Reactive trace gases measured in the interstitialair of surface snow at Summit, Greenland
|
|
|
- Hugo Walsh
- 6 years ago
- Views:
Transcription
1 AE International Polar Regions Atmospheric Environment 38 (2004) Reactive trace gases measured in the interstitialair of surface snow at Summit, Greenland Hans-Werner Jacobi a,b, *, Roger C. Bales a,1, Richard E. Honrath c, Matthew C. Peterson c, Jack E. Dibb d, Aaron L. Swanson e,2, Mary R. Albert f a Department of Hydrology and Water Resources, University of Arizona, 1133 E. North Campus Drive, Tucson, AZ 85721, USA b Alfred Wegener Institute for Polar and Marine Research, Am Handelshafen 12, Bremerhaven, Germany c Department of Civil and Environmental Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, USA d Climate Change Research Center, Institute for the Study of Earth, Oceans and Space, University of New Hampshire, 39 College Road, Durham, NH 03824, USA e Department of Chemistry, University of California at Irvine, 516 Rowland Hall, Irvine, CA 92697, USA f US Army Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH 03755, USA Received 3 March 2003; received in revised form 22 December 2003; accepted 7 January 2004 Abstract Concentration measurements of nitric oxide (NO), nitrogen dioxide (NO 2 ), nitrous acid (HONO), nitric acid (HNO 3 ), formaldehyde (HCHO), hydrogen peroxide (H 2 O 2 ), formic acid (HCOOH) and acetic acid (CH 3 COOH) were performed in air filtered through the pore spaces of the surface snowpack (firn air) at Summit, Greenland, in summer In general, firn air concentrations of NO, NO 2, HONO, HCHO, HCOOH, and CH 3 COOH were enhanced compared to concentrations in the atmospheric boundary layer above the snow. Only HNO 3 and H 2 O 2 normally exhibited lower concentrations in the firn air. In most cases differences were highest during the day and lowest during nighttime hours. Shading experiments showed a good agreement with a photochemicalno x source in the surface snow. Patterns of H 2 O 2,CH 3 COOH, and HNO 3 observed within the surface snow-firn air system imply that the number of molecules in the snow greatly exceeded that in the firn air. Deduced partitioning indicates that the largest fractions of the acids were present at the ice grain air interface. In all cases, the number of molecules located at the interface was significantly higher than the amount in the firn air. Therefore, snow surface area and surface coverage are important parameters, which must be considered for the interpretation of firn air concentrations. r 2004 Elsevier Ltd. All rights reserved. Keywords: Trace gases; Air-snow exchange; Firn air; Polar atmospheric chemistry; Greenland *Corresponding author. Alfred Wegener Institute for Polar and Marine Research, Am Handelshafen 12, Bremerhaven, Germany. Tel.: ; fax: address: hwjacobi@awi-bremerhaven.de (H.-W. Jacobi). 1 Present address: University of California, P.O. Box 2039, Merced, CA 95344, USA. 2 Present address: Cooperative Institute for Research in Environmental Sciences, Colorado University, 1850 Table Mesa Drive, Boulder, CO 80309, USA. 1. Introduction Recent experiments have demonstrated that surface snow in polar regions can act as a photochemical reactor influencing concentrations of a wide variety of important tropospheric trace gases like ozone and nitrogen containing compounds in the atmospheric boundary layer (ABL) over snow-covered regions (e.g. Honrath et al., 2000a, 2002; Jones et al., 2000, 2001; Peterson and Honrath, 2001). Moreover, the exchange of trace gases present in the snow (e.g. H 2 O 2 ) with the ABL is of great /$ - see front matter r 2004 Elsevier Ltd. All rights reserved. doi: /j.atmosenv
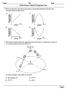
2 1688 ARTICLE IN PRESS H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) importance for the interpretation of firn and ice core profiles of these gases (McConnell et al., 1997; Hutterli et al., 1999, 2001). Since firn air constitutes the link between the ABL and the snow, gases exchanging between the snow and the atmosphere pass through the firn air. Firn air comprises only a small portion of the ABL-snow system, making it sensitive to even small changes in temperature (through firn-air partitioning) or atmospheric concentrations. In order to gain further insight into the role of the firn air, simultaneous measurements of key species in the firn air were performed during the summer 2000 on the Greenland ice sheet. In this paper, we report the first simultaneous observations of nitric oxide (NO), nitrogendioxide (NO 2 ), nitrous acid (HONO), nitric acid (HNO 3 ), formaldehyde (HCHO), hydrogen peroxide (H 2 O 2 ), formic acid (HCOOH), and acetic acid (CH 3 COOH) in the firn air at multiple depths with varying temperatures and radiation levels and investigate the extent to which concentrations are controlled by the partitioning between surface snow and firn air. 2. Experimental Firn air was intensively sampled from 19 to 23 June, 2000 at the Summit EnvironmentalObservatory on top of the Greenland ice sheet (72.6 N, 38.5 W, 3200 m elevation) using three different inlet lines. For the NO and NO 2 measurements a PFA-Teflon tube terminated with a PFA-Teflon filter pack was positioned at the bottom of a narrow hole, which was refilled with excavated snow. Firn air samples for the measurements of H 2 O 2 and HCHO were drawn through a heated and insulated inlet line (0.635 cm ID PFA tubes) mounted in a PVC tube (6.03 cm OD, 5.08 cm ID) with a length of 43 cm. Another piece of the same PVC tube was used to drill holes into the surface snow. After placing the PVC tube with the inlet line into the hole, it was carefully sealed with surface snow (Fig. 1). A similar set-up with a 2-m length of heated 0.95 cm OD PFA tubing with a Teflon pre-filter was used for firn air sampling of HONO, HNO 3, HCOOH, and CH 3 COOH (Dibb and Arsenault, 2002). The distance between the inlets was o2 m. Applied flow rates were on the order of 20 l min 1 for the acids and o2 lmin 1 for the other compounds. Measurements were made at depths of 10 and 30 cm below the snow surface. In each case, a second similar inlet line was used to sample either ambient air or, in the case of NO x for certain periods, firn air at a different depth. Measurements were made with instruments described in detail previously, using a chemiluminescence technique for NO and NO 2 (Honrath et al., 2002), fluorometric detection for HCHO and H 2 O 2 (Jacobi et al., 2002), and mist chamber sampling followed by ion chromatographic detection for HONO, HNO 3, 6.03 cm OD PVC tube Insulation cm ID PFA tube Re-filled snow Surface snow Air flow Fig. 1. Schematic drawing of the inlet line used for sampling H 2 O 2 and HCHO in the firn air. HCOOH, and CH 3 COOH (Dibb and Arsenault, 2002; Dibb et al., 2002). NO 2 photodissociation rate constants were determined with a 2-p Metcon filterradiometer (Yang et al., 2002). Even with a perfect sealat the sampler snow interface, significant amounts of atmospheric air is drawn down through the surface snow into the sample inlets (Albert et al., 2002). Air reaching the inlets has been filtered through firn at a range of depths and layers, and does not represent simply air that has been in contact with distinct snow layers (Bales et al., 1995a). In addition, the sampler-induced flow rates are at least an order of magnitude larger than flows induced by natural ventilation, and are many orders of magnitude higher than movement from diffusion (Albert et al., 2002); thus we cannot use models based on diffusion for quantitative interpretation of these data. Moreover, the inlets for different samplers have vastly different flow rates and were at different locations in the snow, inducing threedimensionalinterstitialflow patterns that further complicate interpretation. In spite of these complications, the data are the first of their kind and do help to give insight into physicaland photochemicalinteractions in the near-surface snow. To investigate the influence of photochemicalprocesses, the sampling area was shaded for periods of 30 min 2 h using pieces (B4m 2 ) of aluminum-covered insulation boards (20 June, 10:56 13:02 and 15:15 17:35; 22 June, 10:11 11:11, 12:22 13:25, 14:42 15:39 and 16:54 17:52), plexiglass (21 June, 8:59 11:04), or polyethylene (PE) film (22 June, 19:06 20:10) mounted B15 cm above the snow surface. After finishing the firn air measurements, a snow pit was dug within the sampling area on 24 June. Snow samples of this pit were analyzed for concentrations of H 2 O 2 and NO 3,
3 H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) density, and surface area. The surface area was determined from quantitative microscopy on twodimensionalthick sections of snow (Albert and Shultz, 2002). While the grain sizes determined by this method agree with visualobservations, the surface area is sometimes affected by three-dimensionaleffects. Preliminary comparison of the side-by-side determination of the surface area of windpack and hoar in a thin snowpack at Alert, Canada by gas adsorption techniques (Domin!e et al., 2002) and by imaging show differences as large as 25% (M. Albert, unpublished data). 3. Results The firn air sampling was done near a tower that had been in place for many years, and the physical characteristics of the snow at the site had different characteristics than snow in undisturbed areas (Albert and Shultz, 2002), primarily due to foot traffic from previous years and drift patterns near the tower. Fig. 2 shows the stratigraphy and permeability profile of the snow at the sampling site. The snow was primarily finegrained wind packed snow interspersed with layers of hoar. No dendritic forms were observed. The packed, low permeability snow below depths of 40 cm was trafficked snow that was deposited in the previous year, and the undisturbed snow from the current year lay above that. The specific surface area steadily decreased from 210 cm 2 g 1 near 13 cm depth to 130 cm 2 g 1 near 28 cm depth. The densities in the subsamples used for surface area determinations were essentially constant in the range g cm 3. Although these densities were lower than those shown in Fig. 2, we used the surface area (210 cm 2 g 1 ) and density (0.22 g cm 3 ) measured on the same sample from 13 depth in further analysis of the firn air measurements at 10 cm depth. H 2 O 2 concentrations in the same snow pit decreased from 17.0 mm at the surface (0 to 3 cm depth) to 4.9 mm in the depth range of 24 to 28 cm. Between 7 and Depth (cm) permeability density Density (g cm -3 ) or Permeability (x10 10 m 2 ) Fig. 2. Density and permeability measured in a snow pit within the firn air sampling area. 13 cm depth, the concentration was 11.5 mm. Similarly, NO 3 decreased from 5.0 mm at the surface to 0.6 mm in the depth range of 24 to 27 cm.between 9 and 12 cm depth, a NO 3 concentration of 2.3 mm was found. Figs. 3 6 show time series of concentration measurements above and below the snow surface for the period June. This period includes six shading experiments with the aluminum-covered insulation boards and single shading experiments using either plexiglass or PE film. The last shading experiment with the PE film, which is partly transparent to UV and visible radiation (the transmission increases from 30% at 350 nm to 50% at 600 nm), was conducted to investigate whether the shading would cause an effect due to a change in the ventilation between firn air and ABL. Since changes in firn air concentrations were not observed during this experiment, changes in air flow patterns due to the shading of the sampling area was neglected. The most pronounced dielcycles in the firn air were observed for NO and NO 2. NO concentrations in the firn air at 10 and 30 cm were similar, and were much higher than ambient concentrations during daytime (Fig. 3a). There were immediate, strong drops in NO at both levels during each shading period. During the longer shading experiments on June 20, the firn air concentrations dropped to ambient levels. NO levels immediately increased upon removing the shading, increasing to levels observed before the shading. NO 2 at 10 cm slowly decreased during the shading experiment whereas NO 2 at 30 cm first jumped to higher values followed by a steady decrease (Fig. 3b). Again, the opposite behavior was found after unshading. Even at night firn air NO 2 was elevated compared to ambient levels. The dielcycle in the H 2 O 2 concentration that was observed in the ABL was attenuated in the firn air at 10 cm (Fig. 4a). Ambient and firn air concentrations were comparable late at night. However, after sunrise ambient concentrations increased more than did those in the firn air, while firn air concentrations at 10 and 30 cm were comparable. Shading experiments did not affect H 2 O 2 concentrations in the firn air. Firn air concentrations of HCHO also exhibited a diel cycle at a depth of 10 cm (Fig. 4b). Late at night firn air and ambient concentrations were comparable. However, after sunrise firn air concentrations increased more than ambient concentrations and peaked around 19:00. At this time ambient and firn air concentrations at 10 cm differed by about 150 pptv. Although firn air concentrations at 30 cm on 22 June were further enhanced compared to firn air concentrations at 10 cm on the previous days, a diel cycle was less obvious. Firn air concentrations at 10 cm dropped by 50 to 60 pptv during the two shading periods on 20 June, while the experiments produced negligible effects on HCHO concentrations at 30 cm on 22 June.
4 1690 ARTICLE IN PRESS H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) Fig. 3. Time series of 10-min averages of (a) NO and (b) NO 2 concentrations above and below the snow surface measured from 19 to 22 June. Also shown is the photolysis rate of NO 2 j(no 2 ) above the snow surface (a). Firn air concentrations during shading experiments are marked by open symbols with beginning and end of each experiment indicated by vertical lines (P: plexiglass, PE: PE film, others: aluminum; see text). Quite high interstitialair concentrations were found for HCOOH and CH 3 COOH (Dibb and Arsenault, 2002). In contrast to ambient concentrations, which exhibited slightly higher concentrations during the day than at night, firn air concentrations were always in the range of pptv for HCOOH and pptv for CH 3 COOH with no distinct dielcycle (Fig. 5). Also, concentrations were not affected during the shading experiments. HONO showed a behavior comparable to the organic acids; however, concentrations were much smaller (Fig. 6a). Shading experiments produced ambiguous results with the first shading experiment on 20 June resulting in a slight increase in HONO at 10 cm and with a strong decrease during the second shading experiment. HNO 3 exhibited the lowest firn air concentrations of all measured compounds (Fig. 6b). At 10 and 30 cm concentrations were comparable and remained below 20 pptv. The largest differences between firn air and ambient concentrations occurred at daytime, owing to the dielcycle of HNO 3 in the ABL. 4. Discussion 4.1. Relationship to fluxes measured above the snow surface Firn air is connected both to the surface snow and the ABL above the snow. Therefore, we can expect that the exchange measured above the snow surface correlates to the gradient between ambient and firn air concentrations. During the summer 2000 field season fluxes above the snow surface of NO x, HONO, HNO 3,H 2 O 2 and HCHO were measured (Honrath et al., 2002; Jacobi et al., 2002). Honrath et al. (2002) reported upward fluxes of NO x and HONO and downward fluxes of HNO 3. The average dielcycle of each compound shows its maximum flux around noon, with negligible exchange during the night. These cycles agree well with the observed elevated firn air concentrations of NO x and HONO and the reduced firn air concentrations of HNO 3. NO 2 and HONO firn air concentrations also remained higher than ambient levels at night and, thus, could cause emissions all day. However, at night very stable conditions normally develop in the ABL at Summit (Cullen and Steffen, 2001) limiting the turbulence to very low values. Therefore, even in the presence of large concentration gradients the exchange can remain negligible, in agreement with the measured fluxes. The dielcycles of the exchange of H 2 O 2 and HCHO followed similar patterns: emissions of both compounds during the day and a slight uptake at night (Jacobi et al., 2002). Daytime gradients of HCHO and nighttime gradients of H 2 O 2 matched the previously reported direction of the fluxes above the snow surface, whereas those at other times did not. The reason for this disagreement could be that the fluxes of H 2 O 2 and
5 H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) Fig. 4. Time series of 10-min averages of (a) H 2 O 2 and (b) HCHO concentrations above and below the snow surface measured from 19 to 22 June. Also shown is the firn air temperature T at 10 cm (a). Firn air concentrations during shading experiments are marked by open symbols with beginning and end of each experiment indicated by vertical lines (P: Plexiglass, PE: PE film, others: aluminum; see text). HCHO are dominated by the exchange with only the top few centimeters or millimeters of the snow surface (Hutterli et al., 2001). The amounts of H 2 O 2 and HCHO stored in the top layer of the snow are sufficient to sustain the measured fluxes to the ABL as well as to deeper layers of the snow (Jacobi et al., 2002). However, differences between firn air and ambient concentrations may also be an artifact of the flow rates and patterns as described above, and points to the need for firn air sampling using much lower flow rates. Thus, although gradients between ambient and firn air at 10 cm are considerably easier to quantify than gradients in the ABL, due to the much higher concentration differences, these gradients appear to be good indicators of fluxes between the surface snow and the ABL only for NO x, HONO, and HNO Photochemistry in the firn air It has been demonstrated that the transfer of different trace gases between snow and air depends on temperature dependent physicaland/or photochemicalprocesses (Bales et al., 1995b; Sumner and Shepson, 1999; Hutterli et al., 1999, 2001; Couch et al., 2000; Honrath et al., 2000a; Jones et al., 2000). We can expect that the same processes also influence firn air concentrations. To investigate the effects of the physicaland photochemical processes, we made a quantitative comparison using maxima of the firn air concentrations and maxima of temperature and radiation in the surface snow. Radiation levels peak between 12:00 and 13:00. In contrast, snow temperatures peaked between 19:00 and 21:00 at 10 cm and between 21:00 and 23:00 at 30 cm.thus, photochemically produced species should exhibit highest firn air concentrations at noon while maxima of species dominated by ice-air partitioning should occur concomitant with the temperature maxima at night. However, since the sampled firn air is not restricted to a distinct layer, the correlation with the temperature is probably rather weak. In contrast, the agreement with the radiation intensity should be much better because the maximum of the photochemicalproduction occurs in all layers at the same time. Accordingly, an unambiguous classification is only possible in the cases of NO and NO 2. Highest firn air concentrations of NO and NO 2 normally occurred around noon (Fig. 3) indicating the photochemical production of NO x (=NO+NO 2 ) in the surface snowpack (Honrath et al., 2000a; Jones et al., 2000, 2001), which has been attributed to the photolysis of the NO 3 dissolved in the snow (Honrath et al., 2000b). Linear regressions of [NO], [NO 2 ], and [NO x ] with the photolysis rate j(no 2 ) measured at 10 cm on 19 June gave correlation coefficients (R 2 ) of 0.89, 0.91, and 0.90, respectively. A similar simple interpretation of the results of the shading experiments is hampered by the
6 1692 ARTICLE IN PRESS H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) Fig. 5. Time series of (a) HCOOH and (b) CH 3 COOH concentrations above and below the snow surface measured from 19 to 22 June. Sampling periods lasted between 19 and 38 min. Firn air concentrations during shading experiments are marked by open symbols with beginning and end of each experiment indicated by vertical lines (P: Plexiglass, PE: PE film, others: aluminum; see text). fact that NO and NO 2 are connected by a very fast photochemical cycle involving the reactions of NO with O 3 and HO 2 and the photolysis of NO 2. However, the NO NO 2 cycling has no effect on NO x, which can be analyzed regarding the effect of the shading experiments. NO x in the firn air decreased during all shading experiments and increased after unshading except after experiments late in the afternoon. These effects were more pronounced at 10 compared to 30 cm and are also in good agreement with a photochemical NO x source in the snowpack. The shading immediately stops photochemicalreactions in the snowpack as wellas the photolysis of NO 2, while the reaction of NO with O 3 is much less affected due to the more steady O 3 concentrations. Therefore, the quick drop in firn air NO and the constant or increasing NO 2 concentrations immediately after shading may be attributed to the shift in the photochemicalno x -O 3 -cycle and the continuing steady decrease of both compounds due to the missing photochemicalproduction in the snowpack. H 2 O 2 and HCHO also exhibited lower firn air concentrations at night versus daytime, indicating a possible photochemical contribution to elevated firn air values. However, maximum concentrations during the afternoon occurred later than the radiation maxima, but prior to temperature maxima. A better correlation might be obscured by the firn air sampling technique, which samples a mixture of ambient air and firn air from shallower depths, where the temperature maxima occur earlier than at 10 and 30 cm.we assume that a combination of chemicaland physicalprocesses determined measured HCHO firn air concentrations. Since H 2 O 2 concentrations in the firn air were lower than ambient values, a significant direct photochemical H 2 O 2 source seems unlikely. For HCOOH, CH 3 COOH, HONO, and HNO 3 the results are more ambiguous because no full diel cycles were measured. The available data show rather constant concentrations for all compounds at 10 and 30 cm.this result is surprising in the case of HONO, which can also be a product of the NO 3 photolysis similar to NO x (Mack and Bolton, 1999) and which normally exhibits higher firn air concentrations with increased radiation levels (Dibb et al., 2002; Zhou et al., 2001). Severaleffects can cause the differences in the behavior of HONO and NO x. First, the very high flow rates for the sampling of the acidic compounds might have obscured any photochemicaleffect by diluting the firn air with a much larger volume of air mostly including ambient air. Second, even if HONO and NO x are produced by the same photochemicalmechanism in the snow, the release of HONO into the firn air could be affected by its properties in the surface region of the ice crystals, which is commonly called quasi-liquid layer (QLL) because it is less ordered and exhibits different properties than the solid ice. Such a QLL could act as a reservoir for HONO, but not for NO and NO 2. Thus, the HONO release could be dominated by
7 H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) Fig. 6. Time series of (a) HONO and (b) HNO 3 concentrations above and below the snow surface measured from 19 to 22 June. Sampling periods lasted between 19 and 38 min. Firn air concentrations during shading experiments are marked by open symbols with beginning and end of each experiment indicated by vertical lines (P: Plexiglass, PE: PE film, others: aluminum; see text). physical equilibria, which follow diel cycles different to the radiation cycles. Third, constant HONO concentrations could result if the most dominant sources and sinks follow similar diel cycles. For example, if the NO 3 photolysis is the most important production process and the HONO photolysis the most important sink, both reactions would exhibit similar diel cycles that vary according to the radiation intensity. Nevertheless, the measurable nighttime HONO concentrations may also indicate a heterogeneous HONO production from NO 2 in the surface snow like observed in previous laboratory experiments (e.g. Finlayson-Pitts et al., 2003) Air snow partitioning In order to further examine the influence of the temperature-dependent equilibrium between snow and firn air, we analyzed the air snow partitioning considering the bulk ice, the ice grain air interface, and the adjacent firn air. We assume that the equilibrium between the firn air and the interface is quickly established. Therefore, we used measured firn air concentrations to calculate surface coverages for the compounds, which have been measured directly in laboratory experiments or can be deduced from partitioning data. With the specific surface areas, the amounts located at the interface are calculated and subtracted from the amounts measured in the snow samples if necessary. The distribution in Table 1 presents the results for H 2 O 2,CH 3 COOH, and HNO 3 following this procedure. The numbers revealsome important features for the air snow partitioning of reactive trace gases. For example, the largest fractions are restricted to the condensed phase, with less than 0.1% present in the firn air in all three cases. The snow air equilibrium constants are in the range of , 230, and M atm 1 for H 2 O 2,CH 3 COOH, and HNO 3. The constant for H 2 O 2 falls well in the range estimated from previous field studies (e.g. McConnell et al., 1997). Practically, all the H 2 O 2 and HNO 3 are located in the bulk ice, while a significant fraction of CH 3 COOH is present at the interface. The different distribution of H 2 O 2 and CH 3 COOH in the condensed phase is somewhat surprising. However, it agrees well with several results of previous studies. First, bulk acetate concentrations in polar snow are very low compared to concentrations in rain in remote regions (Chebbi and Carlier, 1996) possibly due to low concentrations in the bulk ice (Table 1). On the other hand, the H 2 O 2 uptake during the formation of fresh snow seems to be dominated by co-condensation leading to uniformly distributed H 2 O 2 concentrations in the bulk ice of fresh snow (Sigg et al., 1992). Second, since large amounts of CH 3 COOH are available at the interface, its degassing from aging snow can be expected to occur much faster than the degassing of H 2 O 2, which is to a large extent limited by the slow diffusion within
8 1694 ARTICLE IN PRESS H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) Table 1 Properties of the ice-firn air system and partitioning of H 2 O 2,CH 3 COOH, and HNO 3 at a depth of 10 cm a Bulk ice Interface Firn air Volume or surface area per volume unit 0.24 cm 3 cm 3b 46 cm 2 cm 3c 0.76 cm 3 cm 3d H 2 O 2 Concentration or surface coverage 11.5 mm e B10 10 cm 2f 560 pptv g Number of molecules per volume unit cm cm cm 3 Fraction CH 3 COOH Concentration or surface coverage 0.38 mm h cm 2i 3000 pptv j Number of molecules per volume unit cm cm cm 3 Fraction HNO 3 Concentration or surface coverage 2.3 mm e 7 pptv k Number of molecules per volume unit cm cm 3l cm 3 Fraction a Calculations for June 19, 15:00 22:00 (T= 19 C; r air = cm 3 ). b Calculated with the measured snow density of 0.22 g cm 3 and the ice density of 0.92 g cm 3 at T= 19 C. c Product of surface area (210 cm 2 g 1 ) and volume density (0.22 g cm 3 ). d (1 0.24) cm 3 cm 3. e Measured bulk snow concentration. f Clegg and Abbatt (2001). g see Fig. 4a. h Calculated with an average CH 3 COOH bulk snow concentration of 0.5 mm (Dibb et al., 1994; De Angelis and Legrand, 1995; Legrand and Mayewski, 1997) taking into account the amount located at the interface. i Sokolov and Abbatt (2002). j see Fig. 5b. k see Fig. 6b. l Calculated with a partitioning coefficient of n interface /n firn air =25 obtained from the adsorption enthalpy and entropy of HNO 3 (Bartels-Rausch et al., 2002). the ice matrix. Accordingly, field studies have shown that the loss of H 2 O 2 from surface snow occurs over weeks to months (e.g. Bales et al., 1995b), while CH 3 COOH in surface snow can significantly decrease within hours to days (Dibb et al., 1994; De Angelis and Legrand, 1995). Molecular dynamics simulations demonstrate that HCOOH and CH 3 COOH are trapped at the ice surface (Compoint et al., 2002). These calculations show that the incorporation of HCOOH in the bulk is more likely compared to CH 3 COOH. Nevertheless, we assume that a somewhat lower, however still significant fraction of HCOOH is located at the interface of the crystals, since HCOOH concentrations in the firn and firn air behave similar to CH 3 COOH. To further investigate photochemicalreactions occurring in the snow, it would be important to know if the reactive compounds are in the solid ice, dissolved in the QLL, or adsorbed on the ice crystal-air interface. For example, Honrath et al. (2000b) discussed that the photochemistry of NO 3 is different in all three environments. Unfortunately, the laboratory studies of Clegg and Abbatt (2001) and Sokolov and Abbatt (2002) regarding the surface coverages of H 2 O 2 and CH 3 COOH were performed at lower temperatures below 245 K and possibly without the presence of a QLL. Thus, these experimental results do not allow distinguishing between adsorbed amounts and amounts present in the QLL. In addition, Bartels-Rausch et al. (2002) stressed that their results are only valid for the amount of undissociated HNO 3 adsorbed at the surface of the ice crystal. Thus, the bulk ice quantity shown for NO 3 in Table 1 includes NO 3 in the QLL as well as in the bulk ice (but does not include adsorbed NO 3 at the ice grain-air interface). Distribution coefficients for the partitioning of HNO 3 between ice and liquid water indicate that most of this NO 3 is likely to be in the QLL. Gross (2003) obtained an average distribution coefficient (ice/liquid water) for NO 3 of , in a series of experiments using NaNO 3 and KNO 3, while Thibert and Domin!e (1998) reported a much smaller distribution coefficient for HNO 3 ( at 19 C). Even if the QLL volume were only B10 4 of the total(equivalent to a layer on the ice surface with a thickness of cm), these distribution coefficients imply that % of the NO 3 is present in the QLL. Although HNO 3 is almost exclusively present in the condensed phase within the firn firn air system, the
9 H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) calculations suggest that by far the largest fraction is present in the QLL and, thus, available for release to the atmosphere. Indeed, substantialpostdepositionallosses from surface snow have been reported (e.g. Fischer et al., 1998; Wagnon et al., 1999). Moreover, the main source of the NO x produced by irradiated surface snow is most probably the photolysis of nitrate present in the QLL rather than the photolysis of nitrate in the bulk ice. Since considerably higher amounts of the trace gases are located at the interface compared to amounts in the firn air, firn air concentrations are expected to be very sensitive to physicalparameters like temperature or surface area. For example, a simultaneous decrease of H 2 O 2 and temperature at 10 cm during the night from 20 to 21 June was observed (Fig. 4a). Between 22:00 and 7:00, the snow temperature dropped from about 18 C to 19 C, while H 2 O 2 declined from around pptv. Laboratory studies gave an enthalpy of DH = 55 kj mol 1 for the temperature dependence of the ice-atmosphere equilibrium of H 2 O 2 below 11 C (Conklin et al., 1993). Therefore, the temperature drop would lead to a concentration decrease of o10% as long as all other parameters remain constant. This is much less than the observed decline of 40%, which would require a temperature drop of 5 K. Measurements of the H 2 O 2 uptake on ice resulted in a more or less constant surface coverage in the temperature range between 218 and 238 K (Clegg and Abbatt, 2001). Nevertheless, a slight temperature dependence of the surface coverage well below the uncertainty of the laboratory experiments (Clegg and Abbatt, 2001) could explain a decrease of H 2 O 2 in the firn air on the order of 200 pptv due to the exceeding amounts present at the surface compared to the firn air. In addition, Dibb and Arsenault (2002) noted that firn air profiles of CH 3 COOH at Summit exhibited large fluctuations of pptv between profiles measured with time lags of 4 5 h. With the surface coverage given in Table 1, a change in the surface area of only 0.2% could explain fluctuations in firn air concentrations on the order of 2200 pptv. Cabanes et al. (2002) studied trends of surface areas of Arctic snow. They found that the surface area of fresh snow decreased significantly with rates between 19 and 730 cm 2 g 1 day 1. However, the surface area of older underlying snow remained rather stable (Domin!e et al., 2002). The temperature gradients during this study were too low to induce metamorphism leading to an increase in the surface area. Therefore, decreasing surface areas can explain observed increases, but not the also observed quick drops in firn air concentrations of CH 3 COOH. More likely are differences in firn air concentrations that may be related to snow characteristics and/or spatial variability at the sampling site. Nevertheless, surface area and surface coverage are possibly as important as the temperature in determining equilibria between snow and firn air and thus firn air concentrations. Cabanes et al. (2002) calculated that the surface area and the temperature-dependent adsorption could affect HNO 3 concentrations not only in the firn air, but even in the boundary layer. As discussed above, complications due to the varying flow rates and flow patterns make detailed model interpretation of the data untenable for the current study. However, small changes in the physical characteristics of snow, especially the surface area, may yield significant differences in firn air concentrations. Exchange with the ABL also affects firn air concentrations. For example, the average H 2 O 2 flux measured above the snow surface (Jacobi et al., 2002) during 20 June, 22:00 21 June, 7:00 resulted in a slight emission, on the order of molecules m 2 s 1, contributing to the drop in the measured firn air concentration at 10 cm.this small flux could remove the entire H 2 O 2 amount in the firn air of the top 30 cm of the snow within o1 h, were it not replenished from the condensed phase. 5. Conclusions The comparison of trace gas concentration profiles above and below the snow surface during day and night and during shading experiments revealed that firn air concentrations are determined by photochemicalreactions, temperature-dependent equilibria between the surface snow and adjacent firn air, and the exchange between the firn air and the air above the snow. Removalof NO x during shading experiments confirms previous results demonstrating that considerable amounts are produced in the surface snow and released to the firn air due to photochemicalprocesses. However, during the shading experiments two different regimes were identified indicating that the release from the snowpack is considerably slower than the rates in the photochemicalno x -O 3 cycle. Nevertheless, features like elevated NO 2 firn air concentrations at night remain unexplained warranting a more detailed study of the mechanism of NO x production in surface snow and the photochemicalno x reactions in the firn air. Mixing ratios of H 2 O 2,CH 3 COOH, and HNO 3 in the firn air mainly depend on gas and snow phase equilibria. The analysis of the firn air data indicate that a large fraction of CH 3 COOH is present at the ice grain air interface making adsorption to the snow and the surface area of the snow very important parameters for firn air concentrations. Despite the lack of laboratory data for HCOOH, we conclude that it is also mainly located in the QLL or at the grain air interface based on the similarities in the behavior of HCOOH and CH 3 COOH in the firn and firn air. However, further laboratory studies of the ice-gas phase equilibria and surface coverage of ice at
10 1696 ARTICLE IN PRESS H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) temperatures close to the freezing point are needed. For example, the results could be used to analyze whether observed diel cycles of alkyl nitrates in the firn air (Swanson et al., 2002) are caused by phase partitioning or photochemicalproduction. Since most of the NO 3 is probably located in the QLL, it seems likely that the NO x production is also governed by reactions in this layer. Therefore, laboratory experiments in a broad range of temperatures could demonstrate the importance of the QLL for the NO 3 photolysis in ice and snow. For the correct description of the conditions within the firn and the firn air, further information about the nature and properties of the QLL of the snow grains is needed. For example, Dubowski et al. (2002) conclude that some properties of the QLL differ from those of supercooled water. The results underline the obvious need to characterize the amount and properties of this layer using for example NMR techniques (Cho et al., 2002) and to include this knowledge in models describing the transfer of trace gases between firn and firn air. Acknowledgements Financialsupport by the NationalScience Foundation (NSF), grants OPP , OPP , OPP , and OPP , is gratefully acknowledged. HWJ thanks the Deutsche Forschungsgemeinschaft (DFG) for a research stipend. The Summit summer crew, VECO Polar Resources, and the Air National Guard provided assistance and equipment during the field experiments. References Albert, M.R., Shultz, E.F., Snow and firn properties and air snow transport processes at Summit, Greenland. Atmospheric Environment 36, Albert, M.R., Grannas, A.M., Bottenheim, J., Shepson, P.B., Perron, F.E., Processes and properties of snow-air transfer in the high Arctic with application to interstitial ozone at Alert, Canada. Atmospheric Environment 36, Bales, R.C., Losleben, M.V., McConnell, J.R., Fuhrer, K., Neftel, A., 1995a. H 2 O 2 in snow, air and open pore space in firn at Summit, Greenland. Geophysical Research Letters 22, Bales, R.C., McConnell, J.R., Losleben, M.V., Conklin, M.H., Fuhrer, K., Neftel, A., Dibb, J.E., Kahl, J.D.W., Stearns, C.R., 1995b. Dielvariations of H 2 O 2 in Greenland: a discussion of the cause and effect relationship. Journal of GeophysicalResearch 100, Bartels-Rausch, T., Eichler, B., Zimmermann, P., G.aggeler, H.W., Ammann, M., The adsorption enthalpy of nitrogen oxides on crystalline ice. Atmospheric Chemistry and Physics 2, Cabanes, A., Legagneux, L., Domin!e, F., Evolution of the specific surface area and of crystalmorphology of Arctic fresh snow during the ALERT 2000 campaign. Atmospheric Environment 36, Chebbi, A., Carlier, P., Carboxylic acids in the troposphere, occurrence, sources, and sinks: a review. Atmospheric Environment 30, Cho, H., Shepson, P.B., Barrie, L.A., Cowin, J.P., Zaveri, R., NMR investigation of the quasi-brine layer in ice/brine mixtures. Journalof PhysicalChemistry B 106, Clegg, S.M., Abbatt, J.P.D., Uptake of gas-phase SO 2 and H 2 O 2 by ice surfaces: dependence on partialpressure, temperature, and surface acidity. Journalof Physical Chemistry A 105, Compoint, M., Toubin, C., Picaud, S., Hoang, P.N.M., Girardet, C., Geometry and dynamics of formic and acetic acids adsorbed on ice. ChemicalPhysics Letters 365, 1 7. Conklin, M.H., Sigg, A., Neftel, A., Bales, R.C., Atmosphere-snow transfer for H 2 O 2 : microphysical considerations. Journalof GeophysicalResearch 98, Couch, T.L., Sumner, A.L., Dassau, T.M., Shepson, P.B., Honrath, R.E., An investigation of the interaction of carbonylcompounds with the snowpack. Geophysical Research Letters 27, Cullen, N.J., Steffen, K., Unstable near-surface boundary conditions in summer on top of the Greenland ice sheet. GeophysicalResearch Letters 28, De Angelis, M., Legrand, M., Preliminary investigations of post depositionaleffects on HCl, HNO 3, and organic acids in polar firn layers. In: Delmas, R. (Ed.), Ice Core Studies of Global Biogeochemical Cycles, NATO ASI Series I, Vol. 30. Springer, Berlin, pp Dibb, J.E., Arsenault, M., Should not snowpacks be a source of monocarboxylic acids? Atmospheric Environment 36, Dibb, J.E., Talbot, R.W., Bergin, M.H., Soluble acidic species in air and snow at Summit, Greenland. Geophysical Research Letters 21, Dibb, J.E., Arsenault, M., Peterson, M.C., Honrath, R.E., Fast nitrogen oxide photochemistry in Summit, Greenland snow. Atmospheric Environment 36, Domin!e, F., Cabanes, A., Legagneux, L., Structure, microphysics, and surface area of the Arctic snowpack near Alert during the ALERT 2000 campaign. Atmospheric Environment 36, Dubowski, Y., Colussi, A.J., Boxe, C., Hoffmann, M.R., Monotonic increase of nitrite yields in the photolysis of nitrate in ice and water between 238 and 294 K. Journalof PhysicalChemistry A 106, Finlayson-Pitts, B.J., Wingen, L.M., Sumner, A.L., Syomin, D., Ramazan, K.A., The heterogeneous hydrolysis of NO 2 in laboratory systems and in outdoor and indoor atmospheres: an integrated mechanism. PhysicalChemistry ChemicalPhysics 5, Fischer, H., Wagenbach, D., Kipfstuhl, J., Sulfate and nitrate firn concentrations on the Greenland ice sheet, 1. Large-scale geographical deposition changes. Journal of GeophysicalResearch 103,
11 H.-W. Jacobi et al. / Atmospheric Environment 38 (2004) Gross, G.W., Nitrates in ice: uptake; dielectric response by the layered capacitor method. Canadian Journal of Physics 81, Honrath, R.E., Peterson, M.C., Dziobak, M.P., Dibb, J.E., Arsenault, M.A., Green, S.A., 2000a. Release of NO x from sunlight-irradiated midlatitude snow. Geophysical Research Letters 27, Honrath, R.E., Guo, S., Peterson, M.C., Dziobak, M.P., Dibb, J.E., Arsenault, M.A., 2000b. Photochemical production of gas phase NO x from ice crystalno 3. Journalof GeophysicalResearch 105, Honrath, R.E., Lu, Y., Peterson, M.C., Dibb, J.E., Arsenault, M.A., Cullen, N.J., Steffen, K., Vertical fluxes of photochemically important compounds above the snowpack at Summit, Greenland. Atmospheric Environment 36, Hutterli, M.A., R.othlisberger, R., Bales, R.C., Atmosphere-to-snow-to-firn transfer studies of HCHO at Summit, Greenland. Geophysical Research Letters 26, Hutterli, M.A., McConnell, J.R., Stewart, R.W., Jacobi, H.-W., Bales, R.C., Impact of temperature-driven cycling of hydrogen peroxide (H 2 O 2 ) between air and snow on the planetary boundary layer. Journal of Geophysical Research 106, Jacobi, H.-W., Frey, M.M., Hutterli, M.A., Bales, R.C., Schrems, O., Cullen, N.J., Steffen, K., Koehler, C., Long-term measurements of hydrogen peroxide and formaldehyde exchange between the atmosphere and surface snow at Summit, Greenland. Atmospheric Environment 36, Jones, A.E., Weller, R., Wolff, E.W., Jacobi, H.-W., Speciation and rate of photochemicalno and NO 2 production in Antarctic snow. GeophysicalResearch Letters 27, Jones, A.E., Weller, R., Anderson, P.S., Jacobi, H.-W., Wolff, E.W., Schrems, O., Miller, H., Measurements of NO x emissions from the antarctic snowpack. GeophysicalResearch Letters 28, Legrand, M., Mayewski, P., Glaciochemistry of polar ice cores: a review. Reviews of Geophysics 35, Mack, J., Bolton, J.R., Photochemistry of nitrite and nitrate in aqueous solution: a review. Journal of Photochemistry and Photobiology A 128, McConnell, J.R., Bales, R.C., Winterle, J.R., Kuhns, H., Stearns, C.R., A lumped parameter model for the atmosphere-to-snow transfer function for hydrogen peroxide. Journalof GeophysicalResearch 102, Peterson, M.C., Honrath, R.E., Observations of rapid photochemicaldestruction of ozone in snowpack interstitial air. GeophysicalResearch Letters 28, Sigg, A., Staffelbach, T., Neftel, A., Gas phase measurements of hydrogen peroxide in Greenland and their meaning for the interpretation of H 2 O 2 records in ice cores. Journal of Atmospheric Chemistry 14, Sokolov, O., Abbatt, J.P.D., Adsorption to ice of n-alcohols (ethanol to 1-hexanol) acetic acid, and hexanal. Journalof PhysicalChemistry A 106, Sumner, A.L., Shepson, P.B., Snowpack production of formaldehyde and its effect on the Arctic troposphere. Nature 398, Swanson, A.L., Blake, N.J., Dibb, J.E., Albert, M.R., Blake, D.R., Rowland, F.S., Photochemically induced production of CH 3 Br, CH 3 I, C 2 H 5 I, ethene, and propene within surface snow at Summit, Greenland. Atmospheric Environment 36, Thibert, E., Domin!e, F., Thermodynamics and kinetics of the solid solution of HNO 3 in ice. Journalof Physical Chemistry B 102, Wagnon, P., Delmas, R.J., Legrand, M., Loss of volatile acid species from upper firn layers at Vostok, Antarctica. Journalof GeophysicalResearch 104, Yang, J., Honrath, R.E., Peterson, M.C., Dibb, J.E., Sumner, A.L., Shepson, P.B., Frey, M., Jacobi, H.-W., Swanson, A., Blake, N., Impacts of snowpack photochemistry on deduced levels of OH and peroxy radicals at Summit, Greenland. Atmospheric Environment 36, Zhou, X., Beine, H.J., Honrath, R.E., Fuentes, J.D., Simpson, W., Shepson, P.B., Bottenheim, J.W., Snowpack photochemicalproduction of HONO: a major source of OH in the Arctic boundary layer in springtime. Geophysical Research Letters 28,
Partitioning of formaldehyde between air and ice at 351C to 51C
 Atmospheric Environment 36 (2002) 2157 2163 Partitioning of formaldehyde between air and ice at 351C to 51C John F. Burkhart*, Manuel A. Hutterli, Roger C. Bales Department of Hydrology and Water Resources,
Atmospheric Environment 36 (2002) 2157 2163 Partitioning of formaldehyde between air and ice at 351C to 51C John F. Burkhart*, Manuel A. Hutterli, Roger C. Bales Department of Hydrology and Water Resources,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D1, 4023, doi: /2002jd002528, 2003
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D1, 4023, doi:10.1029/2002jd002528, 2003 Sensitivity of hydrogen peroxide (H 2 O 2 ) and formaldehyde (HCHO) preservation in snow to changing environmental
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D1, 4023, doi:10.1029/2002jd002528, 2003 Sensitivity of hydrogen peroxide (H 2 O 2 ) and formaldehyde (HCHO) preservation in snow to changing environmental
First observations of atmospheric Hydrogen Peroxide (H 2. ) and Methylhydroperoxide (CH 3
 First observations of atmospheric Hydrogen Peroxide (H 2 ) and Methylhydroperoxide (CH 3 OOH) in West Antarctica: comparison of experiment and model results Markus M. Frey a, Richard W. Stewart b, Joseph
First observations of atmospheric Hydrogen Peroxide (H 2 ) and Methylhydroperoxide (CH 3 OOH) in West Antarctica: comparison of experiment and model results Markus M. Frey a, Richard W. Stewart b, Joseph
PROBLEMS Sources of CO Sources of tropospheric ozone
 220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
Microstructure and permeability in the near-surface firn near a potential US deep-drilling site in West Antarctica
 62 Annals of Glaciology 39 2004 Microstructure and permeability in the near-surface firn near a potential US deep-drilling site in West Antarctica Ursula K. RICK, 1 Mary R. ALBERT 1,2 1 Thayer School of
62 Annals of Glaciology 39 2004 Microstructure and permeability in the near-surface firn near a potential US deep-drilling site in West Antarctica Ursula K. RICK, 1 Mary R. ALBERT 1,2 1 Thayer School of
Studies of Peroxyacetyl nitrate (PAN) and its interaction with the snowpack at Summit, Greenland
 University of New Hampshire University of New Hampshire Scholars' Repository Earth Sciences Scholarship Earth Sciences 5-27-2002 Studies of Peroxyacetyl nitrate (PAN) and its interaction with the snowpack
University of New Hampshire University of New Hampshire Scholars' Repository Earth Sciences Scholarship Earth Sciences 5-27-2002 Studies of Peroxyacetyl nitrate (PAN) and its interaction with the snowpack
Measurements of Ozone. Why is Ozone Important?
 Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
CHM 5423 Atmospheric Chemistry Notes on reactions of organics in the troposphere (Chapter 5)
 CHM 5423 Atmospheric Chemistry Notes on reactions of organics in the troposphere (Chapter 5) 5.1 Introduction In general, the lifetime of a molecule in the troposphere is governed by a variet of processes.
CHM 5423 Atmospheric Chemistry Notes on reactions of organics in the troposphere (Chapter 5) 5.1 Introduction In general, the lifetime of a molecule in the troposphere is governed by a variet of processes.
CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION
 i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
A reassessment of Antarctic plateau reactive nitrogen based on ANTCI 2003 airborne and ground based measurements
 ARTICLE IN PRESS Atmospheric Environment 42 (2008) 2831 2848 www.elsevier.com/locate/atmosenv A reassessment of Antarctic plateau reactive nitrogen based on ANTCI 2003 airborne and ground based measurements
ARTICLE IN PRESS Atmospheric Environment 42 (2008) 2831 2848 www.elsevier.com/locate/atmosenv A reassessment of Antarctic plateau reactive nitrogen based on ANTCI 2003 airborne and ground based measurements
Tananyag fejlesztés idegen nyelven
 Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Fundamentals to atmospheric chemical reactions. The stratospheric
Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Fundamentals to atmospheric chemical reactions. The stratospheric
Chapter 1. Introduction
 Chapter 1 Introduction I-2 Faraday was the first to propose that water ice was not an absolutely rigid solid as assumed by most scientists of his era. 1-4 However, over the last 150 years a much greater
Chapter 1 Introduction I-2 Faraday was the first to propose that water ice was not an absolutely rigid solid as assumed by most scientists of his era. 1-4 However, over the last 150 years a much greater
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
8.2 Tropospheric ozone
 8.2 Tropospheric ozone Prev Chapter 8. Ozone Next 8.2 Tropospheric ozone Tropospheric ozone is only about 10% of the total amount of ozone contained in a vertical column in the atmosphere. However, this
8.2 Tropospheric ozone Prev Chapter 8. Ozone Next 8.2 Tropospheric ozone Tropospheric ozone is only about 10% of the total amount of ozone contained in a vertical column in the atmosphere. However, this
N10/4/CHEMI/SP2/ENG/TZ0/XX CHEMISTRY STANDARD LEVEL PAPER 2. Thursday 11 November 2010 (afternoon) Candidate session number.
 N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
EVALUATION OF ATMOSPHERIC PROCESSES FOR OZONE FORMATION FROM VEHICLE EMISSIONS
 EVALUATION OF ATMOSPHERIC PROCESSES FOR OZONE FORMATION FROM VEHICLE EMISSIONS by WILLIAM P. L. CARTER STATEWIDE AIR POLLUTION RESEARCH CENTER, and COLLEGE OF ENGINEERING CENTER FOR ENVIRONMENTAL RESEARCH
EVALUATION OF ATMOSPHERIC PROCESSES FOR OZONE FORMATION FROM VEHICLE EMISSIONS by WILLIAM P. L. CARTER STATEWIDE AIR POLLUTION RESEARCH CENTER, and COLLEGE OF ENGINEERING CENTER FOR ENVIRONMENTAL RESEARCH
Tropospheric OH chemistry
 Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Isotopic ratios in gas-phase HNO 3 and snow nitrate at Summit, Greenland
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114,, doi:10.1029/2009jd012134, 2009 Isotopic ratios in gas-phase HNO 3 and snow nitrate at Summit, Greenland Julia C. Jarvis, 1 Meredith G. Hastings, 2 Eric J. Steig,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114,, doi:10.1029/2009jd012134, 2009 Isotopic ratios in gas-phase HNO 3 and snow nitrate at Summit, Greenland Julia C. Jarvis, 1 Meredith G. Hastings, 2 Eric J. Steig,
CHEM/ENVS 380 S14, Midterm Exam ANSWERS 1 Apr 2014
 PART- A. Multiple Choice Questions (5 points each): Each question may have more than one correct answer. You must select ALL correct answers, and correct answers only, to receive full credit. 1. Which
PART- A. Multiple Choice Questions (5 points each): Each question may have more than one correct answer. You must select ALL correct answers, and correct answers only, to receive full credit. 1. Which
Arctic Halogen Chemistry Part II
 Arctic Halogen Chemistry Part II Kerri A. Pratt Department of Chemistry Dept. of Earth & Environmental Sciences University of Michigan 2017 Connaught Summer Institute on Arctic Science Ozone Reminders
Arctic Halogen Chemistry Part II Kerri A. Pratt Department of Chemistry Dept. of Earth & Environmental Sciences University of Michigan 2017 Connaught Summer Institute on Arctic Science Ozone Reminders
Photochemically induced production of CH 3 Br, CH 3 I, C 2 H 5 I, ethene, and propene within surface snow at Summit, Greenland
 Atmospheric Environment 36 (2002) 2671 2682 Photochemically induced production of CH 3 Br, CH 3 I, C 2 H 5 I, ethene, and propene within surface snow at Summit, Greenland Aaron L. Swanson a, Nicola J.
Atmospheric Environment 36 (2002) 2671 2682 Photochemically induced production of CH 3 Br, CH 3 I, C 2 H 5 I, ethene, and propene within surface snow at Summit, Greenland Aaron L. Swanson a, Nicola J.
Research. Rate of Evolution of the Specific Surface Area of Surface Snow Layers. 2. Snow Sampling. 1. Introduction
 Research Rate of Evolution of the Specific Surface Area of Surface Snow Layers AXEL CABANES, LOÏC LEGAGNEUX, AND FLORENT DOMINEÄ * CNRS, Laboratoire de Glaciologie et Géophysique de l Environnement, B.P.
Research Rate of Evolution of the Specific Surface Area of Surface Snow Layers AXEL CABANES, LOÏC LEGAGNEUX, AND FLORENT DOMINEÄ * CNRS, Laboratoire de Glaciologie et Géophysique de l Environnement, B.P.
CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION
 1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
Unique nature of Earth s atmosphere: O 2 present photosynthesis
 Atmospheric composition Major components N 2 78% O 2 21% Ar ~1% Medium components CO 2 370 ppmv (rising about 1.5 ppmv/year) CH 4 1700 ppbv H 2 O variable Trace components H 2 600 ppbv N 2 O 310 ppbv CO
Atmospheric composition Major components N 2 78% O 2 21% Ar ~1% Medium components CO 2 370 ppmv (rising about 1.5 ppmv/year) CH 4 1700 ppbv H 2 O variable Trace components H 2 600 ppbv N 2 O 310 ppbv CO
ATM 507 Lecture 5. Text reading Chapter 4 Problem Set #2 due Sept. 20 Today s topics Photochemistry and Photostationary State Relation
 ATM 507 Lecture 5 Text reading Chapter 4 Problem Set #2 due Sept. 20 Today s topics Photochemistry and Photostationary State Relation Beer-Lambert Law (for the absorption of light) Used to describe the
ATM 507 Lecture 5 Text reading Chapter 4 Problem Set #2 due Sept. 20 Today s topics Photochemistry and Photostationary State Relation Beer-Lambert Law (for the absorption of light) Used to describe the
AQRP Project Implementation and evaluation of new HONO mechanisms in a 3-D Chemical Transport Model for Spring 2009 in Houston
 AQRP Project 12-028 Implementation and evaluation of new HONO mechanisms in a 3-D Chemical Transport Model for Spring 2009 in Houston Barry Lefer 1, Prakash Karamchandani 2, Chris Emery 2, Jochen Stutz
AQRP Project 12-028 Implementation and evaluation of new HONO mechanisms in a 3-D Chemical Transport Model for Spring 2009 in Houston Barry Lefer 1, Prakash Karamchandani 2, Chris Emery 2, Jochen Stutz
Test Booklet. Subject: SC, Grade: HS CST High School Chemistry Part 2. Student name:
 Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
P2.11 THE LAKE SHADOW EFFECT OF LAKE BREEZE CIRCULATIONS AND RECENT EXAMPLES FROM GOES VISIBLE SATELLITE IMAGERY. Frank S. Dempsey
 P2.11 THE LAKE SHADOW EFFECT OF LAKE BREEZE CIRCULATIONS AND RECENT EXAMPLES FROM GOES VISIBLE SATELLITE IMAGERY Frank S. Dempsey 1. ABSTRACT The lake shadow effect is a component of the lake breeze circulation
P2.11 THE LAKE SHADOW EFFECT OF LAKE BREEZE CIRCULATIONS AND RECENT EXAMPLES FROM GOES VISIBLE SATELLITE IMAGERY Frank S. Dempsey 1. ABSTRACT The lake shadow effect is a component of the lake breeze circulation
7.2 NORTHEAST OXIDANT AND PARTICLE STUDY (NEOPS): PRELIMINARY RESULTS FROM THE CENTERTON, NEW JERSEY, FIELD SITE
 7.2 NORTHEAST OXIDANT AND PARTICLE STUDY (NEOPS): PRELIMINARY RESULTS FROM THE CENTERTON, NEW JERSEY, FIELD SITE Nancy A. Marley* and Jeffrey S. Gaffney Environmental Research Division Argonne National
7.2 NORTHEAST OXIDANT AND PARTICLE STUDY (NEOPS): PRELIMINARY RESULTS FROM THE CENTERTON, NEW JERSEY, FIELD SITE Nancy A. Marley* and Jeffrey S. Gaffney Environmental Research Division Argonne National
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 $
 Atmospheric Environment 42 (2008) 2849 2863 www.elsevier.com/locate/atmosenv Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 $ Yuhang Wang a,, Yunsoo Choi a,
Atmospheric Environment 42 (2008) 2849 2863 www.elsevier.com/locate/atmosenv Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 $ Yuhang Wang a,, Yunsoo Choi a,
AN EXPERIMENTAL INVESTIGATION OF BOILING HEAT CONVECTION WITH RADIAL FLOW IN A FRACTURE
 PROCEEDINGS, Twenty-Fourth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 25-27, 1999 SGP-TR-162 AN EXPERIMENTAL INVESTIGATION OF BOILING HEAT CONVECTION
PROCEEDINGS, Twenty-Fourth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 25-27, 1999 SGP-TR-162 AN EXPERIMENTAL INVESTIGATION OF BOILING HEAT CONVECTION
Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations
 Introduction Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations Fraunhofer Institut Atmosphärische Umweltforschung, IFU Kreuzeckbahnstr.
Introduction Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations Fraunhofer Institut Atmosphärische Umweltforschung, IFU Kreuzeckbahnstr.
MAHESH TUTORIALS I.C.S.E.
 MAHESH TUTORIALS I.C.S.E. GRADE - X (2017-2018) Exam No. : MT/ICSE/SEMI PRELIM - I-SET -A 008 Sulphuric acid, Ammonia, Analytical Chemistry, Organic Chemistry HCl, Nitric acid, Metallurgy Chemistry SCIENCE
MAHESH TUTORIALS I.C.S.E. GRADE - X (2017-2018) Exam No. : MT/ICSE/SEMI PRELIM - I-SET -A 008 Sulphuric acid, Ammonia, Analytical Chemistry, Organic Chemistry HCl, Nitric acid, Metallurgy Chemistry SCIENCE
Calorimetry, Heat and ΔH Problems
 Calorimetry, Heat and ΔH Problems 1. Calculate the quantity of heat involved when a 70.0g sample of calcium is heated from 22.98 C to 86.72 C. c Ca= 0.653 J/g C q = 2.91 kj 2. Determine the temperature
Calorimetry, Heat and ΔH Problems 1. Calculate the quantity of heat involved when a 70.0g sample of calcium is heated from 22.98 C to 86.72 C. c Ca= 0.653 J/g C q = 2.91 kj 2. Determine the temperature
Arctic Oxidation Chemistry
 19 July 2016 Connaught Summer Institute 1 Arctic Oxidation Chemistry Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
19 July 2016 Connaught Summer Institute 1 Arctic Oxidation Chemistry Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
The Atmosphere. All of it. In one hour. Mikael Witte 10/27/2010
 The Atmosphere All of it. In one hour. Mikael Witte 10/27/2010 Outline Structure Dynamics - heat transport Composition Trace constituent compounds Some Atmospheric Processes Ozone destruction in stratosphere
The Atmosphere All of it. In one hour. Mikael Witte 10/27/2010 Outline Structure Dynamics - heat transport Composition Trace constituent compounds Some Atmospheric Processes Ozone destruction in stratosphere
Today. Events. Terrestrial Planet Atmospheres (continued) Homework DUE. Review next time? Exam next week
 Today Terrestrial Planet Atmospheres (continued) Events Homework DUE Review next time? Exam next week Planetary Temperature A planet's surface temperature is determined by the balance between energy from
Today Terrestrial Planet Atmospheres (continued) Events Homework DUE Review next time? Exam next week Planetary Temperature A planet's surface temperature is determined by the balance between energy from
Surprisingly small HONO emissions from snow surfaces at Browning Pass, Antarctica
 Atmos. Chem. Phys., 6, 569 58, 6 www.atmos-chem-phys.net/6/569/6/ Author(s) 6. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Surprisingly small HONO emissions
Atmos. Chem. Phys., 6, 569 58, 6 www.atmos-chem-phys.net/6/569/6/ Author(s) 6. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Surprisingly small HONO emissions
Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003
 Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 Yuhang Wang 1, Yunsoo Choi 1, Tao Zeng 1, Douglas Davis 1, Martin Buhr 2, L. Gregory Huey 1, and William Neff
Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 Yuhang Wang 1, Yunsoo Choi 1, Tao Zeng 1, Douglas Davis 1, Martin Buhr 2, L. Gregory Huey 1, and William Neff
molality: m = = 1.70 m
 C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? Miscible: two or more substances blend together for form a solution
C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? Miscible: two or more substances blend together for form a solution
Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO
 Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO Brian G. Heikes, Center for Atmospheric Chemical Studies, Graduate School of Oceanography, University of Rhode Island
Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO Brian G. Heikes, Center for Atmospheric Chemical Studies, Graduate School of Oceanography, University of Rhode Island
Chapter 14. Liquids and Solids
 Chapter 14 Liquids and Solids Section 14.1 Water and Its Phase Changes Reviewing What We Know Gases Low density Highly compressible Fill container Solids High density Slightly compressible Rigid (keeps
Chapter 14 Liquids and Solids Section 14.1 Water and Its Phase Changes Reviewing What We Know Gases Low density Highly compressible Fill container Solids High density Slightly compressible Rigid (keeps
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION
 CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time
 Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Chapter The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d.
 Chapter-6 Multiple Choice Questions 1. The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d. deposition 2. The movement of water among the great global
Chapter-6 Multiple Choice Questions 1. The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d. deposition 2. The movement of water among the great global
ATOC 3500/CHEM 3151 Week 9, 2016 The Game Changer. Some perspective The British Antarctic Survey The Ozone Hole International Regulations
 ATOC 3500/CHEM 3151 Week 9, 2016 The Game Changer Some perspective The British Antarctic Survey The Ozone Hole International Regulations Rowland (1974): The work is going very well, but it may mean the
ATOC 3500/CHEM 3151 Week 9, 2016 The Game Changer Some perspective The British Antarctic Survey The Ozone Hole International Regulations Rowland (1974): The work is going very well, but it may mean the
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1
 ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. a. The gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. a. The gas
Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003
 Atmospheric Environment 41 (2007) 3944 3958 www.elsevier.com/locate/atmosenv Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 Yuhang Wang a,, Yunsoo Choi a, Tao
Atmospheric Environment 41 (2007) 3944 3958 www.elsevier.com/locate/atmosenv Assessing the photochemical impact of snow NO x emissions over Antarctica during ANTCI 2003 Yuhang Wang a,, Yunsoo Choi a, Tao
Kinetics & Equilibrium Review Packet. Standard Level. 1. Which quantities in the enthalpy level diagram are altered by the use of a catalyst?
 Kinetics & Equilibrium Review Packet Standard Level 1. Which quantities in the enthalpy level diagram are altered by the use of a catalyst? Enthalpy I II III Time A. I and II only B. I and III only C.
Kinetics & Equilibrium Review Packet Standard Level 1. Which quantities in the enthalpy level diagram are altered by the use of a catalyst? Enthalpy I II III Time A. I and II only B. I and III only C.
Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet
 C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? 2. What is saturated? Unsaturated? Supersaturated? 3. How does
C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? 2. What is saturated? Unsaturated? Supersaturated? 3. How does
Frey, et al. Climate sensitivity of the H 2 O 2 ice core record 1
 Frey, et al. Climate sensitivity of the H 2 O 2 ice core record 1 Climate sensitivity of the century-scale hydrogen peroxide (H 2 O 2 ) record preserved in 23 ice cores from West Antarctica Markus M. Frey
Frey, et al. Climate sensitivity of the H 2 O 2 ice core record 1 Climate sensitivity of the century-scale hydrogen peroxide (H 2 O 2 ) record preserved in 23 ice cores from West Antarctica Markus M. Frey
The German Contribution to the WMO GAW Programme:
 WORLD METEOROLOGICAL ORGANIZATION GLOBAL ATMOSPHERE WATCH The German Contribution to the WMO GAW Programme: Upon the 225 th Anniversary of GAW Hohenpeissenberg Observatory No. 167 Monitoring and Research
WORLD METEOROLOGICAL ORGANIZATION GLOBAL ATMOSPHERE WATCH The German Contribution to the WMO GAW Programme: Upon the 225 th Anniversary of GAW Hohenpeissenberg Observatory No. 167 Monitoring and Research
Measurements of gas-phase and particle-phase water-soluble organic carbon
 University of New Hampshire University of New Hampshire Scholars' Repository Master's Theses and Capstones Student Scholarship Winter 2007 Measurements of gas-phase and particle-phase water-soluble organic
University of New Hampshire University of New Hampshire Scholars' Repository Master's Theses and Capstones Student Scholarship Winter 2007 Measurements of gas-phase and particle-phase water-soluble organic
Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. A. ammonia D. HFCs B. CFCs E. NONE of these
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. A. ammonia D. HFCs B. CFCs E. NONE of these
Chemistry 471/671. Atmospheric Chemistry III: Stratospheric Ozone Depletion
 Chemistry 471/671 Atmospheric Chemistry III: Stratospheric Ozone Depletion 2 The Chapman Mechanism O 2 + hn 2 O( 1 D) O( 1 D) + O 2 + M O 3 + M Exothermic O( 1 D) + O 3 2 O 2 O 3 + hn O( 1 D) + O 2 ( 1
Chemistry 471/671 Atmospheric Chemistry III: Stratospheric Ozone Depletion 2 The Chapman Mechanism O 2 + hn 2 O( 1 D) O( 1 D) + O 2 + M O 3 + M Exothermic O( 1 D) + O 3 2 O 2 O 3 + hn O( 1 D) + O 2 ( 1
Geographic variability of nitrate deposition and preservation over the Greenland Ice Sheet.
 Geographic variability of nitrate deposition and preservation over the Greenland Ice Sheet. John F. Burkhart 1,2, Roger C. Bales 2, Joseph R McConnell 1,4, Manuel A Hutterli 1,5, Neil Moore 3, Randall
Geographic variability of nitrate deposition and preservation over the Greenland Ice Sheet. John F. Burkhart 1,2, Roger C. Bales 2, Joseph R McConnell 1,4, Manuel A Hutterli 1,5, Neil Moore 3, Randall
Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles
 Air Pollution XIII 519 Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles K. D. Stewart & J. M. Andino Department of Environmental Engineering
Air Pollution XIII 519 Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles K. D. Stewart & J. M. Andino Department of Environmental Engineering
Name Per Date Earth Science Climate & Insolation Test
 Name Per Date Earth Science Climate & Insolation Test 1) Which graph best represents the general relationship between latitude and average surface temperature? 2) The diagram below shows the apparent path
Name Per Date Earth Science Climate & Insolation Test 1) Which graph best represents the general relationship between latitude and average surface temperature? 2) The diagram below shows the apparent path
Measurement of the specific surface area of 176 snow samples using methane adsorption at 77 K
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D17, 4335, doi:10.1029/2001jd001016, 2002 Measurement of the specific surface area of 176 snow samples using methane adsorption at 77 K Loïc Legagneux, Axel
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D17, 4335, doi:10.1029/2001jd001016, 2002 Measurement of the specific surface area of 176 snow samples using methane adsorption at 77 K Loïc Legagneux, Axel
Lecture 3. - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years
 Lecture 3 - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years METR 113/ENVS 113 Spring Semester 2011 March 1, 2011 Suggested Reading
Lecture 3 - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years METR 113/ENVS 113 Spring Semester 2011 March 1, 2011 Suggested Reading
Ozone production in the upper troposphere and the influence of aircraft during SONEX: approach of NO x -saturated conditions
 Ozone production in the upper troposphere and the influence of aircraft during SONEX: approach of NO x -saturated conditions The Harvard community has made this article openly available. Please share how
Ozone production in the upper troposphere and the influence of aircraft during SONEX: approach of NO x -saturated conditions The Harvard community has made this article openly available. Please share how
Experiment Initial [A] Initial [B] Initial Rate
![Experiment Initial [A] Initial [B] Initial Rate Experiment Initial [A] Initial [B] Initial Rate](/thumbs/77/75974637.jpg) Chem 120 Practice Final Winter 2014 1 of 14 1. The following are initial rate data for: A + 2 B C + 2 D Experiment Initial [A] Initial [B] Initial Rate 1 0.10 0.10 0.300 2 0.20 0.10 0.600 3 0.10 0.20 1.200
Chem 120 Practice Final Winter 2014 1 of 14 1. The following are initial rate data for: A + 2 B C + 2 D Experiment Initial [A] Initial [B] Initial Rate 1 0.10 0.10 0.300 2 0.20 0.10 0.600 3 0.10 0.20 1.200
Cherry Hill Tuition A Level Chemistry OCR (A) Paper 9 THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
Unit 12: Chemical Kinetics
 Unit 12: Chemical Kinetics Author: S. Michalek Introductory Resources: Zumdahl v. 5 Chapter 12 Main Ideas: Integrated rate laws Half life reactions Reaction Mechanisms Model for chemical kinetics Catalysis
Unit 12: Chemical Kinetics Author: S. Michalek Introductory Resources: Zumdahl v. 5 Chapter 12 Main Ideas: Integrated rate laws Half life reactions Reaction Mechanisms Model for chemical kinetics Catalysis
(03) WMP/Jun10/CHEM4
 Thermodynamics 3 Section A Answer all questions in the spaces provided. 1 A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may
Thermodynamics 3 Section A Answer all questions in the spaces provided. 1 A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
Name: Regents Review Quiz #1 2016
 Name: Regents Review Quiz #1 2016 1. Which two particle diagrams represent mixtures of diatomic elements? A) A and B B) A and C C) B and C D) B and D 2. At STP, which physical property of aluminum always
Name: Regents Review Quiz #1 2016 1. Which two particle diagrams represent mixtures of diatomic elements? A) A and B B) A and C C) B and C D) B and D 2. At STP, which physical property of aluminum always
6.3. Theories of Reaction Rates. Collision Theory. The Effect of Concentration on Reactant Rates
 Theories of Reaction Rates 6.3 In section 6.2, you explored the rate law, which defines the relationship between the concentrations of reactants and reaction rate. Why, however, does the rate of a reaction
Theories of Reaction Rates 6.3 In section 6.2, you explored the rate law, which defines the relationship between the concentrations of reactants and reaction rate. Why, however, does the rate of a reaction
Measuring Total Reactive N and its Composition
 Measuring Total Reactive N and its Composition Bret A. Schichtel 1, Katie Benedict 2, Christian M. Carrico 2, Anthony Prenni 2, Jr. 2, Ezra Levin 2, Derek Day 3, Doris Chen 2, John Ray 1, William C. Malm
Measuring Total Reactive N and its Composition Bret A. Schichtel 1, Katie Benedict 2, Christian M. Carrico 2, Anthony Prenni 2, Jr. 2, Ezra Levin 2, Derek Day 3, Doris Chen 2, John Ray 1, William C. Malm
Outline. Chemical lifetime. Photochemistry. Ozone chemistry Chapman model Catalytic cycles Ozone hole. Institute of Applied Physics University of Bern
 Institute of Applied Physics University of Bern Outline Introduction Chemical reactions between stable molecules are quite slow in planetary s Absorption of solar UV-radiation leads to the production of
Institute of Applied Physics University of Bern Outline Introduction Chemical reactions between stable molecules are quite slow in planetary s Absorption of solar UV-radiation leads to the production of
UNIT TEST PRACTICE TEST
 Page 1 of 1 Directions: Match the best answer to complete each question. Some words may be used more than once and some may not be used at all. e 1. The condition of Earth s atmosphere at a given time
Page 1 of 1 Directions: Match the best answer to complete each question. Some words may be used more than once and some may not be used at all. e 1. The condition of Earth s atmosphere at a given time
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2013 Triailscrúdú na hardteistiméireachta, 2013 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2013 Triailscrúdú na hardteistiméireachta, 2013 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
The Meteorological Observatory from Neumayer Gert König-Langlo, Bernd Loose Alfred-Wegener-Institut, Bremerhaven, Germany
 The Meteorological Observatory from Neumayer Gert König-Langlo, Bernd Loose Alfred-Wegener-Institut, Bremerhaven, Germany History of Neumayer In March 1981, the Georg von Neumayer Station (70 37 S, 8 22
The Meteorological Observatory from Neumayer Gert König-Langlo, Bernd Loose Alfred-Wegener-Institut, Bremerhaven, Germany History of Neumayer In March 1981, the Georg von Neumayer Station (70 37 S, 8 22
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
ClO + O -> Cl + O 2 Net: O 3 + O -> O 2 + O 2
 Lecture 36. Stratospheric ozone chemistry. Part2: Threats against ozone. Objectives: 1. Chlorine chemistry. 2. Volcanic stratospheric aerosols. 3. Polar stratospheric clouds (PSCs). Readings: Turco: p.
Lecture 36. Stratospheric ozone chemistry. Part2: Threats against ozone. Objectives: 1. Chlorine chemistry. 2. Volcanic stratospheric aerosols. 3. Polar stratospheric clouds (PSCs). Readings: Turco: p.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117, D00R12, doi: /2011jd016639, 2012
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117,, doi:10.1029/2011jd016639, 2012 Hydroxyl radical and NO x production rates, black carbon concentrations and light-absorbing impurities in snow from field measurements
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117,, doi:10.1029/2011jd016639, 2012 Hydroxyl radical and NO x production rates, black carbon concentrations and light-absorbing impurities in snow from field measurements
Frey, et al. Climate sensitivity of the H 2 O 2 ice core record 1
 Frey, et al. Climate sensitivity of the H 2 O 2 ice core record 1 Climate sensitivity of the century-scale hydrogen peroxide (H 2 O 2 ) record preserved in 23 ice cores from West Antarctica Markus M. Frey
Frey, et al. Climate sensitivity of the H 2 O 2 ice core record 1 Climate sensitivity of the century-scale hydrogen peroxide (H 2 O 2 ) record preserved in 23 ice cores from West Antarctica Markus M. Frey
Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple
 Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple How can you describe Earth? What are the composition and the structure of the atmosphere? How
Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple How can you describe Earth? What are the composition and the structure of the atmosphere? How
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... )
 Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... ) 4 Land, biosphere, cryosphere 1. Introduction 2. Atmosphere 3. Ocean 4. Land, biosphere, cryosphere 4.1 Land
Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... ) 4 Land, biosphere, cryosphere 1. Introduction 2. Atmosphere 3. Ocean 4. Land, biosphere, cryosphere 4.1 Land
H 2 O 2 (ppbv) at z = 11.5 m.s.l.
 THE INFLUENCE OF CLOUD PROCESSES ON THE DISTRIBUTION OF CHEMICAL SPECIES FOR THE 10 JULY 1996 STERAO/DEEP CONVECTION STORM Mary C. Barth 1, William C. Skamarock 1,andAmy L. Stuart 2 1 National Center for
THE INFLUENCE OF CLOUD PROCESSES ON THE DISTRIBUTION OF CHEMICAL SPECIES FOR THE 10 JULY 1996 STERAO/DEEP CONVECTION STORM Mary C. Barth 1, William C. Skamarock 1,andAmy L. Stuart 2 1 National Center for
John Abbott College Department of Chemistry Chemistry 202-NYB-05 Sample Final Exam
 John Abbott College Department of Chemistry Chemistry 202-NYB-05 Sample Final Exam Please Note: 1. Available space for answers has been removed from some questions to conserve space. 2. The questions begin
John Abbott College Department of Chemistry Chemistry 202-NYB-05 Sample Final Exam Please Note: 1. Available space for answers has been removed from some questions to conserve space. 2. The questions begin
DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW UNIVERSITY OF VICTORIA. CHEMISTRY 102 Midterm Test 1 February 1, pm (60 minutes)
 SECTION: (circle one): A01 MR (Dr. Lipson) A02 (Dr. Briggs) A03 MWR (Dr. Brolo) NAME Student No. V0 (Please print clearly.) DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW Version A UNIVERSITY
SECTION: (circle one): A01 MR (Dr. Lipson) A02 (Dr. Briggs) A03 MWR (Dr. Brolo) NAME Student No. V0 (Please print clearly.) DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW Version A UNIVERSITY
Weather and Climate Change
 Weather and Climate Change What if the environmental lapse rate falls between the moist and dry adiabatic lapse rates? The atmosphere is unstable for saturated air parcels but stable for unsaturated air
Weather and Climate Change What if the environmental lapse rate falls between the moist and dry adiabatic lapse rates? The atmosphere is unstable for saturated air parcels but stable for unsaturated air
Brass, a solid solution of Zn and Cu, is used to make musical instruments and many other objects.
 Brass, a solid solution of Zn and Cu, is used to make musical instruments and many other objects. 14.1 General Properties of Solutions 14.2 Solubility 14.3 Rate of Dissolving Solids 14.4 Concentration
Brass, a solid solution of Zn and Cu, is used to make musical instruments and many other objects. 14.1 General Properties of Solutions 14.2 Solubility 14.3 Rate of Dissolving Solids 14.4 Concentration
AlCl3(aq) + 3 NaOH(aq) Al(OH)3(s) + 3 NaCl(aq)
 1. Under which conditions does a real gas behave most like an ideal gas? A) at low temperatures and high pressures B) at low temperatures and low pressures C) at high temperatures and high pressures D)
1. Under which conditions does a real gas behave most like an ideal gas? A) at low temperatures and high pressures B) at low temperatures and low pressures C) at high temperatures and high pressures D)
Chemistry of SO 2 in tropospheric volcanic plumes
 Chemistry of SO 2 in tropospheric volcanic plumes by Dr. Lizzette A. Rodríguez Iglesias Department of Geology University of Puerto Rico Mayagüez Campus Photo: L. Rodriguez http://volcano-pictures.info/glossary/volcanic_gas.html
Chemistry of SO 2 in tropospheric volcanic plumes by Dr. Lizzette A. Rodríguez Iglesias Department of Geology University of Puerto Rico Mayagüez Campus Photo: L. Rodriguez http://volcano-pictures.info/glossary/volcanic_gas.html
Due Date: First day of school if you miss the first day of school, you must send a scanned/pdf copy to Mr. Mejia:
 Name: Date: AP Chemistry Summer Assignment Due Date: First day of school if you miss the first day of school, you must send a scanned/pdf copy to Mr. Mejia: jmejia@cboek12.org Assessment: Within the first
Name: Date: AP Chemistry Summer Assignment Due Date: First day of school if you miss the first day of school, you must send a scanned/pdf copy to Mr. Mejia: jmejia@cboek12.org Assessment: Within the first
Chem GENERAL CHEMISTRY II MIDTERM EXAMINATION
 Concordia University CHEM 206 Winter 2009, Dr. C. Rogers, Section 01 LAST NAME: FIRST NAME: STUDENT ID: Chem 206 - GENERAL CHEMISTRY II MIDTERM EXAMINATION INSTRUCTIONS: PLEASE READ THIS PAGE WHILE WAITING
Concordia University CHEM 206 Winter 2009, Dr. C. Rogers, Section 01 LAST NAME: FIRST NAME: STUDENT ID: Chem 206 - GENERAL CHEMISTRY II MIDTERM EXAMINATION INSTRUCTIONS: PLEASE READ THIS PAGE WHILE WAITING
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *156507654 3 * CHEMISTRY 9701/21 Paper 2 Structured Questions AS Core May/June
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *156507654 3 * CHEMISTRY 9701/21 Paper 2 Structured Questions AS Core May/June
An Introduction to Chemical Kinetics
 An Introduction to Chemical Kinetics Michel Soustelle WWILEY Table of Contents Preface xvii PART 1. BASIC CONCEPTS OF CHEMICAL KINETICS 1 Chapter 1. Chemical Reaction and Kinetic Quantities 3 1.1. The
An Introduction to Chemical Kinetics Michel Soustelle WWILEY Table of Contents Preface xvii PART 1. BASIC CONCEPTS OF CHEMICAL KINETICS 1 Chapter 1. Chemical Reaction and Kinetic Quantities 3 1.1. The
GCE O' LEVEL PURE CHEMISTRY (5073/02) Suggested Answers for 2016 O Level Pure Chemistry Paper 2
 Section A (50 M) Aa) trend The number of electron shell increases The number of valence electrons increases Proton number increases There is a change in character from metallic to non-metallic Only true
Section A (50 M) Aa) trend The number of electron shell increases The number of valence electrons increases Proton number increases There is a change in character from metallic to non-metallic Only true
Most hand warmers work by using the heat released from the slow oxidation of iron: The amount your hand temperature rises depends on several factors:
 Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
DO NOT OPEN THE EXAM UNTIL THE PROCTOR TELLS YOU TO DO SO
 CHEM 1021, Spring 2000 Exam #2 March 15, 2000 Prof. J. T. Roberts DO NOT OPEN THE EXAM UNTIL THE PROCTOR TELLS YOU TO DO SO Instructions: 1. Fill in the bubble sheet with your name (last name first), ID
CHEM 1021, Spring 2000 Exam #2 March 15, 2000 Prof. J. T. Roberts DO NOT OPEN THE EXAM UNTIL THE PROCTOR TELLS YOU TO DO SO Instructions: 1. Fill in the bubble sheet with your name (last name first), ID
Gas Laws. Bonding. Solutions M= moles solute Mass %= mass solute x 100. Acids and Bases. Thermochemistry q = mc T
 Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
Stoichiometric relationships 1
 Stoichiometric relationships 1 Chapter outline Describe the three states of matter. Recall that atoms of diff erent elements combine in fi xed ratios to form compounds which have diff erent properties
Stoichiometric relationships 1 Chapter outline Describe the three states of matter. Recall that atoms of diff erent elements combine in fi xed ratios to form compounds which have diff erent properties
Polar Meteorology Group, Byrd Polar Research Center, The Ohio State University, Columbus, Ohio
 JP2.14 ON ADAPTING A NEXT-GENERATION MESOSCALE MODEL FOR THE POLAR REGIONS* Keith M. Hines 1 and David H. Bromwich 1,2 1 Polar Meteorology Group, Byrd Polar Research Center, The Ohio State University,
JP2.14 ON ADAPTING A NEXT-GENERATION MESOSCALE MODEL FOR THE POLAR REGIONS* Keith M. Hines 1 and David H. Bromwich 1,2 1 Polar Meteorology Group, Byrd Polar Research Center, The Ohio State University,
Lecture Presentation. Chapter 6. Thermochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
