McCord CH301 Exam 3 Oct 26, 2017
|
|
|
- Adele Anthony
- 6 years ago
- Views:
Transcription
1 483 version last name first name signature McCord C301 Exam 3 Oct 26, Thursday Remember to refer to the Periodic Table handout that is separate from this exam copy. NOTE: Please keep this exam copy intact (all pages still stapled - including this cover page). You must turn in ALL the materials that were distributed. This means that you turn in your exam copy (name and signature included), bubble sheet, periodic table handout, and all scratch paper. Please also have your UT ID card ready to show as well.
2 Version 483 Exam 3 - F17 mccord (50070) 2 This print-out should have 20 questions. Multiple-choice questions may continue on the next column or page find all choices before answering points In an acceptable Lewis structure for NO 3, whatistheformalchargeonthecentralatom? correct 6. None of the above Formal charge can be expressed with the following basic formula: F.C. = V.E. (Bonds+L.E.) The nitrogen has no lone pairs and is connected to 4 bonds total, resulting in a formal charge of points Whenamoleculeorionisshownviaresonance structures, the proposed structures I. can have any formal charge on the individualatomsaslongastheformalcharge of the molecule is less than 2 II. should be averaged to best depict the bonding III. depict the physical vibrations of the bonds IV. show the various bonding extremes that resonate back and forth 1. I, II, III and IV 2. II only correct 3. I and II only 4. II and III only 5. None of the above 6. I and IV only 7. II and IV only 8. IV only The numerous resonance structures represent the set of bonding extremes of which the average is the best indication of the actual bonding. To create an acceptable resonance structure, you must ensure that all atoms have a formal charge less than points Consider the following potential energy (E p ) vs internuclear distance (r) plot for the interaction between two hydrogen atoms. E p r(pm) What will happen if you place two hydrogen atoms at an internuclear distance of 115 pm? 1. Attractions will dominate until the internuclear radius is equal to 0 2. There will be no attractions or repulsions at this distance 3. Attractions will dominate until potential energy is minimized correct 4. Repulsions will dominate until potential energy is minimized 5. Repulsions will dominate until the internuclear radius is infinite
3 Version 483 Exam 3 - F17 mccord (50070) 3 6. Attractions will dominate until potential energy is zero The bond length (minimum potential energy)isequaltoabout75pm. Atanydistance greater than 75 pm, attractions will dominate to reach minimum potential energy. At any distance less than 75 pm, repulsions will dominate to reach minimum potential energy points The lattice energy for N 4 O is given by which reaction? 1.2N 4 O(s) 2N 4 (g)+o 2 (g)+ 2 (g) 2. N 4 O(s) N + 4 (g) + O (g) correct 3.2N 4 O(s) N 2 (g)+4 2 (g)+ 2 O(g) 4. N 4 O(s) N 4 (g) + O(g) 5.N 4 O(s) N 3+ (g)+5 + (g)+o 2 (g) The lattice energy is the energy needed to overcome the ionic bond (and only the ionic bond) of an ionic compound. Therefore, the reaction is N 4 O(s) N + 4 (g) + O (g) points Rank the following ionic compounds from least to greatest lattice energy: MgCl 2, CaO, CaCO 3, NaCl 1. CaO < NaCl < MgCl 2 < CaCO 3 2. CaCO 3 < MgCl 2 < CaO < NaCl 3.NaCl<MgCl 2 <CaCO 3 <CaOcorrect 4. CaCO 3 < MgCl 2 < NaCl < CaO 5. NaCl < MgCl 2 < CaO < CaCO 3 Lattice energy depends on charge and ionic radius. Rank first based on a charge so that the largest charges have the highest lattice energy. The smallest lattice energy in the list isnacland thehighest latticeenergy iscao. If two compounds have the same charges, the highest lattice energy will be the smaller ionic compound. This gives a final answer of: NaCl < MgCl 2 < CaCO 3 < CaO 006 (part 1 of 3) 5.0 points The molecule shown below is a rare alkaloid found in plants native to South America with profound cultural significance. O a F N What is the correct empirical formula for this compound? 1. C FN 2 O 2. C FN 2 O 3. C FN 2 O 4. C FN 2 O correct 5. C FN 2 O 6. C N 2 O The empirical formula is C FN 2 O. 007 (part 2 of 3) 5.0 points Which of the following best represents the bond angle labeled a? correct N b c
4 Version 483 Exam 3 - F17 mccord (50070) There are two (not shown) lone pairs on the O there which means there are 4 electron regions and therefore a tetrahedral electronic geometry which has a bond angle of The lone pairs are more repulsive than the bonding pairs and therefore pushes the angles down to about (part 3 of 3) 5.0 points What is the electronic geometry around the central atom labeled b? 1. Trigonal planar correct 2. Seesaw 3. Bent 4. Trigonal pyramid 5. Tetrahedral In the line drawing, b has an understood hydrogen atom attached, giving the carbon a trigonal planar structure. The nitrogen labeled c has an understood lone pair, which gives it a tetrahedral electronic geometry points What are the bond angles around the central atom of CF 4? correct ThestructureofCF 4 hasfourbondsaround carbon, resulting in a tetrahedral electronic geometry. The bond angles are degrees points Which of the following choices is the correct line structure for propionaldehyde, C 3 C 2 CO? : O: 1. C C C 2. C C O C : O 3. C C C 4. C C C O : O: 5. C C C 6. C C O C 7. C O C Acetone: : O: C C C correct
5 Version 483 Exam 3 - F17 mccord (50070) 5 Propionaldehyde: Propanol: : O: C C C C C C O points Consider the following molecules: CO, CO 2, CO 2 3 Which molecule has the strongest covalent bonds? 1. All carbon-oxygen bonds have the same strength bonds 2. CO CO 2 4. Both CO 2 3 and CO 2 have the strongest bonds 5. CO correct Carbon monoxide contains a triple bond between carbon and oxygen. Carbon dioxide contains a double bond between the carbon and oxygen atoms. Carbonate is a resonance structure that contains a double bond and two single bonds. Averaged, this results in approximate 1.33 bonds between the carbon and oxygen atoms. Therefore, carbon monoxide has the strongest and shortest bonds. Carbonate has the weakest and longest bonds points Ozone (O 3 ) is. 1. a non-polar molecule with polar bonds. 2. a polar molecule with polar bonds. 3. a polar molecule with non-polar bonds. correct 4. a polar molecule with both polar and non-polar bonds. 5. a non-polar molecule with non-polar bonds. 6. a non-polar molecule with both polar and non-polar bonds. Ozone is only oxygen, so all the bonds are non-polar. owever, the molecule itself is bent with lone pairs that contribute to the asymmetry (and therefore polarity) of the overall molecule points Which of the following molecules is nonpolar? 1. C 3 Cl 2. BeF 2 correct 3. N O 5. PCl 3 BeF 2 has polar bonds, but they are canceled out by its symmetric molecular and electronic structure. 014 (part 1 of 3) 5.0 points For the next three questions, classify the bonds according to their relative difference in electronegativity. Cl contains a(n). 1. Nonpolar covalent bond 2. Polar covalent bond correct 3. Ionic bond Cl is a polar covalent bond.
6 Version 483 Exam 3 - F17 mccord (50070) (part 2 of 3) 5.0 points Ag 2 CO 3 contains. 1. Only polar covalent bonds 2. Ionic and polar covalent bonds correct 3. Nonpolar and polar covalent bonds 4. Only ionic bonds 5. Ionic and nonpolar covalent bonds Ag 2 CO 3 is an ionic molecule. owever, there is also a polyatomic ion with a polar covalent bonds. 016 (part 3 of 3) 5.0 points Cl 2 contains a(n). 1. Polar covalent bond 2. Nonpolar covalent bond correct 3. Ionic bond Cl 2 contains only a nonpolar covalent bond with a difference of electronegativity equal to zero points Consider the Lewis Structure drawn below: : 2 Ȯ. : S S Ȯ. : : O : Is this an acceptable structure for thiosulfate (S 2 O 2 3 )? If not, choose the answer that best explains why this is an unacceptable drawing. 1. This is not an acceptable structure because the formal charge on the atoms does not add up to the formal charge of the polyatomic ion 2. This is not an acceptable structure because it has the wrong number of electrons 3. This is not an acceptable structure because at least one atom disobeys the octet rule 4. This structure is unacceptable because of ALL reasons listed (formal charge, number of electrons, AND the octet rule are violated) correct 5. This is an acceptable structure Considering the top oxygen, we can see that the octet rule is violated. The total charge on the molecule adds up to negative four. The structure uses two electrons more than are available. Therefore, this structure is unacceptable, and violates all rules listed points Consider the following molecule: :Cl X Cl : : Cl : : Cl : Whatistheidentifyofthecentralatom,X? 1. Oxygen 2. Selenium correct 3. Chlorine 4. Nitrogen 5. Carbon 6. Phosphorus The molecule shown has a central atom with a formal charge equal to 0. You can solve for the valence using the formula: F.C. = V.E. (Bonds+L.E.) 0 = V.E. 6
7 Version 483 Exam 3 - F17 mccord (50070) 7 Therefore, your central atom must be in group 6. It is also exhibiting expanded valence, meaning it must be at least a 3rd period element. X = Selenium. be slightly less than predicted by the electronic geometry. Molecular orbitals do hybridize, but thisisnot an explanationfor why a molecule will form imperfect bond angles points What is the electronic geometry of the polyatomic ion IF 4? 1. Octahedral correct 2. Tetrahedral 3. T-shaped 4. Square pyramid 5. Square planar 6. None of the above 7. Linear 8. Trigonal bipyramid The structure of IF 4 has four bonding regions and two lone pairs. This corresponds to an octahedral geometry points VSEPR theory can estimate imperfect bond angles based on the fact that 1. bonding regions attract each other while lone pair regions repel each other 2. lone pair regions are more repulsive than bonding regions correct 3. molecular orbitals hybridize 4. bonding regions are more repulsive than lone pair regions Lone pair regions on the central atom are pulled closer toward the nucleus. Therefore, the repulsive forces are stronger than in a bonding region. This causes bond angles to
Chapter 13: Phenomena
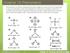 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Form J. Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004
 Form J Chemistry 1441-023 Name (please print) Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004 Instructions: 1. This exam consists of 27 questions. 2. No scratch paper is allowed.
Form J Chemistry 1441-023 Name (please print) Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004 Instructions: 1. This exam consists of 27 questions. 2. No scratch paper is allowed.
CHEM 110 Exam 2 - Practice Test 1 - Solutions
 CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
Ch 6 Chemical Bonding
 Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
AP Chemistry- Practice Bonding Questions for Exam
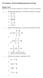 AP Chemistry- Practice Bonding Questions for Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is a correct Lewis structure for
AP Chemistry- Practice Bonding Questions for Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is a correct Lewis structure for
Exam 2. Remember to refer to the Periodic Table handout that is separate from this exam copy.
 001 version last name first name signature McCord CH301 unique: 49885 TTh 9:30 am - 11 am Exam 2 Oct 15, 2018 Monday 7:30-9:00 PM A - Mi in BUR 106 Mo - Z in JES A121A Remember to refer to the Periodic
001 version last name first name signature McCord CH301 unique: 49885 TTh 9:30 am - 11 am Exam 2 Oct 15, 2018 Monday 7:30-9:00 PM A - Mi in BUR 106 Mo - Z in JES A121A Remember to refer to the Periodic
McCord CH301 Exam 3 Fall 2016
 483 version last name first name signature McCord CH301 Exam 3 Fall 2016 49970 / 49975 Remember that the bubble sheet has the periodic table on the back. NOTE: Please keep your Exam copy intact (all pages
483 version last name first name signature McCord CH301 Exam 3 Fall 2016 49970 / 49975 Remember that the bubble sheet has the periodic table on the back. NOTE: Please keep your Exam copy intact (all pages
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Exam 2. Remember to refer to the Periodic Table handout that is separate from this exam copy.
 001 version last name first name signature McCord CH301 unique: 49885 TTh 9:30 am - 11 am Exam 2 Oct 15, 2018 Monday 7:30-9:00 PM A - Mi in BUR 106 Mo - Z in JES A121A Remember to refer to the Periodic
001 version last name first name signature McCord CH301 unique: 49885 TTh 9:30 am - 11 am Exam 2 Oct 15, 2018 Monday 7:30-9:00 PM A - Mi in BUR 106 Mo - Z in JES A121A Remember to refer to the Periodic
Chapters 9&10 Structure and Bonding Theories
 Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
What is Bonding? The Octet Rule. Getting an Octet. Chemical Bonding and Molecular Shapes. (Chapter Three, Part Two)
 Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Chemical Bonding II. Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds Hybridization MO theory
 Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Helpful Hints Lewis Structures Octet Rule For Lewis structures of covalent compounds least electronegative
 Helpful Hints Lewis Structures Octet Rule Lewis structures are a basic representation of how atoms are arranged in compounds based on bond formation by the valence electrons. A Lewis dot symbol of an atom
Helpful Hints Lewis Structures Octet Rule Lewis structures are a basic representation of how atoms are arranged in compounds based on bond formation by the valence electrons. A Lewis dot symbol of an atom
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Molecular Geometry and intermolecular forces. Unit 4 Chapter 9 and 11.2
 1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
Lewis Structure. Lewis Structures & VSEPR. Octet & Duet Rules. Steps for drawing Lewis Structures
 Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
CHEM PRACTICE EXAM IV CLASS - SPRING 2017 ANSWER KEY
 CHEM 1031 - PRACTICE EXAM IV CLASS - SPRING 2017 ANSWER KEY 1. When Group 1A (except for H) and Group 17 (7A) elements react with each other, they are most likely to form: A. Covalent or ionic bonds B.
CHEM 1031 - PRACTICE EXAM IV CLASS - SPRING 2017 ANSWER KEY 1. When Group 1A (except for H) and Group 17 (7A) elements react with each other, they are most likely to form: A. Covalent or ionic bonds B.
CHEMICAL BONDING. Valence Electrons. Chapter Ten
 CHEMICAL BONDING Chapter Ten Valence Electrons! The electrons occupying the outermost energy level of an atom are called the valence electrons; all other electrons are called the core electrons.! The valence
CHEMICAL BONDING Chapter Ten Valence Electrons! The electrons occupying the outermost energy level of an atom are called the valence electrons; all other electrons are called the core electrons.! The valence
VSEPR. Valence Shell Electron Pair Repulsion Theory
 VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED
 CHM-201 General Chemistry and Laboratory I Unit #4 Take Home Test Due December 13, 2018 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
CHM-201 General Chemistry and Laboratory I Unit #4 Take Home Test Due December 13, 2018 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
Name Date Class MOLECULAR COMPOUNDS. Distinguish molecular compounds from ionic compounds Identify the information a molecular formula provides
 8.1 MOLECULAR COMPOUNDS Section Review Objectives Distinguish molecular compounds from ionic compounds Identify the information a molecular formula provides Vocabulary covalent bond molecule diatomic molecule
8.1 MOLECULAR COMPOUNDS Section Review Objectives Distinguish molecular compounds from ionic compounds Identify the information a molecular formula provides Vocabulary covalent bond molecule diatomic molecule
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chemical Bonding Chapter 8
 Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
RESONANCE STRUCTURE When a molecule has more than one possible structure. Draw all possible structures and place a double end arrow ( ) in between.
 CHEMISTRY NOTES 6.1 COVALENT BONDS Objectives Explain the role and location of electrons in a covalent bond. Describe the change in energy and stability that takes place as a covalent bond forms. Distinguish
CHEMISTRY NOTES 6.1 COVALENT BONDS Objectives Explain the role and location of electrons in a covalent bond. Describe the change in energy and stability that takes place as a covalent bond forms. Distinguish
Chapter 7 Chemical Bonding
 Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
Chemical Bonding. 5. _c Atoms with a strong attraction for electrons they share with another atom exhibit
 CHAPTER 6 REVIEW Chemical Bonding SHORT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence electrons and of
CHAPTER 6 REVIEW Chemical Bonding SHORT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence electrons and of
Test Bank for Introductory Chemistry Essentials 5th Edition by Tro
 Test Bank for Introductory Chemistry Essentials 5th Edition by Tro Sample Introductory Chemistry, 5e (Tro) Chapter 10 Chemical Bonding 10.1 True/False Questions 1) Bonding theories are used to predict
Test Bank for Introductory Chemistry Essentials 5th Edition by Tro Sample Introductory Chemistry, 5e (Tro) Chapter 10 Chemical Bonding 10.1 True/False Questions 1) Bonding theories are used to predict
Version 001 Practice Final Exam 1
 Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points ow many moles
Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points ow many moles
Chapter 13: Phenomena
 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
REVIEW: VALENCE ELECTRONS CHEMICAL BONDS: LEWIS SYMBOLS: CHEMICAL BONDING. What are valence electrons?
 REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
Chem 121 Exam 4 Practice Exam
 Chem 121 Exam 4 Practice Exam 1. What is the correct electron configuration for bromine? b. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2 4p 6 c. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 d. 1s 2 2s 2 2p 6 3s 2 3p
Chem 121 Exam 4 Practice Exam 1. What is the correct electron configuration for bromine? b. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2 4p 6 c. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 d. 1s 2 2s 2 2p 6 3s 2 3p
Unit Six --- Ionic and Covalent Bonds
 Unit Six --- Ionic and Covalent Bonds Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples
Unit Six --- Ionic and Covalent Bonds Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples
Version 001 Practice Final Exam 1
 Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points Thesecond
Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points Thesecond
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS
 CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Group 8. Na Mg Al Si P S Cl Ar
 CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) Question 1 An atom loses an electron to another atom. Is this an example of a physical or chemical change? Question 2 Physical
www.tutor-homework.com (for tutoring, homework help, or help with online classes) Question 1 An atom loses an electron to another atom. Is this an example of a physical or chemical change? Question 2 Physical
Chapter 16 Covalent Bonding
 Chemistry/ PEP Name: Date: Chapter 16 Covalent Bonding Chapter 16: 1 26; 28, 30, 31, 35-37, 40, 43-46, Extra Credit: 50-53, 55, 56, 58, 59, 62-67 Section 16.1 The Nature of Covalent Bonding Practice Problems
Chemistry/ PEP Name: Date: Chapter 16 Covalent Bonding Chapter 16: 1 26; 28, 30, 31, 35-37, 40, 43-46, Extra Credit: 50-53, 55, 56, 58, 59, 62-67 Section 16.1 The Nature of Covalent Bonding Practice Problems
Chapter 4 Lecture Outline. Copyright McGraw-Hill Education. Permission required for reproduction or display.
 Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
CHAPTER 12 CHEMICAL BONDING
 CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
Ex. 1) F F bond in F = 0 < % covalent, no transfer of electrons
 #60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
#60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
Lewis Structure and Electron Dot Models
 Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Lewis Theory of Shapes and Polarities of Molecules
 Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Chemical Bonding AP Chemistry Ms. Grobsky
 Chemical Bonding AP Chemistry Ms. Grobsky What Determines the Type of Bonding in Any Substance? Why do Atoms Bond? The key to answering the first question are found in the electronic structure of the atoms
Chemical Bonding AP Chemistry Ms. Grobsky What Determines the Type of Bonding in Any Substance? Why do Atoms Bond? The key to answering the first question are found in the electronic structure of the atoms
: Bond Order = 1.5 CHAPTER 5. Practice Questions
 CAPTER 5 Practice Questions 5.1 5.3 S 5.5 Ethane is symmetrical, so does not have a dipole moment. owever, ethanol has a polar group at one end and so has a dipole moment. 5.7 xygen has the valence electron
CAPTER 5 Practice Questions 5.1 5.3 S 5.5 Ethane is symmetrical, so does not have a dipole moment. owever, ethanol has a polar group at one end and so has a dipole moment. 5.7 xygen has the valence electron
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
Chapter 12. Chemical Bonding
 Chapter 12 Chemical Bonding Chapter 12 Introduction to Chemical Bonding Chemical Bonding Valence electrons are the electrons in the outer shell (highest energy level) of an atom. A chemical bond is a mutual
Chapter 12 Chemical Bonding Chapter 12 Introduction to Chemical Bonding Chemical Bonding Valence electrons are the electrons in the outer shell (highest energy level) of an atom. A chemical bond is a mutual
Check Your Solution A comparison with the figures in Figure 4.31 on page 234 of the student textbook confirms the results.
 Predicting the Shape of a Molecule (Student textbook page 236) 11. What molecular shape is represented by each of the following VSEPR notations? a. AX 3 b. AX 5 E You need to assign a molecular shape that
Predicting the Shape of a Molecule (Student textbook page 236) 11. What molecular shape is represented by each of the following VSEPR notations? a. AX 3 b. AX 5 E You need to assign a molecular shape that
CP Covalent Bonds Ch. 8 &
 CP Covalent Bonds Ch. 8 & 9 2015-2016 Why do atoms bond? Atoms want stability- to achieve a noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons For covalent
CP Covalent Bonds Ch. 8 & 9 2015-2016 Why do atoms bond? Atoms want stability- to achieve a noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons For covalent
Review questions CHAPTER 5. Practice exercises 5.1 F F 5.3
 CHAPTER 5 Practice exercises 5.1 S 5.3 5.5 Ethane is symmetrical, so does not have a dipole moment. However, ethanol has a polar H group at one end and so has a dipole moment. 5.7 xygen has the valence
CHAPTER 5 Practice exercises 5.1 S 5.3 5.5 Ethane is symmetrical, so does not have a dipole moment. However, ethanol has a polar H group at one end and so has a dipole moment. 5.7 xygen has the valence
Chemical Bonds. Chapter 6
 Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Version 001 Practice Final Exam 1
 Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points Thesecond
Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points Thesecond
CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
(A) 1 bonding pair (B) 1 bonding pair and 1 lone pair (C) 2 bonding pairs (D) 2 bonding pairs and 2 lone pairs
 AP Chemistry - Problem Drill 13: Lewis Structures and VSPER No. 1 of 10 1. Lewis structure is used to model covalent bonds of a molecule or ion. Covalent bonds are a type of chemical bonding formed by
AP Chemistry - Problem Drill 13: Lewis Structures and VSPER No. 1 of 10 1. Lewis structure is used to model covalent bonds of a molecule or ion. Covalent bonds are a type of chemical bonding formed by
Molecular Compounds Compounds that are bonded covalently (like in water, or carbon dioxide) are called molecular compounds
 Chapter 8: Covalent Bonding Section 1: Molecular Compounds Bonds are Forces that hold groups of atoms together and make them function as a unit. Two types: Ionic bonds transfer of electrons (gained or
Chapter 8: Covalent Bonding Section 1: Molecular Compounds Bonds are Forces that hold groups of atoms together and make them function as a unit. Two types: Ionic bonds transfer of electrons (gained or
Chapter 8 : Covalent Bonding. Section 8.1: Molecular Compounds
 Chapter 8 : Covalent Bonding Section 8.1: Molecular Compounds What is a molecule? A molecular compound? A molecule is a neutral group of atoms joined together by covalent bonds A molecular compound is
Chapter 8 : Covalent Bonding Section 8.1: Molecular Compounds What is a molecule? A molecular compound? A molecule is a neutral group of atoms joined together by covalent bonds A molecular compound is
Chapter 8 Covalent Boding
 Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 6 PRETEST: Chemical Bonding
 Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
Name. CHM 115 EXAM #2 Practice KEY. a. N Cl b. N F c. F F d. I I e. N Br. a. K b. Be c. O d. Al e. S
 Name CHM 115 EXAM #2 Practice KEY Circle the correct answer. (numbers 1-8, 2.5 points each) 1. Which of the following bonds should be the most polar? a. N Cl b. N F c. F F d. I I e. N Br 2. Choose the
Name CHM 115 EXAM #2 Practice KEY Circle the correct answer. (numbers 1-8, 2.5 points each) 1. Which of the following bonds should be the most polar? a. N Cl b. N F c. F F d. I I e. N Br 2. Choose the
Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules
 Fructose Water Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules Carbon Dioxide Ammonia Title and Highlight TN Ch 10.1 Topic: EQ: Right Side NOTES
Fructose Water Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules Carbon Dioxide Ammonia Title and Highlight TN Ch 10.1 Topic: EQ: Right Side NOTES
Introduction to VSEPR Theory 1
 1 Class 8: Introduction to VSEPR Theory Sec 10.2 VSEPR Theory: The Five Basic Shapes Two Electron Groups: Linear Geometry Three Electron Groups: Trigonal Planar Geometry Four Electron Groups: Tetrahedral
1 Class 8: Introduction to VSEPR Theory Sec 10.2 VSEPR Theory: The Five Basic Shapes Two Electron Groups: Linear Geometry Three Electron Groups: Trigonal Planar Geometry Four Electron Groups: Tetrahedral
Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry Periodic Trends in Atomic Properties Learning Objective
 Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic
Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic
Molecular Geometry & Polarity
 Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
CHM2045 F13--Exam # MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 6 Chemistry Review
 Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Notes: Covalent Bonding
 Name Chemistry Pre-AP Notes: Covalent Bonding Period The main focus of this unit is on the covalent bond; however, we will briefly treat the ionic and metallic bond as well. I. Chemical Bonding Overview
Name Chemistry Pre-AP Notes: Covalent Bonding Period The main focus of this unit is on the covalent bond; however, we will briefly treat the ionic and metallic bond as well. I. Chemical Bonding Overview
Activity Formal Charge and VSEPR Theory for Expanded Octets
 Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
Chapter 8 The Concept of the Chemical Bond
 Chapter 8 The Concept of the Chemical Bond Three basic types of bonds: Ionic - Electrostatic attraction between ions (NaCl) Metallic - Metal atoms bonded to each other Covalent - Sharing of electrons Ionic
Chapter 8 The Concept of the Chemical Bond Three basic types of bonds: Ionic - Electrostatic attraction between ions (NaCl) Metallic - Metal atoms bonded to each other Covalent - Sharing of electrons Ionic
Ch. 12 Section 1: Introduction to Chemical Bonding
 Name Period Date Chemical Bonding & Intermolecular Forces (Chapter 12, 13 &14) Fill-in the blanks during the PowerPoint presentation in class. Ch. 12 Section 1: Introduction to Chemical Bonding Chemical
Name Period Date Chemical Bonding & Intermolecular Forces (Chapter 12, 13 &14) Fill-in the blanks during the PowerPoint presentation in class. Ch. 12 Section 1: Introduction to Chemical Bonding Chemical
What Do Molecules Look Like?
 What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
Ch 13: Covalent Bonding
 Ch 13: Covalent Bonding Section 13: Valence-Shell Electron-Pair Repulsion 1. Recall the rules for drawing Lewis dot structures 2. Remember the special situations: - Resonance structures - ormal charges
Ch 13: Covalent Bonding Section 13: Valence-Shell Electron-Pair Repulsion 1. Recall the rules for drawing Lewis dot structures 2. Remember the special situations: - Resonance structures - ormal charges
Version 001 Practice Final Exam 1
 Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points ow many moles
Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points ow many moles
CHM 151LL: Geometry of Covalent Compounds
 CM 151LL: Geometry of Covalent Compounds Introduction Octet Rule A Lewis structure (or electrondot formula) is a twodimensional structural formula showing the arrangement of electrons around atoms in covalently
CM 151LL: Geometry of Covalent Compounds Introduction Octet Rule A Lewis structure (or electrondot formula) is a twodimensional structural formula showing the arrangement of electrons around atoms in covalently
Periodic Trends. Homework: Lewis Theory. Elements of his theory:
 Periodic Trends There are various trends on the periodic table that need to be understood to explain chemical bonding. These include: Atomic/Ionic Radius Ionization Energy Electronegativity Electron Affinity
Periodic Trends There are various trends on the periodic table that need to be understood to explain chemical bonding. These include: Atomic/Ionic Radius Ionization Energy Electronegativity Electron Affinity
COVALENT BONDING CHEMICAL BONDING I: LEWIS MODEL. Chapter 7
 Chapter 7 P a g e 1 COVALENT BONDING Covalent Bonds Covalent bonds occur between two or more nonmetals. The two atoms share electrons between them, composing a molecule. Covalently bonded compounds are
Chapter 7 P a g e 1 COVALENT BONDING Covalent Bonds Covalent bonds occur between two or more nonmetals. The two atoms share electrons between them, composing a molecule. Covalently bonded compounds are
Na Cl Wants to lose ONE electron! Na Cl Ionic Bond TRANSFER of electrons between atoms. Ionic Bonding. Ionic Bonding.
 BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity
 Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Ionic and Covalent Bonding
 1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
Lab #11- Molecular Geometry
 Objectives Chesapeake Campus Chemistry 111 Laboratory Lab #11- Molecular Geometry Determine the shape of a molecule using the VSEPR. Draw the Lewis structures of a molecule including bond angles and formal
Objectives Chesapeake Campus Chemistry 111 Laboratory Lab #11- Molecular Geometry Determine the shape of a molecule using the VSEPR. Draw the Lewis structures of a molecule including bond angles and formal
CHAPTER 12: CHEMICAL BONDING
 CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
Covalent Bonds Ch. Why do atoms bond? Atoms want noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons
 Covalent Bonds Ch. Why do atoms bond? Atoms want noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons For covalent bonds there is a of electrons to get an
Covalent Bonds Ch. Why do atoms bond? Atoms want noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons For covalent bonds there is a of electrons to get an
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory
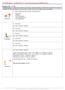 AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
***Occurs when atoms of elements combine together to form compounds.*****
 CHEMICAL BONDING ***Occurs when atoms of elements combine together to form compounds.***** Formation of compounds involve adjustments in the position of one or more valence electrons. PE is lower in bonded
CHEMICAL BONDING ***Occurs when atoms of elements combine together to form compounds.***** Formation of compounds involve adjustments in the position of one or more valence electrons. PE is lower in bonded
Ionic Bond TRANSFER of electrons between atoms. Ionic Bonding. Ionic Bonding. Ionic Bonding. Attraction that holds atoms together
 BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY
 Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Chemistry 105: General Chemistry I Dr. Gutow and Dr. Matsuno Spring 2004 Page 1
 Page 1 1) Name You are to keep this copy of the test. Your name is in case you leave it behind. 2) Use only a #2 pencil on the answer sheet. 3) Before starting the exam fill in your student ID# (not your
Page 1 1) Name You are to keep this copy of the test. Your name is in case you leave it behind. 2) Use only a #2 pencil on the answer sheet. 3) Before starting the exam fill in your student ID# (not your
Test Review # 4. Chemistry: Form TR4.11A
 Chemistry: Form TR4.11 REVIEW Name Date Period Test Review # 4 Bonding. The electrons of one atom are attracted to the protons of another. When atoms combine, there is a tug of war over the valence electrons.
Chemistry: Form TR4.11 REVIEW Name Date Period Test Review # 4 Bonding. The electrons of one atom are attracted to the protons of another. When atoms combine, there is a tug of war over the valence electrons.
electronegativity difference greater than or equal to Ionic Bonding occurs between a metal and a nonmetal when there is an
 Chemistry Unit 4 Review Packet Sweeeeeeeettt ANSWER KEY For the following compounds identify the bond types as one of the following: Ionic, Metallic, Polar Covalent, Non Polar Covalent, and Moderately
Chemistry Unit 4 Review Packet Sweeeeeeeettt ANSWER KEY For the following compounds identify the bond types as one of the following: Ionic, Metallic, Polar Covalent, Non Polar Covalent, and Moderately
Lewis Structures and Molecular Shapes
 Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Fill in the chart below to determine the valence electrons of elements 3-10
 Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chem 11 Unit 4 POLARITY, MOLECULE SHAPE, and BEHAVIOUR
 Chem 11 Unit 4 POLARITY, MOLECULE SHAPE, and BEHAVIOUR Polarity is unequal distribution of a charge on a molecule caused by: 1) some degree of ionic character in the bonding (i.e. unequal electron sharing)
Chem 11 Unit 4 POLARITY, MOLECULE SHAPE, and BEHAVIOUR Polarity is unequal distribution of a charge on a molecule caused by: 1) some degree of ionic character in the bonding (i.e. unequal electron sharing)
Introduction to Chemical Bonding
 Chemical Bonding Introduction to Chemical Bonding Chemical bond! is a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together Why are most
Chemical Bonding Introduction to Chemical Bonding Chemical bond! is a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together Why are most
Unit 9: CHEMICAL BONDING
 Unit 9: CHEMICAL BONDING 1 Unit 9: Bonding: 1. Electronegativity 2. Intramolecular Bonding 3. Intermolecular Bonding 4. Drawing Lewis Structures 5. Lewis Structures for Polyatomic Ions 6. Exceptions to
Unit 9: CHEMICAL BONDING 1 Unit 9: Bonding: 1. Electronegativity 2. Intramolecular Bonding 3. Intermolecular Bonding 4. Drawing Lewis Structures 5. Lewis Structures for Polyatomic Ions 6. Exceptions to
Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories
 C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
CHEMICAL BONDS A CHEMICAL BOND IS A FORCE OF ATTRACTION HOLDING THE ATOMS OR IONS TOGETHER.
 CHEMICAL BONDS A CHEMICAL BOND IS A FORCE OF ATTRACTION HOLDING THE ATOMS OR IONS TOGETHER. q Elements tend to enter into chemical reaction to gain stability q This is satisfied by completing the octet
CHEMICAL BONDS A CHEMICAL BOND IS A FORCE OF ATTRACTION HOLDING THE ATOMS OR IONS TOGETHER. q Elements tend to enter into chemical reaction to gain stability q This is satisfied by completing the octet
