Astonomy 62 Lecture #10. Last Time. Applications of Stefan-Boltzmann Law Color Magnitudes Color Index
|
|
|
- Hilary Ward
- 5 years ago
- Views:
Transcription
1 Last Time Applications of Stefan-Boltzmann Law Color Magnitudes Color Index
2 Standard Visual Band Filters U B V R I
3 Flux through filter X: F x = 0 F S x d F x F x W x Apparent Color Magnitude: m x,1 m x,2 =2.5log F x,2 F x,1 m x,1 m x,2 2.5 log F, 2 x F,1 x x U B V R I (A) W x (A) Å=0.1nm=10 10 m
4 Color Index: B V=2.5 log[ F V F B ] C B V
5 Color Color Diagram for Normal Stars From C&O (Fig. 3.10) T
6 Topics for Today Spectral Types of Stars Kirchhoff's Laws Bohr Atomic Model Reading for today: Reading for next lecture: 8.1
7 Spectral Types of Stars T Credit & Copyright: KPNO 0.9m Telescope, AURA, NOAO, NSF APOD
8 Solar spectrum Spectrum of Planetary Nebula NGC7662
9 Spectroscopy Timeline 1802 Wollaston discovers dark absorption lines in the solar spectrum... gaps separating the colors of the Sun... William Wollaston ( )
10 Spectroscopy Timeline 1802 Wollaston discovers dark absorption lines in the solar spectrum 1814 Fraunhofer catalogs 475 lines and identifies the Na line at ~590 nm. Joseph Fraunhofer ( )
11 Spectroscopy Timeline 1802 Wollaston discovers dark absorption lines in the solar spectrum 1814 Fraunhofer catalogs 475 lines and identifies the Na line at ~590 nm.
12 Spectroscopy Timeline 1802 Wollaston discovers dark absorption lines in the solar spectrum 1814 Fraunhofer catalogs 475 lines and identifies the Na line at ~5900A Fraunhofer observes that spectra of other stars are different from that of the Sun, indicating that the lines cannot have terrestrial origin. 1850's Kirchhoff and Bunsen design a spectrograph. Kirchhoff identifies 70 lines of Fe in the solar spectrum Kirchhoff and Bunsen publish "Chemical Analysis by Spectral Observations". Elements have unique spectral "fingerprint". Absorption and emission lines have the same λ Joseph Lockyer discovered He in the Sun
13 Solar Spectrum λ Element K 3934 A Ca II H 3968 A Ca II G 4340 A H I (H ) F 4861 A H I (H ) b 5184, 5173 A Mg I E 5270 A Fe I D 5896, 5890 A Na I C 6563 A H I (H )
14 Kirchhoff's Laws A hot, dense gas or solid object produces a continuous spectrum with no dark spectral lines. A hot, diffuse gas produces bright spectral lines (emission lines). A cool, diffuse gas in front of a source of continuous spectrum produces dark spectral lines (absorption lines) in the continuous spectrum.
15 Spectroscopy Timeline 1890's Pickering and his assistants cataloged thousands of stellar spectra, labeling them according to strength of hydrogen lines: A, B,... Annie Jump Cannon ( )
16 Spectral Types of Stars T Credit & Copyright: KPNO 0.9m Telescope, AURA, NOAO, NSF APOD
17 Spectroscopy Timeline 1890's Pickering and his assistants cataloged thousands of stellar spectra, labeling them according to strength of hydrogen lines: A, B, Annie Jump Cannon placed the spectra in a temperature sequence: Oh Be A Fine Girl/uy Kiss Me Now
18 Understanding Stellar Spectra 1. Why do atoms emit discreet spectral lines? 2. Do differences between spectral types reflect differences in stellar chemical composition? 3. What role does temperature play in spectral line formation?
19 Spectroscopy Timeline 1885 Johann Balmer found the Balmer formula for H lines 1 =R H n 2 R H = m 1 Rydberg constant 1890's Pickering and his assistants cataloged thousands of stellar spectra, labeling them according to strength of hydrogen lines: A, B, Thomson discovered an electron 1901 Annie Jump Cannon placed the spectra in a temperature sequence 1911 Rutherford discovered an atomic nucleus
20 Spectroscopy Timeline 1913 Bohr develops his atomic model A physicist is just an atom's way of looking at itself. Niels Bohr
21
22 H line wavelengths according to Bohr Atomic Model 1 =R H 1 m 2 1 n 2 m=1, 2, 3,... n= m 1, m 2,... R H = e ħ 3 c = m 1 = m e m p m e m p reduced mass
23 Kirchhoff's Laws A hot, dense gas or solid object produces a continuous spectrum with no dark spectral lines. A hot, diffuse gas produces bright spectral lines (emission lines). A cool, diffuse gas in front of a source of continuous spectrum produces dark spectral lines (absorption lines) in the continuous spectrum.
24 The dependence of spectral line strength on temperature Credit & Copyright: C&O, figure 8.11
Astronomy 1 Winter 2011
 Astronomy 1 Winter 2011 Lecture 8; January 24 2011 Previously on Astro 1 Light as a wave The Kelvin Temperature scale What is a blackbody? Wien s law: λ max (in meters) = (0.0029 K m)/t. The Stefan-Boltzmann
Astronomy 1 Winter 2011 Lecture 8; January 24 2011 Previously on Astro 1 Light as a wave The Kelvin Temperature scale What is a blackbody? Wien s law: λ max (in meters) = (0.0029 K m)/t. The Stefan-Boltzmann
A Stellar Spectra 3. Stars shine at night (during the day too!). A star is a self-luminous sphere of gas. Stars are held together by gravity.
 Stellar Spectra Relativity and Astrophysics Lecture 12 Terry Herter Outline What is a star? Stellar Spectra Kirchhoff s Laws Spectral Classification Spectral Types: O B A F G K M L T Stellar Photometry
Stellar Spectra Relativity and Astrophysics Lecture 12 Terry Herter Outline What is a star? Stellar Spectra Kirchhoff s Laws Spectral Classification Spectral Types: O B A F G K M L T Stellar Photometry
Part I. Quantum Mechanics. 2. Is light a Wave or Particle. 3a. Electromagnetic Theory 1831 Michael Faraday proposes Electric and Magnetic Fields
 Quantized Radiation (Particle Theory of Light) Dr. Bill Pezzaglia Part I 1 Quantum Mechanics A. Classical vs Quantum Theory B. Black Body Radiation C. Photoelectric Effect 2 Updated: 2010Apr19 D. Atomic
Quantized Radiation (Particle Theory of Light) Dr. Bill Pezzaglia Part I 1 Quantum Mechanics A. Classical vs Quantum Theory B. Black Body Radiation C. Photoelectric Effect 2 Updated: 2010Apr19 D. Atomic
Chapter 4. Spectroscopy. Dr. Tariq Al-Abdullah
 Chapter 4 Spectroscopy Dr. Tariq Al-Abdullah Learning Goals: 4.1 Spectral Lines 4.2 Atoms and Radiation 4.3 Formation of the Spectral Lines 4.4 Molecules 4.5 Spectral Line Analysis 2 DR. T. AL-ABDULLAH
Chapter 4 Spectroscopy Dr. Tariq Al-Abdullah Learning Goals: 4.1 Spectral Lines 4.2 Atoms and Radiation 4.3 Formation of the Spectral Lines 4.4 Molecules 4.5 Spectral Line Analysis 2 DR. T. AL-ABDULLAH
LIGHT. Question. Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light.
 LIGHT Question Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light. What kind of information can we get from light? 1 Light
LIGHT Question Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light. What kind of information can we get from light? 1 Light
Today. Kirchoff s Laws. Emission and Absorption. Stellar Spectra & Composition. Doppler Effect & Motion. Extrasolar Planets
 Today Kirchoff s Laws Emission and Absorption Stellar Spectra & Composition Doppler Effect & Motion Extrasolar Planets Three basic types of spectra Continuous Spectrum Intensity Emission Line Spectrum
Today Kirchoff s Laws Emission and Absorption Stellar Spectra & Composition Doppler Effect & Motion Extrasolar Planets Three basic types of spectra Continuous Spectrum Intensity Emission Line Spectrum
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Sir Joseph John Thomson J. J. Thomson 1856-1940 Discovered the electron Did extensive work with cathode ray deflections 1906 Nobel Prize for discovery of electron Early Models
Chapter 28 Atomic Physics Sir Joseph John Thomson J. J. Thomson 1856-1940 Discovered the electron Did extensive work with cathode ray deflections 1906 Nobel Prize for discovery of electron Early Models
Stellar Astrophysics: The Classification of Stellar Spectra
 Stellar Astrophysics: The Classification of Stellar Spectra Temperature and Color The intensity of light emitted by three hypothetical stars is plotted against wavelength The range of visible wavelengths
Stellar Astrophysics: The Classification of Stellar Spectra Temperature and Color The intensity of light emitted by three hypothetical stars is plotted against wavelength The range of visible wavelengths
The History and Philosophy of Astronomy
 Astronomy 350L (Fall 2006) The History and Philosophy of Astronomy (Lecture 16: Birth of Astrophysics I) Instructor: Volker Bromm TA: Jarrett Johnson The University of Texas at Austin Big Q: What is the
Astronomy 350L (Fall 2006) The History and Philosophy of Astronomy (Lecture 16: Birth of Astrophysics I) Instructor: Volker Bromm TA: Jarrett Johnson The University of Texas at Austin Big Q: What is the
ASTR-1010: Astronomy I Course Notes Section IV
 ASTR-1010: Astronomy I Course Notes Section IV Dr. Donald G. Luttermoser Department of Physics and Astronomy East Tennessee State University Edition 2.0 Abstract These class notes are designed for use
ASTR-1010: Astronomy I Course Notes Section IV Dr. Donald G. Luttermoser Department of Physics and Astronomy East Tennessee State University Edition 2.0 Abstract These class notes are designed for use
Today. Spectra. Thermal Radiation. Wien s Law. Stefan-Boltzmann Law. Kirchoff s Laws. Emission and Absorption. Spectra & Composition
 Today Spectra Thermal Radiation Wien s Law Stefan-Boltzmann Law Kirchoff s Laws Emission and Absorption Spectra & Composition Spectrum Originally, the range of colors obtained by passing sunlight through
Today Spectra Thermal Radiation Wien s Law Stefan-Boltzmann Law Kirchoff s Laws Emission and Absorption Spectra & Composition Spectrum Originally, the range of colors obtained by passing sunlight through
Which property of a star would not change if we could observe it from twice as far away? a) Angular size b) Color c) Flux d) Parallax e) Proper Motion
 Exam #1 is in class next monday 25 multiple-choice questions 50 minutes Similar to questions asked in class Review sheet to be posted this week. We will have two 1-hour review sessions Friday 5-6pm (with
Exam #1 is in class next monday 25 multiple-choice questions 50 minutes Similar to questions asked in class Review sheet to be posted this week. We will have two 1-hour review sessions Friday 5-6pm (with
Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I
 Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I Purpose Investigate Kirchhoff s Laws for continuous, emission and absorption spectra Analyze the solar spectrum and identify unknown lines
Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I Purpose Investigate Kirchhoff s Laws for continuous, emission and absorption spectra Analyze the solar spectrum and identify unknown lines
10/31/2018. Chapter 7. Atoms Light and Spectra. Thursday Lab Announcement. Topics For Today s Class Black Body Radiation Laws
 Phys1411 Introductory Astronomy Instructor: Dr. Goderya Chapter 7 Atoms Light and Spectra Thursday Lab Announcement Jonah will start the Lab at 6:00 PM. Two pieces of Glass and HST Lunar Phases Topics
Phys1411 Introductory Astronomy Instructor: Dr. Goderya Chapter 7 Atoms Light and Spectra Thursday Lab Announcement Jonah will start the Lab at 6:00 PM. Two pieces of Glass and HST Lunar Phases Topics
The colors correspond to wavelength of the light, with longer wavelengths for the red light and shorter for
 Classifying Stellar Spectra Printable form Stellar spectra tell us the temperatures and the luminosities of the stars. In this exercise, you will be looking at the data that lead people to classify stars
Classifying Stellar Spectra Printable form Stellar spectra tell us the temperatures and the luminosities of the stars. In this exercise, you will be looking at the data that lead people to classify stars
3/26/2018. Atoms Light and Spectra. Topics For Today s Class. Reminder. Topics For Today s Class. The Atom. Phys1403 Stars and Galaxies
 Foundations of Astronomy 13e Seeds Foundations of Astronomy 13e Seeds Phys1403 Stars and Galaxies Instructor: Dr. Goderya Chapter 7 Atoms Light and Spectra Reminder Homework for Chapter 5, 6 and 7 is posted
Foundations of Astronomy 13e Seeds Foundations of Astronomy 13e Seeds Phys1403 Stars and Galaxies Instructor: Dr. Goderya Chapter 7 Atoms Light and Spectra Reminder Homework for Chapter 5, 6 and 7 is posted
Spectroscopy in Astronomy
 Spectroscopy in Astronomy History 1814 German optician Joseph von Fraunhofer sun with 600+ spectral lines; now we know more than 3000 lines 1860 German chemists Gustav Kirchhoff and Robert W. Bunsen Chemical
Spectroscopy in Astronomy History 1814 German optician Joseph von Fraunhofer sun with 600+ spectral lines; now we know more than 3000 lines 1860 German chemists Gustav Kirchhoff and Robert W. Bunsen Chemical
ASTRO Fall 2012 LAB #7: The Electromagnetic Spectrum
 ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
Review of Star Intro. PHYSICS 162 Lecture 7a 1
 Review of Star Intro Parallax - geometric method of determining star distance Absolute and apparent luminosity. Temperature Spectrum: What characterizes the star s surface Is related to its temperature
Review of Star Intro Parallax - geometric method of determining star distance Absolute and apparent luminosity. Temperature Spectrum: What characterizes the star s surface Is related to its temperature
Light and Atoms
 Light and Atoms ASTR 170 2010 S1 Daniel Zucker E7A 317 zucker@science.mq.edu.au ASTR170 Introductory Astronomy: II. Light and Atoms 1 Overview We ve looked at telescopes, spectrographs and spectra now
Light and Atoms ASTR 170 2010 S1 Daniel Zucker E7A 317 zucker@science.mq.edu.au ASTR170 Introductory Astronomy: II. Light and Atoms 1 Overview We ve looked at telescopes, spectrographs and spectra now
The Main Point. How do light and matter interact? Lecture #7: Radiation and Spectra II. How is light absorbed and emitted?
 Lecture #7: Radiation and Spectra II How is light absorbed and emitted? Models of Atomic Structure. Formation of Spectral Lines. Doppler Shift. Applications in Solar System Studies Detecting gaseous phases
Lecture #7: Radiation and Spectra II How is light absorbed and emitted? Models of Atomic Structure. Formation of Spectral Lines. Doppler Shift. Applications in Solar System Studies Detecting gaseous phases
Ohio University - Lancaster Campus slide 1 of 47 Spring 2009 PSC 100. A star s color, temperature, size, brightness and distance are all related!
 Ohio University - Lancaster Campus slide 1 of 47 A star s color, temperature, size, brightness and distance are all related! Ohio University - Lancaster Campus slide 2 of 47 The Beginnings Late 1800 s,
Ohio University - Lancaster Campus slide 1 of 47 A star s color, temperature, size, brightness and distance are all related! Ohio University - Lancaster Campus slide 2 of 47 The Beginnings Late 1800 s,
Everything that can be invented has been invented.
 Everything that can be invented has been invented. attributed to Charles H. Duell, Commissioner, U.S. patent office, 1899, in letter to President McKinley. 1835 philosopher Auguste Comte considered the
Everything that can be invented has been invented. attributed to Charles H. Duell, Commissioner, U.S. patent office, 1899, in letter to President McKinley. 1835 philosopher Auguste Comte considered the
Light & Matter Interactions
 Light & Matter Interactions. Spectral Lines. Kirchoff's Laws 2. Photons. Inside atoms 2. Classical Atoms 3. The Bohr Model 4. Lowest energy 5. Kirchoff's laws, again 3. Quantum Theory. de Broglie wavelength
Light & Matter Interactions. Spectral Lines. Kirchoff's Laws 2. Photons. Inside atoms 2. Classical Atoms 3. The Bohr Model 4. Lowest energy 5. Kirchoff's laws, again 3. Quantum Theory. de Broglie wavelength
Taking Fingerprints of Stars, Galaxies, and Other Stuff. The Bohr Atom. The Bohr Atom Model of Hydrogen atom. Bohr Atom. Bohr Atom
 Periodic Table of Elements Taking Fingerprints of Stars, Galaxies, and Other Stuff Absorption and Emission from Atoms, Ions, and Molecules Universe is mostly (97%) Hydrogen and Helium (H and He) The ONLY
Periodic Table of Elements Taking Fingerprints of Stars, Galaxies, and Other Stuff Absorption and Emission from Atoms, Ions, and Molecules Universe is mostly (97%) Hydrogen and Helium (H and He) The ONLY
Chapter 6. Atoms and Starlight
 Chapter 6 Atoms and Starlight What is light? Light is an electromagnetic wave. Wavelength and Frequency wavelength frequency = speed of light = constant Particles of Light Particles of light are called
Chapter 6 Atoms and Starlight What is light? Light is an electromagnetic wave. Wavelength and Frequency wavelength frequency = speed of light = constant Particles of Light Particles of light are called
Hertzsprung-Russell Diagram, Flux, Luminosity, Magnitude 10 Oct
 Russell Diagram, Flux, Luminosity, Magnitude 10 Oct Outline Review of 7 Oct Thermal radiation Wien s Law Stefan Boltzmann Law How to measure temperature of stars. AJ Cannon s method of classifying spectra.
Russell Diagram, Flux, Luminosity, Magnitude 10 Oct Outline Review of 7 Oct Thermal radiation Wien s Law Stefan Boltzmann Law How to measure temperature of stars. AJ Cannon s method of classifying spectra.
The Temperatures of Stars. Image credit: NOAO
 The Temperatures of Stars Image credit: NOAO Understanding Stars Starlight contains a lot of information. This information doesn t decay or expire as the light travels through space. By examining the light
The Temperatures of Stars Image credit: NOAO Understanding Stars Starlight contains a lot of information. This information doesn t decay or expire as the light travels through space. By examining the light
Introduction to Stellar Spectra
 Introduction to Stellar Spectra We are about to start down a long road as we search for the chemical composition of the stars. The journey won't be short, or easy, but along the way you'll meet a number
Introduction to Stellar Spectra We are about to start down a long road as we search for the chemical composition of the stars. The journey won't be short, or easy, but along the way you'll meet a number
Stars: some basic characteristics
 Stars: some basic characteristics Stars! How bright are they? How massive are they? What are the different types? How long do they live? How hot are they? Stellar brightness and luminosity The apparent
Stars: some basic characteristics Stars! How bright are they? How massive are they? What are the different types? How long do they live? How hot are they? Stellar brightness and luminosity The apparent
The Nature of Light. Chapter Five
 The Nature of Light Chapter Five Guiding Questions 1. How fast does light travel? How can this speed be measured? 2. Why do we think light is a wave? What kind of wave is it? 3. How is the light from an
The Nature of Light Chapter Five Guiding Questions 1. How fast does light travel? How can this speed be measured? 2. Why do we think light is a wave? What kind of wave is it? 3. How is the light from an
Pictures of Atoms. 48 iron atoms on copper Made with a scanning tunnelling microscope
 A-toms = Not Cutable Democritus 420BC popularized the theory matter was made of Atoms: Too small to be seen Indivisible Surrounded by a void Solid No internal structure Pictures of Atoms 48 iron atoms
A-toms = Not Cutable Democritus 420BC popularized the theory matter was made of Atoms: Too small to be seen Indivisible Surrounded by a void Solid No internal structure Pictures of Atoms 48 iron atoms
We now realize that the phenomena of chemical interactions, and, ultimately life itself, are to be understood in terms of electromagnetism".
 We now realize that the phenomena of chemical interactions, and, ultimately life itself, are to be understood in terms of electromagnetism". -Richard Feynman Quantum H Atom Review Radia Wave Function (1s):
We now realize that the phenomena of chemical interactions, and, ultimately life itself, are to be understood in terms of electromagnetism". -Richard Feynman Quantum H Atom Review Radia Wave Function (1s):
Taking fingerprints of stars, galaxies, and interstellar gas clouds
 - - Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules Periodic Table of Elements The universe is mostly hydrogen H and helium He
- - Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules Periodic Table of Elements The universe is mostly hydrogen H and helium He
AGA5802 Spectroscopy II Prism Gratings Applications
 AGA5802 Spectroscopy II Prism Gratings Applications Bibliography: To Measure the Sky, Kitchin, Lena and others... Prof. Jorge Meléndez 1 Slit Basic components of the Spectrograph Prism or grating Roy &
AGA5802 Spectroscopy II Prism Gratings Applications Bibliography: To Measure the Sky, Kitchin, Lena and others... Prof. Jorge Meléndez 1 Slit Basic components of the Spectrograph Prism or grating Roy &
AST 105 Intro Astronomy The Solar System. MIDTERM II: Tuesday, April 5 [covering Lectures 10 through 16]
![AST 105 Intro Astronomy The Solar System. MIDTERM II: Tuesday, April 5 [covering Lectures 10 through 16] AST 105 Intro Astronomy The Solar System. MIDTERM II: Tuesday, April 5 [covering Lectures 10 through 16]](/thumbs/78/77847540.jpg) AST 105 Intro Astronomy The Solar System MIDTERM II: Tuesday, April 5 [covering Lectures 10 through 16] REVIEW Light as Information Bearer We can separate light into its different wavelengths (spectrum).
AST 105 Intro Astronomy The Solar System MIDTERM II: Tuesday, April 5 [covering Lectures 10 through 16] REVIEW Light as Information Bearer We can separate light into its different wavelengths (spectrum).
I. Light & Spectra. I. Light and Spectra. 1. The Speed of Light. A. The Nature of LIght. b). Olaf Roemer s Experiment. a) Galileo couldn t meaure it
 Part II: Astrophysics 1 I. Light and Spectra 2 Dr. Bill Pezzaglia A. Nature of Light I. Light & Spectra B. Black Body Radiation C. Atomic Physics Updated: 2011Feb24 A. The Nature of LIght 3 1. The Speed
Part II: Astrophysics 1 I. Light and Spectra 2 Dr. Bill Pezzaglia A. Nature of Light I. Light & Spectra B. Black Body Radiation C. Atomic Physics Updated: 2011Feb24 A. The Nature of LIght 3 1. The Speed
Taking fingerprints of stars, galaxies, and interstellar gas clouds. Absorption and emission from atoms, ions, and molecules
 Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules 1 Periodic Table of Elements The universe is mostly hydrogen H and helium He
Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules 1 Periodic Table of Elements The universe is mostly hydrogen H and helium He
Topics Covered in Chapter. Light and Other Electromagnetic Radiation. A Subatomic Interlude II. A Subatomic Interlude. A Subatomic Interlude III
 Light and Other Electromagnetic Radiation Topics Covered in Chapter 1.Structure of Atoms 2.Origins of Electromagnetic Radiation 3.Objects with Different Temperature and their Electromagnetic Radiation
Light and Other Electromagnetic Radiation Topics Covered in Chapter 1.Structure of Atoms 2.Origins of Electromagnetic Radiation 3.Objects with Different Temperature and their Electromagnetic Radiation
Light and Other Electromagnetic Radiation
 Light and Other Electromagnetic Radiation 1 Topics Covered in Chapter 1.Structure of Atoms 2.Origins of Electromagnetic Radiation 3.Objects with Different Temperature and their Electromagnetic Radiation
Light and Other Electromagnetic Radiation 1 Topics Covered in Chapter 1.Structure of Atoms 2.Origins of Electromagnetic Radiation 3.Objects with Different Temperature and their Electromagnetic Radiation
Stellar Nucleosynthesis
 Stellar Nucleosynthesis What makes the sun shine? Gravita7onal contrac7on Chemical reac7ons Nucleosynthesis Stellar Nucleosynthesis The PP chain The CNO cycle The Triple alpha process and on to Fe Stellar
Stellar Nucleosynthesis What makes the sun shine? Gravita7onal contrac7on Chemical reac7ons Nucleosynthesis Stellar Nucleosynthesis The PP chain The CNO cycle The Triple alpha process and on to Fe Stellar
Photosphere. Bob Stein s simulation movie. Chromosphere. Corona. Solar wind
 Photosphere Layer from which light escapes directly into space. Photosphere is what we see. Light from lower layers scatters. Q: Suppose we observe the neutrinos from the sun. The size of the sun when
Photosphere Layer from which light escapes directly into space. Photosphere is what we see. Light from lower layers scatters. Q: Suppose we observe the neutrinos from the sun. The size of the sun when
Parallax: Space Observatories. Stars, Galaxies & the Universe Announcements. Stars, Galaxies & Universe Lecture #7 Outline
 Stars, Galaxies & the Universe Announcements HW#4: posted Thursday; due Monday (9/20) Reading Quiz on Ch. 16.5 Monday (9/20) Exam #1 (Next Wednesday 9/22) In class (50 minutes) first 20 minutes: review
Stars, Galaxies & the Universe Announcements HW#4: posted Thursday; due Monday (9/20) Reading Quiz on Ch. 16.5 Monday (9/20) Exam #1 (Next Wednesday 9/22) In class (50 minutes) first 20 minutes: review
Distances to the stars Friedrich Bessel Cygni 10 light years. Just beat Struve and Henderson who measured Vega and α Centauri respectively.
 Distances to the stars Friedrich Bessel 1838 61 Cygni 10 light years. Just beat Struve and Henderson who measured Vega and α Centauri respectively. Distances to the stars the technique p < 1arcsecond d
Distances to the stars Friedrich Bessel 1838 61 Cygni 10 light years. Just beat Struve and Henderson who measured Vega and α Centauri respectively. Distances to the stars the technique p < 1arcsecond d
Light or the Electromagnetic spectrum.
 Light or the Electromagnetic spectrum www.nasa.gov Diffraction and Light When passed through a prism or grating, light is separated into its component wavelengths This looks like a rainbow in visible light
Light or the Electromagnetic spectrum www.nasa.gov Diffraction and Light When passed through a prism or grating, light is separated into its component wavelengths This looks like a rainbow in visible light
Light III The Atom & Spectra. February 12, 2012
 Light III The Atom & Spectra February 12, 2012 Average: 65 20 Test 1 15 10 5 0 0-50 50-60 60-70 70-80 80-90 90-100 Takeaway Message: YOU NEED TO STUDY MORE you need to come to class EVERY DAY (TPS questions
Light III The Atom & Spectra February 12, 2012 Average: 65 20 Test 1 15 10 5 0 0-50 50-60 60-70 70-80 80-90 90-100 Takeaway Message: YOU NEED TO STUDY MORE you need to come to class EVERY DAY (TPS questions
General Physics (PHY 2140)
 General Physics (PHY 140) Lecture 33 Modern Physics Atomic Physics Atomic spectra Bohr s theory of hydrogen http://www.physics.wayne.edu/~apetrov/phy140/ Chapter 8 1 Lightning Review Last lecture: 1. Atomic
General Physics (PHY 140) Lecture 33 Modern Physics Atomic Physics Atomic spectra Bohr s theory of hydrogen http://www.physics.wayne.edu/~apetrov/phy140/ Chapter 8 1 Lightning Review Last lecture: 1. Atomic
The Physics of Light, part 2. Astronomy 111
 Lecture 7: The Physics of Light, part 2 Astronomy 111 Spectra Twinkle, twinkle, little star, How I wonder what you are. Every type of atom, ion, and molecule has a unique spectrum Ion: an atom with electrons
Lecture 7: The Physics of Light, part 2 Astronomy 111 Spectra Twinkle, twinkle, little star, How I wonder what you are. Every type of atom, ion, and molecule has a unique spectrum Ion: an atom with electrons
Light, Spectra, and Ma1er
 Light, Spectra, and Ma1er 1 Ideal Black Bodies Amount of light thermally radiated: j = σt 4 Where j = total intensity, T is temperature, and σ = 5.67 x 10-8 W m - 2 K - 4 (constant) Wavelength (nm) 2 SoluOon
Light, Spectra, and Ma1er 1 Ideal Black Bodies Amount of light thermally radiated: j = σt 4 Where j = total intensity, T is temperature, and σ = 5.67 x 10-8 W m - 2 K - 4 (constant) Wavelength (nm) 2 SoluOon
Lecture 16 Atomic Spectra Doppler Effect October 29, 2018
 Lecture 16 Atomic Spectra Doppler Effect October 29, 2018 1 4 Comet 96P/Machholz October 2017 Comet 96P does this every 5.24 years. It is a short period comet that experiences frequent blasts of solar
Lecture 16 Atomic Spectra Doppler Effect October 29, 2018 1 4 Comet 96P/Machholz October 2017 Comet 96P does this every 5.24 years. It is a short period comet that experiences frequent blasts of solar
Review: Properties of a wave
 Radiation travels as waves. Waves carry information and energy. Review: Properties of a wave wavelength (λ) crest amplitude (A) trough velocity (v) λ is a distance, so its units are m, cm, or mm, etc.
Radiation travels as waves. Waves carry information and energy. Review: Properties of a wave wavelength (λ) crest amplitude (A) trough velocity (v) λ is a distance, so its units are m, cm, or mm, etc.
Sun. Sirius. Tuesday, February 21, 2012
 Spectral Classification of Stars Sun Sirius Stellar Classification Spectral Lines H Fe Na H Ca H Spectral Classification of Stars Timeline: 1890s Edward C. Pickering (1846-1919) and Williamina P. Fleming
Spectral Classification of Stars Sun Sirius Stellar Classification Spectral Lines H Fe Na H Ca H Spectral Classification of Stars Timeline: 1890s Edward C. Pickering (1846-1919) and Williamina P. Fleming
Experiment 7: Spectrum of the Hydrogen Atom
 Experiment 7: Spectrum of the Hydrogen Nate Saffold nas2173@columbia.edu Office Hour: Mondays, 5:30-6:30PM INTRO TO EXPERIMENTAL PHYS-LAB 1493/1494/2699 Introduction The physics behind: The spectrum of
Experiment 7: Spectrum of the Hydrogen Nate Saffold nas2173@columbia.edu Office Hour: Mondays, 5:30-6:30PM INTRO TO EXPERIMENTAL PHYS-LAB 1493/1494/2699 Introduction The physics behind: The spectrum of
Stars - spectral types
 Stars - spectral types 1901: Led by Annie Jump Cannon, Harvard astronomers looked at the spectra of >200,000 stars. Classified them as A, B, C etc. Cannon rearranged them into OBAFGKM based on how lines
Stars - spectral types 1901: Led by Annie Jump Cannon, Harvard astronomers looked at the spectra of >200,000 stars. Classified them as A, B, C etc. Cannon rearranged them into OBAFGKM based on how lines
The Amazing Power of Starlight
 The Amazing Power of Starlight Chapter 6 Just by analyzing the light received from a star, astronomers can retrieve information about a star s Starlight and Atoms 1. Total energy output 2. Surface temperature
The Amazing Power of Starlight Chapter 6 Just by analyzing the light received from a star, astronomers can retrieve information about a star s Starlight and Atoms 1. Total energy output 2. Surface temperature
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
aka Light Properties of Light are simultaneously
 Today Interaction of Light with Matter Thermal Radiation Kirchhoff s Laws aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves).
Today Interaction of Light with Matter Thermal Radiation Kirchhoff s Laws aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves).
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 32 Modern Physics Atomic Physics Early models of the atom Atomic spectra http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 28 1 If you want to know your progress
General Physics (PHY 2140) Lecture 32 Modern Physics Atomic Physics Early models of the atom Atomic spectra http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 28 1 If you want to know your progress
15.1 Properties of Stars
 Surveying the Stars 15.1 Properties of Stars Our goals for learning: How do we measure stellar luminosities? How do we measure stellar temperatures? How do we measure stellar masses? How do we measure
Surveying the Stars 15.1 Properties of Stars Our goals for learning: How do we measure stellar luminosities? How do we measure stellar temperatures? How do we measure stellar masses? How do we measure
Light & Matter Interactions
 Light & Matter Interactions. Spectral Lines. Kirchoff's Laws 2. Inside atoms 3. Classical Atoms 4. The Bohr Model 5. Lowest energy 6. Kirchoff's laws, again 2. Quantum Theory. The Photoelectric Effect
Light & Matter Interactions. Spectral Lines. Kirchoff's Laws 2. Inside atoms 3. Classical Atoms 4. The Bohr Model 5. Lowest energy 6. Kirchoff's laws, again 2. Quantum Theory. The Photoelectric Effect
Lights. And God said, "Let there be light"; and there was light. And God saw that the light was good; (Bible: Genesis I)
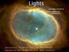 Lights Astronomy is based on observing lights from celestial bodies. And God said, "Let there be light"; and there was light. And God saw that the light was good; (Bible: Genesis I) Cat's Eye planetary
Lights Astronomy is based on observing lights from celestial bodies. And God said, "Let there be light"; and there was light. And God saw that the light was good; (Bible: Genesis I) Cat's Eye planetary
The Hertzprung-Russell Diagram. The Hertzprung-Russell Diagram. Question
 Key Concepts: Lecture 21: Measuring the properties of stars (cont.) The Hertzsprung-Russell (HR) Diagram (L versus T) The Hertzprung-Russell Diagram The Stefan-Boltzmann Law: flux emitted by a black body
Key Concepts: Lecture 21: Measuring the properties of stars (cont.) The Hertzsprung-Russell (HR) Diagram (L versus T) The Hertzprung-Russell Diagram The Stefan-Boltzmann Law: flux emitted by a black body
Models of the Atom. Spencer Clelland & Katelyn Mason
 Models of the Atom Spencer Clelland & Katelyn Mason First Things First Electrons were accepted to be part of the atom structure by scientists in the1900 s. The first model of the atom was visualized as
Models of the Atom Spencer Clelland & Katelyn Mason First Things First Electrons were accepted to be part of the atom structure by scientists in the1900 s. The first model of the atom was visualized as
[10] Spectroscopy (9/28/17)
![[10] Spectroscopy (9/28/17) [10] Spectroscopy (9/28/17)](/thumbs/85/91855577.jpg) 1 [10] Spectroscopy (9/28/17) Upcoming Items 1. Homework #5 due on Tuesday 2. Midterm #1 October 10 3. Read Ch. 6.2 & 6.3 by next class (skim the rest of Ch. 6). Do the selfstudy quizzes APOD 9/28/16 2
1 [10] Spectroscopy (9/28/17) Upcoming Items 1. Homework #5 due on Tuesday 2. Midterm #1 October 10 3. Read Ch. 6.2 & 6.3 by next class (skim the rest of Ch. 6). Do the selfstudy quizzes APOD 9/28/16 2
Electromagnetic waves
 Electromagnetic waves University of Pécs, Faculty of Medicines, Dept. Biophysics Scientists physicists, chemists, astronomers Sir Isaac Newton Sir William Herschel Johann Wilhelm Ritter Joseph von Fraunhofer
Electromagnetic waves University of Pécs, Faculty of Medicines, Dept. Biophysics Scientists physicists, chemists, astronomers Sir Isaac Newton Sir William Herschel Johann Wilhelm Ritter Joseph von Fraunhofer
Stellar Nucleosynthesis
 Stellar Nucleosynthesis What makes the sun shine? Gravita7onal contrac7on Chemical reac7ons Nucleosynthesis Stellar Nucleosynthesis The PP chain The CNO cycle The Triple alpha process and on to Fe Stellar
Stellar Nucleosynthesis What makes the sun shine? Gravita7onal contrac7on Chemical reac7ons Nucleosynthesis Stellar Nucleosynthesis The PP chain The CNO cycle The Triple alpha process and on to Fe Stellar
Family of stars. Fred Sarazin Physics Department, Colorado School of Mines. PHGN324: Family of stars
 Family of stars Reminder: the stellar magnitude scale In the 1900 s, the magnitude scale was defined as follows: a difference of 5 in magnitude corresponds to a change of a factor 100 in brightness. Dm
Family of stars Reminder: the stellar magnitude scale In the 1900 s, the magnitude scale was defined as follows: a difference of 5 in magnitude corresponds to a change of a factor 100 in brightness. Dm
Chapter 6. Quantum Theory and the Electronic Structure of Atoms Part 1
 Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Spectroscopy. Materials Diffraction grating Grating tube Spectrometer Incandescent light source
 Name: Date: Spectroscopy Hazards: The power supply used to run the lights is HIGH VOLTAGE. You should not need to change any tubes, but if you do please call the instructor over for assistance, and turn
Name: Date: Spectroscopy Hazards: The power supply used to run the lights is HIGH VOLTAGE. You should not need to change any tubes, but if you do please call the instructor over for assistance, and turn
Lecture 2. In this lecture we will go through the chronological development of the Atomic physics.
 Lecture 2 TITLE: A brief history of the development of structure of atom Page 1 Objectives In this lecture we will go through the chronological development of the Atomic physics. We will find out the thoughts
Lecture 2 TITLE: A brief history of the development of structure of atom Page 1 Objectives In this lecture we will go through the chronological development of the Atomic physics. We will find out the thoughts
The Hydrogen Spectrum
 1 The Hydrogen Spectrum PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In last week's laboratory experiment on diffraction, you should have noticed that the light from the mercury discharge
1 The Hydrogen Spectrum PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In last week's laboratory experiment on diffraction, you should have noticed that the light from the mercury discharge
2/28/2016 ATOMS) ATOMS. Physics 2D. PHYS 342 Modern Physics Atom I: Rutherford Model and Bohr Model
 PHYS 342 Modern Physics Atom I: Rutherford Model and Bohr Model Today Contents: a) Basic Properties of Atoms b) Rutherford Model and Scattering Experiments c) Bohr Model and Line Spectra Physics 2D Subfields
PHYS 342 Modern Physics Atom I: Rutherford Model and Bohr Model Today Contents: a) Basic Properties of Atoms b) Rutherford Model and Scattering Experiments c) Bohr Model and Line Spectra Physics 2D Subfields
Astrophysical Techniques. opt nd Year Astrophysics Group Research Project
 Astrophysical Techniques opt.292 2 nd Year Astrophysics Group Research Project Paul O Brien & Rhaana Starling For this lecture and lab handout see http://www2.star.le.ac.uk/pto/astrolab2.html Aim of this
Astrophysical Techniques opt.292 2 nd Year Astrophysics Group Research Project Paul O Brien & Rhaana Starling For this lecture and lab handout see http://www2.star.le.ac.uk/pto/astrolab2.html Aim of this
c = l Light: The Cosmic Messenger 1/23/18
 Reading for today s and Thur class: ASTR 1040 Stars & Galaxies SDO: Post-flare ejection from solar surface Prof. Juri Toomre TAs: Peri Johnson, Ryan Horton Lecture 3 Tues 23 Jan 2018 zeus.colorado.edu/astr1040-toomre
Reading for today s and Thur class: ASTR 1040 Stars & Galaxies SDO: Post-flare ejection from solar surface Prof. Juri Toomre TAs: Peri Johnson, Ryan Horton Lecture 3 Tues 23 Jan 2018 zeus.colorado.edu/astr1040-toomre
Physics 1C Lecture 29A. Finish off Ch. 28 Start Ch. 29
 Physics 1C Lecture 29A Finish off Ch. 28 Start Ch. 29 Particle in a Box Let s consider a particle confined to a one-dimensional region in space. Following the quantum mechanics approach, we need to find
Physics 1C Lecture 29A Finish off Ch. 28 Start Ch. 29 Particle in a Box Let s consider a particle confined to a one-dimensional region in space. Following the quantum mechanics approach, we need to find
Stars, Galaxies & the Universe Announcements. Stars, Galaxies & the Universe Observing Highlights. Stars, Galaxies & the Universe Lecture Outline
 Stars, Galaxies & the Universe Announcements Lab Observing Trip Next week: Tues (9/28) & Thurs (9/30) let me know ASAP if you have an official conflict (class, work) - website: http://astro.physics.uiowa.edu/~clang/sgu_fall10/observing_trip.html
Stars, Galaxies & the Universe Announcements Lab Observing Trip Next week: Tues (9/28) & Thurs (9/30) let me know ASAP if you have an official conflict (class, work) - website: http://astro.physics.uiowa.edu/~clang/sgu_fall10/observing_trip.html
Stars III The Hertzsprung-Russell Diagram
 Stars III The Hertzsprung-Russell Diagram Attendance Quiz Are you here today? (a) yes Here! (b) no (c) here is such a 90 s concept Today s Topics (first half) Spectral sequence and spectral types Spectral
Stars III The Hertzsprung-Russell Diagram Attendance Quiz Are you here today? (a) yes Here! (b) no (c) here is such a 90 s concept Today s Topics (first half) Spectral sequence and spectral types Spectral
Lab: Excited Electrons
 Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
! Finish Ch. 4.! Start Chapter 10: The Sun.! Homework Due: Oct. 10
 ! Finish Ch. 4! Start Chapter 10: The Sun! Homework Due: Oct. 10 A Spectral Mystery Mystery: Why do so many stars have an absorption spectrum (with certain colors missing)? The atoms that make up these
! Finish Ch. 4! Start Chapter 10: The Sun! Homework Due: Oct. 10 A Spectral Mystery Mystery: Why do so many stars have an absorption spectrum (with certain colors missing)? The atoms that make up these
The Duality of Light. Electromagnetic Radiation. Light as a Wave
 In this unit, you will be introduced to the dual nature of light, the quantum theory and Bohr s planetary atomic model. The planetary model was an improvement on the nuclear model and attempted to answer
In this unit, you will be introduced to the dual nature of light, the quantum theory and Bohr s planetary atomic model. The planetary model was an improvement on the nuclear model and attempted to answer
EVOLUTION OF STARS HERTZSPRUNG-RUSSELL DIAGRAM
 VISUAL PHYSICS ONLINE EVOLUTION OF STARS HERTZSPRUNG-RUSSELL DIAGRAM The total power radiated by a star is called its intrinsic luminosity L (luminosity). The apparent brightness (apparent luminosity)
VISUAL PHYSICS ONLINE EVOLUTION OF STARS HERTZSPRUNG-RUSSELL DIAGRAM The total power radiated by a star is called its intrinsic luminosity L (luminosity). The apparent brightness (apparent luminosity)
What is LIGHT? Reading Question
 Reading Question What is LIGHT? A. Light is a wave, like sound only much faster. B. Light is like little particles. Each one is a photon. C. Light is the absence of dark. D. A kind of energy we model with
Reading Question What is LIGHT? A. Light is a wave, like sound only much faster. B. Light is like little particles. Each one is a photon. C. Light is the absence of dark. D. A kind of energy we model with
Next quiz: Monday, October 24
 No homework for Wednesday Read Chapter 8! Next quiz: Monday, October 24 1 Chapter 7 Atoms and Starlight Types of Spectra: Pictorial Some light sources are comprised of all colors (white light). Other light
No homework for Wednesday Read Chapter 8! Next quiz: Monday, October 24 1 Chapter 7 Atoms and Starlight Types of Spectra: Pictorial Some light sources are comprised of all colors (white light). Other light
Review: Light and Spectra. Absorption and Emission Lines
 1 Review: Light and Spectra Light is a wave It undergoes diffraction and other wave phenomena. But light also is made of particles Energy is carried by photons 1 Wavelength energy of each photon Computer
1 Review: Light and Spectra Light is a wave It undergoes diffraction and other wave phenomena. But light also is made of particles Energy is carried by photons 1 Wavelength energy of each photon Computer
Lecture #8. Light-matter interaction. Kirchoff s laws
 1 Lecture #8 Light-matter interaction Kirchoff s laws 2 Line emission/absorption Atoms: release and absorb photons with a predefined set of energies (discrete). The number of protons determine the chemical
1 Lecture #8 Light-matter interaction Kirchoff s laws 2 Line emission/absorption Atoms: release and absorb photons with a predefined set of energies (discrete). The number of protons determine the chemical
Astronomy 122. Lunar Eclipse. Make sure to pick up a grating from Emily! You need to give them back after class.
 Astronomy 122 Make sure to pick up a grating from Emily! You need to give them back after class. This Class (Lecture 11): Twinkle, Twinkle, Little Star Next Class: Stellar Evolution: The Main Sequence
Astronomy 122 Make sure to pick up a grating from Emily! You need to give them back after class. This Class (Lecture 11): Twinkle, Twinkle, Little Star Next Class: Stellar Evolution: The Main Sequence
= λ. Light: The Cosmic Messenger. Continuing Topics for Today 1/24/17. Your account on Mastering Astronomy. ASTR 1040 Stars & Galaxies
 REMINDER Your account on Mastering Astronomy ASTR 1040 Stars & Galaxies SDO: Post-flare ejection from solar surface Prof. Juri Toomre TAs: Piyush Agrawal, Connor Bice Lecture 3 Tues 24 Jan 2017 zeus.colorado.edu/astr1040-toomre
REMINDER Your account on Mastering Astronomy ASTR 1040 Stars & Galaxies SDO: Post-flare ejection from solar surface Prof. Juri Toomre TAs: Piyush Agrawal, Connor Bice Lecture 3 Tues 24 Jan 2017 zeus.colorado.edu/astr1040-toomre
Astronomy The Nature of Light
 Astronomy The Nature of Light A. Dayle Hancock adhancock@wm.edu Small 239 Office hours: MTWR 10-11am Measuring the speed of light Light is an electromagnetic wave The relationship between Light and temperature
Astronomy The Nature of Light A. Dayle Hancock adhancock@wm.edu Small 239 Office hours: MTWR 10-11am Measuring the speed of light Light is an electromagnetic wave The relationship between Light and temperature
Chapter 38. The End of Classical Physics
 Chapter 38. The End of Classical Physics Studies of the light emitted by gas discharge tubes helped bring classical physics to an end. Chapter Goal: To understand how scientists discovered the properties
Chapter 38. The End of Classical Physics Studies of the light emitted by gas discharge tubes helped bring classical physics to an end. Chapter Goal: To understand how scientists discovered the properties
History of the Atomic Model
 Chapter 5 Lecture Chapter 5 Electronic Structure and Periodic Trends 5.1 Electromagnetic Radiation Learning Goal Compare the wavelength, frequency, and energy of electromagnetic radiation. Fifth Edition
Chapter 5 Lecture Chapter 5 Electronic Structure and Periodic Trends 5.1 Electromagnetic Radiation Learning Goal Compare the wavelength, frequency, and energy of electromagnetic radiation. Fifth Edition
10/29/2018. Chapter 7. Atoms Light and Spectra. Reminders. Topics For Today s Class. Hydrogen Atom. The Atom. Phys1411 Introductory Astronomy
 Phys1411 Introductory Astronomy Instructor: Dr. Goderya Chapter 7 Atoms Light and Spectra Reminders Topics For Today s Class Project 1 due November 12 th after and during Lab. Extra-credit Homework online.
Phys1411 Introductory Astronomy Instructor: Dr. Goderya Chapter 7 Atoms Light and Spectra Reminders Topics For Today s Class Project 1 due November 12 th after and during Lab. Extra-credit Homework online.
Optical/IR Observational Astronomy Spectroscopy. David Buckley, SALT
 David Buckley, SALT 1 Background is really just monochromatic photometry History 1637 Descartes explained the origin of the rainbow. 1666 Newton s classic experiments on the nature of colour. 1752 Melvil
David Buckley, SALT 1 Background is really just monochromatic photometry History 1637 Descartes explained the origin of the rainbow. 1666 Newton s classic experiments on the nature of colour. 1752 Melvil
Observing Habitable Environments Light & Radiation
 Homework 1 Due Thurs 1/14 Observing Habitable Environments Light & Radiation Given what we know about the origin of life on Earth, how would you recognize life on another world? Would this require a physical
Homework 1 Due Thurs 1/14 Observing Habitable Environments Light & Radiation Given what we know about the origin of life on Earth, how would you recognize life on another world? Would this require a physical
Discussion. Summary Clicker -- Solar Wind. What are effects of solar activity on our technological society? A. Auroral
 ASTR 1040 Accel Astro: Stars & Galaxies Prof. Juri Toomre TA: Nicholas Nelson Lecture 10 Thur 10 Feb 11 zeus.colorado.edu/astr1040-toomre toomre Planetary Nebula NGC 3132 Today What can we measure in other
ASTR 1040 Accel Astro: Stars & Galaxies Prof. Juri Toomre TA: Nicholas Nelson Lecture 10 Thur 10 Feb 11 zeus.colorado.edu/astr1040-toomre toomre Planetary Nebula NGC 3132 Today What can we measure in other
Physics 1C. Lecture 28D
 Physics 1C Lecture 28D "I ask you to look both ways. For the road to a knowledge of the stars leads through the atom; and important knowledge of the atom has been reached through the stars." --Sir Arthur
Physics 1C Lecture 28D "I ask you to look both ways. For the road to a knowledge of the stars leads through the atom; and important knowledge of the atom has been reached through the stars." --Sir Arthur
P O G I L E L E C T R O N E N E R G Y A N D L I G H T
 South Pasadena Honors Chemistry Name 9 Atomic Structure Period Date Why? P O G I L E L E C T R O N E N E R G Y A N D L I G H T How does light reveal the behavior of electrons in an atom? From fireworks
South Pasadena Honors Chemistry Name 9 Atomic Structure Period Date Why? P O G I L E L E C T R O N E N E R G Y A N D L I G H T How does light reveal the behavior of electrons in an atom? From fireworks
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Hertzsprung-Russell Diagram 7 Oct
 Hertzsprung-Russell Diagram 7 Oct Outline Thermal radiation Wien s Law Stefan Boltzmann Law Hertzsprung Russell diagram There are 3 types of stars: main sequence or dwarfs, giants, white dwarfs Missouri
Hertzsprung-Russell Diagram 7 Oct Outline Thermal radiation Wien s Law Stefan Boltzmann Law Hertzsprung Russell diagram There are 3 types of stars: main sequence or dwarfs, giants, white dwarfs Missouri
AST 301, Lecture 2. James Lattimer. Department of Physics & Astronomy 449 ESS Bldg. Stony Brook University. January 29, 2019
 AST 301, Lecture 2 James Lattimer Department of Physics & Astronomy 449 ESS Bldg. Stony Brook University January 29, 2019 Cosmic Catastrophes (AKA Collisions) james.lattimer@stonybrook.edu Properties of
AST 301, Lecture 2 James Lattimer Department of Physics & Astronomy 449 ESS Bldg. Stony Brook University January 29, 2019 Cosmic Catastrophes (AKA Collisions) james.lattimer@stonybrook.edu Properties of
