includes low energy particles
CHAPTER 5 Electrons in Atoms
 CHAPTER 5 Electrons in Atoms 5.1 Light & Quantized Energy Was the Nuclear Atomic model incomplete? To most scientists, the answer was yes. The arrangement of electrons was not determined > Remember...the
CHAPTER 5 Electrons in Atoms 5.1 Light & Quantized Energy Was the Nuclear Atomic model incomplete? To most scientists, the answer was yes. The arrangement of electrons was not determined > Remember...the
Question: Can we use our simple shell model of the atom to make some predictions?
 During Class Invention Question: Can we use our simple shell model of the atom to make some predictions? 1. Describe the nature of the interaction between protons and electrons in an atom? Consider using
During Class Invention Question: Can we use our simple shell model of the atom to make some predictions? 1. Describe the nature of the interaction between protons and electrons in an atom? Consider using
Chapter 7 Introduction to Spectroscopy
 Name AP CHEM / / Chapter 7 Introduction to Spectroscopy Spectroscopy is the study of the interaction of radiant energy and matter. The electromagnetic spectrum diagram is shown below to use a reference.
Name AP CHEM / / Chapter 7 Introduction to Spectroscopy Spectroscopy is the study of the interaction of radiant energy and matter. The electromagnetic spectrum diagram is shown below to use a reference.
He, Ne, and Ar have shells.
 5.111 Lecture Summary #8 Monday September 22, 2014 Readings for today: Section 1.14 Electronic Structure and the Periodic Table, Section 1.15, 1.16, 1.17, 1.18, and 1.20 - The Periodicity of Atomic Properties.
5.111 Lecture Summary #8 Monday September 22, 2014 Readings for today: Section 1.14 Electronic Structure and the Periodic Table, Section 1.15, 1.16, 1.17, 1.18, and 1.20 - The Periodicity of Atomic Properties.
1.1 Atomic structure. The Structure of the Atom Mass Spectrometry Electronic Structure Ionisation Energies
 1.1 Atomic structure The Structure of the Atom Mass Spectrometry Electronic Structure Ionisation Energies a) Protons, neutrons and electrons THE STRUCTURE OF THE ATOM Atoms are made up of three fundamental
1.1 Atomic structure The Structure of the Atom Mass Spectrometry Electronic Structure Ionisation Energies a) Protons, neutrons and electrons THE STRUCTURE OF THE ATOM Atoms are made up of three fundamental
Periodic Trends. objectives: Atomic Radius Ionization Energy Reactivity
 objectives: Periodic Trends I can determine parts (see vocab list) of the periodic table. (with stepline) I can apply Coulomb's law to attraction of electrons to the nucleus. I can analyze data or use
objectives: Periodic Trends I can determine parts (see vocab list) of the periodic table. (with stepline) I can apply Coulomb's law to attraction of electrons to the nucleus. I can analyze data or use
2. Atomic Structure and Periodic Table Details of the three Sub-atomic (fundamental) Particles
 2. Atomic Structure and Periodic Table Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 Electron Orbitals 1/184-1
2. Atomic Structure and Periodic Table Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 Electron Orbitals 1/184-1
6.1.5 Define frequency and know the common units of frequency.
 CHM 111 Chapter 6 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the corresponding
CHM 111 Chapter 6 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the corresponding
Electromagnetic Radiation. Chapter 12: Phenomena. Chapter 12: Quantum Mechanics and Atomic Theory. Quantum Theory. Electromagnetic Radiation
 Chapter 12: Phenomena Phenomena: Different wavelengths of electromagnetic radiation were directed onto two different metal sample (see picture). Scientists then recorded if any particles were ejected and
Chapter 12: Phenomena Phenomena: Different wavelengths of electromagnetic radiation were directed onto two different metal sample (see picture). Scientists then recorded if any particles were ejected and
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET
 EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET 1.) Look at the EM spectrum below to answer this question. As you move across the visible light spectrum from red to violet (A) Does the wavelength
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET 1.) Look at the EM spectrum below to answer this question. As you move across the visible light spectrum from red to violet (A) Does the wavelength
Electrons in Atoms. Section 5.1 Light and Quantized Energy
 Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Chem 6 Practice Exam 2
 These problems are from past Chem 6 exams. Each exam contained a page of universal constant values and common equations; yours will, too, along with a Periodic Table! The answers follow after all the questions.
These problems are from past Chem 6 exams. Each exam contained a page of universal constant values and common equations; yours will, too, along with a Periodic Table! The answers follow after all the questions.
1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons.
 1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. (i) 9.11 10-28 g is the mass of 1 electron No. of electrons 1 g
1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. (i) 9.11 10-28 g is the mass of 1 electron No. of electrons 1 g
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Chemistry 121: Atomic and Molecular Chemistry Topic 3: Atomic Structure and Periodicity
 Text Chapter 2, 8 & 9 3.1 Nature of light, elementary spectroscopy. 3.2 The quantum theory and the Bohr atom. 3.3 Quantum mechanics; the orbital concept. 3.4 Electron configurations of atoms 3.5 The periodic
Text Chapter 2, 8 & 9 3.1 Nature of light, elementary spectroscopy. 3.2 The quantum theory and the Bohr atom. 3.3 Quantum mechanics; the orbital concept. 3.4 Electron configurations of atoms 3.5 The periodic
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Modern Atomic Theory. (a.k.a. the electron chapter!) Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11
 Modern Atomic Theory (a.k.a. the electron chapter!) 1 Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11 ELECTROMAGNETIC RADIATION 2 Electromagnetic radiation. 3 4 Electromagnetic Radiation
Modern Atomic Theory (a.k.a. the electron chapter!) 1 Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11 ELECTROMAGNETIC RADIATION 2 Electromagnetic radiation. 3 4 Electromagnetic Radiation
Periodic Trends. 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
 Periodic Trends objectives: (#2 3) How do the properties of electrons and the electron shells contribute to the periodic trends? 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
Periodic Trends objectives: (#2 3) How do the properties of electrons and the electron shells contribute to the periodic trends? 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
Unit 2 - Electrons and Periodic Behavior
 Unit 2 - Electrons and Periodic Behavior Models of the Atom I. The Bohr Model of the Atom A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The
Unit 2 - Electrons and Periodic Behavior Models of the Atom I. The Bohr Model of the Atom A. Electron Orbits, or Energy Levels 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The
Atomic Structure and Periodicity
 Atomic Structure and Periodicity Atoms and isotopes: Isotopes-#p + same for all but mass number is different b/c of # n o Average atomic mass is weighted average of all the isotopes for an element Average
Atomic Structure and Periodicity Atoms and isotopes: Isotopes-#p + same for all but mass number is different b/c of # n o Average atomic mass is weighted average of all the isotopes for an element Average
PHOTOELECTRON SPECTROSCOPY PROBLEMS:
 PHOTOELECTRON SPECTROSCOPY PROBLEMS: 1. A hydrogen atom in its ground state is irradiated with 80 nm radiation. What is the kinetic energy (in ev) of the ejected photoelectron? Useful formulae: ν = c/λ
PHOTOELECTRON SPECTROSCOPY PROBLEMS: 1. A hydrogen atom in its ground state is irradiated with 80 nm radiation. What is the kinetic energy (in ev) of the ejected photoelectron? Useful formulae: ν = c/λ
McCord CH301 Exam 2 Oct 10, 2017
 452 version last name first name signature McCord CH301 Exam 2 Oct 10, 2017 50070 Tuesday Remember to refer to the Periodic Table handout that is separate from this exam copy. There are many conversion
452 version last name first name signature McCord CH301 Exam 2 Oct 10, 2017 50070 Tuesday Remember to refer to the Periodic Table handout that is separate from this exam copy. There are many conversion
Atoms with More than One Electron
 Fun with the Periodic Table Activity 6 Atoms with More than One Electron GOALS In this activity you will: View the spectra of various materials. Graphically analyze patterns in the amounts of energy required
Fun with the Periodic Table Activity 6 Atoms with More than One Electron GOALS In this activity you will: View the spectra of various materials. Graphically analyze patterns in the amounts of energy required
8. Which of the following could be an isotope of chlorine? (A) 37 Cl 17 (B) 17 Cl 17 (C) 37 Cl 17 (D) 17 Cl 37.5 (E) 17 Cl 37
 Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Name: Date: Blk: Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed:
 Name: Date: Blk: NOTES: PERIODIC TRENDS Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed: I. ATOMIC RADIUS (Size) Going from
Name: Date: Blk: NOTES: PERIODIC TRENDS Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed: I. ATOMIC RADIUS (Size) Going from
PART 2 Electronic Structure and the Periodic Table. Reference: Chapter 7 8 in textbook
 PART 2 Electronic Structure and the Periodic Table Reference: Chapter 7 8 in textbook 1 Early Atomic Models 2 Thomson s 1904 Model of the Atom Plumb Pudding Model He discovered the electron, a discovery
PART 2 Electronic Structure and the Periodic Table Reference: Chapter 7 8 in textbook 1 Early Atomic Models 2 Thomson s 1904 Model of the Atom Plumb Pudding Model He discovered the electron, a discovery
Bonds in molecules are formed by the interactions between electrons.
 CHEM 2060 Lecture 6: Electrostatic Interactions L6-1 PART TWO: Electrostatic Interactions In the first section of this course, we were more concerned with structural aspects of molecules. In this section
CHEM 2060 Lecture 6: Electrostatic Interactions L6-1 PART TWO: Electrostatic Interactions In the first section of this course, we were more concerned with structural aspects of molecules. In this section
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
Chapter 4 Arrangement of Electrons in Atoms. 4.1 The Development of a New Atomic Model
 Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Name Class Date. Chapter: Arrangement of Electrons in Atoms
 Assessment Chapter Test A Chapter: Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the
Assessment Chapter Test A Chapter: Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Atomic Spectroscopy. Objectives
 Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
1 of 43 Boardworks Ltd Chemistry 11. Chemical Bonding
 1 of 43 Boardworks Ltd 2009 Chemistry 11 Chemical Bonding 2 of 43 Boardworks Ltd 2009 Electrostatic Forces An electrostatic force is a forces existing as a result of the attraction or repulsion between
1 of 43 Boardworks Ltd 2009 Chemistry 11 Chemical Bonding 2 of 43 Boardworks Ltd 2009 Electrostatic Forces An electrostatic force is a forces existing as a result of the attraction or repulsion between
Quantum Mechanics/Trends Lab
 NAME Quantum Mechanics/Trends Lab Quantum mechanics: The electron orbit model (Bohr Model) that was proposed for hydrogen, does not work for any other atom. This model does give us the idea of quantization:
NAME Quantum Mechanics/Trends Lab Quantum mechanics: The electron orbit model (Bohr Model) that was proposed for hydrogen, does not work for any other atom. This model does give us the idea of quantization:
When I lecture we will add more info, so leave spaces in your notes
 Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write out the notes from my website. Use different
Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write out the notes from my website. Use different
Electron Configuration & Periodicity Unit 3
 Name: Electron Configuration & Periodicity Unit 3 (seven class periods) Unit 3.1: First Ionization Energy & Photoelectron Spectroscopy 1) Coulombs Law a) The force of attraction between two charged objects
Name: Electron Configuration & Periodicity Unit 3 (seven class periods) Unit 3.1: First Ionization Energy & Photoelectron Spectroscopy 1) Coulombs Law a) The force of attraction between two charged objects
THE STRUCTURE OF ATOMS. ATOMS Atoms consist of a number of fundamental particles, the most important ones are...
 Atomic Structure THE STRUCTURE OF ATOMS ATOMS Atoms consist of a number of fundamental particles, the most important ones are... Mass / kg Charge / C Relative mass Relative Charge PROTON NEUTRON ELECTRON
Atomic Structure THE STRUCTURE OF ATOMS ATOMS Atoms consist of a number of fundamental particles, the most important ones are... Mass / kg Charge / C Relative mass Relative Charge PROTON NEUTRON ELECTRON
Electrons, Energy, & the Electromagnetic Spectrum Notes
 Electrons, Energy, & the Electromagnetic Spectrum Notes Bohr Model Diagram Interpretation What form of EM radiation is released when an electron in a hydrogen atom falls from the 5 th energy level to the
Electrons, Energy, & the Electromagnetic Spectrum Notes Bohr Model Diagram Interpretation What form of EM radiation is released when an electron in a hydrogen atom falls from the 5 th energy level to the
Atomic Structure and Atomic Spectra
 Atomic Structure and Atomic Spectra Atomic Structure: Hydrogenic Atom Reading: Atkins, Ch. 10 (7 판 Ch. 13) The principles of quantum mechanics internal structure of atoms 1. Hydrogenic atom: one electron
Atomic Structure and Atomic Spectra Atomic Structure: Hydrogenic Atom Reading: Atkins, Ch. 10 (7 판 Ch. 13) The principles of quantum mechanics internal structure of atoms 1. Hydrogenic atom: one electron
Notes: Electrons and Periodic Table (text Ch. 4 & 5)
 Name Per. Notes: Electrons and Periodic Table (text Ch. 4 & 5) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to
Name Per. Notes: Electrons and Periodic Table (text Ch. 4 & 5) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to
The Periodic Table. Atoms, Elements, and the Periodic Table
 Atoms, Elements, and the Periodic Table Element: a pure substance that cannot be broken down into simpler substances by a chemical reaction. Each element is identified by a one- or two-letter symbol. Elements
Atoms, Elements, and the Periodic Table Element: a pure substance that cannot be broken down into simpler substances by a chemical reaction. Each element is identified by a one- or two-letter symbol. Elements
A.P. Chemistry Practice Test - Ch. 7, Atomic Structure and Periodicity
 A.P. Chemistry Practice Test - Ch. 7, Atomic Structure and Periodicity 1) Ham radio operators often broadcast on the 6-meter band. The frequency of this electromagnetic radiation is MHz. A) 50 B) 20 C)
A.P. Chemistry Practice Test - Ch. 7, Atomic Structure and Periodicity 1) Ham radio operators often broadcast on the 6-meter band. The frequency of this electromagnetic radiation is MHz. A) 50 B) 20 C)
Sparks CH301. Quantum Mechanics. Waves? Particles? What and where are the electrons!? UNIT 2 Day 3. LM 14, 15 & 16 + HW due Friday, 8:45 am
 Sparks CH301 Quantum Mechanics Waves? Particles? What and where are the electrons!? UNIT 2 Day 3 LM 14, 15 & 16 + HW due Friday, 8:45 am What are we going to learn today? The Simplest Atom - Hydrogen Relate
Sparks CH301 Quantum Mechanics Waves? Particles? What and where are the electrons!? UNIT 2 Day 3 LM 14, 15 & 16 + HW due Friday, 8:45 am What are we going to learn today? The Simplest Atom - Hydrogen Relate
Getting to know the Periodic Table: Recall: Elements are organized based on atomic number and similar properties
 Getting to know the Periodic Table: Recall: Elements are organized based on atomic number and similar properties 1. Find your staircase on the right side of the periodic table. Feel free to make the lines
Getting to know the Periodic Table: Recall: Elements are organized based on atomic number and similar properties 1. Find your staircase on the right side of the periodic table. Feel free to make the lines
Light. Light (con t.) 2/28/11. Examples
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Atoms and Periodic Properties
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Unit 01 (Chp 6,7): Atoms and Periodic Properties John D. Bookstaver St. Charles Community College
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Unit 01 (Chp 6,7): Atoms and Periodic Properties John D. Bookstaver St. Charles Community College
LECTURE 4: HOW TO GENERATE ELECTRONIC CONFIGURATIONS FOR ATOMS AND IONS
 LECTURE 4: HOW TO GENERATE ELECTRONIC CONFIGURATIONS FOR ATOMS AND IONS We are about to do something incredibly useful create the electronic configurations for every kind of neutral or charged atom we
LECTURE 4: HOW TO GENERATE ELECTRONIC CONFIGURATIONS FOR ATOMS AND IONS We are about to do something incredibly useful create the electronic configurations for every kind of neutral or charged atom we
Exercise 1 Atomic line spectra 1/9
 Exercise 1 Atomic line spectra 1/9 The energy-level scheme for the hypothetical one-electron element Juliettium is shown in the figure on the left. The potential energy is taken to be zero for an electron
Exercise 1 Atomic line spectra 1/9 The energy-level scheme for the hypothetical one-electron element Juliettium is shown in the figure on the left. The potential energy is taken to be zero for an electron
Topic 3: Periodic Trends and Atomic Spectroscopy
 Topic 3: Periodic Trends and Atomic Spectroscopy Introduction Valence Electrons are those in the outer most shell of an element and are responsible for the bonding characteristics of that element. Core
Topic 3: Periodic Trends and Atomic Spectroscopy Introduction Valence Electrons are those in the outer most shell of an element and are responsible for the bonding characteristics of that element. Core
Modern Physics Lesson 1: Intro to Quantum Mechanics and Photoelectric Effect Lesson 2: Energy Levels Lesson 3: E = mc^2 Lesson 4: Standard Model of
 Modern Physics Lesson 1: Intro to Quantum Mechanics and Photoelectric Effect Lesson 2: Energy Levels Lesson 3: E = mc^2 Lesson 4: Standard Model of Particle Physics Pass up your homework Energy is Quantized
Modern Physics Lesson 1: Intro to Quantum Mechanics and Photoelectric Effect Lesson 2: Energy Levels Lesson 3: E = mc^2 Lesson 4: Standard Model of Particle Physics Pass up your homework Energy is Quantized
Chem Unit: Part I Atoms and the EM Force
 Chem Unit: Part I Atoms and the EM Force I. Electric Charge & the EM Force of Attraction and Repulsion Some of the fundamental particles that make up the STUFF in this universe have a property called electric
Chem Unit: Part I Atoms and the EM Force I. Electric Charge & the EM Force of Attraction and Repulsion Some of the fundamental particles that make up the STUFF in this universe have a property called electric
Chem 111 Exam #2 November 8, J h = c E = h E. ΔH = energies of bonds broken - energies of bonds formed SHOW ALL WORK
 General Chemistry I NAME: Answer Key Chem 111 Exam #2 November 8, 2013 Some Equations and Constants for your use: -18-2.18 10 J h = c E = h E n = = 2 n mv o ΔH = energies of bonds broken - energies of
General Chemistry I NAME: Answer Key Chem 111 Exam #2 November 8, 2013 Some Equations and Constants for your use: -18-2.18 10 J h = c E = h E n = = 2 n mv o ΔH = energies of bonds broken - energies of
Name: Unit 3 Guide-Electrons In Atoms
 Name: Unit 3 Guide-Electrons In Atoms Importance of Electrons Draw a complete Bohr model of the atom. Write an element s electron configuration. Know how the symbols used in ECs relate to electron properties
Name: Unit 3 Guide-Electrons In Atoms Importance of Electrons Draw a complete Bohr model of the atom. Write an element s electron configuration. Know how the symbols used in ECs relate to electron properties
X-Ray transitions to low lying empty states
 X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
Interactive Periodic Trends: A Graphical Experience
 CHM 101 Name Interactive Periodic Trends: A Graphical Experience This activity explores a variety of properties of the chemical elements as they vary based on position on the periodic table. You should
CHM 101 Name Interactive Periodic Trends: A Graphical Experience This activity explores a variety of properties of the chemical elements as they vary based on position on the periodic table. You should
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A
 CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
6.3 Periodic Trends > Chapter 6 The Periodic Table. 6.3 Periodic Trends. 6.1 Organizing the Elements. 6.2 Classifying the Elements
 1 63 Periodic Trends > Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends 2 63 Periodic Trends > CHEMISTRY & YOU How are trends in the weather similar
1 63 Periodic Trends > Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends 2 63 Periodic Trends > CHEMISTRY & YOU How are trends in the weather similar
Trends in Atomic Size. What are the trends among the elements for atomic size? The distances between atoms in a molecule are extremely small.
 63 Periodic Trends > 63 Periodic Trends > CHEMISTRY & YOU Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends How are trends in the weather similar to
63 Periodic Trends > 63 Periodic Trends > CHEMISTRY & YOU Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends How are trends in the weather similar to
Chapter 7. Characteristics of Atoms. 7.1 Electromagnetic Radiation. Chapter 7 1. The Quantum Mechanical Atom. Atoms: How do we study atoms?
 Chapter 7 The Quantum Mechanical Atom 1 Characteristics of Atoms Atoms: possess mass contain positive nuclei contain electrons occupy volume have various properties attract one another combine to form
Chapter 7 The Quantum Mechanical Atom 1 Characteristics of Atoms Atoms: possess mass contain positive nuclei contain electrons occupy volume have various properties attract one another combine to form
PHOTOELECTRON SPECTROSCOPY
 PHOTOELECTRON SPECTROSCOPY Background Information: In photoelectron spectroscopy (PES), one can determine the identity of a substance by measuring the amount of energy needed to remove electrons from the
PHOTOELECTRON SPECTROSCOPY Background Information: In photoelectron spectroscopy (PES), one can determine the identity of a substance by measuring the amount of energy needed to remove electrons from the
Mendeleev s Periodic Law
 Mendeleev s Periodic Law Periodic Law When the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically. Mendeleev s Periodic Law allows us to predict what
Mendeleev s Periodic Law Periodic Law When the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically. Mendeleev s Periodic Law allows us to predict what
