CEM 251, Fall 2011 Sections Tuesday Thursday 6:00 7:30 pm Midterm #2 October 27, 2011
|
|
|
- Shanna Jackson
- 5 years ago
- Views:
Transcription
1 CEM 251, Fall 2011 Sections Tuesday Thursday 6:00 7:30 pm ctober 27, 2011 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only what is written in the boxes will be graded. You have 80 minutes to complete the test. Section 25 Thu 9:10 10:00 AM Roozbeh Section 26 Thu 4:10 5:00 PM Roozbeh Section 27 Tue 4:10 5:00 PM Mercy Section 28 Mon 3:00 3:50 PM Atefeh Section 29 Tue 10:20 11:10 AM Mercy Section 30 Thu 1:50 2:40 PM Atefeh Section 31 Thu 4:10 5:00 PM Camille Section 32 Tue 3:00 3:50 PM Roozbeh Section 33 Tue 4:10 5:00 PM Camille Section 34 Thu 4:10 5:00 PM Atefeh Section 35 Thu 3:00 3:50 PM Camille Section 36 Tue 9:10 10:00 AM Mercy Question Points Score Bonus 6 Total 100 1
2 1. Provide a name or a structure for the following compounds (Indicate stereochemistry where appropriate)(10 pts, 2 pts each box) a. (3R, 4S) 1,1 dibromo-3,4 diethyl-pentane b. F (1S, 3S) 1 fluoro, 3 methyl cyclohexane Indicate Stereochemistry R, S c. (3R, 5R)-3-bromo-5-chloro-octane d. (2R, 5R) 2 chloro, 5 methyl heptane Indicate Stereochemistry R, S e. Vinyl Iodide I 2
3 2. Artemisinin is a widely used antimalarial drug. Indicate with an asterisk (*) all the chiral carbons on this molecule and clearly define if they are R or S (10 pts). R S* S * * R * * H S 3. The shrub ma huang contains two biologically active isomers, as shown below: Ephedrine and Pseudoephedrin. C1 HN C2 1R, 2S Ephedrine (performance enhancing drug) 1S, 2S Pseudoephedrine (nasal decongestant) a. Draw the structure of both the 1R,2S (Ephedrine) and the 1S, 2S (Pseudoephedrine) isomers. (8 pts) HN HN 1R, 2S 1S, 2S b. What is the relationship between the above isomers? (2 pts) Diastereomers 3
4 Reaction Progress CEM251, Fall (12 pts) For the following reaction: S N 1 two products a. Draw the products for this reaction and label the chiral carbon as (R) or (S). (4 pts) R S b. Draw the structure of the intermediate of the rate determining step (RDS, slow step) of this reaction (2 pts). c. Draw an energy diagram of the above reaction assuming an exothermic reaction. (You must clearly indicate the position of reactants, products, transition state(s), change in enthalpy, activation energies and intermediate) (6 pts). TS 1 TS 2 Ea 1 Ea 2 Energy Intermediate Starting Material 4!G o,!h o <0 Product
5 5. Circle the correct answer. (2 pts each, 12 pts) a. How many possible stereoisomers are there for the following compound? a. 2 b. 4 c. 6 d. 8 b. Which of the following bromides cannot undergo any Substitution reactions? c. What is a racemic mixture? a. 50:50 mixture of diastereomers b. 50:50 mixture of enantiomers c. ptically active solution d. Mixture of optically active compounds d. Which of the following radicals is the most stable? e. Which of the following alkenes is the most stable? f. Which of the following is a meso compound? 5
6 6. assify each transformation as: Substitution, Elimination or Addition (10 pts, 2 pts each) H Elimination H Substitution Addition Addition Substitution 6
7 7. How are the compounds in each pair related to each other? Are they Identical, Enantiomers, Diastereomers, Constitutional Isomers or Not Isomers of each other? (10 pts) Constitutional Isomers Enantiomers Enantiomers Diastereomers H Identical 7
8 8. Draw all possible elimination products for the following reaction and circle the most stable one (8 pts). - K + E2 9. For the following reactions determine the mechanism of nucleophilic substitution (S N 1 or S N2 ) and draw the products (it could be more than one per box). Be sure to depict proper stereochemistry if appropriate (18 pts, 3 pts each reaction) H 2 S N 2 8
9 NH 2 HN HN S N 1 F H 2 H S N 1 H H S N 2 CH 3 S N 1 S N 2 9
10 Bonus Points (6 pts, 1 pt each): Determine if the indicated atoms are Nucleophiles or Electrophiles. Nucleophile Electrophile Electrophile NH Electrophile Nucleophile Electrophile 10
11 Scratch Sheet: Needs to be turned in with the test (if missing, the test will not be graded) 11
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 FALL 2018 CEM 251: Organic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
FALL 2018 CEM 251: Organic Chemistry I Sections Oct 23 rd, 2018
 FALL 2018 CEM 251: Organic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM Oct 23 rd, 2018 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM Oct 23 rd, 2018 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
1. (3 pts) Circle the highest priority substituent of the following list:
 Ch 334 Midterm #3 November 17, 2006 Code 1. (3 pts) Circle the highest priority substituent of the following list: 2. (4 pts) Rank the following groups in order of increasing priority. Place the letter
Ch 334 Midterm #3 November 17, 2006 Code 1. (3 pts) Circle the highest priority substituent of the following list: 2. (4 pts) Rank the following groups in order of increasing priority. Place the letter
Organic Chemistry 1 CHM 2210 Exam 3 (November 9, 2001)
 Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide
 CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
STEREOCHEMISTRY A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
CH 253, Fall Question Points Possible Points Received
 CH 253, Fall 2000 Exam #3 - M. Schwartz November 17, 2000 Name (Print Clearly) Question Points Possible Points Received 1 20 2 15 3 8 4 5 5 4 6 6 7 14 8 8 9 5 10 21 Subtotal 106 Extra Credit 2 Total 108
CH 253, Fall 2000 Exam #3 - M. Schwartz November 17, 2000 Name (Print Clearly) Question Points Possible Points Received 1 20 2 15 3 8 4 5 5 4 6 6 7 14 8 8 9 5 10 21 Subtotal 106 Extra Credit 2 Total 108
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
CHEM Exam 2 October 25, 2007
 CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
CEM 351 2nd EXAM/Version A Friday, October 17, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
(S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer
 C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
CHEM Exam 2 ANSWER KEY
 CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
Due Date: 2) What is the relationship between the following compounds?
 Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
= ( 3 ) + 2 ( 3 ) = ( ) = = ( ) = 20 H H
 Columbia University 92CORG12.DOC CEM S3443D Summer 1992 Professor Grace B. Borowitz Exam No. 2 June 17, 1992 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Columbia University 92CORG12.DOC CEM S3443D Summer 1992 Professor Grace B. Borowitz Exam No. 2 June 17, 1992 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
CHE 321 Summer 2012 Exam 2 Form Select the correct number of chirality centers in heroin, the illicit drug shown below.
 Multiple Choice Questions: 50 points 1. Select the correct number of chirality centers in heroin, the illicit drug shown below. A 4 B 5 C 6 D 7 E 8 F more than 8 2. Choose the correct IUPAC name for the
Multiple Choice Questions: 50 points 1. Select the correct number of chirality centers in heroin, the illicit drug shown below. A 4 B 5 C 6 D 7 E 8 F more than 8 2. Choose the correct IUPAC name for the
Chemistry 201. MW 12:00pm 1:15pm Examination #2 August 15 th Bronco ID. Question Score Possible Points. 1 (12pts) 2 (24pts) 3 (25pts)
 Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chem ORGANIC CHEMISTRY I
 ORGANIC CHEMISTRY I CHEM 221 /4 02 Final Examination April 20, 2005 0900-1200 Dr. Cerrie ROGERS x x periodic table & pk a data table provided non-programmable calculators allowed molecular model kits allowed
ORGANIC CHEMISTRY I CHEM 221 /4 02 Final Examination April 20, 2005 0900-1200 Dr. Cerrie ROGERS x x periodic table & pk a data table provided non-programmable calculators allowed molecular model kits allowed
Chem ORGANIC CHEMISTRY I
 ORGANIC CHEMISTRY I CHEM 221 /2 01 Final Examination December 20, 2005 1400-1700 Dr. Cerrie ROGERS x x molecular model kits (without instructions) programmable calculators must be reset Chem 221 --- ORGANIC
ORGANIC CHEMISTRY I CHEM 221 /2 01 Final Examination December 20, 2005 1400-1700 Dr. Cerrie ROGERS x x molecular model kits (without instructions) programmable calculators must be reset Chem 221 --- ORGANIC
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
More Tutorial at
 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
Final Exam. Your lab section and TA name: (if you are not in a lab section write no lab ) Instructions:
 CHEM 232 Final Exam May 10, 2014 RedID number: Signature: Your lab section and TA name: (if you are not in a lab section write no lab ) Instructions: 1. In fairness to all, do not open this test until
CHEM 232 Final Exam May 10, 2014 RedID number: Signature: Your lab section and TA name: (if you are not in a lab section write no lab ) Instructions: 1. In fairness to all, do not open this test until
Chem 3371 Fall 2013 Exam II
 Page (1 of 12) Grade Sheet: Page 3: Page 4: Page 5: Page 6: Page 7: Page 8: Page 9: Page 10: Page 11: Page 12: Total: Page (2 of 12) 1. (2 pts) Classify the reaction below. (circle the best answer) A.
Page (1 of 12) Grade Sheet: Page 3: Page 4: Page 5: Page 6: Page 7: Page 8: Page 9: Page 10: Page 11: Page 12: Total: Page (2 of 12) 1. (2 pts) Classify the reaction below. (circle the best answer) A.
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
HO HO. 1) BH 3 HCl. + enantiomer either one may be drawn 4. + enantiomer either one may be drawn 4 1) O 3
 . (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
. (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Partial Periodic Table
 CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
CHEM 3311 Exam 2 ANSWER KEY
 CEM 3311 Exam 2 ANSWER KEY March 11, 2014 Time: 2 ours Please sign the onor Pledge h in this sheet with your scantron. I pledge that n my honor, as a University of Colorado-Boulder student, I have neither
CEM 3311 Exam 2 ANSWER KEY March 11, 2014 Time: 2 ours Please sign the onor Pledge h in this sheet with your scantron. I pledge that n my honor, as a University of Colorado-Boulder student, I have neither
Exam 3 November 17, 2005 Professor Rebecca Hoenigman
 EM 3311-200, Fall 2005 Exam 3 November 17, 2005 Professor Rebecca oenigman Average Score = 54.8 igh Score = 98 Low Score = 8 I pledge to uphold the U onor ode: Signature Name (printed) Last four digits
EM 3311-200, Fall 2005 Exam 3 November 17, 2005 Professor Rebecca oenigman Average Score = 54.8 igh Score = 98 Low Score = 8 I pledge to uphold the U onor ode: Signature Name (printed) Last four digits
Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm
 - 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
- 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
EXAM 2 CHEMISTRY 220a Friday, October 14, Section Day: Section Time:
 EXAM 2 CHEMISTRY 220a Friday, October 14, 2005 NAME (print): Your ID#: TA: Section Day: Section Time: No Calculators! Take a few moments to look over the exam. Answer each question on the exam paper. Important
EXAM 2 CHEMISTRY 220a Friday, October 14, 2005 NAME (print): Your ID#: TA: Section Day: Section Time: No Calculators! Take a few moments to look over the exam. Answer each question on the exam paper. Important
350 Organic Chemistry I Winona State University
 350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
KEY Page 1. Name H H H 3 C C 3. Cl CH. AlCl 3 H H. + enantiomer H 3 C CH 3. Cl CH3 HNO 3. AlCl NO 2 CH 3 1) O 3 2) H 2 O 2 OH H 3 C C.
 ame Page 1 I. (8 points) omplete the following reactions as directed. Transformations requiring sequential experimental steps should be numbered appropriately. Show the major organic product(s) unless
ame Page 1 I. (8 points) omplete the following reactions as directed. Transformations requiring sequential experimental steps should be numbered appropriately. Show the major organic product(s) unless
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
Columbia University C99ORG12.DOC S3443D Summer 99 Professor Grace B. Borowitz Exam No. 2 June 14, 1999
 olumbia University 99RG12.D SD Summer 99 Professor Grace B. Borowitz Exam No. 2 June 1, 1999 Name: Sterie hemistry Grade: Please use a non-red pen. Answer questions in the provided space. If you write
olumbia University 99RG12.D SD Summer 99 Professor Grace B. Borowitz Exam No. 2 June 1, 1999 Name: Sterie hemistry Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 8, 2004 ANSWERS
 Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Chemistry 233 Exam 3. The Periodic Table
 Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Partial Periodic Table
 Easily Legible Printed Name: CEM 3351 (100), Fall 2015 Professor Walba econd our Exam ctober 20, 2015 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither given
Easily Legible Printed Name: CEM 3351 (100), Fall 2015 Professor Walba econd our Exam ctober 20, 2015 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither given
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
CHEM 2423 Unit 8 Homework Answers
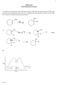 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Chemistry 51 Summer 2006 Final Exam
 Chemistry 51 Summer 2006 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
Chemistry 51 Summer 2006 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
E30 ENANTIOMERS Chirality in organic chemistry
 E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
C sp2 trigonal planar trigonal planar 3
 I. ( points) ame Page A. Rocuronium bromide (Zemuron) is a muscle relaxant that is used in the application of anesthesia. It is used most often to facilitate endotracheal intubation because of its powerful
I. ( points) ame Page A. Rocuronium bromide (Zemuron) is a muscle relaxant that is used in the application of anesthesia. It is used most often to facilitate endotracheal intubation because of its powerful
Enantiomers. nonsuperimposable mirror image Both Configuration will be opposite. Both Configuration will be opposite
 Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Final Exam December 13, 2005 Professor Rebecca Hoenigman
 CEM 3311-200, all 2005 inal Exam December 13, 2005 Professor Rebecca oenigman Average core = 158 igh core = 276 Low core = 20 I pledge to uphold the CU onor Code: ignature Name (printed) Last four digits
CEM 3311-200, all 2005 inal Exam December 13, 2005 Professor Rebecca oenigman Average core = 158 igh core = 276 Low core = 20 I pledge to uphold the CU onor Code: ignature Name (printed) Last four digits
FOURTH EXAMINATION. (1)..20 pts... (2)..24 pts... (3)..18 pts... (4)..12 pts... (5)..12 pts... (6).. 6 pts... (7).. 8 pts... Bonus 4 pts...
 Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
STEREOCHEMISTRY. 2. Define the following, and tell whether or not a given compound or structure fits the description or possesses the feature.
 A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
CHEM 3311 (Richardson) Third Exam Nov. 27, 2018
 CHEM 3311 (Richardson) Third Exam Nov. 27, 2018 Your Name: Question Score Out of 1 15 Student ID: 2 20 3 20 Recitation (check one) O 10:00 Mon (Shafer Soars) 4 10 O 11:00 Mon (Matthew Farmer) O 1:00 Mon
CHEM 3311 (Richardson) Third Exam Nov. 27, 2018 Your Name: Question Score Out of 1 15 Student ID: 2 20 3 20 Recitation (check one) O 10:00 Mon (Shafer Soars) 4 10 O 11:00 Mon (Matthew Farmer) O 1:00 Mon
Chemistry 143 Exam #2 November 15, Name: Student Number: Section Number: TA: INSTRUCTIONS:
 Chemistry 143 Exam #2 November 15, 2017 Name: Student Number: Section Number: TA: INSTRUCTINS: This exam consists of 32 questions on 9 pages. Please make certain that your exam is complete. Write your
Chemistry 143 Exam #2 November 15, 2017 Name: Student Number: Section Number: TA: INSTRUCTINS: This exam consists of 32 questions on 9 pages. Please make certain that your exam is complete. Write your
Problem Set 7: Stereochemistry-ANSWER KEY
 Problem Set 7: Stereochemistry-ANSWER KEY Chemistry 260 Organic Chemistry 1. The answer is (2). Circled isomers have a stereogenic carbon (*) and hence a stereogenic centre. C 2 C 2 C 2 C 2 C 2 C 2 C 2
Problem Set 7: Stereochemistry-ANSWER KEY Chemistry 260 Organic Chemistry 1. The answer is (2). Circled isomers have a stereogenic carbon (*) and hence a stereogenic centre. C 2 C 2 C 2 C 2 C 2 C 2 C 2
Assign (R) or (S) configurations to the chiral carbons in the following molecules: enantiomers
 CAPTER 5: STERECEMISTRY (cont.) Assign (R) or (S) configurations to the chiral carbons in the following molecules: 3 C 3 C N 2 Molecules With Multiple Chiral Atoms. 1-chloro-2-methylcyclohexane has four
CAPTER 5: STERECEMISTRY (cont.) Assign (R) or (S) configurations to the chiral carbons in the following molecules: 3 C 3 C N 2 Molecules With Multiple Chiral Atoms. 1-chloro-2-methylcyclohexane has four
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
Chemistry 3A. Midterm 2. Dr. Steven Pedersen November 9, Student name: ANSWERS Student signature:
 r. Steven Pedersen ovember 9, 2015 Chemistry 3A Midterm 2 Student name: ASWERS Student signature: Problem 1 Problem 2 Problem 3 Problem 4 Problem 5 Problem 6 Problem 7 (18 pts) (30 pts) (32 pts) (18 pts)
r. Steven Pedersen ovember 9, 2015 Chemistry 3A Midterm 2 Student name: ASWERS Student signature: Problem 1 Problem 2 Problem 3 Problem 4 Problem 5 Problem 6 Problem 7 (18 pts) (30 pts) (32 pts) (18 pts)
Exam 2 Chem 109a Fall 2004
 Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
1. The barrier to rotation around the C-C bonds for 2-methylpropane and 2,2-dimethylpropane are shown below.
 1. The barrier to rotation around the C-C bonds for 2-methylpropane and 2,2-dimethylpropane are shown below. E Rot = 14.2 kj/mol E Rot = 19.6 kj/mol a. Why does the potential energy of a molecule increase
1. The barrier to rotation around the C-C bonds for 2-methylpropane and 2,2-dimethylpropane are shown below. E Rot = 14.2 kj/mol E Rot = 19.6 kj/mol a. Why does the potential energy of a molecule increase
FINAL EXAMINATION Chemistry 3A
 1 FINAL EXAMINATION Chemistry 3A Key Name: Print first name before second! Use capital letters! SID #: Peter Vollhardt May 11, 2016 GSI (if you are taking Chem 3AL): Please provide the following information
1 FINAL EXAMINATION Chemistry 3A Key Name: Print first name before second! Use capital letters! SID #: Peter Vollhardt May 11, 2016 GSI (if you are taking Chem 3AL): Please provide the following information
Answer the following questions 1. Define the following : [ ( 6x2) + ( 2x4)= 20 mark]
![Answer the following questions 1. Define the following : [ ( 6x2) + ( 2x4)= 20 mark] Answer the following questions 1. Define the following : [ ( 6x2) + ( 2x4)= 20 mark]](/thumbs/89/97487476.jpg) Benha University Time : 2 hrs. Faculty of Science 1 st Term (2014/2015) Chemistry Department Date : 1 /1/2015 (Jun.2014) Organic photo and Stereochemistry Final Exam. ( 415 Ch.) ; for 4 th level Answer
Benha University Time : 2 hrs. Faculty of Science 1 st Term (2014/2015) Chemistry Department Date : 1 /1/2015 (Jun.2014) Organic photo and Stereochemistry Final Exam. ( 415 Ch.) ; for 4 th level Answer
Problem Set 8: Substitution Reactions-ANSWER KEY. (b) nucleophile NH 3 H C. (d) H 3 N
 Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Time: 3 hours (180 minutes) Marking Scheme For The Exam
 hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
9. Stereochemistry: Introduction to Using Molecular Models
 9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
CH Organic Chemistry I (Katz) Practice Exam #2- Fall 2015 (With Answers)
 2710 - rganic hemistry I (Katz) Practice Exam #2- all 2015 (With nswers) Name: Score: Part I - hoose the best answer and write the letter of your choice in the space provided. 1. The Newman projection
2710 - rganic hemistry I (Katz) Practice Exam #2- all 2015 (With nswers) Name: Score: Part I - hoose the best answer and write the letter of your choice in the space provided. 1. The Newman projection
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
NAME: STUDENT NUMBER: Page 1 of 4
 NAME: STUDENT NUMBER: Page 1 of 4 Chemistry 2.339 Midterm Test Friday ctober 28, 2005 This test is worth 40 Marks. You must complete all work within the class period. Put all answers in the space provided.
NAME: STUDENT NUMBER: Page 1 of 4 Chemistry 2.339 Midterm Test Friday ctober 28, 2005 This test is worth 40 Marks. You must complete all work within the class period. Put all answers in the space provided.
If Compound 2 (below) was used in the above reaction, how might things change? Name Page 1. I. (23 points)
 I. (2 points) ame Page itrogen atoms in amines can undergo substitution reactions repeatedly under mildly basic conditions. Provide the structure of the starting amine and supply the missing reagent(s).
I. (2 points) ame Page itrogen atoms in amines can undergo substitution reactions repeatedly under mildly basic conditions. Provide the structure of the starting amine and supply the missing reagent(s).
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 FALL 2018 CEM 251: rganic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 ame: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
FALL 2018 CEM 251: rganic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 ame: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
Homework Problem Set 7 Iverson CH320M/328M Due Friday, Nov. 2
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. Brent Iverson 7th omework October 29, 2018 Please print the first three letters of your last name in the three boxes 1 Score: 2 1. For the following reactions,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. Brent Iverson 7th omework October 29, 2018 Please print the first three letters of your last name in the three boxes 1 Score: 2 1. For the following reactions,
CHEM 232 Exam Two April 5, 2010
 CEM 232 Exam Two April 5, 2010 Name (-1 pt) TA Name (-1 pt) UIN (-1 pt) Question 1 [Qualitative anking, 40 points] A significant amount of time has been devoted to describing the physical and chemical
CEM 232 Exam Two April 5, 2010 Name (-1 pt) TA Name (-1 pt) UIN (-1 pt) Question 1 [Qualitative anking, 40 points] A significant amount of time has been devoted to describing the physical and chemical
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Experiment 8 Optical Isomers. In this experiment you will be given the opportunity to see the 3-dimensional aspects of
 Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept
Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept
Homework - Review of Chem 2310
 omework - Review of Chem 2310 ame 1. Fill in the following partial Periodic Table of Table of Elements. Do as much as you can from memory. 2. Using the Table above, circle the more electronegative atom
omework - Review of Chem 2310 ame 1. Fill in the following partial Periodic Table of Table of Elements. Do as much as you can from memory. 2. Using the Table above, circle the more electronegative atom
Practice Exam #1. Massachusetts Institute of Technology. Chemistry 5.43 February 21, Professor M. Movassaghi. /08 points.
 Massachusetts Institute of Technology Chemistry 5.43 February 21, 2007 Professor M. Movassaghi Practice Exam #1 Question 1a Question 1l Question 1b /02 points Question 1m Question 1c /03 points Question
Massachusetts Institute of Technology Chemistry 5.43 February 21, 2007 Professor M. Movassaghi Practice Exam #1 Question 1a Question 1l Question 1b /02 points Question 1m Question 1c /03 points Question
STEREOCHEMISTRY A STUDENT WHO HAS MASTERED THE MATERIAL IN THIS SECTION SHOULD BE ABLE TO:
 STEREOEMISTRY A STUDENT WO AS MASTERED TE MATERIAL IN TIS SETION SOULD BE ABLE TO: 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of
STEREOEMISTRY A STUDENT WO AS MASTERED TE MATERIAL IN TIS SETION SOULD BE ABLE TO: 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of
tbuo OtBu Br 1) B 2 H 6 /THF OH 2) OH - + H 2 O 2 NaNH 2 NH3 Na NH 3 /-35 C CH 3 CCH 2 CHCH 3
 EM 12A Name KEY EXAM II all 2003 1.(16 pts) Draw the structure of the major product expected from each of the following sets of reactants. Specify stereochemistry where appropriate. + 2 Pt + 2 2 2 + enantiomer
EM 12A Name KEY EXAM II all 2003 1.(16 pts) Draw the structure of the major product expected from each of the following sets of reactants. Specify stereochemistry where appropriate. + 2 Pt + 2 2 2 + enantiomer
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
NAME (Print): Chemistry 610A/618A Dr. Brent Iverson 2nd Exam Oct. 29, 2003 SIGNATURE:
 NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
O N N. electrons in ring
 ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
This is a long exam. Most students will not finish each problem. Carefully select your problems to work on.
 Chemistry 51 Exam 1, Spring 2008 =============================================== This is a closed book exam. The exam lasts 75 minutes. All answers must appear on the answer sheet. Only the answer sheet
Chemistry 51 Exam 1, Spring 2008 =============================================== This is a closed book exam. The exam lasts 75 minutes. All answers must appear on the answer sheet. Only the answer sheet
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Organic Chemistry 3540
 rganic Chemistry 3540 ctober 27, 2004 (6 Pages, 13 Parts) Name 1. (10%) Draw structures of the following showing stereochemistry if appropriate: a. s -butyl alcohol b. isobutyl isopropyl ether c. p-nitrophenoxide
rganic Chemistry 3540 ctober 27, 2004 (6 Pages, 13 Parts) Name 1. (10%) Draw structures of the following showing stereochemistry if appropriate: a. s -butyl alcohol b. isobutyl isopropyl ether c. p-nitrophenoxide
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
Chapter 6. Isomers and Stereochemistry
 hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
CHE 321 Summer 2010 Exam 2 Form Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III
 CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
1. Draw line-angle pictures of each of the following (include dashes/wedges where necessary to show stereochemistry).
 1. Draw line-angle pictures of each of the following (include dashes/wedges where necessary to show stereochemistry). (C 3 ) 2 CCC 2 C C C N C C 3-bromo-2-methyl-2-hexene (2R,3S)-2,3-dichloropentane cis-1,3-dimethylcyclohexane
1. Draw line-angle pictures of each of the following (include dashes/wedges where necessary to show stereochemistry). (C 3 ) 2 CCC 2 C C C N C C 3-bromo-2-methyl-2-hexene (2R,3S)-2,3-dichloropentane cis-1,3-dimethylcyclohexane
4 1,2,3 - Clockwise 1,2,3 - Counterclockwise S
 Assigning Stereochemistry using Fischer Projections: Fischer projections can be used to assign stereochemistry. If the th (lowest) priority group is vertical the other three groups will show clockwise
Assigning Stereochemistry using Fischer Projections: Fischer projections can be used to assign stereochemistry. If the th (lowest) priority group is vertical the other three groups will show clockwise
CH Organic Chemistry I (Katz) Exam #2- Fall 2012
 2710 - rganic hemistry I (Katz) Exam #2- Fall 2012 Name: Score: Part I - hoose the best answer write the letter of your choice in the space provided. (30 pts) 1. The Newman projection at the right represents:
2710 - rganic hemistry I (Katz) Exam #2- Fall 2012 Name: Score: Part I - hoose the best answer write the letter of your choice in the space provided. (30 pts) 1. The Newman projection at the right represents:
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
