Lecture 15 February 15, 2013 Transition metals
|
|
|
- Lucinda Hancock
- 5 years ago
- Views:
Transcription
1 Lecture 15 February 15, 2013 Transition metals Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a Hours: 2-3pm Monday, Wednesday, Friday William A. Goddard, III, 316 Beckman Institute, x3093 Charles and Mary Ferkel Professor of Chemistry, Materials Science, and Applied Physics, California Institute of Technology Teaching Assistants: Ross Fu Fan Liu Ch120a-1
2 Transition metals (4s,3d) Sc---Cu (5s,4d) Y-- Ag (6s,5d) (La or Lu), Ce-Au 2
3 Ground states of neutral atoms Sc (4s)2(3d)1 Sc ++ (3d)1 Ti (4s)2(3d)2 Ti ++ (3d)2 V (4s)2(3d)3 V ++ (3d)3 Cr (4s)1(3d)5 Cr ++ (3d)4 Mn (4s)2(3d)5 Mn ++ (3d)5 Fe (4s)2(3d)6 Fe ++ (3d)6 Co (4s)2(3d)7 Co ++ (3d)7 Ni (4s)2(3d)8 Ni ++ (3d)8 Cu (4s)1(3d)10 Cu ++ (3d)10 3
4 Hemoglobin Blood has 5 billion erythrocytes/ml Each erythrocyte contains 280 million hemoglobin (Hb) molecules Each Hb has MW=64500 Dalton (diameter ~ 60A) Four subunits (a1, a2, b1, b2) each with one heme subunit Hb Each subunit resembles myoglobin (Mb) which has one heme Mb 4
5 The action is at the heme or Fe-Porphyrin molecule Essentially all action occurs at the heme, which is basically an Fe-Porphyrin molecule The rest of the Mb serves mainly to provide a hydrophobic envirornment at the Fe and to protect the heme 5
6 The heme group The net charge of the Fe-heme is zero. The VB structure shown is one of several, all of which lead to two neutral N and two negative N. Thus we consider that the Fe is Fe 2+ with a d 6 configuration Each N has a doubly occupied sp 2 s orbital pointing at it. 6
7 Energies of the 5 Fe 2+ d orbitals x 2 -y 2 z 2 =2z 2 -x 2 -y 2 yz xz xy 7
8 Exchange stabilizations 8
9 Ferrous Fe II x 2 -y 2 destabilized by heme N lone pairs z 2 destabilized by 5 th ligand imidazole or 6 th ligand CO y x 9
10 Four coordinate Fe- Heme High spin case, S=2 or q The 5 th axial ligand will destabilize q2 since dz2 is doubly occupied A pi acceptor would stabilize q1 wrt q2 Bonding O2 to 5 coordinate will stabilize q3 wrt q1 Future discuss only q1 copyright and denote 2011 William as A. Goddard q III, all rights reserved 10
11 Four coordinate Fe-Heme Intermediate spin, S=1 or t 11
12 Four coordinate Fe-Heme Low spin case, S=0 or s 12
13 Out of plane motion of Fe 4 coordinate 13
14 Add axial base N-N Nonbonded interactions push Fe out of plane is antibonding 14
15 Summary 4 coord and 5 coord states 15
16 Free atom to 4 coord to 5 coord Net effect due to five N ligands is to squish the q, t, and s states by a factor of 3 This makes all three available as possible ground states depending on the 6 th ligand 16
17 Bonding of O 2 with O to form ozone O 2 has available a ps orbital for a s bond to a ps orbital of the O atom And the 3 electron p system for a p bond to a pp orbital of the O atom 17
18 Bond O 2 to Mb Simple VB structures get S=1 or triplet state In fact MbO 2 is singlet Why? 18
19 change in exchange terms when Bond O 2 to Mb O 2 ps O 2 pp Assume perfect VB spin pairing Then get 4 cases Thus average K dd is ( )/4 = K dd 5*4/2 up spin down spin 7 K dd 4*3/2 + 2*1/2 7 K dd 4*3/2 + 2*1/2 6 K dd 3*2/2 + 3*2/2 19
20 Bonding O 2 to Mb Exchange loss on bonding O 2 20
21 Modified exchange energy for q state But expected t binding to be 2*22 = 44 kcal/mol stronger than q What happened? Binding to q would have DH = = + 11 kcal/mol Instead the q state retains the high spin pairing so that there is no exchange loss, but now the coupling of Fe to O 2 does not gain the full VB strength, leading to bond of only 8kcal/mol instead of 33 21
22 Bond CO to Mb H 2 O and N 2 do not bond strongly enough to promote the Fe to an excited state, thus get S=2 22
23 compare bonding of CO and O2 to Mb 23
24 GVB orbitals for bonds to Ti Ti ds character, 1 elect H 1s character, 1 elect Covalent 2 electron TiH bond in Cl 2 TiH 2 Think of as bond from Tidz2 to H1s Covalent 2 electron CH bond in CH 4 Csp 3 character 1 elect H 1s character, 1 elect 24
25 Bonding at a transition metaal Bonding to a transition metals can be quite covalent. Examples: (Cl 2 )Ti(H 2 ), (Cl 2 )Ti(C 3 H 6 ), Cl 2 Ti=CH 2 Here the two bonds to Cl remove ~ 1 to 2 electrons from the Ti, making is very unwilling to transfer more charge, certainly not to C or H (it would be the same for a Cp (cyclopentadienyl ligand) Thus TiCl 2 group has ~ same electronegativity as H or CH 3 The covalent bond can be thought of as Ti(dz2-4s) hybrid spin paired with H1s A{[(Tids)(H1s)+ (H1s)(Tids)](ab-ba)} 25
26 But TM-H bond can also be s-like Cl 2 TiH + Ti (4s) 2 (3d) 2 The 2 Cl pull off 2 e from Ti, leaving a d 1 configuration Ti-H bond character 1.07 Tid+0.22Tisp+0.71H ClMnH Mn (4s) 2 (3d) 5 The Cl pulls off 1 e from Mn, leaving a d 5 s 1 configuration H bonds to 4s because of exchange stabilization of d 5 Mn-H bond character 0.07 Mnd+0.71Mnsp+1.20H 26
27 Bond angle at a transition metal For two p orbitals expect 90, HH nonbond repulsion increases it What angle do two d orbitals want H-Ti-H plane 76 Metallacycle plane 27
28 Best bond angle for 2 pure Metal bonds using d orbitals Assume that the first bond has pure d z2 or ds character to a ligand along the z axis Can we make a 2 nd bond, also of pure ds character (rotationally symmetric about the z axis) to a ligand along some other axis, call it z. For pure p systems, this leads to = 90 For pure d systems, this leads to = 54.7 (or ), this is ½ the tetrahedral angle of (also the magic spinning angle for solid state NMR). 28
29 Best bond angle for 2 pure Metal bonds using d orbitals Problem: two electrons in atomic d orbitals with same spin lead to 5*4/2 = 10 states, which partition into a 3 F state (7) and a 3 P state (3), with 3 F lower. This is because the electron repulsion between say a d xy and d x2-y2 is higher than between sasy d z2 and d xy. Best is ds with dd because the electrons are farthest apart This favors = 90, but the bond to the dd orbital is not as good Thus expect something between 53.7 and 90 Seems that ~76 is often best 29
30 How predict character of Transition metal bonds? Start with ground state atomic configuration Ti (4s) 2 (3d) 2 or Mn (4s) 2 (3d) 5 Consider that bonds to electronegative ligands (eg Cl or Cp) take electrons from 4s easiest to ionize, also better overlap with Cl or Cp, also leads to less reduction in dd exchange (3d) 2 (4s)(3d) 5 Now make bond to less electronegative ligands, H or CH 3 Use 4s if available, otherwise use d orbitals 30
31 But TM-H bond can also be s-like Cl 2 TiH + Ti (4s) 2 (3d) 2 The 2 Cl pull off 2 e from Ti, leaving a d 1 configuration Ti-H bond character 1.07 Tid+0.22Tisp+0.71H ClMnH Mn (4s) 2 (3d) 5 The Cl pulls off 1 e from Mn, leaving a d 5 s 1 configuration H bonds to 4s because of exchange stabilization of d 5 Mn-H bond character 0.07 Mnd+0.71Mnsp+1.20H 31
32 Example (Cl) 2 VH 3 + resonance configuration 32
33 Example ClMometallacycle butadiene 33
34 Example [Mn CH] 2+ 34
35 Summary: start with Mn + s 1 d 5 dy2 s bond to H1s dx2-x2 non bonding dyz p bond to CH dxz p bond to CH dxy non bonding 4sp hybrid s bond to CH 35
36 Summary: start with Mn + s 1 d 5 dy2 s bond to H1s dx2-x2 non bonding dyz p bond to CH dxz p bond to CH dxy non bonding 4sp hybrid s bond to CH 36
Lecture 16 February 20 Transition metals, Pd and Pt
 Lecture 16 February 20 Transition metals, Pd and Pt Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course
Lecture 16 February 20 Transition metals, Pd and Pt Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course
Lecture 11 January 30, Transition metals, Pd and Pt
 Lecture 11 January 30, 2011 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a Hours:
Lecture 11 January 30, 2011 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a Hours:
Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
 Lecture 13, October 31, 2016 Transition metals, Pd and Pt Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Lecture 13, October 31, 2016 Transition metals, Pd and Pt Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
 Lecture 12, October 21, 2016 Transition metals Heme-Fe Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Lecture 12, October 21, 2016 Transition metals Heme-Fe Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Lecture 13 February 1, 2011 Pd and Pt, MH + bonding, metathesis
 Lecture 13 February 1, 2011 Pd and Pt, MH + bonding, metathesis Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and
Lecture 13 February 1, 2011 Pd and Pt, MH + bonding, metathesis Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and
Lecture 17 February 14, 2013 MH + bonding, metathesis
 Lecture 17 February 14, 2013 MH + bonding, metathesis Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Lecture 17 February 14, 2013 MH + bonding, metathesis Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Lecture 9-10 January 25-27, 2012 Rules for Chem. React. - Woodward-Hoffmann
 Lecture 9-10 January 25-27, 2012 Rules for Chem. React. - Woodward-Hoffmann Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic
Lecture 9-10 January 25-27, 2012 Rules for Chem. React. - Woodward-Hoffmann Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic
Lecture 14 February 3, 2014 Rules for Chem. React. - Woodward-Hoffmann
 Lecture 14 February 3, 2014 Rules for Chem. React. - Woodward-Hoffmann Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Lecture 14 February 3, 2014 Rules for Chem. React. - Woodward-Hoffmann Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Lecture 8 January 24, 2013 GaAs crystal surfaces, n-p dopants Si
 Lecture 8 January 24, 2013 Ga crystal surfaces, n-p dopants Si Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinornic chemistry, and
Lecture 8 January 24, 2013 Ga crystal surfaces, n-p dopants Si Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinornic chemistry, and
Lecture 16, February 25, 2015 Metallic bonding
 Lecture 16, February 25, 2015 Metallic bonding Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 115 BI Hours: 11-11:50am
Lecture 16, February 25, 2015 Metallic bonding Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 115 BI Hours: 11-11:50am
Lecture 6 January 18, 2012 CC Bonds diamond, ΔHf, Group additivity
 Lecture 6 January 18, 2012 CC Bonds diamond, ΔHf, Group additivity Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Lecture 6 January 18, 2012 CC Bonds diamond, ΔHf, Group additivity Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Molecular Orbital Theory (MOT)
 Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
B. Electron Deficient (less than an octet) H-Be-H. Be does not need an octet Total of 4 valence electrons
 B. Electron Deficient (less than an octet) e.g. BeH 2 H-Be-H Be does not need an octet Total of 4 valence electrons Not the same as unsaturated systems that achieve the 8e - (octet) through the formation
B. Electron Deficient (less than an octet) e.g. BeH 2 H-Be-H Be does not need an octet Total of 4 valence electrons Not the same as unsaturated systems that achieve the 8e - (octet) through the formation
Lecture February 13-15, Silicon crystal surfaces
 Lecture 18-19 February 13-15, 2012 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a
Lecture 18-19 February 13-15, 2012 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a
Coordination Chemistry: Bonding Theories. Crystal Field Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
Lecture 4; January Electrons in Atoms: Magnetism; Term Symbols, Z eff, and Other Properties
 Lecture 4; January 2017 Electrons in Atoms: Magnetism; Term Symbols, Z eff, and Other Properties Three prototypical kinds of Magnetic Behavior Paramagnetism: atoms, molecules, and solids with unpaired
Lecture 4; January 2017 Electrons in Atoms: Magnetism; Term Symbols, Z eff, and Other Properties Three prototypical kinds of Magnetic Behavior Paramagnetism: atoms, molecules, and solids with unpaired
Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
 Lecture 22, November 16, 2016 Graphite, graphene, bucky balls, bucky tubes Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Lecture 22, November 16, 2016 Graphite, graphene, bucky balls, bucky tubes Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Transition Metals and Coordination Chemistry
 Transition Metals and Coordination Chemistry Transition Metals Similarities within a given period and within a given group. Last electrons added are inner electrons (d s, f s). 20_431 Ce Th Pr Pa d U
Transition Metals and Coordination Chemistry Transition Metals Similarities within a given period and within a given group. Last electrons added are inner electrons (d s, f s). 20_431 Ce Th Pr Pa d U
Lecture 18, March 2, 2015 graphene, bucky balls, bucky tubes
 Lecture 18, March 2, 2015 graphene, bucky balls, bucky tubes Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 115 BI
Lecture 18, March 2, 2015 graphene, bucky balls, bucky tubes Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 115 BI
Inorganic Chemistry with Doc M. Fall Semester, 2011 Day 19. Transition Metals Complexes IV: Spectroscopy
 Inorganic Chemistry with Doc M. Fall Semester, 011 Day 19. Transition Metals Complexes IV: Spectroscopy Name(s): lement: Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields. Term symbols
Inorganic Chemistry with Doc M. Fall Semester, 011 Day 19. Transition Metals Complexes IV: Spectroscopy Name(s): lement: Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields. Term symbols
Lecture 14 February 7, 2014 Symmetry
 Lecture 14 February 7, 2014 Symmetry Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a
Lecture 14 February 7, 2014 Symmetry Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a
If you put an electron into the t 2g, like that for Ti 3+, then you stabilize the barycenter of the d orbitals by 0.4 D o.
 Crystal Field Stabilization Energy Week 2-1 Octahedral Symmetry (O h ) If you put an electron into the t 2g, like that for Ti 3+, then you stabilize the barycenter of the d orbitals by 0.4 D o. Each additional
Crystal Field Stabilization Energy Week 2-1 Octahedral Symmetry (O h ) If you put an electron into the t 2g, like that for Ti 3+, then you stabilize the barycenter of the d orbitals by 0.4 D o. Each additional
Lecture February 8-10, NiCHx
 Lecture 16-17 February 8-10, 2011 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a
Lecture 16-17 February 8-10, 2011 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch120a
Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy
 Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields 2. Term symbols and the method of microstates
Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields 2. Term symbols and the method of microstates
Lecture 26 March 8, 2010 Heterogeneous Catalysis
 Lecture 26 March 8, 2010 Heterogeneous Catalysis Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy William
Lecture 26 March 8, 2010 Heterogeneous Catalysis Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy William
Electronic structure / bonding in d-block complexes
 LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Molecular Orbital Theory and Charge Transfer Excitations
 Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Molecular Orbital Theory and Charge Transfer Excitations
 Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Chapter 20 d-metal complexes: electronic structures and properties
 CHEM 511 Chapter 20 page 1 of 21 Chapter 20 d-metal complexes: electronic structures and properties Recall the shape of the d-orbitals... Electronic structure Crystal Field Theory: an electrostatic approach
CHEM 511 Chapter 20 page 1 of 21 Chapter 20 d-metal complexes: electronic structures and properties Recall the shape of the d-orbitals... Electronic structure Crystal Field Theory: an electrostatic approach
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
PAPER No. 7: Inorganic chemistry II MODULE No. 5: Molecular Orbital Theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Bonding in Octahedral and Tetrahedral Metal Complexes. Predict how the d orbitals are affected by the Metal- Ligand Bonding
 Bonding in Octahedral and Tetrahedral Metal Complexes 327 Molecular Orbital Theory and Crystal Field/Ligand Field Theory Predict how the d orbitals are affected by the Metal- Ligand Bonding d z 2, d x
Bonding in Octahedral and Tetrahedral Metal Complexes 327 Molecular Orbital Theory and Crystal Field/Ligand Field Theory Predict how the d orbitals are affected by the Metal- Ligand Bonding d z 2, d x
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
 Lecture 19, ovember 9, 2016 complexes C4 activation, functionalization ature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Lecture 19, ovember 9, 2016 complexes C4 activation, functionalization ature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry,
Lecture 8 January 28, Silicon crystal surfaces
 Lecture 8 January 28, 203 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch20a Hours:
Lecture 8 January 28, 203 Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course number: Ch20a Hours:
Chapter 21: Transition Metals and Coordination Chemistry
 Chapter 21: Transition Metals and Coordination Chemistry Mg, Cr, V, Co Pt Fe complexes O2 Mo and Fe complexes: nitrogen fixation Zn: 150 Cu, Fe: Co: B12 21.1 Transition Metals show great similarities within
Chapter 21: Transition Metals and Coordination Chemistry Mg, Cr, V, Co Pt Fe complexes O2 Mo and Fe complexes: nitrogen fixation Zn: 150 Cu, Fe: Co: B12 21.1 Transition Metals show great similarities within
Chapter 9. Chemical Bonding II: Molecular Geometry and Bonding Theories
 Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Lecture 14 February 7, 2011 Reactions O2, Woodward-Hoffmann
 Lecture 14 February 7, 2011 Reactions O2, Woodward-Hoffmann Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Lecture 14 February 7, 2011 Reactions O2, Woodward-Hoffmann Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy
Ch125a-1. copyright 2015 William A. Goddard III, all rights reserved
 Lecture, October 28, 205: Si, Ga crystal surfaces Ch 25a: Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Ch 20a:Nature of the Chemical bond Room
Lecture, October 28, 205: Si, Ga crystal surfaces Ch 25a: Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Ch 20a:Nature of the Chemical bond Room
Bonding in Coordination Compounds. Crystal Field Theory. Bonding in Transition Metal Complexes
 Bonding in Transition Metal Complexes 1) Crystal Field Theory (ligand field theory) Crystal Field Theory Treat igands as negative charges (they repel the e- in the d orbitals deals only with d orbitals
Bonding in Transition Metal Complexes 1) Crystal Field Theory (ligand field theory) Crystal Field Theory Treat igands as negative charges (they repel the e- in the d orbitals deals only with d orbitals
 lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
Mo 2+, Mo 2+, Cr electrons. Mo-Mo quadruple bond.
 Problem 1 (2 points) 1. Consider the MoMoCr heterotrimetallic complex shown below (Berry, et. al. Inorganica Chimica Acta 2015, p. 241). Metal-metal bonds are not drawn. The ligand framework distorts this
Problem 1 (2 points) 1. Consider the MoMoCr heterotrimetallic complex shown below (Berry, et. al. Inorganica Chimica Acta 2015, p. 241). Metal-metal bonds are not drawn. The ligand framework distorts this
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Topics Coordination Complexes Chemistry 1B-AL, Fall 2016
 Chemistry 1B Fall 2016 LISTEN UP!!! Topics Lectures 17-18 Coordination Chemistry WE WILL ONLY COVER LIMITED PARTS OF CAPTER 19 (940-944;952-954;963-970) 1 2 good reasons for studying coordination chemistry
Chemistry 1B Fall 2016 LISTEN UP!!! Topics Lectures 17-18 Coordination Chemistry WE WILL ONLY COVER LIMITED PARTS OF CAPTER 19 (940-944;952-954;963-970) 1 2 good reasons for studying coordination chemistry
Chemistry 1B. Fall Topics Lectures Coordination Chemistry
 Chemistry 1B Fall 2016 Topics Lectures 17-18 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (940-944;952-954;963-970) 2 good reasons for studying coordination chemistry
Chemistry 1B Fall 2016 Topics Lectures 17-18 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (940-944;952-954;963-970) 2 good reasons for studying coordination chemistry
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
Covalent Bonding: Orbitals
 Hybridization and the Localized Electron Model Covalent Bonding: Orbitals A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal
Hybridization and the Localized Electron Model Covalent Bonding: Orbitals A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal
Atomic Structure and Bonding. Chapter 1 Organic Chemistry, 8 th Edition John McMurry
 Atomic Structure and Bonding Chapter 1 Organic Chemistry, 8 th Edition John McMurry 1 Common Elements Groups First row Second row In most organic molecules carbon is combined with relatively few elements
Atomic Structure and Bonding Chapter 1 Organic Chemistry, 8 th Edition John McMurry 1 Common Elements Groups First row Second row In most organic molecules carbon is combined with relatively few elements
Exam 2 Review Problems
 09-107 onors Chemistry Carnegie Mellon University Exam 2 Review Problems Exam 2 will be held on Tuesday, ctober 30, in Porter all A18C. We will be there from 2:45 to 4:45 pm. Disclaimer: This review sheet
09-107 onors Chemistry Carnegie Mellon University Exam 2 Review Problems Exam 2 will be held on Tuesday, ctober 30, in Porter all A18C. We will be there from 2:45 to 4:45 pm. Disclaimer: This review sheet
Carbon and Its Compounds
 Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Topics Coordination Complexes Chemistry 1B-AL, Fall 2016
 Chemistry 1B Fall 2016 Topics Lectures 17-18 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (940-944;952-954;963-970) 2 Page 1 good reasons for studying coordination
Chemistry 1B Fall 2016 Topics Lectures 17-18 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (940-944;952-954;963-970) 2 Page 1 good reasons for studying coordination
Chemistry 1B. Fall Lectures Coordination Chemistry
 Chemistry 1B Fall 2013 Lectures 13-14 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (940-944;952-954;963-970) 2 good reasons for studying coordination chemistry a
Chemistry 1B Fall 2013 Lectures 13-14 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (940-944;952-954;963-970) 2 good reasons for studying coordination chemistry a
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Chapter 24. Transition Metals and Coordination Compounds. Lecture Presentation. Sherril Soman Grand Valley State University
 Lecture Presentation Chapter 24 Transition Metals and Coordination Compounds Sherril Soman Grand Valley State University Gemstones The colors of rubies and emeralds are both due to the presence of Cr 3+
Lecture Presentation Chapter 24 Transition Metals and Coordination Compounds Sherril Soman Grand Valley State University Gemstones The colors of rubies and emeralds are both due to the presence of Cr 3+
Hybridization of Orbitals
 Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
2018 Ch112 problem set 6 Due: Thursday, Dec. 6th. Problem 1 (2 points)
 Problem 1 (2 points) a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions (υ1 and
Problem 1 (2 points) a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions (υ1 and
UNIT III Chemical Bonding There are two basic approaches to chemical bonding based on the results of quantum mechanics. These are the Valence Bond
 UNIT III Chemical Bonding There are two basic approaches to chemical bonding based on the results of quantum mechanics. These are the Valence Bond Theory (VB) and the Molecular Orbital theory (MO). 1)
UNIT III Chemical Bonding There are two basic approaches to chemical bonding based on the results of quantum mechanics. These are the Valence Bond Theory (VB) and the Molecular Orbital theory (MO). 1)
Chemistry 1B. Fall Lectures Coordination Chemistry
 Chemistry 1B Fall 2012 Lectures 13-14 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (pp. 933-937; 946-948; 958-966) [940-944;952-954;963-970] 7th 2 good reasons for
Chemistry 1B Fall 2012 Lectures 13-14 Coordination Chemistry 1 LISTEN UP!!! WE WILL ONLY COVER LIMITED PARTS OF CHAPTER 19 (pp. 933-937; 946-948; 958-966) [940-944;952-954;963-970] 7th 2 good reasons for
Chem 104A, UC, Berkeley. Example: Z eff. Chem 104A, UC, Berkeley. Be:
 Example: 4 O: 1s s p p Z eff (0.85) 5(0.35) 4.55 Z 8 4.55 3.45 Be: B: C: 1s s 1 1s s p 1s s p Hund s rule: For any set of orbitals of equal energy (degenerate orbitals), the electron configuration with
Example: 4 O: 1s s p p Z eff (0.85) 5(0.35) 4.55 Z 8 4.55 3.45 Be: B: C: 1s s 1 1s s p 1s s p Hund s rule: For any set of orbitals of equal energy (degenerate orbitals), the electron configuration with
EPSC501 Crystal Chemistry WEEK 5
 EPSC501 Crystal Chemistry WEEK 5 Oxidation states of transition elements (many more in aqueous solutions than in the common rock-forming minerals) Notice that almost every transition metal has a +2 oxidation
EPSC501 Crystal Chemistry WEEK 5 Oxidation states of transition elements (many more in aqueous solutions than in the common rock-forming minerals) Notice that almost every transition metal has a +2 oxidation
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Molecular Structure and Bonding- 2. Assis.Prof.Dr.Mohammed Hassan Lecture 3
 Molecular Structure and Bonding- 2 Assis.Prof.Dr.Mohammed Hassan Lecture 3 Hybridization of atomic orbitals Orbital hybridization was proposed to explain the geometry of polyatomic molecules. Covalent
Molecular Structure and Bonding- 2 Assis.Prof.Dr.Mohammed Hassan Lecture 3 Hybridization of atomic orbitals Orbital hybridization was proposed to explain the geometry of polyatomic molecules. Covalent
Chapter 7: Chemical Bonding and Molecular Structure
 Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
Chemistry Instrumental Analysis Lecture 11. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 11 Molar Absorptivities Range 0 to 10 5 Magnitude of e depends on capture cross section of the species and probability of the energy-absorbing transition. e
Chemistry 4631 Instrumental Analysis Lecture 11 Molar Absorptivities Range 0 to 10 5 Magnitude of e depends on capture cross section of the species and probability of the energy-absorbing transition. e
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 10. Geometry
 Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious).
 Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Lecture 4, January 12, 2015 Bonding in H2
 Lecture 4, January 12, 2015 Bonding in H2 Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 147 Noyes Hours: 11-11:50am
Lecture 4, January 12, 2015 Bonding in H2 Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 147 Noyes Hours: 11-11:50am
Chemistry 121: Topic 4 - Chemical Bonding Topic 4: Chemical Bonding
 Topic 4: Chemical Bonding 4.0 Ionic and covalent bonds; Properties of covalent and ionic compounds 4.1 Lewis structures, the octet rule. 4.2 Molecular geometry: the VSEPR approach. Molecular polarity.
Topic 4: Chemical Bonding 4.0 Ionic and covalent bonds; Properties of covalent and ionic compounds 4.1 Lewis structures, the octet rule. 4.2 Molecular geometry: the VSEPR approach. Molecular polarity.
Chem Spring, 2017 Assignment 5 - Solutions
 Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Lecture 3, January 9, 2015 Bonding in H2+
 Lecture 3, January 9, 2015 Bonding in H2+ Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 147 Noyes Hours: 11-11:50am
Lecture 3, January 9, 2015 Bonding in H2+ Elements of Quantum Chemistry with Applications to Chemical Bonding and Properties of Molecules and Solids Course number: Ch125a; Room 147 Noyes Hours: 11-11:50am
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Bonding/Lewis Dots Lecture Page 1 of 12 Date. Bonding. What is Coulomb's Law? Energy Profile: Covalent Bonds. Electronegativity and Linus Pauling
 Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Where have we been? Lectures 1 and 2 Bohr s Model/ Wave Mechanics/ Radial and Angular Wavefunctions/ Radial Distribution Functions/ s and p orbitals
 Where have we been? Lectures 1 and 2 Bohr s Model/ Wave Mechanics/ Radial and Angular Wavefunctions/ Radial Distribution unctions/ s and p orbitals Where are we going? Lecture 3 Brief wavefunction considerations:
Where have we been? Lectures 1 and 2 Bohr s Model/ Wave Mechanics/ Radial and Angular Wavefunctions/ Radial Distribution unctions/ s and p orbitals Where are we going? Lecture 3 Brief wavefunction considerations:
Coordination Chemistry: Bonding Theories. Molecular Orbital Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
K + 09/04/2018. Structure of Organic Molecules. Ionic bond. The compound potassium fluoride consists of potassium (K+) ions and fluoride (F-) ions
 Structure of rganic Molecules Ref. books: 1. A text book of rganic Chemistry - B.S. Bahl and Arun Bahl 2. rganic Chemistry - R.T. Morrison and R. N. Boyd Atom: The smallest part of an element that can
Structure of rganic Molecules Ref. books: 1. A text book of rganic Chemistry - B.S. Bahl and Arun Bahl 2. rganic Chemistry - R.T. Morrison and R. N. Boyd Atom: The smallest part of an element that can
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
TYPES OF SYMMETRIES OF MO s s-s combinations of orbitals: , if they are antibonding. s-p combinatinos of orbitals: CHEMICAL BONDING.
 TYPES OF SYMMETRIES OF MO s s-s combinations of : Orbitals Molecular Orbitals s s Node s s (g) (g) Bonding orbital Antibonding orbital (u) 4 (u) s-s combinations of atomic In the bonding MO there is increased
TYPES OF SYMMETRIES OF MO s s-s combinations of : Orbitals Molecular Orbitals s s Node s s (g) (g) Bonding orbital Antibonding orbital (u) 4 (u) s-s combinations of atomic In the bonding MO there is increased
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chemical bonding in complexes of transition metals
 Chemical bonding in complexes of transition metals Chem 202, Sept. 28, 2010 What are transition elements? Electronic structure of atoms Naming delocalized molecular orbitals: tetrahedral and octahedral
Chemical bonding in complexes of transition metals Chem 202, Sept. 28, 2010 What are transition elements? Electronic structure of atoms Naming delocalized molecular orbitals: tetrahedral and octahedral
CH1010 Exam #2 Study Guide For reference see Chemistry: An Atoms-focused Approach by Gilbert, Kirss, and Foster
 CH1010 Exam #2 Study Guide For reference see Chemistry: An Atoms-focused Approach by Gilbert, Kirss, and Foster Chapter 3: Atomic Structure, Explaining the Properties of Elements Trends to know (and be
CH1010 Exam #2 Study Guide For reference see Chemistry: An Atoms-focused Approach by Gilbert, Kirss, and Foster Chapter 3: Atomic Structure, Explaining the Properties of Elements Trends to know (and be
(b) electrovalent and covalent (c) electrovalent and co-ordinate (d) covalent and co-ordinate 10. Which pair is different from others (a) Li Mg (b)
 1. Following triads have approximately equal size (a) Na+, Mg 2+, Al 3+ (iso-electronic) (b) F, Ne, O 2 (iso-electronic) (c) Fe, Co, Ni (d) Mn+, Fe 2+, Cr (iso-electronic) 2. Which of the following halides
1. Following triads have approximately equal size (a) Na+, Mg 2+, Al 3+ (iso-electronic) (b) F, Ne, O 2 (iso-electronic) (c) Fe, Co, Ni (d) Mn+, Fe 2+, Cr (iso-electronic) 2. Which of the following halides
Constructing a MO of NH 3. Nitrogen AO symmetries are
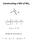 Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Electronic structure Crystal-field theory Ligand-field theory. Electronic-spectra electronic spectra of atoms
 Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Molecular Shapes and VSEPR (Valence Shell Electron Pair Repulsion Theory)
 AP Chemistry Ms. Ye Name Date Block Molecular Shapes and VSEPR (Valence Shell Electron Pair Repulsion Theory) Go to bit.ly/vseprshapes Introduction Atoms bond to satisfy their need for more electrons.
AP Chemistry Ms. Ye Name Date Block Molecular Shapes and VSEPR (Valence Shell Electron Pair Repulsion Theory) Go to bit.ly/vseprshapes Introduction Atoms bond to satisfy their need for more electrons.
Lecture 9 Electronic Spectroscopy
 Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
Bonding in transition metal complexes
 rystal Field Theory(FT) Bonding in transition metal complexes Assumes electrostatic(ionic) interactions between ligands and metal ions Useful for understanding magnetism and electronic spectra Valence
rystal Field Theory(FT) Bonding in transition metal complexes Assumes electrostatic(ionic) interactions between ligands and metal ions Useful for understanding magnetism and electronic spectra Valence
Review Outline Chemistry 1B, Fall 2012
 Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
5.61 Physical Chemistry Exam III 11/29/12. MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry Physical Chemistry.
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry - 5.61 Physical Chemistry Exam III (1) PRINT your name on the cover page. (2) It is suggested that you READ THE ENTIRE EXAM before
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry - 5.61 Physical Chemistry Exam III (1) PRINT your name on the cover page. (2) It is suggested that you READ THE ENTIRE EXAM before
8. Relax and do well.
 CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
Organometallic Chemistry
 Organometallic Chemistry Organometallic compounds combine an organic moiety with a metal in a molecule that has direct metal-carbon bonds. Ferrocene, first prepared in 1951, ushered in the modern era of
Organometallic Chemistry Organometallic compounds combine an organic moiety with a metal in a molecule that has direct metal-carbon bonds. Ferrocene, first prepared in 1951, ushered in the modern era of
