Theoretical Studies of the Correlations in Moderately Asymmetric Binary Hard-Sphere Solid Mixtures
|
|
|
- Jeffrey Webb
- 5 years ago
- Views:
Transcription
1 Ames Laboratory Publications Ames Laboratory Theoretical Studies of the Correlations in Moderately Asymmetric Binary Hard-Sphere Solid Mixtures Vadim B. Warshavsky Iowa State University Xueyu Song Iowa State University, Follow this and additional works at: Part of the Chemistry Commons The complete bibliographic information for this item can be found at ameslab_pubs/377. For information on how to cite this item, please visit howtocite.html. This Article is brought to you for free and open access by the Ames Laboratory at Iowa State University Digital Repository. It has been accepted for inclusion in Ames Laboratory Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact
2 Theoretical Studies of the Correlations in Moderately Asymmetric Binary Hard-Sphere Solid Mixtures Abstract A theoretical approach to calculate the correlation functions in binary hard-sphere (HS) solid mixtures is developed. The method is motivated by the theory of correlations in one-component HS solids [Rascon et al., Phys. Rev. E 54, 161 (1996)] and the Kirkwood-Buff theory for solutions. Combined with the fundamental measure density functional theory the approach provides correlation functions in good agreement with the numerical simulations results for the moderately asymmetric mixtures. Disciplines Chemistry Comments This article is from Physical Review E 77 (008): , doi: /physreve Posted with permission. This article is available at Iowa State University Digital Repository:
3 PHYSICAL REVIEW E 77, Theoretical studies of the correlations in moderately asymmetric binary hard-sphere solid mixtures Vadim B. Warshavsky and Xueyu Song Ames Laboratory and Department of Chemistry, Iowa State University, Ames, Iowa 50011, USA Received 4 January 008; published 9 May 008 A theoretical approach to calculate the correlation functions in binary hard-sphere HS solid mixtures is developed. The method is motivated by the theory of correlations in one-component HS solids Rascon et al., Phys. Rev. E 54, and the Kirkwood-Buff theory for solutions. Combined with the fundamental measure density functional theory the approach provides correlation functions in good agreement with the numerical simulations results for the moderately asymmetric mixtures. DOI: /PhysRevE PACS numbers: h, g I. INTRODUCTION Density functional theories DFT have become a very useful tool in the description of thermodynamics and structure of solid and liquid phases of hard spheres HS 1. In general, the results obtained by the DFT are very accurate, usually within a few percent when compared with the ones from computer simulations. To describe the properties of more realistic systems perturbative theories for free energies have been widely used 4. Within this approach the free energy is computed as a sum of the free energy of a reference system plus a perturbative term, which is calculated from correlation functions of the reference system. Typically, the HS system is chosen as a reference system, as it describes many properties of the reference repulsive potentials accurately. The perturbative theory used for single-component systems became especially reliable with the combination of the fundamental measure FM DFT 5,6 and the Rascon- Mederos-Navascues RMN theory for correlation functions in solids 7 9. The essential inputs of these theories are the solid lattice type and the choice of single particle density distribution. In the widely used Gaussian model the inhomogeneous density profile of a crystal is expressed as a sum of normalized Gaussian functions centered at the crystal lattice sites. In the framework of FM DFT, which is based on the geometrical properties of hard aspheres, the free energy is a functional of weighted densities obtained as integral convolutions of a density profile with density-independent weight functions. This type of DFT provides accurate predictions of the solid equation of state and also the Lindemann parameters 10,11. The RMN approach for correlation functions in solids parametrizes the HS radial distribution function as a summation from various coordination shells; the contributions from the second and higher-order coordination shells are treated in a mean-field fashion whereas the contribution from the nearest neighbors are corrected using essentially exact sum rules. In our previous study 9 we have shown that the single-component HS correlation functions obtained with the theory of Ref. 7 using the fundamental measure density functional led to accurate free energies of Lennard- Jones systems. Within a single theoretical framework phase diagrams of the Lennard-Jones system can be obtained accurately without inputs from computer simulations 9. In this paper we extend the RMN approach to the hardsphere solid mixtures as the correlation functions of HS mixtures are essential inputs to the calculations of thermodynamic properties of mixtures with realistic interactions within the perturbative approach 1,13. For HS liquid mixtures the free energy is given by the very accurate Boublik- Mansoori-Carnahan-Starling-Leland BMCSL formula 14,15 and there are a few theoretical approaches 16,17 and computer simulations 18,19 for the correlation functions of HS liquid mixtures. Previously several types of DFT have been generalized to the binary HS solid mixture systems 0 3. For such systems the densities of two species are also approximated by Gaussian profiles with different widths. The stable equilibrium free energy for given mixture parameters is then determined by the minimization of the free energy functional as a function of the widths of the Gaussian profiles. On the other hand, the correlation function calculations of the hard-sphere solid mixtures are much more demanding. To our knowledge only two computer simulations were performed 4,5 and no theoretical approaches have been reported yet. In the present study we have extended the theory of Ref. 7 for the correlations in singlecomponent HS solids to the case of binary HS solid mixtures. In the mixtures there is a set of the distribution functions, describing the correlations between different mixture species unlike the one component system with a single pair distribution function. Therefore the main task is to find enough sum rules for the parametrization of the correlation functions. In this work a set of the Kirkwood-Buff KB equations 6, which connect the volume integrals of correlation functions to the thermodynamical parameters in the mixture, will be used to motivate the calculation of correlation functions in solid HS mixtures. We use FM DFT to obtain the contact values of correlation functions and Gaussian widths as essential inputs to our theory for correlation functions. In the case of homogeneous binary liquid the FM free energy functional 10,11 reduces to the accurate BMCSL free energy; accurate correlation functions in the HS liquid mixture can be found from the solution of integral equations based on the FM free energy functional 16. Therefore the calculation of the solid and liquid properties based on the same theoretical formulation may lead to the cancellation of a similar source of errors both for the liquid and solid phases, thus more accurate liquidsolid coexistence results. In this work we considered a single perfect fcc lattice with all the particles randomly distributed /008/775/ The American Physical Society
4 VADIM B. WARSHAVSKY AND XUEYU SONG over the lattice sites, i.e., a disordered fcc solid solution, although our method can be generalized to other types of crystal lattice as well. It should be noted that such theoretical approaches are essential for the study of phase behaviors of more realistic systems, such as alloys. For example, when the concentration of one type of atoms is small, simulations of correlation functions become computationally prohibitive, on the other hand the theoretical approaches have no such limitations. The rest of the paper is organized as follows. In Sec. II we briefly outline the fundamental measure density functional theory for the solid mixtures, the conditions at contact for correlation functions, the Kirkwood-Buff theory for solutions, and describe a theoretical method to calculate the correlation functions in solid mixtures. In Sec. III we present the results for the correlation functions, which are compared with the corresponding ones from numerical simulations. Some concluding remarks are given in Sec. IV. II. THEORY We consider a binary solid mixture composed of two types of hard spheres with diameters d ii and bulk number densities i i=1,. The index denotes the larger sphere, thus d 11 d. The collision diameter for the additive mixture is d ij =d ji =d ii +d jj /, and the intermolecular pair potential of interactions of hard spheres being = u ij r +, r d. ij 1 0, r d ij The concentration of the i component is x i = i / = 1 +,sox 1 +x =1. The total packing fraction the fraction of volume occupied by spheres and the densities are then simply related by = 6 1d d 3 = 6 3, where the parameter =x 1 d x d 3 1/3 is an effective diameter. Thus an HS binary mixture can be uniquely characterized by three quantities: total packing fraction, concentration of the larger particles x=x 0x1, which are the thermodynamical variables, and the HS diameter ratio =d 11 /d 1, which is simply a geometric parameter. The total local number density of a binary mixture is expressed as a sum of the components r= 1 r+ r, where i r denotes the local number density of species i i =1,. We will parametrize the local one-body inhomogeneous density in a solid by a sum of Gaussian density distributions centered at the crystal lattice sites. In this study we consider only a substitutionally disordered fcc solid solution. Since for the disordered fcc HS solid solution the concentration of defects is very small we will assume a given site to be occupied by the spheres of both components with the probability being equal to the corresponding concentration x i the defects in the crystal are mostly vacancies as the presence of interstitials is several orders of magnitude less probable than vacancies. Thus i r = x i i 3/ e i r R k i =1,, 3 R k where 1 and are the parameters of the Gaussian distributions; the sum runs over all the fcc lattice sites R k. A. Density functional theory for HS solid mixtures To obtain the equilibrium local density distributions the solid free-energy functional is minimized with respect to the parameters of the local densities. The Helmholtz free energy of the binary HS system F is expressed as a functional of density profiles i r, dr i rlog i r 3 i 1 F 1 r, r = k B T i=1 + F ex 1 r, r, 4 where the first term has the form of the ideal gas free energy, although it still depends on the interparticle interaction potentials via the density profiles i r, i is the thermal de Broglie wavelength, k B the Boltzmann constant, T the temperature, and F ex the excess free-energy functional. The arguments in the square brackets denote the functional dependence on the inhomogeneous densities 1 r and r. Inthe applications below we will use the expression for free-energy functional in the form dr i log i 3 i 1 F 1 r, r = k B T i=1 + F ex 1 r, r, 5 where the first term is the ideal gas contribution in the absence of interactions and F ex the excess free-energy functional, which accounts for intermolecular interactions. Different types of DFT were previously reported to approximate the excess part of free energy F ex for HS solid mixtures 0 3. We computed the thermodynamic properties of HS solid binary mixtures using the fundamental measure FM free-energy functional 10,11, because this functional is shown to be able to reliably describe the properties of a single-component solid. It is also very accurate to describe the equation of state and correlation functions of HS liquid mixtures 16. Thus we can choose FM DFT to treat both HS binary solid and liquid phases using the same theoretical framework, which is essential for the calculation of reliable liquid-solid phase diagrams. In the framework of the FM DFT, the excess part of the free energy is written as F ex = k B T i=1 PHYSICAL REVIEW E 77, dr i rlog i r/ i + k B T drn r, where the expression for in the right-hand side rhs of Eq. 6 is provided in Refs. 10,
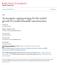
5 THEORETICAL STUDIES OF THE CORRELATIONS IN PHYSICAL REVIEW E 77, = n 0 ln1 n 3 + n 1n n v1 n v + 1 n 1 n 3 8n n 3 +ln1 n 3 n v nˆn v n n v trnˆ 3 + n trnˆ. 7 ασ α 1 σ α σ α 1 σ α σ α 1 σ α σ δ=0.85 δ=0.85 δ=0.90 δ=0.90 δ=0.95 δ=0.95 The scalar n 0, n 1, n, n 3, vector n v1, n v, and tensor nˆ ij weighted densities in this expression are given by the following integral convolutions: 100 n r = i=1 dr i r i r r of the densities i r with the corresponding densityindependent weight functions i r, i r = d ii r, 3 i r = d ii r, 0 i r = 1 d i r, 1 i r = 1 ii d i r, ii v i r = r d ii r r, v i r = 1 v d i r, ii 8 ˆ ijk r = r jr d k ii r r. 9 In these expressions r is the Dirac delta function, r the Heaviside step function, and r i the components of vector r. For solid mixtures with the density distribution of Eq. 3, all the weighted densities n can be found in analytical forms from Eqs. 8 and 9. The volume integration in Eq. 6 is done over a small volume simplex reflecting the symmetry of the crystal for details of the calculations in the singlecomponent case see Refs. 9,8. Thus with the density parametrization of Eq. 3 the free-energy functional F 1 r, r becomes a function of 1 and : F =F,x,; 1,. Finally, for given values of mixture parameters, x, and the minimization of the free energy with 1 and yields the equilibrium values of the free energy F and the Gaussian parameters 1, of the density profile. In Fig. 1 we show the dimensionless Gaussian localization parameters 1, as a function of the concentration of the larger sphere x for =0.85, 0.90, 0.95 and = It can be seen that for given, x, and the condition 1 is always satisfied. This condition reflects the fact that the smaller particles are less localized around the fcc crystal sites compared to the larger ones, and the density distributions of the smaller spheres around the lattice sites at Eq. 3 are broader compared to the ones of the larger spheres. It also can be seen that when x 1 the values of for all the HS diameters ratios reduces to the same single-component limit; a similar conclusion is valid for the values of 1 when x B. Correlation functions in solid mixtures In this section we define the correlation functions g ij r in solid mixtures. Let the first j-type particle be fixed at r 1, whereas the second i-type particle is at r i, j=1,. In this case a pair distribution function ij r 1,r can be expressed as a conditional density distribution of i-type particles with respect to the density distribution of j-type particles j r 1. Instead of this pair distribution function ij r 1,r, which depends on six variables, it is convenient to introduce a pair distribution function averaged over all the locations of the first particle and the orientations of the second particle g ij r = 1 4V i j dr 1 d ij r 1,r 1 + r, 10 where V is the volume, i =1/Vdr i r the bulk density of the i-type particles, and the solid angle of the direction from the first particle to the second particle. Now the correlation functions g ij r depend only on one variable r and is the correlation function used in the perturbative theory for free-energy calculations 1,13. If the first particle is not fixed then the pair distribution function ij r 1,r is equal to j r 1 i r and the corresponding averaged correlation function g ij 0 r is g ij 0 r = 1 4V i j dr 1 d j r 1 i r 1 + r. 11 In this case the short-ranged correlations between the particles are not accounted for. The functions g ij 0 r can be found analytically. Indeed, substituting Eq. 3 into Eq. 11 and some algebraic transformations lead to g ij 0 r = g k,ij 0 r, 1 k0 where x FIG. 1. Dimensionless Gaussian localization parameters 1, vs concentration of larger spheres x for =0.85, 0.90, 0.95 and = g 0,ij 0 r = 1 3/ i j i e i + j i j / i + j r ij,
6 VADIM B. WARSHAVSKY AND XUEYU SONG g k,ij 0 r = 1/ n k i j 4rR k e i + j i j / i + j r R k e i j / i + j r + R k k 0, 14 where 1+ i,j=1 PHYSICAL REVIEW E 77, x i x j drg ij r g ij 0 r = N, 17 where k runs over the number of the successive lattice shells, n k is the coordination number of the kth shell, and R k the lattice vector magnitude of the kth shell. C. Kirkwood-Buff theory for solutions The Kirkwood-Buff KB theory of solutions 6 provides the connection between the correlation functions in the form of so-called Kirkwood-Buff integrals KBIs G ij =drg ij r g ij 0 r and the thermodynamic values in an open system. Some transformations of the KB equations give us the following relations: where G ij ij + i drg ij r g ij 0 r = G ij, V G i V j ij = ik B T T + i, j =1,, i i. V i j are the dimensionless KBIs, T is the isothermal compressibility, V i are the partial molar volumes, and ij the derivatives of chemical potentials with respect to the numbers of particles when the pressure is fixed. Only three of these values are independent because of the additional thermodynamic conditions 6,7: i=1 i V i=1 and i=1 i ij =1 Gibbs-Duhem relation j=1,. For the HS mixture the expressions for T, V i, and ij can be written as combinations of second derivatives of free energy 1 NF,x, with respect to and x. Thus if the dependence of the free energy on, x, and is known all the KBIs G ij in Eq. 16 can be calculated numerically. The physical meaning of the KBIs G ij in an open system can be viewed as the following. The volume integral i g ij 0 rdr is the mean number of i-type particles of the system before fixing any j-type particle at the origin, whereas the volume integral i g ij rdr is the mean number of i-type particles of the system minus ij because of selfexclusion after fixing a j-type particle at the origin. Thus the left-hand side lhs of Eq. 15 is the average number N ij of i-type particles coming into the system plus ij when one j-type particle is fixed at the origin, i.e., N ij =G ij. The KBIs, G ij, can be either positive or negative so the number of i-type particles in the system can increase or decrease when a j particle is fixed. The number of particles N i of i type coming into the system after fixing a particle either first or second type is N i = j=1 x j N ij the coefficient x j in front of N ij is a probability of finding a j-type particle at the origin, and the total number of particles coming into the system N= i=1 N i. Now from Eqs. 15 and 16 we have N = k B T T + 1/ V. 18 V From the stability conditions of the system it follows that the values T and are positive 7; therefore N is always positive. Where are the N particles accommodated in the crystal lattice? It is known that the equilibrium HS binary mixture crystal has a small concentration of vacancies. The probability to have a vacancy at a given lattice site in the disordered fcc HS crystal is about , which gives the number of vacancies per lattice shell From Eq. 18 we estimate N10 ; therefore to accommodate N particles coming into the system after fixing one particle at the origin, approximately 10 lattice shells are needed. Following the arguments in Ref. 7 we note that fixing a particle at the origin introduces short-ranged correlations, which affects mostly the nearest neighbors the particles in the first coordination shell. The contributions to the correlation functions g ij r from the lattice shells higher than the first shell are the same as the ones to g ij 0 r with the only difference that no vacancies are needed to be taken into consideration. As a result, the correlation functions g ij r can be approximated as g ij r = g 1,ij r + g k,ij 0 r. 19 k Thus all of the correlations induced by fixing a particle at the origin are accounted for by the first coordination shell. D. Conditions for the first peaks of the correlation functions To find the first peak contribution g 1,ij r in the above expression we propose the following function form: /r r 1 e ij g 1,ij r = A ij, r d ij 0 r with g 1,ij r=0 for rd ij. In the single-component case the expression for the first peak Eq. 0 reduces to the function proposed in Ref. 7. To find the seven parameters A ij,, ij and r 1 in Eq. 0 seven independent conditions are needed. The first three conditions are from the contact values of the correlation functions. The three values of correlation functions g ij r at contact g 11 d 11, g 1 d 1, and g d g 1 d 1 =g 1 d 1 can be found from the conditions for the derivatives of 1 N F ex with respect to HS diameters d 11 and d, which are provided by the Hamad-Mansoori theory in Refs. 9,30. d ii F ex N T,,x,d ii = k=1 x i x k d ik g ik d ik
7 THEORETICAL STUDIES OF THE CORRELATIONS IN i = 1,; i i. 1 Changing the variables d 11 and d to =x 1 d x d 3 1/3 and =d 11 /d in the lhs of these equations yields a virial theorem for HS mixtures P =1+ 3 i,j=1 x i x j d 3 ij g ij d ij and another exact condition F ex = d x 1 x N,x x x x 1d 11 g 11 d 11 + x x 1 d 1 g 1 d 1 x d g d. 3 In Eq. P is the pressure. The lhs of Eq. is a compressibility factor F ex P =1+. 4 N Equations and 3 are exact, which are related to the two zero-separation theorems for the cavity functions in HS liquid mixtures 17 and to the criteria for HS mixtures 3. An approximate closure is needed to determine the three contact values 31 and a reasonable choice is the one suggested in Ref. 4. g 1 d 1 = g 11 d 11 g d 1/. 5 This closure becomes more accurate when the dissimilarity between the mixture components is not too high, i.e., for The next group of conditions are the normalization conditions for the functions g 1,ij r to the nearest-neighbors number drg 1,ij r = n 1. 6 Indeed, as we discussed in the previous section we can neglect the vacancies in the first lattice shell. Therefore the functions g 1,ij r are normalized to give the exact number of the nearest neighbors n 1. Neglecting the vacancies in the first shell gives also the condition, which follows from the approximation for the mean location of the nearest neighbors r, x i x j drrg 1,ij r = x i x i,j=1 i,j=1 j drrg 1,ij 0 r = n 1 r. 7 In the single-component case in the close-packing limit this condition is exact and is quite accurate over all density ranges 7. To sum up, to find the seven parameters of the first peaks of the correlation functions A ij,, ij and r 1 at Eq. 0 we have seven conditions: three equations from the contact values of the correlation functions Eqs. 5, three conditions for the number of nearest neighbors Eq. 6 and a g(r) PHYSICAL REVIEW E 77, r/σ formula for the mean location of the nearest neighbors Eq. 7. These conditions are the key relations of our approach. III. RESULTS Using the method outlined in the previous section, results for the correlation functions g ij r in HS binary solid mixtures will be presented. First of all, for the given mixture parameters, x, and the equilibrium values of Gaussian parameters 1, are numerically obtained to calculate the higher number shell contributions in Eq. 14. The contact values of correlation functions g ij d ij are found from the calculated dimensionless compressibility factor P, the derivative F ex N, and the approximate closure Eq. 5. Using these data the solution of a set of algebraic equations from Eqs. 0, 6, and 7 yields all the parameters of the first peak A ij,, ij and r 1. Finally, the resulting correlation functions g ij r are found from Eqs. 14, 19, and 0. Comparisons with the simulation ones 4 are shown in Figs. 6 for the correlation functions g ij r at a single packing fraction = and various x and we study one value of the packing fraction = since the correlation functions from the numerical experiment are only available for that value 4. It can be seen that the present theory correctly reproduces the main qualitative features of the correlation functions from simulations. In general, the quantitative differences between the results of the present theory and simulations are small, especially when is close to 1, i.e., =0.95 and 0.90 see Figs. and 3. The differences may become more pronounced for =0.85 see Figs. 4 6, particularly in the first peaks. To elucidate the sources of errors we summarize all of the approximations used in our approach. The first one is the approximate nature of FM density functional at Eq. 7, which is used to calculate P and F ex N. The second one is the approximate closure expression of Eq. 5. Both of these approximations may result in some error for the obtained g 11 Theory g 1 Theory g Theory g 11 MCS g 1 MCS g MCS FIG.. The dependence of the correlation functions ij x i x j g ij on the scaled distance r/ as a result of the present theory and MC simulations 4. The mixture parameters are =0.5707, x=0.037, and =
8 VADIM B. WARSHAVSKY AND XUEYU SONG PHYSICAL REVIEW E 77, FIG. 3. The same as Fig.. The mixture parameters are =0.5707, x=0.037, and =0.90. The inset shows first peaks of the correlation functions obtained with contact values input from simulations 4 see explanations in text. FIG. 5. Same as Fig. 4, but for x= contact values of the correlation functions. To eliminate this source of errors in the calculations of the first peaks we can, in our approach, directly use the contact values obtained from simulations 4. In this case, in the set of equations for the first peaks the only approximate one is Eq. 7. We show the resulting first peaks in the insets of Figs It can be seen that excellent agreements in the first peaks are obtained between the theory and the simulations for all the values of the mixture parameters. Therefore the approximate condition in Eq. 7 turns out to be a very accurate one. The parametric form of the first peak in Eq. 0 is also very accurate, as it reproduces accurately the simulation results using correct parameters in Eq. 0. We also notice that the Gaussian distributions in Eq. 3 are reasonable representations of one-body densities for the disordered fcc solid. As a matter of fact, the part of correlation functions g ij r in the bulk which is not affected by the short-ranged correlations is simply given by a sum of onebody density convolutions in the rhs of Eq. 19. The contributions to the sum g k,ij 0 r k from Eq. 14 depend on the Gaussians parameters 1 and. It can be seen in Figs. 6 that when r =r/ is larger than the minimum between the first and second peaks of correlation functions the agreement between the correlation functions obtained from the present theory and from the simulations is sufficiently good for some mixture parameters the quantitative difference between the corresponding peaks with k obtained by the theory and simulations are due to the approximate 1 and values. This conclusion is in contradiction to the DFT study of Ref. 33 where it was found that the larger particles are showing structure very similar to the one obtained using Gaussian densities, whereas the peaks of densities of smaller particles reveal a rich structure with the splitting of the major peaks at fcc lattice sites, such as the emerging multiple peaks. A possible explanation of such contradictions is that Ref. 33 used an early Ramakrishnan- Yussouff RY DFT and also the Percus-Yevick PY equations to calculate the direct correlation function in liquids which are the inputs to the RY free-energy density functional. This approach is not accurate enough to describe the highly inhomogeneous densities in crystals with high packing fractions in contrast to the fundamental measure DFT used in our study. FIG. 4. The same as Fig.. The mixture parameters are =0.5707, x=0.037, and =0.85. FIG. 6. Same as Fig. 4, but for x=
9 THEORETICAL STUDIES OF THE CORRELATIONS IN IV. CONCLUSIONS A theory to study correlations in a single-component HS crystal has been developed previously by Rascon, Mederos, and Navascues in Ref. 7. In that method the contributions to the correlation function from second and higher lattice shells are accounted for in a mean-field fashion, whereas the parameters of the first peak contributions from nearest neighbors are found from the sum rules. We have recently applied that method in combination with the fundamental measure free-energy functional and a perturbative theory to obtain accurate solid-liquid coexistence conditions of Lennard-Jones systems 9. In the present study the calculation of the correlation functions is extended to binary mixtures of hard spheres. The binary system is characterized by three parameters, namely the total packing fraction, the composition of larger spheres x, and the HS diameters ratio. Following Ref. 7, we consider the interaction of the fixed particle at the origin with the particles from the successive lattice shells in such a manner that the short-ranged correlations mostly affect the nearest neighbors. The method used for the calculation of the free energy is a fundamental measure DFT 10,11, although any other relevant DFT could be used 0 3. We have considered just one type of crystal structure for the binary mixtures, namely disordered fcc structure as this is the only one with extensive studies from computer simulations. The defects were not accounted for in simulations of Ref. 4. Thus to compare our results with the ones of Ref. 4 we did not account for defects in our theoretical calculations, though it is interesting to estimate the effects of defects on the thermodynamics and structure of solid mixture 34,35 with FM DFT 36. In the present work the one-body density used is a sum of Gaussian distributions around crystal sites. The crystal structure and density distribution are inputs to the FM free-energy functional to provide all conditions to calculate the correlation functions. We have found that the obtained correlation functions are in good agreements with the ones from numerical simulations 4 for = and a variety of parameters and x. It will be interesting to have more extensive numerical simulations studies of the correlation functions in HS solid mixtures with various packing fraction and different types of crystal lattice to compare with our theoretical predictions of the dependence of the correlation functions on the mixture parameters. PHYSICAL REVIEW E 77, The quality of our theory is getting a bit worse with the increase of disparity between the two species decreasing the parameter, especially for 0.90, this is largely due to the approximate functional in Eq. 7 and closure Eq. 5 used in the calculations as shown in the previous section. We have applied our methodology to compute the free energies of liquid and solid phases of an Au-Cu alloy using a perturbative theory 37, which is the original motivation of the current work. We found that the typical range of parameters of reference HS solid mixture systems near melting temperatures are and , i.e., the range where our theory provides the reliable results for correlation functions in HS solid mixtures. The resulting melting temperatures for different compositions are in a good agreement within 6% with the ones from simulations. Our method to calculate correlation functions in binary HS solid mixtures can be straightforwardly generalized to ternary mixtures. Indeed, the Rosenfeld fundamental measure density functional theory 5, Kirkwood-Buff theory 6, and the Hamad-Mansoori theory 9, which are inputs to our approach, were initially formulated for multicomponent mixtures. It should be interesting to try to find an exact or, at least, a very accurate closure formula instead of Eq. 5 using the principles of statistical mechanics or scaled particle theory. Better closures in combination with exact equations and 3 may provide more accurate contact values of correlation functions in a HS solid mixture, which are essential for the first peaks of the correlation functions. Another challenging task is to the improve FM free-energy functional in Eq. 7 to have more accurate values for compressibility factor P, derivative of free energy F ex N, and Gaussian parameters 1, in HS solid mixtures. It may lead to better correlation functions, especially for largely dissimilar hard-sphere diameters. ACKNOWLEDGMENTS This research was sponsored in part by the Division of Materials Sciences and Engineering, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract No. W-7405-ENG-8 with Iowa State University V.B.W. and X.S. and by NSF Grant No. CHE X.S.. We are grateful to Alan Denton for providing a copy of the thesis cited in Ref R. Evans, in Fundamentals of Inhomogeneous Fluid, edited by D. Henderson Wiley, New York, 199. J. D. Weeks, D. Chandler, and H. C. Andersen, J. Chem. Phys. 54, D. Chandler, J. D. Weeks, and H. C. Andersen, Science 0, C. Rascon, L. Mederos, and G. Navascues, Phys. Rev. Lett. 77, Y. Rosenfeld, Phys. Rev. Lett. 63, Y. Rosenfeld, M. Schmidt, H. Lowen, and P. Tarazona, Phys. Rev. E 55, C. Rascon, L. Mederos, and G. Navascues, Phys. Rev. E 54, C. Rascon, L. Mederos, and G. Navascues, J. Chem. Phys. 105, V. B. Warshavsky and X. Song, Phys. Rev. E 69, R. Roth, R. Evans, A. Lang, and G. Kahl, J. Phys.: Condens. Matter 14, P. Tarazona, Physica A 306, G. Kahl and J. Hafner, J. Phys. F: Met. Phys. 15, D. Saumon, G. Chabrier, and J. J. Weis, J. Chem. Phys. 90,
10 VADIM B. WARSHAVSKY AND XUEYU SONG T. Boublik, J. Chem. Phys. 53, G. A. Mansoori, N. F. Carnahan, K. E. Starling, and T. W. Leland, Jr., J. Chem. Phys. 54, Y. Yu and J. Wu, J. Chem. Phys. 117, E. W. Grundke and D. Henderson, Mol. Phys. 4, L. L. Lee and D. Levesque, Mol. Phys. 6, J. A. Barker and D. Henderson, Mol. Phys. 1, J. L. Barrat, M. Baus, and J. P. Hansen, J. Phys. C 0, S. W. Rick and A. D. J. Haymet, J. Phys. Chem. 94, X. C. Zeng and D. W. Oxtoby, J. Chem. Phys. 93, A. R. Denton and N. W. Ashcroft, Phys. Rev. A 4, W. Kranendonk and D. Frenkel, Mol. Phys. 7, ; W. G. T. Kranendonk, Ph.D. thesis, University of Utrecht, 1990 unpublished. PHYSICAL REVIEW E 77, T. Gruhn and P. A. Monson, Phys. Rev. E 64, J. G. Kirkwood and F. P. Buff, J. Chem. Phys. 19, A. Ben-Naim, Statistical Thermodynamics for Chemists and Biochemists Plenum, New York, B. Groh and B. Mulder, Phys. Rev. E 61, E. Z. Hamad and G. A. Mansoori, Fluid Phase Equilib. 51, E. Z. Hamad, J. Chem. Phys. 101, E. Z. Hamad, J. Chem. Phys. 107, W. R. Smith and S. Labik, Mol. Phys. 80, M. Valera, R. F. Bielby, F. J. Pinski, and D. D. Johnson, J. Chem. Phys. 115, S. P. Singh and P. Das Shankar, J. Chem. Phys. 16, S. P. Singh and P. Das Shankar, Phys. Rev. B 75, B. Groh, Phys. Rev. E 61, V. B. Warshavsky and X. Song unpublished
Perturbation theory calculations of model pair potential systems
 Graduate Theses and Dissertations Graduate College 2016 Perturbation theory calculations of model pair potential systems Jianwu Gong Iowa State University Follow this and additional works at: http://lib.dr.iastate.edu/etd
Graduate Theses and Dissertations Graduate College 2016 Perturbation theory calculations of model pair potential systems Jianwu Gong Iowa State University Follow this and additional works at: http://lib.dr.iastate.edu/etd
Theoretical Calculation of the Melting Curve of Cu-Zr Binary Alloys
 Ames Laboratory Publications Ames Laboratory 11-2014 Theoretical Calculation of the Melting Curve of Cu-Zr Binary Alloys Koralagamage Gunawardana Iowa State University, korala@ameslab.gov Seth R. Wilson
Ames Laboratory Publications Ames Laboratory 11-2014 Theoretical Calculation of the Melting Curve of Cu-Zr Binary Alloys Koralagamage Gunawardana Iowa State University, korala@ameslab.gov Seth R. Wilson
Geometry explains the large difference in the elastic properties of fcc and hcp crystals of hard spheres Sushko, N.; van der Schoot, P.P.A.M.
 Geometry explains the large difference in the elastic properties of fcc and hcp crystals of hard spheres Sushko, N.; van der Schoot, P.P.A.M. Published in: Physical Review E DOI: 10.1103/PhysRevE.72.067104
Geometry explains the large difference in the elastic properties of fcc and hcp crystals of hard spheres Sushko, N.; van der Schoot, P.P.A.M. Published in: Physical Review E DOI: 10.1103/PhysRevE.72.067104
Binary Hard-Sphere Mixtures Within Spherical Pores
 Journal of the Korean Physical Society, Vol. 35, No. 4, October 1999, pp. 350 354 Binary Hard-Sphere Mixtures Within Spherical Pores Soon-Chul Kim Department of Physics, Andong National University, Andong
Journal of the Korean Physical Society, Vol. 35, No. 4, October 1999, pp. 350 354 Binary Hard-Sphere Mixtures Within Spherical Pores Soon-Chul Kim Department of Physics, Andong National University, Andong
Perturbation approach for equation of state for hard-sphere and Lennard Jones pure fluids
 PRAMANA c Indian Academy of Sciences Vol. 76, No. 6 journal of June 2011 physics pp. 901 908 Perturbation approach for equation of state for hard-sphere and Lennard Jones pure fluids S B KHASARE and M
PRAMANA c Indian Academy of Sciences Vol. 76, No. 6 journal of June 2011 physics pp. 901 908 Perturbation approach for equation of state for hard-sphere and Lennard Jones pure fluids S B KHASARE and M
UNIVERSITY OF CINCINNATI
 UNIVERSITY OF CINCINNATI DATE: August 14, 2002 I, Manuel Valera, hereby submit this as part of the requirements for the degree of: DOCTORATE OF PHILOSOPHY (Ph.D.) in: Physics It is entitled: Density Functional
UNIVERSITY OF CINCINNATI DATE: August 14, 2002 I, Manuel Valera, hereby submit this as part of the requirements for the degree of: DOCTORATE OF PHILOSOPHY (Ph.D.) in: Physics It is entitled: Density Functional
Phase Field Crystal (PFC) Model and Density Functional Theory (DFT) of Freezing
 Phase Field Crystal (PFC) Model and Density Functional Theory (DFT) of Freezing Pyrite Project Meeting October 14 th 2010 Arvind Baskaran John Lowengrub Density functional Theory of Freezing [ Ramakrishnan
Phase Field Crystal (PFC) Model and Density Functional Theory (DFT) of Freezing Pyrite Project Meeting October 14 th 2010 Arvind Baskaran John Lowengrub Density functional Theory of Freezing [ Ramakrishnan
Equation of state of additive hard-disk fluid mixtures: A critical analysis of two recent proposals
 PHYSICAL REVIEW E 66, 0310 00 Equation of state of additive hard-disk fluid mixtures: A critical analysis of two recent proposals M. López de Haro* Centro de Investigación en Energía, UNAM, Temixco, Morelos
PHYSICAL REVIEW E 66, 0310 00 Equation of state of additive hard-disk fluid mixtures: A critical analysis of two recent proposals M. López de Haro* Centro de Investigación en Energía, UNAM, Temixco, Morelos
Density-functional theory for classical fluids and solids
 Density-functional theory for classical fluids and solids C. Ebner Department of Physics, Ohio State University, Columbus, Ohio 43210 H. R. Krishnamurthy and Rahul Pandit Department of Physics, Indian
Density-functional theory for classical fluids and solids C. Ebner Department of Physics, Ohio State University, Columbus, Ohio 43210 H. R. Krishnamurthy and Rahul Pandit Department of Physics, Indian
A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform Phase Fluid
 Commun. Theor. Phys. (Beijing, China) 39 (2003) pp. 231 237 c International Academic Publishers Vol. 39, No. 2, February 15, 2003 A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform
Commun. Theor. Phys. (Beijing, China) 39 (2003) pp. 231 237 c International Academic Publishers Vol. 39, No. 2, February 15, 2003 A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform
Elastic constants and the effect of strain on monovacancy concentration in fcc hard-sphere crystals
 PHYSICAL REVIEW B 70, 214113 (2004) Elastic constants and the effect of strain on monovacancy concentration in fcc hard-sphere crystals Sang Kyu Kwak and David A. Kofke Department of Chemical and Biological
PHYSICAL REVIEW B 70, 214113 (2004) Elastic constants and the effect of strain on monovacancy concentration in fcc hard-sphere crystals Sang Kyu Kwak and David A. Kofke Department of Chemical and Biological
Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid
 Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid Jeff B. Schulte, Patrick A. Kreitzberg, Chris V. Haglund, and David Roundy Department of Physics,
Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid Jeff B. Schulte, Patrick A. Kreitzberg, Chris V. Haglund, and David Roundy Department of Physics,
Three Semi-empirical Analytic Expressions for the Radial Distribution Function of Hard Spheres
 Commun. Theor. Phys. (Beijing, China) 4 (2004) pp. 400 404 c International Academic Publishers Vol. 4, No. 3, March 5, 2004 Three Semi-empirical Analytic Expressions for the Radial Distribution Function
Commun. Theor. Phys. (Beijing, China) 4 (2004) pp. 400 404 c International Academic Publishers Vol. 4, No. 3, March 5, 2004 Three Semi-empirical Analytic Expressions for the Radial Distribution Function
Adsorption properties of a colloid-polymer mixture confined in a slit pore
 PHYSICAL REVIEW E, VOLUME 64, 041507 Adsorption properties of a colloid-polymer mixture confined in a slit pore Soon-Chul Kim* Department of Physics, Andong National University, Andong, 760-749 Korea Peter
PHYSICAL REVIEW E, VOLUME 64, 041507 Adsorption properties of a colloid-polymer mixture confined in a slit pore Soon-Chul Kim* Department of Physics, Andong National University, Andong, 760-749 Korea Peter
Weighted density functional theory of the solvophobic effect
 PHYSICAL REVIEW E, VOLUME 64, 021512 Weighted density functional theory of the solvophobic effect Sean X. Sun Department of Chemistry, University of California, Berkeley, California 94720 Received 24 July
PHYSICAL REVIEW E, VOLUME 64, 021512 Weighted density functional theory of the solvophobic effect Sean X. Sun Department of Chemistry, University of California, Berkeley, California 94720 Received 24 July
Theory of Interfacial Tension of Partially Miscible Liquids
 Theory of Interfacial Tension of Partially Miscible Liquids M.-E. BOUDH-HIR and G.A. MANSOORI * University of Illinois at Chicago (M/C 063) Chicago, Illinois USA 60607-7052 Abstract The aim of this work
Theory of Interfacial Tension of Partially Miscible Liquids M.-E. BOUDH-HIR and G.A. MANSOORI * University of Illinois at Chicago (M/C 063) Chicago, Illinois USA 60607-7052 Abstract The aim of this work
RESEARCH NOTE. DOUGLAS HENDERSON? Department of Chemistry and Biochemistry, Brigham Young University, Provo, Utah USA
 MOLECULAR PHYSICS, 1999, VOL. 96, No. 7, 1145-1149 RESEARCH NOTE A simple theory for the partial molar volumes of a binary mixture DOUGLAS HENDERSON? Department of Chemistry and Biochemistry, Brigham Young
MOLECULAR PHYSICS, 1999, VOL. 96, No. 7, 1145-1149 RESEARCH NOTE A simple theory for the partial molar volumes of a binary mixture DOUGLAS HENDERSON? Department of Chemistry and Biochemistry, Brigham Young
Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures
 PHYSICAL REVIEW E 70, 031109 (2004) Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures Zhidong Li and Jianzhong Wu*
PHYSICAL REVIEW E 70, 031109 (2004) Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures Zhidong Li and Jianzhong Wu*
Lennard-Jones as a model for argon and test of extended renormalization group calculations
 JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 2 22 NOVEMBER 1999 Lennard-Jones as a model for argon and test of extended renormalization group calculations John A. White Department of Physics, American
JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 2 22 NOVEMBER 1999 Lennard-Jones as a model for argon and test of extended renormalization group calculations John A. White Department of Physics, American
Melting line of the Lennard-Jones system, infinite size, and full potential
 THE JOURNAL OF CHEMICAL PHYSICS 127, 104504 2007 Melting line of the Lennard-Jones system, infinite size, and full potential Ethan A. Mastny a and Juan J. de Pablo b Chemical and Biological Engineering
THE JOURNAL OF CHEMICAL PHYSICS 127, 104504 2007 Melting line of the Lennard-Jones system, infinite size, and full potential Ethan A. Mastny a and Juan J. de Pablo b Chemical and Biological Engineering
Global Phase Diagrams and Critical Phenomena of Binary Mixtures. Ji Lin Wang
 Global Phase Diagrams and Critical Phenomena of Binary Mixtures Ji Lin Wang Dissertation Submitted in fulfilment of requirements for the degree of Doctor of Philosophy Centre for Molecular Simulation School
Global Phase Diagrams and Critical Phenomena of Binary Mixtures Ji Lin Wang Dissertation Submitted in fulfilment of requirements for the degree of Doctor of Philosophy Centre for Molecular Simulation School
A patching model for surface tension of spherical droplet and Tolman length. II
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry -5-999 A patching model for surface tension of
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry -5-999 A patching model for surface tension of
On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models
 JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
A patching model for surface tension and the Tolman length
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 8-22-1999 A patching model for surface tension
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 8-22-1999 A patching model for surface tension
Computer simulation of solid-liquid coexistence in binary hard sphere mixtures
 MOLECULAR HYSICS, 1991, VOL. 72, No. 3, 679-697 Computer simulation of solid-liquid coexistence in binary hard sphere mixtures By W. G. T. KRANENDONK and D. FRENKEL FOM Institute for Atomic and Molecular
MOLECULAR HYSICS, 1991, VOL. 72, No. 3, 679-697 Computer simulation of solid-liquid coexistence in binary hard sphere mixtures By W. G. T. KRANENDONK and D. FRENKEL FOM Institute for Atomic and Molecular
Enhanced KR-Fundamental Measure Functional for Inhomogeneous Binary and Ternary Hard Sphere Mixtures
 Commun. Theor. Phys. 55 (2011) 46 58 Vol. 55, No. 1, January 15, 2011 Enhanced KR-Fundamental Measure Functional for Inhomogeneous Binary and Ternary Hard Sphere Mixtures ZHOU Shi-Qi ( Ð) State Key Laboratory
Commun. Theor. Phys. 55 (2011) 46 58 Vol. 55, No. 1, January 15, 2011 Enhanced KR-Fundamental Measure Functional for Inhomogeneous Binary and Ternary Hard Sphere Mixtures ZHOU Shi-Qi ( Ð) State Key Laboratory
BEHAVIOR OF THE CONFINED HARD-SPHERE FLUID WITHIN NANOSLITS: A FUNDAMENTAL-MEASURE DENSITY-FUNCTIONAL THEORY STUDY
 International Journal of Nanoscience Vol. 7, Nos. 4 & 5 (2008) 245 253 c World Scientific Publishing Company BEHAVIOR OF THE CONFINED HARD-SPHERE FLUID WITHIN NANOSLITS: A FUNDAMENTAL-MEASURE DENSITY-FUNCTIONAL
International Journal of Nanoscience Vol. 7, Nos. 4 & 5 (2008) 245 253 c World Scientific Publishing Company BEHAVIOR OF THE CONFINED HARD-SPHERE FLUID WITHIN NANOSLITS: A FUNDAMENTAL-MEASURE DENSITY-FUNCTIONAL
Gibbs ensemble simulation of phase equilibrium in the hard core two-yukawa fluid model for the Lennard-Jones fluid
 MOLECULAR PHYSICS, 1989, VOL. 68, No. 3, 629-635 Gibbs ensemble simulation of phase equilibrium in the hard core two-yukawa fluid model for the Lennard-Jones fluid by E. N. RUDISILL and P. T. CUMMINGS
MOLECULAR PHYSICS, 1989, VOL. 68, No. 3, 629-635 Gibbs ensemble simulation of phase equilibrium in the hard core two-yukawa fluid model for the Lennard-Jones fluid by E. N. RUDISILL and P. T. CUMMINGS
A density-functional theory for bulk and inhomogeneous Lennard-Jones fluids from the energy route
 JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 14 8 OCTOBER 2003 A density-functional theory for bulk and inhomogeneous Lennard-Jones fluids from the energy route Yiping Tang a) Honeywell Hi-Spec Solutions,
JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 14 8 OCTOBER 2003 A density-functional theory for bulk and inhomogeneous Lennard-Jones fluids from the energy route Yiping Tang a) Honeywell Hi-Spec Solutions,
Model Calculations of Thermodynamic Mixture Properties from Direct Correlation Integrals
 Zeitschrift fiir Physikalische Chemie Neue Folge, Bd. 166, S. 63-69 (1990) Vol. 166, Part 1, Pages 63-69, 1999 Model Calculations of Thermodynamic Mixture Properties from Direct Correlation Integrals By
Zeitschrift fiir Physikalische Chemie Neue Folge, Bd. 166, S. 63-69 (1990) Vol. 166, Part 1, Pages 63-69, 1999 Model Calculations of Thermodynamic Mixture Properties from Direct Correlation Integrals By
Excess Entropy, Diffusion Coefficient, Viscosity Coefficient and Surface Tension of Liquid Simple Metals from Diffraction Data
 Materials Transactions, Vol. 43, No. 1 (2002) pp. 67 to 72 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Excess Entropy, Diffusion Coefficient, Viscosity Coefficient and Surface Tension
Materials Transactions, Vol. 43, No. 1 (2002) pp. 67 to 72 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Excess Entropy, Diffusion Coefficient, Viscosity Coefficient and Surface Tension
Supplemental Material for Temperature-sensitive colloidal phase behavior induced by critical Casimir forces
 Supplemental Material for Temperature-sensitive colloidal phase behavior induced by critical Casimir forces Minh Triet Dang, 1 Ana Vila Verde, 2 Van Duc Nguyen, 1 Peter G. Bolhuis, 3 and Peter Schall 1
Supplemental Material for Temperature-sensitive colloidal phase behavior induced by critical Casimir forces Minh Triet Dang, 1 Ana Vila Verde, 2 Van Duc Nguyen, 1 Peter G. Bolhuis, 3 and Peter Schall 1
CH.7 Fugacities in Liquid Mixtures: Models and Theories of Solutions
 CH.7 Fugacities in Liquid Mixtures: Models and Theories of Solutions The aim of solution theory is to express the properties of liquid mixture in terms of intermolecular forces and liquid structure. The
CH.7 Fugacities in Liquid Mixtures: Models and Theories of Solutions The aim of solution theory is to express the properties of liquid mixture in terms of intermolecular forces and liquid structure. The
arxiv: v1 [cond-mat.stat-mech] 1 Oct 2009
![arxiv: v1 [cond-mat.stat-mech] 1 Oct 2009 arxiv: v1 [cond-mat.stat-mech] 1 Oct 2009](/thumbs/74/70932354.jpg) Shell Structure of Confined Charges at Strong Coupling J. Wrighton, J. Dufty, M. Bonitz, 2 and H. Kählert 2 Department of Physics, University of Florida, Gainesville, FL 326 arxiv:9.76v [cond-mat.stat-mech]
Shell Structure of Confined Charges at Strong Coupling J. Wrighton, J. Dufty, M. Bonitz, 2 and H. Kählert 2 Department of Physics, University of Florida, Gainesville, FL 326 arxiv:9.76v [cond-mat.stat-mech]
Direct determination of the Tolman length from the bulk pressures of liquid drops via molecular dynamics simulations
 Direct determination of the Tolman length from the bulk pressures of liquid drops via molecular dynamics simulations Alan E. van Giessen, and Edgar M. Blokhuis Citation: The Journal of Chemical Physics
Direct determination of the Tolman length from the bulk pressures of liquid drops via molecular dynamics simulations Alan E. van Giessen, and Edgar M. Blokhuis Citation: The Journal of Chemical Physics
Hard-sphere fluids in contact with curved substrates
 Hard-sphere fluids in contact with curved substrates P. Bryk, 1,2,3 R. Roth, 2,3 K. R. Mecke, 2,3 and S. Dietrich 2,3 1 Department for the Modeling of Physico-Chemical Processes, Maria Curie-Skłodowska
Hard-sphere fluids in contact with curved substrates P. Bryk, 1,2,3 R. Roth, 2,3 K. R. Mecke, 2,3 and S. Dietrich 2,3 1 Department for the Modeling of Physico-Chemical Processes, Maria Curie-Skłodowska
Improved association in a classical density functional theory for water. Abstract
 Improved association in a classical density functional theory for water Eric J. Krebs, Jeff B. Schulte, and David Roundy Department of Physics, Oregon State University, Corvallis, OR 97331, USA Abstract
Improved association in a classical density functional theory for water Eric J. Krebs, Jeff B. Schulte, and David Roundy Department of Physics, Oregon State University, Corvallis, OR 97331, USA Abstract
Thermodynamics of Three-phase Equilibrium in Lennard Jones System with a Simplified Equation of State
 23 Bulletin of Research Center for Computing and Multimedia Studies, Hosei University, 28 (2014) Thermodynamics of Three-phase Equilibrium in Lennard Jones System with a Simplified Equation of State Yosuke
23 Bulletin of Research Center for Computing and Multimedia Studies, Hosei University, 28 (2014) Thermodynamics of Three-phase Equilibrium in Lennard Jones System with a Simplified Equation of State Yosuke
Voronoi neighbor statistics of hard-disks and hard-spheres
 THE JOURNAL OF CHEMICAL PHYSICS 123, 074502 2005 Voronoi neighbor statistics of hard-disks and hard-spheres V. Senthil Kumar and V. Kumaran a Department of Chemical Engineering, Indian Institute of Science,
THE JOURNAL OF CHEMICAL PHYSICS 123, 074502 2005 Voronoi neighbor statistics of hard-disks and hard-spheres V. Senthil Kumar and V. Kumaran a Department of Chemical Engineering, Indian Institute of Science,
arxiv: v1 [cond-mat.stat-mech] 11 Nov 2015
![arxiv: v1 [cond-mat.stat-mech] 11 Nov 2015 arxiv: v1 [cond-mat.stat-mech] 11 Nov 2015](/thumbs/84/89151318.jpg) Equation of state and critical point behavior of hard-core double-yukawa fluids J. Montes, 1, a) M. Robles, 1, b) 1, c) and M. López de Haro Instituto de Energías Renovables, Universidad Nacional Autóinoma
Equation of state and critical point behavior of hard-core double-yukawa fluids J. Montes, 1, a) M. Robles, 1, b) 1, c) and M. López de Haro Instituto de Energías Renovables, Universidad Nacional Autóinoma
The inverse problem for simple classical liquids: a density functional approach
 J. Phys.: Condens. Matter 9 (1997) L89 L98. Printed in the UK PII: S0953-8984(97)79859-X LETTER TO THE EDITOR The inverse problem for simple classical liquids: a density functional approach Yaakov Rosenfeld
J. Phys.: Condens. Matter 9 (1997) L89 L98. Printed in the UK PII: S0953-8984(97)79859-X LETTER TO THE EDITOR The inverse problem for simple classical liquids: a density functional approach Yaakov Rosenfeld
Fluid Phase Equilibria Journal
 205 Fluid Phase Equilibria Journal Volume 43, Pages 205-212, 1988 STATISTICAL MECHANICAL TEST OF MHEMHS MODEL OF THE INTERACTION THIRD VIRIAL COEFFICIENTS ESAM Z. HAMAD and G. ALI MANSOORI Department of
205 Fluid Phase Equilibria Journal Volume 43, Pages 205-212, 1988 STATISTICAL MECHANICAL TEST OF MHEMHS MODEL OF THE INTERACTION THIRD VIRIAL COEFFICIENTS ESAM Z. HAMAD and G. ALI MANSOORI Department of
510 Subject Index. Hamiltonian 33, 86, 88, 89 Hamilton operator 34, 164, 166
 Subject Index Ab-initio calculation 24, 122, 161. 165 Acentric factor 279, 338 Activity absolute 258, 295 coefficient 7 definition 7 Atom 23 Atomic units 93 Avogadro number 5, 92 Axilrod-Teller-forces
Subject Index Ab-initio calculation 24, 122, 161. 165 Acentric factor 279, 338 Activity absolute 258, 295 coefficient 7 definition 7 Atom 23 Atomic units 93 Avogadro number 5, 92 Axilrod-Teller-forces
Density functional theory for inhomogeneous mixtures of polymeric fluids
 JOURNAL OF CHEICAL PHYSICS VOLUE 117, NUBER 5 1 AUGUST 2002 Density functional theory for inhomogeneous mixtures of polymeric fluids Yang-Xin Yu a) and Jianzhong Wu b) Department of Chemical and Environmental
JOURNAL OF CHEICAL PHYSICS VOLUE 117, NUBER 5 1 AUGUST 2002 Density functional theory for inhomogeneous mixtures of polymeric fluids Yang-Xin Yu a) and Jianzhong Wu b) Department of Chemical and Environmental
of Nebraska - Lincoln
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 10-1-2006 Homogeneous nucleation at high supersaturation
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 10-1-2006 Homogeneous nucleation at high supersaturation
Peter A. Monson. Department of Chemical Engineering, University of Massachusetts, Amherst, MA 01003, USA. Acknowledgements: John Edison (Utrecht)
 Understanding Adsorption/Desorption Hysteresis for Fluids in Mesoporous Materials using Simple Molecular Models and Classical Density Functional Theory Peter A. Monson Department of Chemical Engineering,
Understanding Adsorption/Desorption Hysteresis for Fluids in Mesoporous Materials using Simple Molecular Models and Classical Density Functional Theory Peter A. Monson Department of Chemical Engineering,
Pre-yield non-affine fluctuations and a hidden critical point in strained crystals
 Supplementary Information for: Pre-yield non-affine fluctuations and a hidden critical point in strained crystals Tamoghna Das, a,b Saswati Ganguly, b Surajit Sengupta c and Madan Rao d a Collective Interactions
Supplementary Information for: Pre-yield non-affine fluctuations and a hidden critical point in strained crystals Tamoghna Das, a,b Saswati Ganguly, b Surajit Sengupta c and Madan Rao d a Collective Interactions
An inorganic capping strategy for the seeded growth of versatile bimetallic nanostructures
 Chemistry Publications Chemistry 2015 An inorganic capping strategy for the seeded growth of versatile bimetallic nanostructures Yuchen Pei Iowa State University Raghu V. Maligal-Ganesh Iowa State University,
Chemistry Publications Chemistry 2015 An inorganic capping strategy for the seeded growth of versatile bimetallic nanostructures Yuchen Pei Iowa State University Raghu V. Maligal-Ganesh Iowa State University,
An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation
 J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
A Hard Convex Core Yukawa Equation of State for Nonassociated Chain Molecules. (Received 10 August 2015, Accepted 17 October 2015)
 Regular Article PHYSICAL CHEMISTRY RESEARCH Published by the Iranian Chemical Society www.physchemres.org info@physchemres.org Phys. Chem. Res., Vol. 3, No. 4, 347-360, December 015. DOI: 10.036/pcr.015.11597
Regular Article PHYSICAL CHEMISTRY RESEARCH Published by the Iranian Chemical Society www.physchemres.org info@physchemres.org Phys. Chem. Res., Vol. 3, No. 4, 347-360, December 015. DOI: 10.036/pcr.015.11597
Theory of infinite dilution chemical potential
 Fluid Phase Equilibria Journal Volume 85, 141-151, 1993 141 Esam Z. Hamada and G.Ali Mansoorib, * a Department of Chemical Engineering, King Fahd University of Petroleum and Minerals, Dhahran 31261 (Saudi
Fluid Phase Equilibria Journal Volume 85, 141-151, 1993 141 Esam Z. Hamada and G.Ali Mansoorib, * a Department of Chemical Engineering, King Fahd University of Petroleum and Minerals, Dhahran 31261 (Saudi
Monte Carlo Simulations for a Soft Sphere Fluid
 Monte Carlo Simulations for a Soft Sphere Fluid Patrick Kreitzberg Advisor: Dr. David Roundy May 8 th, 2015 1 Contents 1 2 3 5 7 Abstract Introduction Methods Results and Discussion Conclusion Acknowledgments
Monte Carlo Simulations for a Soft Sphere Fluid Patrick Kreitzberg Advisor: Dr. David Roundy May 8 th, 2015 1 Contents 1 2 3 5 7 Abstract Introduction Methods Results and Discussion Conclusion Acknowledgments
LOCAL PRESSURE OF CONFINED FLUIDS INSIDE NANOSLIT PORES (A DENSITY FUNCTIONAL THEORY PREDICTION)
 International Journal of Theoretical Physics, Group Theory, and Nonlinear Optics Volume 17, Numer, pp.101-119 (01) ISSN 154 4674 LOCAL PRESSURE OF CONFINED FLUIDS INSIDE NANOSLIT PORES Fatemeh Heidari
International Journal of Theoretical Physics, Group Theory, and Nonlinear Optics Volume 17, Numer, pp.101-119 (01) ISSN 154 4674 LOCAL PRESSURE OF CONFINED FLUIDS INSIDE NANOSLIT PORES Fatemeh Heidari
arxiv: v1 [cond-mat.soft] 20 Jun 2008
![arxiv: v1 [cond-mat.soft] 20 Jun 2008 arxiv: v1 [cond-mat.soft] 20 Jun 2008](/thumbs/87/97245012.jpg) Accurate determination of crystal structures based on averaged local bond order parameters Wolfgang Lechner and Christoph Dellago Faculty of hysics, University of Vienna, Boltzmanngasse, 19 Vienna, Austria
Accurate determination of crystal structures based on averaged local bond order parameters Wolfgang Lechner and Christoph Dellago Faculty of hysics, University of Vienna, Boltzmanngasse, 19 Vienna, Austria
Author copy. Structure factors of binary liquid metal alloys within the square-well model. 1. Introduction. Central European Journal of Physics
 Cent. Eur. J. Phys. 7(3) 2009 584-590 DOI: 10.2478/s11534-009-0064-2 Central European Journal of Physics Structure factors of binary liquid metal alloys within the square-well model Research Article Nikolay
Cent. Eur. J. Phys. 7(3) 2009 584-590 DOI: 10.2478/s11534-009-0064-2 Central European Journal of Physics Structure factors of binary liquid metal alloys within the square-well model Research Article Nikolay
Variational Approach to the Equilibrium Thermodynamic Properties of Simple Liquids. I*
 THE JOURNAL OF CHEMICAL PHYSICS VOLUME 51, NUMBER 11 1 DECEMBER 1969 Variational Approach to the Equilibrium Thermodynamic Properties of Simple Liquids. I* G.Ali MANSOORi (1) AND Frank B. CANFIELD (2)
THE JOURNAL OF CHEMICAL PHYSICS VOLUME 51, NUMBER 11 1 DECEMBER 1969 Variational Approach to the Equilibrium Thermodynamic Properties of Simple Liquids. I* G.Ali MANSOORi (1) AND Frank B. CANFIELD (2)
Free energy of the Lennard-Jones solid
 Free energy of the Lennard-Jones solid van der Hoef, M.A. Published in: Journal of Chemical Physics DOI: 10.106/1.11442 Published: 01/01/2000 Document Version Publisher s PDF, also known as Version of
Free energy of the Lennard-Jones solid van der Hoef, M.A. Published in: Journal of Chemical Physics DOI: 10.106/1.11442 Published: 01/01/2000 Document Version Publisher s PDF, also known as Version of
Point Defects in Hard-Sphere Crystals
 6722 J. Phys. Chem. B 2001, 105, 6722-6727 Point Defects in Hard-Sphere Crystals Sander Pronk and Daan Frenkel* FOM Institute for Atomic and Molecular Physics, Kruislaan 407 1098 SJ Amsterdam, The Netherlands
6722 J. Phys. Chem. B 2001, 105, 6722-6727 Point Defects in Hard-Sphere Crystals Sander Pronk and Daan Frenkel* FOM Institute for Atomic and Molecular Physics, Kruislaan 407 1098 SJ Amsterdam, The Netherlands
Calculating thermodynamic properties from perturbation theory I. An analytic representation of square-well potential hard-sphere perturbation theory
 Ž. Fluid Phase Equilibria 154 1999 1 1 Calculating thermodynamic properties from perturbation theory I. An analytic representation of square-well potential hard-sphere perturbation theory Bing-Jian Zhang
Ž. Fluid Phase Equilibria 154 1999 1 1 Calculating thermodynamic properties from perturbation theory I. An analytic representation of square-well potential hard-sphere perturbation theory Bing-Jian Zhang
arxiv: v1 [cond-mat.soft] 23 Nov 2018
![arxiv: v1 [cond-mat.soft] 23 Nov 2018 arxiv: v1 [cond-mat.soft] 23 Nov 2018](/thumbs/91/104979968.jpg) Bulk structural informations from density functionals for patchy particles arxiv:8.9388v [cond-mat.soft] 3 Nov 8 Daniel Stopper,, Frank Hirschmann, Martin Oettel,, and Roland Roth Institute for Theoretical
Bulk structural informations from density functionals for patchy particles arxiv:8.9388v [cond-mat.soft] 3 Nov 8 Daniel Stopper,, Frank Hirschmann, Martin Oettel,, and Roland Roth Institute for Theoretical
Citation PHYSICAL REVIEW E (2002), 65(6) RightCopyright 2002 American Physical So
 TitleStatistical mechanics of two hard d Author(s) Munakata, T; Hu, G Citation PHYSICAL REVIEW E (2002), 65(6) Issue Date 2002-06 URL http://hdl.handle.net/2433/50310 RightCopyright 2002 American Physical
TitleStatistical mechanics of two hard d Author(s) Munakata, T; Hu, G Citation PHYSICAL REVIEW E (2002), 65(6) Issue Date 2002-06 URL http://hdl.handle.net/2433/50310 RightCopyright 2002 American Physical
arxiv: v2 [cond-mat.stat-mech] 30 May 2013
![arxiv: v2 [cond-mat.stat-mech] 30 May 2013 arxiv: v2 [cond-mat.stat-mech] 30 May 2013](/thumbs/82/85489539.jpg) Chemical-potential route for multicomponent fluids Andrés Santos Departamento de Física, Universidad de Extremadura, Badajoz, E-671, Spain arxiv:1.1155v [cond-mat.stat-mech May 1 René D. Rohrmann Instituto
Chemical-potential route for multicomponent fluids Andrés Santos Departamento de Física, Universidad de Extremadura, Badajoz, E-671, Spain arxiv:1.1155v [cond-mat.stat-mech May 1 René D. Rohrmann Instituto
Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems
 THE JOURNAL OF CHEMICAL PHYSICS 124, 184101 2006 Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems Igor V. Pivkin and George E. Karniadakis a Division of Applied Mathematics,
THE JOURNAL OF CHEMICAL PHYSICS 124, 184101 2006 Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems Igor V. Pivkin and George E. Karniadakis a Division of Applied Mathematics,
Thermodynamic expansion of nucleation free-energy barrier and size of critical nucleus near the vapor-liquid coexistence
 JOURNAL OF CHEMICAL PHYSICS VOLUME 110, NUMBER 7 15 FEBRUARY 1999 hermodynamic expansion of nucleation free-energy barrier and size of critical nucleus near the vapor-liquid coexistence Kenichiro Koga
JOURNAL OF CHEMICAL PHYSICS VOLUME 110, NUMBER 7 15 FEBRUARY 1999 hermodynamic expansion of nucleation free-energy barrier and size of critical nucleus near the vapor-liquid coexistence Kenichiro Koga
Local pressure of confined fluids inside nanoslit pores (A density functional theory prediction)
 Local pressure of confined fluids inside nanoslit pores (A density functional theory prediction) y F. Heidari, G. A. Mansoori and E. Keshavarzi Department of BioEngineering, University of Illinois at Chicago,
Local pressure of confined fluids inside nanoslit pores (A density functional theory prediction) y F. Heidari, G. A. Mansoori and E. Keshavarzi Department of BioEngineering, University of Illinois at Chicago,
arxiv: v1 [cond-mat.soft] 28 Jul 2018
![arxiv: v1 [cond-mat.soft] 28 Jul 2018 arxiv: v1 [cond-mat.soft] 28 Jul 2018](/thumbs/92/109519388.jpg) The pair-potential system. I. Fluid phase isotherms, isochores, and quasiuniversality Andreas Kvist Bacher, Thomas B. Schrøder, and Jeppe C. Dyre Glass and Time, IMFUFA, Department of Science and Environment,
The pair-potential system. I. Fluid phase isotherms, isochores, and quasiuniversality Andreas Kvist Bacher, Thomas B. Schrøder, and Jeppe C. Dyre Glass and Time, IMFUFA, Department of Science and Environment,
Self-assembly of the simple cubic lattice with an isotropic potential
 Self-assembly of the simple cubic lattice with an isotropic potential Mikael C. Rechtsman, 1 Frank H. Stillinger, 2 and Salvatore Torquato 2,3,4,5, * 1 Department of Physics, Princeton University, Princeton,
Self-assembly of the simple cubic lattice with an isotropic potential Mikael C. Rechtsman, 1 Frank H. Stillinger, 2 and Salvatore Torquato 2,3,4,5, * 1 Department of Physics, Princeton University, Princeton,
Statistical Mechanics of a Thin Film on a Solid Substrate
 Statistical Mechanics of a Thin Film on a Solid Substrate arxiv:1406.0698v2 [cond-mat.stat-mech] 6 Jan 2017 Diploma Thesis, revised version Andreas Nold submitted at the Technische Universität Darmstadt
Statistical Mechanics of a Thin Film on a Solid Substrate arxiv:1406.0698v2 [cond-mat.stat-mech] 6 Jan 2017 Diploma Thesis, revised version Andreas Nold submitted at the Technische Universität Darmstadt
Phase transitions of quadrupolar fluids
 Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Advantages of a Finite Extensible Nonlinear Elastic Potential in Lattice Boltzmann Simulations
 The Hilltop Review Volume 7 Issue 1 Winter 2014 Article 10 December 2014 Advantages of a Finite Extensible Nonlinear Elastic Potential in Lattice Boltzmann Simulations Tai-Hsien Wu Western Michigan University
The Hilltop Review Volume 7 Issue 1 Winter 2014 Article 10 December 2014 Advantages of a Finite Extensible Nonlinear Elastic Potential in Lattice Boltzmann Simulations Tai-Hsien Wu Western Michigan University
Chapter 2 Experimental sources of intermolecular potentials
 Chapter 2 Experimental sources of intermolecular potentials 2.1 Overview thermodynamical properties: heat of vaporization (Trouton s rule) crystal structures ionic crystals rare gas solids physico-chemical
Chapter 2 Experimental sources of intermolecular potentials 2.1 Overview thermodynamical properties: heat of vaporization (Trouton s rule) crystal structures ionic crystals rare gas solids physico-chemical
B.K. Singh et al./ BIBECHANA 14 (2017) : RCOST p.16 (Online Publication: Dec., 2016)
 B.K. Singh et al./ BIBECHANA 14 (2017) 16-29 : RCOST p.16 (Online Publication: Dec., 2016) BIBECHANA A Multidisciplinary Journal of Science, Technology and Mathematics ISSN 2091-0762 (Print), 2382-5340
B.K. Singh et al./ BIBECHANA 14 (2017) 16-29 : RCOST p.16 (Online Publication: Dec., 2016) BIBECHANA A Multidisciplinary Journal of Science, Technology and Mathematics ISSN 2091-0762 (Print), 2382-5340
An explicit expression for finite-size corrections to the chemical potential
 J. Phys.: Condens. Matter 1 (1989) 8659-8665. Printed in the UK An explicit expression for finite-size corrections to the chemical potential B Smitt and D Frenkelt t Koninklijke/Shell-Laboratorium, Amsterdam
J. Phys.: Condens. Matter 1 (1989) 8659-8665. Printed in the UK An explicit expression for finite-size corrections to the chemical potential B Smitt and D Frenkelt t Koninklijke/Shell-Laboratorium, Amsterdam
Classical Density Functional Theory
 Classical Density Functional Theory Towards interfaces in crystalline materials Gunnar Pruessner and Adrian Sutton Department of Physics Imperial College London INCEMS M12 meeting, Imperial College London,
Classical Density Functional Theory Towards interfaces in crystalline materials Gunnar Pruessner and Adrian Sutton Department of Physics Imperial College London INCEMS M12 meeting, Imperial College London,
Advances in developing multiscale flaw models for eddy-current NDE
 Center for Nondestructive Evaluation Conference Papers, Posters and Presentations Center for Nondestructive Evaluation 7-2011 Advances in developing multiscale flaw models for eddy-current NDE R. Kim Murphy
Center for Nondestructive Evaluation Conference Papers, Posters and Presentations Center for Nondestructive Evaluation 7-2011 Advances in developing multiscale flaw models for eddy-current NDE R. Kim Murphy
On the Calculation of the Chemical Potential. Using the Particle Deletion Scheme
 On the Calculation of the Chemical Potential Using the Particle Deletion Scheme Georgios C. Boulougouris,2, Ioannis G. Economou and Doros. Theodorou,3,* Molecular Modelling of Materials Laboratory, Institute
On the Calculation of the Chemical Potential Using the Particle Deletion Scheme Georgios C. Boulougouris,2, Ioannis G. Economou and Doros. Theodorou,3,* Molecular Modelling of Materials Laboratory, Institute
Numerical Aspects of the SAFT Equation of State
 University of Rhode Island DigitalCommons@URI Senior Honors Projects Honors Program at the University of Rhode Island 006 Numerical Aspects of the SAFT Equation of State Leah M. Octavio University of Rhode
University of Rhode Island DigitalCommons@URI Senior Honors Projects Honors Program at the University of Rhode Island 006 Numerical Aspects of the SAFT Equation of State Leah M. Octavio University of Rhode
Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension
 7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
Competition between face-centered cubic and icosahedral cluster structures
 Competition between face-centered cubic and icosahedral cluster structures R. S. Berry University of Chicago, Chicago, IL 60637, USA B. M. Smyrnov, and A. Yu. Strizhev High-Temperatures Institute, Russian
Competition between face-centered cubic and icosahedral cluster structures R. S. Berry University of Chicago, Chicago, IL 60637, USA B. M. Smyrnov, and A. Yu. Strizhev High-Temperatures Institute, Russian
II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics
 II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics Notes by ChangHoon Lim (and MZB) Open circuit voltage of galvanic cell is To understand compositional effects on, we need to consider
II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics Notes by ChangHoon Lim (and MZB) Open circuit voltage of galvanic cell is To understand compositional effects on, we need to consider
602 ZHANG Zhi and CHEN Li-Rong Vol Gibbs Free Energy From the thermodynamics, the Gibbs free energy of the system can be written as G = U ; TS +
 Commun. Theor. Phys. (Beijing, China) 37 (2002) 601{606 c International Academic Publishers Vol. 37, No. 5, May 15, 2002 The 2D Alternative Binary L J System: Solid-Liquid Phase Diagram ZHANG Zhi and CHEN
Commun. Theor. Phys. (Beijing, China) 37 (2002) 601{606 c International Academic Publishers Vol. 37, No. 5, May 15, 2002 The 2D Alternative Binary L J System: Solid-Liquid Phase Diagram ZHANG Zhi and CHEN
Effect of the range of attractive interactions on crystallization, metastable phase transition, and percolation in colloidal dispersions
 PHYSICAL REVIEW E 68, 011403 2003 Effect of the range of attractive interactions on crystallization, metastable phase transition, and percolation in colloidal dispersions Dong Fu and Yigui Li Department
PHYSICAL REVIEW E 68, 011403 2003 Effect of the range of attractive interactions on crystallization, metastable phase transition, and percolation in colloidal dispersions Dong Fu and Yigui Li Department
Disordered Hyperuniformity: Liquid-like Behaviour in Structural Solids, A New Phase of Matter?
 Disordered Hyperuniformity: Liquid-like Behaviour in Structural Solids, A New Phase of Matter? Kabir Ramola Martin Fisher School of Physics, Brandeis University August 19, 2016 Kabir Ramola Disordered
Disordered Hyperuniformity: Liquid-like Behaviour in Structural Solids, A New Phase of Matter? Kabir Ramola Martin Fisher School of Physics, Brandeis University August 19, 2016 Kabir Ramola Disordered
Monte Carlo Study of the Crystalline and Amorphous NaK Alloy
 Available online at www.sciencedirect.com ScienceDirect Procedia Computer Science 08C (207) 25 22 International Conference on Computational Science, ICCS 207, 2-4 June 207, Zurich, Switzerland Monte Carlo
Available online at www.sciencedirect.com ScienceDirect Procedia Computer Science 08C (207) 25 22 International Conference on Computational Science, ICCS 207, 2-4 June 207, Zurich, Switzerland Monte Carlo
Thermodynamics and structural properties of the dipolar Yukawa fluid. Citation Journal Of Chemical Physics, 1999, v. 111 n. 1, p.
 Title Thermodynamics and structural properties of the dipolar Yukawa fluid Author(s) Szalai, I; Henderson, D; Boda, D; Chan, KY Citation Journal Of Chemical Physics, 1999, v. 111 n. 1, p. 337-344 Issued
Title Thermodynamics and structural properties of the dipolar Yukawa fluid Author(s) Szalai, I; Henderson, D; Boda, D; Chan, KY Citation Journal Of Chemical Physics, 1999, v. 111 n. 1, p. 337-344 Issued
Statistical Geometry and Lattices
 Journal of Statistical Physics, Vol. 96, Nos. 56, 1999 Statistical Geometry and Lattices Gerardo Soto-Campos, 1 Richard Bowles, 1 Andrey Itkin, 1 and Howard Reiss 1 Received January 11, 1999; final May
Journal of Statistical Physics, Vol. 96, Nos. 56, 1999 Statistical Geometry and Lattices Gerardo Soto-Campos, 1 Richard Bowles, 1 Andrey Itkin, 1 and Howard Reiss 1 Received January 11, 1999; final May
RESEARCH ARTICLE On the critical non-additivity driving segregation of asymmetric binary hard sphere fluids
 Molecular Physics Vol. 18, No. 1, 1 January 21, 97 14 RESEARCH ARTICLE On the critical non-additivity driving segregation of asymmetric binary hard sphere fluids Per Sillre n a * and Jean-Pierre Hansen
Molecular Physics Vol. 18, No. 1, 1 January 21, 97 14 RESEARCH ARTICLE On the critical non-additivity driving segregation of asymmetric binary hard sphere fluids Per Sillre n a * and Jean-Pierre Hansen
Structure and phase behaviour of colloidal dispersions. Remco Tuinier
 Structure and phase behaviour of colloidal dispersions Remco Tuinier Yesterday: Phase behaviour of fluids and colloidal dispersions Colloids are everywhere Hard sphere fluid at the base understanding fluids
Structure and phase behaviour of colloidal dispersions Remco Tuinier Yesterday: Phase behaviour of fluids and colloidal dispersions Colloids are everywhere Hard sphere fluid at the base understanding fluids
Molecular Dynamics Simulations of Glass Formation and Crystallization in Binary Liquid Metals
 Citation & Copyright (to be inserted by the publisher ) Molecular Dynamics Simulations of Glass Formation and Crystallization in Binary Liquid Metals Hyon-Jee Lee 1,2, Tahir Cagin 2, William A. Goddard
Citation & Copyright (to be inserted by the publisher ) Molecular Dynamics Simulations of Glass Formation and Crystallization in Binary Liquid Metals Hyon-Jee Lee 1,2, Tahir Cagin 2, William A. Goddard
The calculation of surface tensien for simple liquids
 J. Phys. A: Gen. Phys., Vol. 5, January 1972. Printed in Great Britain The calculation of surface tensien for simple liquids M V BERRY, R F DURRANST and R EVANS H H Wills Physics Laboratory, Tyndall Avenue,
J. Phys. A: Gen. Phys., Vol. 5, January 1972. Printed in Great Britain The calculation of surface tensien for simple liquids M V BERRY, R F DURRANST and R EVANS H H Wills Physics Laboratory, Tyndall Avenue,
SUPPLEMENTARY INFORMATION
 Supplementary Information DNA-Programmable Nanoparticle Crystallization Sung Yong Park,* 1 Abigail K. R. Lytton-Jean,* 1 Byeongdu Lee 2, Steven Weigand 3, George C. Schatz 1 and Chad A. Mirkin 1 1 Department
Supplementary Information DNA-Programmable Nanoparticle Crystallization Sung Yong Park,* 1 Abigail K. R. Lytton-Jean,* 1 Byeongdu Lee 2, Steven Weigand 3, George C. Schatz 1 and Chad A. Mirkin 1 1 Department
ENTHALPY, ENTROPY AND HELMHOLTZ FREE ENERGY OF TRANSITION AND RARE EARTH LIQUID METALS
 Digest Journal of Nanomaterials and iostructures Vol. 5, No, April 010, p. 317 3 ENTHALPY, ENTROPY AND HELMHOLTZ FREE ENERGY OF TRANSITION AND RARE EARTH LIQUID METALS J. K. ARIA a, A. R. JANI b a V. P.
Digest Journal of Nanomaterials and iostructures Vol. 5, No, April 010, p. 317 3 ENTHALPY, ENTROPY AND HELMHOLTZ FREE ENERGY OF TRANSITION AND RARE EARTH LIQUID METALS J. K. ARIA a, A. R. JANI b a V. P.
Fig. 3.1? Hard core potential
 6 Hard Sphere Gas The interactions between the atoms or molecules of a real gas comprise a strong repulsion at short distances and a weak attraction at long distances Both of these are important in determining
6 Hard Sphere Gas The interactions between the atoms or molecules of a real gas comprise a strong repulsion at short distances and a weak attraction at long distances Both of these are important in determining
Entropic wetting and the fluid fluid interface of a model colloid polymer mixture
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS: CONDENSED MATTER J. Phys.: Condens. Matter 4 (22) L L8 PII: S953-8984(2)28952-3 LETTER TO THE EDITOR Entropic wetting and the fluid fluid interface of
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS: CONDENSED MATTER J. Phys.: Condens. Matter 4 (22) L L8 PII: S953-8984(2)28952-3 LETTER TO THE EDITOR Entropic wetting and the fluid fluid interface of
Yang-Xin Yu* and Feng-Qi You. Yiping Tang. Guang-Hua Gao and Yi-Gui Li
 334 J. Phys. Chem. B 2006, 110, 334-341 Structure and Adsorption of A Hard-Core Multi-Yukawa Fluid Confined in A Slitlike Pore: Grand Canonical Monte Carlo Simulation and Density Functional Study Yang-Xin
334 J. Phys. Chem. B 2006, 110, 334-341 Structure and Adsorption of A Hard-Core Multi-Yukawa Fluid Confined in A Slitlike Pore: Grand Canonical Monte Carlo Simulation and Density Functional Study Yang-Xin
THE JOURNAL OF CHEMICAL PHYSICS 126,
 THE JOURNAL OF CHEMICAL PHYSICS 16 44503 007 Development of an equation of state for electrolyte solutions by combining the statistical associating fluid theory and the mean spherical approximation for
THE JOURNAL OF CHEMICAL PHYSICS 16 44503 007 Development of an equation of state for electrolyte solutions by combining the statistical associating fluid theory and the mean spherical approximation for
A theory for calculating the number density distribution of. small particles on a flat wall from pressure between the two
 theory for calculating the number density distribution of small particles on a flat wall from pressure between the two walls Kota Hashimoto a and Ken-ichi mano a a Department of Energy and Hydrocarbon
theory for calculating the number density distribution of small particles on a flat wall from pressure between the two walls Kota Hashimoto a and Ken-ichi mano a a Department of Energy and Hydrocarbon
Mixtures, I. Hard Sphere Mixtures*
 Proceedings of the Natioruil Academy of Scienccs Vol. 67, No. 4, pp. 1818-1823, December 1970 One- and Two-Fluid van der Waals Theories of Liquid Mixtures, I. Hard Sphere Mixtures* Douglas Henderson and
Proceedings of the Natioruil Academy of Scienccs Vol. 67, No. 4, pp. 1818-1823, December 1970 One- and Two-Fluid van der Waals Theories of Liquid Mixtures, I. Hard Sphere Mixtures* Douglas Henderson and
Monte Carlo simulations of alloy segregation in PtAg octahedral nanoparticles
 Monte Carlo simulations of alloy segregation in PtAg octahedral nanoparticles Louis C. Jones 6/8/12 Abstract Simulations were carried out to investigate phase segregation of insoluble alloy nanoparticles
Monte Carlo simulations of alloy segregation in PtAg octahedral nanoparticles Louis C. Jones 6/8/12 Abstract Simulations were carried out to investigate phase segregation of insoluble alloy nanoparticles
From the ideal gas to an ideal glass: a thermodynamic route to random close packing.
 Leslie V. Woodcock From the ideal gas to an ideal glass: a thermodynamic route to random close packing. Department of Chemical and Biomolecular Engineering National University of Singapore, Singapore 117576
Leslie V. Woodcock From the ideal gas to an ideal glass: a thermodynamic route to random close packing. Department of Chemical and Biomolecular Engineering National University of Singapore, Singapore 117576
