MISUSE OF DRUGS (DESIGNATION) (JERSEY) ORDER 1989
|
|
|
- Dwayne Griffith
- 6 years ago
- Views:
Transcription
1 MISUSE OF DRUGS (DESIGNATION) (JERSEY) ORDER 1989 Revised Edition Showing the law as at 1 January 2013 This is a revised edition of the law
2
3 Misuse of Drugs (Designation) (Jersey) Order 1989 Arrangement MISUSE OF DRUGS (DESIGNATION) (JERSEY) ORDER 1989 Arrangement Article 1 Specified drugs Citation... 5 SCHEDULE 6 PART 1 6 CONTROLLED DRUGS TO WHICH ARTICLE 12(4) OF THE LAW APPLIES 6 PART 2 11 CONTROLLED DRUGS EXCEPTED FROM PART 1 11 Supporting Documents ENDNOTES 12 Table of Legislation History Table of Renumbered Provisions Table of Endnote References Revised Edition 1 January 2013 Page - 3
4
5 Misuse of Drugs (Designation) (Jersey) Order 1989 Article 1 MISUSE OF DRUGS (DESIGNATION) (JERSEY) ORDER 1989 THE HEALTH AND SOCIAL SERVICES COMMITTEE, in pursuance of Articles 12 and 27 of the Misuse of Drugs (Jersey) Law and after consultation with the Advisory Council on the Misuse of Drugs, orders as follows Commencement [see endnotes] 1 Specified drugs (1) The controlled drugs specified in Part 1 of the Schedule are designated as drugs to which Article 12(4) of the Misuse of Drugs (Jersey) Law applies. (2) The controlled drugs specified in Part 2 of the Schedule are excepted from Part 1 of that Schedule. 2 Citation This Order may be cited as the Misuse of Drugs (Designation) (Jersey) Order Revised Edition 1 January 2013 Page - 5
6 SCHEDULE Misuse of Drugs (Designation) (Jersey) Order 1989 SCHEDULE PART 1 3 CONTROLLED DRUGS TO WHICH ARTICLE 12(4) OF THE LAW APPLIES 1 The following substances and products, namely (a) 1,4-butanediol 1-(3,4-Methylenedioxybenzyl)butyl-(ethyl)amine 1-(3,4-Methylenedioxybenzyl)butyl-(methyl)amine 2-(1,4-Dimethoxy-2-naphthyl)-1-methylethylamine 2-(1,4-Dimethoxy-2-naphthyl)ethylamine 2-(1,4-Dimethoxy-5,6,7,8-tetrahydro-2-naphthyl)-1- methylethylamine 2-(1,4-Dimethoxy-5,6,7,8-tetrahydro-2-naphthyl)ethylamine 2-(1,4-Methano-5,8-dimethoxy-1,2,3,4-tetrahydro-6-naphthyl)-1- methylethylamine 2-(1,4-Methano-5,8-dimethoxy-1,2,3,4-tetrahydro-6- naphthyl)ethylamine 2-(2,5-Dimethoxy-4-methylphenyl)cyclopropylamine 2-(4,7-Dimethoxy-2,3-dihydro-1H-indan-5-yl)-1-methylethylamine 2-(4,7-Dimethoxy-2,3-dihydro-1H-indan-5-yl)ethylamine 2-(5-Methoxy-2,2-dimethyl-2,3-dihydrobenzo[b]furan-6-yl)-1- methylethylamine 2-(5-Methoxy-2-methyl-2,3-dihydrobenzo[b]furan-6-yl)-1- methylethylamine 2-(α-Methyl-3,4-methylenedioxyphenethylamino)ethanol 2,5-Dimethoxy-α, 4-dimethyl-phenethylamine 2-Amino-1-(2,5-dimethoxy-4-methylphenyl)ethanol 2-Amino-1-(3,4-dimethoxyphenyl)ethanol 2-Methoxyethyl(α-methyl-3,4-methylenedioxyphenethyl)amine 4-Bromo- β,2,5-trimethoxyphenethylamine 4-Bromo-2, 5-dimethoxy- methyl-phenethylamine 4-Iodo-2,5-dimethoxy-α-methylphenethyl(dimethyl)amine 4-Methyl-aminorex Allyl(α-methyl-3,4-methylenedioxyphenethyl)amine Amphetamine Benzyl(α-methyl-3,4-methylenedioxyphenethyl)amine Bufotenine Page - 6 Revised Edition 1 January 2013
7 Misuse of Drugs (Designation) (Jersey) Order 1989 SCHEDULE Cannabinol Cannabinol derivatives except dronabinol and except any sterioisomer of dronabinol Cannabis Cannabis resin Cathinone Coca leaf Concentrate of poppy-straw Cyclopropylmethyl(α-methyl-3,4-methylenedioxyphenethyl)amine Dihydroetorphine Dimethyl(α-methyl-3,4-methylenedioxyphenethyl)amine Eticyclidine Etryptamine Gamma-butyrolactone 4-hydroxybutanoic acid (4-hydroxy-n-butyric acid; gammahydroxybutyric acid) Lysergamide Lysergide and other N-alkyl derivatives of lysergamide Mescaline Methcathinone Methylamphetamine N-(2,5-Dimethoxy-4-propylthiophenethyl)hydroxyl-amine N-(4-Ethylthio-2,5-dimethoxyphenethyl)hydroxyl-amine N-(4-sec-Butylthio-2,5-dimethoxyphenethyl)hydroxyl-amine N.N-Diethyltryptamine N.N-Dimethyltryptamine N-Hydroxy-tenamphetamine N-Methyl-N-(α-methyl-3,4-methylenedioxyphenethyl)hydroxylamine O-Methyl-N-(α-methyl-3,4-methylenedioxyphenethyl)hydroxylamine Psilocin Raw opium Rolicyclidine Tenocyclidine α, α-dimethyl-3,4-methylenedioxyphenethyl (methyl)amine α, α-dimethyl-3,4-methylenedioxyphenethylamine α-methyl-3,4-methylenedioxyphenethyl(prop-2-ynyl)amine Revised Edition 1 January 2013 Page - 7
8 SCHEDULE Misuse of Drugs (Designation) (Jersey) Order 1989 (b) (c) (d) (e) α-methyl-4-(methylthio)phenethylamine (also known as 4- Methylthioamphetamine) α-methylphenethylhydroxylamine (also known as N- Hydroxyamphetamine) β -Methoxy-3,4-methylenedioxyphenethylamine β,2,5-trimethoxy-4-methylphenethylamine β,3,4,5-tetramethoxyphenethylamine; any compound (not being a compound for the time being specified in sub-paragraph (a) above) structurally derived from tryptamine or from a ring-hydroxy tryptamine by substitution at the nitrogen atom of the sidechain with one or more alkyl substituents but no other substituent; any compound (not being methoxyphenamine or a compound for the time being specified in sub-paragraph (a) above) structurally derived from phenethylamine, an N-alkylphenethylamine, - methylphenethylamine, an N-alkyl- -methylphenethylamine, - ethylphenethylamine, or an N-alkyl- -ethylphenethylamine by substitution in the ring to any extent with alkyl, alkoxy, alkylenedioxy or halide substituents, whether or not further substituted in the ring by one or more other univalent substituents; any compound (not being a compound for the time being specified in Part II of this Schedule) structurally derived from fentanyl by modification in any of the following ways, that is to say (ii) (iii) (iv) (v) (vi) by replacement of the phenyl portion of the phenethyl group by any heteromonocycle whether or not further substituted in the heterocycle, by substitution in the phenethyl group with alkyl, alkenyl, alkoxy, hydroxy, halogeno, haloalkyl, amino or nitro groups, by substitution in the piperidine ring with alkyl or alkenyl groups, by substitution in the aniline ring with alkyl, alkoxy, alkylenedioxy, halogeno or haloalkyl groups, by substitution at the 4-position of the piperidine ring with any alkoxycarbonyl or alkoxyalkyl or acyloxy group, by replacement of the N-propionyl group by another acyl group; any compound (not being a compound for the time being specified in Part II of this Schedule) that is structurally derived from pethidine by modification in any of the following ways, that is to say (ii) by replacement of the 1-methyl group by an acyl alkyl whether or not unsaturated, benzyl or phenethyl group, whether or not further substituted, by substitution in the piperidine ring with alkyl or alkenyl groups or with a propano bridge, whether or not further substituted, Page - 8 Revised Edition 1 January 2013
9 Misuse of Drugs (Designation) (Jersey) Order 1989 SCHEDULE (f) (g) (h) (iii) (iv) (v) by substitution in the 4-phenyl ring with alkyl, alkoxy, aryloxy, halogeno or haloalkyl groups, by replacement of the 4-ethoxycarbonyl by any other alkoxycarbonyl or any alkoxyalkyl or acyloxy group, by formation of an N-oxide or of a quaternary base; any compound (not being bupropion, cathinone, diethylpropion, pyrovalerone or a compound for the time being specified in subparagraph (a)) structurally derived from 2-amino-1-phenyl-1- propanone by modification in any of the following ways, that is to say (ii) (iii) by substitution in the phenyl ring to any extent with alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents, whether or not further substituted in the phenyl ring by one or more other univalent substituents, by substitution at the 3-position with an alkyl substituent, by substitution at the nitrogen atom with alkyl or dialkyl groups, or by inclusion of the nitrogen atom in a cyclic structure; any compound structurally derived from 2-aminopropan-1-one by substitution at the 1-position with any monocyclic, or fusedpolycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), whether or not the compound is further modified in any of the following ways, that is to say (ii) (iii) by substitution in the ring system to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents, by substitution at the 3-position with an alkyl substituent, by substitution at the 2-amino nitrogen atom with alkyl or dialkyl groups, or by inclusion of the 2-amino nitrogen atom in a cyclic structure; 1-benzylpiperazine; any compound (not being a compound for the time being specified in Part 2 of this Schedule) structurally derived from 1- benzylpiperazine or 1-phenylpiperazine by modification in any of the following ways (ii) by substitution at the second nitrogen atom of the piperazine ring with alkyl, benzyl, haloalkyl or phenyl substituents, by substitution in the aromatic ring to any extent with alkyl, alkoxy, alkylenedioxy, halide or haloalkyl substituents; (j) any compound structurally derived from 3-(1-naphthoyl)indole or 1H-indol-3-yl-(1-naphthyl)methane by substitution at the nitrogen atom of the indole ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the Revised Edition 1 January 2013 Page - 9
10 SCHEDULE Misuse of Drugs (Designation) (Jersey) Order 1989 indole ring to any extent and whether or not substituted in the naphthyl ring to any extent; (k) any compound structurally derived from 3-(1-naphthoyl)pyrrole by substitution at the nitrogen atom of the pyrrole ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent; (l) any compound structurally derived from 1-(1-naphthylmethyl)indene by substitution at the 3-position of the indene ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent; (m) any compound structurally derived from 3-phenylacetylindole by substitution at the nitrogen atom of the indole ring with alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent; (n) any compound structurally derived from 2-(3-hydroxycyclohexyl)phenol by substitution at the 5-position of the phenolic ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the cyclohexyl ring to any extent; (o) Any compound (not being pipradrol) structurally derived from piperidine, pyrrolidine, azepane, morpholine or pyridine by substitution at a ring carbon atom with a diphenylmethyl group, whether or not the compound is further modified in any of the following ways, that is to say (ii) (iii) by substitution in any of the phenyl rings to any extent with alkyl, alkoxy, haloalkyl or halide groups, by substitution at the methyl carbon atom with an alkyl, hydroxyalkyl or hydroxy group, by substitution at the ring nitrogen atom with an alkyl, alkenyl, haloalkyl or hydroxyalkyl group. 2 Any stereoisomeric form of a substance specified in paragraph 1 above. 3 Any ester or ether of a substance specified in paragraph 1 or 2 above. 4 Any salt of a substance specified in any of paragraphs 1 to 3 above. 5 Any preparation or other product containing a substance or product specified in any of paragraphs 1 to 4 above. Page - 10 Revised Edition 1 January 2013
11 Misuse of Drugs (Designation) (Jersey) Order 1989 SCHEDULE PART 2 4 CONTROLLED DRUGS EXCEPTED FROM PART 1 1 The compounds referred to in Part 1, paragraph 1(d) of this Schedule are Alfentanil Carfentanil Lofentanil Remifentanil Sufentanil. 2 The compounds referred to in Part 1, paragraph 1(e) of this Schedule are Allylprodine Alphameprodine Alphaprodine Anileridine Betameprodine Betaprodine Hydroxypethidine Properidine Trimeperidine. 3 The compounds referred to in Part 1, paragraph 1 of this Schedule are 1-(3-chlorophenyl)piperazine 1-(3-chlorophenyl)-4-(3-chlorophenyl)piperazine. Revised Edition 1 January 2013 Page - 11
12 Endnotes Misuse of Drugs (Designation) (Jersey) Order 1989 ENDNOTES Table of Legislation History Legislation Year and Number Commencement Misuse of Drugs (Designation) R&O March 1989 (Jersey) Order 1989 Misuse of Drugs (Designation) R&O September 1991 (Amendment) (Jersey) Order 1991 Misuse of Drugs (Designation) R&O May 1992 (Amendment No. 2) (Jersey) Order 1992 Misuse of Drugs (Designation) R&O June 1999 (Amendment No. 3) (Jersey) Order 1999 Misuse of Drugs (Designation) R&O.55/ June 2002 (Amendment No. 4) (Jersey) Order 2002 Misuse of Drugs (Designation) R&O.8/ March 2003 (Amendment No. 5) (Jersey) Order 2003 Misuse of Drugs (Miscellaneous R&O.94/ September 2010 Amendments) (Jersey) (Order) 2010 Misuse of Drugs (Miscellaneous Amendments) (No. 2) (Jersey) (Order) 2012 R&O.106/ September 2012 Table of Renumbered Provisions Table of Endnote References Original Current 3 2 SCHEDULE SCHEDULE PART I PART 1 PART II PART 2 1 chapter chapter Schedule Part 1 amended by R&O.8247, R&O.8350, R&O.9376, R&O.55/2002, R&O.8/2003, R&O.94/2010, R&O.106/ Schedule Part 2 amended by R&O.8/2003, R&O.94/2010 Page - 12 Revised Edition 1 January 2013
MISUSE OF DRUGS (MISCELLANEOUS AMENDMENTS) (No. 5) (JERSEY) ORDER 2014
 Arrangement MISUSE OF DRUGS (MISCELLANEOUS AMENDMENTS) (No. 5) (JERSEY) ORDER 2014 Arrangement Article 1 Misuse of Drugs (Jersey) Law 1978 amended... 3 2 Misuse of Drugs (Designation) (Jersey) Order 1989
Arrangement MISUSE OF DRUGS (MISCELLANEOUS AMENDMENTS) (No. 5) (JERSEY) ORDER 2014 Arrangement Article 1 Misuse of Drugs (Jersey) Law 1978 amended... 3 2 Misuse of Drugs (Designation) (Jersey) Order 1989
SENATE, No STATE OF NEW JERSEY. 215th LEGISLATURE INTRODUCED MARCH 8, 2012
 SENATE, No. STATE OF NEW JERSEY th LEGISLATURE INTRODUCED MARCH, 0 Sponsored by: Senator SHIRLEY K. TURNER District (Hunterdon and Mercer) Senator CHRISTOPHER "KIP" BATEMAN District (Hunterdon, Mercer,
SENATE, No. STATE OF NEW JERSEY th LEGISLATURE INTRODUCED MARCH, 0 Sponsored by: Senator SHIRLEY K. TURNER District (Hunterdon and Mercer) Senator CHRISTOPHER "KIP" BATEMAN District (Hunterdon, Mercer,
THE GENERAL ASSEMBLY OF PENNSYLVANIA HOUSE BILL
 PRIOR PRINTER'S NO. PRINTER'S NO. 10 THE GENERAL ASSEMBLY OF PENNSYLVANIA HOUSE BILL No. Session of 01 INTRODUCED BY STERN, MILLARD, PICKETT, SCHLOSSBERG, R. BROWN, HEFFLEY, GRELL, V. BROWN, D. COSTA,
PRIOR PRINTER'S NO. PRINTER'S NO. 10 THE GENERAL ASSEMBLY OF PENNSYLVANIA HOUSE BILL No. Session of 01 INTRODUCED BY STERN, MILLARD, PICKETT, SCHLOSSBERG, R. BROWN, HEFFLEY, GRELL, V. BROWN, D. COSTA,
1 HB By Representative Todd. 4 RFD: Judiciary. 5 First Read: 13-FEB-14. Page 0
 1 HB487 2 156923-1 3 By Representative Todd 4 RFD: Judiciary 5 First Read: 13-FEB-14 Page 0 1 156923-1:n:01/17/2014:JMH/tan LRS2014-305 2 3 4 5 6 7 8 SYNOPSIS: This bill would provide that industrial hemp
1 HB487 2 156923-1 3 By Representative Todd 4 RFD: Judiciary 5 First Read: 13-FEB-14 Page 0 1 156923-1:n:01/17/2014:JMH/tan LRS2014-305 2 3 4 5 6 7 8 SYNOPSIS: This bill would provide that industrial hemp
MANUFACTURING OF A CONTROLLED DANGEROUS SUBSTANCE (SYNTHETIC CANNABINOID) 1 (N.J.S.A. 2C:35-5.3b)
 1 Approved 2/13/17 Count of the indictment charges the defendant as follows: (Read Indictment) The pertinent part of the statute on which this indictment is based reads as follows: It is a crime for any
1 Approved 2/13/17 Count of the indictment charges the defendant as follows: (Read Indictment) The pertinent part of the statute on which this indictment is based reads as follows: It is a crime for any
A Bill Regular Session, 2013 HOUSE BILL 1415
 Stricken language would be deleted from and underlined language would be added to present law. Act of the Regular Session 0 State of Arkansas th General Assembly A Bill Regular Session, HOUSE BILL By:
Stricken language would be deleted from and underlined language would be added to present law. Act of the Regular Session 0 State of Arkansas th General Assembly A Bill Regular Session, HOUSE BILL By:
CHAPTER 513 Drug Abuse
 24G CHAPTER 513 Drug Abuse 513.01 Prohibition on the manufacture, possession, use, exchange, sale and distribution of synthetic cannabinoids, synthetic cathinones and other synthetic drugs, their derivatives
24G CHAPTER 513 Drug Abuse 513.01 Prohibition on the manufacture, possession, use, exchange, sale and distribution of synthetic cannabinoids, synthetic cathinones and other synthetic drugs, their derivatives
Title 17-A: MAINE CRIMINAL CODE
 Maine Revised Statutes Title 17-A: MAINE CRIMINAL CODE Chapter 45: DRUGS 1102. SCHEDULES W, X, Y AND Z For the purposes of defining crimes under this chapter and of determining the penalties therefor,
Maine Revised Statutes Title 17-A: MAINE CRIMINAL CODE Chapter 45: DRUGS 1102. SCHEDULES W, X, Y AND Z For the purposes of defining crimes under this chapter and of determining the penalties therefor,
SYNTHETIC CANNABINOID TRADE NAME AND CHEMICAL COMPOUND CHART
 SYNTHETIC CANNABINOID TRADE NAME AND CHEMICAL COMPOUND CHART The following chart may not include all trade or other names for the designated substances. It also may not include all variations in the chemical
SYNTHETIC CANNABINOID TRADE NAME AND CHEMICAL COMPOUND CHART The following chart may not include all trade or other names for the designated substances. It also may not include all variations in the chemical
NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONSOLIDATION) (AMENDMENT OF SCHEDULE) ORDER 2017
 Public Instrument 7 of 2017 Published in Gazette No. 1836 of 02 June 2017 NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONSOLIDATION) (AMENDMENT OF SCHEDULE) ORDER 2017 The Administrator makes the following
Public Instrument 7 of 2017 Published in Gazette No. 1836 of 02 June 2017 NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONSOLIDATION) (AMENDMENT OF SCHEDULE) ORDER 2017 The Administrator makes the following
HOUSE BILL 12. By Shipley BE IT ENACTED BY THE GENERAL ASSEMBLY OF THE STATE OF TENNESSEE:
 HOUSE BILL 12 By Shipley AN ACT to amend Tennessee Code Annotated, Title 39, Chapter 17, Part 4 and Title 55, Chapter 10, Part 4, relative to illegal drugs and analogues of drugs. BE IT ENACTED BY THE
HOUSE BILL 12 By Shipley AN ACT to amend Tennessee Code Annotated, Title 39, Chapter 17, Part 4 and Title 55, Chapter 10, Part 4, relative to illegal drugs and analogues of drugs. BE IT ENACTED BY THE
Chapter 15 Amines Amines 15.2 Naming Amines 15.3 Physical Properties of Amines
 Chapter 15 Amines 15.1 Amines 15.2 Naming Amines 15.3 Physical Properties of Amines 1 Amines Amines are organic compounds in which one or more H in ammonia, NH 3, is replaced with alkyl or aromatic groups.
Chapter 15 Amines 15.1 Amines 15.2 Naming Amines 15.3 Physical Properties of Amines 1 Amines Amines are organic compounds in which one or more H in ammonia, NH 3, is replaced with alkyl or aromatic groups.
Article 1. New Psychoactive Substances Act 1 (Neue-psychoaktive-Stoffe-Gesetz) (NpSG)
 Act to combat the distribution of new psychoactive substances * of 21 November 2016 The Bundestag has adopted the following Act: Article 1 New Psychoactive Substances Act 1 (Neue-psychoaktive-Stoffe-Gesetz)
Act to combat the distribution of new psychoactive substances * of 21 November 2016 The Bundestag has adopted the following Act: Article 1 New Psychoactive Substances Act 1 (Neue-psychoaktive-Stoffe-Gesetz)
WEIGHTS AND MEASURES (INTERNATIONAL DEFINITIONS) (JERSEY) ORDER 1968
 WEIGHTS AND MEASURES (INTERNATIONAL DEFINITIONS) (JERSEY) ORDER 1968 Revised Edition Showing the law as at 31 August 2004 This is a revised edition of the law Weights and Measures (International Definitions)
WEIGHTS AND MEASURES (INTERNATIONAL DEFINITIONS) (JERSEY) ORDER 1968 Revised Edition Showing the law as at 31 August 2004 This is a revised edition of the law Weights and Measures (International Definitions)
MEDICINAL CHEMISTRY I EXAM #1
 MEDICIAL CEMISTRY I EXAM #1 1 September 30, 2005 ame SECTI A. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 50 points
MEDICIAL CEMISTRY I EXAM #1 1 September 30, 2005 ame SECTI A. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 50 points
Isomerism CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12. Constitutional isomers...
 Isomerism 4 2 6 3 8 4 10 5 12 onstitutional isomers... 3 8 Positional isomers... Functional isomers... ow many constitutional isomers are there for the formula 4 8? arbon atoms are often classified as
Isomerism 4 2 6 3 8 4 10 5 12 onstitutional isomers... 3 8 Positional isomers... Functional isomers... ow many constitutional isomers are there for the formula 4 8? arbon atoms are often classified as
Chemistry 506: Allied Health Chemistry 2. Chapter 15: Amines and Amides. Functional Groups with Single Bonds to Nitrogen
 Chemistry 506 Dr. Hunter s Class Chapter 15. Chemistry 506: Allied Health Chemistry 2 1 Chapter 15: Amines and Amides Functional Groups with Single Bonds to Nitrogen Introduction to General, Organic &
Chemistry 506 Dr. Hunter s Class Chapter 15. Chemistry 506: Allied Health Chemistry 2 1 Chapter 15: Amines and Amides Functional Groups with Single Bonds to Nitrogen Introduction to General, Organic &
.. Amines CHAPTER SUMMARY
 10 N.. N.. CHAPTER SUMMARY 10.1 Structure of are derivatives of ammonia in which one or more hydrogens have been replaced by organic groups. Replacement of one hydrogen results in a primary amine. In a
10 N.. N.. CHAPTER SUMMARY 10.1 Structure of are derivatives of ammonia in which one or more hydrogens have been replaced by organic groups. Replacement of one hydrogen results in a primary amine. In a
Organic Chemistry. A. Introduction
 Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
The Principles of Heterocyclic Chemistry
 The Principles of Heterocyclic Chemistry ALAN R. KATRITZKY M.A., D.PHIL., PH.D., SC.D. Dean of the School of Chemical Sciences, University of East Anglia, Norwich, England and J. M. LAGOWSKI B.S., M.S.,
The Principles of Heterocyclic Chemistry ALAN R. KATRITZKY M.A., D.PHIL., PH.D., SC.D. Dean of the School of Chemical Sciences, University of East Anglia, Norwich, England and J. M. LAGOWSKI B.S., M.S.,
ANALYSIS OF DESIGNER STIMULANTS BY GC/MS. Application Compendium
 ANALYSIS OF DESIGNER STIMULANTS BY GC/MS Application Compendium 1 This page intentionally blank Table of Contents Introduction...4 Sample Preparation/Extraction...5 Gas Chromatograph and Mass Spectrometer
ANALYSIS OF DESIGNER STIMULANTS BY GC/MS Application Compendium 1 This page intentionally blank Table of Contents Introduction...4 Sample Preparation/Extraction...5 Gas Chromatograph and Mass Spectrometer
Organic Chemistry II KEY March 27, 2013
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
Organic Chemistry II KEY March 27, Which of the following reaction intermediates will form the fastest in the reaction below?
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
Functional Groups. Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity.
 Functional Groups Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity. Organic halides: a hydrogen is replaced by a halogen fluoro-,
Functional Groups Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity. Organic halides: a hydrogen is replaced by a halogen fluoro-,
Chapter 21. Organic Chemistry 4 th Edition Paula Yurkanis Bruice. More About Amines. Heterocyclic Compounds
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 21 More About Amines. Heterocyclic Compounds Irene Lee Case Western Reserve University Cleveland, OH 2004, Prentice Hall Amines An amine is
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 21 More About Amines. Heterocyclic Compounds Irene Lee Case Western Reserve University Cleveland, OH 2004, Prentice Hall Amines An amine is
Chapter 20 Amines 胺类
 Amines ORGAIC CEMISTRY 20.1 omenclature ORGAIC CEMISTRY Amines are classified as primary (R 2 ), secondary (R 2 ), or tertiary (R 3 ), depending on the number of organic substituents attached to nitrogen.
Amines ORGAIC CEMISTRY 20.1 omenclature ORGAIC CEMISTRY Amines are classified as primary (R 2 ), secondary (R 2 ), or tertiary (R 3 ), depending on the number of organic substituents attached to nitrogen.
FAMILIES of ORGANIC COMPOUNDS
 1 SCH4U October 2016 Organic Chemistry Chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 - ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen
1 SCH4U October 2016 Organic Chemistry Chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 - ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Examples of Substituted Benzenes
 Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
10. Amines (text )
 2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Unit 5: Organic Chemistry
 Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
 Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with HNO2 at a low temperature to
Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with HNO2 at a low temperature to
Ch 16 Amines. Organic and Biochemsity
 Ch 16 Amines Organic and Biochemsity Amines Section 16.1 Introduction itrogen found in amine group is the fourth most common element in organic chemistry Known for their basicity Section 16.2 Classification
Ch 16 Amines Organic and Biochemsity Amines Section 16.1 Introduction itrogen found in amine group is the fourth most common element in organic chemistry Known for their basicity Section 16.2 Classification
Properties of Amines
 Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
TOWN OF LITTLETON ORDINANCE PROHIBITION OF THE POSSESSION OR SALE OF SYNTHETIC CANNABINOIDS
 TOWN OF LITTLETON ORDINANCE PROHIBITION OF THE POSSESSION OR SALE OF SYNTHETIC CANNABINOIDS Purpose and intent It has been determined that certain businesses and/or individuals within the Town of Littleton
TOWN OF LITTLETON ORDINANCE PROHIBITION OF THE POSSESSION OR SALE OF SYNTHETIC CANNABINOIDS Purpose and intent It has been determined that certain businesses and/or individuals within the Town of Littleton
22.1 Amine Nomenclature
 Chapter 22 Amines 22.1 Amine Nomenclature Classification of Amines Alkylamine N attached to alkyl group Arylamine N attached to aryl group Primary, secondary, or tertiary determined by number of carbon
Chapter 22 Amines 22.1 Amine Nomenclature Classification of Amines Alkylamine N attached to alkyl group Arylamine N attached to aryl group Primary, secondary, or tertiary determined by number of carbon
BENZENE & AROMATIC COMPOUNDS
 BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine.
 The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
Lecture 27 Organic Chemistry 1
 CHEM 232 rganic Chemistry I at Chicago Lecture 27 rganic Chemistry 1 Professor Duncan Wardrop April 20, 2010 1 Self Test Question Nitrosonium (not nitronium) cations can be generated by treating sodium
CHEM 232 rganic Chemistry I at Chicago Lecture 27 rganic Chemistry 1 Professor Duncan Wardrop April 20, 2010 1 Self Test Question Nitrosonium (not nitronium) cations can be generated by treating sodium
FIRST ENGROSSMENT. A BILL for an Act to amend and reenact sections , , ,
 .00.0000 of North Dakota FIRST ENGROSSMENT ENGROSSED SENATE BILL NO. Introduced by Judiciary Committee (At the request of the State Board of Pharmacy) A BILL for an Act to amend and reenact sections -0.-0,
.00.0000 of North Dakota FIRST ENGROSSMENT ENGROSSED SENATE BILL NO. Introduced by Judiciary Committee (At the request of the State Board of Pharmacy) A BILL for an Act to amend and reenact sections -0.-0,
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
All Classes of Organic Compounds
 Amines All Classes of Organic Compounds ydrocarbons Functionalized ydrocarbons F,Cl,Br O,S, Alkanes Alkenes Alkynes Aromatics alides -O- -S- -- Alcohols Phenols Ethers Thiols Dissulfides Amines O C O C
Amines All Classes of Organic Compounds ydrocarbons Functionalized ydrocarbons F,Cl,Br O,S, Alkanes Alkenes Alkynes Aromatics alides -O- -S- -- Alcohols Phenols Ethers Thiols Dissulfides Amines O C O C
FARMINGDALE STATE COLLEGE DEPARTMENT OF CHEMISTRY. CONTACT HOURS: Lecture: 3 Laboratory: 4
 FARMINGDALE STATE COLLEGE DEPARTMENT OF CHEMISTRY COURSE OUTLINE: COURSE TITLE: Prepared by: Dr. M. DeCastro September 2011 Organic Chemistry II COURSE NUMBER: CHM 271 CREDITS: 5 CONTACT HOURS: Lecture:
FARMINGDALE STATE COLLEGE DEPARTMENT OF CHEMISTRY COURSE OUTLINE: COURSE TITLE: Prepared by: Dr. M. DeCastro September 2011 Organic Chemistry II COURSE NUMBER: CHM 271 CREDITS: 5 CONTACT HOURS: Lecture:
Seminar_3. 1. Substituded derivatives of benzene and their nomenclature
 1. Substituded derivatives of benzene and their nomenclature 2. Reactions of arenes. Electrophilic aromatic substitutions 3. Activating substituents. Orientation in the aromatic ring Seminar_3 TEST - Aromatic
1. Substituded derivatives of benzene and their nomenclature 2. Reactions of arenes. Electrophilic aromatic substitutions 3. Activating substituents. Orientation in the aromatic ring Seminar_3 TEST - Aromatic
RODD'S CHEMISTRY OF CARBON COMPOUNDS
 Supplements to the 2nd Edition of RODD'S CHEMISTRY OF CARBON COMPOUNDS VOLUME I ALIPHATIC COMPOUNDS VOLUME II ALICYCLIC COMPOUNDS VOLUME III AROMATIC COMPOUNDS VOLUME IV HETEROCYCLIC COMPOUNDS VOLUME V
Supplements to the 2nd Edition of RODD'S CHEMISTRY OF CARBON COMPOUNDS VOLUME I ALIPHATIC COMPOUNDS VOLUME II ALICYCLIC COMPOUNDS VOLUME III AROMATIC COMPOUNDS VOLUME IV HETEROCYCLIC COMPOUNDS VOLUME V
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
CHAPTER 22: Amines R 2 H N N N R 1. cadeverine N CH 3. nicotine
 Based on notes by JZ updated 4/2018 Amines are Biologically Important CAPTE 22: Amines Amino acids are the basis of all peptides and proteins. These are the tissue building blocks and nature's catalysts
Based on notes by JZ updated 4/2018 Amines are Biologically Important CAPTE 22: Amines Amino acids are the basis of all peptides and proteins. These are the tissue building blocks and nature's catalysts
BRCC CHM102 Chapter 16 Notes Class Notes Page 1 of 7 H 3 C N CH 3 CH 3 CHCH 3
 BR M102 hapter 16 otes lass otes Page 1 of 7 hapter 16 Amines Amines derivatives of ammonia R 2 primary amine 3 R 1 R 2 secondary amine 3 R 1 R 2 R 3 tertiary amine 3 * Remember that classification of
BR M102 hapter 16 otes lass otes Page 1 of 7 hapter 16 Amines Amines derivatives of ammonia R 2 primary amine 3 R 1 R 2 secondary amine 3 R 1 R 2 R 3 tertiary amine 3 * Remember that classification of
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
Ch15_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch15_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name of the compound shown? 1) N-methylethylamine 2-propylamine 1-methylethylamine
Ch15_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name of the compound shown? 1) N-methylethylamine 2-propylamine 1-methylethylamine
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
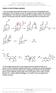 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
OH OH OH CH 2 CH 2 C(CH 3 ) 2 (a) CH 3 CHCH 2 CHCH(CH 3 ) 2. (b)
 hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
Chapter 11. Introduction to Organic Chemistry
 hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
Evans Fine Chem.
 +91-8048602255 Evans Fine Chem https://www.indiamart.com/evansfinechem/ We are a prime manufacturer, exporter and trader of varied kinds of Fine Chemicals and Specialized Molecules. Our products playing
+91-8048602255 Evans Fine Chem https://www.indiamart.com/evansfinechem/ We are a prime manufacturer, exporter and trader of varied kinds of Fine Chemicals and Specialized Molecules. Our products playing
Topic 10.1: Fundamentals of Organic Chemistry Notes
 Topic 10.1: Fundamentals of Organic Chemistry Notes Terminology Hydrocarbon: compounds that contain mostly hydrogen and carbon Homologous Series: compounds with the same general formula Molecular Formula:
Topic 10.1: Fundamentals of Organic Chemistry Notes Terminology Hydrocarbon: compounds that contain mostly hydrogen and carbon Homologous Series: compounds with the same general formula Molecular Formula:
Chemistry of Heterocyclic Compounds
 Chemistry of Heterocyclic Compounds What s Heterocyclic Compound? A heterocyclic compound is one that contain rings made up, in addition to carbon, one or more heteroatoms like 0,N,S,P. etc. Heterocyclic
Chemistry of Heterocyclic Compounds What s Heterocyclic Compound? A heterocyclic compound is one that contain rings made up, in addition to carbon, one or more heteroatoms like 0,N,S,P. etc. Heterocyclic
ORDINANCE
 ORDINANCE 2015-03-02 AN ORDINANCE OF THE CITY COUNCIL OF THE CITY OF ANAHUAC, TEXAS, ADMENDING TITLE XIII GENERAL OFFENSES OF THE CODE OF ORDINANCES, ANAHUAC, TEXAS, TO ADD SECTION 131 THE PROHIBITION
ORDINANCE 2015-03-02 AN ORDINANCE OF THE CITY COUNCIL OF THE CITY OF ANAHUAC, TEXAS, ADMENDING TITLE XIII GENERAL OFFENSES OF THE CODE OF ORDINANCES, ANAHUAC, TEXAS, TO ADD SECTION 131 THE PROHIBITION
Lecture Notes Chemistry 342 Mukund P. Sibi Lecture 33 Amines
 Lecture otes Chemistry 342 Mukund P. Sibi Amines Amines are organic compounds derived from ammonia. The nitrogen in amines contains a lone pair of electrons. The presence of this lone pair of electrons
Lecture otes Chemistry 342 Mukund P. Sibi Amines Amines are organic compounds derived from ammonia. The nitrogen in amines contains a lone pair of electrons. The presence of this lone pair of electrons
Changes to State Controlled Substance Schedules: Bill Status Update
 Changes to State Controlled Substance Schedules: Bill Status Update Research current through March 24, 2015. This project was supported by Grant No. G1399ONDCP03A, awarded by the Office of National Drug
Changes to State Controlled Substance Schedules: Bill Status Update Research current through March 24, 2015. This project was supported by Grant No. G1399ONDCP03A, awarded by the Office of National Drug
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
 . 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
. 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
AMINES. 3. Secondary When two hydrogen atoms are replaced by two alkyl or aryl groups.
 AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
3) Oxidation of tertiary alcohol yields A) Aldehyde B) No reaction C) Ketone D) Carboxylic acid
 ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
(The original text has been published in the Federal Law Gazette (Bundesgesetzblatt - BGBl.) I 2001, )
 Annex I (ad section 1 subsection 1) nonmarketable narcotic drugs (The original text has been published in the Federal Law Gazette (Bundesgesetzblatt BGBl.) I 2001, 1180 1186) Column 1 Column 2 Column 3
Annex I (ad section 1 subsection 1) nonmarketable narcotic drugs (The original text has been published in the Federal Law Gazette (Bundesgesetzblatt BGBl.) I 2001, 1180 1186) Column 1 Column 2 Column 3
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Nomenclature of Organic Compounds Identification of Functional Groups
 Hydrocarbons Nomenclature of Organic ompounds Identification of Functional Groups Alkanes - also known as saturated hydrocarbons or the paraffin series because all bond sites between carbon atoms and between
Hydrocarbons Nomenclature of Organic ompounds Identification of Functional Groups Alkanes - also known as saturated hydrocarbons or the paraffin series because all bond sites between carbon atoms and between
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 5 N S. Typical Reactivity of Pyridines, Quinolines and Isoquinolines
 Chapter 5 S Typical Reactivity of Pyridines, Quinolines and Isoquinolines 1 2 Typical Reactivity of Pyridines pyridines are much less susceptible to electrophilic substitution than benzene and much more
Chapter 5 S Typical Reactivity of Pyridines, Quinolines and Isoquinolines 1 2 Typical Reactivity of Pyridines pyridines are much less susceptible to electrophilic substitution than benzene and much more
GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 2017 H 3 HOUSE BILL 464* Committee Substitute Favorable 4/5/17 Committee Substitute #2 Favorable 4/19/17
 GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 0 H HOUSE BILL * Committee Substitute Favorable // Committee Substitute # Favorable // Short Title: Revise Schedule of Controlled Substances. (Public) Sponsors:
GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 0 H HOUSE BILL * Committee Substitute Favorable // Committee Substitute # Favorable // Short Title: Revise Schedule of Controlled Substances. (Public) Sponsors:
Infrared Characteristic Group Frequencies
 Infrared Characteristic Group Frequencies Tables and Charts Second Edition GEORGE SOCRATES Brunei, The University of West London, Middlesex, United Kingdom JOHN WILEY & SONS Chichester New York Brisbane
Infrared Characteristic Group Frequencies Tables and Charts Second Edition GEORGE SOCRATES Brunei, The University of West London, Middlesex, United Kingdom JOHN WILEY & SONS Chichester New York Brisbane
House Language UES REVISOR FULL-TEXT SIDE-BY-SIDE ARTICLE CONTROLLED SUBSTANCES
 103.22 ARTICLE 8 103.23 CONTROLLED SUBSTANCES 103.24 Section 1. Minnesota Statutes 2016, section 152.02, subdivision 2, is amended to read: 103.25 Subd. 2. Schedule I. (a) Schedule I consists of the substances
103.22 ARTICLE 8 103.23 CONTROLLED SUBSTANCES 103.24 Section 1. Minnesota Statutes 2016, section 152.02, subdivision 2, is amended to read: 103.25 Subd. 2. Schedule I. (a) Schedule I consists of the substances
AMINES. 3. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
2. Match the following NMR spectra with one of the following substances. Write you answer in the box along side the spectrum. (16 points) A B C D E F
 2. Match the following NM spectra with one of the following substances. Write you answer in the box along side the spectrum H H (16 points) A B C H D E F 1. Match the following compounds with their 13
2. Match the following NM spectra with one of the following substances. Write you answer in the box along side the spectrum H H (16 points) A B C H D E F 1. Match the following compounds with their 13
INTRODUCTION TO ORGANIC CHEMISTRY
 INTRODUTION TO ORGANI EMISTRY GENERAL DESRIPTION OF ORGANI EMISTRY The Study of arbon ompounds GENERAL DESRIPTION OF ORGANI EMISTRY The Study of arbon ompounds Organic Man-made Substances Plant or Animal
INTRODUTION TO ORGANI EMISTRY GENERAL DESRIPTION OF ORGANI EMISTRY The Study of arbon ompounds GENERAL DESRIPTION OF ORGANI EMISTRY The Study of arbon ompounds Organic Man-made Substances Plant or Animal
1. LiAlH4 :.. :.. 2. H3O +
 Ch 24 Amines Description of Amines - An amine is a compound with a nitrogen atom that has single bonds to carbon and hydrogen atoms. - An uncharged nitrogen atom normally has three bonds and a lone pair.
Ch 24 Amines Description of Amines - An amine is a compound with a nitrogen atom that has single bonds to carbon and hydrogen atoms. - An uncharged nitrogen atom normally has three bonds and a lone pair.
Organic Chemistry. FAMILIES of ORGANIC COMPOUNDS
 1 SCH4U September 2017 Organic Chemistry Is the chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 2- ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen,
1 SCH4U September 2017 Organic Chemistry Is the chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 2- ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen,
IUPAC Nomenclature Chem12A, Organic Chemistry I
 IUPAC Nomenclature ChemA, rganic Chemistry I IUPAC PEFIXES Prefix Substituent Group Number of Carbons meth- methyl eth- ethyl prop- propyl but- butyl pent- pentyl hex- hexyl hept- heptyl 7 oct- octyl 8
IUPAC Nomenclature ChemA, rganic Chemistry I IUPAC PEFIXES Prefix Substituent Group Number of Carbons meth- methyl eth- ethyl prop- propyl but- butyl pent- pentyl hex- hexyl hept- heptyl 7 oct- octyl 8
Short Summary of IUPAC Nomenclature of Organic Compounds
 Short Summary of IUPA Nomenclature of rganic ompounds Introduction The purpose of the IUPA system of nomenclature is to establish an international standard of naming compounds to facilitate communication.
Short Summary of IUPA Nomenclature of rganic ompounds Introduction The purpose of the IUPA system of nomenclature is to establish an international standard of naming compounds to facilitate communication.
Chapter 9. Organic Chemistry: The Infinite Variety of Carbon Compounds. Organic Chemistry
 Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
ORGANIC - BRUICE 8E CH.3 - AN INTRODUCTION TO ORGANIC COMPOUNDS
 !! www.clutchprep.com CONCEPT: INDEX OF HYDROGEN DEFICIENCY (STRUCTURAL) A saturated molecule is any molecule that has the maximum number of hydrogens possible for its chemical structure. The rule that
!! www.clutchprep.com CONCEPT: INDEX OF HYDROGEN DEFICIENCY (STRUCTURAL) A saturated molecule is any molecule that has the maximum number of hydrogens possible for its chemical structure. The rule that
Identification of functional groups in the unknown Will take in lab today
 Qualitative Analysis of Unknown Compounds 1. Infrared Spectroscopy Identification of functional groups in the unknown Will take in lab today 2. Elemental Analysis Determination of the Empirical Formula
Qualitative Analysis of Unknown Compounds 1. Infrared Spectroscopy Identification of functional groups in the unknown Will take in lab today 2. Elemental Analysis Determination of the Empirical Formula
Chapter 13. Alcohols, Diols, and Ethers
 hem 215 F07 otes Dr. Masato Koreeda - Page 1 of 5. Date: September 12, 2007 hapter 13. lcohols, Diols, and Ethers verview: hemistry and reactions of sp 3 oxygen groups, particularly oxidation of an alcohol,
hem 215 F07 otes Dr. Masato Koreeda - Page 1 of 5. Date: September 12, 2007 hapter 13. lcohols, Diols, and Ethers verview: hemistry and reactions of sp 3 oxygen groups, particularly oxidation of an alcohol,
12.01 Organic Chemistry
 12.01 rganic hemistry hemistry of arbon An Introduction to nomenclatures, structures and reactions Dr. Fred mega Garces hemistry 100 Miramar ollege 1 rganic hemistry What is rganic hemistry? rganic hemistry:
12.01 rganic hemistry hemistry of arbon An Introduction to nomenclatures, structures and reactions Dr. Fred mega Garces hemistry 100 Miramar ollege 1 rganic hemistry What is rganic hemistry? rganic hemistry:
Chapter 15 Benzene and Aromaticity
 Chapter 15 Benzene and Aromaticity Aromatic Compounds Aromatic Originally used to describe fragrant substances Refers to a class of compounds that meets Hückel criteria for aromaticity 2 Aromatic Compounds
Chapter 15 Benzene and Aromaticity Aromatic Compounds Aromatic Originally used to describe fragrant substances Refers to a class of compounds that meets Hückel criteria for aromaticity 2 Aromatic Compounds
Technical Report. Comprehensive Detection and Structural Elucidation of Synthetic Cathinones Using GC-MS/MS. Abstract:
 C146-E327 Technical Report Comprehensive Detection and Structural Elucidation of Synthetic Cathinones Using GC-MS/MS Yuki Sakamoto 1, aruhiko Miyagawa 1 Abstract: A rapid analytical method that allows
C146-E327 Technical Report Comprehensive Detection and Structural Elucidation of Synthetic Cathinones Using GC-MS/MS Yuki Sakamoto 1, aruhiko Miyagawa 1 Abstract: A rapid analytical method that allows
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS
 APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS You must exercise extreme caution when ope at the scene of an illegal drug lab. Do not walk into, touch, or move chemicals or spilled material. Avoid
APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS You must exercise extreme caution when ope at the scene of an illegal drug lab. Do not walk into, touch, or move chemicals or spilled material. Avoid
SORAN UNIVERSITY FACULTY OF EDUCATION BASIC EDUCATION SCHOOL DEPARTMENT OF GENERAL SCIENCES
 SORAN UNIVERSITY FACULTY OF EDUCATION BASIC EDUCATION SCHOOL DEPARTMENT OF GENERAL SCIENCES SUBJECT OUTLINE 2014-2015 Subject title: Practical organic Chemistry Credit hours: Units: (Practical ) (2)Units
SORAN UNIVERSITY FACULTY OF EDUCATION BASIC EDUCATION SCHOOL DEPARTMENT OF GENERAL SCIENCES SUBJECT OUTLINE 2014-2015 Subject title: Practical organic Chemistry Credit hours: Units: (Practical ) (2)Units
Aromatic Aldehydes (Benzaldehydes and Related Compounds)
 Aromatic Aldehydes (Benzaldehydes and Related Compounds) 1 Benzaldehyde, 3,5-diethynyl- A038A C 11 6 153390-76-2 A038B C 17 22 Si 2 Si Si 3,5-di C01A C 7 4 2 17352-25-9 Benzaldehyde, 3,5-bis[2- (trimethylsilyl)ethynyl]-
Aromatic Aldehydes (Benzaldehydes and Related Compounds) 1 Benzaldehyde, 3,5-diethynyl- A038A C 11 6 153390-76-2 A038B C 17 22 Si 2 Si Si 3,5-di C01A C 7 4 2 17352-25-9 Benzaldehyde, 3,5-bis[2- (trimethylsilyl)ethynyl]-
A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D. III > IV > I > II
 Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
The International Union of Pure and Applied Chemistry has developed a system of rules for naming organic molecules.
 HYDRCARBNS AND THEIR DERIVATIVES The field of organic chemistry includes the study of hydrocarbons (compounds composed of carbon and hydrogen atoms covalently bonded together) and their derivatives (variations
HYDRCARBNS AND THEIR DERIVATIVES The field of organic chemistry includes the study of hydrocarbons (compounds composed of carbon and hydrogen atoms covalently bonded together) and their derivatives (variations
Hydrocarbons and their Functional Groups
 Hydrocarbons and their Functional Groups Organic chemistry is the study of compounds in which carbon is the principal element. carbon atoms form four bonds long chains, rings, spheres, sheets, and tubes
Hydrocarbons and their Functional Groups Organic chemistry is the study of compounds in which carbon is the principal element. carbon atoms form four bonds long chains, rings, spheres, sheets, and tubes
 Question 13.1: Classify the following amines as primary, secondary or tertiary: (i) (ii) (iii) (C 2 H 5 ) 2 CHNH 2 (iv) (C 2 H 5 ) 2 NH Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii) Question 13.2:
Question 13.1: Classify the following amines as primary, secondary or tertiary: (i) (ii) (iii) (C 2 H 5 ) 2 CHNH 2 (iv) (C 2 H 5 ) 2 NH Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii) Question 13.2:
Naming Aromatic Compounds (Benzene as the Parent)
 aming Aromatic Compounds (Benzene as the Parent) If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes are named the same way as other hydrocarbons using benzene
aming Aromatic Compounds (Benzene as the Parent) If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes are named the same way as other hydrocarbons using benzene
9. PRECURSOR TRENDS AND MANUFACTURING METHODS
 9. PRECURSOR TREDS AD MAUFACTURIG METHODS Amphetamine-type-stimulants (ATS) comprise a number of substances under international control, primarily amphetamine, methamphetamine, ecstasy-group substances
9. PRECURSOR TREDS AD MAUFACTURIG METHODS Amphetamine-type-stimulants (ATS) comprise a number of substances under international control, primarily amphetamine, methamphetamine, ecstasy-group substances
