24. Atomic Spectra, Term Symbols and Hund s Rules
|
|
|
- Lucas Butler
- 6 years ago
- Views:
Transcription
1 Page of 4. Atomc Spectra, Term Symbols and Hund s Rules Date: 5 October 00 Suggested Readng: Chapters 8-8 to 8- of the text. Introducton Electron confguratons, at least n the forms used n general chemstry are ambguous. For example for carbon s s p, where to the two electrons n the p orbtals resde? In whch of the three p orbtals, p -, p 0, p +? What s the spn on the electrons. As we saw n the last lecture, all of the varous quantum numbers affect the energy of the state, not just the prncple quantum number, n mult-electron systems. We need a new way to desgnate a state unambguously. The scheme presented here s based on the total angular momentum, J, formed by addng the total orbtal angular momentum, L, and the total spn angular momentum, S. The symbol used to desgnate a state by ths means s called a term symbol and s of the form S + LJ (4-)
2 Introducton to Quantum Chemstry Lecture # 4 Page of The scheme s called Russell-Saunders couplng and s performed as follows: The total orbtal angular momentum s gven by L j l j (4-) a vector addton. The total spn angular momentum s gven by S j s j (4-3) another vector addton. The z components of L and S are gven by the scalar sums L z l z, m M L (4-4) and S z s z, m s, M S (4-5) Thus there are L + values of M L spannng -L, -L+, -L+,..., L-, L and S + values of M S for S spannng -S, -S+, -S+,..., S-, S.
3 Introducton to Quantum Chemstry Lecture # 4 Page 3 of The leadng S+ superscrpt n the term symbol s called the spn multplcty. Table 4-: Names for the leadng superscrpts of atomc term symbols. S+ Name snglet doublet 3 trplet The value of L s substtuted as n the hydrogen-lke orbtals but wth a captal letter. That s, Table 4-: Letter conversons for atomc term symbols. Value of L Letter 0 S P D 3 F 4 G 5 H The value of J s kept as a number. Thus for example: 3 S - trplet S one D 3 - snglet D three
4 Introducton to Quantum Chemstry Lecture # 4 Page 4 of Examples Hydrogen: H s, The maxmum value of M S s M Smax, m s, -- (4-6) to gve S /. The maxmum value of L s M Lmax, m 0 (4-7) thus L 0 and J L + S /. The term symbol for the ground state of hydrogen s S -- (4-8) The ground state of He, s : M Smax, m s, (4-9)
5 Introducton to Quantum Chemstry Lecture # 4 Page 5 of M Lmax, m (4-0) Therefore, the term symbol s S0 (4-) An excted state of He, s s : M Smax, m s, (4-) therefore M S, 0, - but what value of S? M Lmax, m (4-3) Mcrostates To answer our queston above, there are two methods. One nvolves nspecton of the problem and works quckly but s not relable, the second s
6 Introducton to Quantum Chemstry Lecture # 4 Page 6 of perhaps tedous, but always works. It s ths second methods that s gven n the text and wll be used here. Lets create a table M L M S 0-0 (0 +,0 + ) (0 +,0 - ), (0 -,0 + ) (0 -,0 - ) The notaton (0 +,0 + ) means that (m 0, m s +/; m 0, m s +/) for the two electrons. (0 +,0 - ) and (0 -,0 + ) are not equvalent because the two electrons are n separate orbtals. The four entres are called mcrostates. Lets loot at L 0, S, L 0 -> M L 0 S -> M S, 0, - Ths corresponds to the mcrostates (0 +,0 + ), (0 +,0 - ) and (0 -,0 - ). We have one unaccounted for mcrostate, (0 -,0 + ). Ths mcrostate has S 0, L 0. Thus there are two possble states for He s s : 3 S S0 (4-4) The allowed values of J are J L+S, L+S-, L+S-,..., L-S.
7 Introducton to Quantum Chemstry Lecture # 4 Page 7 of Beryllum s s : M Smax, m s, (4-5) therefore M S 0. M Lmax, m (4-6) therefore the term symbol s S 0 (4-7) Flled subshells have total angular momentum of zero and make no contrbuton to the determnaton of the term symbol. Back to carbon. We have sx possble spn orbtals n whch to place the two electrons p 0 α, p 0 β, p - α, p - β, p α, p β Ths s 6 choose
8 Introducton to Quantum Chemstry Lecture # 4 Page 8 of 6 6! !4! 65 ( ) (4-8) There wll be 5 mcrostates to deal wth. In general, f we have j spn orbtals and need to place k electrons we have j j! k k! ( j k)! (4-9) mcrostates. For example 3d has 0 0! !8! 45 (4-0) mcrostates. How about 3d 8? 0 0! !! 45 (4-) mcrostates. d 0-j has the same number of mcrostates as d j. Back to carbon: The two electrons may go n the m -, 0, orbtals. Thus M L ranges from to -. The spns may be pared or unpared, hence M S ->, 0, -.
9 Introducton to Quantum Chemstry Lecture # 4 Page 9 of M L M S 0 - ( +, - ) (0 +, + ) ( +,0 - ), (0 +, - ) (0 -, - ) 0 ( +,- + ) ( +,- - ), (- +, - ), (0 +,0 - ) ( -,- - ) - (0 +,- + ) (- +,0 - ), (0 +,- - ) (0 -,- - ) - (- +,- - ) Snce the electrons are n the same shell ( +, - ) and ( -, + ) are ndstngushable and hence only one s ncluded. Combnatons that are excluded by the Paul Prncple do not make up the table entres. to gve The largest value of M L s and has M S 0, therefore L, S 0, J D (4-) Ths state has M S 0, M L,, 0, -, - for fve mcrostates. The next largest value of M L remanng s M L. The largest value of M S assocated wth ths s M S. Therefor L, S 3 P (4-3)
10 Introducton to Quantum Chemstry Lecture # 4 Page 0 of wth M L, 0, - and M S, 0, - for nne mcrostates. The possble values of J are J (+), (+-), (-) or J,, 0 hence we have 3 P, 3 P and 3 P 0 wth 5, 3, and mcrostates respectvely. Ths leaves a sngle mcrostate remanng wth M L 0 and M S 0 to gve S0 (4-4) Thus the states for the ground state confguraton of carbon are D, 3 P, 3 P and 3 P 0, S 0 The degeneracy of each state s J+ for 5,, 3, 5, for 5 mcrostates. The term symbols for a gven electron confguraton correspond to that wth the same number of holes. For example p and p 4 or 3d 4 and 3d 6 (see Table 8.4 n the text). Hund s Rules Each of the states desgnated by a term symbol corresponds to a determnantal wave functon that s an egenfuncton of Lˆ and Ŝ. Thus each state corresponds to a gven energy. The spectroscopst Fredrch Hund worked out a set of emprcal rules to order the energes of the states.
11 Introducton to Quantum Chemstry Lecture # 4 Page of. The state wth the largest value of S s the most stable (has the lowest energy) and stablty decreases wth decreasng S.. For states wth the same value of S, the state wth the largest value of L s the most stable. 3. If the states have the same value of L and S, then for a subshell that s less than half flled, the state wth the smallest value of J s the most stable. For a subshell that s more than half flled, the state wth the largest value of J s the most stable. For example for carbon, 3 P 0 s the lowest energy state. [As an exercse work out the state for d usng the mcrostate method.] The term symbols are used to descrbe atomc spectra. Thus far n our development of the Hamltonan operator for atoms we have ncluded knetc energy and electrostatc potental energy terms. That s, Ĥ -- j j j Z r j ---- r j < j (4-5) We need to nclude spn and magnetc terms as well. The most mportant s the spn-orbt nteracton term whch results from the nteracton of the magnetc moment assocated wth the spn of an electron wth the magnetc feld generated by the electrc current produced by the electron s own orbtal moton. Other terms nclude spn-spn and orbt-orbt nteracton (n mult-electron systems) but spn-orbt nteractons are the most mportant.
12 Introducton to Quantum Chemstry Lecture # 4 Page of Ĥ -- j j j Z r j + ξ( r r j )l j s j j < j j (4-6) If the effect of the spn-orbt couplng s small, as t s for lght atoms (approxmately Z < 30) the effect may be treated as a perturbaton. For heaver atoms, the effect s not small and must be dealt wth explctly. Selecton Rules for Atomc Spectra Transtons nvolvng lght are allowed wth atoms for the followng L ± S 0 J 0, ± (4-7) expect that a transton from a state wth J 0 to another state wth J 0 s not allowed (forbdden). Suggested Readng for Next Lecture: Chapter 9 of the text.
5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 2 May 11: Ligand Field Theory
 5.03, Inorganc Chemstry Prof. Danel G. Nocera Lecture May : Lgand Feld Theory The lgand feld problem s defned by the followng Hamltonan, h p Η = wth E n = KE = where = m m x y z h m Ze r hydrogen atom
5.03, Inorganc Chemstry Prof. Danel G. Nocera Lecture May : Lgand Feld Theory The lgand feld problem s defned by the followng Hamltonan, h p Η = wth E n = KE = where = m m x y z h m Ze r hydrogen atom
Complex Atoms; The Exclusion Principle and the Periodic System
 Complex Atoms; The Excluson Prncple and the Perodc System In order to understand the electron dstrbutons n atoms, another prncple s needed. Ths s the Paul excluson prncple: No two electrons n an atom can
Complex Atoms; The Excluson Prncple and the Perodc System In order to understand the electron dstrbutons n atoms, another prncple s needed. Ths s the Paul excluson prncple: No two electrons n an atom can
Multi-electron atoms (11) 2010 update Extend the H-atom picture to more than 1 electron: H-atom sol'n use for N-elect., assume product wavefct.
 Mult-electron atoms (11) 2010 update Extend the H-atom pcture to more than 1 electron: VII 33 H-atom sol'n use for -elect., assume product wavefct. n ψ = φn l m where: ψ mult electron w/fct φ n l m one
Mult-electron atoms (11) 2010 update Extend the H-atom pcture to more than 1 electron: VII 33 H-atom sol'n use for -elect., assume product wavefct. n ψ = φn l m where: ψ mult electron w/fct φ n l m one
5.76 Lecture #5 2/07/94 Page 1 of 10 pages. Lecture #5: Atoms: 1e and Alkali. centrifugal term ( +1)
 5.76 Lecture #5 /07/94 Page 1 of 10 pages 1e Atoms: H, H + e, L +, etc. coupled and uncoupled bass sets Lecture #5: Atoms: 1e and Alkal centrfugal term (+1) r radal Schrödnger Equaton spn-orbt l s r 3
5.76 Lecture #5 /07/94 Page 1 of 10 pages 1e Atoms: H, H + e, L +, etc. coupled and uncoupled bass sets Lecture #5: Atoms: 1e and Alkal centrfugal term (+1) r radal Schrödnger Equaton spn-orbt l s r 3
The Dirac Equation for a One-electron atom. In this section we will derive the Dirac equation for a one-electron atom.
 The Drac Equaton for a One-electron atom In ths secton we wll derve the Drac equaton for a one-electron atom. Accordng to Ensten the energy of a artcle wth rest mass m movng wth a velocty V s gven by E
The Drac Equaton for a One-electron atom In ths secton we wll derve the Drac equaton for a one-electron atom. Accordng to Ensten the energy of a artcle wth rest mass m movng wth a velocty V s gven by E
Note on the Electron EDM
 Note on the Electron EDM W R Johnson October 25, 2002 Abstract Ths s a note on the setup of an electron EDM calculaton and Schff s Theorem 1 Basc Relatons The well-known relatvstc nteracton of the electron
Note on the Electron EDM W R Johnson October 25, 2002 Abstract Ths s a note on the setup of an electron EDM calculaton and Schff s Theorem 1 Basc Relatons The well-known relatvstc nteracton of the electron
ψ ij has the eigenvalue
 Moller Plesset Perturbaton Theory In Moller-Plesset (MP) perturbaton theory one taes the unperturbed Hamltonan for an atom or molecule as the sum of the one partcle Foc operators H F() where the egenfunctons
Moller Plesset Perturbaton Theory In Moller-Plesset (MP) perturbaton theory one taes the unperturbed Hamltonan for an atom or molecule as the sum of the one partcle Foc operators H F() where the egenfunctons
CHAPTER 14 GENERAL PERTURBATION THEORY
 CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
Non-interacting Spin-1/2 Particles in Non-commuting External Magnetic Fields
 EJTP 6, No. 0 009) 43 56 Electronc Journal of Theoretcal Physcs Non-nteractng Spn-1/ Partcles n Non-commutng External Magnetc Felds Kunle Adegoke Physcs Department, Obafem Awolowo Unversty, Ile-Ife, Ngera
EJTP 6, No. 0 009) 43 56 Electronc Journal of Theoretcal Physcs Non-nteractng Spn-1/ Partcles n Non-commutng External Magnetc Felds Kunle Adegoke Physcs Department, Obafem Awolowo Unversty, Ile-Ife, Ngera
Yukawa Potential and the Propagator Term
 PHY304 Partcle Physcs 4 Dr C N Booth Yukawa Potental an the Propagator Term Conser the electrostatc potental about a charge pont partcle Ths s gven by φ = 0, e whch has the soluton φ = Ths escrbes the
PHY304 Partcle Physcs 4 Dr C N Booth Yukawa Potental an the Propagator Term Conser the electrostatc potental about a charge pont partcle Ths s gven by φ = 0, e whch has the soluton φ = Ths escrbes the
CHE450G Final Exam. CP-109 December 11, :30-12:30 AM
 CH450G Fnal xam CP-09 December, 2006 0:30-2:30 AM Last name Frst Name Score [ /5] 00 = % () Construct a physcally realstc molecular orbtal dagram for CS. Draw all SALC s, molecular orbtals, and provde
CH450G Fnal xam CP-09 December, 2006 0:30-2:30 AM Last name Frst Name Score [ /5] 00 = % () Construct a physcally realstc molecular orbtal dagram for CS. Draw all SALC s, molecular orbtals, and provde
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Probabilistic method to determine electron correlation energy
 Probablstc method to determne electron elaton energy T.R.S. Prasanna Department of Metallurgcal Engneerng and Materals Scence Indan Insttute of Technology, Bombay Mumba 400076 Inda A new method to determne
Probablstc method to determne electron elaton energy T.R.S. Prasanna Department of Metallurgcal Engneerng and Materals Scence Indan Insttute of Technology, Bombay Mumba 400076 Inda A new method to determne
XII. The Born-Oppenheimer Approximation
 X. The Born-Oppenhemer Approxmaton The Born- Oppenhemer (BO) approxmaton s probably the most fundamental approxmaton n chemstry. From a practcal pont of vew t wll allow us to treat the ectronc structure
X. The Born-Oppenhemer Approxmaton The Born- Oppenhemer (BO) approxmaton s probably the most fundamental approxmaton n chemstry. From a practcal pont of vew t wll allow us to treat the ectronc structure
Module 9. Lecture 6. Duality in Assignment Problems
 Module 9 1 Lecture 6 Dualty n Assgnment Problems In ths lecture we attempt to answer few other mportant questons posed n earler lecture for (AP) and see how some of them can be explaned through the concept
Module 9 1 Lecture 6 Dualty n Assgnment Problems In ths lecture we attempt to answer few other mportant questons posed n earler lecture for (AP) and see how some of them can be explaned through the concept
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Negative-energy contributions to transition amplitudes in heliumlike ions
 PHYSICAL REVIEW A VOLUME 58, NUMBER 6 DECEMBER 998 Negatve-energy contrbutons to transton ampltudes n helumlke ons A. Derevanko, Igor M. Savukov, and W. R. Johnson Department of Physcs, Notre Dame Unversty,
PHYSICAL REVIEW A VOLUME 58, NUMBER 6 DECEMBER 998 Negatve-energy contrbutons to transton ampltudes n helumlke ons A. Derevanko, Igor M. Savukov, and W. R. Johnson Department of Physcs, Notre Dame Unversty,
A how to guide to second quantization method.
 Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Electron-Impact Double Ionization of the H 2
 I R A P 6(), Dec. 5, pp. 9- Electron-Impact Double Ionzaton of the H olecule Internatonal Scence Press ISSN: 9-59 Electron-Impact Double Ionzaton of the H olecule. S. PINDZOLA AND J. COLGAN Department
I R A P 6(), Dec. 5, pp. 9- Electron-Impact Double Ionzaton of the H olecule Internatonal Scence Press ISSN: 9-59 Electron-Impact Double Ionzaton of the H olecule. S. PINDZOLA AND J. COLGAN Department
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Dynamics of a Superconducting Qubit Coupled to an LC Resonator
 Dynamcs of a Superconductng Qubt Coupled to an LC Resonator Y Yang Abstract: We nvestgate the dynamcs of a current-based Josephson juncton quantum bt or qubt coupled to an LC resonator. The Hamltonan of
Dynamcs of a Superconductng Qubt Coupled to an LC Resonator Y Yang Abstract: We nvestgate the dynamcs of a current-based Josephson juncton quantum bt or qubt coupled to an LC resonator. The Hamltonan of
Level Crossing Spectroscopy
 Level Crossng Spectroscopy October 8, 2008 Contents 1 Theory 1 2 Test set-up 4 3 Laboratory Exercses 4 3.1 Hanle-effect for fne structure.................... 4 3.2 Hanle-effect for hyperfne structure.................
Level Crossng Spectroscopy October 8, 2008 Contents 1 Theory 1 2 Test set-up 4 3 Laboratory Exercses 4 3.1 Hanle-effect for fne structure.................... 4 3.2 Hanle-effect for hyperfne structure.................
Intermolecular force fields and how they can be determined
 Intermolecular force felds and how they can be determned Ad van der Avord Unversty of Njmegen Han-sur-Lesse, December 23 p.1 Equaton of state (Van der Waals) of non-deal gas ( p + a )( ) V 2 V b = kt repulson
Intermolecular force felds and how they can be determned Ad van der Avord Unversty of Njmegen Han-sur-Lesse, December 23 p.1 Equaton of state (Van der Waals) of non-deal gas ( p + a )( ) V 2 V b = kt repulson
V.C The Niemeijer van Leeuwen Cumulant Approximation
 V.C The Nemejer van Leeuwen Cumulant Approxmaton Unfortunately, the decmaton procedure cannot be performed exactly n hgher dmensons. For example, the square lattce can be dvded nto two sublattces. For
V.C The Nemejer van Leeuwen Cumulant Approxmaton Unfortunately, the decmaton procedure cannot be performed exactly n hgher dmensons. For example, the square lattce can be dvded nto two sublattces. For
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Born-Oppenheimer Approximation
 5.76 Lecture otes /16/94 Page 1 The Born-Oppenhemer Approxmaton For atoms we use SCF to defne 1e eff orbtals. Get V for each e n feld of e s n all other occuped () r orbtals. ψ ( r) = φ 1( r1) φ( r ) sngle
5.76 Lecture otes /16/94 Page 1 The Born-Oppenhemer Approxmaton For atoms we use SCF to defne 1e eff orbtals. Get V for each e n feld of e s n all other occuped () r orbtals. ψ ( r) = φ 1( r1) φ( r ) sngle
PHYS 215C: Quantum Mechanics (Spring 2017) Problem Set 3 Solutions
 PHYS 5C: Quantum Mechancs Sprng 07 Problem Set 3 Solutons Prof. Matthew Fsher Solutons prepared by: Chatanya Murthy and James Sully June 4, 07 Please let me know f you encounter any typos n the solutons.
PHYS 5C: Quantum Mechancs Sprng 07 Problem Set 3 Solutons Prof. Matthew Fsher Solutons prepared by: Chatanya Murthy and James Sully June 4, 07 Please let me know f you encounter any typos n the solutons.
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Complex atoms and the Periodic System of the elements
 Complex atoms and the Peodc System of the elements Non-cental foces due to electon epulson Cental feld appoxmaton electonc obtals lft degeneacy of l E n l = R( hc) ( n δ ) l Aufbau pncple Lectue Notes
Complex atoms and the Peodc System of the elements Non-cental foces due to electon epulson Cental feld appoxmaton electonc obtals lft degeneacy of l E n l = R( hc) ( n δ ) l Aufbau pncple Lectue Notes
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
THERMAL DISTRIBUTION IN THE HCL SPECTRUM OBJECTIVE
 ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
GREENBERGER- HORNE- ZEILINGER (GHZ) STATES IN QUANTUM DOT MOLECULE
 GREENERGER- ORNE- ZEILINGER (GZ) STATES IN QUANTUM DOT MOLECULE A. SARMA and P. AWRYLAK QUANTUM TEORY GROUP INSTITUTE FOR MICROSTRUCTURAL SCIENCES NATIONAL RESEARC COUNCIL OF CANADA OTTAWA, KAOR,CANADA
GREENERGER- ORNE- ZEILINGER (GZ) STATES IN QUANTUM DOT MOLECULE A. SARMA and P. AWRYLAK QUANTUM TEORY GROUP INSTITUTE FOR MICROSTRUCTURAL SCIENCES NATIONAL RESEARC COUNCIL OF CANADA OTTAWA, KAOR,CANADA
where the sums are over the partcle labels. In general H = p2 2m + V s(r ) V j = V nt (jr, r j j) (5) where V s s the sngle-partcle potental and V nt
 Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Physcs 543 Quantum Mechancs II Fall 998 Hartree-Fock and the Self-consstent Feld Varatonal Methods In the dscusson of statonary perturbaton theory, I mentoned brey the dea of varatonal approxmaton schemes.
Lagrangian Field Theory
 Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Lagrangan Feld Theory Adam Lott PHY 391 Aprl 6, 017 1 Introducton Ths paper s a summary of Chapter of Mandl and Shaw s Quantum Feld Theory [1]. The frst thng to do s to fx the notaton. For the most part,
Introduction to Density Functional Theory. Jeremie Zaffran 2 nd year-msc. (Nanochemistry)
 Introducton to Densty Functonal Theory Jereme Zaffran nd year-msc. (anochemstry) A- Hartree appromatons Born- Oppenhemer appromaton H H H e The goal of computatonal chemstry H e??? Let s remnd H e T e
Introducton to Densty Functonal Theory Jereme Zaffran nd year-msc. (anochemstry) A- Hartree appromatons Born- Oppenhemer appromaton H H H e The goal of computatonal chemstry H e??? Let s remnd H e T e
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
Applied Nuclear Physics (Fall 2004) Lecture 23 (12/3/04) Nuclear Reactions: Energetics and Compound Nucleus
 .101 Appled Nuclear Physcs (Fall 004) Lecture 3 (1/3/04) Nuclear Reactons: Energetcs and Compound Nucleus References: W. E. Meyerhof, Elements of Nuclear Physcs (McGraw-Hll, New York, 1967), Chap 5. Among
.101 Appled Nuclear Physcs (Fall 004) Lecture 3 (1/3/04) Nuclear Reactons: Energetcs and Compound Nucleus References: W. E. Meyerhof, Elements of Nuclear Physcs (McGraw-Hll, New York, 1967), Chap 5. Among
Spin. Introduction. Michael Fowler 11/26/06
 Spn Mchael Fowler /6/6 Introducton The Stern Gerlach experment for the smplest possble atom, hydrogen n ts ground state, demonstrated unambguously that the component of the magnetc moment of the atom along
Spn Mchael Fowler /6/6 Introducton The Stern Gerlach experment for the smplest possble atom, hydrogen n ts ground state, demonstrated unambguously that the component of the magnetc moment of the atom along
Lecture 20: Noether s Theorem
 Lecture 20: Noether s Theorem In our revew of Newtonan Mechancs, we were remnded that some quanttes (energy, lnear momentum, and angular momentum) are conserved That s, they are constant f no external
Lecture 20: Noether s Theorem In our revew of Newtonan Mechancs, we were remnded that some quanttes (energy, lnear momentum, and angular momentum) are conserved That s, they are constant f no external
At zero K: All atoms frozen at fixed positions on a periodic lattice.
 September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
Electronic Structure for Excited States (multiconfigurational methods) Spiridoula Matsika
 Electronc Structure for Excted States (multconfguratonal methods) Sprdoula Matska Excted Electronc States Theoretcal treatment of excted states s needed for: UV/Vs electronc spectroscopy Photochemstry
Electronc Structure for Excted States (multconfguratonal methods) Sprdoula Matska Excted Electronc States Theoretcal treatment of excted states s needed for: UV/Vs electronc spectroscopy Photochemstry
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Chapter 11 Coordination Chemistry III: Electronic Spectra
 Chapter Coordination Chemistry III: Electronic Spectra - Absorption of Light -2 Quantum Numbers of Multielectron Atoms - Electronic Spectra of Coordination Compounds Chapter Coordination Chemistry III:
Chapter Coordination Chemistry III: Electronic Spectra - Absorption of Light -2 Quantum Numbers of Multielectron Atoms - Electronic Spectra of Coordination Compounds Chapter Coordination Chemistry III:
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Physics 141. Lecture 14. Frank L. H. Wolfs Department of Physics and Astronomy, University of Rochester, Lecture 14, Page 1
 Physcs 141. Lecture 14. Frank L. H. Wolfs Department of Physcs and Astronomy, Unversty of Rochester, Lecture 14, Page 1 Physcs 141. Lecture 14. Course Informaton: Lab report # 3. Exam # 2. Mult-Partcle
Physcs 141. Lecture 14. Frank L. H. Wolfs Department of Physcs and Astronomy, Unversty of Rochester, Lecture 14, Page 1 Physcs 141. Lecture 14. Course Informaton: Lab report # 3. Exam # 2. Mult-Partcle
Errors in Nobel Prize for Physics (7) Improper Schrodinger Equation and Dirac Equation
 Errors n Nobel Prze for Physcs (7) Improper Schrodnger Equaton and Drac Equaton u Yuhua (CNOOC Research Insttute, E-mal:fuyh945@sna.com) Abstract: One of the reasons for 933 Nobel Prze for physcs s for
Errors n Nobel Prze for Physcs (7) Improper Schrodnger Equaton and Drac Equaton u Yuhua (CNOOC Research Insttute, E-mal:fuyh945@sna.com) Abstract: One of the reasons for 933 Nobel Prze for physcs s for
Solutions to Problems Fundamentals of Condensed Matter Physics
 Solutons to Problems Fundamentals of Condensed Matter Physcs Marvn L. Cohen Unversty of Calforna, Berkeley Steven G. Loue Unversty of Calforna, Berkeley c Cambrdge Unversty Press 016 1 Acknowledgement
Solutons to Problems Fundamentals of Condensed Matter Physcs Marvn L. Cohen Unversty of Calforna, Berkeley Steven G. Loue Unversty of Calforna, Berkeley c Cambrdge Unversty Press 016 1 Acknowledgement
CI/CEPA. Introduction CI Size Consistency Derivation CEPA EPV Results Remarks
 CI/CEPA Introducton CI Sze Consstency Dervaton CEPA EPV Results Remarks 1 HY=EY H e Ψ e = E e Ψe Expanson of the Many-Electron Wave Functon Ψ HF Ψ e = Φ o + a a C Φ + ab ab C j Φ j +... Φo = A φ 1 φ 2...
CI/CEPA Introducton CI Sze Consstency Dervaton CEPA EPV Results Remarks 1 HY=EY H e Ψ e = E e Ψe Expanson of the Many-Electron Wave Functon Ψ HF Ψ e = Φ o + a a C Φ + ab ab C j Φ j +... Φo = A φ 1 φ 2...
Matrix Mechanics Exercises Using Polarized Light
 Matrx Mechancs Exercses Usng Polarzed Lght Frank Roux Egenstates and operators are provded for a seres of matrx mechancs exercses nvolvng polarzed lght. Egenstate for a -polarzed lght: Θ( θ) ( ) smplfy
Matrx Mechancs Exercses Usng Polarzed Lght Frank Roux Egenstates and operators are provded for a seres of matrx mechancs exercses nvolvng polarzed lght. Egenstate for a -polarzed lght: Θ( θ) ( ) smplfy
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Workshop: Approximating energies and wave functions Quantum aspects of physical chemistry
 Workshop: Approxmatng energes and wave functons Quantum aspects of physcal chemstry http://quantum.bu.edu/pltl/6/6.pdf Last updated Thursday, November 7, 25 7:9:5-5: Copyrght 25 Dan Dll (dan@bu.edu) Department
Workshop: Approxmatng energes and wave functons Quantum aspects of physcal chemstry http://quantum.bu.edu/pltl/6/6.pdf Last updated Thursday, November 7, 25 7:9:5-5: Copyrght 25 Dan Dll (dan@bu.edu) Department
Chapter 1. Probability
 Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Chapter. Probablty Mcroscopc propertes of matter: quantum mechancs, atomc and molecular propertes Macroscopc propertes of matter: thermodynamcs, E, H, C V, C p, S, A, G How do we relate these two propertes?
Density matrix. c α (t)φ α (q)
 Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
Densty matrx Note: ths s supplementary materal. I strongly recommend that you read t for your own nterest. I beleve t wll help wth understandng the quantum ensembles, but t s not necessary to know t n
8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS
 SECTION 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS 493 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS All the vector spaces you have studed thus far n the text are real vector spaces because the scalars
SECTION 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS 493 8.4 COMPLEX VECTOR SPACES AND INNER PRODUCTS All the vector spaces you have studed thus far n the text are real vector spaces because the scalars
E For K > 0. s s s s Fall Physical Chemistry (II) by M. Lim. singlet. triplet
 Eneges of He electonc ψ E Fo K > 0 ψ = snglet ( )( ) s s+ ss αβ E βα snglet = ε + ε + J s + Ks Etplet = ε + ε + J s Ks αα ψ tplet = ( s s ss ) ββ ( αβ + βα ) s s s s s s s s ψ G = ss( αβ βα ) E = ε + ε
Eneges of He electonc ψ E Fo K > 0 ψ = snglet ( )( ) s s+ ss αβ E βα snglet = ε + ε + J s + Ks Etplet = ε + ε + J s Ks αα ψ tplet = ( s s ss ) ββ ( αβ + βα ) s s s s s s s s ψ G = ss( αβ βα ) E = ε + ε
8.022 (E&M) Lecture 4
 Topcs: 8.0 (E&M) Lecture 4 More applcatons of vector calculus to electrostatcs: Laplacan: Posson and Laplace equaton url: concept and applcatons to electrostatcs Introducton to conductors Last tme Electrc
Topcs: 8.0 (E&M) Lecture 4 More applcatons of vector calculus to electrostatcs: Laplacan: Posson and Laplace equaton url: concept and applcatons to electrostatcs Introducton to conductors Last tme Electrc
Mechanics Physics 151
 Mechancs Physcs 151 Lecture 3 Lagrange s Equatons (Goldsten Chapter 1) Hamlton s Prncple (Chapter 2) What We Dd Last Tme! Dscussed mult-partcle systems! Internal and external forces! Laws of acton and
Mechancs Physcs 151 Lecture 3 Lagrange s Equatons (Goldsten Chapter 1) Hamlton s Prncple (Chapter 2) What We Dd Last Tme! Dscussed mult-partcle systems! Internal and external forces! Laws of acton and
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Canonical transformations
 Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Physics 30 Lesson 31 The Bohr Model of the Atom
 Physcs 30 Lesson 31 The Bohr Model o the Atom I. Planetary models o the atom Ater Rutherord s gold ol scatterng experment, all models o the atom eatured a nuclear model wth electrons movng around a tny,
Physcs 30 Lesson 31 The Bohr Model o the Atom I. Planetary models o the atom Ater Rutherord s gold ol scatterng experment, all models o the atom eatured a nuclear model wth electrons movng around a tny,
Department of Chemistry Purdue University Garth J. Simpson
 Objectves: 1. Develop a smple conceptual 1D model for NLO effects. Extend to 3D and relate to computatonal chemcal calculatons of adabatc NLO polarzabltes. 2. Introduce Sum-Over-States (SOS) approaches
Objectves: 1. Develop a smple conceptual 1D model for NLO effects. Extend to 3D and relate to computatonal chemcal calculatons of adabatc NLO polarzabltes. 2. Introduce Sum-Over-States (SOS) approaches
Lecture 14: Forces and Stresses
 The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
The optimal delay of the second test is therefore approximately 210 hours earlier than =2.
 THE IEC 61508 FORMULAS 223 The optmal delay of the second test s therefore approxmately 210 hours earler than =2. 8.4 The IEC 61508 Formulas IEC 61508-6 provdes approxmaton formulas for the PF for smple
THE IEC 61508 FORMULAS 223 The optmal delay of the second test s therefore approxmately 210 hours earler than =2. 8.4 The IEC 61508 Formulas IEC 61508-6 provdes approxmaton formulas for the PF for smple
Quantum Mechanics I Problem set No.1
 Quantum Mechancs I Problem set No.1 Septembe0, 2017 1 The Least Acton Prncple The acton reads S = d t L(q, q) (1) accordng to the least (extremal) acton prncple, the varaton of acton s zero 0 = δs = t
Quantum Mechancs I Problem set No.1 Septembe0, 2017 1 The Least Acton Prncple The acton reads S = d t L(q, q) (1) accordng to the least (extremal) acton prncple, the varaton of acton s zero 0 = δs = t
12. The Hamilton-Jacobi Equation Michael Fowler
 1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
1. The Hamlton-Jacob Equaton Mchael Fowler Back to Confguraton Space We ve establshed that the acton, regarded as a functon of ts coordnate endponts and tme, satsfes ( ) ( ) S q, t / t+ H qpt,, = 0, and
DEMO #8 - GAUSSIAN ELIMINATION USING MATHEMATICA. 1. Matrices in Mathematica
 demo8.nb 1 DEMO #8 - GAUSSIAN ELIMINATION USING MATHEMATICA Obectves: - defne matrces n Mathematca - format the output of matrces - appl lnear algebra to solve a real problem - Use Mathematca to perform
demo8.nb 1 DEMO #8 - GAUSSIAN ELIMINATION USING MATHEMATICA Obectves: - defne matrces n Mathematca - format the output of matrces - appl lnear algebra to solve a real problem - Use Mathematca to perform
Poisson brackets and canonical transformations
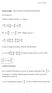 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Foundations of Arithmetic
 Foundatons of Arthmetc Notaton We shall denote the sum and product of numbers n the usual notaton as a 2 + a 2 + a 3 + + a = a, a 1 a 2 a 3 a = a The notaton a b means a dvdes b,.e. ac = b where c s an
Foundatons of Arthmetc Notaton We shall denote the sum and product of numbers n the usual notaton as a 2 + a 2 + a 3 + + a = a, a 1 a 2 a 3 a = a The notaton a b means a dvdes b,.e. ac = b where c s an
Transition State. Transition State. Reactants Products. Reaction Coordinate
 Ab Into Calculaton of xcted States from Quartc Potental Surfaces John L. Dasson Ncole R. Brnmann and Wllam F. Pol Department of Chemstry Hope College Holland Mchgan 494 Introducton The oerall goal of our
Ab Into Calculaton of xcted States from Quartc Potental Surfaces John L. Dasson Ncole R. Brnmann and Wllam F. Pol Department of Chemstry Hope College Holland Mchgan 494 Introducton The oerall goal of our
Section 3.6 Complex Zeros
 04 Chapter Secton 6 Comple Zeros When fndng the zeros of polynomals, at some pont you're faced wth the problem Whle there are clearly no real numbers that are solutons to ths equaton, leavng thngs there
04 Chapter Secton 6 Comple Zeros When fndng the zeros of polynomals, at some pont you're faced wth the problem Whle there are clearly no real numbers that are solutons to ths equaton, leavng thngs there
Molecular magnetic properties
 Molecular magnetc propertes Trygve Helgaker Centre for Theoretcal and Computatonal Chemstry Department of Chemstry, Unversty of Oslo, Norway European Summer School n Quantum Chemstry (ESQC) 2011 Torre
Molecular magnetc propertes Trygve Helgaker Centre for Theoretcal and Computatonal Chemstry Department of Chemstry, Unversty of Oslo, Norway European Summer School n Quantum Chemstry (ESQC) 2011 Torre
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
SINGLE EVENTS, TIME SERIES ANALYSIS, AND PLANETARY MOTION
 SINGLE EVENTS, TIME SERIES ANALYSIS, AND PLANETARY MOTION John N. Harrs INTRODUCTION The advent of modern computng devces and ther applcaton to tme-seres analyses permts the nvestgaton of mathematcal and
SINGLE EVENTS, TIME SERIES ANALYSIS, AND PLANETARY MOTION John N. Harrs INTRODUCTION The advent of modern computng devces and ther applcaton to tme-seres analyses permts the nvestgaton of mathematcal and
B-spline-based complex-rotation method with spin-dependent interaction
 PHYSICAL REVIEW A 76, 012721 2007 B-splne-based complex-rotaton method wth spn-dependent nteracton T. K. Fang 1, * and T.. Chang 2,3 1 Department of Physcs, Fu Jen Catholc Unversty, Tape, Tawan 242, Republc
PHYSICAL REVIEW A 76, 012721 2007 B-splne-based complex-rotaton method wth spn-dependent nteracton T. K. Fang 1, * and T.. Chang 2,3 1 Department of Physcs, Fu Jen Catholc Unversty, Tape, Tawan 242, Republc
C/CS/Phy191 Problem Set 3 Solutions Out: Oct 1, 2008., where ( 00. ), so the overall state of the system is ) ( ( ( ( 00 ± 11 ), Φ ± = 1
 C/CS/Phy9 Problem Set 3 Solutons Out: Oct, 8 Suppose you have two qubts n some arbtrary entangled state ψ You apply the teleportaton protocol to each of the qubts separately What s the resultng state obtaned
C/CS/Phy9 Problem Set 3 Solutons Out: Oct, 8 Suppose you have two qubts n some arbtrary entangled state ψ You apply the teleportaton protocol to each of the qubts separately What s the resultng state obtaned
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
Some Comments on Accelerating Convergence of Iterative Sequences Using Direct Inversion of the Iterative Subspace (DIIS)
 Some Comments on Acceleratng Convergence of Iteratve Sequences Usng Drect Inverson of the Iteratve Subspace (DIIS) C. Davd Sherrll School of Chemstry and Bochemstry Georga Insttute of Technology May 1998
Some Comments on Acceleratng Convergence of Iteratve Sequences Usng Drect Inverson of the Iteratve Subspace (DIIS) C. Davd Sherrll School of Chemstry and Bochemstry Georga Insttute of Technology May 1998
LECTURE 9 CANONICAL CORRELATION ANALYSIS
 LECURE 9 CANONICAL CORRELAION ANALYSIS Introducton he concept of canoncal correlaton arses when we want to quantfy the assocatons between two sets of varables. For example, suppose that the frst set of
LECURE 9 CANONICAL CORRELAION ANALYSIS Introducton he concept of canoncal correlaton arses when we want to quantfy the assocatons between two sets of varables. For example, suppose that the frst set of
) is the unite step-function, which signifies that the second term of the right-hand side of the
 Casmr nteracton of excted meda n electromagnetc felds Yury Sherkunov Introducton The long-range electrc dpole nteracton between an excted atom and a ground-state atom s consdered n ref. [1,] wth the help
Casmr nteracton of excted meda n electromagnetc felds Yury Sherkunov Introducton The long-range electrc dpole nteracton between an excted atom and a ground-state atom s consdered n ref. [1,] wth the help
14 The Postulates of Quantum mechanics
 14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
14 The Postulates of Quantum mechancs Postulate 1: The state of a system s descrbed completely n terms of a state vector Ψ(r, t), whch s quadratcally ntegrable. Postulate 2: To every physcally observable
Structure and spectra of polyatomic molecules
 Chapter 5 Structure and spectra of polyatomc molecules 5.1 Structure of polyatomc molecules The same approxmatons can be used for the statonary states of a polyatomc molecule as for a datomc molecule,
Chapter 5 Structure and spectra of polyatomc molecules 5.1 Structure of polyatomc molecules The same approxmatons can be used for the statonary states of a polyatomc molecule as for a datomc molecule,
Supplemental document
 Electronc Supplementary Materal (ESI) for Physcal Chemstry Chemcal Physcs. Ths journal s the Owner Socetes 01 Supplemental document Behnam Nkoobakht School of Chemstry, The Unversty of Sydney, Sydney,
Electronc Supplementary Materal (ESI) for Physcal Chemstry Chemcal Physcs. Ths journal s the Owner Socetes 01 Supplemental document Behnam Nkoobakht School of Chemstry, The Unversty of Sydney, Sydney,
Spin-rotation coupling of the angularly accelerated rigid body
 Spn-rotaton couplng of the angularly accelerated rgd body Loua Hassan Elzen Basher Khartoum, Sudan. Postal code:11123 E-mal: louaelzen@gmal.com November 1, 2017 All Rghts Reserved. Abstract Ths paper s
Spn-rotaton couplng of the angularly accelerated rgd body Loua Hassan Elzen Basher Khartoum, Sudan. Postal code:11123 E-mal: louaelzen@gmal.com November 1, 2017 All Rghts Reserved. Abstract Ths paper s
PSEUDO ORBIT EXPANSION FOR THE RESONANCE CONDITION ON QUANTUM GRAPHS AND THE RESONANCE ASYMPTOTICS
 PSEUDO ORBIT EXPANSION FOR THE RESONANCE CONDITION ON QUANTUM GRAPHS AND THE RESONANCE ASYMPTOTICS JIŘÍ LIPOVSKÝ Department of Physcs, Faculty of Scence, Unversty of Hradec Králové, Roktanského 6, 500
PSEUDO ORBIT EXPANSION FOR THE RESONANCE CONDITION ON QUANTUM GRAPHS AND THE RESONANCE ASYMPTOTICS JIŘÍ LIPOVSKÝ Department of Physcs, Faculty of Scence, Unversty of Hradec Králové, Roktanského 6, 500
Generalized Linear Methods
 Generalzed Lnear Methods 1 Introducton In the Ensemble Methods the general dea s that usng a combnaton of several weak learner one could make a better learner. More formally, assume that we have a set
Generalzed Lnear Methods 1 Introducton In the Ensemble Methods the general dea s that usng a combnaton of several weak learner one could make a better learner. More formally, assume that we have a set
Structure and Drive Paul A. Jensen Copyright July 20, 2003
 Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
SUPPLEMENTARY INFORMATION
 do: 0.08/nature09 I. Resonant absorpton of XUV pulses n Kr + usng the reduced densty matrx approach The quantum beats nvestgated n ths paper are the result of nterference between two exctaton paths of
do: 0.08/nature09 I. Resonant absorpton of XUV pulses n Kr + usng the reduced densty matrx approach The quantum beats nvestgated n ths paper are the result of nterference between two exctaton paths of
Classical Field Theory
 Classcal Feld Theory Before we embark on quantzng an nteractng theory, we wll take a dverson nto classcal feld theory and classcal perturbaton theory and see how far we can get. The reader s expected to
Classcal Feld Theory Before we embark on quantzng an nteractng theory, we wll take a dverson nto classcal feld theory and classcal perturbaton theory and see how far we can get. The reader s expected to
Lecture 10 Support Vector Machines. Oct
 Lecture 10 Support Vector Machnes Oct - 20-2008 Lnear Separators Whch of the lnear separators s optmal? Concept of Margn Recall that n Perceptron, we learned that the convergence rate of the Perceptron
Lecture 10 Support Vector Machnes Oct - 20-2008 Lnear Separators Whch of the lnear separators s optmal? Concept of Margn Recall that n Perceptron, we learned that the convergence rate of the Perceptron
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Atomic Term Symbols and Energy Splitting. λ=5890 Å
 Chemistry 362 Spring 2018 Dr. Jean M. Standard April 18, 2018 Atomic Term Symbols and Energy Splitting 1. Atomic Term Symbols and the Sodium D-Line The sodium D-line is responsible for the familiar orange
Chemistry 362 Spring 2018 Dr. Jean M. Standard April 18, 2018 Atomic Term Symbols and Energy Splitting 1. Atomic Term Symbols and the Sodium D-Line The sodium D-line is responsible for the familiar orange
5. THE ADIABATIC APPROXIMATION
 5. THE ADIABATIC APPROXIMATION In quantum mechancs, the adabatc approxmaton refers to those solutons to the Schrödnger equaton that make use of a tme-scale separaton between fast and slow degrees of freedom,
5. THE ADIABATIC APPROXIMATION In quantum mechancs, the adabatc approxmaton refers to those solutons to the Schrödnger equaton that make use of a tme-scale separaton between fast and slow degrees of freedom,
Textbook Problem 4.2: The theory in question has two scalar fields Φ(x) and φ(x) and the Lagrangian. 2 Φ ( µφ) 2 m2
 PHY 396 K. Solutons for problem set #11. Textbook Problem 4.2: The theory n queston has two scalar felds Φx) and φx) and the Lagrangan L 1 2 µφ) 2 M2 2 Φ2 + 1 2 µφ) 2 m2 2 φ2 µφφ 2, S.1) where the frst
PHY 396 K. Solutons for problem set #11. Textbook Problem 4.2: The theory n queston has two scalar felds Φx) and φx) and the Lagrangan L 1 2 µφ) 2 M2 2 Φ2 + 1 2 µφ) 2 m2 2 φ2 µφφ 2, S.1) where the frst
4.1. Lecture 4: Fitting distributions: goodness of fit. Goodness of fit: the underlying principle
 Lecture 4: Fttng dstrbutons: goodness of ft Goodness of ft Testng goodness of ft Testng normalty An mportant note on testng normalty! L4.1 Goodness of ft measures the extent to whch some emprcal dstrbuton
Lecture 4: Fttng dstrbutons: goodness of ft Goodness of ft Testng goodness of ft Testng normalty An mportant note on testng normalty! L4.1 Goodness of ft measures the extent to whch some emprcal dstrbuton
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Lecture 16. Chapter 11. Energy Dissipation Linear Momentum. Physics I. Department of Physics and Applied Physics
 Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
Amplification and Relaxation of Electron Spin Polarization in Semiconductor Devices
 Amplfcaton and Relaxaton of Electron Spn Polarzaton n Semconductor Devces Yury V. Pershn and Vladmr Prvman Center for Quantum Devce Technology, Clarkson Unversty, Potsdam, New York 13699-570, USA Spn Relaxaton
Amplfcaton and Relaxaton of Electron Spn Polarzaton n Semconductor Devces Yury V. Pershn and Vladmr Prvman Center for Quantum Devce Technology, Clarkson Unversty, Potsdam, New York 13699-570, USA Spn Relaxaton
Difference Equations
 Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
