a ^^a^avnhj Gob Title) (Job Summary) phu.viu3i.aim b <w#ivitijw^^<9<uvmn tl^ij^^tuqifn^ ^Tuen^uiiju ^Tumivifr^aija ua - ^aaa^iia^tuq'ami ^Tuem"5biiai
|
|
|
- Piers Ford
- 5 years ago
- Views:
Transcription
1 phu.u3i.aim b a ^^a^avnhj Gob Title) ki (Job Summary) b en tl^ij^^tuqifn^ ^Tuen^uiiju ^Tumifr^aija ua ^TUiilvniyhliJ i<waei<u<u?f<u'un't3<ij^ij^-^tuh(,tl'ul'll aan^flij^^bb/n'w ua^anwa^wiu^^i.^^a'ueniiu^ii^ vtrnvrn^^h ^u^u^iau^ avimii'^ajjaabw tanecr^ ^n^tuvfu^fia wnu^sma^jqb^j^ij^i.'haei^^nnwafn^^'uvn uasi^^^ anfn<uvn-3in<bfn'5 <w#itijw^^<9<uvmn tl^^ijw-^tu^itum^'w'^a^tuaiy'wnvrusu.asaotu i,^la?('u<uei<u'ufn^'ijfi'li^3n^<^a^3w'u?vni^nam^ma^3 ^ d^ <U u^sfni\j^<uw<n<uwnjjutau'ia^^a^!sij<ufib a/ - ^aaa^iia^tuq'ami ^Tuem"5biiai '^TUiJru^n'^iaii^ ^mnvmvnhj a'iu'i'5nnm<um^l^nwa-^ vrunan - ^aa^s^ia^a^ja vsaijnifl^ni.chjfm m^^tunul^on^a-a mucfi^ ^n^ii^idaijen'a'u'j'aa! - ^aaasiia-afn's^ni.^iifn^^tufn^'d^a atuatut^twusiufisaatu anuma ^nmufnil^'m'ui.'jan uasanwa-awnw s: ^iuua'uijrn^a^iipi^aufn^iij^is^ u.^sa^vf^na-aturn^ ijf^^bjj'^l^fujjausjna i^iaifn^il^^'tjsji.tl'ulijw^a ^msji.iau^aau.assanstuium^'ij^s'wjj da 1 - ^sj^jfmjjani^a^ia-^m^i^ni^'umiavi il^isj'qjjvtl^fujja'uuia
2 -to Id ^n ^^waij^^atu'^tumj^flpi^/n^l^'ii'jei^tui.lla^n'uia <iba-n'uvknen6ua-3 ^ahmiij^ijw-nuiliilil^'jej FlTttJ^TUTU wmfuw^'fla'uvmn HulnTa^auaufiwajn^^^'a^Twrn^ iaivfl^fu^iaua 11 V V asihiluilista'w'uui^a'hj w^^n.anefta^i^'^ ^aenluei'u'ufn'j'ij^iji^tu^nu/n^n^ aa^vni^a^nu u.asij^ijpi'una^i.na^^^a^^sjl^tu - faaas^ia^rn^tj^sfitu-^tu aiut^a - fa^^^pmnw^aia^ia^wtuuifni - faa^^^^njj^'wala^ub^wfuiiini^ uauuia (Job Specifications) n.. vffwg. b.. en. 9vm. en. si..
3 -tia^iaynhj (Job Title) to v<un^fmajfu^^<aa'u'[^i^a^tl Gob Summary) en n. vt'unf'uw^iifa'u^n Q/ fi V la an ij^ii^^i'u^ma^mj-^i'u'uivn'aynlij ^ahm^ifi'u'uei'u'u ni'3ria'!ua^i^3j'u^1 i.il'ul'ija^n^^n^i.it u.a^ ^il^i^^bfni^ ^n^^ipi^si'ma^^^'u^sj'liajj^ m^^mj/mna^fma^ uw'una^^^a-^eitu^itbm'^ ^aecuiiei'u'ufnm-auw'u niwulaui^ uwwiu uwuwihsjj'iaj vtiat^^fn^ H ui^^ /n^ina'mn'i'u^hua^m^iwamjqbg^^^ ^^ins^^ w^^nu il^s^uw^rn^^ni^ufn^^jjuwu^tu/ Ipii-^mi/fian's'sjj ^awrnii^nm'um^s'ijii^^bfn ua^t^awa^itia^nmih^ny^fri'u^^k ^fn^n ^ifiins^ ^in^aad nm4ma-3lia-3^i,n^^mj-^tu tilvmvnuj maa^\ji,1a^i,ecua^nijji,'m<u^iaf SJ'U^iTl'u - iiaaa'?ja^tuvti,n^inij-3tu'uiivn'5 vnltl ^^ni.^^fn<5'l^fjn^a-3 vmnai - ^^^ufl^^u^u^fl^ja^fm^^vh u,astp^-3fn?/n^ni^ii ma^^a^ - ^^^j^muanifa^ia-^fn^a^t u,astf^fm/n^fmjj ma^^a-j - ^aaas^a-ai.ia'^a^'ij^'^nui'w'u ^^i^um^l^fin^a-3 vfuuan
4 vnjrmfuw^^'fla'uvmn ^jjqn^uwun^n^ijjvtiai^^^niiuawu^^-atu ^as unt^^yvnt^fn^il^'ij^^'vu iaifn'^^nm'u-anultlpnu ttliuiej ^a^waa^iqbfh'u^^ a/ ^f a/ - is^jfltiu^fni^^fl^a^fn'^^nnnuwu nant5jj1atf^m<3'iua^3vribej-n<u VI k) 'un^uw^i'aa'u^n <Ll'5^?n'Ufn"ji-3i<u<L^^Ciu'uil'um^iHfniu^'uu,fi 9/ in^a^a-^ iahm^fmu'j^jjsa u,awa^u^5 ht^u^i H'tfapi^i.'M'uu.aK^^nu'usj'uniila-^W'u tn^^nu^iam^^l^ uas^sm^il^ijw-atu^nu-^n^uivn^vhlij u,as-nu Qt^iis^'M^iIainaua^iiw'u nnij^iann^l<u^n^u,a^ vni^a^tumat^a^ ^ahtn^^n'iu^it.a ila^an^ia-3 sn^ahi.n^um'u^ WTH^fl - ^aaa^^inu6?ni!a<?ia-3mtihs:^tun'ii i^on-^i<umj<u^iann^'l'u?^nvi u,as vni^a-3tui.f!a^^^a-3 - ^aaaspmu^fni.i^^ia-^nti'lvraafi^i.'mj uasflnu'usuni^^a^iw'u ftlnjpiann^lu ^n^iu.^^vni^a^tui.na'^^^a^ ^ihtuw^^'ba'uvmn a/ ^a/ Is an W^Ti^nw^ uii^iln ^aijtlfyvniaas^^^u.a'^i.naimj ^Tunii^liJ u^sj-atu^ifl^ns'w'uta'unau.asuwu iahflpmui fni^mla u^^^iin'aa'unt.'ijil^'u^^tu Han^a^pnuvt^nmtu'wl^ ^^nw ^i^^ns^ uasw^n^^sanitiwu'^'iu iam^ u i,wau <w^m^i>1au ^3ntiina'3mj'anu^Tu-anuu1vn^vh1liJ u^^-^i'u^ifiin^'m'uia^nau.asjuwu i^jjmvru^u'ut^n^fn^^^n^su'ui.flt'uiaseniieiiii.vn'i u,fisis'uiitu^aij^ ia'w^atufniiij^istnajiwus'baija crifuwff'uta - lay^^pm^^'^ata^ua-awfuuimi - ^^^j^nn^ani.^a^oa-^fn'aa^'mihsa fit^il^^ijl-^tu uasjiana^waui - iaoa^pniu^<wa1^<iia<iwtuu1fn^ - lu^u^majaii^a'ua-^m^iavi'^'i'a^uu ifil'uiasat^a'ui.pi - atutuwmhiistj'u^iu'wajja
5 - en - ghti < flfliaaj'uw'^^ni^^'uiun'u (Job Specifications) Sii n/w. mvmw gfou si. pmuifniu^njj'iiinl^fiiiw^'i'ui^^u^a-3fni is 1ib is is en. v^aw^fn^fituq^ui^u^a-3mi is si. ihv^i is. mnjimi^^i^s^u^a^nii is en. nma'aajjpniji^a^^nfyi'u'n'uan^i^mj^^a-am's is si. m^is^jt'ui'u^n'iu^nwa-^^^a'ub^ju.^^^i^qi'ajj^^irua-3ni^ is si. mir^^i.fi^s'm?s^j^a-3m^i is is. fitajja-a/n'wa'af^^iij^^^jvl^a^mi is en. m^auienstbaiiai
6 8 n a ifaiavnltl (Job Title) i9909i5-3ii9l,ij 115x1, x0iihnij0i5 3iii5vn5vnliJ la (Job Summary) i <Ha^mi?i<u<ufnina'^a'af3J<uw^ en n. ^n.ynn51l^ib.nn5 'untuw^'wa^^n OS M^S Is en si U^^i^-^^tii.n^nmj^Tu^Jn^a^in^fnii ua^ ^n'uiaun'unn^ t^lahm5e('uue!'u'ufn'3n^<ioa-95 jj'u^5 uns^nijn'^nui.ilylijaan-^nn^a'^ vmnan ^^i nifi5ns5^ ^55^^91 naiun5a^3i.5a-3i,njnmj-3it! "^n^a^in^mi ^aatuiia-u^iaflnnm'm^afii'u^ii 1ynn5ni9aa ^n^n m^3awi ^ni,u'ufn5^h<u<niu^bejan<unejfn5 a^^ ^111,9^)11955 i,awm53iiaiifn5n9na-35 ii05 u,9t^3oiin-3iii,ililijaii-39n0a-3 0ii5sii,ijiani,na!ei HI ^^ ,I,3X-311'^5915 ^aw ll5x6aivi5a~311^^ el 1559 i09il5x3-ami9xiih^ii&jv)9ii0h - 5ai9xiia-3-3iiib&jaini9i5 u,3s 3111,91)11915 Y)011,119151, vfin9i - 5aEJ9X05113O1l59D93l,5a-3Vi95ll 05111,11-59J9XD9-3'311ii5jail5J915 U9X-311 I.9U11915 ^0H,11915l0O ,591-5X0105H91159D X^ '3IJ,9X-311<^5015
7 -te vrunfuw^i'wa'uvran ii^m-mw^nant^ajiatm^m^a^i'iby-^'u ua^ Via s) i ci <^c ^ o 1/ o ^=* Hi umj^yvnt'umtujj'u^^i'u ^ialm^mun'ulilw'iij iihina u^swaaii^bfn'u^ a/ ^o/ - ^^^j^niu^ni,^a<?ia-^m<3a^i'wiuw^! nan^jj1aifs)'5-^rn'5tua'3vi'ii'3a-3tu 'un^tuw^i^ia'uffn V va^/ te Uiisen'Ufnin-^TuI^aS^JVi'U'i'l'umiHpn'ijJi'M'uuas a/" ^^iu.^sii'il^ia-^^'u ^^umj^^^ann^^-^n^i ua^ vfu^a-atuifia^^a-^ ^^alvtma^msj^usa u.a wa^iqsfhvrufl W'fiafl^iM'uij.asflnu'u^'iIniiia^w'u ma^mj'lial'^aa^ i.i.^i^snt^ij^^'u^^tu^i'tu^aa^i'u^ani's ntiij^i^nn^lu a^nviu.ajivni^a^tuina'j^a^ iahm^pm^imla u,a^n^a-^wnuani,n^unnvru^ - ^aa^sj^ijj^ni^a^ia^m'a'ij'j^en'unta vfn-31'umj'u^^ann^i'u^n^ uas; vniqej-an^vttnen'ua-a - ^aaa^^nsj^ni.^atia^m^lvraa^^^i'm'u uasmu'usj'uima^^'u nu'u^icnni'l'u a-^n^a^^u^^-nuwien'iia^ ^. to ^untuw^^'aa'uiin H^ilin'fcn u^siln ^ia<uilujvnila^^ua-^i,na'3n'u^tu ^iw3ejatu^eifm ia'lspmjj? ^inumta uas eiijjtsa'unlil'ij^'u^^i'uwcin^^^^njjvi^nma!^ ^^ntn ^t^^nti'm u.asj^^^nhsamiwbj'u'i'^tu tam^ Lwa^^m'jtia'uf^TU'MLna^mj^nu^iTu^aa^TU^ani^ v ^ as ^IQUQ^ - faa^^^nijj^a'la'^a^wtu'uim'a - ^s^<uflqiuf?ni^^<oa-ann?^i'm'ifljja fn^j^'u^^tu uaslan?n^iwou^ - laaasj^nijj^ala'ua^wfu'uim^
8 10 gbu a: ^suaaji^vmiil'utu-n'u (Job Specifications) ^il n.^. fmajffmajaianin vftms lo. b. ^^n'wsfmhfn'fcna-anq^ en. vfftttkfn^^iinni<5^mjwa'3fn^i b si. vfnw^n'i^^vifn^asja^s^uwa-afm b. b. mifuimi^i en. fnia-^aupmu^at^n^jlii'^tuan^^"^s^uwa^fn? b si. ni'38^aj'<ul^ifsmjj^]n^ia-^eaa'u5'5^3ji.i^^^flij5'3<5u^^ja^nt5 b si. n'itm<nmtl'uij s^v en. m'^^^^^eia'upnnjj^n^a^^njjn^utu^tu^^jwa-^rnii b
9 11 OI k>o a (Job Title) la (Job Summary) u^siln 61 O 0/0/ uwu-nu n. Tfl'uiTUN^^'wa'uvran a/ 40/ Is ^ipcai^ nfujn^a^ ei'jijifi^apnnjjpi^it'u^tia-iiia^ yjn^^ ^a-^i^m ^a-a^apmjj'w^ai.^a t^a-^en^apniii pi^ii.'m<u<?ia-3i)'5'b'iww'uiei<uapiafs3j<u1 ^^i Bipins^ nmjnia-i p^upi^t^aija ei^ilienja pnn^ji.'m'ut'ufn^^'ii.^'umii^i^ m^^mjmi^a^vjn^^ ^a-itiau ^a^apmw^^'jai^a ^<uli<^it3i<^nt5 pitijj fuw^^'ni-i^udpi'^a-^an'vru'iyi ^n^n^^m^^a-avn pi^ai^ji m^^it^'upi^^awii^a^i'u u^^fuinapn^ lupi^'wsws'u^a^n^s^n-i^nwbmii u.^^i^u'u^i -?e^as^a-^m^fm1a-3ia<mmj ^a-aibaii la^^apniaj^j'^ai^a iia-i ifi'uapnijjpipii.'mul^ianl^a-i wunan - ^aa^^^ia-im'a^n^'ufn^il^i^! v\ ma^mjnii^a-^yjn'^ fa^lia^ fa-i^a pni^^ai^a Tua^mwii mijj fuw^vn-^^^is^ha-^^'i'un ^nm^nts^ia-ivnpipiai^jn m? ^^n^umiwnumjwa'u^a-apn^l^i an^fa^i vrunan
10 n. (^^a) 12 an vnh Tuw^^^ja'uvmn ^i^^^^sjfl^najfliiiima^iia^a-avjm) fa^uiau uas ua-^hw^a^iinu ua^^ifl^ijm^^^n^un'ui.nen'ira-a mjfifi a^^vms^jirciasjaemamf'i ^as^i^^mia^yma^ a/ ^^^a/ - fa^as^a-am^^^i^nsjpnijjplii'un Tia-uia-a - a^mj^msj^ilfa'^ia^mia^n 5na-^TUTO^i^?n^?i<ui,Piu^^m'9 ^^Mi^ia^Tui^a-^^^a-^yjn^ hua^ivh ilfu-u^^ i^ai.ei'uau'u'avn^'ufni il^u^i^tui'ui.'^a^m'aa^n^u ^a ivmtstl^i)^tujj "Uiisjfisfn'wa-a^i'u - ^s^j^mjj^'^ai^^ia^w'uivniclwa <u m'atj^'u^^i'utui.ia^fa^n^ a/ ^ oi o (9) w'u'itijw^^'wa'uvmn il/9atum<3vin-3n<ul'unau'3'i^ifui1a-3ii't3fa-3n^ T^a i5'33j^aua^mjq3^n3jm'u^h W3tfJfl - <9^^umiuanLia^n3JT'^^il'9t:a-3^a-3 ^TUvfl^Tuuauajna Is W^a^^i.'M'uia^Li'UJi'un^a^^'ui.ifi'UPiainila vni^a-^utna'a^a-^ ^a^^n^^^iumt^ ua^pmjj ^'asjsaiufn^^n^'u-^'iin^nsjvfl^fuwa'usjna - ^aaa^^a^m^^nm^tu^iaj ^^\J'3^a-3^<iU0-3^aTum^fujja"u3jn^
11 - en - 13 a. vn!htuw^^'a'uvmn iwhijf f^^n wu^ih ^^aii^lqjvnu^^mi.a-at'ui.ia-a maimj^'i'u'l'u^i^n^jfuw^^aiiun'ii^a^i'uii'i^fn'a lamm tl^^i^vnhj mahw^m^i^a-^l^^'nij'tiaajci u,^un(^^^^^munmigis;vnha^n<u - '3^mjmiaj^ala<iya^wfuu1m^i^a ^a^^l^fu a/ ^a/ to iwaui^^a^^^n^ai^ifa^rma^vjn^vktknb^l^ eh^^qjiimiijl^a^^^^i^^ 3j^1 ^ai^^m^mi,^ ^^n^^nisjmlaunij^ji^m^b'uvnlil - -s^^j^^^nu^nt^a'^^a^ni'siwaim'j ^au^^i'n^n'alia-^ii^i'a-^n^u^ (Job Specifications) n.. nnu^ nw. V ^4tu V^^ V. to. en. to. en. si.. en.
S U E K E AY S S H A R O N T IM B E R W IN D M A R T Z -PA U L L IN. Carlisle Franklin Springboro. Clearcreek TWP. Middletown. Turtlecreek TWP.
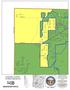 F R A N K L IN M A D IS O N S U E R O B E R T LE IC H T Y A LY C E C H A M B E R L A IN T W IN C R E E K M A R T Z -PA U L L IN C O R A O W E N M E A D O W L A R K W R E N N LA N T IS R E D R O B IN F
F R A N K L IN M A D IS O N S U E R O B E R T LE IC H T Y A LY C E C H A M B E R L A IN T W IN C R E E K M A R T Z -PA U L L IN C O R A O W E N M E A D O W L A R K W R E N N LA N T IS R E D R O B IN F
CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A. Ήχος Πα. to os se e e na aș te e e slă ă ă vi i i i i
 CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A Ήχος α H ris to os s n ș t slă ă ă vi i i i i ți'l Hris to o os di in c ru u uri, în tâm pi i n ți i'l Hris
CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A Ήχος α H ris to os s n ș t slă ă ă vi i i i i ți'l Hris to o os di in c ru u uri, în tâm pi i n ți i'l Hris
Fun and Fascinating Bible Reference for Kids Ages 8 to 12. starts on page 3! starts on page 163!
 F a Faa R K 8 12 a a 3! a a 163! 2013 a P, I. ISN 978-1-62416-216-9. N a a a a a, a,. C a a a a P, a 500 a a aa a. W, : F G: K Fa a Q &, a P, I. U. L aa a a a Fa a Q & a. C a 2 (M) Ta H P M (K) Wa P a
F a Faa R K 8 12 a a 3! a a 163! 2013 a P, I. ISN 978-1-62416-216-9. N a a a a a, a,. C a a a a P, a 500 a a aa a. W, : F G: K Fa a Q &, a P, I. U. L aa a a a Fa a Q & a. C a 2 (M) Ta H P M (K) Wa P a
P a g e 5 1 of R e p o r t P B 4 / 0 9
 P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o
 I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o u l d a l w a y s b e t a k e n, i n c l u d f o l
I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o u l d a l w a y s b e t a k e n, i n c l u d f o l
Trade Patterns, Production networks, and Trade and employment in the Asia-US region
 Trade Patterns, Production networks, and Trade and employment in the Asia-U region atoshi Inomata Institute of Developing Economies ETRO Development of cross-national production linkages, 1985-2005 1985
Trade Patterns, Production networks, and Trade and employment in the Asia-U region atoshi Inomata Institute of Developing Economies ETRO Development of cross-national production linkages, 1985-2005 1985
Ranking accounting, banking and finance journals: A note
 MPRA Munich Personal RePEc Archive Ranking accounting, banking and finance ournals: A note George Halkos and Nickolaos Tzeremes University of Thessaly, Department of Economics January 2012 Online at https://mpra.ub.uni-muenchen.de/36166/
MPRA Munich Personal RePEc Archive Ranking accounting, banking and finance ournals: A note George Halkos and Nickolaos Tzeremes University of Thessaly, Department of Economics January 2012 Online at https://mpra.ub.uni-muenchen.de/36166/
K E L LY T H O M P S O N
 K E L LY T H O M P S O N S E A O LO G Y C R E ATO R, F O U N D E R, A N D PA R T N E R K e l l y T h o m p s o n i s t h e c r e a t o r, f o u n d e r, a n d p a r t n e r o f S e a o l o g y, a n e x
K E L LY T H O M P S O N S E A O LO G Y C R E ATO R, F O U N D E R, A N D PA R T N E R K e l l y T h o m p s o n i s t h e c r e a t o r, f o u n d e r, a n d p a r t n e r o f S e a o l o g y, a n e x
A L A BA M A L A W R E V IE W
 A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
Towards Healthy Environments for Children Frequently asked questions (FAQ) about breastfeeding in a contaminated environment
 Ta a i f i Fu a ui (FQ) abu bafi i a aia i Su b i abu i ia i i? Y; u b i. ia aia a aui a u i; ia aii, bafi u a a aa i a ai f iiai f i ia i i. If ifa b a, a i, u fi i a b bu f iuia i iui ii, PB, u, aa,
Ta a i f i Fu a ui (FQ) abu bafi i a aia i Su b i abu i ia i i? Y; u b i. ia aia a aui a u i; ia aii, bafi u a a aa i a ai f iiai f i ia i i. If ifa b a, a i, u fi i a b bu f iuia i iui ii, PB, u, aa,
c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f
![c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f](/thumbs/87/95703678.jpg) Essential Skills Chapter f ( x + h) f ( x ). Simplifying the difference quotient Section. h f ( x + h) f ( x ) Example: For f ( x) = 4x 4 x, find and simplify completely. h Answer: 4 8x 4 h. Finding the
Essential Skills Chapter f ( x + h) f ( x ). Simplifying the difference quotient Section. h f ( x + h) f ( x ) Example: For f ( x) = 4x 4 x, find and simplify completely. h Answer: 4 8x 4 h. Finding the
Scripture quotations marked cev are from the Contemporary English Version, Copyright 1991, 1992, 1995 by American Bible Society. Used by permission.
 N Ra: E K B Da a a B a a, a-a- a aa, a a. T, a a. 2009 Ba P, I. ISBN 978-1-60260-296-0. N a a a a a, a,. C a a a Ba P, a 500 a a aa a. W, : F K B Da, Ba P, I. U. S a a a a K Ja V B. S a a a a N K Ja V.
N Ra: E K B Da a a B a a, a-a- a aa, a a. T, a a. 2009 Ba P, I. ISBN 978-1-60260-296-0. N a a a a a, a,. C a a a Ba P, a 500 a a aa a. W, : F K B Da, Ba P, I. U. S a a a a K Ja V B. S a a a a N K Ja V.
4.01 Elements, Symbols and Periodic Table
 .0 Elements, Symbols and Periodic Table Dr. Fred O. Garces Chemistry 00 Miramar College.0 Elements, symbols and the Periodic Table Aug The Elements: Building block of Matter The periodic table of the chemical
.0 Elements, Symbols and Periodic Table Dr. Fred O. Garces Chemistry 00 Miramar College.0 Elements, symbols and the Periodic Table Aug The Elements: Building block of Matter The periodic table of the chemical
T h e C S E T I P r o j e c t
 T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
-Z ONGRE::IONAL ACTION ON FY 1987 SUPPLEMENTAL 1/1
 -Z-433 6 --OGRE::OA ATO O FY 987 SUPPEMETA / APPR)PRATO RfQUEST PAY AD PROGRAM(U) DE ARTMET OF DEES AS O' D 9J8,:A:SF ED DEFS! WA-H ODM U 7 / A 25 MRGOPf RESOUTO TEST HART / / AD-A 83 96 (~Go w - %A uj
-Z-433 6 --OGRE::OA ATO O FY 987 SUPPEMETA / APPR)PRATO RfQUEST PAY AD PROGRAM(U) DE ARTMET OF DEES AS O' D 9J8,:A:SF ED DEFS! WA-H ODM U 7 / A 25 MRGOPf RESOUTO TEST HART / / AD-A 83 96 (~Go w - %A uj
ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom.
 178 (MAGNETIC) SPIN QUANTUM NUMBER: "spin down" or "spin up" - An ORBITAL (region with fixed "n", "l" and "ml" values) can hold TWO electrons. ORBITAL DIAGRAM - A graphical representation of the quantum
178 (MAGNETIC) SPIN QUANTUM NUMBER: "spin down" or "spin up" - An ORBITAL (region with fixed "n", "l" and "ml" values) can hold TWO electrons. ORBITAL DIAGRAM - A graphical representation of the quantum
VIIIA H PREDICTING CHARGE
 58 IA PREDICTING CHARGE VIIIA H IIA IIIA IVA VA VIA VIIA You can reliably determine the charge using our method for Groups IA, IIA, IIIB, Aluminum, and the Group VA, VIA, and VIIA NONMETALS Li Be B C N
58 IA PREDICTING CHARGE VIIIA H IIA IIIA IVA VA VIA VIIA You can reliably determine the charge using our method for Groups IA, IIA, IIIB, Aluminum, and the Group VA, VIA, and VIIA NONMETALS Li Be B C N
Software Process Models there are many process model s in th e li t e ra t u re, s om e a r e prescriptions and some are descriptions you need to mode
 Unit 2 : Software Process O b j ec t i ve This unit introduces software systems engineering through a discussion of software processes and their principal characteristics. In order to achieve the desireable
Unit 2 : Software Process O b j ec t i ve This unit introduces software systems engineering through a discussion of software processes and their principal characteristics. In order to achieve the desireable
Beechwood Music Department Staff
 Beechwood Music Department Staff MRS SARAH KERSHAW - HEAD OF MUSIC S a ra h K e rs h a w t r a i n e d a t t h e R oy a l We ls h C o l le g e of M u s i c a n d D ra m a w h e re s h e ob t a i n e d
Beechwood Music Department Staff MRS SARAH KERSHAW - HEAD OF MUSIC S a ra h K e rs h a w t r a i n e d a t t h e R oy a l We ls h C o l le g e of M u s i c a n d D ra m a w h e re s h e ob t a i n e d
H STO RY OF TH E SA NT
 O RY OF E N G L R R VER ritten for the entennial of th e Foundin g of t lair oun t y on ay 8 82 Y EEL N E JEN K RP O N! R ENJ F ] jun E 3 1 92! Ph in t ed b y h e t l a i r R ep u b l i c a n O 4 1922
O RY OF E N G L R R VER ritten for the entennial of th e Foundin g of t lair oun t y on ay 8 82 Y EEL N E JEN K RP O N! R ENJ F ] jun E 3 1 92! Ph in t ed b y h e t l a i r R ep u b l i c a n O 4 1922
Executive Committee and Officers ( )
 Gifted and Talented International V o l u m e 2 4, N u m b e r 2, D e c e m b e r, 2 0 0 9. G i f t e d a n d T a l e n t e d I n t e r n a t i o n a2 l 4 ( 2), D e c e m b e r, 2 0 0 9. 1 T h e W o r
Gifted and Talented International V o l u m e 2 4, N u m b e r 2, D e c e m b e r, 2 0 0 9. G i f t e d a n d T a l e n t e d I n t e r n a t i o n a2 l 4 ( 2), D e c e m b e r, 2 0 0 9. 1 T h e W o r
WRITING AN IONIC FORMULA
 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example: If
WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example: If
Example: If a simple ionic compound is made of these two ions, what is its formula? In the final formula, don't write the charges on the ions!
 88 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example:
88 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example:
176 5 t h Fl oo r. 337 P o ly me r Ma te ri al s
 A g la di ou s F. L. 462 E l ec tr on ic D ev el op me nt A i ng er A.W.S. 371 C. A. M. A l ex an de r 236 A d mi ni st ra ti on R. H. (M rs ) A n dr ew s P. V. 326 O p ti ca l Tr an sm is si on A p ps
A g la di ou s F. L. 462 E l ec tr on ic D ev el op me nt A i ng er A.W.S. 371 C. A. M. A l ex an de r 236 A d mi ni st ra ti on R. H. (M rs ) A n dr ew s P. V. 326 O p ti ca l Tr an sm is si on A p ps
STEEL PIPE NIPPLE BLACK AND GALVANIZED
 Price Sheet Effective August 09, 2018 Supersedes CWN-218 A Member of The Phoenix Forge Group CapProducts LTD. Phone: 519-482-5000 Fax: 519-482-7728 Toll Free: 800-265-5586 www.capproducts.com www.capitolcamco.com
Price Sheet Effective August 09, 2018 Supersedes CWN-218 A Member of The Phoenix Forge Group CapProducts LTD. Phone: 519-482-5000 Fax: 519-482-7728 Toll Free: 800-265-5586 www.capproducts.com www.capitolcamco.com
VIIIA H PREDICTING CHARGE
 58 IA PREDICTING CHARGE VIIIA H IIA IIIA IVA VA VIA VIIA You can reliably determine the charge using our method for Groups IA, IIA, IIIB, Aluminum, and the Group VA, VIA, and VIIA NONMETALS Li Be B C N
58 IA PREDICTING CHARGE VIIIA H IIA IIIA IVA VA VIA VIIA You can reliably determine the charge using our method for Groups IA, IIA, IIIB, Aluminum, and the Group VA, VIA, and VIIA NONMETALS Li Be B C N
EXAMPLES. He VIA VIIA Li Be B C N O F Ne
 59 IA EXAMPLES VIIIA H IIA IIIA IVA VA He VIA VIIA Li Be B C N O F Ne Na Mg IIIB IVB VB Al Si P VIB VIIB VIIIB IB IIB S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru
59 IA EXAMPLES VIIIA H IIA IIIA IVA VA He VIA VIIA Li Be B C N O F Ne Na Mg IIIB IVB VB Al Si P VIB VIIB VIIIB IB IIB S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru
CHEMICAL COMPOUNDS MOLECULAR COMPOUNDS
 48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
WRITING AN IONIC FORMULA
 55 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example:
55 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example:
Vlaamse Overheid Departement Mobiliteit en Openbare Werken
 Vlaamse Overheid Departement Mobiliteit en Openbare Werken Waterbouwkundig Laboratorium Langdurige metingen Deurganckdok: Opvolging en analyse aanslibbing Bestek 16EB/05/04 Colofon Ph o to c o ve r s h
Vlaamse Overheid Departement Mobiliteit en Openbare Werken Waterbouwkundig Laboratorium Langdurige metingen Deurganckdok: Opvolging en analyse aanslibbing Bestek 16EB/05/04 Colofon Ph o to c o ve r s h
CHEMICAL COMPOUNDS MOLECULAR COMPOUNDS
 48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom.
 160 ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom. 4p 3d 4s 3p 3s 2p 2s 1s Each blank represents an ORBITAL, and can hold two electrons. The 4s subshell
160 ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom. 4p 3d 4s 3p 3s 2p 2s 1s Each blank represents an ORBITAL, and can hold two electrons. The 4s subshell
~ ~ ~.._--- Harappan Weights
 Harappan Weights The data of Marshall (1934) about Harappan weights shows that they were standardised with an uncertainty of 6%. This fluctuation within each weight class may be a result of weathering
Harappan Weights The data of Marshall (1934) about Harappan weights shows that they were standardised with an uncertainty of 6%. This fluctuation within each weight class may be a result of weathering
fl W12111 L5N
 fl 41@ W1111 471,1516In15 o (1@ ) Imn5o td&& -miet9cqi c, 1119- bdo&-).6)./ 'MI 9 tg&&d L5N li@wymut4lp51:nfrthnnhiict46n-m'imilimlingnywimtpuctvuivi iru o cinuniulviu 1:411.,IJJIMg11.7f1Y91 11?ITri nct
fl 41@ W1111 471,1516In15 o (1@ ) Imn5o td&& -miet9cqi c, 1119- bdo&-).6)./ 'MI 9 tg&&d L5N li@wymut4lp51:nfrthnnhiict46n-m'imilimlingnywimtpuctvuivi iru o cinuniulviu 1:411.,IJJIMg11.7f1Y91 11?ITri nct
-"l" also contributes ENERGY. Higher values for "l" mean the electron has higher energy.
 175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
E5 Lewis Acids and Bases: lab 2. Session two lab Parts 2B, 3, and 4. Session one lab Parts 1and 2A. Aquo Complex Ions
 E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Session two lab Parts 2B, 3, and 4 Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing
E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Session two lab Parts 2B, 3, and 4 Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
gender mains treaming in Polis h practice
 gender mains treaming in Polis h practice B E R L IN, 1 9-2 1 T H A P R IL, 2 O O 7 Gender mains treaming at national level Parliament 25 % of women in S ejm (Lower Chamber) 16 % of women in S enat (Upper
gender mains treaming in Polis h practice B E R L IN, 1 9-2 1 T H A P R IL, 2 O O 7 Gender mains treaming at national level Parliament 25 % of women in S ejm (Lower Chamber) 16 % of women in S enat (Upper
WRITING AN IONIC FORMULA
 55 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example:
55 WRITING AN IONIC FORMULA - if you know the ions that make up a compound, all you need to do is find the smallest ratio of cation to anion the compound needs to have an overall charge of zero Example:
Halogens HALOGENS. Parts 2A and 2B. Chem : Feb. 19, 20 and March 3. Compare the properties and reactivity of the halogens and halides
 Chem. 125-126: Feb. 19, 20 and March 3 Experiment 3 Session 2 (Three hour lab) Complete Experiment 3 Parts 2B and 3 Complete team report Complete discussion presentation Parts 2A and 2B Compare the properties
Chem. 125-126: Feb. 19, 20 and March 3 Experiment 3 Session 2 (Three hour lab) Complete Experiment 3 Parts 2B and 3 Complete team report Complete discussion presentation Parts 2A and 2B Compare the properties
E5 Lewis Acids and Bases: lab 2. Session two lab Parts 2B, 3, and 4. Session one lab Parts 1and 2A. Aquo Complex Ions. Aquo Complex Ion Reactions
 E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing pre-transition, transition, and post-transition
E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing pre-transition, transition, and post-transition
Table of C on t en t s Global Campus 21 in N umbe r s R e g ional Capac it y D e v e lopme nt in E-L e ar ning Structure a n d C o m p o n en ts R ea
 G Blended L ea r ni ng P r o g r a m R eg i o na l C a p a c i t y D ev elo p m ent i n E -L ea r ni ng H R K C r o s s o r d e r u c a t i o n a n d v e l o p m e n t C o p e r a t i o n 3 0 6 0 7 0 5
G Blended L ea r ni ng P r o g r a m R eg i o na l C a p a c i t y D ev elo p m ent i n E -L ea r ni ng H R K C r o s s o r d e r u c a t i o n a n d v e l o p m e n t C o p e r a t i o n 3 0 6 0 7 0 5
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
& Ö IN 4> * o»'s S <«
 & Ö IN 4 0 8 i I *»'S S s H WD 'u H 0 0D fi S «a 922 6020866 Aessin Number: 690 Publiatin Date: Mar 28, 1991 Title: Briefing T mbassy Representatives n The Refused Strategi Defense Initiative Persnal Authr:
& Ö IN 4 0 8 i I *»'S S s H WD 'u H 0 0D fi S «a 922 6020866 Aessin Number: 690 Publiatin Date: Mar 28, 1991 Title: Briefing T mbassy Representatives n The Refused Strategi Defense Initiative Persnal Authr:
CHEMICAL COMPOUNDS. - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds!
 69 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
69 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
Student Jobs Fairs. YOUR ticket to student recruitment in Birmingham 2015 Version 1
 S J Fi YOUR i i i Biih 215 Vi 1 Wl D vi -i/l vi vi v 28,? Th l fh h J Z h Gil f S h vi f f l vii ii i il Wl W 214 i i f 6, v 12, i hh h Gil, f h hi f -i/l vi ii h hil h Th i i vi j ii hi i Oii h J Z, Gil
S J Fi YOUR i i i Biih 215 Vi 1 Wl D vi -i/l vi vi v 28,? Th l fh h J Z h Gil f S h vi f f l vii ii i il Wl W 214 i i f 6, v 12, i hh h Gil, f h hi f -i/l vi ii h hil h Th i i vi j ii hi i Oii h J Z, Gil
WH2. Queensville West PS to WH2 WH2. Queensville West Pumping Station. Queensville Sid. Concession Road 2. Figure B.13
 Quvill Si Quvill W S a Quvill W upi Sai xc_x25_1_fcai_pfil p u Quvil W upi Sai Map L ' ci a 2 Fiu B.13 a Suiabl f Wa Rclaai 12 buff f Gbl Naual Hia S Quvill W upi Sai 12 buff f Siifica W a UYSS Svic a
Quvill Si Quvill W S a Quvill W upi Sai xc_x25_1_fcai_pfil p u Quvil W upi Sai Map L ' ci a 2 Fiu B.13 a Suiabl f Wa Rclaai 12 buff f Gbl Naual Hia S Quvill W upi Sai 12 buff f Siifica W a UYSS Svic a
CHEMICAL COMPOUNDS MOLECULAR COMPOUNDS
 48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
- A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged.
 14 POLARITY and shape: - A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged. POLARITY influences several easily observable properties.
14 POLARITY and shape: - A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged. POLARITY influences several easily observable properties.
CLEARLY SHOW ALL WORK AND REASONING.
 Name (print clearly) Seat number Quiz 4 (100 points) Chemistry 1303.002 October 17, 2018 1. (25points) Using the information below, find the enthalpy of formation ( H f ) for gaseous hydrogen chloride.
Name (print clearly) Seat number Quiz 4 (100 points) Chemistry 1303.002 October 17, 2018 1. (25points) Using the information below, find the enthalpy of formation ( H f ) for gaseous hydrogen chloride.
IONIC COMPOUNDS. - USUALLY form from metals combining with nonmetals, or from metals combining with metalloids
 52 IONIC COMPOUNDS - USUALLY form from metals combining with nonmetals, or from metals combining with metalloids Examples: - almost always solid at room temperature, and usually have relatively high melting
52 IONIC COMPOUNDS - USUALLY form from metals combining with nonmetals, or from metals combining with metalloids Examples: - almost always solid at room temperature, and usually have relatively high melting
Lewis dot structures for molecules
 1 Lewis dot structures for molecules In the dot structure of a molecule, - SHARED valence electrons are shown with dashes - one per pair. - UNSHARED valence electrons ("lone pairs") are represented by
1 Lewis dot structures for molecules In the dot structure of a molecule, - SHARED valence electrons are shown with dashes - one per pair. - UNSHARED valence electrons ("lone pairs") are represented by
INSTRUCTIONS: 7. Relax and do well.
 EM 1314 Name Exam III TA Name John III. Gelder November 16, 1992 Lab Section INSTRUTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and some
EM 1314 Name Exam III TA Name John III. Gelder November 16, 1992 Lab Section INSTRUTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and some
CHEMICAL COMPOUNDS MOLECULAR COMPOUNDS
 48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
LU N C H IN C LU D E D
 Week 1 M o n d a y J a n u a ry 7 - C o lo u rs o f th e R a in b o w W e w ill b e k ic k in g o ff th e h o lid a y s w ith a d a y fu ll o f c o lo u r! J o in u s fo r a ra n g e o f a rt, s p o rt
Week 1 M o n d a y J a n u a ry 7 - C o lo u rs o f th e R a in b o w W e w ill b e k ic k in g o ff th e h o lid a y s w ith a d a y fu ll o f c o lo u r! J o in u s fo r a ra n g e o f a rt, s p o rt
Atomic weight: This is a decimal number, but for radioactive elements it is replaced with a number in parenthesis.
 47 Blocks on the periodic table 11 Sodium 22.99 Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name
47 Blocks on the periodic table 11 Sodium 22.99 Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name
- When atoms share electrons, the electrons might not be EVENLY shared. Shared electrons may spend more time around one atomic nucleus than the other.
 228 POLARITY - When atoms share electrons, the electrons might not be EVENLY shared. Shared electrons may spend more time around one atomic nucleus than the other. - When electrons are shared UNEVENLY,
228 POLARITY - When atoms share electrons, the electrons might not be EVENLY shared. Shared electrons may spend more time around one atomic nucleus than the other. - When electrons are shared UNEVENLY,
-"l" also contributes ENERGY. Higher values for "l" mean the electron has higher energy.
 170 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
170 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
BENEFITS OF COMPLETING COLLEGE Lesson Plan #1
 BNFITS OF COMPLTING COLLG L P #1 Ti: Bi Ci C: Ovvi & B J Hi Py P: ( y, i i i /i) S i i v i i i ii i ii i. Li O(): ( i /k y ) I ii i i i i, i ii i y i ii ii i. Ti i iii i y i y i iky i j y jy i ki y v.
BNFITS OF COMPLTING COLLG L P #1 Ti: Bi Ci C: Ovvi & B J Hi Py P: ( y, i i i /i) S i i v i i i ii i ii i. Li O(): ( i /k y ) I ii i i i i, i ii i y i ii ii i. Ti i iii i y i y i iky i j y jy i ki y v.
4.06 Periodic Table and Periodic Trends
 4.06 Periodic Table and Periodic Trends Dr. Fred Omega Garces Chemistry 100, Miramar College 1 4.06 Periodic Table and Periodic Trend The Periodic Table and the Elements What is the periodic table? What
4.06 Periodic Table and Periodic Trends Dr. Fred Omega Garces Chemistry 100, Miramar College 1 4.06 Periodic Table and Periodic Trend The Periodic Table and the Elements What is the periodic table? What
Results as of 30 September 2018
 rt Results as of 30 September 2018 F r e e t r a n s l a t ion f r o m t h e o r ig ina l in S p a n is h. I n t h e e v e n t o f d i s c r e p a n c y, t h e Sp a n i s h - la n g u a g e v e r s ion
rt Results as of 30 September 2018 F r e e t r a n s l a t ion f r o m t h e o r ig ina l in S p a n is h. I n t h e e v e n t o f d i s c r e p a n c y, t h e Sp a n i s h - la n g u a g e v e r s ion
REPRESENTATIONS FOR A SPECIAL SEQUENCE
 REPRESENTATIONS FOR A SPECIAL SEQUENCE L. CARLITZ* RICHARD SCOVILLE Dyke University, Durham,!\!orth Carolina VERNERE.HOGGATTJR. San Jose State University, San Jose, California Consider the sequence defined
REPRESENTATIONS FOR A SPECIAL SEQUENCE L. CARLITZ* RICHARD SCOVILLE Dyke University, Durham,!\!orth Carolina VERNERE.HOGGATTJR. San Jose State University, San Jose, California Consider the sequence defined
S ca le M o d e l o f th e S o la r Sy ste m
 N a m e ' D a t e ' S ca le M o d e l o f th e S o la r Sy ste m 6.1 I n t r o d u c t i o n T h e S olar System is large, at least w hen com pared to distances we are fam iliar w ith on a day-to-day basis.
N a m e ' D a t e ' S ca le M o d e l o f th e S o la r Sy ste m 6.1 I n t r o d u c t i o n T h e S olar System is large, at least w hen com pared to distances we are fam iliar w ith on a day-to-day basis.
Atomic weight: This is a decimal number, but for radioactive elements it is replaced with a number in parenthesis.
 47 Blocks on the periodic table 11 Sodium 22.99 Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name
47 Blocks on the periodic table 11 Sodium 22.99 Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name
I N A C O M P L E X W O R L D
 IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
- A CHEMICAL BOND is a strong attractive force between the atoms in a compound. attractive forces between oppositely charged ions
 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride Covalent
CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride Covalent
(Portfolio) iili?if\j?iwfi'5t4nll0uvima^^nwltj^^ ^ V1I0
 m's'a'u jjvihqianaa'jiieilin'yeu d'asaniln'i^^n'fe^n lad^bia lauvi Portfolio ^QyjJilQlEJ'l^EIQaEjSn'fc^CuS'uIll'UiyTU'Uni.'afJ'UlWf^EUfl'lJll'LllSll^T.t^ll.'^IIPinw'l'UWllQIEJnaEi- T^Ejam^cu *5011^ s)
m's'a'u jjvihqianaa'jiieilin'yeu d'asaniln'i^^n'fe^n lad^bia lauvi Portfolio ^QyjJilQlEJ'l^EIQaEjSn'fc^CuS'uIll'UiyTU'Uni.'afJ'UlWf^EUfl'lJll'LllSll^T.t^ll.'^IIPinw'l'UWllQIEJnaEi- T^Ejam^cu *5011^ s)
A = (a + 1) 2 = a 2 + 2a + 1
 A = (a + 1) 2 = a 2 + 2a + 1 1 A = ( (a + b) + 1 ) 2 = (a + b) 2 + 2(a + b) + 1 = a 2 + 2ab + b 2 + 2a + 2b + 1 A = ( (a + b) + 1 ) 2 = (a + b) 2 + 2(a + b) + 1 = a 2 + 2ab + b 2 + 2a + 2b + 1 3 A = (
A = (a + 1) 2 = a 2 + 2a + 1 1 A = ( (a + b) + 1 ) 2 = (a + b) 2 + 2(a + b) + 1 = a 2 + 2ab + b 2 + 2a + 2b + 1 A = ( (a + b) + 1 ) 2 = (a + b) 2 + 2(a + b) + 1 = a 2 + 2ab + b 2 + 2a + 2b + 1 3 A = (
Figure 7: Boat Houses in the Thousand Islands. Sheet 1 of 1. March 2015
 T f Alxaia/Villag f Alxaia cal af vializai Pla T f Alxaia & Villag f Alxaia Jff u, N Y Figu 7: a u i h Thua Ila h f ach 5 N: Thi figu a a f h N Y a a f a ih fu vi u Til f h Evial Pci Fu. uc:. c-ea Oai
T f Alxaia/Villag f Alxaia cal af vializai Pla T f Alxaia & Villag f Alxaia Jff u, N Y Figu 7: a u i h Thua Ila h f ach 5 N: Thi figu a a f h N Y a a f a ih fu vi u Til f h Evial Pci Fu. uc:. c-ea Oai
Example: Helium has an atomic number of 2. Every helium atom has two protons in its nucleus.
 59 Atomic terms - ATOMIC NUMBER: The number of protons in the atomic nucleus. Each ELEMENT has the SAME NUMBER OF PROTONS in every nucleus. In neutral atoms, the number of ELECTRONS is also equal to the
59 Atomic terms - ATOMIC NUMBER: The number of protons in the atomic nucleus. Each ELEMENT has the SAME NUMBER OF PROTONS in every nucleus. In neutral atoms, the number of ELECTRONS is also equal to the
2 tel
 Us. Timeless, sophisticated wall decor that is classic yet modern. Our style has no limitations; from traditional to contemporar y, with global design inspiration. The attention to detail and hand- craf
Us. Timeless, sophisticated wall decor that is classic yet modern. Our style has no limitations; from traditional to contemporar y, with global design inspiration. The attention to detail and hand- craf
... but using electron configurations to describe how aluminum bromide forms is a bit cumbersome! Can we simplify the picture a bit?
 193... but using electron configurations to describe how aluminum bromide forms is a bit cumbersome! Can we simplify the picture a bit? LEWIS NOTATION / ELECTRON-DOT NOTATION - Lewis notation represents
193... but using electron configurations to describe how aluminum bromide forms is a bit cumbersome! Can we simplify the picture a bit? LEWIS NOTATION / ELECTRON-DOT NOTATION - Lewis notation represents
Knowledge Fusion: An Approach to Time Series Model Selection Followed by Pattern Recognition
 LA-3095-MS Knwledge Fusin: An Appch t Tie Seies Mdel Selectin Fllwed by Ptten Recgnitin Ls Als N A T I O N A L L A B O R A T O R Y Ls Als Ntinl Lbty is peted by the Univesity f Clifni f the United Sttes
LA-3095-MS Knwledge Fusin: An Appch t Tie Seies Mdel Selectin Fllwed by Ptten Recgnitin Ls Als N A T I O N A L L A B O R A T O R Y Ls Als Ntinl Lbty is peted by the Univesity f Clifni f the United Sttes
1.02 Elements, Symbols and Periodic Table
 .0 Elements, Symbols and Periodic Table Dr. Fred O. Garces Chemistry Miramar College.0 Elements, Symbols and the Periodic Table January 0 The Elements: Building block of Matter The periodic table of the
.0 Elements, Symbols and Periodic Table Dr. Fred O. Garces Chemistry Miramar College.0 Elements, Symbols and the Periodic Table January 0 The Elements: Building block of Matter The periodic table of the
Microsoft Excel Directions
 Microsoft Excel Directions 1. Working in groups of two, log onto a computer. 2. Create a folder on the desktop a. Right click anywhere on the desktop new folder Name the folder Chemistry 3. Open MS Excel
Microsoft Excel Directions 1. Working in groups of two, log onto a computer. 2. Create a folder on the desktop a. Right click anywhere on the desktop new folder Name the folder Chemistry 3. Open MS Excel
- Atomic line spectra are UNIQUE to each element. They're like atomic "fingerprints".
 - Atomic line spectra are UNIQUE to each element. They're like atomic "fingerprints". - Problem was that the current model of the atom completely failed to explain why atoms emitted these lines. An orbit
- Atomic line spectra are UNIQUE to each element. They're like atomic "fingerprints". - Problem was that the current model of the atom completely failed to explain why atoms emitted these lines. An orbit
SHAPES OF EXPANDED VALENCE MOLECULES
 228 SHAPES OF EXPANDED VALENCE MOLECULES There are five atoms bonded to the central phosphorus atom, and they will attempt to get as far apart as possible from one another! The top and bottom atoms are
228 SHAPES OF EXPANDED VALENCE MOLECULES There are five atoms bonded to the central phosphorus atom, and they will attempt to get as far apart as possible from one another! The top and bottom atoms are
(please print) (1) (18) H IIA IIIA IVA VA VIA VIIA He (2) (13) (14) (15) (16) (17)
 CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
h : sh +i F J a n W i m +i F D eh, 1 ; 5 i A cl m i n i sh» si N «q a : 1? ek ser P t r \. e a & im a n alaa p ( M Scanned by CamScanner
 m m i s t r * j i ega>x I Bi 5 n ì r s w «s m I L nk r n A F o n n l 5 o 5 i n l D eh 1 ; 5 i A cl m i n i sh» si N «q a : 1? { D v i H R o s c q \ l o o m ( t 9 8 6) im a n alaa p ( M n h k Em l A ma
m m i s t r * j i ega>x I Bi 5 n ì r s w «s m I L nk r n A F o n n l 5 o 5 i n l D eh 1 ; 5 i A cl m i n i sh» si N «q a : 1? { D v i H R o s c q \ l o o m ( t 9 8 6) im a n alaa p ( M n h k Em l A ma
Experiment Three. Lab two: Parts 2B and 3. Halogens used in Parts 2 and 3. Lab one: Parts 1 and 2A. Halogens (Family VIIA) used in Parts 2 and 3
 Experiment Three Lab one: Parts 1 and 2A Lab two: Parts 2B and 3 1 1A 1 H 1s 1 2 IIA 3 Li 2s 1 1 1 Na 3s 1 1 9 K 4s 1 3 7 Rb 5s 1 5 5 Cs 6s 1 8 7 Fr 7s 1 4 Be 2s 2 1 2 Mg 3s 2 3 IIIB 4 IVB 5 VB 6 VIB 7
Experiment Three Lab one: Parts 1 and 2A Lab two: Parts 2B and 3 1 1A 1 H 1s 1 2 IIA 3 Li 2s 1 1 1 Na 3s 1 1 9 K 4s 1 3 7 Rb 5s 1 5 5 Cs 6s 1 8 7 Fr 7s 1 4 Be 2s 2 1 2 Mg 3s 2 3 IIIB 4 IVB 5 VB 6 VIB 7
Agenda Rationale for ETG S eek ing I d eas ETG fram ew ork and res u lts 2
 Internal Innovation @ C is c o 2 0 0 6 C i s c o S y s t e m s, I n c. A l l r i g h t s r e s e r v e d. C i s c o C o n f i d e n t i a l 1 Agenda Rationale for ETG S eek ing I d eas ETG fram ew ork
Internal Innovation @ C is c o 2 0 0 6 C i s c o S y s t e m s, I n c. A l l r i g h t s r e s e r v e d. C i s c o C o n f i d e n t i a l 1 Agenda Rationale for ETG S eek ing I d eas ETG fram ew ork
8. Relax and do well.
 EM 1515.001-006 Exam II John II. Gelder March 5, 2002 Name TA's Name Section INSTRUTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic table, a
EM 1515.001-006 Exam II John II. Gelder March 5, 2002 Name TA's Name Section INSTRUTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic table, a
Assumptions for the theoretical properties of the
 Statistica Sinica: Supplement IMPUTED FACTOR REGRESSION FOR HIGH- DIMENSIONAL BLOCK-WISE MISSING DATA Yanqing Zhang, Niansheng Tang and Annie Qu Yunnan University and University of Illinois at Urbana-Champaign
Statistica Sinica: Supplement IMPUTED FACTOR REGRESSION FOR HIGH- DIMENSIONAL BLOCK-WISE MISSING DATA Yanqing Zhang, Niansheng Tang and Annie Qu Yunnan University and University of Illinois at Urbana-Champaign
ph = - log [H3O+] Example: ph 7 = - log [ 1 x 10-7] [H3O+] = mole/liter units ph values are unitless
![ph = - log [H3O+] Example: ph 7 = - log [ 1 x 10-7] [H3O+] = mole/liter units ph values are unitless ph = - log [H3O+] Example: ph 7 = - log [ 1 x 10-7] [H3O+] = mole/liter units ph values are unitless](/thumbs/80/82250423.jpg) E4 Acids, Bases, and Salts Oct. 1517 and Oct. 2728* Session One of two session lab Complete Parts 1 and 2 in lab. Part 1. Structure and AcidBase Properties Q. Are properties of a compound predictable from
E4 Acids, Bases, and Salts Oct. 1517 and Oct. 2728* Session One of two session lab Complete Parts 1 and 2 in lab. Part 1. Structure and AcidBase Properties Q. Are properties of a compound predictable from
E4 Acids, Bases, and Salts
 E4 Acids, Bases, and Salts Session One of two session lab Complete Parts 1 and 2 in lab. If time allows, start or complete Part 3. Acids and Bases Q. Are acid-base properties of substances predictable
E4 Acids, Bases, and Salts Session One of two session lab Complete Parts 1 and 2 in lab. If time allows, start or complete Part 3. Acids and Bases Q. Are acid-base properties of substances predictable
Periodic Table. - Mendeleev was able to predict the properties of previously unknown elements using his "periodic law" Modern periodic table
 74 Periodic Table - Mendeleev (1869): --- When atoms are arranged in order of their atomic weight, some of their chemical and physical properties repeat at regular intervals (periods) --- Some of the physical
74 Periodic Table - Mendeleev (1869): --- When atoms are arranged in order of their atomic weight, some of their chemical and physical properties repeat at regular intervals (periods) --- Some of the physical
MEDWAY SPORTS DEVELOPMENT
 PB 2:L 1 13:36 P 1 MEDWAY SPTS DEVELPMENT.m.v./vlm A i 11 Bfil Sl i P-16 FE C? D v i i? T l i i S Li Pmm? B fi Ti iq l vlm mm ill l vl fi, mivi ill vii flli ii: l Nm S l : W Tm li ii (ili m fi i) j l i?
PB 2:L 1 13:36 P 1 MEDWAY SPTS DEVELPMENT.m.v./vlm A i 11 Bfil Sl i P-16 FE C? D v i i? T l i i S Li Pmm? B fi Ti iq l vlm mm ill l vl fi, mivi ill vii flli ii: l Nm S l : W Tm li ii (ili m fi i) j l i?
Partial Periodic Table of the Elements
 Name (print clearly) Seat number Quiz 3 (100 points) Chemistry 1303.002 September 12, 2018 1. (15 points) One of the following reactions will occur, one will not. Determine which one occurs and then a.
Name (print clearly) Seat number Quiz 3 (100 points) Chemistry 1303.002 September 12, 2018 1. (15 points) One of the following reactions will occur, one will not. Determine which one occurs and then a.
- A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged.
 POLARITY and shape: - A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged. POLARITY influences several easily observable properties. -
POLARITY and shape: - A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged. POLARITY influences several easily observable properties. -
Course theme. Three hours of lab Complete E1 (Parts 1, 2, 3, 4, and 5B) Prepare discussion presentation Prepare team report.
 Experiment Session 2 Electrons and Solution Color Three hours of lab Complete E (Parts, 2, 3, 4, and 5B) Prepare discussion presentation Prepare team report. Course theme There are structure and property
Experiment Session 2 Electrons and Solution Color Three hours of lab Complete E (Parts, 2, 3, 4, and 5B) Prepare discussion presentation Prepare team report. Course theme There are structure and property
Three hours of lab Complete E1 (Parts 1, 2, 3, 4, and 5B) Prepare discussion presentation Prepare team report. Course theme
 Experiment 1 Session 2 Electrons and Solution Color Three hours of lab Complete E1 (Parts 1, 2, 3, 4, and 5B) Prepare discussion presentation Prepare team report. Course theme There are structure and property
Experiment 1 Session 2 Electrons and Solution Color Three hours of lab Complete E1 (Parts 1, 2, 3, 4, and 5B) Prepare discussion presentation Prepare team report. Course theme There are structure and property
Putting it together... - In the early 20th century, there was a debate on the structure of the atom. Thin gold foil
 36 Putting it together... - In the early 20th century, there was a debate on the structure of the atom. RUTHERFORD EXPERIMENT Where do the particles go? Radioactive material A few bounce back A few particles
36 Putting it together... - In the early 20th century, there was a debate on the structure of the atom. RUTHERFORD EXPERIMENT Where do the particles go? Radioactive material A few bounce back A few particles
15.083J/6.859J Integer Optimization. Lecture 10: Solving Relaxations
 15.083J/6.859J Integer Optimization Lecture 10: Solving Relaxations 1 Outline The key geometric result behind the ellipsoid method Slide 1 The ellipsoid method for the feasibility problem The ellipsoid
15.083J/6.859J Integer Optimization Lecture 10: Solving Relaxations 1 Outline The key geometric result behind the ellipsoid method Slide 1 The ellipsoid method for the feasibility problem The ellipsoid
i.ea IE !e e sv?f 'il i+x3p \r= v * 5,?: S i- co i, ==:= SOrq) Xgs'iY # oo .9 9 PE * v E=S s->'d =ar4lq 5,n =.9 '{nl a':1 t F #l *r C\ t-e
 fl ) 2 ;;:i c.l l) ( # =S >' 5 ^'R 1? l.y i.i.9 9 P * v ,>f { e e v? 'il v * 5,?: S 'V i: :i (g Y 1,Y iv cg G J :< >,c Z^ /^ c..l Cl i l 1 3 11 5 (' \ h 9 J :'i g > _ ^ j, \= f{ '{l #l * C\? 0l = 5,
fl ) 2 ;;:i c.l l) ( # =S >' 5 ^'R 1? l.y i.i.9 9 P * v ,>f { e e v? 'il v * 5,?: S 'V i: :i (g Y 1,Y iv cg G J :< >,c Z^ /^ c..l Cl i l 1 3 11 5 (' \ h 9 J :'i g > _ ^ j, \= f{ '{l #l * C\? 0l = 5,
CMSC 313 Lecture 17 Postulates & Theorems of Boolean Algebra Semiconductors CMOS Logic Gates
 CMSC 313 Lecture 17 Postulates & Theorems of Boolean Algebra Semiconductors CMOS Logic Gates UMBC, CMSC313, Richard Chang Last Time Overview of second half of this course Logic gates &
CMSC 313 Lecture 17 Postulates & Theorems of Boolean Algebra Semiconductors CMOS Logic Gates UMBC, CMSC313, Richard Chang Last Time Overview of second half of this course Logic gates &
A THREE-VALUED, NON-L E V E L L E D L OGIC CONSISTENT FO R A L L SELF-REFERENCE M. J. OVARROLL 1. INTRODUCTION
 A THREE-VALUED, NON-L E V E L L E D L OGIC CONSISTENT FO R A L L SELF-REFERENCE M. J. OVARROLL 1. INTRODUCTION In classical logic, some self-referring statements may be "liarparadoxical" (such as S : S)
A THREE-VALUED, NON-L E V E L L E D L OGIC CONSISTENT FO R A L L SELF-REFERENCE M. J. OVARROLL 1. INTRODUCTION In classical logic, some self-referring statements may be "liarparadoxical" (such as S : S)
8. Relax and do well.
 EM 1515.001-009 Exam II John II. Gelder March 5, 2003 Name TA's Name Section INSTRUTIONS: 1. This examination consists of a total of 9 different pages. The last four pages include a periodic table; useful
EM 1515.001-009 Exam II John II. Gelder March 5, 2003 Name TA's Name Section INSTRUTIONS: 1. This examination consists of a total of 9 different pages. The last four pages include a periodic table; useful
- A CHEMICAL BOND is a strong attractive force between the atoms in a compound. attractive forces between oppositely charged ions
 191 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride
191 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Periodic Table. Modern periodic table
 41 Periodic Table - Mendeleev (1869): --- When atoms are arranged in order of their atomic weight, some of their chemical and physical properties repeat at regular intervals (periods) --- Some of the physical
41 Periodic Table - Mendeleev (1869): --- When atoms are arranged in order of their atomic weight, some of their chemical and physical properties repeat at regular intervals (periods) --- Some of the physical
