Figure I. A. Look at the atomic sizes shown in the table and describe all the trends that you see in the space below.
|
|
|
- Anissa Beatrix Alexander
- 6 years ago
- Views:
Transcription
1 Question: How do we use the model of the electronic structure of the atom to understand periodic trends of the elements? I. Data Collection Using a web browser access the following address: When the animation starts you will see Figure I below: Figure I. A. Look at the atomic sizes shown in the table and describe all the trends that you see in the space below. Based on the elements that are populating the periodic table it appears that the atomic radius decreases going from let to right across periods, and increases from top to bottom in rows. 11/25/10 1 Periodicity Activity
2 Using the mouse, click on an atom in the row of atoms above the periodic table and drag them to their respective location in the periodic table. Complete the same information by listing the atoms and their atomic radii in the Periodic Table in Figure I above. II. Data Analysis and Interpretation A. Select one of the Groups (1, 2, 16 or 17) in Atom View and complete the following the Table below: (a) Element symbol (b) Atomic Radii (c) (# of protons) (d) Total # of 11/25/10 2 Periodicity Activity (e) (f) # of inner core (g) # of (h) Level the electron(s) occupy Electron configuration Li 1.34 Å 3 3 1s 2 2s n = 2 Na 1.54 Å s 2 2s 1 2p 6 3s n = 3 K 1.96 Å s 2 2s 1 2p 6 3s 1 3p 6 4s n = 4 Rb 2.11 Å s 2 2s 1 2p 6 3s 1 3p 6 4s 1 3d 10 4p 6 5s 1 Table I n = 5 After completing Table I. work with several other students or participate in a class discussion and answer parts B - E below. B. Which columns (c h) in Table I. cannot explain the trend in atomic radii observed in your group and why? Column c does not by itself explain the trend in atomic radius going down a group. In Column c the total nuclear charge increases as the atomic radius increases. Since the atomic radius is getting larger as the nuclear charge gets larger it is difficult to relate the trend in atomic radius with total nuclear charge. Also considering Column d the total number of one must take care in connecting the increase in total with the increasing radius. The reason is that as we go across a period the total number of increases, yet the atomic radius decreases. So we would not want to believe that it is just the total number of that are responsible for the increasing atomic radius. Column g does not help because the number of is the same for each element. C. Which columns (c h) in Table I. might explain the trend in atomic radii observed in your group and why? Column e (and the related column h) is our best evidence for understanding the trend in atomic radii going down a group. When considering the electron configuration for each element we should note the location of the outer most electron. We focus on that electron because of all the in an atom, it is the outermost, or, that are responsible for determining the radius of an atom. So our best explanation for the observed increase in atomic radius going down a group in the periodic table is the location of the electron. Going down a group the electron is located in a different, higher shell (n value). Based on our knowledge of the hydrogen atom, as the e value increases the size of the shell increases, so the electron get further from the nucleus and the atomic radius increases. Also considering Column d the total number of one might think that it should be considered as a possible explanation for the trend. However, we must
3 take care in connecting the increase in total with the increasing radius. The reason is that as we go across a period the total number of increases, yet the atomic radius decreases. So we would not want to believe that it is just the total number of that are responsible for the increasing atomic radius. D. Which column (c h) in Table I. best explains the trend in atomic radii observed in your group and why? Column e (and the related column h) is our best evidence for understanding the trend in atomic radii going down a group. When considering the electron configuration for each element we should note the location of the outer most electron. We focus on that electron because of all the in an atom, it is the outermost, or, that are responsible for determining the radius of an atom. So our best explanation for the observed increase in atomic radius going down a group in the periodic table is the location of the electron. Going down a group the electron is located in a different, higher shell (n value). Based on our knowledge of the hydrogen atom, as the e value increases the size of the shell increases, so the electron get further from the nucleus and the atomic radius increases. E. Write a general statement that explains the trend in atomic radius that applies to any Group in the Periodic Table. When going down a group in the periodic table the electron(s) are located in a shell with a larger and larger n value. As the n value for the shell that contains the electron(s) increases the shell gets larger and larger, and therefore the electron gets further from the nucleus and the atomic radius increases. For a specific comparison of atoms in the same group the best explanation will include the electron configuration for each atom, the identification of the n value (level) where the are located, and then the statement that as the n value for the shell that contains the electron(s) increases the shell gets larger and larger, and therefore the electron gets further from the nucleus and the atomic radius increases. 11/25/10 3 Periodicity Activity
4 III. Data Analysis and Interpretation A. Select one of the periods in the atom view and complete the table below. (a) Element symbol (b) Atomic Radii (c) (# of protons) (d) Total # of (e) (f) # of inner core (g) # of (h) Level the electron(s) occupy Electron configuration Na 1.54 Å s 2 2s 2 2p 6 3s n = 3 Mg 1.30 Å s 2 2s 2 2p 6 3s n = 3 Si 1.11 Å s 2 2s 2 2p 6 3s 2 3p n = 3 S 1.02 Å s 2 2s 2 2p 6 3s 2 3p n = 3 Cl 0.98 Å s 2 2s 2 2p 6 3s 2 3p n = 3 Table II. After completing Table II. work with several other students or participate in a class discussion and answer parts B - E below. B. Which column(s) (c h) in Table II. cannot explain the trend in atomic radii observed in your period and why? Column (d) cannot explain the trend in atomic radii going across the period. The increase in the total number of proceeding from left to right does not agree with a decrease in the atomic radius. Column (f) in and of itself cannot explain the trend in atomic radii, however, as will be discussed later the number of inner core can be used to understand the trend in atomic radii going across a period. Column (g) cannot explain the trend in atomic radii going across a period from left to right. The number of increases as we go across the period, yet the atomic radius is decreasing. So Column (g) would not be useful. Column (h) in and of itself can not explain the trend in atomic radius. However, it is used in combination with other columns to explain the trend. This will be explained below. C. Which column(s) (c h) in Table II. might explain the trend in atomic radii observed in your period and why? Column (c) might be used to explain the trend in atomic radii going across a period. As the total nuclear charge increases the atomic radius decreases, so there might be a connection. Column (e) might be important to help understand the trend in atomic radii going across a period. D. Which column(s) (c h) in Table II. best explains the trend in atomic radii observed in your group and why? To explain the trend in atomic radii going across the period Columns (e), (g), (f) and (c) must be combined to best explain the observed trend in atomic radii going across a period. 11/25/10 4 Periodicity Activity
5 Looking at Column (e), the electron configuration, the are in the same shell (in this example the shell with an n value of 3). So all of the (Column (g)) are shielded by the same number of (Column (f)). Since the total nuclear charge is increasing (Column (c)) the experience a greater attraction to the nucleus and the atomic radius decreases going across the period. E. Write a general statement that explains the trend in atomic radius that applies to any Group in the Periodic Table. Looking at Column (e), the electron configuration, the are in the same shell (in this example the shell with an n value of 3). So all of the (Column (g)) are shielded by the same number of (Column (f)). Since the total nuclear charge is increasing (Column (c)) the experience a greater attraction to the nucleus and the atomic radius decreases going across the period. For a specific comparison of the atomic radii of two elements in the same period, the best explanation will include the electron configuration for each atom, the identification of the n value (level) where the are located, and then the statement that as the nuclear charge increase is larger for one atom compared to the other and the number of that shield the from the nucleus are the same, that the in the atom further to the right experience a greater attraction to the nucleus compared to the atom to the left, and therefore the atom further to the right in the period has a smaller atomic radius compared to the atom further to the left in the period. 11/25/10 5 Periodicity Activity
6 IV. Data Analysis and Interpretation A. Effective nuclear charge (ENC) is a term used to describe the amount of nuclear charge experienced by a particular electron in an atom. charge is the total positive charge in the nucleus. For example the nuclear charge for a carbon atom is +6. The nuclear charge is equivalent to the atomic number for an element. Carbon has six protons so consider the following information; (a) Element symbol (b) (# of protons) (c) Total # of (d) Electron configuration (e) # of inner core (f) # of (g) Level the electron(s) occupy (h) Effective (ENC) for the C s 2 2s 2 2p If we focus on the electron configuration for carbon we see there are 4 in the second level and two in the first level. Electrons in the second level are further away from the nucleus. The 2 in the first level (inner core ) partially shield the from some of the nuclear charge. To a first approximation we will assume each inner core electron shields one proton from the in the outer most level ( ). The ENC is calculated by subtracting the number of inner core (IC) from the total nuclear charge (Z): ENC = Z IC. In the case of carbon, ENC = +6 2 = +4. Transfer columns (a h) from Table I and Table II into Table III below. (a) Group Element symbol (b) Atomic Radii (c) (# of protons) (d) Total # of (e) Electron configuration (f) # of inner core (g) # of (h) Level the electron(s) occupy (i) Effective (ENC) for the Be 0.90 Å 4 4 1s 2 2s n = 2 +2 Mg 1.30 Å s 2 2s 1 2p 6 3s n = 3 +2 Cs 1.74 Å s 2 2s 1 2p 6 3s 2 3p n = s 2 Sr 1.92 Å s 2 2s 1 2p 6 3s 2 3p n = s 2 3d 10 4p 6 5s 2 Period Element symbol Na 1.54 Å s 2 2s 2 2p 6 3s n = 3 +1 Mg 1.30 Å s 2 2s 2 2p 6 3s n = 3 +2 Si 1.11 Å s 2 2s 2 2p 6 3s 2 2p n = /25/10 6 Periodicity Activity
7 S 1.02 Å s 2 2s 2 2p 6 3s 2 2p n = 3 +6 Cl 0.98 Å s 2 2s 2 2p 6 3s 2 2p n = 3 +7 Table III. B. Complete column (i) in Table IV for each element. C. In the space below show the calculation for ENC for the for any two of the elements in Table III. The effective nuclear charge on a electron in Mg: ENC = Z IC = = +2 The effective nuclear charge on a electron in S: ENC = Z IC = = +6 After completing Table III. work with several other students or participate in a class discussion and answer parts D - F below. D. Is there a relationship between ENC experienced by the and the trend in atomic radius for the elements in the Group from Table I? Explain. The ENC for all of the elements in Group IA are the same (+1). The atomic radii increases going down a Group, so there does not appear to be any relationship between the ENC and the atomic radii in a Group in the Periodic Table. E. Is there a relationship between ENC and the trend in atomic radius for the elements in the period from Table II? Explain. The ENC for the elements in the second period increases from left to right. The atomic radii also increases going across a period from left to right, so there does appear to be a relationship between the ENC and the atomic radii in a Period in the Periodic Table. Atomic radius does decrease as the ENC increases however it is critical that we remember that in a particular row in a period all the are in the same shell. Under such circumstances the number of shielding the is a constant, so as the nuclear charge increases the experience a greater attraction to the nucleus and the atomic radius will decrease. NOTE: Students should never say a particular atom has an effective nuclear charge, or it has an effective nuclear charge. Students must identify what electron they are talking about and what its specific effective nuclear charge is. 11/25/10 7 Periodicity Activity
8 F. Hypothetical statement made by a student to another student: When I look at Figure I. it appears to me that as the nuclear charge increases the atomic size increases. Cite evidence that supports and/or refutes this student s statement. The statement is true, however it is critical that the student include in the statement that this is the case only when the atoms that are being compared, are in the same Group. Consider what happens to the nuclear charge when going across a period from left to right, it increases. However, the atomic radius decreases. So the student s statement only works going down a Group when the are in different shells. Going across a period the are in the same shell. 11/25/10 8 Periodicity Activity
9 V. Data Collection Switch to the Ion View. Figure II. Using the mouse, click on an ion in the row of ions above the periodic table and drag them to their respective location in the periodic table. Complete the same information by listing the ions and their ionic radii in the Periodic Table in Figure II above. 11/25/10 9 Periodicity Activity
10 VI. Data Analysis and Interpretation A. Using the atomic and ion size information from Figure I and Figure II. select one metal from Group 1 or 2 and select one nonmetal from Group 16 or 17 and complete Table IV. In Table IV. enter the metal you selected in the first row and complete the row of information. In the next row below enter the cation for that metal and complete the information in the row. Do the same for the nonmetal you selected. (a) (b) (c) (# of (d) Atomic Total # # of inner # of Element /ion of core symbol Radii protons) Electron configuration Mg 1.30 Å s 2 2s 2 2p 6 3s n = 3 (e) (f) (g) (h) Level the electron(s) occupy Mg Å s 2 2s 2 2p (in the n = 2 Cl 0.98 Å s 2 2s 2 2p 6 3s 2 3p rd shell) 7 n = 3 Cl Å s 2 2s 2 2p 6 3s 2 3p n = 3 Table IV. After completing Table IV. work with several other students or participate in a class discussion and answer parts B - F below. B. Which columns (c h) in Table III. cannot explain the trend in radii observed for the metal atom and its cation and why? Column (f) cannot explain the observed change in the radii of the metal and the metal ion. Column (g) does not help explain the observed trend in the radii of the metal and the metal ion. C Which columns (c h) in Table III. explain the trend in radii observed for the metal atom and its cation and why? When comparing the atomic radius of the metal with its ion, consider Column (e) the electron configuration and notice the metal has two in the n= 3 level (Column h). The electron configuration for the metal ion has no in the n = 3 level. The outer most in Mg 2+ are in the n = 2 level. Electrons in the n = 3 level are further from the nucleus compared to in the n = 2 level, so the metal is larger than the metal ion. Columns (c) and (d). 11/25/10 10 Periodicity Activity
11 D. In general what is the relationship between the radius for the neutral metal atom and its cation? After writing the electron configuration of both the metal and its ion, one can identify what level the are located for each. In the case of the metal ion the will typically be located in one shell lower compared to the of the metal. E. Which column (c h) in Table III. best explain the trend in radii observed for the metal atom and its cation and why? Columns (e) and (h) are the most important, while columns (c) and (d) are supporting. For metal ions the outer most are in a shell that is one less than the outer most shell in the metal atom. The smaller the n value occupied by the outer most the smaller the radius of the atom or ion. F. Write a general statement that explains the trend in radius between a metal atom and its ion that applies to any combination of metal and its cation. Based on the electron configuration the outer most in a metal ion will be one level less than the outer most in the metal. The smaller the value of n for the shell the closer the are to the nucleus and the smaller the radius compared to in a higher value of n. G. Which columns (c h) in Table III. cannot explain the trend in radii observed for the nonmetal atom and its anion and why? Column (f) cannot explain the observed change in the radii of the metal and the metal ion. Column (h) in and of itself does not explain the observed trend in the radii of the metal and the metal ion. 11/25/10 11 Periodicity Activity
12 H. Which columns (c h) in Table III. explain the trend in radii observed for the nonmetal atom and its anion and why? When comparing the atomic radius of the nonmetal with its ion, consider Column (e) the electron configuration and notice the nonmetal and its ion have in the n= 3 level (Column h). Since the for the nonmetal and its ion are in the same level (Column (h)), and both the nonmetal and its ion have the same number of protons (Column (c)) there will be greater electron-electron repulsions in the nonmetal ion compared to the nonmetal and the radius of the nonmetal ion will be greater than the radius of the nonmetal. I. In general what is the relationship between the radius for the neutral nonmetal atom and its anion? The radius of the nonmetal ion will be larger than the radius of the nonmetal. j. Which column (c h) in Table III. best explains the trend in radii observed for the nonmetal atom and its anion and why? Columns (e), (h), and (c) are most important, while Columns (d) and (g) play a supportive role. K. Write a general statement that explains the trend in radius between a nonmetal atom and its anion that applies to any combination of nonmetal atom and its anion. Based on the electron configuration of the nonmetal and its ion the occupy the same shell. Since the nonmetal and its ion have the same nuclear charge, the electron-electron repulsions are greater for the nonmetal ion compared to the nonmetal so the radius of the nonmetal ion will be larger than the radius of the nonmetal. 11/25/10 12 Periodicity Activity
13 Complete the Table below. Element symbol Ion Radii (c) (# of protons) (d) Total # of (e) Electron configuration (f) # of inner core (g) # of (h) Level the electron(s ) occupy (i) Effective (ENC) on the outermost S Å s 2 2s 2 2p 6 3s 2 3p n = 3 +6 Cl 1.81 Å s 2 2s 2 2p 6 3s 2 3p n = 3 +7 K Å s 2 2s 2 2p 6 3s 2 3p n = 3 +9 Ca Å s 2 2s 2 2p 6 3s 2 3p n = Table V. Which columns (c h) in Table V. cannot explains the trend in ion radii observed in this selection of ions and why? Columns (d), (f), (g) or (h) by themselves cannot explain the trend in the radius of the ions in this set. Which columns (c h) in Table V. explain the trend in ion radii observed in this selection of ions and why? Columns (e), (h), (i) and (c) can be used to explain the trend in the radii of this set of ions. After writing the electron configuration for each of the ions, the are all in the same (Column (h)) shell. So all of the (Column (g)) are shielded by the same number of inner core (Column (f)). So as the nuclear charge increases (Column (c)), the experience a greater attraction to the nucleus (Column (i)), and the radius gets smaller. Which column(s) (c h) in Table V. best explains the trend in ion radii observed in this selection of ions and why? Columns (e), (h), (i) and (c) can be used to explain the trend in the radii of this set of ions. After writing the electron configuration for each of the ions, the are all in the same (Column (h)) shell. So all of the (Column (g)) are shielded by the same number of inner core (Column (f)). So as the nuclear charge increases (Column (c)), the experience a greater attraction to the nucleus (Column (i)), and the radius gets smaller. 11/25/10 13 Periodicity Activity
14 Write a general statement that explains the trend in ion radius that applies to a related (isoelectronic) set of ions in the Periodic Table. Write the electron configuration for each of the ions, then identify shell where the are located. When the are in the same shell, and shielded by the same number of inner core, the greater the nuclear charge the greater the effective nuclear charge experienced by the and the smaller the radius of the ion. 11/25/10 14 Periodicity Activity
John Gelder 7/14/17 1 Periodicity Activity
 Question: How do we use the model of the electronic structure of the atom to understand periodic trends of the elements? I. Data Collection Using a web browser access the following address: http://group.chem.iastate.edu/greenbowe/sections/projectfolder/flashfiles/matters/periodictbl2.html
Question: How do we use the model of the electronic structure of the atom to understand periodic trends of the elements? I. Data Collection Using a web browser access the following address: http://group.chem.iastate.edu/greenbowe/sections/projectfolder/flashfiles/matters/periodictbl2.html
Name(s) with Lab section in Group. How do we use the model of the electronic structure of the atom to understand periodic trends of the elements?
 Chem 1314 Sections 14 and 15 Periodicity Laboratory Fall 2004 Monday, November 22, 2004 Name(s) with Lab section in Group How do we use the model of the electronic structure of the atom to understand periodic
Chem 1314 Sections 14 and 15 Periodicity Laboratory Fall 2004 Monday, November 22, 2004 Name(s) with Lab section in Group How do we use the model of the electronic structure of the atom to understand periodic
Question: How do we use the model of the electronic structure of the atom to understand periodic trends of the elements?
 Chemistry: Periodicity Activity Names Question: How do we use the model of the electronic structure of the atom to understand periodic trends of the elements? I. Data Collection Using a web browser access
Chemistry: Periodicity Activity Names Question: How do we use the model of the electronic structure of the atom to understand periodic trends of the elements? I. Data Collection Using a web browser access
CHAPTER 6. Chemical Periodicity
 CHAPTER 6 Chemical Periodicity 1 Chapter Goals 1. More About the Periodic Table Periodic Properties of the Elements 2. Atomic Radii 3. Ionization Energy (IE) 4. Electron Affinity (EA) 5. Ionic Radii 6.
CHAPTER 6 Chemical Periodicity 1 Chapter Goals 1. More About the Periodic Table Periodic Properties of the Elements 2. Atomic Radii 3. Ionization Energy (IE) 4. Electron Affinity (EA) 5. Ionic Radii 6.
Explaining Periodic Trends. Saturday, January 20, 18
 Explaining Periodic Trends Many observable trends in the chemical and physical properties of elements are observable in the periodic table. Let s review a trend that you should already be familiar with,
Explaining Periodic Trends Many observable trends in the chemical and physical properties of elements are observable in the periodic table. Let s review a trend that you should already be familiar with,
Periodic Table Trends. Atomic Radius Ionic Radius Ionization Energy Electronegativity
 Periodic Table Trends Atomic Radius Ionic Radius Ionization Energy Electronegativity 1. Atomic Radius Atomic Radius - distance from nucleus to outermost atom Measured by dividing the distance between 2
Periodic Table Trends Atomic Radius Ionic Radius Ionization Energy Electronegativity 1. Atomic Radius Atomic Radius - distance from nucleus to outermost atom Measured by dividing the distance between 2
Trends in the Periodic Table
 Trends in the Periodic Table OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret group and period trends in atomic radii, ionization energies and electronegativity The Periodic Table
Trends in the Periodic Table OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret group and period trends in atomic radii, ionization energies and electronegativity The Periodic Table
Chemical symbols. Know names and symbols of elements #1 30, plus. Rb, Cs, Sr, Ba, Ag, Au, Cd, Hg, Pt, Ga, Ge, As, Sn, Pb, Se, Br, I, and U
 Chemical symbols Know names and symbols of elements #1 30, plus Rb, Cs, Sr, Ba, Ag, Au, Cd, Hg, Pt, Ga, Ge, As, Sn, Pb, Se, Br, I, and U Coulomb s Law F = attractive/repulsive force Q 1, Q 2 = charges
Chemical symbols Know names and symbols of elements #1 30, plus Rb, Cs, Sr, Ba, Ag, Au, Cd, Hg, Pt, Ga, Ge, As, Sn, Pb, Se, Br, I, and U Coulomb s Law F = attractive/repulsive force Q 1, Q 2 = charges
Topic 3 Periodicity 3.2 Physical Properties. IB Chemistry T03D02
 Topic 3 Periodicity 3.2 Physical Properties IB Chemistry T03D02 3.1 Physical Properties hrs 3.2.1 Define the terms first ionization energy and electronegativity. (1) 3.2.2 Describe and explain the trends
Topic 3 Periodicity 3.2 Physical Properties IB Chemistry T03D02 3.1 Physical Properties hrs 3.2.1 Define the terms first ionization energy and electronegativity. (1) 3.2.2 Describe and explain the trends
Electron Configuration and Periodic Trends - Chapter 5 section 3 Guided Notes
 Electron Configuration and Periodic Trends - Chapter 5 section 3 Guided Notes There are several important atomic characteristics that show predictable that you should know. Atomic Radius The first and
Electron Configuration and Periodic Trends - Chapter 5 section 3 Guided Notes There are several important atomic characteristics that show predictable that you should know. Atomic Radius The first and
Electron configurations follow the order of sublevels on the periodic table.
 Electron configurations follow the order of sublevels on the periodic table. 1 The periodic table consists of sublevel blocks arranged in order of increasing energy. Groups 1A(1)-2A(2) = s level Groups
Electron configurations follow the order of sublevels on the periodic table. 1 The periodic table consists of sublevel blocks arranged in order of increasing energy. Groups 1A(1)-2A(2) = s level Groups
Chapter 7. Generally, the electronic structure of atoms correlates w. the prop. of the elements
 Chapter 7 Periodic Properties of the Elements I) Development of the P.T. Generally, the electronic structure of atoms correlates w. the prop. of the elements - reflected by the arrangement of the elements
Chapter 7 Periodic Properties of the Elements I) Development of the P.T. Generally, the electronic structure of atoms correlates w. the prop. of the elements - reflected by the arrangement of the elements
Electron Configurations and the Periodic Table
 Electron Configurations and the Periodic Table The periodic table can be used as a guide for electron configurations. The period number is the value of n. Groups 1A and 2A have the s-orbital filled. Groups
Electron Configurations and the Periodic Table The periodic table can be used as a guide for electron configurations. The period number is the value of n. Groups 1A and 2A have the s-orbital filled. Groups
Na Mg Al Si P S Cl Ar
 Section 14.2 Periodic Trends OBJECTIVES: Interpret group trends in atomic radii, ionic radii, ionization energies, and electronegativities. Interpret period trends in atomic radii, ionic radii, ionization
Section 14.2 Periodic Trends OBJECTIVES: Interpret group trends in atomic radii, ionic radii, ionization energies, and electronegativities. Interpret period trends in atomic radii, ionic radii, ionization
Chemical Periodicity. Periodic Table
 Chemical Periodicity Periodic Table Classification of the Elements OBJECTIVES: Explain why you can infer the properties of an element based on those of other elements in the periodic table. Classification
Chemical Periodicity Periodic Table Classification of the Elements OBJECTIVES: Explain why you can infer the properties of an element based on those of other elements in the periodic table. Classification
Sparks CH301 EFFECTIVE NUCLEAR CHARGE AND PERIODIC TRENDS. Why is strontium so dangerous? UNIT 2 Day 5
 Sparks CH301 EFFECTIVE NUCLEAR CHARGE AND PERIODIC TRENDS Why is strontium so dangerous? UNIT 2 Day 5 How many electrons in Na have l=0? QUIZ QUESTION: INDIVIDUAL WORK, NO TALKING What are we going to
Sparks CH301 EFFECTIVE NUCLEAR CHARGE AND PERIODIC TRENDS Why is strontium so dangerous? UNIT 2 Day 5 How many electrons in Na have l=0? QUIZ QUESTION: INDIVIDUAL WORK, NO TALKING What are we going to
Hydrogen (H) Nonmetal (none)
 Honors Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Metal, Nonmetal or Group/Family Name Semi-metal (Metalloid)? Group 1, Period 1 Hydrogen
Honors Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Metal, Nonmetal or Group/Family Name Semi-metal (Metalloid)? Group 1, Period 1 Hydrogen
Topic 3: Periodicity OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret trends in atomic radii, ionic radii, ionization energies &
 Topic 3: Periodicity OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret trends in atomic radii, ionic radii, ionization energies & electronegativity The Periodic Table What is the periodic
Topic 3: Periodicity OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret trends in atomic radii, ionic radii, ionization energies & electronegativity The Periodic Table What is the periodic
Trends in Atomic Size. Atomic Radius-one half the distance between the nuclei of two atoms of the same element when the atoms are joined
 Periodic trends Trends in Atomic Size Atomic Radius-one half the distance between the nuclei of two atoms of the same element when the atoms are joined Trends in Atomic Size Group Trend: Atomic radii of
Periodic trends Trends in Atomic Size Atomic Radius-one half the distance between the nuclei of two atoms of the same element when the atoms are joined Trends in Atomic Size Group Trend: Atomic radii of
Periodic Relationships
 Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
PowerPoint to accompany. Chapter 6. Periodic Properties of the Elements
 PowerPoint to accompany Chapter 6 Periodic Properties of the Elements Development of the Periodic Table Elements in the same group generally have similar chemical properties. Properties are not identical,
PowerPoint to accompany Chapter 6 Periodic Properties of the Elements Development of the Periodic Table Elements in the same group generally have similar chemical properties. Properties are not identical,
Periodic Trends. 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
 Periodic Trends objectives: (#2 3) How do the properties of electrons and the electron shells contribute to the periodic trends? 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
Periodic Trends objectives: (#2 3) How do the properties of electrons and the electron shells contribute to the periodic trends? 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
Shielding & Atomic Radius, Ions & Ionic Radius. Chemistry AP
 Shielding & Atomic Radius, Ions & Ionic Radius Chemistry AP Periodic Table Periodic Table Elements in same column have similar properties Column # (IA-VIIIA) gives # valence electrons All elements in column
Shielding & Atomic Radius, Ions & Ionic Radius Chemistry AP Periodic Table Periodic Table Elements in same column have similar properties Column # (IA-VIIIA) gives # valence electrons All elements in column
Supplemental Activities. Module: Atomic Theory. Section: Periodic Properties and Trends - Key
 Supplemental Activities Module: Atomic Theory Section: Periodic Properties and Trends - Key Periodic Table and Reactivity Activity 1 1. Consider lithium metal. a. Why don t we find lithium metal in its
Supplemental Activities Module: Atomic Theory Section: Periodic Properties and Trends - Key Periodic Table and Reactivity Activity 1 1. Consider lithium metal. a. Why don t we find lithium metal in its
6.3 Periodic Trends > Chapter 6 The Periodic Table. 6.3 Periodic Trends. 6.1 Organizing the Elements. 6.2 Classifying the Elements
 1 63 Periodic Trends > Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends 2 63 Periodic Trends > CHEMISTRY & YOU How are trends in the weather similar
1 63 Periodic Trends > Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends 2 63 Periodic Trends > CHEMISTRY & YOU How are trends in the weather similar
Trends in Atomic Size. What are the trends among the elements for atomic size? The distances between atoms in a molecule are extremely small.
 63 Periodic Trends > 63 Periodic Trends > CHEMISTRY & YOU Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends How are trends in the weather similar to
63 Periodic Trends > 63 Periodic Trends > CHEMISTRY & YOU Chapter 6 The Periodic Table 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends How are trends in the weather similar to
1 of 43 Boardworks Ltd Chemistry 11. Chemical Bonding
 1 of 43 Boardworks Ltd 2009 Chemistry 11 Chemical Bonding 2 of 43 Boardworks Ltd 2009 Electrostatic Forces An electrostatic force is a forces existing as a result of the attraction or repulsion between
1 of 43 Boardworks Ltd 2009 Chemistry 11 Chemical Bonding 2 of 43 Boardworks Ltd 2009 Electrostatic Forces An electrostatic force is a forces existing as a result of the attraction or repulsion between
Periods: horizontal rows (# 1-7) 2. Periodicity the of the elements in the same group is explained by the arrangement of the around the nucleus.
 The Modern Periodic Table 1. An arrangement of the elements in order of their numbers so that elements with properties fall in the same column (or group). Groups: vertical columns (#1-18) Periods: horizontal
The Modern Periodic Table 1. An arrangement of the elements in order of their numbers so that elements with properties fall in the same column (or group). Groups: vertical columns (#1-18) Periods: horizontal
Periodic Trends (Section 5.3)
 Periodic Trends (Section 5.3) Periodic Trends (Section 5.3) 1. Atomic Radius: Periodic Trends (Section 5.3) 1. Atomic Radius: The distance from the nucleus to the outermost electrons. (See Figure 3.2,
Periodic Trends (Section 5.3) Periodic Trends (Section 5.3) 1. Atomic Radius: Periodic Trends (Section 5.3) 1. Atomic Radius: The distance from the nucleus to the outermost electrons. (See Figure 3.2,
Periodic Properties of the Elements
 Chapter 7 Periodic Properties of the Elements DEVELOPMENT OF THE PERIODIC TABLE Elements in the same group generally have similar chemical properties. Properties are not identical, however. Brown, LeMay,
Chapter 7 Periodic Properties of the Elements DEVELOPMENT OF THE PERIODIC TABLE Elements in the same group generally have similar chemical properties. Properties are not identical, however. Brown, LeMay,
Trends in the Periodic Table
 Trends in the Periodic Table A trend is a predictable change in a particular direction. Example: There is a trend in the alkali metals to increase in reactivity as you move down a group. Atomic Radius
Trends in the Periodic Table A trend is a predictable change in a particular direction. Example: There is a trend in the alkali metals to increase in reactivity as you move down a group. Atomic Radius
Explaining Periodic Trends
 Explaining Periodic Trends! Many observable trends in the chemical and physical properties of elements are observable in the periodic table.! On trends you may be familiar with is reactivity, which is
Explaining Periodic Trends! Many observable trends in the chemical and physical properties of elements are observable in the periodic table.! On trends you may be familiar with is reactivity, which is
Shapes of the orbitals
 Electrons Review and Periodic Table Trends Unit 7 Electrons Shapes of the orbitals Electron Configuration Electrons spin in opposite direction Background Electrons can jump between shells (Bohr s model
Electrons Review and Periodic Table Trends Unit 7 Electrons Shapes of the orbitals Electron Configuration Electrons spin in opposite direction Background Electrons can jump between shells (Bohr s model
Why is it called a periodic table?
 The Periodic Table Why is it called a periodic table? The properties of the elements in the table repeat in a "periodic" way (specific pattern). Periodic law: There is a periodic repetition of chemical
The Periodic Table Why is it called a periodic table? The properties of the elements in the table repeat in a "periodic" way (specific pattern). Periodic law: There is a periodic repetition of chemical
Atomic Theory and Periodic Trends Practice AP Chemistry Questions
 AP Chemistry/1516 Atomic Theory and Periodic Trends Practice AP Chemistry Questions 1. 2007 B, question #2 Answer the following problems about gases. (b) A major line in the emission spectrum of neon corresponds
AP Chemistry/1516 Atomic Theory and Periodic Trends Practice AP Chemistry Questions 1. 2007 B, question #2 Answer the following problems about gases. (b) A major line in the emission spectrum of neon corresponds
The Shell Model (II)
 22 ChemActivity 5 The Shell Model (II) Model 1: Valence Electrons, Inner-Shell Electrons, and Core Charge. The electrons in the outermost shell of an atom are referred to as valence electrons. Electrons
22 ChemActivity 5 The Shell Model (II) Model 1: Valence Electrons, Inner-Shell Electrons, and Core Charge. The electrons in the outermost shell of an atom are referred to as valence electrons. Electrons
SCH3U- R. H. KING ACADEMY ATOMIC STRUCTURE HANDOUT NAME:
 Particle Theory of Matter Matter is anything that has and takes up. All matter is made up of very small. Each pure substance has its of particle, from the particles of other pure substances. Particles
Particle Theory of Matter Matter is anything that has and takes up. All matter is made up of very small. Each pure substance has its of particle, from the particles of other pure substances. Particles
CHEM 115 Electron Configurations and
 CHEM 115 Electron Configurations and Periodic Trends Lecture 20 Prof. Sevian 1 Agenda Electron configurations Ground state vs. excited state Periodic properties Ionization energy Atomic radius Others Interpreting
CHEM 115 Electron Configurations and Periodic Trends Lecture 20 Prof. Sevian 1 Agenda Electron configurations Ground state vs. excited state Periodic properties Ionization energy Atomic radius Others Interpreting
Chapter 6 The Periodic Table The how and why History. Mendeleev s Table
 Chapter 6 The Periodic Table The how and why History 1829 German J. W. Dobereiner grouped elements into triads Three elements with similar properties Properties followed a pattern The same element was
Chapter 6 The Periodic Table The how and why History 1829 German J. W. Dobereiner grouped elements into triads Three elements with similar properties Properties followed a pattern The same element was
CHEM 103 Quantum Mechanics and Periodic Trends
 CHEM 103 Quantum Mechanics and Periodic Trends Lecture Notes April 11, 2006 Prof. Sevian Agenda Predicting electronic configurations using the QM model Group similarities Interpreting measured properties
CHEM 103 Quantum Mechanics and Periodic Trends Lecture Notes April 11, 2006 Prof. Sevian Agenda Predicting electronic configurations using the QM model Group similarities Interpreting measured properties
Chapter 7. Electron Configuration and the Periodic Table
 Chapter 7 Electron Configuration and the Periodic Table Topics Development of the periodic table The modern periodic table Effective nuclear charge Periodic trends in properties of elements Electron configuration
Chapter 7 Electron Configuration and the Periodic Table Topics Development of the periodic table The modern periodic table Effective nuclear charge Periodic trends in properties of elements Electron configuration
Activity 06.3a Periodic Trends Inquiry
 Background In this investigation you will examine several periodic trends, including atomic radius, ionization energy and ionic radius. You will be asked to interact with select atoms as you investigate
Background In this investigation you will examine several periodic trends, including atomic radius, ionization energy and ionic radius. You will be asked to interact with select atoms as you investigate
Chemical Bonding. Nuclear Charge. Nuclear Charge. Trends of the Periodic Table. Down the Table (from Top to Bottom):
 Trends of the Periodic Table Chemical Bonding TRENDS OF THE PERIODIC TABLE CHEM ISTRY 11 3 factors are usually discussed when explaining trends nuclear charge n value (outer most filled shell) Inter-electron
Trends of the Periodic Table Chemical Bonding TRENDS OF THE PERIODIC TABLE CHEM ISTRY 11 3 factors are usually discussed when explaining trends nuclear charge n value (outer most filled shell) Inter-electron
Chapter 7 Electron Configuration and the Periodic Table
 Chapter 7 Electron Configuration and the Periodic Table Copyright McGraw-Hill 2009 1 7.1 Development of the Periodic Table 1864 - John Newlands - Law of Octaves- every 8th element had similar properties
Chapter 7 Electron Configuration and the Periodic Table Copyright McGraw-Hill 2009 1 7.1 Development of the Periodic Table 1864 - John Newlands - Law of Octaves- every 8th element had similar properties
POST TRANSITION METALS
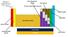 Hydrogen is considered to be a group on its own POST TRANSITION METALS NON METALS NOBLE GASSES HALOGENS TRANSITION METALS ALKALI METALS ALKALINE EARTH METALS LANTHANIDES ACTINIDES SEMI METALS TRENDS OF
Hydrogen is considered to be a group on its own POST TRANSITION METALS NON METALS NOBLE GASSES HALOGENS TRANSITION METALS ALKALI METALS ALKALINE EARTH METALS LANTHANIDES ACTINIDES SEMI METALS TRENDS OF
Periodic Trends. Name: Class: Date: ID: A. Matching
 Name: Class: Date: Periodic Trends Matching Match each item with the correct statement below. a. electronegativity f. periodic law b. ionization energy g. atomic mass c. atomic radius h. period d. metal
Name: Class: Date: Periodic Trends Matching Match each item with the correct statement below. a. electronegativity f. periodic law b. ionization energy g. atomic mass c. atomic radius h. period d. metal
October 05, Periodic_Trends_Presentation student notes.notebook. Periodic Trends. Periodic Trends: Effective Nuclear Charge
 Periodic Trends: tomic Radius Ionization nergy lectronegativity Metallic haracter Ionic Radius Periodic Trends Five main trends in the periodic table will be discussed: The sizes of atoms Ionization energy
Periodic Trends: tomic Radius Ionization nergy lectronegativity Metallic haracter Ionic Radius Periodic Trends Five main trends in the periodic table will be discussed: The sizes of atoms Ionization energy
Chapter 7 Electron Configuration and the Periodic Table
 Chapter 7 Electron Configuration and the Periodic Table Copyright McGraw-Hill 2009 1 7.1 Development of the Periodic Table 1864 - John Newlands - Law of Octaves- every 8 th element had similar properties
Chapter 7 Electron Configuration and the Periodic Table Copyright McGraw-Hill 2009 1 7.1 Development of the Periodic Table 1864 - John Newlands - Law of Octaves- every 8 th element had similar properties
Periodic Trends. 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
 Periodic Trends objectives: (#2 3) How do the properties of electrons and the electron shells contribute to the periodic trends? 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
Periodic Trends objectives: (#2 3) How do the properties of electrons and the electron shells contribute to the periodic trends? 1. (#2 3a) I can determine how gaining or losing electrons affects the atomic
POGIL 5 KEY Periodic Table Trends (Part 1)
 Honors Chem Block Name POGIL 5 KEY Periodic Table Trends (Part 1) The periodic table is often considered to be the best friend of chemists and chemistry students alike. It includes information about atomic
Honors Chem Block Name POGIL 5 KEY Periodic Table Trends (Part 1) The periodic table is often considered to be the best friend of chemists and chemistry students alike. It includes information about atomic
Periodic Relationships
 Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
Periodic Trends. More than 20 properties change in predictable way based location of elements on PT
 Periodic Trends Periodic Trends More than 20 properties change in predictable way based location of elements on PT Some properties: Density Melting point/boiling point Atomic radius Ionization energy Electronegativity
Periodic Trends Periodic Trends More than 20 properties change in predictable way based location of elements on PT Some properties: Density Melting point/boiling point Atomic radius Ionization energy Electronegativity
Topic : Periodic Trends
 Topic 3.1-3.2: Periodic Trends Essential Ideas: 3.1: The arrangement of elements in the Periodic Table helps to predict their electron configurations 3.2: Elements show trends in their physical and chemical
Topic 3.1-3.2: Periodic Trends Essential Ideas: 3.1: The arrangement of elements in the Periodic Table helps to predict their electron configurations 3.2: Elements show trends in their physical and chemical
2011 CHEM 120: CHEMICAL REACTIVITY
 2011 CHEM 120: CHEMICAL REACTIVITY INORGANIC CHEMISTRY SECTION Lecturer: Dr. M.D. Bala Textbook by Petrucci, Harwood, Herring and Madura 15 Lectures (4/10-29/10) 3 Tutorials 1 Quiz 1 Take-home test https://chemintra.ukzn.ac.za/
2011 CHEM 120: CHEMICAL REACTIVITY INORGANIC CHEMISTRY SECTION Lecturer: Dr. M.D. Bala Textbook by Petrucci, Harwood, Herring and Madura 15 Lectures (4/10-29/10) 3 Tutorials 1 Quiz 1 Take-home test https://chemintra.ukzn.ac.za/
Periodic Variations in Element Properties
 OpenStax-CNX module: m51042 1 Periodic Variations in Element Properties OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end
OpenStax-CNX module: m51042 1 Periodic Variations in Element Properties OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end
8.6,8.7 Periodic Properties of the Elements
 Pre -AP Chemistry 8.6,8.7 Periodic Properties of the Elements READ p. 305 315, 294-296 Practice Problems Pg 315 -Exercise 8.9 Pg 318-321 #36, 55, 64, 66, 67, 69, 72, 80 Periodic Trends are predictable
Pre -AP Chemistry 8.6,8.7 Periodic Properties of the Elements READ p. 305 315, 294-296 Practice Problems Pg 315 -Exercise 8.9 Pg 318-321 #36, 55, 64, 66, 67, 69, 72, 80 Periodic Trends are predictable
Periodic Trends. The trends we will study all have to do with the valence electrons in one way or another. Two key ideas:
 Periodic Trends The trends we will study all have to do with the valence electrons in one way or another. Two key ideas: Nuclear Charge = the number of protons in the nucleus. This is the positive charge
Periodic Trends The trends we will study all have to do with the valence electrons in one way or another. Two key ideas: Nuclear Charge = the number of protons in the nucleus. This is the positive charge
List how many protons, neutrons, and electrons in the following isotopes
 List how many protons, neutrons, and electrons in the following isotopes Silver 109 47 p + and e ; 62 n 0 Molybdenum 96 42 p + and e ; 54 n 0 Scandium 45 21 p + and e ; 24 n 0 1of 26 2of 26 Review: Which
List how many protons, neutrons, and electrons in the following isotopes Silver 109 47 p + and e ; 62 n 0 Molybdenum 96 42 p + and e ; 54 n 0 Scandium 45 21 p + and e ; 24 n 0 1of 26 2of 26 Review: Which
Electron Configuration and Chemical Periodicity
 Electron Configuration and Chemical Periodicity The Periodic Table Periodic law (Mendeleev, Meyer, 1870) periodic reoccurrence of similar physical and chemical properties of the elements arranged by increasing
Electron Configuration and Chemical Periodicity The Periodic Table Periodic law (Mendeleev, Meyer, 1870) periodic reoccurrence of similar physical and chemical properties of the elements arranged by increasing
Ø Draw the Bohr Diagrams for the following atoms: Sodium Potassium Rubidium
 Chemistry 11 Atomic Theory V Name: Date: Block: 1. Atomic Radius 2. Ionization Energy 3. Electronegativity 4. Chemical Bonding Atomic Radius Periodic Trends Ø As we move across a period or down a chemical
Chemistry 11 Atomic Theory V Name: Date: Block: 1. Atomic Radius 2. Ionization Energy 3. Electronegativity 4. Chemical Bonding Atomic Radius Periodic Trends Ø As we move across a period or down a chemical
Name: Block: Date: Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom.
 Name: Block: Date: Chemistry 11 Trends Activity Assignment Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom. Ionic Radius: the distance from the center
Name: Block: Date: Chemistry 11 Trends Activity Assignment Atomic Radius: the distance from the center of the nucleus to the outer most electrons in an atom. Ionic Radius: the distance from the center
Chapter 3 Classification of Elements and Periodicity in Properties
 Question 3.1: What is the basic theme of organisation in the periodic table? The basic theme of organisation of elements in the periodic table is to classify the elements in periods and groups according
Question 3.1: What is the basic theme of organisation in the periodic table? The basic theme of organisation of elements in the periodic table is to classify the elements in periods and groups according
How do elements join together to form chemical bonds?
 How do elements join together to form chemical bonds? Do you agree or disagree? 1. Chemical bonds that form between atoms involve electrons. 2. The atoms in a water molecule are more chemically stable
How do elements join together to form chemical bonds? Do you agree or disagree? 1. Chemical bonds that form between atoms involve electrons. 2. The atoms in a water molecule are more chemically stable
Lesson 14: Periodic Trends
 Lesson 14: Periodic Trends Review: Cations and Anions negative positive electrons n anion cation Metals lose electrons when they undergo chemical reactions. Na will always lose one electron. Nonmetals
Lesson 14: Periodic Trends Review: Cations and Anions negative positive electrons n anion cation Metals lose electrons when they undergo chemical reactions. Na will always lose one electron. Nonmetals
Professor K. Section 8 Electron Configuration Periodic Table
 Professor K Section 8 Electron Configuration Periodic Table Schrödinger Cannot be solved for multielectron atoms We must assume the orbitals are all hydrogen-like Differences In the H atom, all subshells
Professor K Section 8 Electron Configuration Periodic Table Schrödinger Cannot be solved for multielectron atoms We must assume the orbitals are all hydrogen-like Differences In the H atom, all subshells
Name: Date: Blk: Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed:
 Name: Date: Blk: NOTES: PERIODIC TRENDS Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed: I. ATOMIC RADIUS (Size) Going from
Name: Date: Blk: NOTES: PERIODIC TRENDS Examine your periodic table to answer these questions and fill-in-the-blanks. Use drawings to support your answers where needed: I. ATOMIC RADIUS (Size) Going from
Chapter 4. Periodic Trends of the Elements. Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby
 Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby Chapter 4 Periodic Trends of the Elements M. Stacey Thomson Pasco-Hernando State College Copyright (c) The McGraw-Hill Companies, Inc.
Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby Chapter 4 Periodic Trends of the Elements M. Stacey Thomson Pasco-Hernando State College Copyright (c) The McGraw-Hill Companies, Inc.
Chapter 7 The Structure of Atoms and Periodic Trends
 Chapter 7 The Structure of Atoms and Periodic Trends Jeffrey Mack California State University, Sacramento Arrangement of Electrons in Atoms Electrons in atoms are arranged as SHELLS (n) SUBSHELLS (l) ORBITALS
Chapter 7 The Structure of Atoms and Periodic Trends Jeffrey Mack California State University, Sacramento Arrangement of Electrons in Atoms Electrons in atoms are arranged as SHELLS (n) SUBSHELLS (l) ORBITALS
The Periodic Table. Beyond protons, neutrons, and electrons
 The Periodic Table Beyond protons, neutrons, and electrons It wasn t always like this Early PT Folks n Johann Dobereiner n Triads- groups of 3 with similarities/ trends n Cl, Br, I the properties of Br
The Periodic Table Beyond protons, neutrons, and electrons It wasn t always like this Early PT Folks n Johann Dobereiner n Triads- groups of 3 with similarities/ trends n Cl, Br, I the properties of Br
Chemistry (www.tiwariacademy.com)
 () Question 3.1: What is the basic theme of organisation in the periodic table? Answer 1.1: The basic theme of organisation of elements in the periodic table is to classify the elements in periods and
() Question 3.1: What is the basic theme of organisation in the periodic table? Answer 1.1: The basic theme of organisation of elements in the periodic table is to classify the elements in periods and
Summation of Periodic Trends
 Summation of Periodic Trends Factors Affecting Atomic Orbital Energies The Effect of Nuclear Charge (Z effective ) Higher nuclear charge lowers orbital energy (stabilizes the system) by increasing nucleus-electron
Summation of Periodic Trends Factors Affecting Atomic Orbital Energies The Effect of Nuclear Charge (Z effective ) Higher nuclear charge lowers orbital energy (stabilizes the system) by increasing nucleus-electron
History German J. W. Dobereiner Grouped elements into triads
 The Periodic Table History 1829 German J. W. Dobereiner Grouped elements into triads One of these triads included chlorine, bromine, and iodine; another consisted of calcium, strontium, and barium. In
The Periodic Table History 1829 German J. W. Dobereiner Grouped elements into triads One of these triads included chlorine, bromine, and iodine; another consisted of calcium, strontium, and barium. In
Summation of Periodic Trends Factors Affecting Atomic Orbital Energies
 Summation of Periodic Trends Factors Affecting Atomic Orbital Energies The Effect of Nuclear Charge (Z effective ) Higher nuclear charge lowers orbital energy (stabilizes the system) by increasing nucleus-electron
Summation of Periodic Trends Factors Affecting Atomic Orbital Energies The Effect of Nuclear Charge (Z effective ) Higher nuclear charge lowers orbital energy (stabilizes the system) by increasing nucleus-electron
Homework Assignment #2 Key
 Homework Assignment #2 Key Chapter 5 21. (a) 16 protons 18 neutrons 15 electrons (b) 41 protons 52 neutrons 41 electrons (c) 13 protons 14 neutrons 13 electrons (d) 29 protons 34 neutrons 28 electrons
Homework Assignment #2 Key Chapter 5 21. (a) 16 protons 18 neutrons 15 electrons (b) 41 protons 52 neutrons 41 electrons (c) 13 protons 14 neutrons 13 electrons (d) 29 protons 34 neutrons 28 electrons
Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals
 Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples Mg: 1s 2 2s 2 2p 6 3s 2 2 valence e
Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples Mg: 1s 2 2s 2 2p 6 3s 2 2 valence e
IONIC AND METALLIC BONDING
 7 IONIC AND METALLIC BONDING Chapter Test B A. Matching Match each term in Column B with the correct description in Column A. Write the letter of the correct term on the line. Column A Column B 1. compound
7 IONIC AND METALLIC BONDING Chapter Test B A. Matching Match each term in Column B with the correct description in Column A. Write the letter of the correct term on the line. Column A Column B 1. compound
Atomic Radius. Half of the distance between two bonding atoms nuclei
 Periodic Trends Atomic Radius Half of the distance between two bonding atoms nuclei Increases Atomic Radius Trend Increases Atomic Radius Across a Period Atomic radius generally decreases in size as you
Periodic Trends Atomic Radius Half of the distance between two bonding atoms nuclei Increases Atomic Radius Trend Increases Atomic Radius Across a Period Atomic radius generally decreases in size as you
CHEM 1305: Introductory Chemistry
 CHEM 1305: Introductory Chemistry The Periodic Table From Chapter 5 Textbook Introductory Chemistry: Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Classification of Elements By 1870,
CHEM 1305: Introductory Chemistry The Periodic Table From Chapter 5 Textbook Introductory Chemistry: Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Classification of Elements By 1870,
4 Periodic Trends. 1.Atomic Radii (AR) 2.Ionization Energy (IE) 3.Ionic Radii (IR) 4.Electronegativity (EN) Periodic Trends > Types of Periodic Trends
 Periodic Trends > Types of Periodic Trends 4 Periodic Trends 1.Atomic Radii (AR) 2.Ionization Energy (IE) 3.Ionic Radii (IR) 4.Electronegativity (EN) 1 of 31 Periodic Trends > Trends in Atomic Size The
Periodic Trends > Types of Periodic Trends 4 Periodic Trends 1.Atomic Radii (AR) 2.Ionization Energy (IE) 3.Ionic Radii (IR) 4.Electronegativity (EN) 1 of 31 Periodic Trends > Trends in Atomic Size The
Problems with the Wave Theory of Light (Photoelectric Effect)
 CHEM101 NOTES Properties of Light Found that the wave theory could not work for some experiments e.g. the photovoltaic effect This is because the classic EM view of light could not account for some of
CHEM101 NOTES Properties of Light Found that the wave theory could not work for some experiments e.g. the photovoltaic effect This is because the classic EM view of light could not account for some of
Periodic Trends. Atomic Radius: The distance from the center of the nucleus to the outer most electrons in an atom.
 Periodic Trends Study and learn the definitions listed below. Then use the definitions and the periodic table provided to help you answer the questions in the activity. By the end of the activity you should
Periodic Trends Study and learn the definitions listed below. Then use the definitions and the periodic table provided to help you answer the questions in the activity. By the end of the activity you should
2. Why do all elements want to obtain a noble gas electron configuration?
 AP Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Electron Configuration Metal, Nonmetal or Semi-metal Metalloid)? Group 1, Period 1 Group 11,
AP Chemistry Ms. Ye Name Date Block Do Now: 1. Complete the table based on the example given Location Element Electron Configuration Metal, Nonmetal or Semi-metal Metalloid)? Group 1, Period 1 Group 11,
Chapter 6 The Periodic Table
 Chapter 6 The Periodic Table Section 6.1 Organizing the Elements OBJECTIVES: Explain how elements are organized in a periodic table. Section 6.1 Organizing the Elements OBJECTIVES: Compare early and modern
Chapter 6 The Periodic Table Section 6.1 Organizing the Elements OBJECTIVES: Explain how elements are organized in a periodic table. Section 6.1 Organizing the Elements OBJECTIVES: Compare early and modern
Periodic Trends. Elemental Properties and Patterns
 Periodic Trends Elemental Properties and Patterns The Periodic Law Dimitri Mendeleev was the first scientist to publish an organized periodic table of the known elements. Henry Moseley Discovered the proton
Periodic Trends Elemental Properties and Patterns The Periodic Law Dimitri Mendeleev was the first scientist to publish an organized periodic table of the known elements. Henry Moseley Discovered the proton
CHEM 1305 Introductory Chemistry
 CHEM 1305 Introductory Chemistry Introductory Chemistry: Concepts and Critical Thinking 7 th Edition, Charles H. Corwin Chapter 12. Chemical Bonding Modified by: Dr. Violeta F. Coarfa 1 Chemical Bond Concept
CHEM 1305 Introductory Chemistry Introductory Chemistry: Concepts and Critical Thinking 7 th Edition, Charles H. Corwin Chapter 12. Chemical Bonding Modified by: Dr. Violeta F. Coarfa 1 Chemical Bond Concept
POGIL 6 Key Periodic Table Trends (Part 2)
 Honors Chem Block Name POGIL 6 Key Periodic Table Trends (Part 2) is a measure of the ability of an atom s nucleus to attract electrons from a different atom within a covalent bond. A higher electronegativity
Honors Chem Block Name POGIL 6 Key Periodic Table Trends (Part 2) is a measure of the ability of an atom s nucleus to attract electrons from a different atom within a covalent bond. A higher electronegativity
Chapter 9 Ionic and Covalent Bonding
 Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Periodicity SL (answers) IB CHEMISTRY SL
 (answers) IB CHEMISTRY SL Syllabus objectives 3.1 Periodic table Understandings: The periodic table is arranged into four blocks associated with the four sublevels s, p, d, and f. The periodic table consists
(answers) IB CHEMISTRY SL Syllabus objectives 3.1 Periodic table Understandings: The periodic table is arranged into four blocks associated with the four sublevels s, p, d, and f. The periodic table consists
7.10: History of the Periodic Table
 7.10: History of the Periodic Table Dmitri Mendeleev given credit for the first periodic table in 1869 Grouped elements with similar chemical & physical properties in rows according to atomic mass He emphasized
7.10: History of the Periodic Table Dmitri Mendeleev given credit for the first periodic table in 1869 Grouped elements with similar chemical & physical properties in rows according to atomic mass He emphasized
Unit 1 Part 2 Atomic Structure and The Periodic Table Introduction to the Periodic Table UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE
 UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 2 INTRODUCTION TO THE PERIODIC TABLE Contents 1. The Structure of the Periodic Table 2. Trends in the Periodic Table Key words: group, period, block,
UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 2 INTRODUCTION TO THE PERIODIC TABLE Contents 1. The Structure of the Periodic Table 2. Trends in the Periodic Table Key words: group, period, block,
PRACTICE EXERCISE Using Figure 7.6, predict which will be greater, the P Br bond length in PBr 3 or the As Cl bond length in AsCl 3.
 SAMPLE EXERCISE 7.1 Bond Lengths in a Molecule Natural gas used in home heating and cooking is odorless. Because natural gas leaks pose the danger of explosion or suffocation, various smelly substances
SAMPLE EXERCISE 7.1 Bond Lengths in a Molecule Natural gas used in home heating and cooking is odorless. Because natural gas leaks pose the danger of explosion or suffocation, various smelly substances
Unit Five Practice Test (Part I) PT C U5 P1
 Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Li or Na Li or Be Ar or Kr Al or Si
 Pre- AP Chemistry 11 Atomic Theory V Name: Date: Block: 1. Atomic Radius/Size 2. Ionization Energy 3. Electronegativity 4. Chemical Bonding Atomic Radius Effective Nuclear Charge (Z eff) Ø Net positive
Pre- AP Chemistry 11 Atomic Theory V Name: Date: Block: 1. Atomic Radius/Size 2. Ionization Energy 3. Electronegativity 4. Chemical Bonding Atomic Radius Effective Nuclear Charge (Z eff) Ø Net positive
Bonding Chapter 7. Bond an attractive force that holds two atoms together. Atoms bond to obtain a more stable electronic configuration.
 Bonding Chapter 7 Bond an attractive force that holds two atoms together. Atoms bond to obtain a more stable electronic configuration. Ionic bonds attraction between oppositely charged atoms/molecules
Bonding Chapter 7 Bond an attractive force that holds two atoms together. Atoms bond to obtain a more stable electronic configuration. Ionic bonds attraction between oppositely charged atoms/molecules
Average values of the Duration of Electronic Transitions
 Average values of the Duration of Electronic Transitions Kid Spoon Forward 18 s Backward 35 s Fever Strip Forward -14 s Backward 22 s Nail Forward 4s Backward 28 s Ironing of color paper Pink paper Forward:
Average values of the Duration of Electronic Transitions Kid Spoon Forward 18 s Backward 35 s Fever Strip Forward -14 s Backward 22 s Nail Forward 4s Backward 28 s Ironing of color paper Pink paper Forward:
CHAPTER 2. Atoms,Elements, Periodic Table
 CHAPTER Atoms,Elements, Periodic Table 1 Vocabulary Chemistry Science that describes matter its properties, the changes it undergoes, and the energy changes that accompany those processes Matter Anything
CHAPTER Atoms,Elements, Periodic Table 1 Vocabulary Chemistry Science that describes matter its properties, the changes it undergoes, and the energy changes that accompany those processes Matter Anything
Periodic Trends. Elemental Properties and Patterns
 Periodic Trends Elemental Properties and Patterns History of the Periodic Table 1871 Mendeleev arranged the elements according to: Increasing atomic mass Elements w/ similar properties were put in the
Periodic Trends Elemental Properties and Patterns History of the Periodic Table 1871 Mendeleev arranged the elements according to: Increasing atomic mass Elements w/ similar properties were put in the
Chapter 3 Atoms and Ionic Bonds
 Chapter 3 Atoms and Ionic Bonds LEARNING OBJECTIVES SUMMARIES 1. Periodic trends: Know how each of the following are affected by the size of the valence shell (n), the nuclear charge (# of protons), and
Chapter 3 Atoms and Ionic Bonds LEARNING OBJECTIVES SUMMARIES 1. Periodic trends: Know how each of the following are affected by the size of the valence shell (n), the nuclear charge (# of protons), and
Electronic Structure and Bonding Review
 Name: Band: Date: Electronic Structure and Bonding Review 1. For electrons: a. What is the relative charge? b. What is the relative mass? c. What is the symbol? d. Where are they located in the modern
Name: Band: Date: Electronic Structure and Bonding Review 1. For electrons: a. What is the relative charge? b. What is the relative mass? c. What is the symbol? d. Where are they located in the modern
Atomic Radius. Atomic Radius. Atomic Radius 10/17/18. Monday October AGENDA YOYO. AIM What are periodic table trends?
 10/17/18 Monday October AGENDA 15 2018 YOYO PUT EVERYTHING AWAY QUIZ TIME! YOYO AIM What are periodic table trends? Quiz Periodic Trend Activity AFTER QUIZ Turn in Quiz and take and start working on Periodic
10/17/18 Monday October AGENDA 15 2018 YOYO PUT EVERYTHING AWAY QUIZ TIME! YOYO AIM What are periodic table trends? Quiz Periodic Trend Activity AFTER QUIZ Turn in Quiz and take and start working on Periodic
