1.5 h; 100 points Signature UM ID # Problem Points Score GSI I 17 II 20 III 20 IV 19 V 24 Total
|
|
|
- Darren Burke
- 6 years ago
- Views:
Transcription
1 hemistry 10 First ame First Examination Last ame May 0, 01 please print clearly 1.5 h; 100 points ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 17 II 0 III 0 IV 19 V 4 Total omplete Lewis structures are required unless you are given other specific instructions. Precision in drawing counts. heck all three-dimensional representations to ensure you are implying an unequivocal direction of bonding. Do not forget to include important features such as nonbonding electron pairs and formal charges when appropriate. Individual point values are given in the corner of each answer space. The exam has pages in addition to this cover page. A pka table is on the last page.
2 I. (17 points) A. ame Page 1 onsider the heterocylic aromatic compounds (the best of a set of possible resonance contributors is shown for each): xazole pk a of conjugate acid is ~ 0.8 Imidazole pk a of conjugate acid is ~ 7 (a) Assuming the hybridization model for all atoms, provide an accurate and complete threedimensional orbital picture for the best resonance contributor of oxazole. As always, you should use lines, dashes, wedges, p orbitals (lone or overlapping) to show the direction of all electrons (or empty orbitals) in your drawing. All bond angles should reflect the appropriate geometry and hybridization chosen for your drawing. (b) omplete the following sentence by circling the best word. xazole is a stronger weaker similar base compared to imidazole. circle one (c) xazole and imidazole will interact via a very strong hydrogen bond. Using the structure of imidazole provided, draw in a molecule of oxazole, and clearly show the best possilbe hydrogen bond that can form between these two using. B. Imidazole is very versatile as a reactant. Predict the product(s) that are most likely in each of the following situations and balance your reactions! (a) K (b) l l Al l
3 II. (0 points) ame Page A. onsider Molecule 1 shown below. For each of the other drawings, pick the letter for the best choice from the list of "Descriptors" to describe if and how the other drawing is related to the structure labeled as 1. 1 Descriptors the same resonance A form as 1 a different resonance B form for 1 D E F a structural isomer of 1 a conjugate acid of 1 a conjugate base of 1 is not related to 1 i) compared to 1, this is: ii) compared to 1, this is: iii) compared to 1, this is: iv) compared to 1, this is: v) compared to 1, this is: vi) compared to 1, this is: B. Working with the same molecular formula as in Molecule 1 above ( 5 ) draw valid and complete Lewis structures (i.e. show all electrons) with the following criteria: This compound contains: 1) all closed shell atoms ) no atoms carry formal charge ) a ring containing only 4 atoms 4) a carboxylic acid functional group This compound contains: 1) all closed shell atoms ) no atoms carry formal charge ) a ring containing only 4 atoms 4) neither oxygen can be a hydrogen bond donor 4 4
4 III. (0 points) ame Page alicylic acid is an important ingredient used in the production of aspirin (aka, acetylsalicylic acid). It is a diprotic acid, and the pk a s of its protons are ~ and ~8. Use your pk a table to assign these actual values to the appropriate acidic protons on salicylic acid. salicylic acid A. (a) Provide the complete curved arrow mechanism, draw the products and evauate the Keq for the reaction that occurs when salicylic acid is treated with one equivalent of the the base diethylamine. curved arrow mechanism (b) K eq = 10 (c) 4 B. (a) In aqueous solutions at a p of 7.5, salicylic acid exists as a mixture of two compounds (protonated and deprotonated forms), one at a higher concentration than the other. Draw these two compounds in their appropriate boxes. higher concentration lower concentration (b) The reaction scheme depicted below is part of the process used in the manufacture of aspirin. Provide the missing information (curved arrows, intermediates as needed) in this chemical transformation. :B and -B + represent general Bronsted bases and acids available and used in every step. B B salicylic acid
5 IV. (19 points) ame Page 4 The chemical imatinib, shown below, has been used to treat a number of cancers (most successfully hronic Myelogenous Leukemia). When evaluating the structure of this compound, please remember to take into account all other major and valid resonance contributors (as always). ATM 1 (a) For each of the atoms indicated with an arrow, provide the atom's most reasonable hybridization as well as its electronic geometry (VEPR), and observable geometry (shape). ATM ybridization Electronic geometry bservable geometry ATM 1 ATM ATM ATM ATM 4 imatinib ATM 4 (b) Ima-1 (shown below) represents a small molecular "section" that is found within imatinib. Its reactivity and structure can be best explained with a set of resonance contributors. Draw all other resonance contributors for this structure that contain all closed shell atoms and atomic formal charges between +1 and -1. Ima-1 (c) Another "section" of imatinab, Ima-, is shown below. ii)t/f More nitrogen atoms carry partial negative charge in Ima- than they do in X. The - bond indicated by the arrow is longer in Ima- than it is in X. - Ima- - X Ima- has units of unsaturation. Ima- and X share the same molecular formula. There are more sp hybridized carbon atoms in Ima- than there are in X. 5
6 V. (4 points) A. ame Page 5 Fill in the missing curved arrow mechanisms for each step in the production of malate, a key reaction in the breakdown of carbohydrates. ote how water plays important roles in this (and many other) biological reactions. (a) (b) B. yanamides are organic compounds used widely in pharmaceuticals. (a) Draw the complete set of Lewis structures (resonance contributors) for cyanimide, the connectivity is shown below. All atoms are closed shell, and atomic formal charges are +1, 0, or -1. connectivity of cyanamide resonance contributors. (b) Use your resonance contributors to make predictions about geometry and hybridization of the atoms indicated with arrows. Assuming the hybridization model for all atoms, provide an accurate and complete three-dimensional orbital picture for the best resonance contributor of cyanamide. As always, you should use lines, dashes, wedges, p orbitals (lone or overlapping) to show the direction of all electrons (or empty orbitals) in your drawing. All bond angles should reflect the appropriate geometry and hybridization chosen for your drawing. ybridization connectivity of cyanamide ybridization VEPR geom
7 AID (trongest Acid) l I l F pk a JUGATE BAE (Weakest Base) -1 l -7. I l -4. F AID l l l pk a JUGATE BAE l l l (Weakest Acid) (trongest Base)
1.5 h; 100 points please print clearly Dr. Kathleen Nolta Signature UM ID # Problem Points Score GSI I 24 II 19 III 20 IV 21 V 16 Total 100
 hemistry 0 First ame First Examination Last ame.5 h; 00 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 4 II 9 III 0 IV V
hemistry 0 First ame First Examination Last ame.5 h; 00 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 4 II 9 III 0 IV V
1.5 h; 100 points please print clearly Dr. Kathleen Nolta Signature UM ID # Problem Points Score GSI I 20 II 20 III 20 IV 17 V 23 Total 100
 hemistry 0 irst ame irst Examination Last ame. h; 00 points please print clearly Dr. athleen olta ignature UM ID # PRIT TE IRT LETTER YUR LAT AME ERE: Problem Points core GI I 0 II 0 III 0 IV 7 V Total
hemistry 0 irst ame irst Examination Last ame. h; 00 points please print clearly Dr. athleen olta ignature UM ID # PRIT TE IRT LETTER YUR LAT AME ERE: Problem Points core GI I 0 II 0 III 0 IV 7 V Total
2 h; 240 points Signature UM ID # Problem Points Score GSI I 42 II 45 III 35 IV 46 V 36 VI 36 Total 240
 hemistry 10 First ame Final Examination Last ame June 5, 01 please print clearly h; 0 points Signature UM ID # PRIT TE FIRST LETTER F YUR LAST AME ERE: Problem Points Score GSI I II 5 III 5 IV V VI Total
hemistry 10 First ame Final Examination Last ame June 5, 01 please print clearly h; 0 points Signature UM ID # PRIT TE FIRST LETTER F YUR LAST AME ERE: Problem Points Score GSI I II 5 III 5 IV V VI Total
2 h; 240 points please print clearly Dr. Kathleen Nolta Signature UM ID # Problem Points Score GSI I 42 II 44 III 40 IV 43 V 31 VI 40 Total 240
 hemistry 10 First ame Final Examination Last ame h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I II III 0 IV V 1 VI 0
hemistry 10 First ame Final Examination Last ame h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I II III 0 IV V 1 VI 0
1.5 h; 120 points Signature. Problem Points Score GSI I 23 II 30 III 23 IV 24 V 20 Total 120
 hemistry 0 econd Examination June, 0 First ame Last ame please print clearly.5 h; 0 points ignature For fastest return, if you are in lab this term. LAB GI: UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE:
hemistry 0 econd Examination June, 0 First ame Last ame please print clearly.5 h; 0 points ignature For fastest return, if you are in lab this term. LAB GI: UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE:
Problem Points Score GSI I 30 II 21 III 28 IV 30 V 31 Total 140
 hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
1.5 h; 120 points please print clearly Dr. Kathleen Nolta Dr. Jordan Walk. Problem Points Score GSI I 23 II 27 III 24 IV 22 V 24 Total 120
 hemistry 10 econd Examination First ame Last ame 1.5 h; 10 points please print clearly Dr. Kathleen olta Dr. Jordan Walk ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I
hemistry 10 econd Examination First ame Last ame 1.5 h; 10 points please print clearly Dr. Kathleen olta Dr. Jordan Walk ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I
2.0 h; 240 points please print clearly Dr. Kathleen Nolta Signature. Problem Points Score GSI I 42 II 35 III 36 IV 46 V 42 VI 39 Total 240
 hemistry 10 irst ame inal Examination Last ame.0 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # Problem Points core GI I II 5 III IV V VI 9 Total 0 Precision in drawing counts. heck
hemistry 10 irst ame inal Examination Last ame.0 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # Problem Points core GI I II 5 III IV V VI 9 Total 0 Precision in drawing counts. heck
1.5 h; 120 points please print clearly Dr. Kathleen Nolta Signature. Problem Points Score GSI I 20 II 27 III 25 IV 22 V 26 Total 120
 hemistry 0 First ame econd Examination Last ame. h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II 7 III IV V Total
hemistry 0 First ame econd Examination Last ame. h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II 7 III IV V Total
Problem Points Score GSI I 22 II 26 III 28 IV 19 V 25 Total 120
 hemistry 0 First ame econd Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I II 6 III 8 IV 9 V 5
hemistry 0 First ame econd Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I II 6 III 8 IV 9 V 5
ATOM O1 ATOM N2 ATOM O2 ATOM N1 ATOM N2 ATOM C
 I. (8 points) ame Page 1 AMPA, shown below, is a synthetic compound that mimics the effects of the neurotransmitter glutamate. When evaluating the structure of this compound, please remember to take into
I. (8 points) ame Page 1 AMPA, shown below, is a synthetic compound that mimics the effects of the neurotransmitter glutamate. When evaluating the structure of this compound, please remember to take into
1.5 h; 140 points please print clearly Dr. Ginger Shultz Dr. Kathleen Nolta. Problem Points Score GSI I 32 II 27 III 32 IV 25 V 24 Total 140
 hemistry 10 Third Examination First ame Last ame 1.5 h; 10 points please print clearly Dr. Ginger hultz Dr. Kathleen olta ignature UM D # PRT TE FRT LETTER F YUR LAT AME ERE: Problem Points core G 7 V
hemistry 10 Third Examination First ame Last ame 1.5 h; 10 points please print clearly Dr. Ginger hultz Dr. Kathleen olta ignature UM D # PRT TE FRT LETTER F YUR LAT AME ERE: Problem Points core G 7 V
(c) Is this the form of MDPV that you would expect to exist in the bloodstream, where the ph is ~7? Why or why not?
 I. ( points) ame Page 1 Methylenedioxypyrovalerone (MDPV) is one terribly dangerous ingredient in the recreational drugs better known as "bath salts." This psychoactive drug acts as a stimulant with dangerous,
I. ( points) ame Page 1 Methylenedioxypyrovalerone (MDPV) is one terribly dangerous ingredient in the recreational drugs better known as "bath salts." This psychoactive drug acts as a stimulant with dangerous,
(a) Draw a circle around the longest C-C bond(s) in serotonin. sp 2. sp 3
 I. ( points) ame Page A. Serotonin is an important neurotransmitter that is thought to play an essential role in the regulation of moods, despite being concentrated in the enterochromaffin cells in the
I. ( points) ame Page A. Serotonin is an important neurotransmitter that is thought to play an essential role in the regulation of moods, despite being concentrated in the enterochromaffin cells in the
sp 2 sp 2 trigonal planar bent 2
 I. ( points) ame Page 1 Methylenedioxypyrovalerone (MDPV) is one terribly dangerous ingredient in the recreational drugs better known as "bath salts." This psychoactive drug acts as a stimulant with dangerous,
I. ( points) ame Page 1 Methylenedioxypyrovalerone (MDPV) is one terribly dangerous ingredient in the recreational drugs better known as "bath salts." This psychoactive drug acts as a stimulant with dangerous,
O N N. electrons in ring
 ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
CHEMpossible. 261 Exam 1 Review
 CHEMpossible 261 Exam 1 Review A. Rank the following carboxylic acids from least acidic to most acidic: B. Draw the line-angle formulas for three acylic (non-cyclic) esters with the molecular formula C
CHEMpossible 261 Exam 1 Review A. Rank the following carboxylic acids from least acidic to most acidic: B. Draw the line-angle formulas for three acylic (non-cyclic) esters with the molecular formula C
stereoselection view Product 1 with its enantiomer
 . ( points) A. Complete the following reactions as directed. (a) Cl C1 C i) major product DBU C C C C C Page 1 ii) Draw a ewman Projection for the conformation of the C1-C bond indicated that specifically
. ( points) A. Complete the following reactions as directed. (a) Cl C1 C i) major product DBU C C C C C Page 1 ii) Draw a ewman Projection for the conformation of the C1-C bond indicated that specifically
"Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. A.
 ame I. (6 points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. (a) ircle all the tetrahedral (chiral) stereocenters in both piperacillin
ame I. (6 points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. (a) ircle all the tetrahedral (chiral) stereocenters in both piperacillin
CHE Organic Chemistry 1 Exam 1
 E 230 001 rganic hemistry 1 Exam 1 September 23, 2013 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials.
E 230 001 rganic hemistry 1 Exam 1 September 23, 2013 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials.
Chemistry /002 Exam 1 Version Green. The Periodic Table
 Name: Last First MI Chemistry 233-001/002 Exam 1 Version Green Fall 2018 Dr. J. sbourn Instructions: The first 13 questions of this exam should be answered on the provided Scantron. You must use a pencil
Name: Last First MI Chemistry 233-001/002 Exam 1 Version Green Fall 2018 Dr. J. sbourn Instructions: The first 13 questions of this exam should be answered on the provided Scantron. You must use a pencil
"Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. A.
 ame I. ( points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. A. piperacillin tazobactam (a) ircle all the tetrahedral (chiral)
ame I. ( points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. A. piperacillin tazobactam (a) ircle all the tetrahedral (chiral)
HO 1:1 1:1. Amines, like cathine, are often excellent nucleophiles Provide the requested information for the following one-step reaction scheme.
 I. (4 points) A. athine (shown at right) is a psychoactive amphetamine drug obtained from the plant atha edulis (also known as khat). View down the 1 - bond indicated ( 1 being the front atom), and answer
I. (4 points) A. athine (shown at right) is a psychoactive amphetamine drug obtained from the plant atha edulis (also known as khat). View down the 1 - bond indicated ( 1 being the front atom), and answer
I. (42 points) (a) Label the indicated sites (circles and oval box) with the appropriate stereochemical designation.
 I. ( points) ame Page A. etamethasone is a powerful anti-inflammatory drug that comes with unfortunate immunosupressive properties. It is often prescribed as a topical cream that can be used to alleviate
I. ( points) ame Page A. etamethasone is a powerful anti-inflammatory drug that comes with unfortunate immunosupressive properties. It is often prescribed as a topical cream that can be used to alleviate
HOMEWORK PROBLEMS: POLAR BONDS, RESONANCE, ACIDS & BASES 1. Which of the following molecules is the most polar?
 CEM 31 MEWRK PRBLEMS: PLAR BDS, RESACE, ACIDS & BASES 1. Which of the following molecules is the most polar? 2. Trans-dichlorodifluoroethylene, C 2 Cl 2 2, has a number of polar bonds but no net dipole
CEM 31 MEWRK PRBLEMS: PLAR BDS, RESACE, ACIDS & BASES 1. Which of the following molecules is the most polar? 2. Trans-dichlorodifluoroethylene, C 2 Cl 2 2, has a number of polar bonds but no net dipole
CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008
 First Three Letters of Last Name NAME Network ID CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed,
First Three Letters of Last Name NAME Network ID CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed,
PLEASE DO NOT WRITE ON THE EXAM (EVEN YOUR NAME) UNTIL TOLD TO START! Student ID # CHEM 8A, Organic Chemistry EXAM 1 (300 points)
 PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
Exam Analysis: Organic Chemistry, Midterm 1
 Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Covalent bonds can have ionic character These are polar covalent bonds
 Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution
Orbital Shapes Carbon: Electron configuration Carbon: Full. Short form. Orbital energy diagram. Orbital energy levels diagram
 rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
Chemistry 250A -- Exam #1 Answer Key -- September 23, 2008 (There are 5 pages.)
 ame: 1 hemistry 250 -- Exam #1 nswer Key -- September 23, 2008 (There are 5 pages.) 1. (15 pts) Show the major product(s) from the following acid-base reactions. If there is no reaction then say o Reaction.
ame: 1 hemistry 250 -- Exam #1 nswer Key -- September 23, 2008 (There are 5 pages.) 1. (15 pts) Show the major product(s) from the following acid-base reactions. If there is no reaction then say o Reaction.
Organic Chem Chapter 3: Acids and Bases
 Organic Chem Chapter 3: Acids and Bases Title and Highlight Right side: NOTES! Topic: EQ: Date NOTES: Write out the notes from my website. Use different types of note-taking methods to help you recall
Organic Chem Chapter 3: Acids and Bases Title and Highlight Right side: NOTES! Topic: EQ: Date NOTES: Write out the notes from my website. Use different types of note-taking methods to help you recall
CHEM 109A Organic Chemistry
 CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
Chemistry 210 Exam 1
 hemistry 210 Exam 1 University of Missouriolumbia Dr. Rainer Glaser September 25, 1992 Name: Answer Key Max. Question 1 (Atoms) 12 Question 2 (Lewis) 13 Question 3 (Resonance) 27 Question 4 (Names) 20
hemistry 210 Exam 1 University of Missouriolumbia Dr. Rainer Glaser September 25, 1992 Name: Answer Key Max. Question 1 (Atoms) 12 Question 2 (Lewis) 13 Question 3 (Resonance) 27 Question 4 (Names) 20
Chapter 6 Molecular Structure
 hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
C sp2 trigonal planar trigonal planar 3
 I. ( points) ame Page A. Rocuronium bromide (Zemuron) is a muscle relaxant that is used in the application of anesthesia. It is used most often to facilitate endotracheal intubation because of its powerful
I. ( points) ame Page A. Rocuronium bromide (Zemuron) is a muscle relaxant that is used in the application of anesthesia. It is used most often to facilitate endotracheal intubation because of its powerful
Hour Examination # 1
 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam # 1 Problem Booklet Page 1 of 11 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam Booklet No. Instructors: Paul Bracher & Erin Whitteck Hour Examination # 1 Wednesday,
CHEM 2410 Organic Chemistry 1 Fall 2017 Exam # 1 Problem Booklet Page 1 of 11 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam Booklet No. Instructors: Paul Bracher & Erin Whitteck Hour Examination # 1 Wednesday,
NAME: SPRING 2015 MIDTERM
 page 1 pts NAME: SPRING 2015 MIDTERM hemistry 231 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 4/6) Do your best on this take-home portion of your mid-term. I may grade
page 1 pts NAME: SPRING 2015 MIDTERM hemistry 231 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 4/6) Do your best on this take-home portion of your mid-term. I may grade
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Chemistry 2541, Fall 2017 Midterm Exam 1 (100 points)
 hemistry 2541, all 2017 Midterm Exam 1 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
hemistry 2541, all 2017 Midterm Exam 1 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
ORGANIC - CLUTCH CH. 1 - A REVIEW OF GENERAL CHEMISTRY.
 !! www.clutchprep.com CONCEPT: WHAT IS ORGANIC CHEMISTRY? Organic Chemistry is the chemistry of life. It consists of the study of molecules that are (typically) created and used by biological systems.
!! www.clutchprep.com CONCEPT: WHAT IS ORGANIC CHEMISTRY? Organic Chemistry is the chemistry of life. It consists of the study of molecules that are (typically) created and used by biological systems.
Partial Periodic Table
 CEM 33-00, Fall 203 Professor Walba First our Exam September 24, 203 scores: ) 20 2) 20 3) 20 4) 20 5) 20 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, I have neither
CEM 33-00, Fall 203 Professor Walba First our Exam September 24, 203 scores: ) 20 2) 20 3) 20 4) 20 5) 20 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, I have neither
Homework 1 Organic Chemistry MCAT Review Summer 2012 Brent Iverson
 omework 1 rganic hemistry MAT Review Summer 2012 Brent Iverson 1 1. The most important question in chemistry is:? 2. Atoms prefer a filled shell of electrons. The vast majority of stable bonding in molecules
omework 1 rganic hemistry MAT Review Summer 2012 Brent Iverson 1 1. The most important question in chemistry is:? 2. Atoms prefer a filled shell of electrons. The vast majority of stable bonding in molecules
Chapter 1 Introduction and Review
 Chapter 1 Introduction and Review Concept to review: It is your responsibility to review the following concepts before the first class to ensure success in understanding new concepts: Atomic structure
Chapter 1 Introduction and Review Concept to review: It is your responsibility to review the following concepts before the first class to ensure success in understanding new concepts: Atomic structure
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE CHEMISTRY 201 FALL 2015 FINAL EXAMINATION
 hem 201 Page 1 of 20 24 TE UNIVERSITY F ALGARY FAULTY F SIENE EMISTRY 201 FALL 2015 FINAL EXAMINATIN Written Mark Written Mark Q19 Q22 Q20 Q23 Q21 DATE: Dec 14 th, 2015 TIME: 3 ours FIRST NAME: LAST NAME:
hem 201 Page 1 of 20 24 TE UNIVERSITY F ALGARY FAULTY F SIENE EMISTRY 201 FALL 2015 FINAL EXAMINATIN Written Mark Written Mark Q19 Q22 Q20 Q23 Q21 DATE: Dec 14 th, 2015 TIME: 3 ours FIRST NAME: LAST NAME:
Many Organic compounds are acids or bases (or both) Many Organic compounds undergo acid-base reactions
 Objective 4 Intro to Reactivity 1: identify acids and bases using Lewis definition. Use curved arrows to show how base reacts with acid. Relate strength to pk a. Determine direction of equilibrium. Use
Objective 4 Intro to Reactivity 1: identify acids and bases using Lewis definition. Use curved arrows to show how base reacts with acid. Relate strength to pk a. Determine direction of equilibrium. Use
CHEM1101 Worksheet 6: Lewis Structures
 CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
CHEMISTRY 31 Name: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds:
 CEMISTRY 31 ame: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds: (1S,3S)-1-bromo-3-butylcyclopentane 3,4-diethyl-2,2-dimethyloctane 1-cyclopropyl-2-methylcyclobutane
CEMISTRY 31 ame: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds: (1S,3S)-1-bromo-3-butylcyclopentane 3,4-diethyl-2,2-dimethyloctane 1-cyclopropyl-2-methylcyclobutane
13. Free Radical Chemistry
 hem 201 Study Session Final Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Various possibilities: Types of Isomers, Degrees of Unsaturation, common nomenclature,
hem 201 Study Session Final Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Various possibilities: Types of Isomers, Degrees of Unsaturation, common nomenclature,
1. methyl 2. methylene 3. methine 4. primary 5. secondary 6. tertiary 7. quarternary 8. isopropyl
 hem 201 Sample Midterm Beauchamp Exams are designed so that no one question will make or break you. The best strategy is to work steadily, starting with those problems you understand best. Make sure you
hem 201 Sample Midterm Beauchamp Exams are designed so that no one question will make or break you. The best strategy is to work steadily, starting with those problems you understand best. Make sure you
Ch 2 Polar Covalent Bonds
 h 2 Polar ovalent Bonds Two primary bond types: ovalent (shared e -1 s) and Ionic (transferred e -1 s) Ionic bonds can have covalent character, such as with Na:l. An e -1 pair on l -1 can fill the 3s orbital
h 2 Polar ovalent Bonds Two primary bond types: ovalent (shared e -1 s) and Ionic (transferred e -1 s) Ionic bonds can have covalent character, such as with Na:l. An e -1 pair on l -1 can fill the 3s orbital
CHM 233 : Fall 2018 Quiz #10 - Answer Key
 M 233 : Fall 2018 Quiz #10 - nswer Key Question 1 M20a Which of the following is The strongest Bronsted cid? R- 2 is a carboxylic acid and R-S 3 is a solfonic acid, you will want to draw these two as Lewis
M 233 : Fall 2018 Quiz #10 - nswer Key Question 1 M20a Which of the following is The strongest Bronsted cid? R- 2 is a carboxylic acid and R-S 3 is a solfonic acid, you will want to draw these two as Lewis
 Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday
Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
C h a p t e r T h r e e: Acids and Bases. 17, 21-Dimethylheptatriacontane, a sex attractant pheromone of the tsetse fly
 C h a p t e r T h r e e: Acids and Bases 17, 21-Dimethylheptatriacontane, a sex attractant pheromone of the tsetse fly CM 321: Summary of Important Concepts YConcepts for Chapter 3: Acids and Bases I.
C h a p t e r T h r e e: Acids and Bases 17, 21-Dimethylheptatriacontane, a sex attractant pheromone of the tsetse fly CM 321: Summary of Important Concepts YConcepts for Chapter 3: Acids and Bases I.
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
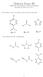 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. cleophiles and Electrophiles V. Acids and Bases What a chemical
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. cleophiles and Electrophiles V. Acids and Bases What a chemical
CHEMISTRY 332 FALL 08 EXAM I September 24-25, 2008
 First Three Letters of Last ame AME etwork ID EMISTRY 332 FALL 08 EXAM I September 24-25, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed, electronic,
First Three Letters of Last ame AME etwork ID EMISTRY 332 FALL 08 EXAM I September 24-25, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed, electronic,
Homework - Chapter 1 Chem 2310
 omework - hapter 1 hem 2310 ame I. Introduction to rganic hemistry 1. Explain in your own words what organic chemistry is, and what it is useful for. 2. Why do you think the field of study that you are
omework - hapter 1 hem 2310 ame I. Introduction to rganic hemistry 1. Explain in your own words what organic chemistry is, and what it is useful for. 2. Why do you think the field of study that you are
Fruit Straight from the Chemistree Thanks to for Ryan F. the title!
 Fruit Straight from the Chemistree Thanks to for Ryan F. the title!! rganic Chemistry I CE 310 002, Exam #1 100 sweet, tree-ripened points September 22, 2017 Rules of the road: 1. There are 10 questions
Fruit Straight from the Chemistree Thanks to for Ryan F. the title!! rganic Chemistry I CE 310 002, Exam #1 100 sweet, tree-ripened points September 22, 2017 Rules of the road: 1. There are 10 questions
Chapter 1 Atomic and Molecular Structure
 Name Date PEP Organic Chemistry Think About It: What is organic chemistry? Chapter 1 Atomic and Molecular Structure Describe some of the ways that ancient civilizations have taken advantage of organic
Name Date PEP Organic Chemistry Think About It: What is organic chemistry? Chapter 1 Atomic and Molecular Structure Describe some of the ways that ancient civilizations have taken advantage of organic
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
CHEM 231 (Davis) Organic Chemistry FINAL EXAM May 15, YOUR NAME (Last, First, M.I.) DISCUSSION SECTION #53 (5 Points)
 CHEM 231 (Davis) rganic Chemistry FINAL EXAM May 15, 2006 YUR NAME (Last, First, M.I.) DISCUSSIN SECTIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and
CHEM 231 (Davis) rganic Chemistry FINAL EXAM May 15, 2006 YUR NAME (Last, First, M.I.) DISCUSSIN SECTIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and
Chemistry 3351: Organic Chemistry Thursday: Sept. 7:00pm 9:00/1 st Exam. Name: (please print)
 Chemistry 3351: Organic Chemistry Thursday: Sept. 23 @ 7:00pm 9:00/1 st Exam 1 Name: (please print) Page Possible Points Score 2 9 3 9 4 8 5 10 6 10 7 4 8 9 9 10 10 10 11 10 12 10 13 10 TOTAL 109 2 1.
Chemistry 3351: Organic Chemistry Thursday: Sept. 23 @ 7:00pm 9:00/1 st Exam 1 Name: (please print) Page Possible Points Score 2 9 3 9 4 8 5 10 6 10 7 4 8 9 9 10 10 10 11 10 12 10 13 10 TOTAL 109 2 1.
What Is Organic Chemistry?
 What Is Organic Chemistry? EQ: What is Organic Chemistry? Read: pages 1-3 Answer the questions in your packet Basics of Organic Chem 1 Chapter 1: Structure and Bonding Key terms Organic Chemistry Inorganic
What Is Organic Chemistry? EQ: What is Organic Chemistry? Read: pages 1-3 Answer the questions in your packet Basics of Organic Chem 1 Chapter 1: Structure and Bonding Key terms Organic Chemistry Inorganic
Lecture Notes Chemistry 342 Mukund P. Sibi Lecture 33 Amines
 Lecture otes Chemistry 342 Mukund P. Sibi Amines Amines are organic compounds derived from ammonia. The nitrogen in amines contains a lone pair of electrons. The presence of this lone pair of electrons
Lecture otes Chemistry 342 Mukund P. Sibi Amines Amines are organic compounds derived from ammonia. The nitrogen in amines contains a lone pair of electrons. The presence of this lone pair of electrons
Introduction to Organic Chemistry
 Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Chemistry 234 Exam 1 (Gray) The Periodic Table
 ame: Last First MI Chemistry 234 Exam 1 (Gray) Summer 2017 Dr. J. sbourn Instructions: The first 21 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling
ame: Last First MI Chemistry 234 Exam 1 (Gray) Summer 2017 Dr. J. sbourn Instructions: The first 21 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling
PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008
 PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008 1 Name SECTION B. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 60
PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008 1 Name SECTION B. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 60
CHEM 3013 ORGANIC CHEMISTRY I LECTURE NOTES CHAPTER 2
 EM 3013 RGANI EMISTRY I LETURE NTES 1 APTER 2 1. ormal harge The Lewis structures we have drawn thus far have all been neutral covalent molecules. owever, some covalently bonded molecules may contain charged
EM 3013 RGANI EMISTRY I LETURE NTES 1 APTER 2 1. ormal harge The Lewis structures we have drawn thus far have all been neutral covalent molecules. owever, some covalently bonded molecules may contain charged
2. How many tertiary carbons are there in Flabelliferin A, shown above? (3 points)
 Page 1 of 8 I. Nomenclature 1. Flabelliferin A, shown below, was isolated from a marine sponge and was found to have cytotoxic activity. (J. Nat. Prod. 2012, 1490). Circle and identify each different functional
Page 1 of 8 I. Nomenclature 1. Flabelliferin A, shown below, was isolated from a marine sponge and was found to have cytotoxic activity. (J. Nat. Prod. 2012, 1490). Circle and identify each different functional
NAME: SUMMER 2015 MIDTERM
 page 1 pts NAME: SUMMER 2015 MIDTERM hemistry 350 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 6/16) Do your best on this take-home portion of your midterm. I may grade
page 1 pts NAME: SUMMER 2015 MIDTERM hemistry 350 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 6/16) Do your best on this take-home portion of your midterm. I may grade
2. Acids and Bases (text )
 2009, Department of hemistry, The University of Western ntario 2.1 2. Acids and Bases (text 2.1 2.6) Acid-base reactions are one of the most important reaction types in organic chemistry and biology, e.g.:
2009, Department of hemistry, The University of Western ntario 2.1 2. Acids and Bases (text 2.1 2.6) Acid-base reactions are one of the most important reaction types in organic chemistry and biology, e.g.:
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts)
 CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
RESONANCE HELPS US TO UNDERSTAND WHAT A STRUCTURE WITH DELOCALIZED ELECTRONS IS ACTUALLY LIKE IN A WAY THAT a (single) LEWIS STRUCTURE CAN'T.
 Resonance Delocalized Electrons 1 Delocalization and onjugation Lewis structures assign ALL ELETRS to specific bonds or as non-bonding pairs localized on specific atoms. The Lewis structure model is powerful,
Resonance Delocalized Electrons 1 Delocalization and onjugation Lewis structures assign ALL ELETRS to specific bonds or as non-bonding pairs localized on specific atoms. The Lewis structure model is powerful,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Practice Hour Examination # 1-1
 CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
Hour Examination # 3
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Exam # 3 Solutions Key Page 1 of 8 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Hour Examination # 3 Tuesday,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Exam # 3 Solutions Key Page 1 of 8 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Hour Examination # 3 Tuesday,
2. Polar Covalent Bonds: Acids and Bases
 2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
Chapter 2: Acids and Bases
 hapter 2: Acids and Bases 32 hapter 2: Acids and Bases Problems 2.1 Write each acid- reaction as a proton-transfer reaction. Label which reactant is the acid and which the, as well as which product is
hapter 2: Acids and Bases 32 hapter 2: Acids and Bases Problems 2.1 Write each acid- reaction as a proton-transfer reaction. Label which reactant is the acid and which the, as well as which product is
Please read and sign the Honor Code statement below:
 CHEM 3311 Exam #1 Name Dr. Minger June 7, 2010 Please read and sign the Honor Code statement below: I pledge that on my honor, as a University of Colorado at Boulder student, I have neither given nor received
CHEM 3311 Exam #1 Name Dr. Minger June 7, 2010 Please read and sign the Honor Code statement below: I pledge that on my honor, as a University of Colorado at Boulder student, I have neither given nor received
10-bromo-1-methyl-1-cyclodecene *** 7. (E)-1-bromo-2-chloro-3-methyl-2-pentene. will accept trans *** 8. (E)-2,4,8-trimethyl-4-nonene
 10-bromo-1-methyl-1-cyclodecene *** 7. (E)-1-bromo-2-chloro-3-methyl-2-pentene will accept trans *** 8. (E)-2,4,8-trimethyl-4-nonene will accept trans B. Determine whether the following pairs of structures
10-bromo-1-methyl-1-cyclodecene *** 7. (E)-1-bromo-2-chloro-3-methyl-2-pentene will accept trans *** 8. (E)-2,4,8-trimethyl-4-nonene will accept trans B. Determine whether the following pairs of structures
2.1 Representing Molecules
 2.1 Representing Molecules Notice that the molecular formula would be inadequate to distinguish between propanol and isopropanol. Practice converting from one type of representation to another with Skillbuilder
2.1 Representing Molecules Notice that the molecular formula would be inadequate to distinguish between propanol and isopropanol. Practice converting from one type of representation to another with Skillbuilder
Chemistry Exam 1. The Periodic Table
 ame: Last First MI Chemistry 234-002 Exam 1 Spring 2017 Dr. J. sbourn Instructions: The first 18 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in
ame: Last First MI Chemistry 234-002 Exam 1 Spring 2017 Dr. J. sbourn Instructions: The first 18 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in
Acid-Base Chemistry. Introduction to Reaction Mechanism
 Acid-Base Chemistry Introduction to Reaction Mechanism What is an acid and what is a base? Bronsted-Lowry definition Acids are proton donors (A-H) e.g. HCl, H 2 SO 4, HBr, HNO 3, HI etc. Bronsted-Lowry
Acid-Base Chemistry Introduction to Reaction Mechanism What is an acid and what is a base? Bronsted-Lowry definition Acids are proton donors (A-H) e.g. HCl, H 2 SO 4, HBr, HNO 3, HI etc. Bronsted-Lowry
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #1 - September 22, 2004 ANSWERS
 ame Department of hemistry SUY/neonta hem 221 - rganic hemistry I Examination #1 - September 22, 2004 ASWERS ISTRUTIS This examination has two parts. The first part is in multiple choice format; the questions
ame Department of hemistry SUY/neonta hem 221 - rganic hemistry I Examination #1 - September 22, 2004 ASWERS ISTRUTIS This examination has two parts. The first part is in multiple choice format; the questions
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): IGATURE: hemistry 320M/328M Dr. Brent Iverson 2nd Midterm ctober 25, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): IGATURE: hemistry 320M/328M Dr. Brent Iverson 2nd Midterm ctober 25, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
Organic Chemistry Peer Tutoring Department University of California, Irvine
 Organic Chemistry Peer Tutoring Department University of California, Irvine Arash Khangholi (akhangho@uci.edu) Cassandra Amezquita (camezqu1@uci.edu) Jiana Machhor (jmachhor@uci.edu) OCHEM 51A Professor
Organic Chemistry Peer Tutoring Department University of California, Irvine Arash Khangholi (akhangho@uci.edu) Cassandra Amezquita (camezqu1@uci.edu) Jiana Machhor (jmachhor@uci.edu) OCHEM 51A Professor
1. Multiple Choice Questions (15 points) Please circle the best answer to each question. Lone pairs are generally not shown.
 Page 2 Name _ANSWER KEY_ 1. Multiple Choice Questions (15 points) Please circle the best answer to each question. Lone pairs are generally not shown. (i) The formal charges on the nitrogen and oxygen atoms
Page 2 Name _ANSWER KEY_ 1. Multiple Choice Questions (15 points) Please circle the best answer to each question. Lone pairs are generally not shown. (i) The formal charges on the nitrogen and oxygen atoms
Chem 3A - Practice Midterm I. Note: This is a slightly modified version of the first midterm exam from Chem 112A Fall 2012
 Chem 3A - Practice Midterm I Note: This is a slightly modified version of the first midterm exam from Chem 112A Fall 2012 Please provide all answers in the space provided. You are not allowed to use a
Chem 3A - Practice Midterm I Note: This is a slightly modified version of the first midterm exam from Chem 112A Fall 2012 Please provide all answers in the space provided. You are not allowed to use a
Exam 1 Chem 3045x Friday, October 1, 1999 ANSWER KEY: Exam 1. C3043. Friday, October 1, 1999 p. 1
 Exam 1 Chem 3045x Friday, ctober 1, 1999 ANSWER KEY: Exam 1. C3043. Friday, ctober 1, 1999 p. 1 1. (10 Points). Consider the composition C 2 N 2. Draw the Lewis structures of 5 constitutional isomers which
Exam 1 Chem 3045x Friday, ctober 1, 1999 ANSWER KEY: Exam 1. C3043. Friday, ctober 1, 1999 p. 1 1. (10 Points). Consider the composition C 2 N 2. Draw the Lewis structures of 5 constitutional isomers which
6.2 Electron Movements in Brønsted Acid Base Reactions. Copyright 2018 by Nelson Education Limited 1
 6.2 Electron Movements in Brønsted Acid Base Reactions Copyright 2018 by Nelson Education Limited 1 Recall: Brønsted Acid Base Reactions often simply termed acid base reactions Recall: H + does not actually
6.2 Electron Movements in Brønsted Acid Base Reactions Copyright 2018 by Nelson Education Limited 1 Recall: Brønsted Acid Base Reactions often simply termed acid base reactions Recall: H + does not actually
Reaction mechanisms offer us insights into how reactions work / how molecules react with one another.
 Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
"Friendship is one mind in two bodies." Mencius
 California State Polytechnic University, Pomona 1 Fall, 2014 Midterm Exam Chem 314 Beauchamp Chem 314 Name Problem Points Credit 1. Nomenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal Charge
California State Polytechnic University, Pomona 1 Fall, 2014 Midterm Exam Chem 314 Beauchamp Chem 314 Name Problem Points Credit 1. Nomenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal Charge
General Class Information.
 General Class Information Instructors: Lectures: Recitations: Text: B. Imperiali & S. 'Connor utline, Syllabus & Suggested Reading on Website Start Second Week; See andout for Policy on Changes "rganic
General Class Information Instructors: Lectures: Recitations: Text: B. Imperiali & S. 'Connor utline, Syllabus & Suggested Reading on Website Start Second Week; See andout for Policy on Changes "rganic
KEY I. (28 points) i) NOTE: sp-hybridized carbanions make good nucleophiles for substitution reactions. Product A C C CH 3. Product B.
 I. (8 points) Page 1 A. omplete the following as necessary NT: sp-hybridized carbanions make good nucleophiles for substitution reactions NaN N Na Na (optional) i equivalents of Product A Product B a)
I. (8 points) Page 1 A. omplete the following as necessary NT: sp-hybridized carbanions make good nucleophiles for substitution reactions NaN N Na Na (optional) i equivalents of Product A Product B a)
Models of Molecular Structure
 19 Models of Molecular tructure Molecular model kits are designed to give a reasonably accurate picture of how atoms are arranged in molecules. They follow common bonding patterns and produce the geometric
19 Models of Molecular tructure Molecular model kits are designed to give a reasonably accurate picture of how atoms are arranged in molecules. They follow common bonding patterns and produce the geometric
