COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
|
|
|
- Spencer McDowell
- 5 years ago
- Views:
Transcription
1 COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
2 DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.) are not fully explained and it is even more difficult to develop medicines for these diseases. Only 1 out of 10,000 molecules synthesized can be used as a drug. It is quite costly. It takes a long time.
3 RATIONAL DRUG DESIGN For all these reasons, it is now necessary to design drugs in a rational way. Understanding of several physiological and biochemical mechanisms and their relation to diseases at the molecular level, clarification of some receptors and structures have contributed to the development of computer-aided drug design methods.
4 COMPUTER-AIDED DRUG DESIGN Quantitative structure activity relationship (QSAR) Molecular modeling studies
5 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPS (QSAR) A QSAR is a mathematical relationship between a biological activity of a molecular system and its geometric and chemical characteristics.
6 QSAR The first study to identify the relationships between chemical structure and biological activity has been done in France in (A. Cros) According to this study, "As the solubility of water in some of the investigated alcohols decreases, the toxic effects on the mammals are increased".
7 QSAR s goal Designing a new compound that can exert better effect using the structure-effect relationship analysis equation developed over a series of compounds, Reducing the toxicity of an existing compound, Optimize to be the leader with the optimum lipophilic property to pass a selected barrier (e.g. blood-brain barrier)
8 Biological Responses Used in QSAR Studies Affinity data: substrate or receptor binding Rate constants: association, dissociation Inhibition constants: IC50, enzyme inhibition values Pharmacokinetic parameters: absorption, distribution, metabolism, excretion In vitro and in vivo biological activity data Pharmacodynamic data of drugs (drug-receptor interaction) Toxic effect parameters
9 Parameters Parameters used in QSAR studies are constants that are used to quantitatively describe intramolecular forces, activities such as transportation, distribution that provide drug receptor interaction.
10 Physicochemical Parameters Used in Structure-effect Studies PHYSICOCHEMICAL PARAMETERS LIPOPHILIC (HYDROPHOBIC) PARAMETERS Partition Coefficient π-substituent Constant Chromatography Distribution Coefficient (Liquid-liquid) Hydrophobic Fragmental Constant SYMBOL Log P, (log P) 2, ( ) 2 R M f ELECTRONIC PARAMETERS Ionization Constant Sigma Aromatic Substituent Constant Modification Aromatic Substituent Constants Sigma Aliphatic Substituent Constant Substituent Resonance Effect Substituent Inductive Effect pk a m, m +, -, 1, R, o * R F
11 Physicochemical Parameters Used in Structure-effect Studies PHYSICOCHEMICAL PARAMETERS QUANTUM MECHANICAL PARAMETERS Atomic Elektron Charge Atomic Elektron Charge Nucleophilic Delocalization State Electrophilic Delocalization State Energy of Lowest Unoccupied Molecular Orbital, electrophilicity Energy of Highest Occupied Molecular Orbital, nucleophilicity SYMBOL q, Q q, Q S r N S r E E LUMO E HOMO STERIC PARAMETERS Steric Substituent Constant Molar Volume Molar Refractivity Substituent Constant Molecular Weight Van der Waals Radii Sterimol Width and Length Parameters E S MV MR MW R L, B 1 -B 4
12 Structural Parameters (Indicator) The structural parameter is used if any position in the molecular structures of the chemical compounds does not include a sufficient number of substituent substitutions. Structural parameters are determined to be "1" or "0", respectively, depending on the presence or absence of the molecular substituent being analyzed.
13 Lipophilic Property The most used physicochemical property in QSAR studies are lipophilic property. Lipophilicity can be defined as the dispersion between water and oil phase. Parameters showing this distribution; Log P R M
14 Log P = Partition Coefficient It is a parameter that expresses the concentration of the chemical compound distributed between the lipid-water layers. For this purpose, it was found that the most suitable solvent system is 1-octanol / water. As the water, the buffer solution is prepared to imitate the physiological ph (ph = 7.4).
15 Why 1-Octanol 1-octanol, due to the long alkyl chain and the polar hydroxyl (OH) group, carries a hydrophobic tail and a polar head. So, it forms a good example of cell membrane lipids. The OH group it carries has a receptor and donor property in the formation of hydrogen bonds and can interact with a wide variety of polar groups. It has low vapor pressure. This allows the measurements to be repeated. It has a broad range of UV transmittance and facilitates the quantitative determination of many compounds dissolved therein.
16 Partition Coefficient (Log P) Calculation 1- Fragmentation Method and Theoretical Log P Calculation Includes the theoretical calculation of the hydrophobic constant (Log P) value of the molecule, taking advantage of the sum of the hydrophobic action values of various atomic and atomic groups (various fragments) calculated by Hanch et al.
17 As the form of mathematical expression, the following symbols and expressions are used. fb = Single bond between fragments of straight chain fb = Single bond between fragments of ring fcbr = Branched chain fgbr = Branched group (used in case of polar fragments instead of H atoms in the structure) f = A fragment attached to an aromatic ring f = A fragment attached to two aromatic rings
18 Regulated Fragment Constants f H = f CH3 = 0.89 f CH2 = f CH3 - f H = 0.66 f CH = f CH2 - f H = 0.43 f b = single bond (between fragments of straight chain) f b = single bond (between fragments of ring) f cbr = (branched chain) f C = f CH - f H = 0.20 f gbr = (branched group)
19 Fragment f f f - Br - Cl - F - I - N(CH 3 ) 2 - NO 2 - O - - S - - NH - - NH OH -CN -C (=O)N(CH 3 ) 2 -C (=O)NH -C 6 H
20 Examples -1: Isobutane 3 f CH3 + 1f CH + 2 f b + f cbr =3(0.89) + (0.43) + 2(-0.12) + (-0.13) = 2.86 (found log P : 2.76) Cyclopropane 3 f CH2 + 2 f b = 3(0.66) +2(-0.09)= 1.80 (found log P : 1.72) Note: f b is used for every single bond after the first C-C bond.
21 Examples -2: 2-phenylethanol f C6H5 + 2 f CH2 + f OH + 2 f b = (1.90) +2 (0.66)+ (-1.64) + 2 (-0.12) = 1.34 (found log P : 1.36) (2-chloroethyl) benzene f C6H5 + 2 f CH2 + f Cl + 2 f b = (1.90) + 2(0.66) + (0.06) + 2(-0.12) =3.04 (found log P : 2.95)
22 Examples -3: Isopropyl alcohol 2 f CH3 + 1 f CH + f OH + 2f b + f gbr = 2(0.89) + (0.43) + (-1.64) + 2(-0.12) + (-0.22) = 0.11 (found log P : 0.05) tert-butylamine 3 f CH3 + 1 f C + f NH2 + 3 f b +2 f gbr = 3(0.89) + (0.20) + (-1.54) + 3(-0.12) + 2(-0.22)= 0.53 (found log P : 0.40)
23 2- LogP Calculation Using Computer Programs Using the ChemSketch drawing program of ACD (Advanced Chemistry Development) / Labs, molecules can be drawn and the Log P values can be calculated from the hydrophobic parameters using Log P mod.
24 Distribution Coefficient Calculation on the Computer Using ChemDraw Ultra Program
25 3-Calculation of Log P Value by Experimental Method 1-Octanol solution (saturated with buffer solution) hazırlanır. Buffer solution (saturated with 1-octanol) Buffer Solution contains; potassium dihydrogen phosphate, Disodium hydrogen phosphate.12h 2 O
26 Experimental Procedure: The compound to be determined by the distribution coefficient is weighed to about 10 mg and is completed 50 ml with 1-octanol. 10 ml of this solution is taken and 10 ml of buffer solution is added. It is stirred for 1 hour in a water bath at 37ºC (body temperature). At the end of this period 1-octanol and water layers are separated. Take 1 ml of the octanol layer and complete 20 ml with 1-octanol. The absorbance value (y1) of the maximum wavelength of this solution in the UV spectrum taken between nm is determined.
27 Preparation of standard solutions and calibration curve 1 ml of solution (A) prepared at the beginning of the experiment is transferred to 3 volumetric flask. The volumetric flasks are completed 20, 30 and 40 ml separately with 1-octanol. The absorbance values (y) of the standard solutions prepared are read in the maximum wavelength at nm in the UV spectra. Two separate studies can be performed using the absorbance values obtained.
28 1-Regression analysis method Prepared at various concentrations, standard solutions absorbance values y = ax + b These solutions concentration values The a and b values found are substituted in the following equation. y1 = ax1 + b UV absorbance value of the standard The concentration of compound remaining in the octanol layer is found
29 2-Graphical Method Measured absorbance values A A 3 A 2 y1 20 ml: A1-C1 30 ml: A2-C2 40 ml: A3-C3 A 1 logp = log Amount, passed into octanol Amount, passed into water C 1 x1 C 2 C 3 C Concentration values of standard solution
30 Example: 5 mg of aspirin was dissolved in 50 ml of 1-octanol, from which 25 ml was taken and mixed with an equal volume of buffer solution for one hour at 37 C in a erlenmeyer with stopper. At the end of the period, 1 ml of 1 octanol layer was taken and was completed 10 ml in volumetric flask and the absorbance value was in UV. Calculate the Log P value of the aspirin. (Mw=180, a= , b= )
31 Solution: (Mw=180, a= , b= ) (Absorbance value: 0,4320) y = ax + b 0,4320 = 9723,57x + (-0,07243) x = 5, (the amount remaining in octanol) 1/10 diluted concentration 5, x10 = 5, (actual concentration remaining in octanol) (starting amount) 5, , =0, (Amount, passed into water) Amount, passed into octanol 5, logp = log = log = 1,14 Amount, passed into water 0,
32 Calculation of They are used to roughly predict the lipophilic properties of the compounds. In the R M assay using the thin layer chromatography (TLC) method it is believed that 1-octanol-saturated plates represent the lipid phase in the organism. R M value is calculated from Rf values. R M = log (1/Rf 1)
33 Structure-Activity Relationships (QSAR) Analysis In the 1960s, two separate quantitative structure activity relationship analysis methods were developed. They were developed by Hansch and Fujita, Free and Wilson. Quantitative structure-activity relationships (QSAR) are the mathematical methods for describing the relationships between molecular properties of chemical compounds (structural / physicochemical properties) and biological activities.
34 Hansch Analysis Method Hansch developed the following formula, expressing that the observed biological effects of the compounds in a homologous series in the method of analysis are a function of the physicochemical properties of these compounds. biological effect = f (hydrophobic) + f (electronic) + f (steric) + c (constant)
35 Log 1 / C = Logarithmic biological effect Independent variables of physicochemical parameters Y (biological activity) = k o + k 1 X 1 + k 2 X k n X n The constant (correlation constant) indicating the contribution of the unexplained residue to the biological activity The constants (regression coefficients) that define (+) or (-) contribution of physicochemical properties to biological activity
36 Regression processing: Correlates the relationship between dependent Y variables (biological activity) and independent X variables (physicochemical parameters) with the least squares method, yielding the most appropriate model in the statistical direction and allowing the QSAR analysis to be resolved. Objective: To determine the correlation equation that quantitative structure-effect relationships provides adequately and the best solution.
37 Correlation Coefficient (R or R 2 ): Provides statistical information on which model is compatible and valid. The less the difference between the observed and the calculated biological effect values of the analyzed compounds, the closer the R is to 1. R 2 : Indicates the percentage of this harmony identified. Standard deviation or error: Indicates whether the model in which the correlation equation emerges corresponds to the statistically. As this value approaches zero, the value of R increases. Fisher Test: Indicates to what degree the model is statistically valid. Statistically the model is considered valid and reliable if p> 95% contains a value above the table probability limits.
38 QSAR Application A series of 2,5-disubstituted benzimidazole, benzoxazole and 2- substituted oxazolo (4,5-b) pyridine derivatives have been synthesized and tested in vitro against K. pneumoniae. The quantitative structure-effect relationships (QSAR) of the compounds are explained by applying the Hansch analysis method using the obtained activity results. R Y X N Z R 1 I = X: NH, Y: CH, Z: CH 2 or II = X: O, Y: CH, Z: CH 2 or III = X: O, Y: N, Z: -
39
40
41
42 Log 1/C = 0,400(±0,015)H ACCEPT, R + 0,332(±0,015)I Y + 0,347 (± 0,013)I Z 0,477(±0,024)F R 0,308(±0,015)I X + 4,245 The most appropriate correlation (linear relationship) was obtained with the equations, R 2 = 97% and s =
43 When the equality is examined, R Y X N Z R 1 Log 1/C = 0,400(±0,015)H ACCEPT, R + 0,332(±0,015)I Y + 0,347 (± 0,013)I Z 0,477(±0,024)F R 0,308(±0,015)I X + 4,245 The R group is important for biological activity, The substituents in the hydrogen trapping group (H ACCEPT ) in the R group increase the activity, The presence of substituents having the negative field effect (F R ) of the R groups increases activity, IX, IY, IZ are also determinants for activity, IX, NH reduces activity, O increases activity, IY, N increases activity, IZ methylene group is important, it increases activity. There is no obvious statistical effect of group R1.
44 Result The 2-benzyl oxazolo (4,5-b) pyridine derivatives which have a negative field effect at R are more effective. This work will lead to the synthesis of compounds which we have found to be more effective. O H 2 N N N CH 2 R 1
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR
 Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Nonlinear QSAR and 3D QSAR
 onlinear QSAR and 3D QSAR Hugo Kubinyi Germany E-Mail kubinyi@t-online.de HomePage www.kubinyi.de onlinear Lipophilicity-Activity Relationships drug receptor Possible Reasons for onlinear Lipophilicity-Activity
onlinear QSAR and 3D QSAR Hugo Kubinyi Germany E-Mail kubinyi@t-online.de HomePage www.kubinyi.de onlinear Lipophilicity-Activity Relationships drug receptor Possible Reasons for onlinear Lipophilicity-Activity
CHEM 4170 Problem Set #1
 CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Electronic Structure. Hammett Correlations. Linear Free Energy Relationships. Quantitative Structure Activity Relationships (QSAR)
 Electronic Structure ammett Correlations Quantitative Structure Activity Relationships (QSAR) Quantitative Structure Activity Relationships (QSAR) We have already seen that by changing groups in a drug
Electronic Structure ammett Correlations Quantitative Structure Activity Relationships (QSAR) Quantitative Structure Activity Relationships (QSAR) We have already seen that by changing groups in a drug
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a
 Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Introduction into Biochemistry. Dr. Mamoun Ahram Lecture 1
 Introduction into Biochemistry Dr. Mamoun Ahram Lecture 1 Course information Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 7 th edition Instructors Dr. Mamoun
Introduction into Biochemistry Dr. Mamoun Ahram Lecture 1 Course information Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 7 th edition Instructors Dr. Mamoun
Statistical concepts in QSAR.
 Statistical concepts in QSAR. Computational chemistry represents molecular structures as a numerical models and simulates their behavior with the equations of quantum and classical physics. Available programs
Statistical concepts in QSAR. Computational chemistry represents molecular structures as a numerical models and simulates their behavior with the equations of quantum and classical physics. Available programs
Solvent & geometric effects on non-covalent interactions
 Solvent & geometric effects on non-covalent interactions Scott L. Cockroft PhysChem Forum 10, Syngenta, Jealott s Hill, 23 rd March 11 QSAR & Physical Organic Chemistry Quantifiable Physicochemical Properties
Solvent & geometric effects on non-covalent interactions Scott L. Cockroft PhysChem Forum 10, Syngenta, Jealott s Hill, 23 rd March 11 QSAR & Physical Organic Chemistry Quantifiable Physicochemical Properties
Acids and Bases. Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago.
 Acids and Bases Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Drug dissolution can impact buffering capacity of the body Most enzymes require
Acids and Bases Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Drug dissolution can impact buffering capacity of the body Most enzymes require
Drug Discovery. Zainab Al Kharusi Office: 33-10
 Drug Discovery Zainab Al Kharusi Office: 33-10 Objectives: To understand the processes of Drug Development To be able to develop a plan for drug discovery Reference: Patrick G L. An Introduction to Medicinal
Drug Discovery Zainab Al Kharusi Office: 33-10 Objectives: To understand the processes of Drug Development To be able to develop a plan for drug discovery Reference: Patrick G L. An Introduction to Medicinal
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
AMINES. 3. Secondary When two hydrogen atoms are replaced by two alkyl or aryl groups.
 AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
Solutions. Experiment 11. Various Types of Solutions. Solution: A homogenous mixture consisting of ions or molecules
 Solutions Solution: A homogenous mixture consisting of ions or molecules -Assignment: Ch 15 Questions & Problems : 5, (15b,d), (17a, c), 19, 21, 23, 27, (33b,c), 39, (43c,d),45b, 47, (49b,d), (55a,b),
Solutions Solution: A homogenous mixture consisting of ions or molecules -Assignment: Ch 15 Questions & Problems : 5, (15b,d), (17a, c), 19, 21, 23, 27, (33b,c), 39, (43c,d),45b, 47, (49b,d), (55a,b),
PHYSIO-CHEMICAL PROPERTIES OF THE DRUG. It is the interaction of the drug with its environment
 PYSIO-CEMICAL PROPERTIES OF TE DRUG It is the interaction of the drug with its environment Reducing the complexity Biological process in drug action Dissolution of drug in gastrointestinal fluids Absorption
PYSIO-CEMICAL PROPERTIES OF TE DRUG It is the interaction of the drug with its environment Reducing the complexity Biological process in drug action Dissolution of drug in gastrointestinal fluids Absorption
Chapter 8. Acidity, Basicity and pk a
 Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chemistry in Biology. Section 1. Atoms, Elements, and Compounds
 Section 1 Atoms, Elements, and Compounds Atoms! Chemistry is the study of matter.! Atoms are the building blocks of matter.! Neutrons and protons are located at the center of the atom.! Protons are positively
Section 1 Atoms, Elements, and Compounds Atoms! Chemistry is the study of matter.! Atoms are the building blocks of matter.! Neutrons and protons are located at the center of the atom.! Protons are positively
HW #3: 14.26, 14.28, 14.30, 14.32, 14.36, 14.42, 14.46, 14.52, 14.56, Alcohols, Ethers and Thiols
 Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Properties of Amines
 Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
PHYSICOCHEMICAL PROPERTIES OF DRUG ACTION
 PHYSICCHEMICAL PRPERTIES F DRUG ACTIN III Year B.Pharm I Sem Subject: Medicinal Chemistry-I Presented by: Mr. N. Uday Kumar M.Pharm (Ph.D) PHYSICCHEMICAL PRINCIPLES F DRUG ACTIN INTRDUCTIN Drug molecules
PHYSICCHEMICAL PRPERTIES F DRUG ACTIN III Year B.Pharm I Sem Subject: Medicinal Chemistry-I Presented by: Mr. N. Uday Kumar M.Pharm (Ph.D) PHYSICCHEMICAL PRINCIPLES F DRUG ACTIN INTRDUCTIN Drug molecules
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
The Chemistry of Life
 The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS
 2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
CHEM1902/4 Worksheet 2: Isomerism and Structure Elucidation Model 4: Isomerism
 CHEM1902/4 Worksheet 2: Isomerism and Structure Elucidation Model 4: Isomerism There are three broad classes of isomers. Use the definitions below to help you decide the type of isomerism shown by each
CHEM1902/4 Worksheet 2: Isomerism and Structure Elucidation Model 4: Isomerism There are three broad classes of isomers. Use the definitions below to help you decide the type of isomerism shown by each
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
Journal of Chemical and Pharmaceutical Research, 2015, 7(2): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Water and solutions. Prof. Ramune Morkuniene, Biochemistry Dept., LUHS
 Water and solutions Prof. Ramune Morkuniene, Biochemistry Dept., LUHS Characteristics of water molecule Hydrophylic, hydrophobic and amphipatic compounds Types of real solutions Electrolytes and non- electrolytes
Water and solutions Prof. Ramune Morkuniene, Biochemistry Dept., LUHS Characteristics of water molecule Hydrophylic, hydrophobic and amphipatic compounds Types of real solutions Electrolytes and non- electrolytes
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
2. Separate the ions based on their mass to charge (m/e) ratio. 3. Measure the relative abundance of the ions that are produced
 I. Mass spectrometry: capable of providing both quantitative and qualitative information about samples as small as 100 pg (!) and with molar masses in the 10 4-10 5 kdalton range A. The mass spectrometer
I. Mass spectrometry: capable of providing both quantitative and qualitative information about samples as small as 100 pg (!) and with molar masses in the 10 4-10 5 kdalton range A. The mass spectrometer
Downloaded from
 1 Class XI Chemistry Ch 13: Hydrocarbons TOP Concepts: 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
1 Class XI Chemistry Ch 13: Hydrocarbons TOP Concepts: 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
Chemistry 216. First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh. Lab section. GSI name. Name Please print.
 Chemistry 216 First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh Lab section GSI name Name Please print Signature Student ID# I 8 II 10 III 6 IV 12 V 12 VI 10 VII 14 VIII 8 Total 80
Chemistry 216 First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh Lab section GSI name Name Please print Signature Student ID# I 8 II 10 III 6 IV 12 V 12 VI 10 VII 14 VIII 8 Total 80
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
 1 TOP Concepts: Class XI Chemistry Ch 13: Hydrocarbons 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
1 TOP Concepts: Class XI Chemistry Ch 13: Hydrocarbons 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Downloaded from
 1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
1. (18) Multiple choice questions. Please place your answer on the line preceding each question.
 CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
Notes of Dr. Anil Mishra at 1
 Introduction Quantitative Structure-Activity Relationships QSPR Quantitative Structure-Property Relationships What is? is a mathematical relationship between a biological activity of a molecular system
Introduction Quantitative Structure-Activity Relationships QSPR Quantitative Structure-Property Relationships What is? is a mathematical relationship between a biological activity of a molecular system
Chapter 2 The Chemistry of Biology. Dr. Ramos BIO 370
 Chapter 2 The Chemistry of Biology Dr. Ramos BIO 370 2 Atoms, Bonds, and Molecules Matter - all materials that occupy space and have mass Matter is composed of atoms. Atom simplest form of matter not divisible
Chapter 2 The Chemistry of Biology Dr. Ramos BIO 370 2 Atoms, Bonds, and Molecules Matter - all materials that occupy space and have mass Matter is composed of atoms. Atom simplest form of matter not divisible
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
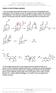 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Organic Chemistry. Introduction to Organic Molecules and Functional Groups
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
H O H. Chapter 3: Outline-2. Chapter 3: Outline-1
 Chapter 3: utline-1 Molecular Nature of Water Noncovalent Bonding Ionic interactions van der Waals Forces Thermal Properties of Water Solvent Properties of Water ydrogen Bonds ydrophilic, hydrophobic,
Chapter 3: utline-1 Molecular Nature of Water Noncovalent Bonding Ionic interactions van der Waals Forces Thermal Properties of Water Solvent Properties of Water ydrogen Bonds ydrophilic, hydrophobic,
1 Answer. 2 Answer A B C D
 216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
5.65 g = kg m = mm 174 ml = L. 711 kg = g 3.79 km = m L = μl g = mg 745 μm = cm 127 μl = ml 302 C = K 185 K = C 100 C = K
 WLHS / AP Bio / UNIT 1 Chemistry of Life Name AP Biology Summer Assignment Use Campbell CH 2-4 Biology is the study of life and living things. Before we can study and understand many biological principles,
WLHS / AP Bio / UNIT 1 Chemistry of Life Name AP Biology Summer Assignment Use Campbell CH 2-4 Biology is the study of life and living things. Before we can study and understand many biological principles,
Introduction to Chemoinformatics and Drug Discovery
 Introduction to Chemoinformatics and Drug Discovery Irene Kouskoumvekaki Associate Professor February 15 th, 2013 The Chemical Space There are atoms and space. Everything else is opinion. Democritus (ca.
Introduction to Chemoinformatics and Drug Discovery Irene Kouskoumvekaki Associate Professor February 15 th, 2013 The Chemical Space There are atoms and space. Everything else is opinion. Democritus (ca.
24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols. + Electron-withdrawing groups make an O
 Chapter 24: Phenols. Alcohols contain an group bonded to an sp 3 -hybridized carbon. Phenols contain an group bonded to an sp 2 -hybridized carbon of a benzene ring 24.1: Nomenclature (please read) 24.2:
Chapter 24: Phenols. Alcohols contain an group bonded to an sp 3 -hybridized carbon. Phenols contain an group bonded to an sp 2 -hybridized carbon of a benzene ring 24.1: Nomenclature (please read) 24.2:
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry
 Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
Quantitative structure activity relationship and drug design: A Review
 International Journal of Research in Biosciences Vol. 5 Issue 4, pp. (1-5), October 2016 Available online at http://www.ijrbs.in ISSN 2319-2844 Research Paper Quantitative structure activity relationship
International Journal of Research in Biosciences Vol. 5 Issue 4, pp. (1-5), October 2016 Available online at http://www.ijrbs.in ISSN 2319-2844 Research Paper Quantitative structure activity relationship
Chemistry 212 Fall Experiment 3: S N 1 Evaluation Summary
 Experiment 3: S N 1 Evaluation Summary Last Name: Atique Date: 11/05/2013 First Name: Anika Lab Day/TA/Group: Wednesday; TA: Patrick Julien; Group C Lab reports must be typed and chemical structures must
Experiment 3: S N 1 Evaluation Summary Last Name: Atique Date: 11/05/2013 First Name: Anika Lab Day/TA/Group: Wednesday; TA: Patrick Julien; Group C Lab reports must be typed and chemical structures must
CHAPTER 6 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIP (QSAR) ANALYSIS
 159 CHAPTER 6 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIP (QSAR) ANALYSIS 6.1 INTRODUCTION The purpose of this study is to gain on insight into structural features related the anticancer, antioxidant
159 CHAPTER 6 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIP (QSAR) ANALYSIS 6.1 INTRODUCTION The purpose of this study is to gain on insight into structural features related the anticancer, antioxidant
Name Date Class HYDROCARBONS
 22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
Ester Synthesis And Analysis: Aspirin and Oil of Wintergreen. Vanessa Jones November 19, 2015 Thursday 8:30 Lab Section Lab Partner: Melissa Blanco
 Ester Synthesis And Analysis: Aspirin and Oil of Wintergreen Vanessa Jones November 19, 2015 Thursday 8:30 Lab Section Lab Partner: Melissa Blanco INTRODUCTION For this lab, students attempted to synthesize
Ester Synthesis And Analysis: Aspirin and Oil of Wintergreen Vanessa Jones November 19, 2015 Thursday 8:30 Lab Section Lab Partner: Melissa Blanco INTRODUCTION For this lab, students attempted to synthesize
Visualization in Chemistry. Giovanni Morelli
 Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
NAME. EXAM I I. / 36 September 25, 2000 Biochemistry I II. / 26 BICH421/621 III. / 38 TOTAL /100
 EXAM I I. / 6 September 25, 2000 Biochemistry I II. / 26 BIH421/621 III. / 8 TOTAL /100 I. MULTIPLE HOIE (6 points) hoose the BEST answer to the question by circling the appropriate letter. 1. An amino
EXAM I I. / 6 September 25, 2000 Biochemistry I II. / 26 BIH421/621 III. / 8 TOTAL /100 I. MULTIPLE HOIE (6 points) hoose the BEST answer to the question by circling the appropriate letter. 1. An amino
The Chemistry and Energy of Life
 2 The Chemistry and Energy of Life Chapter 2 The Chemistry and Energy of Life Key Concepts 2.1 Atomic Structure Is the Basis for Life s Chemistry 2.2 Atoms Interact and Form Molecules 2.3 Carbohydrates
2 The Chemistry and Energy of Life Chapter 2 The Chemistry and Energy of Life Key Concepts 2.1 Atomic Structure Is the Basis for Life s Chemistry 2.2 Atoms Interact and Form Molecules 2.3 Carbohydrates
Plan. Day 2: Exercise on MHC molecules.
 Plan Day 1: What is Chemoinformatics and Drug Design? Methods and Algorithms used in Chemoinformatics including SVM. Cross validation and sequence encoding Example and exercise with herg potassium channel:
Plan Day 1: What is Chemoinformatics and Drug Design? Methods and Algorithms used in Chemoinformatics including SVM. Cross validation and sequence encoding Example and exercise with herg potassium channel:
Bio10 Cell and Molecular Lecture Notes SRJC
 Basic Chemistry Atoms Smallest particles that retain properties of an element Made up of subatomic particles: Protons (+) Electrons (-) Neutrons (no charge) Isotopes Atoms of an element with different
Basic Chemistry Atoms Smallest particles that retain properties of an element Made up of subatomic particles: Protons (+) Electrons (-) Neutrons (no charge) Isotopes Atoms of an element with different
Alkanes, Alkenes and Alkynes
 Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Many Organic compounds are acids or bases (or both) Many Organic compounds undergo acid-base reactions
 Objective 4 Intro to Reactivity 1: identify acids and bases using Lewis definition. Use curved arrows to show how base reacts with acid. Relate strength to pk a. Determine direction of equilibrium. Use
Objective 4 Intro to Reactivity 1: identify acids and bases using Lewis definition. Use curved arrows to show how base reacts with acid. Relate strength to pk a. Determine direction of equilibrium. Use
CHEM 109A Organic Chemistry
 CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
Hydrogen Bonding & Molecular Design Peter
 Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 01
 Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Organic Chemistry, 7 L. G. Wade, Jr. 2010, Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 16 Aromatic Compounds 2010, Prentice Hall Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 16 Aromatic Compounds 2010, Prentice Hall Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Experiment : Reduction of Ethyl Acetoacetate
 Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
ASSESSING THE DRUG ABILITY OF CHALCONES USING IN- SILICO TOOLS
 Page109 IJPBS Volume 5 Issue 1 JAN-MAR 2015 109-114 Research Article Pharmaceutical Sciences ASSESSING THE DRUG ABILITY OF CHALCONES USING IN- SILICO TOOLS Department of Pharmaceutical Chemistry, Institute
Page109 IJPBS Volume 5 Issue 1 JAN-MAR 2015 109-114 Research Article Pharmaceutical Sciences ASSESSING THE DRUG ABILITY OF CHALCONES USING IN- SILICO TOOLS Department of Pharmaceutical Chemistry, Institute
Chemistry 283g Experiment 4
 Chemistry 283g xperiment 4 XPRIMNT 4: lectrophilic Aromatic Substitution: A Friedel-Craft Acylation Reaction Relevant sections in the text: Fox & Whitesell, 3 rd d. Chapter 11, especially pg. 524-526,
Chemistry 283g xperiment 4 XPRIMNT 4: lectrophilic Aromatic Substitution: A Friedel-Craft Acylation Reaction Relevant sections in the text: Fox & Whitesell, 3 rd d. Chapter 11, especially pg. 524-526,
MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS
 MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS The following questions illustrate the range of the test in terms of the abilities measured, the disciplines covered, and the difficulty of the questions
MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS The following questions illustrate the range of the test in terms of the abilities measured, the disciplines covered, and the difficulty of the questions
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
CHAPTER 2: Structure and Properties of Organic Molecules
 1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
Introductory Biochemistry
 Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
Iodination of Salicylamide
 Iodination of Salicylamide lectrophilic Aromatic Substitution Aromatic compounds are unusually stable because of the delocalization of their electrons. Given that the cloud is so stable, aromatic compounds
Iodination of Salicylamide lectrophilic Aromatic Substitution Aromatic compounds are unusually stable because of the delocalization of their electrons. Given that the cloud is so stable, aromatic compounds
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction.
 Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
3) Accounts for strands of DNA being held together into a double helix. 7) Accounts for the cohesive nature of water and its high surface tension
 AP Chemistry Test (Chapter 11) Multiple Choice (50%) Please use the following choices to answer questions 1-7. A) London dispersion forces B) Ion-ion attractions C) Dipole-dipole attractions D) Dipole-ion
AP Chemistry Test (Chapter 11) Multiple Choice (50%) Please use the following choices to answer questions 1-7. A) London dispersion forces B) Ion-ion attractions C) Dipole-dipole attractions D) Dipole-ion
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Chimica Farmaceutica
 Chimica Farmaceutica Drug Targets Why should chemicals, some of which have remarkably simple structures, have such an important effect «in such a complicated and large structure as a human being? The answer
Chimica Farmaceutica Drug Targets Why should chemicals, some of which have remarkably simple structures, have such an important effect «in such a complicated and large structure as a human being? The answer
There are two main electronic effects that substituents can exert:
 Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
8. Methods in Developing Mobile Phase Condition for C18 Column
 I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
King Saud University College of Pharmacy Department of Pharmaceutics. Biopharmaceutics PHT 414. Laboratory Assignments 2010 G 1431 H
 King Saud University College of Pharmacy Department of Pharmaceutics Biopharmaceutics PHT 414 Laboratory Assignments 20 G 1431 H Department of Pharmaceutics Biopharmaceutics PHT -414 Laboratory Assignments
King Saud University College of Pharmacy Department of Pharmaceutics Biopharmaceutics PHT 414 Laboratory Assignments 20 G 1431 H Department of Pharmaceutics Biopharmaceutics PHT -414 Laboratory Assignments
Atoms. Atoms 9/9/2015
 The Chemistry of Life The Nature of Matter, Water,Carbon Compounds, Chemical Reactions and Enzymes The Nature of Matter B.1.9 Both living and nonliving things are composed of compounds, which are themselves
The Chemistry of Life The Nature of Matter, Water,Carbon Compounds, Chemical Reactions and Enzymes The Nature of Matter B.1.9 Both living and nonliving things are composed of compounds, which are themselves
Foundations in Microbiology Seventh Edition
 Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 2 The Chemistry of Biology Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 2 The Chemistry of Biology Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
R R CH. Some reactions of alcohols vary depending on their classification as 1º, 2º, or 3º alcohols.
 Experiment: Alcohol Reactions Alcohols are important organic molecules characterized by an alkyl group covalently bonded to a hydroxyl group. They may be classified as primary, secondary, or tertiary,
Experiment: Alcohol Reactions Alcohols are important organic molecules characterized by an alkyl group covalently bonded to a hydroxyl group. They may be classified as primary, secondary, or tertiary,
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water
 Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Chemistry in Biology Section 1 Atoms, Elements, and Compounds
 Name Chemistry in Biology Section 1 Atoms, Elements, and Compounds Date Main Idea Details Scan the headings and boldfaced words in Section 1 of the chapter. Predict two things that you think might be discussed.
Name Chemistry in Biology Section 1 Atoms, Elements, and Compounds Date Main Idea Details Scan the headings and boldfaced words in Section 1 of the chapter. Predict two things that you think might be discussed.
Groups to the Extractability of
 129 Contribution of Substituent Phenols and Benzoic Acids Groups to the Extractability of Takuyuki HAGIWARA and Shoji MOTOMIzu Department of Chemistry, Faculty of Science,Okayama University, Tsushimanaka,
129 Contribution of Substituent Phenols and Benzoic Acids Groups to the Extractability of Takuyuki HAGIWARA and Shoji MOTOMIzu Department of Chemistry, Faculty of Science,Okayama University, Tsushimanaka,
Unit 10: Part 1: Polarity and Intermolecular Forces
 Unit 10: Part 1: Polarity and Intermolecular Forces Name: Block: Intermolecular Forces of Attraction and Phase Changes Intramolecular Bonding: attractive forces that occur between atoms WITHIN a molecule;
Unit 10: Part 1: Polarity and Intermolecular Forces Name: Block: Intermolecular Forces of Attraction and Phase Changes Intramolecular Bonding: attractive forces that occur between atoms WITHIN a molecule;
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008
 Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes
 Name Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes Atoms, Elements, and Chemical Bonding I can draw atom models and identify the # protons, # neutrons, and # electrons in an atom. I can identify
Name Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes Atoms, Elements, and Chemical Bonding I can draw atom models and identify the # protons, # neutrons, and # electrons in an atom. I can identify
Conjugated Dienes and Ultraviolet Spectroscopy
 Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
