Supporting Information. Substitutent Rate Effects
|
|
|
- Blake Wilkins
- 5 years ago
- Views:
Transcription
1 Supporting Information Gosteli Claisen Rearrangement: DFT Study of Substitutent Rate Effects Julia Rehbein* and Martin Hiersemann* Fakultät Chemie, Technische Universität Dortmund, Dortmund, Germany SI- Figure SI-1 free energy profile for (E,E)-1a SI-4 Figure SI-2 free energy profile for (E,Z)-1b SI-5 Figure SI- free energy profile for (Z,E)-1c SI-6 Figure SI-4 free energy profile for (Z,Z)-1a SI-7 Table SI-1 geometric data for (E,E)-1a SI-8 Table SI-2 geometric data for (E,Z)-1b SI-9 Table SI- geometric data for (Z,E)-1c SI-10 Table SI-4 geometric data for (Z,Z)-1d SI-11 SI-12 Table SI-5 Table SI-6 BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane) free energies of 1a-d BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane) free energies of [1a-d] SI-1 Table SI-7 geometric data for 2a,b
2 SI-14 SI-15 SI-16 SI-17 SI-18 SI-19 SI-20 SI-21 SI-22 Table SI-8 Table SI-9 Figure SI-5 Figure SI-6 Figure SI-7 Figure SI-8 Figure SI-9 Figure SI-10 Figure SI-11 BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane) energies and geometric data for [st-1a-d] BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane) energies and geometric data for st-1a-d at K BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane)-relative free energies G rel for st- 1a-d and [st-1a-d] Newman projection of [st-1a-d], k rel,exp, G ts rel and ΔG rel BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane)-mulliken atomic charges BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane)-structures for (E,E)-1a BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane)-structures for (E,Z)-1b BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane)-structures for (Z,E)-1c BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane)-structures for (Z,Z)-1d SI-2
3 [st-(e,e)-1a] BLYP/6-1G* i-pr [sc-(e,e)-1a] i-pr BLYP/6-1G* S R sc-syn-2a i-pr sc-2a* could not be located on PES st-(e,e)-1a* st-(e,e)-1a i-pr sc-(e,e)-1a i-pr sc-(e,e)-1a* 1. G rel [kcal/mol] BLYP/6-1G* 2. G [Hartree] BLYP/6-1G*. G rel [kcal/mol] BLYP/6-1G*+PCM(1,2-dichloroethane) 4. G [Hartree] BLYP/6-1G*+PCM (1,2-dichloroethane) i-pr st-2a* S R st-syn-2a i-pr s-trans pathway s-cis pathway FIGURE SI-1. Calculated free energy profile for the Gosteli Claisen rearrangement of (E,E)-1a. SI-
4 [st-(e,z)-1b] BLYP/6-1G* i-pr [sc-(e,z)-1b] i-pr BLYP/6-1G* i-pr 4R R sc-anti-2b sc-2b* could not be located on PES converged to s-trans s-trans pathway st-(e,z)-1b* st-(e,z)-1b i-pr sc-(e,z)-1b i-pr sc-(e,z)-1b* G rel [kcal/mol] BLYP/6-1G* 2. G [Hartree] BLYP/6-1G*. G rel [kcal/mol] BLYP/6-1G*+PCM(1,2-dichloroethane) 4. G [Hartree] BLYP/6-1G*+PCM (1,2-dichloroethane) i-pr st-2b* s-cis pathway R R st-anti-2b i-pr FIGURE SI-2. Calculated free energy profile for the Gosteli Claisen rearrangement of (E,Z)-1b. SI-4
5 i-pr 4R S sc-anti-2b [st-(z,e)-1c] sc-2b* BLYP/6-1G* st-(z,e)-1c* st-(z,e)-1c i-pr sc-(z,e)-1c i-pr [sc-(z,e)-1c] i-pr sc-(z,e)-1c* G rel [kcal/mol] BLYP/6-1G* 2. G [Hartree] BLYP/6-1G*. G rel [kcal/mol] BLYP/6-1G*+PCM(1,2-dichloroethane) 4. G [Hartree] BLYP/6-1G*+PCM (1,2-dichloroethane) i-pr st-2b* i-pr BLYP/6-1G* R S i-pr st-anti-2b s-trans pathway s-cis pathway FIGURE SI-. Calculated free energy profile for the Gosteli Claisen rearrangement of (Z,E)-1c. SI-5
6 i-pr i-pr [st-(z,z)-1d] BLYP/6-1G* [sc-(z,z)-1d] BLYP/6-1G* R S sc-syn-2a i-pr sc-2a* st-(z,z)-1d* st-(z,z)-1d i-pr sc-(z,z)-1d G rel [kcal/mol] BLYP/6-1G* 2. G [Hartree] BLYP/6-1G*. G rel [kcal/mol] BLYP/6-1G*+PCM(1,2-dichloroethane) 4. G [Hartree] BLYP/6-1G*+PCM(1,2-dichloroethane) i-pr sc-(z,z)-1d* i-pr st-2a* R S i-pr st-syn-2a s-trans pathway s-cis pathway FIGURE SI-4. Calculated free energy profile for the Gosteli Claisen rearrangement of (Z,Z)-1d. SI-6
7 TABLE SI-1. Gosteli Claisen rearrangement of the (E,E)-configured allyl vinyl ether 1a. BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane) bond order of the breaking (d 1 C1 ) and the forming bond (d C C ), dihedral angles ω, charge separation δ between the allylic and oxallylic segment and dipole moments μ for the allyl vinyl ethers and transition states. BLYP/6-1G*-values in parenthesis. d 1 C1 d C C ω c δ d μ e entry cmpd [Å] n a [Å] n b [ ] [AU] [D] 1 sc-1a (1.428) (5.256) (+1) (0.5) (2.) [sc-1a] (2.021) (0.7) (2.27) (0.28) ( 8) (0.4) (2.6) st-2a 4 st-1a (5.409) (1.556) ( 156) (1.7) (1.427) (5.258) ( 154) (0.45) (2.1) [st-1a] (2.027) (0.7) (2.57) (0.28) (+124) (0.45) (.2) 6 sc-2a (5.1) (1.55) (+4) (4.) a Bond order n in % calculated according to Pauling: n = exp[(d ave d ts ) / 0.6]. b Bond order n in % calculated according to Pauling: n = exp[(d α- ke d ts ) / 0.6]. c as defined in Table SI-5. d Mulliken charge separation between the allylic and oxallylic segment of the allyl vinyl ether as defined in Figure SI-7. e Dipole moments calculated by Mulliken Population Analysis (MPA) 1 or Natural Population Analysis (NPA) 2, were identical. SI-7
8 TABLE SI-2. Gosteli Claisen rearrangement of the (E,Z)-configured allyl vinyl ether 1b. BLYP/6-1G*+PCM(solvent = 1,2- dichloroethane) bond lengths of the breaking (d 1 C1 ) and the forming bond (d C C ), dihedral angles ω, charge separation δ between the allylic and oxallylic segment and dipole moments μ for the allyl vinyl ethers and transition states. BLYP/6-1G*-values in parenthesis. d 1 C1 d C C ω c δ d μ e entry cmpd [Å] n a [Å] n b [ ] [AU] [D] 1 sc-1b (1.427) (5.18) +11 (0.5) (2.2) ( 1) [sc-1b] (1.986) (0.9) (2.2) (0.27) ( 7) (0.44) (2.5) st-2b 4 st-1b (5.154) (1.551) ( 174) (1.5) (1.427) (5.22) (+155) (0.54) (2.4) [st-1b] (1.99) (0.9) (2.47) (0.28) (+11) (0.46) (.1) 6 sc-2b (5.1) (1.55) (+4) (4.) a Bond order n in % calculated according to Pauling: n = exp[(d ave d ts ) / 0.6]. b Bond order n in % calculated according to Pauling: n = exp[(d α- ke d ts ) / 0.6]. c as defined in Table SI-5. d Mulliken charge separation between the allylic and oxallylic segment of the allyl vinyl ether as defined in Figure SI-7. e Dipole moments calculated by Mulliken Population Analysis (MPA) 1 or Natural Population Analysis (NPA) 2, were identical. SI-8
9 TABLE SI-. Gosteli Claisen rearrangement of the (Z,E)-configured allyl vinyl ether 1c. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) bond lengths of the breaking (d 1 C1 ) and the forming bond (d C C ), dihedral angles ω, charge separation δ between the allylic and oxallylic segment and dipole moments μ for the allyl vinyl ethers and transition states. BLYP/6-1G*-values in parenthesis. entry cmpd d 1 C1 d C C ω c δ d μ e [Å] n a [Å] n b [ ] [AU] [D] 1 sc-1c (1.44) (5.526) ( 7) (0.52) (2.1) [sc-1c] (2.017) (0.8) (2.64) (0.26) ( 16) (0.44) (2.2) st-2c 4 st-1c (5.154) (1.551) (+174) (1.5) (1.446) 0.6 (5.548) 0.24 (+17) (0.54) (2.0) (0.6) (0.24) [st-1c] (2.04) (2.70) (+145) (0.46) (.6) 6 sc-2c (5.1) (1.55) (+4) (4.) a calculated according to Bond order n in % calculated according to Pauling: n = exp[(d ave d ts ) / 0.6]. b Bond order n in % calculated according to Pauling: n = exp[(d α-ke d ts ) / 0.6]. c As defined in Table SI-5. d Mulliken charge separation between the allylic and oxallylic segment of the allyl vinyl ether as defined in Figure SI-7. e Dipole moments calculated by Mulliken Population Analysis (MPA) 1 or Natural Population Analysis (NPA) 2, were identical. SI-9
10 TABLE SI-4. Gosteli Claisen rearrangement of the (Z,Z)-configured allyl vinyl ether 1d. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) bond lengths of the breaking (d 1 C1 ) and the forming bond (d C C ), dihedral angles ω, charge separation δ between the allylic and oxallylic segment and dipole moments μ for the allyl vinyl ethers and transition states. BLYP/6-1G*-values in parenthesis. entry cmpd d 1 C1 d C C ω c δ d μ e [Å] n a [Å] n b [ ] [AU] [D] 1 sc-1d (1.44) (5.585) (+7) (0.52) (2.0) [sc-1d] (2.000) (0.40) (2.82) (0.25) ( 16) (0.45) (2.2) st-2a 4 st-1d (5.409) (1.556) ( 156) (1.5) (1.446) (5.578) (+172) (0.54) (2.) [st-1d] (2.005) (0.9) (2.75) (0.25) ( 150) (0.46) (.5) 6 sc-2a (5.1) (1.55) (+4) (4.) a calculated according to Bond order n in % calculated according to Pauling: n = exp[(d ave d ts ) / 0.6]. b Bond order n in % calculated according to Pauling: n = exp[(d α-ke d ts ) / 0.6]. c as defined in Table SI-5. d Mulliken charge separation between the allylic and oxallylic segment of the allyl vinyl ether as defined in Figure SI-7. e Dipole moments calculated by Mulliken Population Analysis (MPA) 1 or Natural Population Analysis (NPA) 2, were identical. SI-10
11 TABLE SI-5. Gosteli Claisen rearrangement of 1a-d. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) free energies of 1a-d at K. i-pr i-pr ω [deg] i-pr st-1a sc-1a st-1b sc-1b i-pr i-pr +ω [deg] st-1c sc-1c st-1d sc-1d entry configuration conformation compound G [Hartree] G relative [Hartree] G relative [kcal/mol] 1 (E,E) s-trans st-1a s-cis sc-1a (E,Z) s-trans st-1b s-cis sc-1b (Z,E) s-trans st-1c s-cis sc-1c (Z,Z) s-trans st-1d s-cis sc-1d SI-11
12 TABLE SI-6. Gosteli Claisen rearrangement of the allyl vinyl ether 1a-d. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) free energies of the transition states [1a-d] at K. [c-st-1a] = chairlike transition state for the Gosteli Claisen rearrangement of the allyl vinyl ether 1a. b = boatlike, sc = s-cis. i-pr i-pr i-pr i-pr [st-1a] [sc-1a] [st-1b] [sc-1b] [st-1c] [sc-1c] [st-1d] [sc-1d] entry configuration conformation compound G [Hartree] G relative [Hartree] G relative [kcal/mol] 1 (E,E) s-trans [st-1a] s-cis [sc-1a] (E,Z) s-trans [st-1b] s-cis [sc-1b] (Z,E) s-trans [st-1c] s-cis [sc-1c] (Z,Z) s-trans [st-1d] s-cis [sc-1d] SI-12
13 TABLE SI-7. Gosteli Claisen rearrangement of the allyl vinyl ether 1a-d: BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) bond length of the newly formed C/C bond (d C C ), dihedral angles ω of the dicarbonyl moiety and, dipole moments μ for the α-keto ester 2a-b. BLYP/6-1G*-values in parenthesis. ϕ [deg] i-pr ' ' i-pr i-pr ' ' i-pr i-pr (R,4S)-st-2a (R,4R)-st-2b (S,4R)-st-2a (S,4S)-st-2b +ϕ [deg] ' i-pr ' i-pr ' i-pr ' i-pr (R,4S)-sc-2a (R,4R)-sc-2b (S,4R)-sc-2a (S,4S)-sc-2b entry cmpd rel. config. d C C [Å] φ [ ] μ [D] 1 (R,4S)-st-2a (1.556) +156 (+159) 2.1 (1.7) 2 (S,4R)-st-2a syn ( ( 16) 2.1 (1.7) (R,4S)-sc-2a (1.556) 25 ( 6) 5.4 (4.1) 4 (S,4R)-sc-2a (1.556) +2 (+5) 5.4 (4.1) 5 (R,4R)-st-2b (1.551)) +171 (+174) 1.8 (1.5) 6 (S,4S)-st-2b anti (1.551) 178 (+179) 1.8 (1.5) 7 (R,4R)-sc-2b 1.55 (1.55).8 ( 4) 5.6 (4.) 8 (S,4S)-sc-2b 1.55 (1.55) 0 ( 2) 5.6 (4.) SI-1
14 TABLE SI-8. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) relative free energy (G ts rel) at K, bond lengths and order of the breaking (d 1 C1 ) and the forming bond (d C C ), dihedral angles ω and dipole moments μ for the transition-state structures [st-1a-d]. entry cmpd double bond configuration G ts rel d 1 C1 d C C ω c μ [kcal/mol] [Å] (n) a [Å] (n) b [ ] [D] 1 [st-1a] (E,E) (0.6) 2.82 (0.25) [st-1b] (E,Z) (0.8) 2.77 (0.25) [st-1c] (Z,E) (0.6) (0.24) [st-1d] (Z,Z) (0.7) (0.24) a Bond order n in % calculated according to Pauling: n = exp[(d ave d ts ) / 0.6] b Bond order n in % calculated according to Pauling: n = exp[(d α-ke d ts ) / 0.6] SI-14
15 TABLE SI-9. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) relative free energies (G ave rel), bond lengths of the breaking (d 1 C1 ) bond, dihedral angles ω and dipole moments μ of the allyl vinyl ethers st-1a-d at K. entry comp config G ave a rel ΔG rel d 1 C1 ω b μ [kcal/mol] [kcal/mol] [Å] [ ] [D] 1 st-1a (E,E) st-1b (E,Z) st-1c (Z,E) st-1d (Z,Z) a Loss of stability on the way to the transition-state, relative to st-1a. Calculated according to: ΔG rel = G ave rel G ts rel for st-1b,c and ΔG rel = G ave rel + G ts rel for st-1d. b as defined in Table SI-5. SI-15
16 [st-1a] ω = +17 μ = 4.8 D 0 [st-1b] [st-1d] ω = +140 ω = +158 μ = 4.6 D μ = 5.1 D [st-1c] ω = +169 μ = 5.4 D G relative [kcal/mol] of the s-trans transition states 25.6 (26.7) (27.4) 28.2 (28.2) ΔG [kcal/mol] calculated for s-trans pathway (experimental) (27.2) ω 1.29 st-1a ω = +156 μ =.1 D.0 st-1b ω = +157 μ =.2 D st-1c ω = +171 μ = 2.9 D G relative [kcal/mol] of the s-trans allyl vinyl ethers st-1d ω = +171 μ =.1 D FIGURE SI-5. Relative free energies G rel for st-1a-d, [st-1a-d] and the calculated (experimental) free energies of activation ΔG at the BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane) level of theory in kcal/mol at K. Definition of the dihedral angle ω. SI-16
17 ' ' ' ' [st-1a] [st-1b] [st-1c] [st-1d] H H H H H H ' H H H H ' H ' H H H ' H H k rel, exp = 8.6 G ts rel = 0 ΔG rel = FIGURE SI-6. Newman projection of [st-1a-d], experimentally determined relative rate constant (k rel,exp ), calculated relative free energies (G ts rel, kcal/mol) and ΔG rel as a measure for the strain energy caused by axial methyl groups. SI-17
18 δ oxallylic = 0.28 δ allylic = sc-1a [sc-1a] 0.2 δ oxallylic = 0.26 δ allylic = δ oxallylic = 0.29 δ allylic = st-1a [st-1a] 0.0 δ oxallylic = 0.26 δ allylic = δoxallylic = 0.29 δ allylic = sc-1b [sc-1b] 0. δ oxallylic = 0.25 δ allylic = δ oxallylic = δ allylic = st-1b [st-1b] δ oxallylic = 0.27 δ allylic = δ oxallylic = 0.29 δ allylic = sc-1c [sc-1c] δ oxallylic = δ allylic = δ oxallylic = 0.29 δ allylic = st-1b [st-1b] δ oxallylic = δ allylic = δ oxallylic = 0.28 δ allylic = δ 0.61 oxallylic = δ oxallylic = δ allylic = δ allylic = sc-1d [sc-1d] st-1d [st-1d] δ oxallylic = δ allylic = FIGURE SI-7. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane)-Mulliken charges; charges on hydrogen atoms have been summed into charges of heavy atoms. SI-18
19 (E,E)-sc-1a (E,E)-sc-1a* (Si,4Si)-[sc-1a] (R,4S)-st-2a* (R,4S)-st-2a could not be located on PES (E,E)-st-1a (E,E)-st-1a* (Si,4Si)-[st-1a] (R,4S)-sc-2a* (R,4S)-sc-2a FIGURE SI-8. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane)-stationary points for the Gosteli Claisen rearrangement of 1a SI-19
20 (E,Z)-sc-1b (E,Z)-sc-1b* (Si,4Re)-[sc-1b] (R,4R)-st-2b* (R,4R)-st-2b could not be located on PES (E,Z)-st-1b (E,Z)-st-1b* (Si,4Re)-[st-1b] (R,4R)-sc-2b* (R,4R)-sc-2b FIGURE SI-9. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane)-stationary points for the Gosteli Claisen rearrangement of 1b SI-20
21 (Z,E)-sc-1c (Z,E)-sc-1c* (Re,4Si)-[sc-1c] (S,4S)-st-2b* (S,4S)-st-2b (Z,E)-st-1c (Z,E)-st-1c* (Re,4Si)-[st-1c] (S,4S)-sc-2b* (S,4S)-sc-2b FIGURE SI-10. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane)-stationary points for the Gosteli Claisen rearrangement of 1c SI-21
22 (Z,Z)-sc-1d (Z,Z)-sc-1d* (Re,4Re)-[sc-1d] (S,4R)-st-2a* (S,4R)-st-2a (Z,Z)-st-1d (Z,Z)-st-1d* (Re,4Re)-[st-1d] (S,4R)-sc-2a* (S,4R)-sc-2a FIGURE SI-11. BLYP/6-1G*+PCM(solvent = 1,2-dichloroethane)-stationary points for the Gosteli Claisen rearrangement of 1d SI-22
23 1 Mulliken, R.S: J. Chem. Phys. 1956, 2, Reed, E. A.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1988, 8, (a) Wiberg, K. B.; Rablem, P. R.; J. Comput. Chem. 199, 14, (b) McAllister, M. A.; Tidwell, T. T. J. rg. Chem. 1994, 59, SI-2
Electronic Supplementary Information. for
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information for Two Chiral Catalysts in Action: Insights on Cooperativity
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information for Two Chiral Catalysts in Action: Insights on Cooperativity
Chem 201 Midterm Winter, 2013 Beauchamp
 hem 0 Midterm Winter, 0 Beauchamp Name Problems Points redit. Functional Group Nomenclature. Degrees of Unsaturation & Functional Groups or Various Nomenclature Terms. D structure, Functional Groups 0.
hem 0 Midterm Winter, 0 Beauchamp Name Problems Points redit. Functional Group Nomenclature. Degrees of Unsaturation & Functional Groups or Various Nomenclature Terms. D structure, Functional Groups 0.
Supplementary Information
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 Supplementary Information
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 Supplementary Information
Theoretical study of the BF 3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates
 Theoretical study of the BF 3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates Margarita M. Vallejos a* and Silvina C. Pellegrinet b* a Laboratorio de Química Orgánica, IQUIBA-NEA,
Theoretical study of the BF 3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates Margarita M. Vallejos a* and Silvina C. Pellegrinet b* a Laboratorio de Química Orgánica, IQUIBA-NEA,
Supporting Information
 Supporting Information Tuning of Second-Order Nonlinear Optical Response Properties of Aryl-Substituted Boron-Dipyrromethene Dyes: Unidirectional Charge Transfer Coupled with Structural Tailoring Ramprasad
Supporting Information Tuning of Second-Order Nonlinear Optical Response Properties of Aryl-Substituted Boron-Dipyrromethene Dyes: Unidirectional Charge Transfer Coupled with Structural Tailoring Ramprasad
California State Polytechnic University, Pomona Nomenclature (one structure) 25
 alifornia State Polytechnic University, Pomona 1 hem 314 Final Exam Spring, 2005 Beauchamp ame Problem Points redit 1. omenclature (one structure) 25 2. 2D Lewis structure (large structure with 20 possible
alifornia State Polytechnic University, Pomona 1 hem 314 Final Exam Spring, 2005 Beauchamp ame Problem Points redit 1. omenclature (one structure) 25 2. 2D Lewis structure (large structure with 20 possible
Theoretical studies on the bifunctionality of chiral thiourea-based organocatalysts: Competing routes to C C bond formation
 Supporting Information Theoretical studies on the bifunctionality of chiral thiourea-based organocatalysts: Competing routes to C C bond formation Andrea Hamza, a Gábor Schubert, a Tibor Soós b and Imre
Supporting Information Theoretical studies on the bifunctionality of chiral thiourea-based organocatalysts: Competing routes to C C bond formation Andrea Hamza, a Gábor Schubert, a Tibor Soós b and Imre
Supporting information
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting information Resonant Raman Spectra of Molecules with Diradical Character:
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting information Resonant Raman Spectra of Molecules with Diradical Character:
Supporting Information I
 Supporting Information I Gosteli-Claisen Rearrangement: Substrate Synthesis, Simple Diastereoselectivity and Kinetic Studies Julia Rehbein*, Sabine Leick, and Martin Hiersemann Fakultät Chemie, Technische
Supporting Information I Gosteli-Claisen Rearrangement: Substrate Synthesis, Simple Diastereoselectivity and Kinetic Studies Julia Rehbein*, Sabine Leick, and Martin Hiersemann Fakultät Chemie, Technische
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
CHEM50002: Orbitals in Organic Chemistry- Stereoelectronics. LECTURE 2 Stereoelectronics of Ground States Conformational Analysis
 CEM50002: rbitals in rganic Chemistry- Stereoelectronics 1 LECTUE 2 Stereoelectronics of Ground States Conformational Analysis Alan C. Spivey a.c.spivey@imperial.ac.uk Feb-Mar 2018 2 Format & scope of
CEM50002: rbitals in rganic Chemistry- Stereoelectronics 1 LECTUE 2 Stereoelectronics of Ground States Conformational Analysis Alan C. Spivey a.c.spivey@imperial.ac.uk Feb-Mar 2018 2 Format & scope of
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Photochemistry of N-Methylformamide: Matrix Isolation and Nonadiabatic Dynamics Rachel Crespo-Otero, [a] Artur Mardyukov,
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Photochemistry of N-Methylformamide: Matrix Isolation and Nonadiabatic Dynamics Rachel Crespo-Otero, [a] Artur Mardyukov,
Thiourea Derivatives as Brønsted Acid Organocatalysts
 Supporting Information Thiourea Derivatives as Brønsted Acid Organocatalysts Ádám Madarász, Zsolt Dósa, Szilárd Varga, * Tibor Soós, Antal Csámpai, Imre Pápai * Institute of Organic Chemistry, Research
Supporting Information Thiourea Derivatives as Brønsted Acid Organocatalysts Ádám Madarász, Zsolt Dósa, Szilárd Varga, * Tibor Soós, Antal Csámpai, Imre Pápai * Institute of Organic Chemistry, Research
Supporting Information
 Supporting Information Synthesis of α-(pentafluorosulfanyl)- and α-(trifluoromethyl)-substituted Carboxylic Acid Derivatives by Ireland-Claisen Rearrangement Anna-Lena Dreier, Bernd Beutel, Christian Mück-Lichtenfeld,
Supporting Information Synthesis of α-(pentafluorosulfanyl)- and α-(trifluoromethyl)-substituted Carboxylic Acid Derivatives by Ireland-Claisen Rearrangement Anna-Lena Dreier, Bernd Beutel, Christian Mück-Lichtenfeld,
Only by constructing a model does one at first appreciate fully how. cyclohexane can exist in a non-planar, beautifully symmetrical, and apparently
 Text Related to Segment 5.05 2002 Claude E. Wintner Only by constructing a model does one at first appreciate fully how cyclohexane can exist in a non-planar, beautifully symmetrical, and apparently entirely
Text Related to Segment 5.05 2002 Claude E. Wintner Only by constructing a model does one at first appreciate fully how cyclohexane can exist in a non-planar, beautifully symmetrical, and apparently entirely
Exam 1 Chem 3045x Friday, October 1, 1999 ANSWER KEY: Exam 1. C3043. Friday, October 1, 1999 p. 1
 Exam 1 Chem 3045x Friday, ctober 1, 1999 ANSWER KEY: Exam 1. C3043. Friday, ctober 1, 1999 p. 1 1. (10 Points). Consider the composition C 2 N 2. Draw the Lewis structures of 5 constitutional isomers which
Exam 1 Chem 3045x Friday, ctober 1, 1999 ANSWER KEY: Exam 1. C3043. Friday, ctober 1, 1999 p. 1 1. (10 Points). Consider the composition C 2 N 2. Draw the Lewis structures of 5 constitutional isomers which
Organic Chemistry 1 Lecture 6
 CEM 232 Organic Chemistry I Illinois at Chicago Organic Chemistry 1 Lecture 6 Instructor: Prof. Duncan Wardrop Time/Day: T & R, 12:30-1:45 p.m. January 28, 2010 1 Self Test Question Which form of strain
CEM 232 Organic Chemistry I Illinois at Chicago Organic Chemistry 1 Lecture 6 Instructor: Prof. Duncan Wardrop Time/Day: T & R, 12:30-1:45 p.m. January 28, 2010 1 Self Test Question Which form of strain
1. Root of name depends on longest chain of C containing the double bond; ends in "ene"
 Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
1. The barrier to rotation around the C-C bonds for 2-methylpropane and 2,2-dimethylpropane are shown below.
 1. The barrier to rotation around the C-C bonds for 2-methylpropane and 2,2-dimethylpropane are shown below. E Rot = 14.2 kj/mol E Rot = 19.6 kj/mol a. Why does the potential energy of a molecule increase
1. The barrier to rotation around the C-C bonds for 2-methylpropane and 2,2-dimethylpropane are shown below. E Rot = 14.2 kj/mol E Rot = 19.6 kj/mol a. Why does the potential energy of a molecule increase
The Silacyclobutene Ring: An Indicator of Triplet State Baird-Aromaticity
 The Silacyclobutene Ring: An Indicator of Triplet State Baird-Aromaticity Rabia Ayub, 1,2 Kjell Jorner, 1,2 and Henrik Ottosson 1,2 * 1 Department of Chemistry - BMC, Uppsala University, Box 576, 751 23
The Silacyclobutene Ring: An Indicator of Triplet State Baird-Aromaticity Rabia Ayub, 1,2 Kjell Jorner, 1,2 and Henrik Ottosson 1,2 * 1 Department of Chemistry - BMC, Uppsala University, Box 576, 751 23
5.6 Long-Range ( 4 J and higher) Proton-Proton Couplings
 5.6 Long-Range ( 4 J and higher) Proton-Proton ouplings opyright ans J. Reich 2010 All Rights Reserved University of Wisconsin Proton-proton couplings over more than three bonds are usually too small to
5.6 Long-Range ( 4 J and higher) Proton-Proton ouplings opyright ans J. Reich 2010 All Rights Reserved University of Wisconsin Proton-proton couplings over more than three bonds are usually too small to
Chapter 2: An Introduction to Organic Compounds
 Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Computational details, X-ray datas and spectral copies of 1 H, 13 C NMR of compounds obtained in this study
 Stereo, Regio-, and Chemoselective [3+2]-Cycloaddition of (2E,4E)-Ethyl 5-(Phenylsulfonyl)penta-2,4-dienoate with Various Azomethine Ylides, Nitrones, and Nitrile Oxides: Synthesis of Pyrrolidine, Isoxazolidine,
Stereo, Regio-, and Chemoselective [3+2]-Cycloaddition of (2E,4E)-Ethyl 5-(Phenylsulfonyl)penta-2,4-dienoate with Various Azomethine Ylides, Nitrones, and Nitrile Oxides: Synthesis of Pyrrolidine, Isoxazolidine,
Computational Chemistry Problem Set 3 Due Monday, February 16, 2009 (at the start of class) Total Possible Points = 69
 Computational Chemistry Problem Set 3 Due Monday, ebruary 16, 2009 (at the start of class) Total Possible Points = 69 Basic Technical Notes: (1) or security reasons, you are allowed to log into the ope
Computational Chemistry Problem Set 3 Due Monday, ebruary 16, 2009 (at the start of class) Total Possible Points = 69 Basic Technical Notes: (1) or security reasons, you are allowed to log into the ope
13. Free Radical Chemistry
 hem 201 Study Session Final Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Various possibilities: Types of Isomers, Degrees of Unsaturation, common nomenclature,
hem 201 Study Session Final Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Various possibilities: Types of Isomers, Degrees of Unsaturation, common nomenclature,
Problems Points Credit
 Chem 201 Midterm Winter, 2018 Beauchamp ame Problems Points Credit 1. Functional Group omenclature (1 large structure) 30 2. Resonance, Formal Charge, Arrows 18 3. Properties of Atoms, Logic Arguments
Chem 201 Midterm Winter, 2018 Beauchamp ame Problems Points Credit 1. Functional Group omenclature (1 large structure) 30 2. Resonance, Formal Charge, Arrows 18 3. Properties of Atoms, Logic Arguments
5. Stereochemical Analysis. 7. Dipole Moments and Inductive versus Resonance Effects. 8. Types of isomers from a given formula. 9. Physical Properties
 hem 201 Sample Midterm Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Lewis Structures, Resonance, Formal harge 3. yclohexane onformations, 2 substituents, ewman
hem 201 Sample Midterm Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Lewis Structures, Resonance, Formal harge 3. yclohexane onformations, 2 substituents, ewman
DAMIETTA UNIVERSITY CHEM-405: PERICYCLIC REACTIONS LECTURE
 DAMIETTA UNIVERSITY CHEM-405: PERICYCLIC REACTIONS LECTURE 8 Dr Ali El-Agamey 1 LEARNING OUTCOMES LECTURE 8 (1) The Woodward-Hoffmann rules for [1,n] sigmatropic rearrangements -[1,2] cationic shift -[1,2]
DAMIETTA UNIVERSITY CHEM-405: PERICYCLIC REACTIONS LECTURE 8 Dr Ali El-Agamey 1 LEARNING OUTCOMES LECTURE 8 (1) The Woodward-Hoffmann rules for [1,n] sigmatropic rearrangements -[1,2] cationic shift -[1,2]
NAME: SPRING 2015 MIDTERM
 page 1 pts NAME: SPRING 2015 MIDTERM hemistry 231 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 4/6) Do your best on this take-home portion of your mid-term. I may grade
page 1 pts NAME: SPRING 2015 MIDTERM hemistry 231 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 4/6) Do your best on this take-home portion of your mid-term. I may grade
Density Functional Theory Study on Mechanism of Forming Spiro-Geheterocyclic Ring Compound from Me 2 Ge=Ge: and Acetaldehyde
 CHINESE JURNAL F CHEMICAL PHYSICS VLUME 26, NUMBER 1 FEBRUARY 27, 2013 ARTICLE Density Functional Theory Study on Mechanism of Forming Spiro-Geheterocyclic Ring Compound from Me 2 Ge=Ge: and Acetaldehyde
CHINESE JURNAL F CHEMICAL PHYSICS VLUME 26, NUMBER 1 FEBRUARY 27, 2013 ARTICLE Density Functional Theory Study on Mechanism of Forming Spiro-Geheterocyclic Ring Compound from Me 2 Ge=Ge: and Acetaldehyde
CHAPTER 8 HW SOLUTIONS: ELIMINATIONS REACTIONS
 APTER 8 W SLUTNS: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult
APTER 8 W SLUTNS: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult
Computational Chemistry Problem Set 3 Due Tuesday, February 15, 2011 (at the start of class) Total Possible Points = 69
 Computational Chemistry Problem Set 3 Due Tuesday, ebruary 15, 2011 (at the start of class) Total Possible Points = 69 Basic Technical Notes: (1) or security reasons, you are allowed to log into the ope
Computational Chemistry Problem Set 3 Due Tuesday, ebruary 15, 2011 (at the start of class) Total Possible Points = 69 Basic Technical Notes: (1) or security reasons, you are allowed to log into the ope
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Constitutional Isomers and Conformations of Alkanes & Cycloalkanes
 Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Constitutional Isomers and Conformations of Alkanes & Cycloalkanes
 Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
- 1 - Institute of Organic Chemistry and Biochemistry AS CR, v.v.i., Flemingovo náměstí 2, CZ, Praha, Czech Republic
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014-1 - The activation of N-glycosidic bond cleavage operated by base-excision repair enzyme hogg1;
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014-1 - The activation of N-glycosidic bond cleavage operated by base-excision repair enzyme hogg1;
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
PLEASE read the questions carefully! Partial Periodic Table
 CEMISTRY 3311, Fall 1997 Professor Walba First our Exam September 25, 1997 ame scores: 1) 2) This is a closed-book "open model" exam. You may use models, but no notes 3) or books. Please put all your answers
CEMISTRY 3311, Fall 1997 Professor Walba First our Exam September 25, 1997 ame scores: 1) 2) This is a closed-book "open model" exam. You may use models, but no notes 3) or books. Please put all your answers
PRESENTATION ISOMERISM. Dr. Susmita Bajpai
 PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
c. Cl H Page 1 of 7 major P (E > Z and more substituted over less substituted alkene) LG must be axial are the same Cl -
 CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
Competitive 1,2-C Atom Shifts in the Strained Carbene. Spiro[3.3]hept-1-ylidene Explained by Distinct Ring-
![Competitive 1,2-C Atom Shifts in the Strained Carbene. Spiro[3.3]hept-1-ylidene Explained by Distinct Ring- Competitive 1,2-C Atom Shifts in the Strained Carbene. Spiro[3.3]hept-1-ylidene Explained by Distinct Ring-](/thumbs/92/109564160.jpg) Competitive 1,2-C Atom Shifts in the Strained Carbene Spiro[3.3]hept-1-ylidene Explained by Distinct Ring- Puckered Conformers Murray G. Rosenberg, Theodor Schrievers, and Udo H. Brinker*,, Institute of
Competitive 1,2-C Atom Shifts in the Strained Carbene Spiro[3.3]hept-1-ylidene Explained by Distinct Ring- Puckered Conformers Murray G. Rosenberg, Theodor Schrievers, and Udo H. Brinker*,, Institute of
Pericyclic Rearrangements of N-Heterocyclic Carbenes of Indazole to Substituted 9-Aminoacridines
 Pericyclic Rearrangements of N-Heterocyclic Carbenes of Indazole to Substituted 9-Aminoacridines Zong Guan, Sascha Wiechmann, Martin Drafz, Eike Hübner, and Andreas Schmidt* Clausthal University of Technology,
Pericyclic Rearrangements of N-Heterocyclic Carbenes of Indazole to Substituted 9-Aminoacridines Zong Guan, Sascha Wiechmann, Martin Drafz, Eike Hübner, and Andreas Schmidt* Clausthal University of Technology,
Supporting Information
 Supporting Information Electronic Origins of the Variable Efficiency of Room-Temperature Methane Activation by Homo- and Heteronuclear Cluster Oxide Cations [XYO 2 ] + (X, Y = Al, Si, Mg): Competition
Supporting Information Electronic Origins of the Variable Efficiency of Room-Temperature Methane Activation by Homo- and Heteronuclear Cluster Oxide Cations [XYO 2 ] + (X, Y = Al, Si, Mg): Competition
More Tutorial at
 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
Chap. 3 Conformational Analysis and Molecular Mechanics
 hap. 3 onformational Analysis and Molecular Mechanics onformation:ne of several different spatial arrangements that a molecule can achieve by rotation about single bonds between atoms. Notations: -sc -ac
hap. 3 onformational Analysis and Molecular Mechanics onformation:ne of several different spatial arrangements that a molecule can achieve by rotation about single bonds between atoms. Notations: -sc -ac
H C H H. sawhorse projection
 Alkanes arbons are sp 3 hybridized. Bonds are σ-bonds. - bonds ~ 1.54Å; - bonds ~ 1.10Å. Bond angles ~ 109 o. Ethane sawhorse projection Newman projection Different arrangements of atoms in a molecule
Alkanes arbons are sp 3 hybridized. Bonds are σ-bonds. - bonds ~ 1.54Å; - bonds ~ 1.10Å. Bond angles ~ 109 o. Ethane sawhorse projection Newman projection Different arrangements of atoms in a molecule
18 Isomerism and stereochemistry
 s manual for Burrows et.al. hemistry Third edition 8 Isomerism and stereochemistry s to worked examples WE 8. Structural isomers (on p. 88 in hemistry ) For the following four compounds, A D, identify
s manual for Burrows et.al. hemistry Third edition 8 Isomerism and stereochemistry s to worked examples WE 8. Structural isomers (on p. 88 in hemistry ) For the following four compounds, A D, identify
Solvent Scales. ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute
 Solvent Scales ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute Water 78 1.17 0.47 DMS 47 0.00 0.76 DM 37 0.00 0.76 Methanol 33 0.93 0.66 MPA 29 0.00 1.05 Acetone 21 0.08 0.43 Methylene
Solvent Scales ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute Water 78 1.17 0.47 DMS 47 0.00 0.76 DM 37 0.00 0.76 Methanol 33 0.93 0.66 MPA 29 0.00 1.05 Acetone 21 0.08 0.43 Methylene
No Title. Li HUANG August 07, 2008
 o Title Li UAG August 07, 2008 utline Bioisostere and Isostere Report on the possibility of thioureas catalyzed Claisen rearrangement Isosteres are DefiniIon of isostere molecules or ions with the same
o Title Li UAG August 07, 2008 utline Bioisostere and Isostere Report on the possibility of thioureas catalyzed Claisen rearrangement Isosteres are DefiniIon of isostere molecules or ions with the same
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
SUPPLEMENTARY INFORMATION
 Calculations predict a stable molecular crystal of N 8 : Barak Hirshberg a, R. Benny Gerber a,b, and Anna I. Krylov c a Institute of Chemistry and The Fritz Haber Center for Molecular Dynamics, The Hebrew
Calculations predict a stable molecular crystal of N 8 : Barak Hirshberg a, R. Benny Gerber a,b, and Anna I. Krylov c a Institute of Chemistry and The Fritz Haber Center for Molecular Dynamics, The Hebrew
Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory
 Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley
Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley
First Name MIKE. Chem 30A Winter 2005 MIDTERM #1 (50 Min) Weds February 2nd
 Last First MI Student ID Number: Total Score Circle the name of your TA: MIKE ROB Discussion Section Day: Time: / 00 Chem 30A Winter 2005 MIDTERM # (50 Min) Weds February 2nd INTERPRETATION OF TE QUESTIONS
Last First MI Student ID Number: Total Score Circle the name of your TA: MIKE ROB Discussion Section Day: Time: / 00 Chem 30A Winter 2005 MIDTERM # (50 Min) Weds February 2nd INTERPRETATION OF TE QUESTIONS
Stereoelectronic Effects Recent Advances and New Insights
 Stereoelectronic Effects Recent Advances and ew Insights An Evans Group Afternoon Seminar Keith Fandrick ctober 10, 2003 I. II. III. IV. Introduction to yperconjugation and B Analysis The Role of yperconjugation
Stereoelectronic Effects Recent Advances and ew Insights An Evans Group Afternoon Seminar Keith Fandrick ctober 10, 2003 I. II. III. IV. Introduction to yperconjugation and B Analysis The Role of yperconjugation
Supporting Information
 Supporting Information Conflict in the Mechanism and Kinetics of the Barrierless Reaction between SH and NO 2 Radicals Ramanpreet Kaur and Vikas * Quantum Chemistry Group, Department of Chemistry & Centre
Supporting Information Conflict in the Mechanism and Kinetics of the Barrierless Reaction between SH and NO 2 Radicals Ramanpreet Kaur and Vikas * Quantum Chemistry Group, Department of Chemistry & Centre
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
Supporting Information
 Supporting Information Wiley-VCH 2005 69451 Weinheim, Germany Synthesis of Homoallylic Sulfones Using a Novel Decarboxylative Claisen Rearrangement Reaction** Damien Bourgeois, Donald Craig,* N. Paul King,
Supporting Information Wiley-VCH 2005 69451 Weinheim, Germany Synthesis of Homoallylic Sulfones Using a Novel Decarboxylative Claisen Rearrangement Reaction** Damien Bourgeois, Donald Craig,* N. Paul King,
Problems Points Credit
 hem 201 Final Fall, 2012 Beauchamp Name Problems Points redit 1. Functional Group Nomenclature (1 large structure) (R/S and E/Z too) 30 2. Types of Isomers, Degrees of Unsaturation 25 3. yclohexane onformations,
hem 201 Final Fall, 2012 Beauchamp Name Problems Points redit 1. Functional Group Nomenclature (1 large structure) (R/S and E/Z too) 30 2. Types of Isomers, Degrees of Unsaturation 25 3. yclohexane onformations,
Computational Studies of Lithium Diisopropylamide Deaggregation. Alexander C. Hoepker and David B. Collum*
 omputational Studies of thium Diisopropylamide Deaggregation Alexander. Hoepker and David B. ollum* Department of hemistry and hemical Biology Baker Laboratory, ornell University, Ithaca, ew York 14853-1301
omputational Studies of thium Diisopropylamide Deaggregation Alexander. Hoepker and David B. ollum* Department of hemistry and hemical Biology Baker Laboratory, ornell University, Ithaca, ew York 14853-1301
eg ethylene (IUPAC: ethene), C 2
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Rapid and precise thermochemical calculations by quantum chemical methods
 Rapid and precise thermochemical calculations by quantum chemical methods Ph.D. thesis By: Adrienn Ruzsinszky Supervisor: Dr. Gábor Csonka Budapest University of Technology and Economics Department of
Rapid and precise thermochemical calculations by quantum chemical methods Ph.D. thesis By: Adrienn Ruzsinszky Supervisor: Dr. Gábor Csonka Budapest University of Technology and Economics Department of
O Enolate. = Electrophile/ Lewis acid. = Electrophile/ Lewis acid O B. Enolate. = Electrophile/ Lewis acid. Enolate. O O Enolate
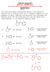 CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
Pericyclic Reactions 6 Lectures Year 3 Handout 2 Michaelmas 2017
 Pericyclic eactions 6 Lectures Year 3 andout 2 Michaelmas 27 π6 a σ2 s Prof Martin Smith CL 3.87 martin.smith@chem.ox.ac.uk http://msmith.chem.ox.ac.uk/ Cycloadditions: oxyallyl cation P46 ω s σ2 s odd
Pericyclic eactions 6 Lectures Year 3 andout 2 Michaelmas 27 π6 a σ2 s Prof Martin Smith CL 3.87 martin.smith@chem.ox.ac.uk http://msmith.chem.ox.ac.uk/ Cycloadditions: oxyallyl cation P46 ω s σ2 s odd
CPT-26 ANSWERS 73. (1) 145. (4) 2. (1) 74. (3) 146. (4) 3. (3) 75. (2) 147. (3) 4. (2) 5. (4) 76. (4) 77. (2) 148. (2) 149. (3) 6. (3) 78.
 1 08/01/2018 COMMON PRACTICE TEST [PMT] : 2017-19 CPT-26 ANSWERS CODE GOL 1. (1) 37. (3) 73. (1) 109. (3) 145. (4) 2. (1) 38. (1) 74. (3) 110. (3) 146. (4) 3. (3) 39. (1) 75. (2) 111. (2) 147. (3) 4. (2)
1 08/01/2018 COMMON PRACTICE TEST [PMT] : 2017-19 CPT-26 ANSWERS CODE GOL 1. (1) 37. (3) 73. (1) 109. (3) 145. (4) 2. (1) 38. (1) 74. (3) 110. (3) 146. (4) 3. (3) 39. (1) 75. (2) 111. (2) 147. (3) 4. (2)
CHEMISTRY 241 Section 004 EXAMINATION I TUESDAY, October 11, :30-11:50 AM Professor William P. Dailey NAME: QUESTIONS POINTS SCORE
 CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Three-Dimensional Structures of Drugs
 Three-Dimensional Structures of Drugs Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Chiral drugs are sometimes sold as one enantiomer (pure
Three-Dimensional Structures of Drugs Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Chiral drugs are sometimes sold as one enantiomer (pure
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Ab Initio Study on the Substituent Effect in the Transition State of Keto-Enol Tautomerism of Acetyl Derivatives
 594 J. Phys. Chem. 1996, 100, 594-600 Ab Initio Study on the Substituent Effect in the Transition State of Keto-Enol Tautomerism of Acetyl Derivatives Chen-Chang Wu and Min-Hsiung Lien* Department of Chemistry,
594 J. Phys. Chem. 1996, 100, 594-600 Ab Initio Study on the Substituent Effect in the Transition State of Keto-Enol Tautomerism of Acetyl Derivatives Chen-Chang Wu and Min-Hsiung Lien* Department of Chemistry,
Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Supporting Information Over or under: Hydride attack at the metal versus the coordinated
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Supporting Information Over or under: Hydride attack at the metal versus the coordinated
Chapter 6 Cyclic urea - a new central unit in bent-core compounds
 82 Chapter 6 Cyclic urea - a new central unit in bent-core compounds A new class of five-ring bent-core molecules with a cyclic urea group as a central unit was synthesized [94]. A significant difference
82 Chapter 6 Cyclic urea - a new central unit in bent-core compounds A new class of five-ring bent-core molecules with a cyclic urea group as a central unit was synthesized [94]. A significant difference
Literature values: ΔH f, gas = % error Source: ΔH f, solid = % error. For comparison, your experimental value was ΔH f = phase:
 1 Molecular Calculations Lab: Some guideline given at the bottom of page 3. 1. Use the semi-empirical AM1 method to calculate ΔH f for the compound you used in the heat of combustion experiment. Be sure
1 Molecular Calculations Lab: Some guideline given at the bottom of page 3. 1. Use the semi-empirical AM1 method to calculate ΔH f for the compound you used in the heat of combustion experiment. Be sure
one ν im: transition state saddle point
 Hypothetical Potential Energy Surface Ethane conformations Hartree-Fock theory, basis set stationary points all ν s >0: minimum eclipsed one ν im: transition state saddle point multiple ν im: hilltop 1
Hypothetical Potential Energy Surface Ethane conformations Hartree-Fock theory, basis set stationary points all ν s >0: minimum eclipsed one ν im: transition state saddle point multiple ν im: hilltop 1
by Iridium Silyl Complexes
 Facile Redistribution of Trialkyl Silanes Catalyzed by Iridium Silyl Complexes Sehoon Park, Bong Gon Kim, Inigo Göttker-Schnetmann, and Maurice Brookhart*, Department of Chemistry, University of North
Facile Redistribution of Trialkyl Silanes Catalyzed by Iridium Silyl Complexes Sehoon Park, Bong Gon Kim, Inigo Göttker-Schnetmann, and Maurice Brookhart*, Department of Chemistry, University of North
with the larger dimerization energy also exhibits the larger structural changes.
 A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
Macrocyclic Oligofurans: A Computational Study
 Supporting Information Macrocyclic Oligofurans: A Computational Study Or Dishi and Ori Gidron* Institute of Chemistry, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem,
Supporting Information Macrocyclic Oligofurans: A Computational Study Or Dishi and Ori Gidron* Institute of Chemistry, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem,
Introduction to organic compounds
 Chapter 2 Introduction to organic compounds Nomenclature Physical properties Conformation Organic compounds Ch 2 #2 in Organic Chemistry 1 hydrocarbons [R] alkanes alkenes alkynes alkyl halides [RX] ethers
Chapter 2 Introduction to organic compounds Nomenclature Physical properties Conformation Organic compounds Ch 2 #2 in Organic Chemistry 1 hydrocarbons [R] alkanes alkenes alkynes alkyl halides [RX] ethers
Potential Energy Surface and Binding Energy in External Electric Field: Modulation of Anion π Interactions for Graphene Based Receptors
 Potential Energy Surface and Binding Energy in External Electric Field: Modulation of Anion π Interactions for Graphene Based Receptors Cina Foroutan Nejad a and Radek Marek a,b a National Center for Biomolecular
Potential Energy Surface and Binding Energy in External Electric Field: Modulation of Anion π Interactions for Graphene Based Receptors Cina Foroutan Nejad a and Radek Marek a,b a National Center for Biomolecular
The Alkaline Hydrolysis of Sulfonate Esters: Challenges in Interpreting
 Supporting Information for: The Alkaline Hydrolysis of Sulfonate Esters: Challenges in Interpreting Experimental and Theoretical Data Fernanda Duarte 1, Ting Geng 1, Gaël Marloie 1, Adel O. Al Hussain
Supporting Information for: The Alkaline Hydrolysis of Sulfonate Esters: Challenges in Interpreting Experimental and Theoretical Data Fernanda Duarte 1, Ting Geng 1, Gaël Marloie 1, Adel O. Al Hussain
ONETEP PB/SA: Application to G-Quadruplex DNA Stability. Danny Cole
 ONETEP PB/SA: Application to G-Quadruplex DNA Stability Danny Cole Introduction Historical overview of structure and free energy calculation of complex molecules using molecular mechanics and continuum
ONETEP PB/SA: Application to G-Quadruplex DNA Stability Danny Cole Introduction Historical overview of structure and free energy calculation of complex molecules using molecular mechanics and continuum
ORGANIC - EGE 5E CH. 5 - ALKANES AND CYCLOALKANES.
 !! www.clutchprep.com CONCEPT: ALKANE NOMENCLATURE Before 1919, chemists literally had to memorize thousands of random (common) chemical names. IUPAC naming provides a systematic method to give every chemical
!! www.clutchprep.com CONCEPT: ALKANE NOMENCLATURE Before 1919, chemists literally had to memorize thousands of random (common) chemical names. IUPAC naming provides a systematic method to give every chemical
The Mechanistic Studies of the Wacker Oxidation. Tyler W. Wilson SED Group Meeting
 The Mechanistic Studies of the Wacker xidation Tyler W. Wilson SE Group Meeting 11.27.2007 Introduction xidation of ethene by (II) chloride solutions (Phillips, 1894) -First used as a test for alkenes
The Mechanistic Studies of the Wacker xidation Tyler W. Wilson SE Group Meeting 11.27.2007 Introduction xidation of ethene by (II) chloride solutions (Phillips, 1894) -First used as a test for alkenes
Renaud Group Exercise Set
 Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
CHEM 545 Theory and Practice of Molecular Electronic Structure. Anna I. Krylov. DON T PANIC.
 CHEM 545 Theory and Practice of Molecular Electronic Structure Anna I. Krylov http://iopenshell.usc.edu/chem545/ DON T PANIC USC Fall 2014 Things to do: 1. Install IQmol (by this Thursday). http://iqmol.org/.
CHEM 545 Theory and Practice of Molecular Electronic Structure Anna I. Krylov http://iopenshell.usc.edu/chem545/ DON T PANIC USC Fall 2014 Things to do: 1. Install IQmol (by this Thursday). http://iqmol.org/.
Keto-Enol Thermodynamics of Breslow Intermediates
 Keto-Enol Thermodynamics of Breslow Intermediates Mathias Paul, Martin Breugst, Jörg-Martin Neudörfl, Raghavan B. Sunoj, and Albrecht Berkessel Computational Section of the Supporting Information 1 Computational
Keto-Enol Thermodynamics of Breslow Intermediates Mathias Paul, Martin Breugst, Jörg-Martin Neudörfl, Raghavan B. Sunoj, and Albrecht Berkessel Computational Section of the Supporting Information 1 Computational
An Introduction to Quantum Chemistry and Potential Energy Surfaces. Benjamin G. Levine
 An Introduction to Quantum Chemistry and Potential Energy Surfaces Benjamin G. Levine This Week s Lecture Potential energy surfaces What are they? What are they good for? How do we use them to solve chemical
An Introduction to Quantum Chemistry and Potential Energy Surfaces Benjamin G. Levine This Week s Lecture Potential energy surfaces What are they? What are they good for? How do we use them to solve chemical
Jack Smith. Center for Environmental, Geotechnical and Applied Science. Marshall University
 Jack Smith Center for Environmental, Geotechnical and Applied Science Marshall University -- Division of Science and Research WV Higher Education Policy Commission WVU HPC Summer Institute June 20, 2014
Jack Smith Center for Environmental, Geotechnical and Applied Science Marshall University -- Division of Science and Research WV Higher Education Policy Commission WVU HPC Summer Institute June 20, 2014
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
List of Figures Page Figure No. Figure Caption No. Figure 1.1.
 List of Figures Figure No. Figure Caption Page No. Figure 1.1. Cation- interactions and their modulations. 4 Figure 1.2. Three conformations of benzene dimer, S is not a minimum on the potential energy
List of Figures Figure No. Figure Caption Page No. Figure 1.1. Cation- interactions and their modulations. 4 Figure 1.2. Three conformations of benzene dimer, S is not a minimum on the potential energy
ORGANIC - BRUICE 8E CH.3 - AN INTRODUCTION TO ORGANIC COMPOUNDS
 !! www.clutchprep.com CONCEPT: INDEX OF HYDROGEN DEFICIENCY (STRUCTURAL) A saturated molecule is any molecule that has the maximum number of hydrogens possible for its chemical structure. The rule that
!! www.clutchprep.com CONCEPT: INDEX OF HYDROGEN DEFICIENCY (STRUCTURAL) A saturated molecule is any molecule that has the maximum number of hydrogens possible for its chemical structure. The rule that
Suggested solutions for Chapter 27
 uggested solutions for Chapter 27 27 PRBLEM 1 uggest a mechanism for this reaction, commenting on the selectivity and the stereochemistry. Me 2 1. t-buk 2. Raney Ni Et The opportunity to explore the consequences
uggested solutions for Chapter 27 27 PRBLEM 1 uggest a mechanism for this reaction, commenting on the selectivity and the stereochemistry. Me 2 1. t-buk 2. Raney Ni Et The opportunity to explore the consequences
Chem 314. Problem Points Credit. 1. Nomenclature D Lewis structures D Structures, Formal Charge & Resonance 34
 alifornia State Polytechnic University, Pomona 1 Spring, 2013 Midterm Exam hem 314 Beauchamp hem 314 ame Problem Points redit 1. omenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal harge
alifornia State Polytechnic University, Pomona 1 Spring, 2013 Midterm Exam hem 314 Beauchamp hem 314 ame Problem Points redit 1. omenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal harge
B. A transition state represents a maximum on the reaction path diagram and can be isolated.
 Practice Hour Exam 2, Chemistry 2210, Organic Chemistry I 1. The most stable carbocation is: 2. Which of the following statements is true of transition states? A. A transition state represents a minimum
Practice Hour Exam 2, Chemistry 2210, Organic Chemistry I 1. The most stable carbocation is: 2. Which of the following statements is true of transition states? A. A transition state represents a minimum
Organic Chemistry The Functional Group Approach. Organic Chemistry The Functional Group Approach
 Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Supporting Information
 Supporting Information Formation of Ruthenium Carbenes by gem-hydrogen Transfer to Internal Alkynes: Implications for Alkyne trans-hydrogenation Markus Leutzsch, Larry M. Wolf, Puneet Gupta, Michael Fuchs,
Supporting Information Formation of Ruthenium Carbenes by gem-hydrogen Transfer to Internal Alkynes: Implications for Alkyne trans-hydrogenation Markus Leutzsch, Larry M. Wolf, Puneet Gupta, Michael Fuchs,
NAME: SUMMER 2015 MIDTERM
 page 1 pts NAME: SUMMER 2015 MIDTERM hemistry 350 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 6/16) Do your best on this take-home portion of your midterm. I may grade
page 1 pts NAME: SUMMER 2015 MIDTERM hemistry 350 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 6/16) Do your best on this take-home portion of your midterm. I may grade
Chemistry 210 Organic Chemistry I Winter Semester 1999 Dr. Rainer Glaser
 Chemistry 210 rganic Chemistry I Winter Semester 1999 Dr. Rainer Glaser Examination #1 Structure, Bonding and Properties of rganic Molecules. Structure, Stereochemistry and Properties of Alkanes. Friday,
Chemistry 210 rganic Chemistry I Winter Semester 1999 Dr. Rainer Glaser Examination #1 Structure, Bonding and Properties of rganic Molecules. Structure, Stereochemistry and Properties of Alkanes. Friday,
hand and delocalization on the other, can be instructively exemplified and extended
 Text Related to Segment 8.0 00 Claude E. Wintner The ideas developed up to this point, concerning stereochemistry on the one hand and delocalization on the other, can be instructively exemplified and extended
Text Related to Segment 8.0 00 Claude E. Wintner The ideas developed up to this point, concerning stereochemistry on the one hand and delocalization on the other, can be instructively exemplified and extended
