Study on Chemical Removal of Nitric Oxide (NO) as a Main Cause of Fine Dust (Air Pollution) and Acid Rain
|
|
|
- Shonda Small
- 6 years ago
- Views:
Transcription
1 Appl. Sci. Converg. Technol. 26(6): (2017) Research Paper Study on Chemical Removal of Nitric Oxide (NO) as a Main Cause of Fine Dust (Air Pollution) and Acid Rain Hyeon Jin Seo a, Rak Hyun Jeong b, Jang-Heon Boo c, Jimin Song c, and Jin-Hyo Boo a,b * a Department of Chemistry, Sungkyunkwan University, Suwon 16419, Korea b Institute of Basic Science, Sungkyunkwan University, Suwon 16419, Korea c Songnae High School, Bucheon 14741, Korea Received September 20, 2017; revised October 18, 2017; accepted November 21, 2017 Abstract This study was conducted to remove NO x, which is the main cause of fine dust and air pollution as well as acid rain. NO x was tested using 3% NO (diluted in He) as a simulated gas. Experiments were sequentially carried out by oxidizing NO to NO 2 and absorbing NO 2. Especially, we focused on the changes of NO oxidation according to both oxidant (NaClO 2 ) concentration change (1~10 M) and oxidant ph change (ph = 1~5) by adding HCl. In addition, we tried to suggest a method to improve NO 2 absorption by conducting NO 2 reduction reaction with reducing agent (NaOH) concentration (40~60%). It was found that NO removal efficiency increased as both concentration of oxidant and flow rate of NO gas increased, and NO decreased more effectively as the ph of hydrochloric acid added to the oxidant was lower. The NO 2 adsorption was also better with increasing NaOH concentration, but the NO removal efficiency was ~20% lower than that of the selective NO reduction. Indeed, this experimental method is expected to be a new method that can be applied to the capture and removal of fine dust caused by air pollution because it is a method that can easily remove NO gas by a simple device without expensive giant equipment. Keywords: NO oxidation, NO 2 absorption, Fine dust, Air pollution, Acid rain I. Introduction Fine dust that is striking our country every spring is often from China, but most of them come from cars using diesel engines [1]. If we do not solve this problem, Korea s fine dust problem will not be solved forever. However, people have not found a solution to the problem other than reducing diesel-powered vehicles, and many still do not see the problem of solving the fine dust problem. Particulate Matter (PM) or dust is an air pollutant containing a large number of air pollutants together with sulfur dioxide, nitrogen oxides, lead, ozone, carbon monoxide, etc. It occurs in automobiles, factories, etc., and it is referred to as PM10. When the particle is 2.5 μm or less, it is referred to as PM 2.5 and called as ultrafine dust [1]. Air pollutants from fossil fuels such as petroleum and coal or from exhaust fumes from automobile soot are known to be the main cause of fine dusts, but methods for completely removing ultrafine dusts have not been developed yet [1,2]. In addition, recently, regulations for pollutant emission at sea have been strengthened, and accordingly, there is a growing interest in pollutant control technology [3,4]. Acid rain is defined as the accumulation of atmospheric acidic *Corresponding author jhboo@skku.edu substances in wet or dry conditions in the ecosystem. Acid rain refers to the ratio of chemicals in the air that combine with the rain to become acidified [1,3]. When the natural rain is in equilibrium with carbon dioxide in the atmosphere, the ph is lower than 5.6. Today, much more acidic rainfall is being observed all over the world. Acid rain is mainly caused by human release of sulfur and nitrogen mixtures that produce acidic reactivity in the atmosphere [5]. Sulfur dioxide and nitric oxide are air pollutants, which combine with the water vapor of the atmosphere to form acidic substances and acid rain. The acidic material is moved by the wind and becomes acid rain. The acid rain falls to a distance of about 4,023 km from where the pollutants are generated [6]. Acid rain made by humans damages lakes, forests, wildlife, and acidifies lakes to kill fish and other aquatic life. As the producer of the food chain is destroyed, the number of birds is reduced. It is because birds' food, insects, plants and aquatic life are killed by acid rain [7]. Not all areas where acid rain comes down have the same results. That is, the potential of the area capable of neutralizing acidic substances determines the amount of damage. Alkaline soil neutralizes acid. Therefore, areas with high alkaline soil are less damaging than those with neutral or acid soil. Several temporary measures have been taken to solve the acid rain problem, such as attempting to neutralize the acid by 218
2 219 Hyeon Jin Seo, Rak Hyun Jeong, Jang-Heon Boo, Jimin Song, and Jin-Hyo Boo adding lime to the contaminated lake, but they have not had much effect. The solution to the acid rain problem is to remove pollutants that cause acid rain. We must reduce the use of fossil fuels such as coal, especially those containing a lot of sulfur. In order to protect forests, it is necessary to reduce the amount of automobile exhaust gas, use alternative energy, and use a lot of public transportation. This process can solve the acid rain problem [8]. The International Maritime Organization (IMO), under the United Nations (UN), announced that from 2016, the emission regulation at sea would be strengthened from the current Tier II to Tier III [9]. Accordingly, research and development on the removal of sulfur oxides (SO x ) and nitrogen oxides (NO x ) contained in gas discharged from vessels have been actively carried out, but there are still many problems to be solved. In addition, various researches to prevent the acidification of the soil due to acid rain and to create a more pleasant and affluent life (well-being culture) by decreasing the atmospheric concentration of sulfur oxides (SO x ) and nitrogen oxides (NO x ) [10]. Therefore, through this study, we tried to suggest a new method to solve the ultrafine dust problem and contribute to solving the acid rain problem. Especially, for smooth NO x treatment, (1) first remove NO by oxidizing it to NO 2, and (2) finally remove NO 2 by absorption reaction using sodium hydroxide solution, which dissolves in alkaline solution, were suggested in this study II. Experimental Background and Procedure 1. NO x generation and reduction principle It is known that various compounds exist in the nitrogen oxides depending on the bonding state of nitrogen and oxygen. Most of what is generated by combustion of fuel is NO and NO 2, and is generally referred to as NO x (hereinafter referred to as NO x ). The ratio of NO/NO x in the combustion gas discharged from the diesel engine combustion equipment is about 90 to 95%, and the NO x in the exhaust gas is mostly NO [11]. NO is a colorless, odorless gas and hardly soluble in water. NO 2 is produced by oxidation of NO in the atmosphere, and it is converted to nitric acid (HNO 3 ) by binding with moisture, which is a main cause of acid rain. NO x can be odorous even in the presence of 1 to 3 ppm, and it inhibits oxygen delivery by diminished immune responses by respiratory diseases and methemoglobin formation by reaction with blood hemoglobin. NO 2 is more soluble than NO, and when it is high, it is a reddish-brown irritant gas, which is five times more toxic than NO. Acute damage causes eye, nasal irritation and pulmonary hyperemia, pulmonary edema, obstructive bronchitis and pneumonia. NO x generated in the combustion process of the fuel is generated by the following two routes. (1) Thermal NO x : Nitrogen and oxygen in combustion air react at high temperature to become NO x. In this case, the higher the combustion temperature, the higher the O 2 in the combustibility, and the longer the residence time of the combustion gas in the higher temperature region, the more NO x is generated [12]. (2) Fuel NO x : When a portion of the nitrogen oxides contained in the fuel burns, it is oxidized to become NO x, and there is almost no nitrogen (N) compound in the gaseous fuel [11,13]. Therefore, the following basic principle is applied to suppress the generation of NO x. 1 Lower the oxygen concentration in the combustion zone. 2 Shorten the residence time of the combustion gas in the high temperature region. 3 Lower combustion temperature. Especially, local high temperature area should not be generated. 4 Using fuel containing less nitrogen 2. Traditional processes of NO x reduction The most commonly used methods for treating nitrogen oxides are Selective Non-Catalytic Reduction (SNCR) and Selective Catalytic Reduction (SCR). (1) Selective non-catalytic reduction (SNCR) [14-16] The selective non-catalytic reduction method is a method in which a reducing agent containing nitrogen such as ammonia (NH 3 ) and urea (NH 2 CONH 2 ) is injected at a temperature of about 870 to 1150 o C, and the reduced reducing agent is ionized to react with nitrogen oxides. The ammonia and urea are separated according to the temperature range of the point where the reducing agent is injected by the method of decomposing into N 2, CO 2 and H 2 O. When ammonia is injected, the reaction occurs at a lower temperature than when the urea is injected. Therefore, when the temperature of the injection point is low, ammonia is used. When this method is used, the removal efficiency of nitrogen oxide is about 50~70% (2) Selective Catalytic Reduction (SCR) [17,18] In the selective catalytic reduction method, exhaust gas and a reducing agent are simultaneously brought into contact with a catalyst layer at a temperature of 300 to 400 o C, thereby, reducing NO x in exhaust gas to nitrogen (N 2 ) and water vapor (H 2 O) by selective reaction with a reducing agent (NH 3 ). The reducing agent used is ammonia water (mainly 25% ammonia solution), urea (50% urea solution), etc., injected into the front end of the SCR catalyst and injected into the exhaust gas. The reduction of NO x in the SCR reactor is achieved by the following reaction. 4NO + 4NH 3 +O 2 4N 2 +6H 2 O (1) 4NH 3 +6NO 5N 2 +6H 2 O (2) Since most of the exhaust gas contains O 2 component, reaction (1) occurs, so NO and NH 3 react with each other
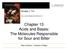
3 Study on Chemical Removal of Nitric Oxide (NO) as a Main Cause of Fine Dust (Air Pollution) and Acid Rain 220 at a ratio of 1:1, and NH 3 is required as much as the NO reduction amount. In the exhaust gas, about 5% of the total NO x is the NO 2 component, and the reaction formula is as follows. 2NO 2 +4NH 3 +O 2 3N 2 +6H 2 O (3) 8NH 3 +6NO 2 7N 2 +6H 2 O (4) According to reaction (3), NH 3 is required twice as much as NO for NO 2 reduction, but the NO 2 content in the exhaust gas is as small as about 5%. 3. Our suggested processes of NO x reduction 3.1 NO oxidation As mentioned above, the NO x in the exhaust gas emitted from the engine is largely divided into nitrogen oxide (NO) and nitrogen dioxide (NO 2 ). Of these, NO emissions account for the majority of NO x emissions. Accordingly, for smooth NO x treatment, NO is oxidized to NO 2 and removed. The oxidizing agent used here is sodium chlorite (NaClO 2 ). The chemical mechanism of the oxidation reaction using NaClO 2 is shown in the following reaction (5). NO + 2NaClO 2FNO 2 + NaClO 3 +NaCl (5) The formation of chlorine dioxide (ClO 2 ) gas is very important for the reaction of the above reaction formula (5) to be activated. It is known that NaClO 2 generates ClO 2 gas in the acidic range below ph 5 [19]. In the acidic region of NaClO 2, the reaction of producing ClO 2 gas is as shown in the following reaction formula (6). 5NaClO 2 +4HClF 4ClO 2 +5NaCl+2H 2 (6) 3.2 NO 2 absorption reaction The NO 2 generated through the oxidation reaction is absorbed into the cleaning liquid to be removed. NO 2 is known to dissolve well in alkaline solution [20]. The mechanism of absorption reaction with sodium hydroxide (NaOH), which is a representative alkali solution, is shown in the following reaction formula (7). NO + NO 2 + 2NaOHF 2NaNO 2 +H 2 O (7) When NO is oxidized to NO 2, a reaction mechanism similar to that of reaction (7) is shown. At this time, nitric acid (HNO 3 ) is produced as a reaction product (see reaction 8). Therefore, in this experiment, the exact removal rate of NO gas was obtained by measuring ph and volume change of aqueous NaOH solution and titrating with standard acid solution (HCl) according to the formation of HNO 3, resulting in determination of both HNO 3 and NO 2 concentrations and then obtaining NO 2 /NO conversion ratio. Figure 1. (a) Schematic diagram of NO oxidation and NO 2 absorption system used in this work, and (b) images of the actual home-made system. 2NO 2 +NaOHF NaNO 2 + HNO 3 (8) 4. Experimental set-up and materials The NO x used in this experiment was simulated using 3% NO+97% He gas mixture in consideration of the health of the experimenter and the laboratory environment. Sodium chlorite (NaClO 2 ), which is an oxidizing agent for NO oxidation, was used by diluting the powder with water to a concentration, and further added hydrochloric acid (HCl) was dissolved appropriately in accordance with ph. For adsorption reaction of NO 2, sodium hydroxide (NaOH) was diluted with 40~60% aqueous solution. The supply of the simulated gas was maintained by MFC (Mass Flow Controller) and the range of NO gas flow rates were kept from 5 to 100 sccm. Figure 1(a) shows the schematic diagram of experimental setup used in this experiment. Figure 1(b) shows the actual home-made system. 3% NO+ 97% He gas mixture container, MFC & controller, NO oxidation chamber and NO 2 adsorption chamber can be seen from left side respectively. 5. Experimental method Experiments were carried out by oxidizing NO to NO 2 and absorbing NO 2 as shown in Section 2.3., respectively. The aim of this study is to present the optimal NO x treatment method by examining the change of NO oxidation reaction depending on oxidant concentration (1~10 M) change and oxidant ph (ph = 1~5) change, which could control by adding HCl into NaClO 2. In addition, we tried to suggest a method to improve NO 2 absorption by conducting NO 2 reduction reaction with reducing agent (NaOH) concentration (40~60%). For second step NO 2 reduction reaction, NO gas was also supplied (see reaction 3). Appl. Sci. Converg. Technol. Vol. 26, No. 6 November 2017
4 221 Hyeon Jin Seo, Rak Hyun Jeong, Jang-Heon Boo, Jimin Song, and Jin-Hyo Boo III. Results and Discussion Figure 2(a) shows the changes of NO 2 /NO conversion ratio according to the concentration of oxidant (NaClO 2 ). As the concentration of NaClO 2 increases under 100 sccm of the NO gas flow rate, NO is more oxidized to NO 2. It is shown that NO oxidation can occur only when NaClO 2 concentration is more than 1 M. Figure 2(b) shows that under 10 M of the NaClO 2 concentration, the NO oxidation reaction occurs linearly with increasing NO gas flow rate, and the NO oxidation reaction can occur only when the NO gas flow rate is at least 5 sccm. Therefore, figures 2 (a) and (b) show that NO 2 /NO conversion ratio of 20% can be obtained when the NaClO 2 concentration is 10 M and the NO gas flow rate is 100 sccm, respectively. However, this is much less efficient than selective non-catalytic reduction (50~60%). Since the wet reaction depends on various parameters (type of oxidizer and reducing agent, concentration, ph, reaction temperature, absorption method and titration method), therefore, we think that the removal efficiency can be greatly improved by adjusting the above variables well. This means that it is still necessary to develop a new concept adsorbent capable of adsorbing / removing the ultrafine dust which does not have the technology to completely remove the ultrafine dust. As shown in Section 2.3, the formation of chlorine dioxide (ClO 2 ) gas, which can generate in the acidic range below ph 5 and control by adding HCl into NaClO 2, is very important for the reaction of NO oxidation. Figure 3 shows that NO is effectively oxidized to NO 2 as the ph of the oxidizing agent decreases with hydrochloric acid added to the oxidizing agent (NaClO 2 ). Also, when the ph is above 5, the NO oxidation reaction hardly occurs. This means that the lower the ph of the oxidant under the optimal conditions (i.e. NaClO 2 concentration: 10 M and NO gas flow rate: 100 sccm) as shown in figure 2, the more ClO 2 is generated and the NO oxidation reaction is further promoted. Figure 4(a) shows that the higher the NaOH concentration, the more the NO 2 absorption (reduction) reaction occurs, and figure 4(b) shows that the ph of NaOH, the NO 2 absorbent, continues to decrease as the NaOH concentration increases. This means that the ph of the NaOH is reduced because the nitric acid (HNO 3 ) resulting from the NO 2 reduction reaction (see reaction (8) in Section 2.3) causes the neutralization reaction with NaOH to produce water continuously. Therefore, it is possible to determine the concentration of HNO 3 produced as a result of the NO 2 absorption reaction if the exact concentration is obtained by titrating with the standard acid solution (HCl) after accurately measuring the volume of the absorbent (NaOH) remaining after the reaction, and further, NO 2 /NO conversion ratio was obtained. Based on these results, it was found that the removal efficiency of NO gas was up to 20% in this study. Figure 5(a), similar to figure 4(b), shows that the ph of the absorbent NaOH decreases gradually as the concentration of oxidizing agent (NaClO 2 ) increases. Figure 5(b) also shows that the concentration of nitric acid (HNO 3 ) increases continuously as the concentration increases. This means that the NO removal efficiency is rather low, but it is a method that can easily remove NO gas with a relatively Figure 2. (a) The change of NO 2 /NO conversion ratio according to the concentration of oxidizing agent, and (b) the change of NO 2 /NO conversion ratio according to the NO gas flow rate at 10 M of oxidizing agent. Figure 4. Changes of (a) NO 2 reduction ratio and (b) ph of NaOH with the NaOH concentrations. Figure 3. NO 2 /NO conversion ratio vs. ph change. Figure 5. Changes of (a) ph of NaOH and (b) concentration of formed HNO 3 according to the concentrations of oxidizing agent.
5 Study on Chemical Removal of Nitric Oxide (NO) as a Main Cause of Fine Dust (Air Pollution) and Acid Rain 222 simple device without existing large-scale expensive equipment. Therefore, our newly developed method can be applied to the capture and removal of ultrafine dust caused by air pollution in the future. IV. Conclusions It was found that NO removal efficiency increased as the concentration of oxidant increased (as the flow rate of NO gas increased by more than 100 sccm), and NO decreased more effectively as the ph of hydrochloric acid added to the oxidant was lower. The NO 2 uptake was better with increasing NaOH concentration, but the molar concentration (M) was more important than the NaOH concentration of 40% or more. It was found that the NO removal efficiency was ~20% lower than that of the selective NO reduction, suggesting that if we could precisely control the variable parameters of influencing the NO removal efficiency, more higher NO removal efficiency (possibly over 40%) than now would get. Indeed, this experimental method is expected to be a new method that can be applied to the capture and removal of ultrafine dust caused by air pollution because it is a method that can easily remove NO gas by a simple device without expensive giant equipment. Acknowledgements This work was partially supported by the CBDRC of the Sungkyunkwan University References [1] D. Kang and J.-E. Kim, J. Korean Med. Sci. 29, 621 (2014). [2] H. Lee, J. Kim, C.-L. Myung, and S. Park, J. of Mechanical Science and Technology 23, 1591 (2009). [3] A. G. Nord and K. Tronner, Water, Air and Soil Pollution 85, 2719 (1995). [4] D. Harikishore, K. Reddy, and S.M. Lee, J. Environ. Anal. Toxicol. 2, 1 (2012). [5] L. Bityukova, Water, Air, and Soil Pollution 172, 239 (2006). [6] D.-S. Kim, J. Jeong, and J. Ahn, J. Korean Soc. Atmos. Environ. 32, 422 (2016). [7] Sunita Bhargava and Sharad Bhargava, J. of Applied Chemistry 5, 19 (2013). [8] İ. A. Reşitoğlu, K. Altinisik, and A. Keskin, Clean Technol. Environ. Policy 17, 15 (2015). [9] UNCTAD, Review of Maritime Transport 2016, United Nations Publications (2016) pp [10] A. Singh and M. Agrawal, J. of Environmental Biology 29, 15 (2008). [11] D. Shimokuri, S. Fukuba, and S. Ishizuka, Proceedings of the Combustion Institute 35, 3573 (2015). [12] J. Odgers and D. Kretschmer, The American Society of Mechanical Engineers 2, 1 (1985). [13] D.W. Pershing and J. O. L. Wendt, Symposium (International) on Combustion 16, 389 (1977). [14] S. Mahmoudi, J. Baeyesns and J. P.K. Seville, Biomass and Bioenergy 34, 1393 (2010). [15] S. W. Bae, S. A. Roh, and S. D. Kim, Chemosphere 65, 170 (2006). [16] X. Han, X. Wei, U. Schnell, and K. R.G. Hein, Combustion and Flame 132, 374 (2003). [17] M. F. Irfan, J. H. Goo, and S. D. Kim, Applied Catalysis B: Environmental 78, 267 (2008). [18] N.W. Cant and A. D. Cowan, Catalysis Letters 46, 207 (1997). [19] H. W. Hsu, C. J. Lee, and K. S. Chou, Chem. Eng. Comm. 170, 67 (1997). [20] C. Baukal, Metal Finishing 103, 18 (2005). Appl. Sci. Converg. Technol. Vol. 26, No. 6 November 2017
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Reference pg and in Textbook
 Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
NATS 101 Section 13: Lecture 31. Air Pollution Part II
 NATS 101 Section 13: Lecture 31 Air Pollution Part II Last time we talked mainly about two types of smog:. 1. London-type smog 2. L.A.-type smog or photochemical smog What are the necessary ingredients
NATS 101 Section 13: Lecture 31 Air Pollution Part II Last time we talked mainly about two types of smog:. 1. London-type smog 2. L.A.-type smog or photochemical smog What are the necessary ingredients
MARIYA INTERNATIONAL SCHOOL. Work sheet III. Term I. Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR
![MARIYA INTERNATIONAL SCHOOL. Work sheet III. Term I. Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR MARIYA INTERNATIONAL SCHOOL. Work sheet III. Term I. Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR](/thumbs/95/123248955.jpg) MARIYA INTERNATIONAL SCHOOL Work sheet III Term I Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR 1. A steel works and a chemical works are built near to a city. The limestone buildings in the
MARIYA INTERNATIONAL SCHOOL Work sheet III Term I Level 8 Chemistry [MCQ] Name: CHEMICAL REACTIONS & SULFUR 1. A steel works and a chemical works are built near to a city. The limestone buildings in the
CHAPTER 3 WATER AND THE FITNESS OF THE ENVIRONMENT. Section B: The Dissociation of Water Molecules
 CHAPTER 3 WATER AND THE FITNESS OF THE ENVIRONMENT Section B: The Dissociation of Water Molecules 1. Organisms are sensitive to changes in ph 2. Acid precipitation threatens the fitness of the environment
CHAPTER 3 WATER AND THE FITNESS OF THE ENVIRONMENT Section B: The Dissociation of Water Molecules 1. Organisms are sensitive to changes in ph 2. Acid precipitation threatens the fitness of the environment
Acids and Bases 2 Science Notes JC-Learn. JC-Learn. Science Notes Acids and Bases 2. 1 P a g e
 JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
Unit 1: Chemistry in Action
 Unit 1: Chemistry in Action Intermediate 1 Chemistry Learning Outcomes Substances Elements Everything in the world is made from about 100 elements. Each element has a name and a symbol. Chemists have arranged
Unit 1: Chemistry in Action Intermediate 1 Chemistry Learning Outcomes Substances Elements Everything in the world is made from about 100 elements. Each element has a name and a symbol. Chemists have arranged
8.8 - Gases. These are assumptions that can be made about 99% of the gases we come in contact with which are called ideal gases.
 Gases The substance that we come in contact with every second of every day is in fact the substance we never think about. This substance is a mixture of gases known as the atmosphere. Gases have the weakest
Gases The substance that we come in contact with every second of every day is in fact the substance we never think about. This substance is a mixture of gases known as the atmosphere. Gases have the weakest
CHAPTER 13: Nitrogen and Sulfur
 CHAPTER 13: Nitrogen and Sulfur 13.1 Nitrogen Compounds 13.2 Environmental Consequences of Using Nitrogen Compounds 13.3 Sulfur Compounds Learning outcomes: (a) explain the lack of reactivity of nitrogen.
CHAPTER 13: Nitrogen and Sulfur 13.1 Nitrogen Compounds 13.2 Environmental Consequences of Using Nitrogen Compounds 13.3 Sulfur Compounds Learning outcomes: (a) explain the lack of reactivity of nitrogen.
1 (a) Describe a chemical test which shows the presence of water. Describe how water is treated before it is supplied to homes and industry.
 1 (a) Describe a chemical test which shows the presence of water. test... colour change if water is present...... [3] (b) How could you show that a sample of water is pure?...[1] (c) Describe how water
1 (a) Describe a chemical test which shows the presence of water. test... colour change if water is present...... [3] (b) How could you show that a sample of water is pure?...[1] (c) Describe how water
ph and poh * OpenStax
 OpenStax-CNX module: m51117 1 ph and poh * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able to:
OpenStax-CNX module: m51117 1 ph and poh * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able to:
Acid-Base Theory. In this lecture the theory of acids and bases will be present along with web sites which discuss acids and bases
 Acid-Base Theory In this lecture the theory of acids and bases will be present along with web sites which discuss acids and bases Lecture Outline: these questions should be answered in this lecture. What
Acid-Base Theory In this lecture the theory of acids and bases will be present along with web sites which discuss acids and bases Lecture Outline: these questions should be answered in this lecture. What
IMADUDDIN SCHOOL Second Term Examination 2017
 Index Register Class number number Name IMADUDDIN SCHOOL Second Term Examination 2017 GRADE 9 CHEMISTRY 5070/02 Paper 2 Theory Nov 2017 TIME 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Write your name,
Index Register Class number number Name IMADUDDIN SCHOOL Second Term Examination 2017 GRADE 9 CHEMISTRY 5070/02 Paper 2 Theory Nov 2017 TIME 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Write your name,
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
CE 370. Disinfection. Location in the Treatment Plant. After the water has been filtered, it is disinfected. Disinfection follows filtration.
 CE 70 Disinfection 1 Location in the Treatment Plant After the water has been filtered, it is disinfected. Disinfection follows filtration. 1 Overview of the Process The purpose of disinfecting drinking
CE 70 Disinfection 1 Location in the Treatment Plant After the water has been filtered, it is disinfected. Disinfection follows filtration. 1 Overview of the Process The purpose of disinfecting drinking
Topic 5 National Chemistry Summary Notes. Acids and Alkalis
 Topic 5 National Chemistry Summary Notes Acids and Alkalis Experiment Collect some samples of rain water LI 1 The ph Scale The ph scale is a continuous range of numbers from below 0 to above 14. Acids
Topic 5 National Chemistry Summary Notes Acids and Alkalis Experiment Collect some samples of rain water LI 1 The ph Scale The ph scale is a continuous range of numbers from below 0 to above 14. Acids
Reactions with Acids. Chemistry 10 Mrs. Page
 Reactions with Acids Chemistry 10 Mrs. Page Learning Objectives Predict the products and balance chemical equations for the reactions with acids involving; reactive metals, metal oxides, metal hydroxides,
Reactions with Acids Chemistry 10 Mrs. Page Learning Objectives Predict the products and balance chemical equations for the reactions with acids involving; reactive metals, metal oxides, metal hydroxides,
In terms of production, nitric acid is the third most widely produced acid across the world.
 In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
Chapter Chemical Elements Matter solid, liquid, and gas elements atoms. atomic symbol protons, neutrons, electrons. atomic mass atomic number
 Chapter 2 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. A. Elements 1. All matter (both living and non-living)
Chapter 2 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. A. Elements 1. All matter (both living and non-living)
Experimental Study on NO Removal Using Non-thermal Plasma Oxidation-alkali Absorption
 Experimental Study on NO Removal Using Non-thermal Plasma Oxidation-alkali Absorption Shuilan Ding 1, Qi Yu, Yingzhou Zhang 1, Yuan Liu 1, Chunxue Xie 1, Gang Yu*, 1, 3 1 School of Environment Engineering,
Experimental Study on NO Removal Using Non-thermal Plasma Oxidation-alkali Absorption Shuilan Ding 1, Qi Yu, Yingzhou Zhang 1, Yuan Liu 1, Chunxue Xie 1, Gang Yu*, 1, 3 1 School of Environment Engineering,
UNIT 10 COMMON ACIDS AND ALKALIS
 ABLE EDUCATION CENTRE UNIT 10 COMMON ACIDS AND ALKALIS A Multiple-choice questions 1 Which of the following are the properties of alkalis? (1) They taste sour. (2) They are slippery. (3) They can turn
ABLE EDUCATION CENTRE UNIT 10 COMMON ACIDS AND ALKALIS A Multiple-choice questions 1 Which of the following are the properties of alkalis? (1) They taste sour. (2) They are slippery. (3) They can turn
Which fertiliser would improve the quality of this soil most effectively?
 1 farmer s soil is very low in both nitrogen (N) and phosphorus (P). Which fertiliser would improve the quality of this soil most effectively? 2 Which compound is not used as a fertiliser? ammonium sulfate
1 farmer s soil is very low in both nitrogen (N) and phosphorus (P). Which fertiliser would improve the quality of this soil most effectively? 2 Which compound is not used as a fertiliser? ammonium sulfate
5 Energy from chemicals
 5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
National 4 Unit Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes. Homework
 National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
1. Base your answer to the following question on information below and on your knowledge of chemistry.
 1. Base your answer to the following question on information below and on your knowledge of A sample of nitric acid contains both ions and ions. This sample has a ph value of 1. Write a name of the positive
1. Base your answer to the following question on information below and on your knowledge of A sample of nitric acid contains both ions and ions. This sample has a ph value of 1. Write a name of the positive
Unit 4: ACIDS, BASES AND SALTS
 ABS - 1 Unit 4: ACIDS, BASES AND SALTS 4.1 Arrhenius Acids and Bases Acids release H + in water Bases release OH - in water Salts are products of an acid-base neutralization reaction. The salt is an ionic
ABS - 1 Unit 4: ACIDS, BASES AND SALTS 4.1 Arrhenius Acids and Bases Acids release H + in water Bases release OH - in water Salts are products of an acid-base neutralization reaction. The salt is an ionic
4.19 Buffer Solutions
 4.19 Buffer Solutions Buffer solution: p.319. (i.e. it minimizes the change in ph when A or B added) Buffers are made by: high conc of a weak acid + equal conc of its conj base Add base in salt form eg)
4.19 Buffer Solutions Buffer solution: p.319. (i.e. it minimizes the change in ph when A or B added) Buffers are made by: high conc of a weak acid + equal conc of its conj base Add base in salt form eg)
AQA A2 CHEMISTRY TOPIC 5.4 TRANSITION METALS PART 2 REDOX REACTIONS AND CATALYSIS BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 5.4 TRANSITION METALS PART 2 REDOX REACTIONS AND CATALYSIS BOOKLET OF PAST EXAMINATION QUESTIONS 1. Chemical reactions can be affected by homogeneous or by heterogeneous catalysts.
AQA A2 CHEMISTRY TOPIC 5.4 TRANSITION METALS PART 2 REDOX REACTIONS AND CATALYSIS BOOKLET OF PAST EXAMINATION QUESTIONS 1. Chemical reactions can be affected by homogeneous or by heterogeneous catalysts.
NITROGEN AND ITS COMPOUNDS Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure.
 NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
Same theme covered in Combined but extra content Extra parts atomic symbols (first 20, Group 1 and Group 7)
 Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Bases = Anti-Acids. The process is called neutralization (neither acidic nor basic) O H 3 2H 2
 Bases = Anti-Acids Example: HCl(aq) + H 2 (l) à H 3 + (aq) + Cl - (aq) NaH(aq) à Na + (aq) + H - (aq) H 3 + (aq) + H - (aq) à 2H 2 (l) Net: HCl(aq) + NaH(aq) à Na + (aq) + Cl - (aq) + H 2 (l) The process
Bases = Anti-Acids Example: HCl(aq) + H 2 (l) à H 3 + (aq) + Cl - (aq) NaH(aq) à Na + (aq) + H - (aq) H 3 + (aq) + H - (aq) à 2H 2 (l) Net: HCl(aq) + NaH(aq) à Na + (aq) + Cl - (aq) + H 2 (l) The process
CHEMICAL REACTIONS & EQUATIONS
 CHEMICAL REACTIONS & EQUATIONS PHYSICAL AND CHEMICAL CHANGE In our daily life many processes occur around us. Some of them do not lead to formation of any new substance, while others may lead to formation
CHEMICAL REACTIONS & EQUATIONS PHYSICAL AND CHEMICAL CHANGE In our daily life many processes occur around us. Some of them do not lead to formation of any new substance, while others may lead to formation
Types of Chemical Reactions
 Types of Chemical Reactions Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Types of Chemical Reactions Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Q1. The chart shows the processes involved in the manufacture of nitric acid from ammonia.
 Q1. The chart shows the processes involved in the manufacture of nitric acid from ammonia. (a) Complete the word equation for the reaction that takes place in the first reaction vessel. ammonia +... nitrogen
Q1. The chart shows the processes involved in the manufacture of nitric acid from ammonia. (a) Complete the word equation for the reaction that takes place in the first reaction vessel. ammonia +... nitrogen
Moles and Stoichiometry 2016
 Name: Moles and Stoichiometry 2016 1. What is the empirical formula of a compound with the molecular formula N2O4? A) NO B) NO2 C) N2O D) N2O3 2. What is the total number of atoms contained in 1 mole of
Name: Moles and Stoichiometry 2016 1. What is the empirical formula of a compound with the molecular formula N2O4? A) NO B) NO2 C) N2O D) N2O3 2. What is the total number of atoms contained in 1 mole of
0620 CHEMISTRY. Mark schemes should be read in conjunction with the question paper and the Principal Examiner Report for Teachers.
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/32 Paper 3 (Extended Theory), maximum raw mark
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/32 Paper 3 (Extended Theory), maximum raw mark
Measurements of Ozone. Why is Ozone Important?
 Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
AS Level Chemistry B (Salters) H033/01 Foundations of chemistry Date Morning/Afternoon You must have: You may use: First name Last name
 AS Level Chemistry B (Salters) H033/01 Foundations of chemistry Sample Question Paper Date Morning/Afternoon Time allowed: 1 hour 30 minutes You must have: the Data Sheet for Chemistry B (Salters) You
AS Level Chemistry B (Salters) H033/01 Foundations of chemistry Sample Question Paper Date Morning/Afternoon Time allowed: 1 hour 30 minutes You must have: the Data Sheet for Chemistry B (Salters) You
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Advanced Chemistry Final Review
 Advanced Chemistry Final Review 1. What are the products of complete combustion of hydrocarbons? Hydrocarbons are compounds made of carbon and oxygen. When they burn (combine with oxygen) they form carbon
Advanced Chemistry Final Review 1. What are the products of complete combustion of hydrocarbons? Hydrocarbons are compounds made of carbon and oxygen. When they burn (combine with oxygen) they form carbon
Water, the SPECIAL Equilibrium
 THE ACID TEST Water, the SPECIAL Equilibrium I. Characteristics of Water A. Water are highly. B. They are in continuous. C. Always. D. Water is dense in the solid phase than in the phase. i.e. ice floats
THE ACID TEST Water, the SPECIAL Equilibrium I. Characteristics of Water A. Water are highly. B. They are in continuous. C. Always. D. Water is dense in the solid phase than in the phase. i.e. ice floats
Combustion Generated Pollutants
 Combustion Generated Pollutants New Delhi Peking Climate change Combustion Generated Pollutants Greenhouse gases: CO 2, methane, N 2 O, CFCs, particulates, etc. Hydrocarbons: Toxins and a major contributor
Combustion Generated Pollutants New Delhi Peking Climate change Combustion Generated Pollutants Greenhouse gases: CO 2, methane, N 2 O, CFCs, particulates, etc. Hydrocarbons: Toxins and a major contributor
Chapter 5 Notes Science 10 Name:
 5.1 Acids and Bases Many familiar compounds are acids or bases. o Classification as acids or bases is based on chemical composition. Acids and bases can be very dangerous. o Both can be very. o NEVER try
5.1 Acids and Bases Many familiar compounds are acids or bases. o Classification as acids or bases is based on chemical composition. Acids and bases can be very dangerous. o Both can be very. o NEVER try
3. Chemical industry. Because of their modular design, the instruments in the TOC-L series can be equipped for any possible measurement
 3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
S4 CHEMISTRY SUMMARY NOTES
 S4 CHEMISTRY SUMMARY NOTES 1. The Mole One mole of a substance = GRAM FORMULA MASS e.g. H 2 SO 4 RAM from databook pg.7 2H 2 x 1 = 2 1S 1 x 32 = 32 4O 4 x 16 = 64 98g Mass = number of moles x Mass of 1
S4 CHEMISTRY SUMMARY NOTES 1. The Mole One mole of a substance = GRAM FORMULA MASS e.g. H 2 SO 4 RAM from databook pg.7 2H 2 x 1 = 2 1S 1 x 32 = 32 4O 4 x 16 = 64 98g Mass = number of moles x Mass of 1
Chemistry. Student Number. Mark / 64. Final Examination Preliminary Course General Instructions. Total Marks 64
 Student Number Mark / 64 Chemistry Final Examination Preliminary Course 2003 General Instructions Reading time 5 minutes Working time 120 minutes Write using black or blue pen Draw diagrams using pencil
Student Number Mark / 64 Chemistry Final Examination Preliminary Course 2003 General Instructions Reading time 5 minutes Working time 120 minutes Write using black or blue pen Draw diagrams using pencil
Chem 150, Spring Unit 4 - Acids & Bases. Introduction
 Chem 150, Spring 2015 Unit 4 - Acids & Bases Introduction Patients with emphysema cannot expel CO2 from their lungs rapidly enough. This can lead to an increase of carbonic (H2CO3) levels in the blood
Chem 150, Spring 2015 Unit 4 - Acids & Bases Introduction Patients with emphysema cannot expel CO2 from their lungs rapidly enough. This can lead to an increase of carbonic (H2CO3) levels in the blood
1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time
 Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Unit 5 Lesson 3 Measuring ph. Copyright Houghton Mifflin Harcourt Publishing Company
 What s Your Number? What is the ph scale? The ph of a solution is a measure of how acidic or basic a solution is. A solution that has a ph value of exactly 7 is neutral neither acidic nor basic. A solution
What s Your Number? What is the ph scale? The ph of a solution is a measure of how acidic or basic a solution is. A solution that has a ph value of exactly 7 is neutral neither acidic nor basic. A solution
What Is an Acid, and How Do Acids Cause Damage? Harmful Effects of Air Pollution Are Far-Flung, a Study Finds
 What Is an Acid, and How Do Acids Cause Damage? Harmful Effects of Air Pollution Are Far-Flung, a Study Finds NEW YORK, NEW YORK Air pollution in the Northeast is not just about lakes without fish. It
What Is an Acid, and How Do Acids Cause Damage? Harmful Effects of Air Pollution Are Far-Flung, a Study Finds NEW YORK, NEW YORK Air pollution in the Northeast is not just about lakes without fish. It
Q1. Ammonia is used in the production of fertilisers. The flow diagram shows the main stages in the manufacture of ammonia.
 Q1. Ammonia is used in the production of fertilisers. The flow diagram shows the main stages in the manufacture of ammonia. Study the flow diagram and then answer the questions. (a) What is the purpose
Q1. Ammonia is used in the production of fertilisers. The flow diagram shows the main stages in the manufacture of ammonia. Study the flow diagram and then answer the questions. (a) What is the purpose
Chapter 14: Acids and Bases
 Chemistry 12 Ch 1 4 : Acids and Bases Page 1 Chapter 14: Acids and Bases Check MasteringChemistry Deadlines Acids and Bases: The sour taste of lemons and lime, the bite of sourdough bread, and the tang
Chemistry 12 Ch 1 4 : Acids and Bases Page 1 Chapter 14: Acids and Bases Check MasteringChemistry Deadlines Acids and Bases: The sour taste of lemons and lime, the bite of sourdough bread, and the tang
Edexcel Chemistry Checklist
 Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
I Write the reference number of the correct answer in the Answer Sheet below.
 (2016) Nationality No. CHEMISTRY Name (Please print full name, underlining family name) Marks I Write the reference number of the correct answer in the Answer Sheet below. (1) Which of the atoms 1) to
(2016) Nationality No. CHEMISTRY Name (Please print full name, underlining family name) Marks I Write the reference number of the correct answer in the Answer Sheet below. (1) Which of the atoms 1) to
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
CHEMICAL REACTIONS. Chapter11 OUTLINE GOALS Gas Fuels Solid Fuels
 OUTLINE Quantitative Chemistry 11.1 Phlogiston 11.2 Oxygen 11.3 The Mole 11.4 Formula Units Chemical Energy 11.5 Exothermic and Endothermic Reactions 11.6 Chemical Energy and Stability 11.7 Activation
OUTLINE Quantitative Chemistry 11.1 Phlogiston 11.2 Oxygen 11.3 The Mole 11.4 Formula Units Chemical Energy 11.5 Exothermic and Endothermic Reactions 11.6 Chemical Energy and Stability 11.7 Activation
(a) graph Y versus X (b) graph Y versus 1/X
 HOMEWORK 5A Barometer; Boyle s Law 1. The pressure of the first two gases below is determined with a manometer that is filled with mercury (density = 13.6 g/ml). The pressure of the last two gases below
HOMEWORK 5A Barometer; Boyle s Law 1. The pressure of the first two gases below is determined with a manometer that is filled with mercury (density = 13.6 g/ml). The pressure of the last two gases below
Coimisiún na Scrúduithe Stáit State Examinations Commission
 Coimisiún na Scrúduithe Stáit State Examinations Commission 2009. M33 LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY - ORDINARY LEVEL TUESDAY, 16 JUNE AFTERNOON 2.00 TO 5.00 400 MARKS Answer eight questions
Coimisiún na Scrúduithe Stáit State Examinations Commission 2009. M33 LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY - ORDINARY LEVEL TUESDAY, 16 JUNE AFTERNOON 2.00 TO 5.00 400 MARKS Answer eight questions
Homework Acids and Alkalis
 S2 Homework Acids and Alkalis This booklet is split into the main areas of the topic. Each main area has three different spice levels which tell you about the difficulty of the homework. Each week your
S2 Homework Acids and Alkalis This booklet is split into the main areas of the topic. Each main area has three different spice levels which tell you about the difficulty of the homework. Each week your
Chapter 13 Acids and Bases: The Molecules Responsible for Sour and Bitter
 Nivaldo J. Tro http://www.cengage.com/chemistry/tro Chapter 13 Acids and Bases: The Molecules Responsible for Sour and Bitter Mark Erickson Hartwick College Acids Sourness in foods is caused by acids,
Nivaldo J. Tro http://www.cengage.com/chemistry/tro Chapter 13 Acids and Bases: The Molecules Responsible for Sour and Bitter Mark Erickson Hartwick College Acids Sourness in foods is caused by acids,
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2007 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2007 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
CHEMISTRY. SCIENCE Paper 2
 CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
E. placing 100 grams of powder in ice water and then stirring. D. placing 20 grams of large crystals in hot water and then stirring
 Name: Date: Chemistry Terms 3 and 4 Review 1) The Law of Conservation of Mass states that when two or more reactants combine to produce a product, the total mass of the product is the same as the sum of
Name: Date: Chemistry Terms 3 and 4 Review 1) The Law of Conservation of Mass states that when two or more reactants combine to produce a product, the total mass of the product is the same as the sum of
Chemical Reactions (Chapter 13)
 Chemical Reactions (Chapter 13) coefficients reactants products https://www.youtube.com/watch?v=dummopdw By4 Student Learning Objectives Utilize chemical equations to determine the amounts of reactants,
Chemical Reactions (Chapter 13) coefficients reactants products https://www.youtube.com/watch?v=dummopdw By4 Student Learning Objectives Utilize chemical equations to determine the amounts of reactants,
For Practice 4.1 Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily HCl, according to the reaction:
 Stoichiometry For Practice 4.1 Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily HCl, according to the reaction: What mass of HCl, in grams, is neutralized
Stoichiometry For Practice 4.1 Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily HCl, according to the reaction: What mass of HCl, in grams, is neutralized
Introduction to Chemical Reactions. Chapter 6
 Introduction to Chemical Reactions Chapter 6 Instructional Goals 1. Given the reactants and product in a chemical reaction, the student will be able to write and balance chemical equations. 2. Identify
Introduction to Chemical Reactions Chapter 6 Instructional Goals 1. Given the reactants and product in a chemical reaction, the student will be able to write and balance chemical equations. 2. Identify
Question 1: Solution 1:
 Book Name: Selina Concise Question 1: Comment, sulphuric acid is referred to as: (a) King of chemicals (b) Oil of vitriol Solution 1: (a) Sulphuric acid is called King of Chemicals because there is no
Book Name: Selina Concise Question 1: Comment, sulphuric acid is referred to as: (a) King of chemicals (b) Oil of vitriol Solution 1: (a) Sulphuric acid is called King of Chemicals because there is no
Chemical Reactions. Chemical changes are occurring around us all the time
 Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Definition of Acid. HCl + H 2 O H 3 O + + Cl
 Acids Definition of Acid Acids are substances that contain H + ions that ionize when dissolved in water. Arrhenius acid: a compound that increases the concentration of H + ions that are present when added
Acids Definition of Acid Acids are substances that contain H + ions that ionize when dissolved in water. Arrhenius acid: a compound that increases the concentration of H + ions that are present when added
C2 Revision Pack (Please keep this pack with you)
 Name: C2 Revision Pack (Please keep this pack with you) Follow all the steps below... 1) Practice all the maths and working scientifically questions PRACTICE ALL THESE QUESTIONS! Maths and Science Skills
Name: C2 Revision Pack (Please keep this pack with you) Follow all the steps below... 1) Practice all the maths and working scientifically questions PRACTICE ALL THESE QUESTIONS! Maths and Science Skills
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Acids and Bases Properties of Acids An acid is any substance that releases hydrogen ions, H +, into water.
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Acids and Bases Properties of Acids An acid is any substance that releases hydrogen ions, H +, into water.
State how a catalyst speeds up a chemical reaction. ...
 Q1. This question is about the use of transition metals as catalysts. (a) State how a catalyst speeds up a chemical reaction. State the characteristic property of transition metals that enables them to
Q1. This question is about the use of transition metals as catalysts. (a) State how a catalyst speeds up a chemical reaction. State the characteristic property of transition metals that enables them to
Chapter 8 Acids, Bases, and Acid-Base Reactions. An Introduction to Chemistry by Mark Bishop
 Chapter 8 Acids, Bases, and Acid-Base Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Arrhenius Base Definitions A base is a substance that generates OH when added to water. A basic solution
Chapter 8 Acids, Bases, and Acid-Base Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Arrhenius Base Definitions A base is a substance that generates OH when added to water. A basic solution
Ch 9 Stoichiometry Practice Test
 Ch 9 Stoichiometry Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A balanced chemical equation allows one to determine the a. mole ratio
Ch 9 Stoichiometry Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A balanced chemical equation allows one to determine the a. mole ratio
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
 THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) Time: 3 Hours Thursday, 06 th November
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) Time: 3 Hours Thursday, 06 th November
AQA Chemistry Checklist
 Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Exampro GCSE Chemistry
 Exampro GCSE Chemistry C Chapter 4 Higher Name: Class: Author: Date: Time: 59 Marks: 59 Comments: Page of 0 Q. The picture shows a lump of phosphate rock. Rob Lavinsky, irocks.com CC-BY-SA-3.0 [CC-BY-SA-3.0],
Exampro GCSE Chemistry C Chapter 4 Higher Name: Class: Author: Date: Time: 59 Marks: 59 Comments: Page of 0 Q. The picture shows a lump of phosphate rock. Rob Lavinsky, irocks.com CC-BY-SA-3.0 [CC-BY-SA-3.0],
Manufacture and uses includes sulfur dioxide questions
 Manufacture and uses includes sulfur dioxide questions Question Paper 3 Level Subject ExamBoard Topic Sub-Topic IGCSE Paper (Extended) Theory Booklet Question Paper 3 Chemistry CIE Sulfur Manufacture and
Manufacture and uses includes sulfur dioxide questions Question Paper 3 Level Subject ExamBoard Topic Sub-Topic IGCSE Paper (Extended) Theory Booklet Question Paper 3 Chemistry CIE Sulfur Manufacture and
Identification of ions and gases
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ Identification Of ions nd Gases Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ Identification Of ions nd Gases Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International
LISTA DE EXERCÍCIOS AULA 06/10/2016
 LISTA DE EXERCÍCIOS AULA 06/10/2016 1- Sodium hypochlorite, the active ingredient in Clorox, can be made by the following reaction: 2 NaOH(aq) + Cl 2 (g) NaCl(aq) + NaClO(aq) + H 2 O(l) If chlorine gas
LISTA DE EXERCÍCIOS AULA 06/10/2016 1- Sodium hypochlorite, the active ingredient in Clorox, can be made by the following reaction: 2 NaOH(aq) + Cl 2 (g) NaCl(aq) + NaClO(aq) + H 2 O(l) If chlorine gas
Atoms, Elements, Atoms, Elements, Compounds and Mixtures. Compounds and Mixtures. Atoms and the Periodic Table. Atoms and the.
 Atoms, Elements, Compounds and Mixtures Explain how fractional distillation can be used to separate a mixture. 1 Atoms, Elements, Compounds and Mixtures Fractional distillation is used to separate components
Atoms, Elements, Compounds and Mixtures Explain how fractional distillation can be used to separate a mixture. 1 Atoms, Elements, Compounds and Mixtures Fractional distillation is used to separate components
EDEXCEL IGCSE chemistry (double award)
 EDEXCEL IGCSE chemistry (double award) Section 1: Principles of chemistry a) States of matter 1.1 understand the three states of matter in terms of the arrangement, movement and energy of the particles
EDEXCEL IGCSE chemistry (double award) Section 1: Principles of chemistry a) States of matter 1.1 understand the three states of matter in terms of the arrangement, movement and energy of the particles
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
FORM 4 CHEMISTRY - SUMMER REVISION WORK
 Form 3 Syllabus: FORM 4 CHEMISTRY - SUMMER REVISION WORK Chapter 1: STATES OF MATTER Converting between the 3 states of matter Application of kinetic theory to changes of state Diffusion Physical and chemical
Form 3 Syllabus: FORM 4 CHEMISTRY - SUMMER REVISION WORK Chapter 1: STATES OF MATTER Converting between the 3 states of matter Application of kinetic theory to changes of state Diffusion Physical and chemical
Ozone in the Atmosphere
 Ozone in the Atmosphere Why are we concerned with ozone? This simple molecule affects us in very important ways. It protects us, as well as all animals and plants on our planet, from the harm that ultraviolet
Ozone in the Atmosphere Why are we concerned with ozone? This simple molecule affects us in very important ways. It protects us, as well as all animals and plants on our planet, from the harm that ultraviolet
For the element X in the ionic compound MX, explain the meaning of the term oxidation state.
 1. (a) By referring to electrons, explain the meaning of the term oxidising agent.... For the element X in the ionic compound MX, explain the meaning of the term oxidation state.... (c) Complete the table
1. (a) By referring to electrons, explain the meaning of the term oxidising agent.... For the element X in the ionic compound MX, explain the meaning of the term oxidation state.... (c) Complete the table
Chapter 4 Lesson 1: Describing Earth s Atmosphere
 Chapter 4 Lesson 1: Describing Earth s Atmosphere Vocabulary Importance of Earth s Atmosphere The atmosphere is a thin layer of gases surrounding Earth. o Contains the oxygen and water needed for life.
Chapter 4 Lesson 1: Describing Earth s Atmosphere Vocabulary Importance of Earth s Atmosphere The atmosphere is a thin layer of gases surrounding Earth. o Contains the oxygen and water needed for life.
Acids and Bases. Properties, Reactions, ph, and Titration
 Acids and Bases Properties, Reactions, ph, and Titration C-19 2017 Properties of acids 1. Taste Sour (don t try this except with foods). 2. Are electrolytes (conduct electricity). Some are strong, some
Acids and Bases Properties, Reactions, ph, and Titration C-19 2017 Properties of acids 1. Taste Sour (don t try this except with foods). 2. Are electrolytes (conduct electricity). Some are strong, some
reacts with ammonium sulfate to form ammonia It reacts with a carbonate to form carbon dioxide. It reacts with an ammonium salt to form ammonia.
 1 Which statements are properties of an acid? 1 reacts with ammonium sulfate to form ammonia 2 turns red litmus blue 1 2 2 Which property is not characteristic of a base? It reacts with a carbonate to
1 Which statements are properties of an acid? 1 reacts with ammonium sulfate to form ammonia 2 turns red litmus blue 1 2 2 Which property is not characteristic of a base? It reacts with a carbonate to
UNIT 2. Chemical Reactions. Chapter 4: Developing Chemical Equations. Chapter 5:Classifying. Chemical Reactions. Chapter 6:Acids and Bases
 UNIT 2 Chemical Reactions Chapter 4: Developing Chemical Equations Chapter 5:Classifying Chemical Reactions Chapter 6:Acids and Bases CHAPTER 5 Classifying Chemical Reactions In this chapter, you will:
UNIT 2 Chemical Reactions Chapter 4: Developing Chemical Equations Chapter 5:Classifying Chemical Reactions Chapter 6:Acids and Bases CHAPTER 5 Classifying Chemical Reactions In this chapter, you will:
Sample Questions Chem 22 Student Chapters Page 1 of 5 Spring 2016
 Sample Questions Chem 22 Student Chapters 13-18 Page 1 of 5 1. The vapor pressure of a liquid is the pressure, at equilibrium, of the a) solid above its liquid. b) liquid above its solid. c) gas above
Sample Questions Chem 22 Student Chapters 13-18 Page 1 of 5 1. The vapor pressure of a liquid is the pressure, at equilibrium, of the a) solid above its liquid. b) liquid above its solid. c) gas above
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1
 ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
Continuous measurement of airborne particles and gases
 Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Chemistry. ANSWERS and MARKING SCHEME. Final Examination Preliminary Course General Instructions. Total Marks 64
 ANSWERS and MARKING SCHEME Chemistry Final Examination Preliminary Course 2003 General Instructions Reading time 5 minutes Working time 120 minutes Write using black or blue pen Draw diagrams using pencil
ANSWERS and MARKING SCHEME Chemistry Final Examination Preliminary Course 2003 General Instructions Reading time 5 minutes Working time 120 minutes Write using black or blue pen Draw diagrams using pencil
GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. Bonding. GCSE OCR Revision Chemistry
 Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON
 Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
AQA Chemistry (Combined Science) Specification Checklists. Name: Teacher:
 AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
Choose words from the list to complete the sentences below. electrical heat light kinetic. an endothermic an exothermic a neutralisation a reduction
 Q1. The diagram shows some magnesium ribbon burning. (a) Choose words from the list to complete the sentences below. electrical heat light kinetic an endothermic an exothermic a neutralisation a reduction
Q1. The diagram shows some magnesium ribbon burning. (a) Choose words from the list to complete the sentences below. electrical heat light kinetic an endothermic an exothermic a neutralisation a reduction
