Late-Stage Deoxyfluorination of Alcohols with PhenoFluor
|
|
|
- Virgil Horn
- 5 years ago
- Views:
Transcription
1 Late-Stage Deoxyfluorination of Alcohols with Phenoluor Sladojevich,.; Arlow, S. I.; Tang, P.; itter, T. J. Am. Chem. Soc. 2012, ASAP DI: /ja Phenoluor 71% predictable and selective deoxyfluorination Kara George osenker Current Literature 23 ebruary 2013
2 luorine in dicinal Chemistry The carbon fluorine bond plays an integral role in agrochemicals, pharmaceuticals, materials, and imaging Approximately 20% of all pharmaceuticals contain fluorine Strategies for the introduction of fluorine atoms in medicinal chemistry: tabolic stability by blocking metabolically labile sites Modulate the physicochemical properties such as lipophilicity or basicity Enhance binding affinity to a target protein on-natural 18 is the most commonly used positron-emitting isotope for molecular positron emission tomography (PET) imaging in oncology Lipitor (Pfizer) $7.7 billion (2011) Lexapro (orest Laboratories) $2.9 billion (2011) (a) Vitaku, E.; Ilardi, E. A.; jarðarson, J. T. Top 200 Pharmaceutical Products by US etail Sales in (b) Böhm,.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; bst-sander, U.; Stahl, M. ChemBioChem 2004, 5, 637. (c) Müller, K.; aeh, C.; Diederich,. Science 2007, 317, (d) Purser, S.; Moore, P..; Swallow, S.; Gouverneur, V. Chem. Soc. ev. 2008, 37, 320. (e) Jens Langner, J.; retrieved from S Advair Diskus (GSK) $4.6 billion
3 Carbon-luorine Bond ormation Despite fluorine s importance, carbon-fluorine bond formation still represents a formidable synthetic challenge nly 21 biosynthesized natural molecules containing fluorine are known and no fluoroperoxidase is known Conventional fluorination reactions are generally limited to very simple molecules, with reliable fluorination of more complex molecules at specific positions being difficult ew methods to incorporate fluorine into complex organic molecules are crucial to the progress of the field 2 C 2 S 2 C C 2 fluoroacetate fluorocitrate nucleocidin 2 ω-fluoro-oleic acid C 2 Böhm,.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; bst-sander, U.; Stahl, M. ChemBioChem 2004, 5, 637. Müller, K.; aeh, C.; Diederich,. Science 2007, 317, Purser, S.; Moore, P..; Swallow, S.; Gouverneur, V. Chem. Soc. ev. 2008, 37, 320. uruya, T.; Kamlet, A. S.; itter, T. ature 2011, 473, 470. agan, D.; arper, D. B. Asymmetric luoroorganic Chemistry; ACS Symposium Series; American Chemical Society: Washington, DC, 1999.
4 Carbon-luorine Bond ormation Despite fluorine s importance, carbon-fluorine bond formation still represents a formidable synthetic challenge nly 21 biosynthesized natural molecules containing fluorine are known and no fluoroperoxidase is known Conventional fluorination reactions are generally limited to very simple molecules, with reliable fluorination of more complex molecules at specific positions being difficult ew methods to incorporate fluorine into complex organic molecules are crucial to the progress of the field Böhm,.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; bst-sander, U.; Stahl, M. ChemBioChem 2004, 5, 637. Müller, K.; aeh, C.; Diederich,. Science 2007, 317, Purser, S.; Moore, P..; Swallow, S.; Gouverneur, V. Chem. Soc. ev. 2008, 37, 320. uruya, T.; Kamlet, A. S.; itter, T. ature 2011, 473, 470. agan, D.; arper, D. B. Asymmetric luoroorganic Chemistry; ACS Symposium Series; American Chemical Society: Washington, DC, 1999.
5 Deoxyfluorination eagents: DAST and Deoxo-luor eported by Middleton in 1975 as the first bench-stable deoxyfluorinating reagent and a useful alternative to S 4 S 3 S Et 2 S Et 2 S Et 2 DAST DAST suffers from poor thermal stability and potentially hazardous scale-up Et 2 S Et 2 S 90 C Deoxo-luor was introduced in 1999 and is a significant competitor to DAST for deoxyfluorination reactions due to its improved thermal stability S 4 Et 2 S Et 2 Explosive S 3 Deoxo-luor 3 S coordination prevents decomposition Middleton, W.J. J. rg. Chem. 1975, 40, 574. Lal, G. S.; Pez, G. P.; Pesaresi,. J.; Prozonic,. M.; Cheng,. J. rg. Chem. 1999, 64, Lal, G. S.; Pez, G. P.; Pesaresi,. J.; Prozonic,. M. Chem. Commun. 1999, 215.
6 Deoxyfluorination eagents: Xtalluor-E and Xtalluor-M S 3 DAST S 3 Deoxo-luor S 2 B4 Xtalluor-E Xtalluor-E S Et 2 S 2 B4 Xtalluor-M [DBU-] + S DBU Et 2 Et 3 3 S Et 2 In 2009, Courturier and co-workers reported the preparation and use of Xtalluor-E and Xtalluor-M Xtalluor-E and Xtalluor-M are crystalline reagents that are relatively safe and costefficient to prepare The reactions require the addition of either an amine reagent or DBU for efficient transformation The Xtalluor reagents are typically more selective and reduce the levels of elimination side products often observed with DAST and Deoxo-luor L eureux, A.; Beaulieu,.; BenneJ, C.; Bill, D..; Clayton, S.; Lalamme,.; Mirmehrabi, M.; Tadayon, S.; Tovell, D.; Couturier, M. J. rg. Chem. 2010, 75, Beaulieu,.; Beauregard, L.- P.; Courchesne, G.; Couturier, M; Lalamme,.; L eureux, A. rg. Le , 11, 5050.
7 Deoxyfluorination eagents: luolead and TEDMA, Yarovenko s and Ishikawa s reagents In 2010, Umemoto and co-workers introduced the second S generation PhS 3, which is marketed as luolead 3 More chemically stable than PhS 3, and more more thermally stable than DAST because of the stronger C-S bond in luolead Ishikawa s, Yarovenko s, and TDMA reagents fluorinate a wide range t Bu luolead of primary and secondary alcohols to provide alkyl fluorides There reagents are generally prepared by the addition of Et 2 to the corresponding halogenated alkene This group of reagents can suffer from formation of ester and amide side products Cl Yarovenko's reagent Ishikawa's reagent Ishikawa's reagent Et 2 C 3 Et 2 C 3 TEDMA Ishikawa's reagent Et 2 C 3 C 3 Et 2 (a) Umemoto, T.; Singh,. P.; Xu, Y.; Saito,. J. Am. Chem. Soc. 2010, 132, (b) Takaoka, A.; Iwakiri,.; Ishikawa,. Bull. Chem. Soc. Jpn. 1979, 52, (c) Petrov, V. A.; Swearingen, S.; ong, W.; Petersen, W. C. J. luorine Chem. 2001, 109, 25. (d) Yarovenko,..; aksha, M. S. Zh. bshch. Khim. 1959, 29, 2159.
8 Deoxyfluorination eagent: Phenoluor Phenoluor Phenoluor was first reported by itter and co-workers in 2011 for deoxyfluorination of phenols Phenoluor is commercially available from Sigma-Aldrich Phenoluor is a crystalline, nonexplosive solid that can be handled in air, but hydrolyzes upon prolonged storage in a wet atmosphere Phenoluor can be stored in a dry toluene solution for at least 2 months without detectable decomposition Phenoluor 3 equiv Cs toluene, 110 C 82% Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2011, 133, Tang, P.; Wang, W.; ijer, T. W 2012/142162
9 Phenoluor: Proposed chanism itter and co-workers propose that the mechanism for fluorination proceeds via a 2- phenoxyimidazolium bifluoride salt 3 equiv Cs toluene, 23 C toluene-d 8, 110 C 67% Ar Ar B 2 Ar Ar Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2011, 133,
10 Phenoluor: Proposed chanism itter and co-workers propose that the mechanism for fluorination proceeds via a 2- phenoxyimidazolium bifluoride salt toluene, 23 C Ar Ar B 2 Ar Ar Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2011, 133,
11 Phenoluor: ydrogen Bonding 3 equiv Cs toluene, 23 C toluene-d 8, 110 C 67% Ar Cl Cl 2 Ar 3 equiv Cs toluene-d 8, 110 C Ar' Ar Ar' 2 Ar 3 equiv Cs toluene-d 8, 110 C Ar P 6 Ar 3 equiv Cs toluene-d 8, 110 C Ar' 2% Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2011, 133,
12 Title Paper: Deoxyfluorination of Aliphatic Alcohols Modifications of the initial reaction conditions allowed for the deoxyfluorination of aliphatic alcohols Phenoluor 74% in dioxane 80% in toluene moc C 2 moc C 2 deoxyfluroinating reagent moc C 2 moc C 2 S 3 S 2 B4 S 3 t Bu DI DAST Xtalluor-M Deoxo-luor luolead S 3 toluene dioxane 2% 11% 10% reported optimized conditions 3% Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
13 Title Paper: Late-Stage Deoxyfluorination of Alcohols Boc C 2 (2S, 4)-4-hydroxy-proline 92% toluene, 80 C 2 1 Phenoluor 2.0 equiv Et equiv K 2-20 h D-allofuranose 83% toluene, 80 C 1 2 parent compound yield% solvent, temperature reserpine 82% 2 C toluene, 80 C artemisinin 79% toluene, 80 C testosterone 88% toluene, 80 C epi-androterone 84% toluene, 80 C 77% toluene, 80 C Chiral secondary alcohols could typically be deoxyfluorinated with inversion Carbonyl functional groups are well tolerated Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
14 Title Paper: Late-Stage Deoxyfluorination of Alcohols 2 1 Phenoluor 2.0 equiv Et equiv K 2-20 h 1 2 parent compound yield% solvent, temperature galantamine 80%, 10:1 dr toluene, 80 C Bz morphine 80% C 2 Cl 2, 23 C methyl α-d-glucopyranoside 43% dioxane, 80 C ajmaline 30% C 2 Cl 2, 50 C ivermectin B 1a 41% toluene, 50 C Secondary allylic alcohols afforded allylic fluorides consistent with an S 2 mechanism Deoxyfluorination is site-selective and predictable Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
15 Title Paper: Site-Selective Late-Stage Deoxyfluorination of Alcohols 2 1 Phenoluor 2.0 equiv Et equiv K 2-20 h 1 2 parent compound yield% solvent, temperature everolimus 83% C 2 Cl 2, 23 C oligomycin A 71% C 2 Cl 2, 23 C to 0 C Deoxyfluorination can be carried out at room temperature, allowing for fluorination of temperature sensitive substrates K was not required Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
16 Title Paper: Site-Selective Late-Stage Deoxyfluorination of Alcohols tertiary alcohol non-allylic β,β'-disubsitution hydrogen bonding β,β'-disubsitution Phenoluor 71% oligomycin A selective fluorination site Primary alcohols are selectively deoxyfluorinated Secondary alcohols react slower or not at all when they are β,β -dibranched, unless it is allylic ydroxyl groups engaged in hydrogen bonding are not reactive Tertiary alcohols do not react, unless they are allylic Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
17 Deoxyfluorination with Phenoluor: chanistic Considerations B 2 The formation of elimination products could be reduced by increasing the reaction temperature from 23 C to 80 C The addition of DIPEA was beneficial to shorten the reaction time K was found to reduce side products resulting from elimination, but was not generally required for the reaction to proceed Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
18 Conclusions and utlook A general method for selective, late-stage deoxyfluorination of complex aliphatic alcohols has been developed 2 1 Phenoluor 2.0 equiv Et equiv K 2-20 h 1 2 The substrate scope and functional group tolerance of this methodology surpass all others reported to date Phenoluor has a better safety profile and higher chemoselectivity than other deoxyfluorination reagents ne drawback is the molar mass (427 g/mol), which is convenient for subgram- and gram-scale reactions, but is wasteful for larger-scale reactions Extending this method to late-stage 18 radiolabeling would be useful for positron emission tomography (PET) applications Sladojevich,.; Arlow, S. I.; Tang, P.; Wang, W.; ijer, T. J. Am. Chem. Soc. 2012, ASAP. DI: /ja
Nucleophilic Fluorination. Souvik Rakshit Burke group Literature Seminar July 13, 2013
 Nucleophilic Fluorination Souvik Rakshit Burke group Literature Seminar July 13, 2013 Relevance 20% of pharmaceuticals contain fluorine 5-fluorouracil Antineoplastic agent, 1957 Lipitor (Atorvastatin)
Nucleophilic Fluorination Souvik Rakshit Burke group Literature Seminar July 13, 2013 Relevance 20% of pharmaceuticals contain fluorine 5-fluorouracil Antineoplastic agent, 1957 Lipitor (Atorvastatin)
Recent Developments in Nucleophilic Fluorination
 ecent Developments in ucleophilic luorination + K + S K + 13 ipr ipr ipr ipr S Vlad Bacauanu MacMillan esearch Group Group eting March 13th, 2018 Importance of ovel luorination Technologies Improved membrane
ecent Developments in ucleophilic luorination + K + S K + 13 ipr ipr ipr ipr S Vlad Bacauanu MacMillan esearch Group Group eting March 13th, 2018 Importance of ovel luorination Technologies Improved membrane
Current Trends in Practical Fluorination Chemistry
 WITEPAPE Current Trends in Practical luorination Chemistry Michael G. Campbell and Professor Tobias itter Department of Chemistry and Chemical Biology arvard University Introduction This white paper briefly
WITEPAPE Current Trends in Practical luorination Chemistry Michael G. Campbell and Professor Tobias itter Department of Chemistry and Chemical Biology arvard University Introduction This white paper briefly
Recent Advancement in Ag Mediated C-F Bond Formation. Chem 535 Literature Seminar Jiabao Zhang 02/21/2017
 ecent Advancement in Ag diated C- Bond ormation Chem 535 Literature Seminar Jiabao Zhang 02/21/2017 1 Why Would You Want luorination? igh redox potential: block possible metabolic oxidation. 2 Why Would
ecent Advancement in Ag diated C- Bond ormation Chem 535 Literature Seminar Jiabao Zhang 02/21/2017 1 Why Would You Want luorination? igh redox potential: block possible metabolic oxidation. 2 Why Would
Current Trends in Practical Fluorination Chemistry
 WHITEPAPE Current Trends in Practical luorination Chemistry Michael G. Campbell and Professor Tobias itter Department of Chemistry and Chemical Biology Harvard University Introduction This white paper
WHITEPAPE Current Trends in Practical luorination Chemistry Michael G. Campbell and Professor Tobias itter Department of Chemistry and Chemical Biology Harvard University Introduction This white paper
Manganese-Catalyzed Late- Stage Aliphatic C H Azidation
 Wipf group current literature Manganese-Catalyzed Late- Stage Aliphatic C H Azidation J. Am. Chem. Soc. 2015, 137, 5300 5303 Xiongyi Huang, Tova M. Bergsten, and John T. Groves Department of Chemistry,
Wipf group current literature Manganese-Catalyzed Late- Stage Aliphatic C H Azidation J. Am. Chem. Soc. 2015, 137, 5300 5303 Xiongyi Huang, Tova M. Bergsten, and John T. Groves Department of Chemistry,
REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!!
 CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Palladium-Catalyzed Electrophilic Aromatic C H Fluorination
 Palladium-Catalyzed Electrophilic Aromatic C luorination +2 Pd II 2 B 4 C (5 mol %) SI (2 eq) MeC, rt 61%, 69:31 o:p C Yamamoto, K; Li, J.; Garber, J. A..; Rolfes, J. D.; Boursalian, G. B.; Borghs, J.
Palladium-Catalyzed Electrophilic Aromatic C luorination +2 Pd II 2 B 4 C (5 mol %) SI (2 eq) MeC, rt 61%, 69:31 o:p C Yamamoto, K; Li, J.; Garber, J. A..; Rolfes, J. D.; Boursalian, G. B.; Borghs, J.
Asymmetric Synthesis of Medium-Sized Rings by Intramolecular Au(I)-Catalyzed Cyclopropanation
 Asymmetric Synthesis of Medium-Sized ings by Intramolecular Au(I)-Catalyzed Cyclopropanation 1 2 Iain D. G. Watson, Stefanie itter, and F. Dean Toste JACS, ASAP, 1/22/2009 DI: 10.1021/ja8085005 2.5 mol%
Asymmetric Synthesis of Medium-Sized ings by Intramolecular Au(I)-Catalyzed Cyclopropanation 1 2 Iain D. G. Watson, Stefanie itter, and F. Dean Toste JACS, ASAP, 1/22/2009 DI: 10.1021/ja8085005 2.5 mol%
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Mild and Selective Hydrozirconation of Amides to Aldehydes Using Cp 2 Zr(H)Cl: Scope and Mechanistic Insight
 Mild and Selective Hydrozirconation of Amides to Aldehydes Using Cp 2 Zr(H)Cl: Scope and Mechanistic Insight Jared T. Spletstoser, Jonathan M. White, Ashok Rao Tunoori, and Gunda I. George J. Am. Chem.
Mild and Selective Hydrozirconation of Amides to Aldehydes Using Cp 2 Zr(H)Cl: Scope and Mechanistic Insight Jared T. Spletstoser, Jonathan M. White, Ashok Rao Tunoori, and Gunda I. George J. Am. Chem.
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent
 Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Abstracts VII. Phthalocyanines and Related Compounds. M. S. Rodr guez-morgade and T. Torres
 VII p1 thalocyanines and Related Compounds 17.9.24 M.. Rodr guez-morgade and T. Torres This review updates the original cience of ynthesis chapter (ection 17.9) on phthalocyanines and various ring-fused,
VII p1 thalocyanines and Related Compounds 17.9.24 M.. Rodr guez-morgade and T. Torres This review updates the original cience of ynthesis chapter (ection 17.9) on phthalocyanines and various ring-fused,
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Hydroboration. Carreira: Chapter 7
 ydroboration Carreira: Chapter 7 ydroboration of alkenes/alkynes is one of the most versatile reactions available. Most commonly, the resulting alkyl borane intermediates are not isolated, but are used
ydroboration Carreira: Chapter 7 ydroboration of alkenes/alkynes is one of the most versatile reactions available. Most commonly, the resulting alkyl borane intermediates are not isolated, but are used
Experiment : Reduction of Ethyl Acetoacetate
 Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Aryl Halides. Structure
 Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
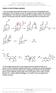 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Intramolecular Ene Reactions Utilizing Oxazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129,
 Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Self-stable Electrophilic Reagents for Trifluoromethylthiolation. Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date:
 Self-stable Electrophilic Reagents for Trifluoromethylthiolation Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date: 2017-12-25 Content Introduction Trifluoromethanesulfenates: Preparation and reactivity
Self-stable Electrophilic Reagents for Trifluoromethylthiolation Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date: 2017-12-25 Content Introduction Trifluoromethanesulfenates: Preparation and reactivity
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Massachusetts Institute of Technology Organic Chemistry Problem Set 1. Functional Group Transformations Study Guide
 Massachusetts Institute of Technology rganic Chemistry 5.511 Problem Set 1 September, 2007 Prof. Rick L. Danheiser Functional Group Transformations Study Guide The purpose of this three-part study guide
Massachusetts Institute of Technology rganic Chemistry 5.511 Problem Set 1 September, 2007 Prof. Rick L. Danheiser Functional Group Transformations Study Guide The purpose of this three-part study guide
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides
 Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Title. Author(s)Kobayashi, Shingo; Yoneda, Atushi; Fukuhara, Tsuyosh. CitationTetrahedron Letters, 45(6): Issue Date
 Title Selective synthesis of fluorinated carbohydrates usi Author(s)Kobayashi, Shingo; Yoneda, Atushi; ukuhara, Tsuyosh CitationTetrahedron Letters, 45(6): 1287-1289 Issue Date 2004-02-02 Doc URL http://hdl.handle.net/2115/576
Title Selective synthesis of fluorinated carbohydrates usi Author(s)Kobayashi, Shingo; Yoneda, Atushi; ukuhara, Tsuyosh CitationTetrahedron Letters, 45(6): 1287-1289 Issue Date 2004-02-02 Doc URL http://hdl.handle.net/2115/576
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine N-oxide
 Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions
 Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
Organic Chemistry I Exam 3 Fall 2001 November 30, Which of the following compounds corresponds to the spectral data given below?
 . Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
. Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide
 General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
September [KV 804] Sub. Code: 3804
![September [KV 804] Sub. Code: 3804 September [KV 804] Sub. Code: 3804](/thumbs/89/97855699.jpg) September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Chemistry 335 Supplemental Slides: Interlude 1. Reduction: addition of hydrogen to the substrate. Oxidation: addition of oxygen to the substrate
 Interlude 1: Oxidations, Reductions & Other Functional Group Interconversions (FGI) 1. Definition of Oxidation and Reduction For practical purposes in organic chemistry, oxidation and reduction are defined
Interlude 1: Oxidations, Reductions & Other Functional Group Interconversions (FGI) 1. Definition of Oxidation and Reduction For practical purposes in organic chemistry, oxidation and reduction are defined
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
Chapter 08 Alcohols, Ethers, and Thiols
 Alcohols, Ethers and Thiols Chapter 08 Alcohols, Ethers, and Thiols CEM 240: Fall 2016 Prof. Greg Cook 2 Alcohol Nomenclature - common imple alcohols are often named using common naming (functional class)
Alcohols, Ethers and Thiols Chapter 08 Alcohols, Ethers, and Thiols CEM 240: Fall 2016 Prof. Greg Cook 2 Alcohol Nomenclature - common imple alcohols are often named using common naming (functional class)
CHEM3331: Fundamentals of Organic Chemistry I Prof. Ognjen Š. Miljanić December 11, 2012
 HEM3331: Fundamentals of rganic hemistry I Final Exam Prof. gnjen Š. Miljanić December 11, 2012 Name: Last First Student ID Number: ead all directions very carefully, think about your answer, and then
HEM3331: Fundamentals of rganic hemistry I Final Exam Prof. gnjen Š. Miljanić December 11, 2012 Name: Last First Student ID Number: ead all directions very carefully, think about your answer, and then
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
L substrate (Leaving group,l)
 Aliphatic Nucleophilic Substitution Nu + Nucleophile L substrate (Leaving group,l) conditions products Nucleophiles are chemical species that react with centers of positive ionic character. When the center
Aliphatic Nucleophilic Substitution Nu + Nucleophile L substrate (Leaving group,l) conditions products Nucleophiles are chemical species that react with centers of positive ionic character. When the center
10. Amines (text )
 2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
1. Which compound would you expect to have the lowest boiling point? A) NH 2 B) NH 2
 MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
1. Theoretical Investigation of Mechanisms and Stereoselectivities of Synthetic Organic Reactions
 1. Theoretical Investigation of Mechanisms and Stereoselectivities of Synthetic Organic Reactions 2. Copper Catalyzed One-Pot Synthesis of Multisubstituted Quinolinones Hao Wang Denmark Group Presentation
1. Theoretical Investigation of Mechanisms and Stereoselectivities of Synthetic Organic Reactions 2. Copper Catalyzed One-Pot Synthesis of Multisubstituted Quinolinones Hao Wang Denmark Group Presentation
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions:
 1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
mcpba e.g. mcpba (major) Section 7: Oxidation of C=X bonds
 Section 7: xidation of C=X bonds Functional Group Interconversions - Lecture 6 7.1 Epoxidation of Alkenes Epoxides are VEY useful in synthesis - the strain of the three membered ring makes these cyclic
Section 7: xidation of C=X bonds Functional Group Interconversions - Lecture 6 7.1 Epoxidation of Alkenes Epoxides are VEY useful in synthesis - the strain of the three membered ring makes these cyclic
Organic Chemistry. Radical Reactions
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Option G: Further organic chemistry (15/22 hours)
 Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways
 Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways Xiao Cheng, Charles Goodhue, and Jin-Quan Yu Brandeis University
Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways Xiao Cheng, Charles Goodhue, and Jin-Quan Yu Brandeis University
ummary Manipulating Radicals
 Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
b.(12) Where is pyrrole protonated under strong acidic conditions? Why this site of protonation?
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank the following dienes by rate of Diels-Alder reaction. The diene which reacts the fastest with an alkene is 1, while the
1. Rank the following compounds in the trend requested. (15 points each) a. Rank the following dienes by rate of Diels-Alder reaction. The diene which reacts the fastest with an alkene is 1, while the
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
GC Derivatization Pierce Applications Handbook & Catalog
 GC Derivatization Pierce 2003-2004 Applications Handbook & Catalog Derivatization The chemical literature contains an abundance of data on derivatization, most of which is relevant to particular compounds,
GC Derivatization Pierce 2003-2004 Applications Handbook & Catalog Derivatization The chemical literature contains an abundance of data on derivatization, most of which is relevant to particular compounds,
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Stille-type cross coupling reactions with tetraalkynyl stannanes
 Stille-type cross coupling reactions with tetraalkynyl stannanes Andrey S. Levashov,* Dmitriy S. Buriy, Valeriy V. Konshin and Alexey A. Andreev (deceased) Kuban State University, 149 Stavropolskaya Str.,
Stille-type cross coupling reactions with tetraalkynyl stannanes Andrey S. Levashov,* Dmitriy S. Buriy, Valeriy V. Konshin and Alexey A. Andreev (deceased) Kuban State University, 149 Stavropolskaya Str.,
Organic Fluorine as a Hydrogen-Bond Acceptor: Recent Examples and Applications
 Organic luorine as a ydrogen-bond Acceptor: Recent Examples and Applications Reporter: Xinjin Li eb 2 nd, 2015 1 Xinjin Li u Group Content 1 Introduction 2 3 Properties of ydrogen Bonds 7 C(sp 2 ) as a
Organic luorine as a ydrogen-bond Acceptor: Recent Examples and Applications Reporter: Xinjin Li eb 2 nd, 2015 1 Xinjin Li u Group Content 1 Introduction 2 3 Properties of ydrogen Bonds 7 C(sp 2 ) as a
CEE 772: INSTRUMENTAL METHODS IN ENVIRONMENTAL ANALYSIS
 David Reckhow CEE 772 #12 1 Updated: 23 ctober 2014 Print version CEE 772: INSTRUMENTAL METHDS IN ENVIRNMENTAL ANALYSIS Lecture #12 Sample Preparation: Basics and Physical Methods (Skoog, nothing) (Harris,
David Reckhow CEE 772 #12 1 Updated: 23 ctober 2014 Print version CEE 772: INSTRUMENTAL METHDS IN ENVIRNMENTAL ANALYSIS Lecture #12 Sample Preparation: Basics and Physical Methods (Skoog, nothing) (Harris,
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
