PHYSICAL REVIEW B VOLUME 59, NUMBER 13
|
|
|
- Tabitha Terry
- 5 years ago
- Views:
Transcription
1 PHYSICAL REVIEW B VOLUME 59, NUMBER 13 1 APRIL 1999-I Orbital contribution to the 3d magnetic moment in ferromagnetic Cu 3 Au-type transition-metal-pt alloys probed by soft-x-ray magnetic circular dichroism S. Imada, T. Muro, T. Shishidou,* and S. Suga Division of Material Physics, Graduate School of Engineering Science, Osaka University, 1-3, Machikaneyama, Toyonaka , Japan H. Maruyama, K. Kobayashi, and H. Yamazaki Faculty of Science, Okayama University, Tsushimanaka, Okayama , Japan T. Kanomata Faculty of Engineering, Tohoku Gakuin University, Chuo, Tagajo , Japan Received 14 October 1998 Magnetic circular dichroism in the soft-x-ray absorption spectrum in the 2p 3d excitation region of the transition-metal element is measured for ferromagnetic Cu 3 Au-type alloys, MnPt 3, Fe 3 Pt and CoPt 3. The electronic state, especially the contribution of the spin and orbital angular momenta to the magnetic moment, is discussed by analyzing the results. The condition for the orbital angular momentum to make a large contribution to the magnetic moment in a metallic system is discussed in terms of the degree of the localization of the 3d orbital, the filling of it, and the symmetry around it. S I. INTRODUCTION 3d transition metals and Pt make binary alloys in a wide range of concentration ratios and these alloys have various magnetic properties. At stoichiometric compositions, there often exist ordered phases. When the transition metal represented by T) is one of the elements among Mn through Ni, ordered TPt has CuAuI-type or L1 0 ) structure and TPt 3 and T 3 Pt have Cu 3 Au-type or L2 1 ) structure. 1,2 Among the Cu 3 Au-type ordered alloys with T Mn Ni, ferromagnetism is found for MnPt 3, 3 Fe 3 Pt, 4 CoPt 3, 5 and Ni 3 Pt. This series is quite interesting in at least two points. The first point is the contrast between TPt 3 and T 3 Pt, namely that the 3d electron of the transition metal is expected to be relatively localized in the former and itinerant in the latter. The second point is the change of the filling of the 3d orbital from T Mn to Ni. From a view point of application, magneto-optical effect and magnetic anisotropy have been studied for films of MnPt 3 and CoPt 3. 6,7 On the other hand, Fe 3 Pt is known to show the Invar effect. 8 Magnetic circular dichroism MCD of core-level absorption spectrum XAS, namely the difference of the spectra between parallel and antiparallel geometries of the sample magnetization and the photon spin of the incident circularly polarized light, has recently been used for the study of ferromagnetic materials. XAS is not only an element-specific method but it also yields information about a specific orbital, to which the core electron is strongly excited due to the selection rule and the transition probability. For the transition metal, the 3d orbital, which is the main origin of the magnetic moment, can be probed by 2p and 3p XAS. The origin of the MCD in XAS can be summarized as follows. 9 First, a circularly polarized photon selectively excites an electron with an orbital angular momentum parallel antiparallel to the photon spin when the azimuthal quantum number l is increased decreased by the photoexcitation. Second, the non-s core level has, in addition, spin-orbit interaction, which aligns the electron s spin and orbital angular momenta parallel or antiparallel to each other. Now, in a ferromagnetic material, a valence orbital has a magnetic moment, which means that the occupation of the orbital is polarized in spin and/or orbital angular momenta. The photoabsorption transition can occur only when the state to which the electron is excited is empty. The unbalance of the occupation of the valence orbital in spin and orbital angular momenta is reflected in the dependence of the absorption intensity on whether the photon spin is parallel or antiparallel to the magnetization. XAS-MCD for a core level with a large spin-orbit splitting, for example, the 2 p level of the transition-metal elements, is particularly a good tool since, in such a case, the circularly polarized photon select both orbital angular momentum and spin of the core electron. The two sum rules known as the l z sum rule 10 and the s z sum rule 11 make use of these features of MCD and yield l z and s z of the valence orbital by integrating the XAS with each circular polarization. The s z sum rule can only be used when spin-orbit splitting of the core level is large. In T-Pt alloys, magnetic moments are carried mainly by T 3d electrons but the contribution of the Pt 5d electrons is not negligible. Spin and orbital angular momenta of the Pt 5d orbital were studied using the MCD in Pt 2p 5d XAS Ref. 12 and Pt 4 f 5d XAS. 13 In this paper, we report MCD in T 2p 3d XAS of MnPt 3,Fe 3 Pt, and CoPt 3. Our aim is to investigate the electronic and magnetic state of the T 3d orbital. By using the sum rules, contributions of the spin and orbital angular momenta to the magnetic moment are estimated. We discuss what determines the degree to which the orbital angular mo /99/59 13 / /$15.00 PRB The American Physical Society
2 PRB ORBITAL CONTRIBUTION TO THE 3d MAGNETIC... mentum contributes to the magnetic moment in a metallic system, and apply this argument to the present systems. II. EXPERIMENT The MnPt 3,Fe 3 Pt, and CoPt 3 samples were prepared by the arc-melting method under an Ar atmosphere. The Cu 3 Au ordered phase was realized by a suitable thermal treatment and was verified by x-ray powder diffraction. The measurements of the MCD in the 2p XAS were performed at the beam-line NE-1B of the Accumulation Ring of the Photon Factory in the High Energy Accelerator Research Organization in Tsukuba, Japan. The clean surface of the sample was obtained by scraping in situ in an ultrahigh vacuum of about Torr. The sample was cooled to below 120 K using a liquid-nitrogen cryostat. The circularly polarized soft x-ray from the helical undulator was monochromatized and incident normally on the sample. The degree of circular polarization was 95 2% according to the calculation. XAS was obtained using the total photoelectron yield method by measuring the sample current and normalizing it by the photon current. The photon current was defined by the total photoelectron yield of the gold mesh just before the measurement chamber. A magnetic field of 1.1 T was applied to the sample during the measurement by a permanent magnet. The magnetic field on the sample was reversed by setting on the optical axis one of the two permanent dipole magnets whose magnetic field is opposite to each other. The magnetic field was either parallel or antiparallel to the photon s k vector Faraday geometry. Magnetization was reversed at each photon energy to cancel a possible drift or fluctuation of the photon current and other factors. III. RESULTS AND DISCUSSIONS In Figs. 1 a 1 c, the observed 2p XAS for the magnetization parallel to the photon spin defined as the I spectrum and that for magnetization antiparallel to the photon spin (I ), and their difference spectrum (I MCD I I ) are shown for MnPt 3 Fig. 1 a,fe 3 Pt Fig. 1 b, and CoPt 3 Fig. 1 c. We note that, by taking the magnetization direction as the quantization axis, the negative spin corresponds to the majority spin in this paper. Each spectrum has a twopeak structure due to the spin-orbit splitting of the 2p core level into j 3/2(2p 3/2 ) and j 1/2(2p 1/2 ) sublevels. The two main peaks mainly represent the 2p 3/2 3d and 2p 1/2 3d excitations. The origin of the relative photon energy was set at the boundary between the 2p 3/2 and 2p 1/2 regions (2p 1/2 onset. The boundary was determined as the energy where the energy derivative of the absorption (di/d ) rises abruptly. I was multiplied by a constant 1 so that I and I have the same intensity in the lowest photon energy region where 2p absorption should be absent. was less than A. Line shape The overall MCD features seen for the three compounds, namely positive MCD in the 2p 3/2 region and negative MCD in the 2p 1/2 region, are the common features for the MCD of the T 2p core in ferromagnets as long as the magnetic moment of the T is in the same direction as the net magnetization. The origin of these features is that the 3d orbitals with negative spin is more occupied than the positive spin. For example, in the 2p 3/2 region, the 2p electron with positive negative spin is mainly excited to the 3d orbital in I (I ) due to the selection rule of the circularly polarized light and the spin-orbit interaction of the 2 p core level. Since transition to an occupied state is forbidden, I is smaller than I in the 2p 3/2 region. Although multiplet structures are much less conspicuous than in ionic compounds, we can still see some remaining structures due to the intra-atomic electron-electron interaction in the final state of the x-ray absorption. In MnPt 3, for example, structures in both XAS and MCD around 7.5 and 4.5 ev in the relative photon energy resemble the structures found in the calculation for Mn 2 (3d 5 ) assuming a moderate crystal-field splitting (10Dq 1 ev). 14 It might seem strange that multiplet structure is quite prominent only in MnPt 3. For Fe 3 Pt and CoPt 3, however, MCD spectra approach zero much more rapidly than the XAS spectra in the higher photon energy region of 2p 3/2. This is not the case in the 2p 1/2 absorption region where both MCD and XAS equally have long tails on the higher photon energy side. These features are similar to those seen in the atomic calculation of MCD for high-spin d 6 and d 7 initial state without considering the 3d spin-orbit interaction (z 0 and 1 in Ref. 14, where MCD changes sign from positive to negative in the higher photon energy region of 2p 3/2 while MCD is monotonously negative for 2p 1/2. From these investigations, we can infer that the long tails seen in the 2p absorption of the metallic transition-metal compounds originate at least partially from the multiplet splitting of the 2p 5 3d n 1 states due to the electron-electron interaction. B. Sum-rule analysis In order to estimate quantitatively the contribution of the spin and orbital angular momenta to the magnetic moment of the transition metal, we analyze the spectra by using the sum rules. 10,11 In the present case of 2p 3d XAS, the l z sum rule can be written as l z 2 d I I d I I I 0, where l z is the average of the magnetic quantum number of the orbital angular momentum per 3d hole. I 0 is the XAS with the linearly polarized light whose polarization vector is parallel to the magnetization. The integration is taken over the whole 2p absorption region. The s z sum rule is written as s z 7 2 t z 1 3 j 3/2 d I I 2 j 1/2 d I I, 2 2 d I I I 0 where t z is the z component of the magnetic dipole operator t s 3r(r s)/ r 2. The integration j 3/2 ( j 1/2 ) is taken only over the 2p 3/2 (2p 1/2 ) absorption region. By dividing Eq. 1 with Eq. 2, we obtain
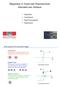
3 8754 S. IMADA et al. PRB 59 FIG. 1. a c Upper panels: 2p core-level absorption spectra of Cu 3 Au-type transition-metal-pt ordered alloys for circularly polarized light with photon spin parallel (I ) and antiparallel (I ) to the magnetization. The background used in the integration is given by the thin solid line. Lower panels: MCD spectra defined by I MCD I I. d f I d and I d : Integration of I and I after subtracting the background. l z and s z : Dependence of the orbital and spin angular momenta per 3d hole estimated by the sum rules on the upper energy limit of the integration of the 2p 1/2 region. In the case of MnPt 3, l z and s z are extrapolated by dashed smooth curves. l z s z 7/2 t z 4 3 d I I j 3/2 d I I 2 j 1/2 d I I. 3 We should note that this equation is quite efficient. This equation stands even if the circular polarization of the light is not perfect in I and I so long as the degree of polarization is the same for both I and I. This equation also stands even if the magnetization of the sample is not saturated. Another advantage of this equation is that it is defined only by the difference spectrum of MCD. It is not affected by how the background of XAS is assumed, while Eqs. 1 and 2 do depend on the background. In order to use Eqs. 1 and 2, we should subtract back-
4 PRB ORBITAL CONTRIBUTION TO THE 3d MAGNETIC... grounds from I and I to obtain intensities of pure 2p 3d photoabsorption. We assume an empirical background that has a step at 2p 3/2 and 2p 1/2 absorption peaks and otherwise is a linear line as shown in Fig. 1. The increase of the absorption is due to the transition except for the 2p 3d transition, e.g., the 2p continuum state is taken into account by the step at the absorption peak. The height ratio between the step for the 2p 3/2 peak and that for 2p 1/2 is assumed to be 2:1,which is just the ratio of the degeneracy of the core hole state. For Fe 3 Pt and CoPt 3, the slope of the background was determined so that the background agrees the absorption in the highest photon energy region. For MnPt 3, whose high-energy part could not be measured due to the lack of beam time, a comparable background was assumed. The boundary between the 2p 3/2 and 2p 1/2 regions was determined and set to the origin of the photon energy as described in the beginning of Sec. III. We also have to determine the point where 2p 3/2 absorption starts. This point was defined as the point where the absorption coincides with the background, namely, 12.9, 14.3, and 16.1 ev, for MnPt 3,Fe 3 Pt, and CoPt 3, respectively. In Figs. 1 d 1 f are shown the integrated values of the absorption after subtraction of the background, I d and I d, as functions of the upper energy limit to which the energy integrations are performed. s z and l z calculated by using these values, or in other words, obtained s z and l z values as functions of the upper energy limit of the integration of the 2p 1/2 region, are also shown. Here, we omit t z for simplicity. Although it has been shown that t z is not negligible in solids, 15 analysis including t z needs band-structure calculation and simulation of the MCD spectrum, which is out of our scope. We will come back to this point in Sec. III D. In the case of MnPt 3, the integration region dependences of s z and l z are extrapolated to higher energies by the use of the smooth dashed curves in Fig. 1 d. In Fig. 2 a, we present the values of l z /( l z 2 s z ), namely the portion of the contribution of the orbital angular momentum in the total magnetic moment of the transition-metal 3d electrons, as functions of the upper energy limit of the integration of the 2p 1/2 absorption region. We note that this quantity has a profound physical meaning. Being a function of l z / s z it is calculated using Eq. 3 and depends only on the MCD spectrum, I I, and does not depend on how we assume the background of the XAS. We also note that this quantity is not affected by the degree of the polarization of the light and the degree of saturation of the magnetization, both of which suppress l z and s z. Hence, we concentrate our discussion on the compound dependence of l z /( l z 2 s z ). Teramura et al. 16 argued the effect of the overlap between 2p 3/2 and 2p 1/2 final states on the s z sum rule. According to their results, the s z sum rule underestimates s z by a factor which depends mainly upon the 3d electron number. The factor of the underestimation is reported to be 0.68, 0.88, 0.92, and 0.92 for 3d electron numbers of 5, 6, 7, and 8, respectively. According to the band-structure calculations by Shirai, 17 occupation of the 3d electronic state, i.e., the 3d electron number divided by the number of the 3d states within the muffin-tin sphere, is 55% for MnPt 3 and 76% for FIG. 2. a The contribution of the orbital angular momentum to the magnetic moment of the 3d electrons estimated by the sum rule as a function of the upper energy limit of the integration of the 2p 1/2 region. b As for a but after the correction of the underestimation of s z due to the overlap between 2p 3/2 and 2p 1/2 regions. s z is assumed to be underestimated by a factor of 0.78, 0.90, and 0.92 in the case of MnPt 3,Fe 3 Pt, and CoPt 3. CoPt 3. We therefore assume that the 3d electron number is 5.5 and 7.6 for MnPt 3 and CoPt 3, respectively. We furthermore assume that it is 6.5 for Fe 3 Pt. By linearly interpolating the values obtained by Teramura et al. we consider the factor of underestimation of s z is 0.78, 0.90, and 0.92 for MnPt 3, Fe 3 Pt, and CoPt 3, respectively. Figure 2 b shows the dependence of the values of l z /( l z 2 s z ) on the integration region after correcting the s z value by dividing it with the factors above. The integration region dependence of s z and l z in Figs. 1 d 1 f indicates that convergence, especially of l z, is quite slow. The value of l z /( l z 2 s z ), therefore, crucially depends upon the integration region. If we take this into account, it is desirable to take as wide an integration region as possible. However, doing so would integrate the possible errors in the normalization and the background. Although l z /( l z 2 s z ) is free from error in the background, it is not free from that in the before-mentioned normalization between I and I. In order to estimate the l z /( l z 2 s z ) value, the integration should hence be cut at some appropriate point. If we set this point to 25 ev above the 2p 1/2 onset and by using the corrected values in Fig. 2 b, l z /( l z 2 s z ) is 0.044, 0.031, and for the transition-metal 3d holes in MnPt 3,Fe 3 Pt, and CoPt 3, respectively. The values of l z /( l z 2 s z ) obtained in this way are not accurate in an absolute sense. However, it is quite meaningful to compare them between different systems.
5 8756 S. IMADA et al. PRB 59 C. What determines the strength of the orbital angular momentum Now, we discuss what determines the degree to which the orbital angular momentum is induced in metallic systems. We treat this issue from the following three points. One is the competition between itinerancy and localization of the 3d electron. Another is the occupancy of the 3d partial density of states, and the other is the symmetry of the system around the T site. First, we consider the case where the system can be assumed to be spherically symmetric around the T atom. In this case, all the 3d orbitals, for example the e g and t 2g, are equivalent to each other. The 3d partial density of states DOS of such a ferromagnet may be represented by a model DOS in the upper panel of Fig. 3 a in the absence of the spin-orbit interaction. Since we are taking the direction of the magnetic field as the quantization axis in this paper, corresponds to the majority spin and to the minority spin. When the spin-orbit interaction is switched on, each majority- and minority-spin partial DOS would split into subbands with a different magnetic quantum number of the orbital angular momentum, l z. Here, we have assumed that the spin is still a good quantum number in the presence of the spin-orbit interaction. In other words, we only consider the l z s z term. This approximation can be justified when the exchange interaction is large enough so that the l s and l s terms that mix the different spin state are negligible. The energy shift of the l z subband would be l z s z where is the spin-orbit interaction constant of the 3d orbital which takes a positive value. The resultant shift of the subbands is illustrated in the lower panel of Fig. 3 a, where the subbands with positive negative l z are represented by the thickest thinnest lines. The consequence of the present shift would be as follows. When the 3d partial DOS is less than half-filled, l z subbands with positive l z and negative majority spin are more occupied than the negative l z bands. Hence, orbital angular momentum with antiparallel direction as the spin is induced in this case. When the 3d partial DOS is nearly half-filled, the positive l z induced in the majority-spin band is cancelled by the negative l z induced in the minority-spin band, which leads to very small orbital angular momentum. When the 3d partial DOS is more than half-filled, negative l z is induced and hence the orbital angular momentum is parallel to the spin. Now, what happens when the degree of itinerancy, and hence the bandwidth, is changed in the present case? The energy splitting due to the spin-orbit coupling is not likely to depend much upon the bandwidth. Therefore, the induced orbital angular momentum would be larger for the narrower band which has larger DOS. In other words, orbital angular momentum is more induced in a system where 3d electrons are more localized. Next, we should consider the case where the symmetry around the T atom is lower than the spherical symmetry. The 3d orbitals are no longer equivalent to each other. Here, we consider as an example that the system is octahedral or tetrahedral around the T atom. The partial density of states of t 2g and that of e g are then different from each other. FIG. 3. Model 3d partial density of states DOS for a a spherically symmetric case, b a case where the symmetry around the transition-metal atom causes the bandwidth of the partial DOS to depend upon l z, and c a case where the symmetry causes a splitting of the partial DOS into subbands. In each case, the upper panel shows the partial DOS without the spin-orbit interaction. The lower panel shows those with the spin-orbit interaction, where the thickest thinnest line corresponds to positive negative l z. In order to discuss the effect of the spin-orbit interaction on such a system, we should recall how the t 2g and e g 3d states are expressed in terms of the eigenfunction of the magnetic quantum number l z, namely the spherical harmonics Y dlz. The three t 2g states are expressed as yz (i/ 2)(Y d1 Y d 1 ), zx (1/ 2)(Y d1 Y d 1 ), and xy (i/ 2)(Y d2 Y d 2 ). The two e g states are expressed as 3z 2 r 2 Y d0 and x 2 y 2 (1/ 2)(Y d2 Y d 2 ). Let us consider the mixing between the 3d and the surrounding orbitals. Either of the t 2g or e g states is mixed more strongly with the surroundings and hence become more itin-
6 PRB ORBITAL CONTRIBUTION TO THE 3d MAGNETIC... erant, depending upon the crystal structure. If the t 2g orbital is more itinerant than the e g orbital, for example, the l z 1 states are the most itinerant since these states consist purely of t 2g states. l z 2, which are linear combinations of the t 2g and e g states, are the next and l z 0 is the most localized. This is schematically shown in the upper panel of Fig. 3 b. When the spin-orbit interaction is switched on, these bands split by energies proportional to l z as shown in the lower panel of Fig. 3 b. In this case, the contribution to the orbital angular momentum comes mostly from l z 2, since the spin-orbit splitting of these states is stronger and the band width is narrower than l z 1. When the ionicity of the system is stronger, the center of gravity of the t 2g and e g partial DOS would be quite different. The upper panel of Fig. 3 c illustrates the partial DOS for a case where the energy of the e g band is significantly lower than that of the t 2g band as when the T atom is tetrahedrally surrounded by negative ions. Linear combinations between two of the t 2g orbitals, yz and zx, lead to states with l z 0. Namely, the l z 1 state is realized as Y d 1 (1/ 2) 1 i yz zx. Therefore, when the spin-orbit interaction is switched on, the t 2g partial DOS split into subbands, namely, Y d1, Y d 1, and xy partial DOS s as shown in the lower panel of Fig. 3 c. On the other hand, l z of any linear combination of e g states is always zero. Therefore, the e g partial DOS would not be affected. In this situation, only the t 2g states contribute to the orbital angular momentum. Therefore, l z is zero if the t 2g band is totally full or totally empty. Discussion about these aspects based on an atomic point of view was reviewed by Kanamori. 18 D. Discussion about the present result Now, we apply the model above to the present systems. In the Cu 3 Au structure, Au is on the corner site of the fcc structure and Cu is on the face-centered site. Therefore, in both the Cu and Au sites, the t 2g orbital hybridizes stronger with the surrounding orbitals than the e g orbitals. Since the structure is nearly close-packed and these systems are presumably quite metallic, the 3d partial DOS is considered to be like Fig. 3 b. This is qualitatively well reproduced by the bandstructure calculation by Shirai. 17 Hence the orbital angular momentum should come mainly from the l z 2 orbitals. This is quite consistent with the argument on TPt 3 by Iwashita et al. 19 They discussed the orbital angular momenta of the T 3d and Pt 5d orbitals on the basis that the l z 2 give the largest contribution. This was deduced from their band-structure calculation based on the scalarrelativistic full-potential linear-augmented plane-wave method within the local-spin-density approximation LSDA with the additional inclusion of the spin-orbit interaction as a perturbation. In MnPt 3 and CoPt 3, all the nearest neighbors of the transition-metal site are Pt sites. On the other hand, in Fe 3 Pt, 2/3 of the nearest neighbors are the Fe sites and the other 1/3 are the Pt sites. Generally speaking, the Pt 5d partial DOS is distributed in the energy region with a significantly larger binding energy than the T 3d partial DOS. Therefore, the 3d electrons are relatively localized in MnPt 3 and CoPt 3 whereas they are quite itinerant in Fe 3 Pt. In MnPt 3, although the 3d orbital is relatively localized, it is nearly half-filled. Therefore, from our discussion in the last section it follows that the orbital angular momentum of the 3d electrons is expected to be small. In Fe 3 Pt, although the 3d orbital is more than half-filled, it is quite itinerant and the orbital angular momentum is also small. In CoPt 3, the 3d orbital is more than half-filled and is relatively localized and therefore the orbital angular momentum is expected to be larger than the other two systems. This argument well explains our experimental results that the contribution of the orbital angular momentum to the magnetic moment of the 3d orbital is larger in CoPt 3 than in MnPt 3 and Fe 3 Pt. Finally, we quantitatively compare the contribution of the orbital angular momentum, l z /( l z 2 s z ), obtained in Sec. III B with that obtained by Iwashita et al. 19 According to their calculation, l z /( l z 2 s z ) values of the 3d electrons are and for MnPt 3 and CoPt 3, respectively. These values are significantly smaller than our values, and 0.063, respectively. One of the origins of this discrepancy is that t z is omitted in our analysis since Wu et al. showed that l z / s z is overestimated by the sum-rule analysis without t z. 15 Another origin, of course, might be that the band-structure calculation within LSDA underestimates l z since it cannot treat l z self-consistently. IV. SUMMARY We measured the MCD in the transition-metal 2 p absorption of the ferromagnetic Cu 3 Au-type transition-metal-pt ordered alloys, MnPt 3,Fe 3 Pt, and CoPt 3. By combining the l z and s z sum rules, we derived l z /( l z 2 s z ), the portion of the orbital angular momentum s contribution to the magnetic moment of the transition-metal 3d orbital, for the three compounds. This quantity is not only physically important but also experimentally more reliable in the sense that it is less affected by possible extrinsic errors such as the ambiguity in the background subtraction and imperfectness in magnetization and circular polarization than individual l z and s z. The estimated l z /( l z 2 s z ) values, 0.044, 0.031, and for MnPt 3, Fe 3 Pt, and CoPt 3, respectively, are still not very reliable in an absolute sense, but they are quite meaningful in a relative sense. What determines the degree of the orbital contribution to the magnetic moment in metallic ferromagnets was discussed. The orbital contribution is enhanced when the 3d electrons are localized and when the 3d filling is far from either empty, half-filled, or fully filled. The fact that the orbital contribution is the largest in CoPt 3 is explained by that the 3d electrons are relatively localized and the 3d orbital is more than half-filled in it, whereas the 3d electron is quite itinerant in Fe 3 Pt and the 3d orbital is nearly half-filled in MnPt 3. ACKNOWLEDGMENTS We would like to thank Professor T. Oguchi and Professor M. Shirai for discussions and for providing the details of their studies. We would also like to thank Professor T. Miyahara and Dr. Y. Kagoshima for their support during the experiments in the Photon Factory.
7 8758 S. IMADA et al. PRB 59 * Present address: Department of Materials Science, Faculty of Science, Hiroshima University, Higashi-Hiroshima 739, Japan. Present address: Fundamental Research Laboratories, NEC Corporation, 34, Miyukigaoka, Tsukuba, Ibaraki , Japan. 1 J. J. M. Franse and R. Gersdorf, in Magnetic Properties of Metals 3d, 4d and 5d Elements, Alloys and Compounds, Landolt-Börnstein, New Series, Vol. 19a, edited by H. P. J. Wijn Springer-Verlag, Berlin, J. G. Booth, in Ferromagnetic Materials, edited by E. P. Wohlfarth and K. H. J. Buschow North-Holland, Amsterdam, 1988, Vol B. Antonini, F. Lucari, F. Menzinger, and A. Paoletti, Phys. Rev. 187, Y. Itoh, T. Sasaki, and T. Mizoguchi, Solid State Commun. 15, F. Menzinger and A. Paoletti, Phys. Rev. 143, E.T. Kulatov, Yu.A. Uspenskii, and S.V. Halilov, J. Magn. Magn. Mater. 163, M. Maret, M.C. Cadeville, R. Poisot, A. Herr, E. Beaurepaire, and C. Monier, J. Magn. Magn. Mater. 166, P. Böni, G. Shirane, B.H. Grier, and Y. Ishikawa, J. Phys. Soc. Jpn. 55, S. Imada and T. Jo, J. Phys. Soc. Jpn. 59, B.T. Thole, P. Carra, F. Sette, and G. van der Laan, Phys. Rev. Lett. 68, P. Carra, B.T. Thole, M. Altarelli, and X. Wang, Phys. Rev. Lett. 70, H. Maruyama, F. Matsuoka, K. Kobayashi, and H. Yamazaki, J. Magn. Magn. Mater , T. Shishidou, S. Imada, T. Muro, F. Oda, A. Kimura, S. Suga, T. Miyahara, T. Kanomata, and T. Kaneko, Phys. Rev. B 55, G. van der Laan and B.T. Thole, Phys. Rev. B 43, R. Wu, D. Wang, and A.J. Freeman, J. Magn. Magn. Mater. 132, Y. Teramura, A. Tanaka, and T. Jo, J. Phys. Soc. Jpn. 65, M. Shirai, Physica B , ; private communication. 18 J. Kanamori, in Magnetism, edited by G. T. Rado and H. Suhl Academic, New York, 1963, p K. Iwashita, T. Oguchi, and T. Jo, Phys. Rev. B 54, ; private communication.
Soft X-ray Physics DELNOR-WIGGINS PASS STATE PARK
 Soft X-ray Physics Overview of research in Prof. Tonner s group Introduction to synchrotron radiation physics Photoemission spectroscopy: band-mapping and photoelectron diffraction Magnetic spectroscopy
Soft X-ray Physics Overview of research in Prof. Tonner s group Introduction to synchrotron radiation physics Photoemission spectroscopy: band-mapping and photoelectron diffraction Magnetic spectroscopy
Self-compensating incorporation of Mn in Ga 1 x Mn x As
 Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
X-Ray Magnetic Circular Dichroism: basic concepts and theory for 4f rare earth ions and 3d metals. Stefania PIZZINI Laboratoire Louis Néel - Grenoble
 X-Ray Magnetic Circular Dichroism: basic concepts and theory for 4f rare earth ions and 3d metals Stefania PIZZINI Laboratoire Louis Néel - Grenoble I) - History and basic concepts of XAS - XMCD at M 4,5
X-Ray Magnetic Circular Dichroism: basic concepts and theory for 4f rare earth ions and 3d metals Stefania PIZZINI Laboratoire Louis Néel - Grenoble I) - History and basic concepts of XAS - XMCD at M 4,5
arxiv: v1 [cond-mat.mtrl-sci] 25 Jun 2017
![arxiv: v1 [cond-mat.mtrl-sci] 25 Jun 2017 arxiv: v1 [cond-mat.mtrl-sci] 25 Jun 2017](/thumbs/86/94556061.jpg) Magnetic anisotropy of L1 0 -ordered FePt thin films studied by Fe and Pt L 2,3 -edges x-ray magnetic circular dichroism K. Ikeda, 1 T. Seki, 2 G. Shibata, 1 T. Kadono, 1 K. Ishigami, 1 Y. Takahashi, 1
Magnetic anisotropy of L1 0 -ordered FePt thin films studied by Fe and Pt L 2,3 -edges x-ray magnetic circular dichroism K. Ikeda, 1 T. Seki, 2 G. Shibata, 1 T. Kadono, 1 K. Ishigami, 1 Y. Takahashi, 1
X-ray Magnetic Circular and Linear Dichroism (XMCD, XMLD) and X-ray Magnetic Imaging (PEEM,...)
 X-ray Magnetic Circular and Linear Dichroism (XMCD, XMLD) and X-ray Magnetic Imaging (PEEM,...) Jan Vogel Institut Néel (CNRS, UJF), Nanoscience Department Grenoble, France - X-ray (Magnetic) Circular
X-ray Magnetic Circular and Linear Dichroism (XMCD, XMLD) and X-ray Magnetic Imaging (PEEM,...) Jan Vogel Institut Néel (CNRS, UJF), Nanoscience Department Grenoble, France - X-ray (Magnetic) Circular
arxiv:cond-mat/ v1 [cond-mat.str-el] 27 Oct 2003
![arxiv:cond-mat/ v1 [cond-mat.str-el] 27 Oct 2003 arxiv:cond-mat/ v1 [cond-mat.str-el] 27 Oct 2003](/thumbs/71/65811887.jpg) Magnetic versus crystal field linear dichroism in NiO thin films arxiv:cond-mat/0310634v1 [cond-mat.str-el] 27 Oct 2003 M. W. Haverkort, 1 S. I. Csiszar, 2 Z. Hu, 1 S. Altieri, 3 A. Tanaka, 4 H. H. Hsieh,
Magnetic versus crystal field linear dichroism in NiO thin films arxiv:cond-mat/0310634v1 [cond-mat.str-el] 27 Oct 2003 M. W. Haverkort, 1 S. I. Csiszar, 2 Z. Hu, 1 S. Altieri, 3 A. Tanaka, 4 H. H. Hsieh,
Making the Invisible Visible: Probing Antiferromagnetic Order in Novel Materials
 Making the Invisible Visible: Probing Antiferromagnetic Order in Novel Materials Elke Arenholz Lawrence Berkeley National Laboratory Antiferromagnetic contrast in X-ray absorption Ni in NiO Neel Temperature
Making the Invisible Visible: Probing Antiferromagnetic Order in Novel Materials Elke Arenholz Lawrence Berkeley National Laboratory Antiferromagnetic contrast in X-ray absorption Ni in NiO Neel Temperature
X-ray absorption spectroscopy.
 X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ Frank de Groot PhD: solid state chemistry U Nijmegen
X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ X-ray absorption spectroscopy www.anorg.chem.uu.nl/people/staff/frankdegroot/ Frank de Groot PhD: solid state chemistry U Nijmegen
Electronic structure of correlated electron systems. G.A.Sawatzky UBC Lecture
 Electronic structure of correlated electron systems G.A.Sawatzky UBC Lecture 6 011 Influence of polarizability on the crystal structure Ionic compounds are often cubic to maximize the Madelung energy i.e.
Electronic structure of correlated electron systems G.A.Sawatzky UBC Lecture 6 011 Influence of polarizability on the crystal structure Ionic compounds are often cubic to maximize the Madelung energy i.e.
Spin-resolved photoelectron spectroscopy
 Spin-resolved photoelectron spectroscopy Application Notes Spin-resolved photoelectron spectroscopy experiments were performed in an experimental station consisting of an analysis and a preparation chamber.
Spin-resolved photoelectron spectroscopy Application Notes Spin-resolved photoelectron spectroscopy experiments were performed in an experimental station consisting of an analysis and a preparation chamber.
Current-driven Magnetization Reversal in a Ferromagnetic Semiconductor. (Ga,Mn)As/GaAs/(Ga,Mn)As Tunnel Junction
 Current-driven Magnetization Reversal in a Ferromagnetic Semiconductor (Ga,Mn)As/GaAs/(Ga,Mn)As Tunnel Junction D. Chiba 1, 2*, Y. Sato 1, T. Kita 2, 1, F. Matsukura 1, 2, and H. Ohno 1, 2 1 Laboratory
Current-driven Magnetization Reversal in a Ferromagnetic Semiconductor (Ga,Mn)As/GaAs/(Ga,Mn)As Tunnel Junction D. Chiba 1, 2*, Y. Sato 1, T. Kita 2, 1, F. Matsukura 1, 2, and H. Ohno 1, 2 1 Laboratory
SUPPLEMENTARY INFORMATION
 Titanium d xy ferromagnetism at the LaAlO 3 /SrTiO 3 interface J.-S. Lee 1,*, Y. W. Xie 2, H. K. Sato 3, C. Bell 3, Y. Hikita 3, H. Y. Hwang 2,3, C.-C. Kao 1 1 Stanford Synchrotron Radiation Lightsource,
Titanium d xy ferromagnetism at the LaAlO 3 /SrTiO 3 interface J.-S. Lee 1,*, Y. W. Xie 2, H. K. Sato 3, C. Bell 3, Y. Hikita 3, H. Y. Hwang 2,3, C.-C. Kao 1 1 Stanford Synchrotron Radiation Lightsource,
Department of Electrical Engineering and Information Systems, Tanaka-Ohya lab.
 Observation of the room-temperature local ferromagnetism and its nanoscale expansion in the ferromagnetic semiconductor Ge 1 xfe x Yuki K. Wakabayashi 1 and Yukio Takahashi 2 1 Department of Electrical
Observation of the room-temperature local ferromagnetism and its nanoscale expansion in the ferromagnetic semiconductor Ge 1 xfe x Yuki K. Wakabayashi 1 and Yukio Takahashi 2 1 Department of Electrical
Chapter 10: Multi- Electron Atoms Optical Excitations
 Chapter 10: Multi- Electron Atoms Optical Excitations To describe the energy levels in multi-electron atoms, we need to include all forces. The strongest forces are the forces we already discussed in Chapter
Chapter 10: Multi- Electron Atoms Optical Excitations To describe the energy levels in multi-electron atoms, we need to include all forces. The strongest forces are the forces we already discussed in Chapter
Spettroscopia risonante di stati elettronici: un approccio impossibile senza i sincrotroni
 Spettroscopia risonante di stati elettronici: un approccio impossibile senza i sincrotroni XAS, XMCD, XES, RIXS, ResXPS: introduzione alle spettroscopie risonanti * Dipartimento di Fisica - Politecnico
Spettroscopia risonante di stati elettronici: un approccio impossibile senza i sincrotroni XAS, XMCD, XES, RIXS, ResXPS: introduzione alle spettroscopie risonanti * Dipartimento di Fisica - Politecnico
First Principles Calculation of Defect and Magnetic Structures in FeCo
 Materials Transactions, Vol. 47, No. 11 (26) pp. 2646 to 26 Special Issue on Advances in Computational Materials Science and Engineering IV #26 The Japan Institute of Metals First Principles Calculation
Materials Transactions, Vol. 47, No. 11 (26) pp. 2646 to 26 Special Issue on Advances in Computational Materials Science and Engineering IV #26 The Japan Institute of Metals First Principles Calculation
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics. Website: Sakai 01:750:228 or
 Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Nuclear Sizes Nuclei occupy the center of the atom. We can view them as being more
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Nuclear Sizes Nuclei occupy the center of the atom. We can view them as being more
PEEM and XPEEM: methodology and applications for dynamic processes
 PEEM and XPEEM: methodology and applications for dynamic processes PEEM methods and General considerations Chemical imaging Magnetic imaging XMCD/XMLD Examples Dynamic studies PEEM and XPEEM methods 1
PEEM and XPEEM: methodology and applications for dynamic processes PEEM methods and General considerations Chemical imaging Magnetic imaging XMCD/XMLD Examples Dynamic studies PEEM and XPEEM methods 1
Paramagnetism and Diamagnetism. Paramagnets (How do paramagnets differ fundamentally from ferromagnets?)
 Paramagnetism and Diamagnetism Paramagnets (How do paramagnets differ fundamentally from ferromagnets?) The study of paramagnetism allows us to investigate the atomic magnetic moments of atoms almost in
Paramagnetism and Diamagnetism Paramagnets (How do paramagnets differ fundamentally from ferromagnets?) The study of paramagnetism allows us to investigate the atomic magnetic moments of atoms almost in
X-ray Magnetic Circular Dichroism (XMCD) : basic concepts and theory for rare earths and 3d metals
 X-ray Magnetic Circular Dichroism () : basic concepts and theory for rare earths and 3d metals Laboratoire Louis Néel Grenoble - Introduction to X-ray absorption spectroscopy and History of - of rare earth
X-ray Magnetic Circular Dichroism () : basic concepts and theory for rare earths and 3d metals Laboratoire Louis Néel Grenoble - Introduction to X-ray absorption spectroscopy and History of - of rare earth
X-Ray Magnetic Circular Dichroism: basic concepts and applications for 3d transition metals. Stefania PIZZINI Laboratoire Louis Néel CNRS- Grenoble
 X-Ray Magnetic Circular Dichroism: basic concepts and applications for 3d transition metals Stefania PIZZINI Laboratoire Louis Néel CNRS- Grenoble I) - Basic concepts of XAS and XMCD - XMCD at L 2,3 edges
X-Ray Magnetic Circular Dichroism: basic concepts and applications for 3d transition metals Stefania PIZZINI Laboratoire Louis Néel CNRS- Grenoble I) - Basic concepts of XAS and XMCD - XMCD at L 2,3 edges
X-Ray Magnetic Dichroism. S. Turchini ISM-CNR
 X-Ray Magnetic Dichroism S. Turchini SM-CNR stefano.turchini@ism.cnr.it stefano.turchini@elettra.trieste.it Magnetism spin magnetic moment direct exchange: ferro antiferro superexchange 3d Ligand 2p 3d
X-Ray Magnetic Dichroism S. Turchini SM-CNR stefano.turchini@ism.cnr.it stefano.turchini@elettra.trieste.it Magnetism spin magnetic moment direct exchange: ferro antiferro superexchange 3d Ligand 2p 3d
Electromagnetism II. Instructor: Andrei Sirenko Spring 2013 Thursdays 1 pm 4 pm. Spring 2013, NJIT 1
 Electromagnetism II Instructor: Andrei Sirenko sirenko@njit.edu Spring 013 Thursdays 1 pm 4 pm Spring 013, NJIT 1 PROBLEMS for CH. 6 http://web.njit.edu/~sirenko/phys433/phys433eandm013.htm Can obtain
Electromagnetism II Instructor: Andrei Sirenko sirenko@njit.edu Spring 013 Thursdays 1 pm 4 pm Spring 013, NJIT 1 PROBLEMS for CH. 6 http://web.njit.edu/~sirenko/phys433/phys433eandm013.htm Can obtain
compound Cs 2 Cu 2 Mo 3 O 12
 133 Cs-NMR study on aligned powder of competing spin chain compound A Yagi 1, K Matsui 1 T Goto 1, M Hase 2 and T Sasaki 3 1 2 Sophia University, Physics Division, Tokyo, 102-8554, Japan National Institute
133 Cs-NMR study on aligned powder of competing spin chain compound A Yagi 1, K Matsui 1 T Goto 1, M Hase 2 and T Sasaki 3 1 2 Sophia University, Physics Division, Tokyo, 102-8554, Japan National Institute
Supplementary Figure S1 The magneto-optical images of Gd24Fe66.5Co9.5
 Supplementary Figure S1 The magneto-optical images of Gd24Fe66.5Co9.5 continuous film obtained after the action of a sequence of the N right-handed (σ+ σ+) σ+ and left-handed (σ σ ) σ circularly-polarized
Supplementary Figure S1 The magneto-optical images of Gd24Fe66.5Co9.5 continuous film obtained after the action of a sequence of the N right-handed (σ+ σ+) σ+ and left-handed (σ σ ) σ circularly-polarized
X-ray magnetic circular dichroism in d and f ferromagnetic materials: recent theoretical progress. Part II
 Fizika Nizkikh Temperatur, 8, v. 34, No., p. 7 47 X-ray magnetic circular dichroism in d and f ferromagnetic materials: recent theoretical progress. Part II (Review Article) V.N. Antonov and A.P. Shpak
Fizika Nizkikh Temperatur, 8, v. 34, No., p. 7 47 X-ray magnetic circular dichroism in d and f ferromagnetic materials: recent theoretical progress. Part II (Review Article) V.N. Antonov and A.P. Shpak
Matter-Radiation Interaction
 Matter-Radiation Interaction The purpose: 1) To give a description of the process of interaction in terms of the electronic structure of the system (atoms, molecules, solids, liquid or amorphous samples).
Matter-Radiation Interaction The purpose: 1) To give a description of the process of interaction in terms of the electronic structure of the system (atoms, molecules, solids, liquid or amorphous samples).
Intermediate valence in Yb Intermetallic compounds
 Intermediate valence in Yb Intermetallic compounds Jon Lawrence University of California, Irvine This talk concerns rare earth intermediate valence (IV) metals, with a primary focus on certain Yb-based
Intermediate valence in Yb Intermetallic compounds Jon Lawrence University of California, Irvine This talk concerns rare earth intermediate valence (IV) metals, with a primary focus on certain Yb-based
PBS: FROM SOLIDS TO CLUSTERS
 PBS: FROM SOLIDS TO CLUSTERS E. HOFFMANN AND P. ENTEL Theoretische Tieftemperaturphysik Gerhard-Mercator-Universität Duisburg, Lotharstraße 1 47048 Duisburg, Germany Semiconducting nanocrystallites like
PBS: FROM SOLIDS TO CLUSTERS E. HOFFMANN AND P. ENTEL Theoretische Tieftemperaturphysik Gerhard-Mercator-Universität Duisburg, Lotharstraße 1 47048 Duisburg, Germany Semiconducting nanocrystallites like
(002)(110) (004)(220) (222) (112) (211) (202) (200) * * 2θ (degree)
 Supplementary Figures. (002)(110) Tetragonal I4/mcm Intensity (a.u) (004)(220) 10 (112) (211) (202) 20 Supplementary Figure 1. X-ray diffraction (XRD) pattern of the sample. The XRD characterization indicates
Supplementary Figures. (002)(110) Tetragonal I4/mcm Intensity (a.u) (004)(220) 10 (112) (211) (202) 20 Supplementary Figure 1. X-ray diffraction (XRD) pattern of the sample. The XRD characterization indicates
Soft X-ray Absorption Spectroscopy Kenta Amemiya (KEK-PF)
 Cheiron School 014 Soft X-ray Absorption Spectroscopy Kenta Amemiya (KEK-PF) 1 Atomic Number Absorption Edges in the Soft X-ray Region M edge L edge K edge. Li Absorption-edge Energy (ev) Studies using
Cheiron School 014 Soft X-ray Absorption Spectroscopy Kenta Amemiya (KEK-PF) 1 Atomic Number Absorption Edges in the Soft X-ray Region M edge L edge K edge. Li Absorption-edge Energy (ev) Studies using
First-Principles Calculation of Exchange Interactions
 Chapter 2 First-Principles Calculation of Exchange Interactions Before introducing the first-principles methods for the calculation of exchange interactions in magnetic systems we will briefly review two
Chapter 2 First-Principles Calculation of Exchange Interactions Before introducing the first-principles methods for the calculation of exchange interactions in magnetic systems we will briefly review two
Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies.
 PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
Atomic Structure. Chapter 8
 Atomic Structure Chapter 8 Overview To understand atomic structure requires understanding a special aspect of the electron - spin and its related magnetism - and properties of a collection of identical
Atomic Structure Chapter 8 Overview To understand atomic structure requires understanding a special aspect of the electron - spin and its related magnetism - and properties of a collection of identical
Moldavian Journal of the Physical Sciences, N2, 2002
 Moldavian Journal of the Physical Sciences, N2, 2 LCTRONIC STRUCTURS AND MAGNTIC PROPRTIS O R n+1 Co n+ 2n (n=, 1, 2, and ) COMPOUNDS WITH R=Y AND P. Vlaic,. urzo aculty of Physics, abes-olyai University,
Moldavian Journal of the Physical Sciences, N2, 2 LCTRONIC STRUCTURS AND MAGNTIC PROPRTIS O R n+1 Co n+ 2n (n=, 1, 2, and ) COMPOUNDS WITH R=Y AND P. Vlaic,. urzo aculty of Physics, abes-olyai University,
Mott insulators. Introduction Cluster-model description Chemical trend Band description Self-energy correction
 Mott insulators Introduction Cluster-model description Chemical trend Band description Self-energy correction Introduction Mott insulators Lattice models for transition-metal compounds Hubbard model Anderson-lattice
Mott insulators Introduction Cluster-model description Chemical trend Band description Self-energy correction Introduction Mott insulators Lattice models for transition-metal compounds Hubbard model Anderson-lattice
Magnetism of Atoms and Nanostructures Adsorbed onto Surfaces
 Magnetism of Atoms and Nanostructures Adsorbed onto Surfaces Magnetism Coordination Small Ferromagnets Superlattices Basic properties of a permanent magnet Magnetization "the strength of the magnet" depends
Magnetism of Atoms and Nanostructures Adsorbed onto Surfaces Magnetism Coordination Small Ferromagnets Superlattices Basic properties of a permanent magnet Magnetization "the strength of the magnet" depends
THE MAGNETO-OPTICAL PROPERTIES OF HEUSLER ALLOYS OF THE TYPE Coz_xCuxMnSn
 Philips J. Res. 42, 429-434, 1987 R 1165 THE MAGNETO-OPTICAL PROPERTIES OF HEUSLER ALLOYS OF THE TYPE Coz_xCuxMnSn by P.P.J. VAN ENGE~EN and K.H.J. BUSCHOW Philips Research Laboratories, 56 JA Eindhoven,
Philips J. Res. 42, 429-434, 1987 R 1165 THE MAGNETO-OPTICAL PROPERTIES OF HEUSLER ALLOYS OF THE TYPE Coz_xCuxMnSn by P.P.J. VAN ENGE~EN and K.H.J. BUSCHOW Philips Research Laboratories, 56 JA Eindhoven,
Creating Energy-Level Diagrams Aufbau (building up) Principle Electrons are added to the lowest energy orbital available.
 3.6 Atomic Structure and the Periodic Table Bohr's Theory Was Incorrect Because... Only explained the line spectrum of hydrogen Position and motion of an e cannot be specified (since the e is so small,
3.6 Atomic Structure and the Periodic Table Bohr's Theory Was Incorrect Because... Only explained the line spectrum of hydrogen Position and motion of an e cannot be specified (since the e is so small,
Probing Matter: Diffraction, Spectroscopy and Photoemission
 Probing Matter: Diffraction, Spectroscopy and Photoemission Anders Nilsson Stanford Synchrotron Radiation Laboratory Why X-rays? VUV? What can we hope to learn? 1 Photon Interaction Incident photon interacts
Probing Matter: Diffraction, Spectroscopy and Photoemission Anders Nilsson Stanford Synchrotron Radiation Laboratory Why X-rays? VUV? What can we hope to learn? 1 Photon Interaction Incident photon interacts
N. Gonzalez Szwacki and Jacek A. Majewski Faculty of Physics, University of Warsaw, ul. Hoża 69, Warszawa, Poland
 Ab initio studies of Co 2 FeAl 1-x Si x Heusler alloys N. Gonzalez Szwacki and Jacek A. Majewski Faculty of Physics, University of Warsaw, ul. Hoża 69, 00-681 Warszawa, Poland Abstract We present results
Ab initio studies of Co 2 FeAl 1-x Si x Heusler alloys N. Gonzalez Szwacki and Jacek A. Majewski Faculty of Physics, University of Warsaw, ul. Hoża 69, 00-681 Warszawa, Poland Abstract We present results
doi: /PhysRevLett
 doi: 1.113/PhysRevLett.9.17 PRL 9, 17 (7) 5 JANUARY 7 Optical Control of the Magnetic Anisotropy of Ferromagnetic Bilayered Manganites S. Tomimoto, 1 M. Matsubara, 1 T. Ogasawara, 1 H. Okamoto, 1, T. Kimura,
doi: 1.113/PhysRevLett.9.17 PRL 9, 17 (7) 5 JANUARY 7 Optical Control of the Magnetic Anisotropy of Ferromagnetic Bilayered Manganites S. Tomimoto, 1 M. Matsubara, 1 T. Ogasawara, 1 H. Okamoto, 1, T. Kimura,
Name: (a) What core levels are responsible for the three photoelectron peaks in Fig. 1?
 Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
An introduction to magnetism in three parts
 An introduction to magnetism in three parts Wulf Wulfhekel Physikalisches Institut, Karlsruhe Institute of Technology (KIT) Wolfgang Gaede Str. 1, D-76131 Karlsruhe 0. Overview Chapters of the three lectures
An introduction to magnetism in three parts Wulf Wulfhekel Physikalisches Institut, Karlsruhe Institute of Technology (KIT) Wolfgang Gaede Str. 1, D-76131 Karlsruhe 0. Overview Chapters of the three lectures
Observation of quadrupole helix chirality and its domain structure in DyFe 3 (BO 3 ) 4
 Observation of quadrupole helix chirality and its domain structure in DyFe 3 (BO 3 ) 4 T. Usui, Y. Tanaka, H. Nakajima, M. Taguchi, A. Chainani, M. Oura, S. Shin, N. Katayama, H. Sawa, Y. Wakabayashi,
Observation of quadrupole helix chirality and its domain structure in DyFe 3 (BO 3 ) 4 T. Usui, Y. Tanaka, H. Nakajima, M. Taguchi, A. Chainani, M. Oura, S. Shin, N. Katayama, H. Sawa, Y. Wakabayashi,
An Introduction to XAFS
 An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
CHEM1902/ N-2 November 2014
 CHEM1902/4 2014-N-2 November 2014 The cubic form of boron nitride (borazon) is the second-hardest material after diamond and it crystallizes with the structure shown below. The large spheres represent
CHEM1902/4 2014-N-2 November 2014 The cubic form of boron nitride (borazon) is the second-hardest material after diamond and it crystallizes with the structure shown below. The large spheres represent
Orbital correlation and magnetocrystalline anisotropy in one-dimensional transition-metal systems
 PHYSICAL REVIEW B VOLUME 60, NUMBER 13 1 OCTOBER 1999-I Orbital correlation and magnetocrystalline anisotropy in one-dimensional transition-metal systems Lei Zhou Dingsheng Wang and Institute of Physics,
PHYSICAL REVIEW B VOLUME 60, NUMBER 13 1 OCTOBER 1999-I Orbital correlation and magnetocrystalline anisotropy in one-dimensional transition-metal systems Lei Zhou Dingsheng Wang and Institute of Physics,
SUPPLEMENTARY INFORMATION
 Materials and Methods Single crystals of Pr 2 Ir 2 O 7 were grown by a flux method [S1]. Energy dispersive x-ray analysis found no impurity phases, no inhomogeneities and a ratio between Pr and Ir of 1:1.03(3).
Materials and Methods Single crystals of Pr 2 Ir 2 O 7 were grown by a flux method [S1]. Energy dispersive x-ray analysis found no impurity phases, no inhomogeneities and a ratio between Pr and Ir of 1:1.03(3).
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS
 2753 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2011 Wednesday, 22 June, 9.30 am 12.30
2753 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2011 Wednesday, 22 June, 9.30 am 12.30
Chapter 4: Bonding in Solids and Electronic Properties. Free electron theory
 Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Lecture 3 continuation from Wednesday
 Lecture 3 continuation from Wednesday Dynamical Matrix - Conduction electrons Importance of el-ph Matrix elements NiAl 50-50 compound Confirmed by H. Chou and S.M.Shapiro Phys. Rev. B48, 16088 (1993) Exp.
Lecture 3 continuation from Wednesday Dynamical Matrix - Conduction electrons Importance of el-ph Matrix elements NiAl 50-50 compound Confirmed by H. Chou and S.M.Shapiro Phys. Rev. B48, 16088 (1993) Exp.
The Gutzwiller Density Functional Theory
 The Gutzwiller Density Functional Theory Jörg Bünemann, BTU Cottbus I) Introduction 1. Model for an H 2 -molecule 2. Transition metals and their compounds II) Gutzwiller variational theory 1. Gutzwiller
The Gutzwiller Density Functional Theory Jörg Bünemann, BTU Cottbus I) Introduction 1. Model for an H 2 -molecule 2. Transition metals and their compounds II) Gutzwiller variational theory 1. Gutzwiller
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
XMCD analysis beyond standard procedures
 XMCD analysis beyond standard procedures H. Wende, A. Scherz,, C. Sorg, K. Baberschke, E.K.U. Gross, H. Appel, K. Burke, J. Minár, H. Ebert, A.L. Ankudinov and J.J. Rehr Fachbereich Physik, Freie Universität
XMCD analysis beyond standard procedures H. Wende, A. Scherz,, C. Sorg, K. Baberschke, E.K.U. Gross, H. Appel, K. Burke, J. Minár, H. Ebert, A.L. Ankudinov and J.J. Rehr Fachbereich Physik, Freie Universität
2.1 Experimental and theoretical studies
 Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Electronic structure of correlated electron systems. Lecture 2
 Electronic structure of correlated electron systems Lecture 2 Band Structure approach vs atomic Band structure Delocalized Bloch states Fill up states with electrons starting from the lowest energy No
Electronic structure of correlated electron systems Lecture 2 Band Structure approach vs atomic Band structure Delocalized Bloch states Fill up states with electrons starting from the lowest energy No
Experiment 7: Understanding Crystal Structures
 Experiment 7: Understanding Crystal Structures To do well in this laboratory experiment you need to be familiar with the concepts of lattice, crystal structure, unit cell, coordination number, the different
Experiment 7: Understanding Crystal Structures To do well in this laboratory experiment you need to be familiar with the concepts of lattice, crystal structure, unit cell, coordination number, the different
Phthalocyanine-Based Single-Component
 Phthalocyanine-Based Single-Component Molecular Conductor [Mn Ⅲ (Pc)(CN)] 2 O Mitsuo Ikeda, Hiroshi Murakawa, Masaki Matsuda, and Noriaki Hanasaki *, Department of Physics, Graduate School of Science,
Phthalocyanine-Based Single-Component Molecular Conductor [Mn Ⅲ (Pc)(CN)] 2 O Mitsuo Ikeda, Hiroshi Murakawa, Masaki Matsuda, and Noriaki Hanasaki *, Department of Physics, Graduate School of Science,
Size- and site-dependence of XMCD spectra of iron clusters from ab-initio calculations
 Size- and site-dependence of XMCD spectra of iron clusters from ab-initio calculations O. Šipr 1 and H. Ebert 2 1 Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 162
Size- and site-dependence of XMCD spectra of iron clusters from ab-initio calculations O. Šipr 1 and H. Ebert 2 1 Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 162
Material Science II. d Electron systems
 Material Science II. d Electron systems 1. Electronic structure of transition-metal ions (May 23) 2. Crystal structure and band structure (June 13) 3. Mott s (June 20) 4. Metal- transition (June 27) 5.
Material Science II. d Electron systems 1. Electronic structure of transition-metal ions (May 23) 2. Crystal structure and band structure (June 13) 3. Mott s (June 20) 4. Metal- transition (June 27) 5.
Local Electronic Structures and Chemical Bonds in Zr-Based Metallic Glasses
 Materials Transactions, Vol. 45, No. 4 (2004) pp. 1172 to 1176 Special Issue on Bulk Amorphous, Nano-Crystalline and Nano-Quasicrystalline Alloys-V #2004 The Japan Institute of Metals Local Electronic
Materials Transactions, Vol. 45, No. 4 (2004) pp. 1172 to 1176 Special Issue on Bulk Amorphous, Nano-Crystalline and Nano-Quasicrystalline Alloys-V #2004 The Japan Institute of Metals Local Electronic
A very brief history of the study of light
 1. Sir Isaac Newton 1672: A very brief history of the study of light Showed that the component colors of the visible portion of white light can be separated through a prism, which acts to bend the light
1. Sir Isaac Newton 1672: A very brief history of the study of light Showed that the component colors of the visible portion of white light can be separated through a prism, which acts to bend the light
QUESTIONS AND ANSWERS
 QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
WORLD SCIENTIFIC (2014)
 WORLD SCIENTIFIC (2014) LIST OF PROBLEMS Chapter 1: Magnetism of Free Electrons and Atoms 1. Orbital and spin moments of an electron: Using the theory of angular momentum, calculate the orbital
WORLD SCIENTIFIC (2014) LIST OF PROBLEMS Chapter 1: Magnetism of Free Electrons and Atoms 1. Orbital and spin moments of an electron: Using the theory of angular momentum, calculate the orbital
Crystal Field Theory
 Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable
Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable
Correlations between spin accumulation and degree of time-inverse breaking for electron gas in solid
 Correlations between spin accumulation and degree of time-inverse breaking for electron gas in solid V.Zayets * Spintronic Research Center, National Institute of Advanced Industrial Science and Technology
Correlations between spin accumulation and degree of time-inverse breaking for electron gas in solid V.Zayets * Spintronic Research Center, National Institute of Advanced Industrial Science and Technology
Tunneling Spectroscopy of Disordered Two-Dimensional Electron Gas in the Quantum Hall Regime
 Tunneling Spectroscopy of Disordered Two-Dimensional Electron Gas in the Quantum Hall Regime The Harvard community has made this article openly available. Please share how this access benefits you. Your
Tunneling Spectroscopy of Disordered Two-Dimensional Electron Gas in the Quantum Hall Regime The Harvard community has made this article openly available. Please share how this access benefits you. Your
201. The Nature o f the Metallic Bond. III
 No. 8] 913 201. The Nature o f the Metallic Bond. III Atomic Interactions in Alloys. I By San-ichiro MIZUSHIMA, M.J.A., and Isao Ichishima Tokyo Research Institute, Yawata Iron and Steel Co. Ltd., Ida,
No. 8] 913 201. The Nature o f the Metallic Bond. III Atomic Interactions in Alloys. I By San-ichiro MIZUSHIMA, M.J.A., and Isao Ichishima Tokyo Research Institute, Yawata Iron and Steel Co. Ltd., Ida,
Photon Interaction. Spectroscopy
 Photon Interaction Incident photon interacts with electrons Core and Valence Cross Sections Photon is Adsorbed Elastic Scattered Inelastic Scattered Electron is Emitted Excitated Dexcitated Stöhr, NEXAPS
Photon Interaction Incident photon interacts with electrons Core and Valence Cross Sections Photon is Adsorbed Elastic Scattered Inelastic Scattered Electron is Emitted Excitated Dexcitated Stöhr, NEXAPS
CHAPTER 6. ELECTRONIC AND MAGNETIC STRUCTURE OF ZINC-BLENDE TYPE CaX (X = P, As and Sb) COMPOUNDS
 143 CHAPTER 6 ELECTRONIC AND MAGNETIC STRUCTURE OF ZINC-BLENDE TYPE CaX (X = P, As and Sb) COMPOUNDS 6.1 INTRODUCTION Almost the complete search for possible magnetic materials has been performed utilizing
143 CHAPTER 6 ELECTRONIC AND MAGNETIC STRUCTURE OF ZINC-BLENDE TYPE CaX (X = P, As and Sb) COMPOUNDS 6.1 INTRODUCTION Almost the complete search for possible magnetic materials has been performed utilizing
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~pchemlab ; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~pchemlab ; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
Metallic and Ionic Structures and Bonding
 Metallic and Ionic Structures and Bonding Ionic compounds are formed between elements having an electronegativity difference of about 2.0 or greater. Simple ionic compounds are characterized by high melting
Metallic and Ionic Structures and Bonding Ionic compounds are formed between elements having an electronegativity difference of about 2.0 or greater. Simple ionic compounds are characterized by high melting
G. Gantefdr and W. Eberhardt Institut fiir Festkiirperforschung, Forschungszentrum Jiilich, 5170 Jiilich, Germany
 Shell structure and s-p hybridization in small aluminum clusters G. Gantefdr and W. Eberhardt Institut fiir Festkiirperforschung, Forschungszentrum Jiilich, 5170 Jiilich, Germany Photoelectron spectra
Shell structure and s-p hybridization in small aluminum clusters G. Gantefdr and W. Eberhardt Institut fiir Festkiirperforschung, Forschungszentrum Jiilich, 5170 Jiilich, Germany Photoelectron spectra
Magnetic Oxides. Gerald F. Dionne. Department of Materials Science and Engineering Massachusetts Institute of Technology
 Magnetic Oxides Gerald F. Dionne Department of Materials Science and Engineering Massachusetts Institute of Technology Spins in Solids Summer School University of Virginia Charlottesville, VA 21 June 2006
Magnetic Oxides Gerald F. Dionne Department of Materials Science and Engineering Massachusetts Institute of Technology Spins in Solids Summer School University of Virginia Charlottesville, VA 21 June 2006
ECE440 Nanoelectronics. Lecture 07 Atomic Orbitals
 ECE44 Nanoelectronics Lecture 7 Atomic Orbitals Atoms and atomic orbitals It is instructive to compare the simple model of a spherically symmetrical potential for r R V ( r) for r R and the simplest hydrogen
ECE44 Nanoelectronics Lecture 7 Atomic Orbitals Atoms and atomic orbitals It is instructive to compare the simple model of a spherically symmetrical potential for r R V ( r) for r R and the simplest hydrogen
Lecture 12 Multiplet splitting
 Lecture 12 Multiplet splitting Multiplet splitting Atomic various L and S terms Both valence and core levels Rare earths Transition metals Paramagnetic free molecules Consider 3s level emission from Mn2+
Lecture 12 Multiplet splitting Multiplet splitting Atomic various L and S terms Both valence and core levels Rare earths Transition metals Paramagnetic free molecules Consider 3s level emission from Mn2+
J 12 J 23 J 34. Driving forces in the nano-magnetism world. Intra-atomic exchange, electron correlation effects: Inter-atomic exchange: MAGNETIC ORDER
 Driving forces in the nano-magnetism world Intra-atomic exchange, electron correlation effects: LOCAL (ATOMIC) MAGNETIC MOMENTS m d or f electrons Inter-atomic exchange: MAGNETIC ORDER H exc J S S i j
Driving forces in the nano-magnetism world Intra-atomic exchange, electron correlation effects: LOCAL (ATOMIC) MAGNETIC MOMENTS m d or f electrons Inter-atomic exchange: MAGNETIC ORDER H exc J S S i j
Optical Pumping in 85 Rb and 87 Rb
 Optical Pumping in 85 Rb and 87 Rb John Prior III*, Quinn Pratt, Brennan Campbell, Kjell Hiniker University of San Diego, Department of Physics (Dated: December 14, 2015) Our experiment aimed to determine
Optical Pumping in 85 Rb and 87 Rb John Prior III*, Quinn Pratt, Brennan Campbell, Kjell Hiniker University of San Diego, Department of Physics (Dated: December 14, 2015) Our experiment aimed to determine
Gilbert Kirss Foster. Chapter3. Atomic Structure. Explaining the Properties of Elements
 Gilbert Kirss Foster Chapter3 Atomic Structure Explaining the Properties of Elements Chapter Outline 3.1 Waves of Light 3.2 Atomic Spectra 3.3 Particles of Light: Quantum Theory 3.4 The Hydrogen Spectrum
Gilbert Kirss Foster Chapter3 Atomic Structure Explaining the Properties of Elements Chapter Outline 3.1 Waves of Light 3.2 Atomic Spectra 3.3 Particles of Light: Quantum Theory 3.4 The Hydrogen Spectrum
PHYSICS 4750 Physics of Modern Materials Chapter 5: The Band Theory of Solids
 PHYSICS 4750 Physics of Modern Materials Chapter 5: The Band Theory of Solids 1. Introduction We have seen that when the electrons in two hydrogen atoms interact, their energy levels will split, i.e.,
PHYSICS 4750 Physics of Modern Materials Chapter 5: The Band Theory of Solids 1. Introduction We have seen that when the electrons in two hydrogen atoms interact, their energy levels will split, i.e.,
Luigi Paolasini
 Luigi Paolasini paolasini@esrf.fr LECTURE 4: MAGNETIC INTERACTIONS - Dipole vs exchange magnetic interactions. - Direct and indirect exchange interactions. - Anisotropic exchange interactions. - Interplay
Luigi Paolasini paolasini@esrf.fr LECTURE 4: MAGNETIC INTERACTIONS - Dipole vs exchange magnetic interactions. - Direct and indirect exchange interactions. - Anisotropic exchange interactions. - Interplay
CHAPTER 2 MAGNETISM. 2.1 Magnetic materials
 CHAPTER 2 MAGNETISM Magnetism plays a crucial role in the development of memories for mass storage, and in sensors to name a few. Spintronics is an integration of the magnetic material with semiconductor
CHAPTER 2 MAGNETISM Magnetism plays a crucial role in the development of memories for mass storage, and in sensors to name a few. Spintronics is an integration of the magnetic material with semiconductor
Magnetic domain theory in dynamics
 Chapter 3 Magnetic domain theory in dynamics Microscale magnetization reversal dynamics is one of the hot issues, because of a great demand for fast response and high density data storage devices, for
Chapter 3 Magnetic domain theory in dynamics Microscale magnetization reversal dynamics is one of the hot issues, because of a great demand for fast response and high density data storage devices, for
Lecture 9 Electronic Spectroscopy
 Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
Some pictures are taken from the UvA-VU Master Course: Advanced Solid State Physics by Anne de Visser (University of Amsterdam), Solid State Course
 Some pictures are taken from the UvA-VU Master Course: Advanced Solid State Physics by Anne de Visser (University of Amsterdam), Solid State Course by Mark Jarrel (Cincinnati University), from Ibach and
Some pictures are taken from the UvA-VU Master Course: Advanced Solid State Physics by Anne de Visser (University of Amsterdam), Solid State Course by Mark Jarrel (Cincinnati University), from Ibach and
X-Ray and Mössbauer Spectra and Electronic Structure of ScFe 2 Si 2 Compound
 Journal of Materials Science and Engineering B 5 (1-2) (2015) 42-49 doi: 10.17265/2161-6221/2015.1-2.004 D DAVID PUBLISHING X-Ray and Mössbauer Spectra and Electronic Structure of ScFe 2 Si 2 Compound
Journal of Materials Science and Engineering B 5 (1-2) (2015) 42-49 doi: 10.17265/2161-6221/2015.1-2.004 D DAVID PUBLISHING X-Ray and Mössbauer Spectra and Electronic Structure of ScFe 2 Si 2 Compound
Supporting information Chemical Design and Example of Transparent Bipolar Semiconductors
 Supporting information Chemical Design and Example of Transparent Bipolar Semiconductors Takeshi Arai 1, Soshi Iimura 1, *, Junghwan Kim 2, Yoshitake Toda 2, Shigenori Ueda 3, 4, and Hideo Hosono 1, 2,
Supporting information Chemical Design and Example of Transparent Bipolar Semiconductors Takeshi Arai 1, Soshi Iimura 1, *, Junghwan Kim 2, Yoshitake Toda 2, Shigenori Ueda 3, 4, and Hideo Hosono 1, 2,
High Resolution Photoemission Study of the Spin-Dependent Band Structure of Permalloy and Ni
 High Resolution Photoemission Study of the Spin-Dependent Band Structure of Permalloy and Ni K. N. Altmann, D. Y. Petrovykh, and F. J. Himpsel Department of Physics, University of Wisconsin, Madison, 1150
High Resolution Photoemission Study of the Spin-Dependent Band Structure of Permalloy and Ni K. N. Altmann, D. Y. Petrovykh, and F. J. Himpsel Department of Physics, University of Wisconsin, Madison, 1150
Photoelectron Spectroscopy
 Stefan Hüfner Photoelectron Spectroscopy Principles and Applications Third Revised and Enlarged Edition With 461 Figures and 28 Tables JSJ Springer ... 1. Introduction and Basic Principles 1 1.1 Historical
Stefan Hüfner Photoelectron Spectroscopy Principles and Applications Third Revised and Enlarged Edition With 461 Figures and 28 Tables JSJ Springer ... 1. Introduction and Basic Principles 1 1.1 Historical
Atomic Structure and Processes
 Chapter 5 Atomic Structure and Processes 5.1 Elementary atomic structure Bohr Orbits correspond to principal quantum number n. Hydrogen atom energy levels where the Rydberg energy is R y = m e ( e E n
Chapter 5 Atomic Structure and Processes 5.1 Elementary atomic structure Bohr Orbits correspond to principal quantum number n. Hydrogen atom energy levels where the Rydberg energy is R y = m e ( e E n
Magnetic linear dichroism effects in reflection spectroscopy: A case study at the Fe M 2,3 edge
 Magnetic linear dichroism effects in reflection spectroscopy: A case study at the Fe M 2,3 edge Hartmut Höchst a, Dennis Rioux b, Dai Zhao a and David L. Huber, a,c a Synchrotron Radiation Center, University
Magnetic linear dichroism effects in reflection spectroscopy: A case study at the Fe M 2,3 edge Hartmut Höchst a, Dennis Rioux b, Dai Zhao a and David L. Huber, a,c a Synchrotron Radiation Center, University
ELEMENTARY BAND THEORY
 ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
Molecular-Orbital Theory
 Prof. Dr. I. Nasser atomic and molecular physics -551 (T-11) April 18, 01 Molecular-Orbital Theory You have to explain the following statements: 1- Helium is monatomic gas. - Oxygen molecule has a permanent
Prof. Dr. I. Nasser atomic and molecular physics -551 (T-11) April 18, 01 Molecular-Orbital Theory You have to explain the following statements: 1- Helium is monatomic gas. - Oxygen molecule has a permanent
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Magnetic Anisotropy. Chapter Introduction
 Chapter 3 Magnetic Anisotropy The work presented in this chapter was published as Large Magnetic Anisotropy of a Single Atomic Spin Embedded in a Surface Molecular Network, by C. F. Hirjibehedin, C.-Y.
Chapter 3 Magnetic Anisotropy The work presented in this chapter was published as Large Magnetic Anisotropy of a Single Atomic Spin Embedded in a Surface Molecular Network, by C. F. Hirjibehedin, C.-Y.
Time Resolved Faraday Rotation Measurements of Spin Polarized Currents in Quantum Wells
 Time Resolved Faraday Rotation Measurements of Spin Polarized Currents in Quantum Wells M. R. Beversluis 17 December 2001 1 Introduction For over thirty years, silicon based electronics have continued
Time Resolved Faraday Rotation Measurements of Spin Polarized Currents in Quantum Wells M. R. Beversluis 17 December 2001 1 Introduction For over thirty years, silicon based electronics have continued
1.1 Units, definitions and fundamental equations. How should we deal with B and H which are usually used for magnetic fields?
 Advance Organizer: Chapter 1: Introduction to single magnetic moments: Magnetic dipoles Spin and orbital angular momenta Spin-orbit coupling Magnetic susceptibility, Magnetic dipoles in a magnetic field:
Advance Organizer: Chapter 1: Introduction to single magnetic moments: Magnetic dipoles Spin and orbital angular momenta Spin-orbit coupling Magnetic susceptibility, Magnetic dipoles in a magnetic field:
Investigation of Ti2AlC and TiC by soft x-ray emission spectroscopy
 Investigation of Ti2AlC and TiC by soft x-ray emission spectroscopy Martin Magnuson Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Martin
Investigation of Ti2AlC and TiC by soft x-ray emission spectroscopy Martin Magnuson Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Martin
Lecture 10. Transition probabilities and photoelectric cross sections
 Lecture 10 Transition probabilities and photoelectric cross sections TRANSITION PROBABILITIES AND PHOTOELECTRIC CROSS SECTIONS Cross section = = Transition probability per unit time of exciting a single
Lecture 10 Transition probabilities and photoelectric cross sections TRANSITION PROBABILITIES AND PHOTOELECTRIC CROSS SECTIONS Cross section = = Transition probability per unit time of exciting a single
