MitoSeminar II: Some calculations in bioenergetics
|
|
|
- Brooke Elliott
- 6 years ago
- Views:
Transcription
1 MitoSeminar II: Some calculations in bioenergetics MUDr. Jan Pláteník, PhD. Ústav lékařské biochemie 1.LF UK Helpful comments of Prof. MUDr. Jiří Kraml, DrSc., are acknowledged. 1 Respiratory chain and oxidative phosphorylation: Summary * Transfer of electrons via electron carriers (respiratory chain) to oxygen in the inner membrane * Proton pumping from the matrix to the cytosolic site of the inner mitochondrial membrane (proton gradient). Protonmotive force = ph gradient + membrane potential * Protons flow through ATP synthase and power the synthesis of ATP. 2 1
2 Stoichiometry of oxidative phosphorylation If F 0 complex has 10 c subunits: one complete turn means flow of 10 protons. The F 1 part has three ATP-producing sites: one complete turn of the shaft means synthesis of three ATP from ADP + Pi At least 3 H + have to be pumped out of the matrix for production of 1 ATP One more H + is consumed for import of phosphate Transport of 2 electrons from NADH to oxygen (complexes I, III, IV) pumps 10 protons, from FADH 2 to oxygen (complexes III, IV) 6 protons. Oxidation of 1 NADH produces 2.5 ATP Oxidation of 1 FADH 2 produces 1.5 ATP 3 G G = H T S The chemical reaction can occur only if the G G is negative. It means: when the products have less free energy than the reactants have. 4 2
3 G G anda chemical equilibrium A + B C + D K eq = [C eq ][D eq ] [A eq ][B eq ] G = RT ln K eq + RT ln [C][D] [A][B] A + B C + D G negative A + B C + D G positive (negative for reverse reaction) A + B C + D G = 0 (equilibrium) 5 Standard free enthalpy G o = RT lnk eq 1.0M, 25 C ph 7.0 R... universal gas constant J mol -1 K -1 T... absolute temperature in Kelvins ( K = 25 o C) G = RT ln K eq + RT ln [C][D] [A][B] G = G o + RT ln [C][D] [A][B] 6 3
4 7 Redox potential X + Y X + Y X X + e Y + e Y Difference in affinities to electrons between two redox couples, in volts. 8 4
5 E o standard redox potential,, 1.0 M, ph 0 E o standard redox potential,, 1.0 M, ph 7 Redox potential Reference hydrogen electrode: E o = 0.0 V E o = 0.42 V 9 (from Harper s Illustrated Biochemistry, 27th edition, McGraw-Hill Co. 2006) 10 5
6 Relationship between free enthalpy and redox potential G G = nf E n number of transfered electrons F... Faraday constant = C /mol C (Coulomb) = J/ V 11 The Nernst equation Tells voltage of galvanic cell, or redox potential, for various concentrations of components. [C][D] G G = G o + RT ln [A][B] + G G = nf E RT [OX] E = E o + ln nf [RED] Walther Hermann Nernst ( ): Nobel Prize 1920 Equation also called Nernst-Peters (Peters applied Nernst equation for redox processes) 12 6
7 Example I: Transfer of electrons from NADH to oxygen: NADH + H + + 1/2O 2 NAD + + H 2 O Redox couples: NADH + H + NAD + + 2H + + 2e E o = 0.320V 1/2O 2 + 2H + + 2e H 2 O E o = V For the whole reaction: E o = 0.816V ( 0.320V )= = 1.136V G o = nf E o G o = 2(96.5 kj V 1 mol 1 )(1.136V) = kj mol 1 13 Example II: Succinate dehydrogenase Redox couples: succinate fumarate + 2H + + 2e E o = V FAD + 2H + + 2e FADH 2 E o = V G o = 2(96.5 kj V 1 mol 1 )( V) = kj mol 1 Reaction cannot proceed??? 14 7
8 Example II: Succinate dehydrogenase Redox couples: succinate fumarate + 2H + + 2e E o = V FAD + 2H + + 2e FADH 2 E o = V But, if fumarate is consumed in the next reaction, and FAD reoxidized, the actual ratios succinate/fumarate and FAD/FADH 2 would not be 1:1 If succinate : fumarate is 500:1, redox potential of the system using the Nernst equation would be: RT [OX] E = E o + ln = ( 0.08)( = 0.05 V nf [RED] And for FAD/FADH 2 500:1 E = = 0.04 V 15 Likewise malate dehydrogenase reaction: Redox couples: malate oxaloacetate + 2H + + 2e E o = V NAD + + 2H + + 2e NADH + H + E o = V 16 8
9 Ionic gradient on a membrane can also do work c( + ) 1 = 120 mmol/l c( + ) 2 = 12 mmol/l G G = c 1 RT ln c 2 = ( ) ln 10 = = 5.7 kj/mol Equation is valid if c 1 > c 2 (diffusion to c 2 ); for 37 C the coefficient is
10 Protonmotive force p = ph + ψ Difference in concentration of protons - about 1 ph unit from Nernst equation is 60 mv Electric potential on the inner membrane. Must be measured, value e.g. 160 mv p = 60 mv mv = 220 mv 19 Efficiency of mitochondrial production of ATP Oxidation of 1 mole of NADH leads to pumping of 10 mol protons and production of 2.5 mol ATP, protonmotive force is 220 mv Oxidation of 1 mol NADH: G o = kj/mol Protonmotive force p = 220 mv corresponds to: µ H + G = µ H + = F p = 21.2 kj/mol For pumping 10 mol protons: G = 10 x (21.2) = 212 kj is electrochemical proton gradient Most of the energy from oxidation is converted to proton gradient 20 10
11 Efficiency of mitochondrial production of ATP Oxidation of 1 mole of NADH leads to pumping of 10 mol protons and production of 2.5 mol ATP, protonmotive force is 220 mv Oxidation of 1 mol NADH: G o = kj/mol Protonmotive force p = 220 mv corresponds to: µ H + G = µ H + = F p = 21.2 kj/mol For pumping 10 mol protons: G = 10 x (21.2) = 212 kj For 1 ATP is needed: G o = 30.5 kj/mol, In the cell really (excess of ATP): G = asi 50 kj/mol 4 protons do work 84.9 kj/mol, which is certainly enough for 1 ATP is electrochemical proton gradient 21 Efficiency of mitochondrial production of ATP Oxidation of 1 mole of NADH leads to pumping of 10 mol protons and production of 2.5 mol ATP, protonmotive force is 220 mv Oxidation of 1 mol NADH: G o = kj/mol Protonmotive force p = 220 mv corresponds to: µ H + G = µ H + = F p = 21.2 kj/mol For pumping 10 mol protons: G = 10 x (21.2) = 212 kj For 1 ATP is needed: G o = 30.5 kj/mol, In the cell really (excess of ATP): G = asi 50 kj/mol is electrochemical proton gradient Oxidation of 1 mol NADH gives theoretically energy for making 4.4 mol ATP, practically 2.5 mol is produced efficiency cca 57 % (for( standard conditions cca 35 %) 22 11
BCH 4054 Spring 2001 Chapter 21 Lecture Notes
 BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
TCA Cycle. Voet Biochemistry 3e John Wiley & Sons, Inc.
 TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
TCA Cycle Voet Biochemistry 3e Voet Biochemistry 3e The Electron Transport System (ETS) and Oxidative Phosphorylation (OxPhos) We have seen that glycolysis, the linking step, and TCA generate a large number
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová
 Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Lecture 10. Proton Gradient-dependent ATP Synthesis. Oxidative. Photo-Phosphorylation
 Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation Model of the Electron Transport Chain (ETC) Glycerol-3-P Shuttle Outer Mitochondrial Membrane G3P DHAP
Change to Office Hours this Friday and next Monday. Tomorrow (Abel): 8:30 10:30 am. Monday (Katrina): Cancelled (05/04)
 Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Change to Office Hours this Friday and next Monday Tomorrow (Abel): 8:30 10:30 am Monday (Katrina): Cancelled (05/04) Lecture 10 Proton Gradient-dependent ATP Synthesis Oxidative Phosphorylation Photo-Phosphorylation
Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
MITOCW watch?v=vykadbjib8a
 MITOCW watch?v=vykadbjib8a The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
MITOCW watch?v=vykadbjib8a The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
All organisms require a constant expenditure of energy to maintain the living state - "LIFE".
 CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
CELLULAR RESPIRATION All organisms require a constant expenditure of energy to maintain the living state - "LIFE". Where does the energy come from and how is it made available for life? With rare exception,
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
number 8 Done by Ali Yaghi Corrected by Mamoon Mohamad Alqtamin Doctor Nafeth Abu Tarboush 0 P a g e Oxidative phosphorylation Oxidative phosphorylation has 3 major aspects: 1. It involves flow of electrons
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
ETC/CHEMIOSIS. By: Leslie, Kelsey, Morgan
 ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
Hg2 2+ (aq) + H2(g) 2 Hg(l) + 2H + (aq)
 The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
ATP. Division Ave. High School AP Biology. Cellular Respiration Stage 4: Electron Transport Chain. Cellular respiration. The point is to make ATP!
 ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
EMA4303/5305 Electrochemical Engineering Lecture 02 Equilibrium Electrochemistry
 EMA4303/5305 Electrochemical Engineering Lecture 02 Equilibrium Electrochemistry Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University 2 Equilibrium Electrochemistry
EMA4303/5305 Electrochemical Engineering Lecture 02 Equilibrium Electrochemistry Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University 2 Equilibrium Electrochemistry
Ch/APh2 Bioenergetics Section Lecture of May 14, The thermodynamics of biological energy production.
 Ch/APh2 Bioenergetics Section Lecture of May 14, 2009 Introduction to bioenergetics. The thermodynamics of biological energy production. Kinetic aspects of bioenergetic processes. The molecular and cellular
Ch/APh2 Bioenergetics Section Lecture of May 14, 2009 Introduction to bioenergetics. The thermodynamics of biological energy production. Kinetic aspects of bioenergetic processes. The molecular and cellular
Homework - I. Hand draw the structure of an eukaryotic cell with all organelles clearly drawn and describe the function of each organelle.
 Homework - I Hand draw the structure of an eukaryotic cell with all organelles clearly drawn and describe the function of each organelle. Please get a book and read the chapter related to cell structure!!!
Homework - I Hand draw the structure of an eukaryotic cell with all organelles clearly drawn and describe the function of each organelle. Please get a book and read the chapter related to cell structure!!!
of catabolic processes, like glycolysis and the Krebs cycle, as hydride ions (H ). This free energy is used to regenerate ATP in the matrix.
 Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely
Biochemistry I Oxidative Phosphorylation I. MITOCHONDRIA Mitochondria are cellular organelles which have two bilayer membranes, and two compartments defined by those membranes. Mitochondria are completely
Table 4.3. Thermo. of Metabolism pp : Like Problem S1. Phosphorylations involving ATP. 361 Lec 21 Fri., 6oct17
 Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Phosphorylations involving ATP 361 Lec 21 Fri., 6oct17 1 Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Oxidations and Reductions
Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Phosphorylations involving ATP 361 Lec 21 Fri., 6oct17 1 Table 4.3 Thermo. of Metabolism pp135-138: Like Problem S1. Oxidations and Reductions
Cellular respiration ATP. Cellular Respiration Stage 4: Electron Transport Chain. AP Biology. The point is to make ATP! What s the point?
 ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Giving you the energy you need!
 Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
Giving you the energy you need! Use your dominant hand Open and close the pin (with your thumb and forefinger) as many times as you can for 20 seconds while holding the other fingers straight out! Repeat
State state describe
 Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Warm-Up State the products of the light-dependent reaction of photosynthesis, state which product has chemical energy, and describe how that product is made. KREBS ETC FADH 2 Glucose Pyruvate H 2 O NADH
Lecture 20. Chemical Potential
 Lecture 20 Chemical Potential Reading: Lecture 20, today: Chapter 10, sections A and B Lecture 21, Wednesday: Chapter 10: 10 17 end 3/21/16 1 Pop Question 7 Boltzmann Distribution Two systems with lowest
Lecture 20 Chemical Potential Reading: Lecture 20, today: Chapter 10, sections A and B Lecture 21, Wednesday: Chapter 10: 10 17 end 3/21/16 1 Pop Question 7 Boltzmann Distribution Two systems with lowest
Courtesy of Elsevier. Used with permission.
 Chemistry 5.07 2013 Problem Set 5 Answers Problem 1 Succinate dehydrogenase (SDH) is a heterotetramer enzyme complex that catalyzes the oxidation of succinate to fumarate with concomitant reduction of
Chemistry 5.07 2013 Problem Set 5 Answers Problem 1 Succinate dehydrogenase (SDH) is a heterotetramer enzyme complex that catalyzes the oxidation of succinate to fumarate with concomitant reduction of
Copyright 2018 Dan Dill 1
 when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
Electrochemistry objectives
 Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
8. ELECTROCHEMICAL CELLS. n Electrode Reactions and Electrode Potentials a. H 2 2H + + 2e. Cl 2 + 2e 2Cl. H 2 + Cl 2 2H + + 2Cl ; z = 2
 8. ELECTROCHEMICAL CELLS n Electrode Reactions and Electrode Potentials 8.1. a. H H + + e Cl + e Cl H + Cl H + + Cl ; z = E = E RT F ln ( a H +a Cl ) b. Hg(l)+ Cl Hg Cl + e H + + e H Hg + H + + Cl Hg Cl
8. ELECTROCHEMICAL CELLS n Electrode Reactions and Electrode Potentials 8.1. a. H H + + e Cl + e Cl H + Cl H + + Cl ; z = E = E RT F ln ( a H +a Cl ) b. Hg(l)+ Cl Hg Cl + e H + + e H Hg + H + + Cl Hg Cl
BIOLOGY 479 INTEGRATED PHYSIOLOGY LABORATORY Dept. of Biological & Allied Health Sciences BLOOMSBURG UNIVERSITY of PENNSYLVANIA.
 BIOLOGY 479 INTEGRATED PHYSIOLOGY LABORATORY Dept. of Biological & Allied Health Sciences BLOOMSBURG UNIVERSITY of PENNSYLVANIA LAB REPORT: Investigating Aerobic Respiration Michael Tekin Lab section:
BIOLOGY 479 INTEGRATED PHYSIOLOGY LABORATORY Dept. of Biological & Allied Health Sciences BLOOMSBURG UNIVERSITY of PENNSYLVANIA LAB REPORT: Investigating Aerobic Respiration Michael Tekin Lab section:
Supplementary thermodynamics as applied to biosystems
 Supplementary thermodynamics as applied to biosystems Glucose is transferred to glucose-6-phosphate, abbreviated here to G6P. The reaction may be written Glucose + phosphate G6P + H 2 O G o = 13.8kJ/mol
Supplementary thermodynamics as applied to biosystems Glucose is transferred to glucose-6-phosphate, abbreviated here to G6P. The reaction may be written Glucose + phosphate G6P + H 2 O G o = 13.8kJ/mol
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
The Proton Motive Force. Overview. Compartmentalization 11/6/2015. Chapter 21 Stryer Short Course. ATP synthesis Shuttles
 The Proton Motive Force Chapter 21 Stryer Short Course Redox reactions Electron transport chain Proton gradient Overview ATP synthesis Shuttles Analogy: How does burning coal put flour in the grocery store?
The Proton Motive Force Chapter 21 Stryer Short Course Redox reactions Electron transport chain Proton gradient Overview ATP synthesis Shuttles Analogy: How does burning coal put flour in the grocery store?
ΔG o' = ηf ΔΕ o' = (#e ( V mol) ΔΕ acceptor
 Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
ΔG = -nfe cell. Electrode Potentials. The cell potential E cell is related to the free energy of the reaction ΔG by:
 Electrode Potentials Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building: 05,
Electrode Potentials Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building: 05,
Sara Khraim. Shaymaa Alnamos ... Dr. Nafeth
 10 Sara Khraim Shaymaa Alnamos... Dr. Nafeth *Requirement of oxidative phosphorylation: 1- Source and target for electrons(nadh+fadh2 >> O2). 2- Electron carriers. 3- Enzymes, like oxidoreductases and
10 Sara Khraim Shaymaa Alnamos... Dr. Nafeth *Requirement of oxidative phosphorylation: 1- Source and target for electrons(nadh+fadh2 >> O2). 2- Electron carriers. 3- Enzymes, like oxidoreductases and
Electrochemistry & Redox. Voltaic Cells. Electrochemical Cells
 Electrochemistry & Redox An oxidation-reduction (redox) reaction involves the transfer of electrons from the reducing agent to the oxidising agent. OXIDATION - is the LOSS of electrons REDUCTION - is the
Electrochemistry & Redox An oxidation-reduction (redox) reaction involves the transfer of electrons from the reducing agent to the oxidising agent. OXIDATION - is the LOSS of electrons REDUCTION - is the
Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain *
 OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m59707 1 Bis2A 5.6: Oxidative Phosphorylation and the Electron Transport Chain * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Chapter 19: Electrochemistry
 Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Electrochemical Cells at Non-Standard Conditions
 Electrochemical Cells at Non-Standard Conditions Oxidation-reduction reactions in the real world rarely occur under standard conditions. Even if the cell started out with all dissolved species at 1M concentration,
Electrochemical Cells at Non-Standard Conditions Oxidation-reduction reactions in the real world rarely occur under standard conditions. Even if the cell started out with all dissolved species at 1M concentration,
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Paolo Sarti 2011 Department of Biochemistry Sapienza REDOX REACTIONS
 REDOX REACTIONS What happens upon diping a needle in a CuSO 4 solution!? Fe + Cu 2+ SO 4 2- Fe 2+ SO 4 2- + Cu In a redox reaction, one (or more) electrons are donated by a reductant to an oxidant Multiple
REDOX REACTIONS What happens upon diping a needle in a CuSO 4 solution!? Fe + Cu 2+ SO 4 2- Fe 2+ SO 4 2- + Cu In a redox reaction, one (or more) electrons are donated by a reductant to an oxidant Multiple
Ch. 13 Fundamentals of Electrochemistry
 Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
( 1 vρ) = ( sec)( N sec m 1 )
 Page 1 of 5 + formula sheet 1. You have isolated a novel protein that has been implicated in a disease and you would like to use what you have learned in Chem/Biochem 471 to deduce some of its properties.
Page 1 of 5 + formula sheet 1. You have isolated a novel protein that has been implicated in a disease and you would like to use what you have learned in Chem/Biochem 471 to deduce some of its properties.
Chemistry 2000 Lecture 15: Electrochemistry
 Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Microbiology II Microbial physiology I Energetics
 Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses
Microbiology II Microbial physiology I Energetics Catabolism Heat Efficiency ~ 60% Efficiency ~ 40% Anabolism Chemical energy (chemotrophic) Light energy (phototrophic) ATP +/ 50 kj/mol ADP + P i Biosyntheses
Ch 18 Electrochemistry OIL-RIG Reactions
 Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Aerobic Cellular Respiration
 Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
The Electron-Transfer Chain and Oxidative Phosphorylation
 The Electron-Transfer Chain and Oxidative Phosphorylation The Mitochondrion - Scene of the Action 1.1 The inner mitochondrial membrane compartmentalizes metabolic functions. Although mitochondria in different
The Electron-Transfer Chain and Oxidative Phosphorylation The Mitochondrion - Scene of the Action 1.1 The inner mitochondrial membrane compartmentalizes metabolic functions. Although mitochondria in different
Department of Chemistry and Biochemistry University of Lethbridge. Biochemistry II. Bioenergetics
 Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
number Done by Corrected by Doctor Nafeth Abu Tarboush
 number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
number 6 Done by أنس القيشاوي Corrected by Zaid Emad Doctor Nafeth Abu Tarboush 1 P a g e In the previous lecture, we talked about redox reactions and the reduction potential briefly and how it can help
A + B C +D ΔG = ΔG + RTlnKp. Me n+ + ne - Me. Me n n
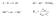 A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions.
 Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Electrochemistry. Goal: Understand basic electrochemical reactions. Half Cell Reactions Nernst Equation Pourbaix Diagrams.
 Electrochemistry Goal: Understand basic electrochemical reactions Concepts: Electrochemical Cell Half Cell Reactions Nernst Equation Pourbaix Diagrams Homework: Applications Battery potential calculation
Electrochemistry Goal: Understand basic electrochemical reactions Concepts: Electrochemical Cell Half Cell Reactions Nernst Equation Pourbaix Diagrams Homework: Applications Battery potential calculation
Transporters and Membrane Motors Nov 15, 2007
 BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
2/18/2013. Spontaneity, Entropy & Free Energy Chapter 16. The Dependence of Free Energy on Pressure Sample Exercises
 Spontaneity, Entropy & Free Energy Chapter 16 16.7 The Dependence of Free Energy on Pressure Why is free energy dependent on pressure? Isn t H, enthalpy independent of pressure at constant pressure? No
Spontaneity, Entropy & Free Energy Chapter 16 16.7 The Dependence of Free Energy on Pressure Why is free energy dependent on pressure? Isn t H, enthalpy independent of pressure at constant pressure? No
Electrochemical System
 Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Washington University in St. Louis Chemistry Tournament Sample Problems for Individual Round #3: Kinetics, Electrochemistry, and Thermodynamics
 Individual Exam #3: Kinetics, Electrochemistry, and Thermodynamics 1) A galvanic cell is to be constructed using the following half reactions under acidic conditions: ε 1.49 V ε 1.66 V Given that the potentials
Individual Exam #3: Kinetics, Electrochemistry, and Thermodynamics 1) A galvanic cell is to be constructed using the following half reactions under acidic conditions: ε 1.49 V ε 1.66 V Given that the potentials
Bioenergetics and high-energy compounds
 Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Bioenergetics and high-energy compounds Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
ELECTROCHEMISTRY Chapter 14
 ELECTROCHEMISTRY Chapter 14 Basic Concepts: Overview of Electrochemical Process at Constant T, P (14-1) ΔG = ΔG o + RT ln Q = w elec (maximum) = qe = ItE (exp) (E intensive parameter, q extensive) = nfe
ELECTROCHEMISTRY Chapter 14 Basic Concepts: Overview of Electrochemical Process at Constant T, P (14-1) ΔG = ΔG o + RT ln Q = w elec (maximum) = qe = ItE (exp) (E intensive parameter, q extensive) = nfe
BIOLOGY 111. CHAPTER 7: Vital Harvest: Deriving Energy From Food
 BIOLOGY 111 CHAPTER 7: Vital Harvest: Deriving Energy From Food Deriving Energy from Food: What is the best carbohydrate source (for energy) in our food? Glucose! Where is the energy stored in glucose?
BIOLOGY 111 CHAPTER 7: Vital Harvest: Deriving Energy From Food Deriving Energy from Food: What is the best carbohydrate source (for energy) in our food? Glucose! Where is the energy stored in glucose?
Biophysics 490M Project
 Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
CHEMISTRY - CLUTCH CH.18 - ELECTROCHEMISTRY.
 !! www.clutchprep.com CONCEPT: OXIDATION-REDUCTION REACTIONS Chemists use some important terminology to describe the movement of electrons. In reactions we have the movement of electrons from one reactant
!! www.clutchprep.com CONCEPT: OXIDATION-REDUCTION REACTIONS Chemists use some important terminology to describe the movement of electrons. In reactions we have the movement of electrons from one reactant
Basic overall reaction for hydrogen powering
 Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Cellular Respiration. Mitochondria Rule! Mr. Kurt Kristensen
 Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
CP Biology Unit 5 Cell Energy Study Guide. Electron Carriers Electron Transport Chain Fermentation Glycolysis Krebs cycle Light-Dependent Reactions
 Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Chapter 13 Principles of Bioenergetics
 Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
Chapter 13 Principles of Bioenergetics 1. Cells need energy to do all their work To generate and maintain its highly ordered structure (biosynthesis of macromolecules) To generate all kinds of movement
ph calculations
 Medical hemistry. Department of uman Physiology and Biochemistry p calculations http://aris.gusc.lv/biothermodynamics/paprekiniuzdld.pdf Universal gas constant R=8.3144 J/(mol K); Faraday s constant F=96485
Medical hemistry. Department of uman Physiology and Biochemistry p calculations http://aris.gusc.lv/biothermodynamics/paprekiniuzdld.pdf Universal gas constant R=8.3144 J/(mol K); Faraday s constant F=96485
Biochemical Thermodynamics
 CHAPTER Biochemical Thermodynamics 1 Learning Objectives 1. Define and use correctly the terms system, closed, open, surroundings, state, energy, temperature, thermal energy, irreversible process, entropy,
CHAPTER Biochemical Thermodynamics 1 Learning Objectives 1. Define and use correctly the terms system, closed, open, surroundings, state, energy, temperature, thermal energy, irreversible process, entropy,
Bis2A 5.1 REDOX Chemistry and the REDOX Tower *
 OpenStax-CNX module: m59682 1 Bis2A 5.1 REDOX Chemistry and the REDOX Tower * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract
OpenStax-CNX module: m59682 1 Bis2A 5.1 REDOX Chemistry and the REDOX Tower * The BIS2A Team This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract
Oxidation number. The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred.
 Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
 Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Division Ave. High School AP Biology
 Overview 10 reactions u convert () to pyruvate (3C) u produces: 4 & NADH u consumes: u net: & NADH C-C-C-C-C-C fructose-1,6bp P-C-C-C-C-C-C-P DHAP P-C-C-C G3P C-C-C-P H P i P i pyruvate C-C-C 4 4 NAD +
Overview 10 reactions u convert () to pyruvate (3C) u produces: 4 & NADH u consumes: u net: & NADH C-C-C-C-C-C fructose-1,6bp P-C-C-C-C-C-C-P DHAP P-C-C-C G3P C-C-C-P H P i P i pyruvate C-C-C 4 4 NAD +
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
AP* Electrochemistry Free Response Questions page 1
 Galvanic (Voltaic) Cells 1988 Average score = 5.02 a) two points Sn ---> Sn 2+ + 2e Ag + + e ---> Ag AP* Electrochemistry Free Response Questions page 1 b) two points 2 Ag + + Sn ---> 2 Ag + Sn 2+ E =
Galvanic (Voltaic) Cells 1988 Average score = 5.02 a) two points Sn ---> Sn 2+ + 2e Ag + + e ---> Ag AP* Electrochemistry Free Response Questions page 1 b) two points 2 Ag + + Sn ---> 2 Ag + Sn 2+ E =
Part One: Introduction. a. Chemical reactions produced by electric current. (electrolysis)
 CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
Electrochemistry. Chapter 18. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
ELECTROCHEMISTRY Chapter 19, 4.9
 ELECTROCHEMISTRY Chapter 19, 4.9 Overview of an Electrochemical Process at Constant T and P ΔG = ΔG o + RT ln Q = welec (maximum) Note: I below stands for current measured in amperes = qecell = ItEcell
ELECTROCHEMISTRY Chapter 19, 4.9 Overview of an Electrochemical Process at Constant T and P ΔG = ΔG o + RT ln Q = welec (maximum) Note: I below stands for current measured in amperes = qecell = ItEcell
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Chapter 2 Energy in Biology Demand and Use
 Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Chapter 2 Energy in Biology Demand and Use A coupled energy source is a prerequisite of sustained dynamics in thermodynamically open systems. Abstract From the point of view of energy management in biological
Free Energy. because H is negative doesn't mean that G will be negative and just because S is positive doesn't mean that G will be negative.
 Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
