Spectre. Users Manual
|
|
|
- Elvin Norman
- 5 years ago
- Views:
Transcription
1 Spectre Users Manual Andrew Boothroyd August 28, 205 Spectre is a program for calculating spectroscopic and magnetic properties of rare-earth ions in crystals. The calculation employs intermediatecoupling basis states, and allows mixing of all terms within the f n configuration. The various electrostatic and spin orbit parameters in the free ion Hamiltonian are taken from Carnall et al. []. The program includes a facility for fitting crystal field parameters to sets of energy levels and transition intensities for one or more ions. The core routines in Spectre are based on a program called Shell, which was developed at Argonne National Laboratory by G. L. Goodman and colleagues. Further revisions were made by R. Osborn. Spectre uses numerical minimization and matrix diagonalisation subroutines from the NAG Fortran Library [2]. How to cite the program: A. T. Boothroyd, Spectre a program for calculating spectroscopic properties of rare earth ions in crystals ( ). Please report bugs to A. T. Boothroyd (a.boothroyd@physics.ox.ac.uk). Disclaimer Spectre is distributed in the hope that it will be useful, but without any guarantee that it is error-free. In no event will the author or his institution be liable to you for damages, including any general, special, incidental or consequential damages arising out of the use or inability to use the program. The author is not responsible for erroneous results obtained with the program.
2 Contents Installation 3 2 Theoretical background 3 3 Getting started 5 4 Calculations 8 4. Eigenvalues, eigenvectors and transition matrix elements Scale CEF parameters to different rare-earth ion Neutron cross-section Fit to observed energies and intensities Specific heat capacity Magnetic susceptibility Magnetisation Magnetic moment Matrix elements of magnetic moment operator References 9 2
3 Installation The current version was compiled under Windows 7 and works on Windows. For Mac OS you ll need to install a Windows emulator. The suite comprises a single executable file Spectre.exe compiled from fortran source code, together with a number of ascii files which contain single-ion parameters and input data. All files must be located in the same folder. The ascii files should under no circumstances be modified because if the formatting is changed then Spectre will not be able to read them. To launch the program, double-click on the file spectre.exe. 2 Theoretical background We assume the magnetism of the rare earth ion derives from n equivalent f electrons (i.e. we work within an f n configuration). The effective single-ion Hamiltonian has the form where H 0 is the free-ion Hamiltonian and H = H 0 + H CF + H ex + H Z, () H CF = 2l +k k=0 q= k B k q C (k) q (2) H ex = 2 H ex S (3) H Z = µ B. (4) Term (3) describes an interaction with an exchange field H ex, and term (4) is the Zeeman interaction between the magnetic moment µ = µ B (L + 2S) and an external magnetic field of flux density B. Equation (2) defines the crystal field Hamiltonian. The Bq k are crystal field parameters, and the C q (k) are the Wybourne tensor operators, which are given by C (k) q (θ, ϕ) = 4π 2k + Y k,q(θ, ϕ), (5) where Y k,q (θ, ϕ) are the spherical harmonic functions. There are several slightly different definitions in use for the spherical harmonics. We use the 3
4 Condon Shortley phase convention, and Y k,q (θ, ϕ) = ( ) q [ 2k + 4π (k q) (k + q) ] 2 P q k (cos θ) exp(iqϕ) (q 0), (6) Y k, q = ( ) q Y k,q (q 0). (7) The P q k (cos θ) are the associated Legendre polynomials. If the multi-electron basis states can be restricted to either a single L term with weak spin orbit coupling, or a single J level, then H CF can be expressed in an alternative form which is often simpler to use in practice: H CF = 2l +k k=0 q= k B q k Oq k, (8) where l is the orbital quantum number of the one-electron orbital (l = 3 for f electrons), and the O q k are known as Stevens operator equivalents and are functions of angular momentum operators: O q k = Oq k (J) in the SLJM J basis, or O q k = Oq k (L) in the SLM SM L basis. Note that the crystal field parameters Bq k in (2) and B q k in (8) are not the same. The indices are interchanged. It is often convenient to group the spherical operators into real combinations. From (6) and (7) it can be seen that a suitable basis of real functions is Zk,q c = 2 {Y k, q + ( ) q } Y k,q } cos qϕ q > 0, Zk,q s = i 2 {Y k, q ( ) q Y k,q } sin qϕ Z k,0 = Y k,0. (9) The Z k,q are known as Tesseral harmonics. Similar real and imaginary combinations of the Wybourne tensors (but without the / 2 pre-factor) are defined: H CF = = 2l k=0 2l k=0 B k 0 C (k) 0 + B k 0 C (k) 0 + 2l k=2 q= 2l k [ ] Bq k (c){c (k) q + ( ) q C (k) } + ibq k (s){c (k) q ( ) q C (k) k k=2 q= q q } Bq k (c)c q (k) (c) + Bq k (s)c q (k) (s). (0) The operators associated with B k q (c) and B k q (s) parameters have cos ϕ and sin ϕ angular dependence, respectively, as can be seen from (9). Likewise, in 4
5 Table : Multiplicative factors λ kq in the relation B q k (c, s) = λ kqθ k B k q (c, s) between the Stevens crystal field parameters in () and the corresponding Wybourne parameters in (0). The θ k are numerical coefficients which have been tabulated for the ground states of the trivalent 4f ions see Tables VI & VII of M.T. Hutchings, Solid State Physics 6 (964) 227. q k terms of Stevens operators, 2l H CF = BkO 0 k 0 + k=0 2l k k=2 q= B q k (c)oq k (c) + Bq k (s)oq k (s). () Note that in the LSJM J basis the matrix elements of the sine operators in (0) and () are imaginary. The crystal field parameters that appear in (0) are not the same as those in () it is unfortunate that B is used for both the Wybourne and Stevens parameters in the literature, but note that the positions of the k and q indices are reversed. Within the reduced basis of states on which the Stevens operators act, the Stevens and Wybourne parameters are related by B q k (c, s) = λ kqθ k B k q (c, s). The λ kq coefficients are given in Table. The parameterisation of the crystal field Hamiltonian in Spectre is that given in eqn (0). 3 Getting started After launching the program you get the following screen, which is the point to which the program returns after completion of any task: CEF Parameters (mev): B(2,0) = 0.533E+0 2 B(4,0) = -0.57E+03 3 B(6,0) = 0.000E+00 4 B(2,)c = 0.000E+00 6 B(2,)s = 0.000E+00 5 B(2,2)c = 0.000E+00 7 B(2,2)s = 0.000E+00 6 B(4,)c = 0.000E+00 8 B(4,)s = 0.000E+00 7 B(4,2)c = 0.000E+00 9 B(4,2)s = 0.000E+00 5
6 8 B(4,3)c = 0.000E B(4,3)s = 0.000E+00 9 B(4,4)c = 0.000E+00 2 B(4,4)s = 0.000E+00 0 B(6,)c = 0.000E B(6,)s = 0.000E+00 B(6,2)c = 0.000E B(6,2)s = 0.000E+00 2 B(6,3)c = 0.000E B(6,3)s = 0.000E+00 3 B(6,4)c = 0.000E B(6,4)s = 0.000E+00 4 B(6,5)c = 0.000E B(6,5)s = 0.000E+00 5 B(6,6)c = 0.000E B(6,6)s = 0.000E+00 Exchange field (He) and applied field (B): 28 Hex = 0.000E Hey = 0.000E Hez = 0.92E+02 mev 3 Bx = 0.000E By = 0.000E Bz = 0.000E+00 Tesla 34 No. f electrons = 5. (select 34 to check free-ion levels also) number... new value (<CR> to end) : The B(k,q) (... 27) are crystal field parameters defined in eqn (0). Parameter numbers are the three orthogonal components of the internal exchange (or molecular) field H ex defined in eqn (3), and are the equivalent components of an externally applied magnetic field B. To change the value of a parameter, enter the parameter number followed by a space then the new value: number... new value (<CR> to end) : <CR> Typing the ENTER or Carriage Return key (here denoted by <CR>) allows you to change another parameter. Typing <CR> without any new data brings up the revised list of all the parameters. Parameter number 34 gives you the following menu: Free ion and submatrix information Sm3+ Truncate free ion basis set? (0=No, =Yes) : 2 Number of CF submatrices (if unsure use ) : 3 q min for CF submatrices (if unsure use ) : 4 No. submatrices per mu (if unsure use ) : 5 Orbital reduction factor (if unsure use ) :.000 number... new value (<CR> to end) : 6
7 The first option allows you to reduce the number of basis states from that in the complete f n basis. This is necessary if the number of basis states exceeds 300, which is the maximum size of the arrays in the routine that diagonalises the Hamiltonian. Truncation means restricting the number of 2S+ L J terms in the basis. Reducing the number of basis states speeds up the calculations. If the truncation flag is then when you hit <CR> you get the following: Use intermediate coupling basis states (N or <CR>)? Normally you will want to use intermediate coupling free-ion basis states, in which case hit <CR>. However, if you want to switch off intermediate coupling and use pure LSJ basis states then answer N. Whichever answer you give you see the next output: J no. J multiplets to keep and skip number... N(keep), ( N(skip), ( N(keep)...)) <CR> to end : This table makes sense if you look at the energy level scheme of Sm 3+ [3]. In the energy range up to.3 ev there are 2 terms, 6 F and 6 H, containing a total of 2 J multiplets. The table contains a list of the 2 J values. Whenever a J value exists in both the 6 F and the 6 H terms the number of kept J multiplets in the table is given as 2. If the J value occurs only once then the entry is. One can now change the number of kept J multiplets in the table. For example, if you want to perform the calculations in the intermediate coupling ground state multiplet 6 H 5/2 then you will set the J = 2.5 keep flag to 7
8 (enter: 2 <CR>) and set all the other keep flags to 0 (0 0<CR>, 0<CR>, etc). Note, however, that when there are two or more terms with the same J they are not always indexed in order of energy. There are two situations when this occurs. One is when there is no truncation of the free-ion basis, i.e. the truncate free ion basis set flag is set to 0, and the other is when there is truncation (the truncate flag is set to ) but the user chooses not to use intermediate coupling basis states. In these two cases the pure LS-coupling basis states are indexed in order of increasing L starting with those of largest S. For example, the 4f 2 configuration (Pr 3+ ) has three terms that include J = 4 and these are indexed in the order () 3 F, (2) 3 H and (3) G. The 3 H is the ground state term, so if you want to do the calculations in 3 H then you can select this by setting the J = 4 keep flag to 2 and the skip flag to. If the truncate flag is set to and intermediate coupling basis states are chosen then the intermediate coupling states are indexed in order of increasing energy. In the case of 4f 2, the intermediate coupling term which derives predominantly from 3 H would then be first. One application of the keep/skip functionality is to perform a calculation that is identical to the Stevens operator method. Taking 4f 2 (Pr 3+ ) as an example, you would set the truncate flag is set to and answer N to the question Use intermediate coupling basis states (N or <CR>)?. You would then set the keep flags for all J 4 to 0. For J = 4 you would set the keep flag to 2 and the skip flag to. Options 2 to 4 in the Free ion and submatrix information menu allow the user to take advantage of symmetry to transform the crystal field matrix into block diagonal form and hence to diagonalise 2 or more sub-matrices rather than the entire matrix. For example, time reversal symmetry causes the crystal field matrix of all Kramers ions to have two blocks. Changing the number of CF sub-matrices to 2 means that sub-matrices corresponding to each block are diagonalised separately. This is faster than diagonalising the complete CF matrix. Note: make sure you know what you are doing if you decide to take advantage of this facility! Option 5 in the Free ion and submatrix information menu makes it possible to take into account covalency effects which effectively reduce the orbital quantum number relative to that of the free ion. For example, the magnetic moment is calculated from µ B (xl + 2S), where 0 < x < is the orbital reduction factor. 4 Calculations When you have finished changing parameters, hit <CR>. You get the menu: 8
9 Calculations: Eigenvalues, eigenvectors and transition matrix elements 2 Scale CEF parameters to different RE ion. 3 Neutron cross-section. 4 Fit to observed energies and intensities 5 Specific heat. 6 Susceptibility. 7 Magnetisation. 8 Magnetic moment. 9 Matrix elements of mu. 99 Quit. Option number (default = ) : You can choose between 9 different calculations, or exit the program by typing: Option number (default = ) : 99 <CR> 4. Eigenvalues, eigenvectors and transition matrix elements If you chose or hit <CR>, you will see the following series of outputs here corresponding to the crystal field splitting of Pr 3+ in Pr 2 Sn 2 O 7, A. J. Princep et al., Phys. Rev. B 88, 0442 (203): EAVE F F F ALPHA BETA GAMMA ZETA M M M P P P6 9
10 MATRIX HAS 952 COEFFICIENTS SUB MTX COEFS COEFS MTX DIM READ WRITTEN COEFFICIENTS READ VECTORS CALCULATED (REAL PART) : etc LOWEST LEVEL MADE 0.0 BY SUBTRACTING 638. Eigenvalues relative to ground state: I 2J 2M Eigenvectors (real part):. :
11 : etc M N Eigenvalue(N) Lgst EVec. comp. 2nd Lgst EVec. comp. ST I 2J 2M ST I 2J 2M ( 0.874) ( 0.409) ( 0.874) (-0.409) ( 0.959) ( 0.65) : etc <n/muperp/m> 2:
12 : etc CEF Parameters (mev): B(2,0) = 0.579E+02 2 B(4,0) = 0.433E+03 3 B(6,0) = 0.45E+03 4 B(2,)c = 0.000E+00 6 B(2,)s = 0.000E+00 5 B(2,2)c = 0.000E+00 7 B(2,2)s = 0.000E+00 6 B(4,)c = 0.000E+00 8 B(4,)s = 0.000E+00 7 B(4,2)c = 0.000E+00 9 B(4,2)s = 0.000E+00 8 B(4,3)c = 0.6E B(4,3)s = 0.000E+00 9 B(4,4)c = 0.000E+00 2 B(4,4)s = 0.000E+00 0 B(6,)c = 0.000E B(6,)s = 0.000E+00 B(6,2)c = 0.000E B(6,2)s = 0.000E+00 2 B(6,3)c = -0.08E B(6,3)s = 0.000E+00 3 B(6,4)c = 0.000E B(6,4)s = 0.000E+00 4 B(6,5)c = 0.000E B(6,5)s = 0.000E+00 5 B(6,6)c = 0.92E B(6,6)s = 0.000E+00 Exchange field (He) and applied field (B): 28 Hex = 0.000E Hey = 0.000E Hez = 0.000E+00 mev 3 Bx = 0.000E By = 0.000E Bz = 0.000E+00 Tesla 34 No. f electrons = 2. (select 34 to check free-ion levels also) number... new value (<CR> to end) : The printout contains the following information (in the order it appears): List of 4 parameters of the atomic Hamiltonian; Pr 3+ has 3 J levels 2
13 Diagnostics relating to the Hamiltonian matrix, which for Pr 3+ dimension 9 has List of eigenvalues of the Hamiltonian, in order of increasing energy Horizontal list of the first 2J + eigenvalues, with E = 0 as the lowest level Eigenvectors of the first 2J + eigenvalues, in the IJM J basis, where I is an index that labels the three J = 4 levels of the 4f 2 configuration of Pr 3+. Note that I = 2 corresponds to the J = 4 level which is lowest in energy. List of energy levels in order of increasing energy, giving the largest two eigenvector components Table of the squared dipole matrix elements of µ these give the transition probabilities for neutron scattering from a powder sample Finally the program returns to the list of crystal field and exchange and magnetic field parameters. During the calculation, the program writes the eigenvectors, eigenvalues and dipole matrix elements to the file CF Levels.dat in the same format as the screen output. Note that the file CF Levels.dat must already exist in the folder, and is overwritten each time the calculation is performed. So remember to copy the results from CF Levels.dat to a separate text file if you want to save them. The program also write some diagnostic data to files called fort.8, fort.6 and fort.2. These files contain lists of intermediate coefficients and can be deleted. 4.2 Scale CEF parameters to different rare-earth ion This option transforms the crystal field parameters from one tripositive rare earth ion to another, assuming the crystal field parameters can be expressed in the form B k q = r k A k q, (2) where r k is the k th radial moment of the 4f electron distribution, and A k q are intrinsic crystal-field parameters independent of the lanthanide ion. 3
14 4.3 Neutron cross-section This option calculates the neutron spectrum in the dipole approximation. The transition intensities are given in absolute units of cross section. The program asks for the temperature, then diagonalises the Hamiltonian and outputs a list of transition energies and their cross sections for neutron scattering: Neutron cross-section: double diff. X-section = kf/ki. F(Q) 2.exp(-2W).S(Q,w) from to w /mev S(Q,w) /mb(sr ion) : etc The program also gives you the option to simulate the spectrum and write it to a file. The transition lines are modelled by Gaussian peaks, all with the same width. You are asked to enter the energy range for the simulation (E min and E max ), the number of points ( 50), and the full width at half maximum (FWHM) of the Gaussian line shape. You are also asked for a file name. Note: there must not already exist a file with the name you choose. 4
15 4.4 Fit to observed energies and intensities This option allows you to fit a CF model to a set of experimental transition energies and relative intensities. If you select this option the first thing you will be asked is Fit with existing data? (N): If you haven t entered any experimental observables then answer no (N or <CR>). If you have entered the experimental observables then answering yes (Y) saves you from entering the observables again and proceeds directly to the fit. This allows you to vary the starting parameters and fit to the same set of observables. Next, you will have the following options for fitting: Enter option (E,C,R,V,G,M,S,X,Q,H(elp),<CR> to fit): Each letter has a different function. If you select H you get a basic statement of what they mean: E - to input transition energies C - to combine peak intensities R - to input peak intensity ratios V - to vary one of the B coefficients G - to fix the ratio of 2 parameters (st determined by 2nd; both.true. in V) M - to fit to magnetic moment S - to include data from >= 2 lanthanide ions X - to change the B coefficient normalising factor Q - to quit the fitting program H - Help; this menu <CR> to fit E this allows you to enter a set of observed transition energies. The energy levels are are number in order of ascending energy, starting with (the ground state). For example, suppose experiments have established that the ground state is a doublet, the first excited state is a singlet at 8 ± 0.5 mev, the second excited state is a doublet at 58 ± mev, etc., you would enter E <CR> then Level, level 2, energy, uncertainty: Level, level 2, energy, uncertainty: Level, level 2, energy, uncertainty: 4 58 Level, level 2, energy, uncertainty: 5 58 Level, level 2, energy, uncertainty: <CR> 5
16 This input implies that you can number the transitions in order of energy, which is not always the case. For example, if you suspected another singlet between the 8 and 58 mev levels then the above numbering would be incorrect. There is no simple way round this problem. You would have to try fitting again with the 58 mev doublet numbered as levels 5 and 6. C if there are overlapping peaks in the experimental spectrum then you can combine their intensities. For example, if you suspect unresolved transitions from the ground state (level ) to singlet levels at 8 and 9 mev (levels 3 and 4) then you can combine their intensities as follows: Enter option (E,C,R,V,G,M,S,X,Q,H(elp),<CR> to fit): C Type in transitions which are to be grouped together (<CR> to finish) Level, level 2: 3 Level, level 2: 4 Level, level 2: <CR> R This allows you to enter experimentally-determined ratios of transition intensities. For example, suppose the intensity of the 8 mev transition (from the ground state doublet to level 3) is 4 ± times larger than the transition from the ground state to the 58 mev doublet (levels 4 and 5), both transitions measured at a temperature of 5 K. First you would select option C and combine the intensities of transitions to 3 and 2 to 3, and levels to 4, to 5, 2 to 4 and 2 to 5. Then Enter option (E,C,R,V,G,M,S,X,Q,H(elp),<CR> to fit): R st peak: Temp., level, level 2 : 5 3 2nd peak: Temp., level, level 2 : 5 4 Ratio, uncertainty : 4 st peak: Temp., level, level 2 : <CR> V This is how you enter which parameters you want to vary in the fitting procedure. If you don t specify that a parameter is to be varied then it will remain fixed at its initial value. Suppose you want to fit the parameters B 4 0, B 6 0, B 4 3 and B 6 3. Do the following: Enter option (E,C,R,V,G,M,S,X,Q,H(elp),<CR> to fit): V Vary which B coeff.? : 2 6
17 Vary which B coeff.? : 3 Vary which B coeff.? : 8 Vary which B coeff.? : 2 Vary which B coeff.? : <CR> You can fit the exchange and magnetic field components, as well as the crystal field parameters. G This allows you to fix the ratio of two parameters. This function is needed if the symmetry of the crystal field constrains two parameters to be in a fixed ratio. For example, in cubic symmetry B 4 4/B 4 0 = ± 70/4 = ± and B 6 4/B 6 0 = 4/2 =.87. You would first use the V option to specify that the variable parameters are B 4 0, B 4 4, B 6 0 and B 6 4, then Enter option (E,C,R,V,G,M,S,X,Q,H(elp),<CR> to fit): G Coeff., coeff.2, Ratio :2 : Coeff., coeff.2, Ratio :2 : Coeff., coeff.2, Ratio :2 : <CR> M This allows you to fit to the magnitude of a magnetic moment induced by an exchange or external magnetic field at a given temperature. S With this option you can fit observables from two or more rare earth ions simultaneously. After specifying the second ion and giving the single-ion levels to include in the basis, you enter the observables for the second ion. You can repeat for a third ion, and so on. X The minimisation routine works most efficiently if the variables are kept in the range [, +]. This is achieved with a normalisation factor, which by default is set to 200 mev. If a larger or small factor would be better then it can be changed via this option. Q Quits without performing the fit. H Prints out the list of fitting options described above. Be aware that you can only fit as many parameters as you have observables. Once you have input all the information for the fit and hit <CR> you will see an iterative improvement in the parameters step by step until the final set of parameters is reached. The quality of the fit is gauged by the usual χ 2 function, and on completion the routine writes out the converged 7
18 values of the fitting parameters and χ 2. There is also a parameter called IFAIL. If the fit reaches a global minimum in χ 2 then IFAIL=0. If not, then IFAIL=...5 and you might want to change the starting parameters and re-run the fit. The results of the fit are written into a file called RESULTS.DAT. A file with this name must exist already before performing the fit. If you perform another fit then the contents of RESULTS.DAT will be overwritten with the results of the new fit. So if you want to keep the results, copy them into another file. At the end of the fit you will see the following on the screen: Details of fit written to file RESULT.DAT Save these best-fit parameters? (N) : If you answer Y then the program will replace the parameters in the starting menu by those calculated here. Otherwise, the original parameters are kept as they were. 4.5 Specific heat capacity With this option you can calculate (but not fit) the specific heat capacity as a function of temperature. The calculation makes use of the eigenvalues but not the eigenfunctions. You need to specify the temperature interval for the calculation [T min, T max ] and the number of points. You are asked whether you wish to save the results to a file. 4.6 Magnetic susceptibility This option calculates the magnetic susceptibility as a function of temperature over the interval [0, T max ], where T max is user-specified. The calculation is performed at 00 temperature values (not changeable). The susceptibility is given in units of cm 3 mol. The program calculates the susceptibility in the directions parallel and perpendicular to the crystal field axis, χ z and (χ x +χ y )/2, respectively, and the powder-averaged susceptibility χ powder. You are asked whether you wish to save the results to a file. 4.7 Magnetisation This option calculates the magnetisation (in µ B ) as a function of applied field at a specified temperature. The direction of the applied field is specified by values B x, B y, B z along the crystal field axes. The magnetisation is 8
19 calculated at 2 field values from 0 to B max, where B max = B 2 x + B 2 y + B 2 z. You are asked whether you wish to save the results to a file. 4.8 Magnetic moment This option calculates the value of the magnetic moment (in µ B ) at a given temperature. 4.9 Matrix elements of magnetic moment operator This function writes out a list of certain matrix elements used by the program. The type of matrix element is chosen from a menu. The function is is mainly for diagnostic purposes, but the default option (option : matrix elements of µ) is useful for calculating the scattering cross-section from single crystals. 5 References References [] W. T. Carnall, G. L. Goodman, K. Rajnak and R. S. Rana, J. Chem Phys. 90, 3443 (989) [2] The Numerical Algorithms Group (NAG), [3] R. Osborn, S. W. Lovesey, A. D. Taylor and E. Balcar, Handbook on the Physics and Chemistry of Rare Earths, eds. K. A. Gschneidner, jr. and L. Eyring, Vol. 4 (North-Holland, Amsterdam, 99) 9
Zeeman Effect Physics 481
 Zeeman Effect Introduction You are familiar with Atomic Spectra, especially the H- atom energy spectrum. Atoms emit or absorb energies in packets, or quanta which are photons. The orbital motion of electrons
Zeeman Effect Introduction You are familiar with Atomic Spectra, especially the H- atom energy spectrum. Atoms emit or absorb energies in packets, or quanta which are photons. The orbital motion of electrons
Electronic Structure and Transition Intensities in Rare-Earth Materials
 Electronic Structure and Transition Intensities in Rare-Earth Materials Michael F Reid Department of Physics and Astronomy and MacDiarmid Institute for Advanced Materials and Nanotechnology University
Electronic Structure and Transition Intensities in Rare-Earth Materials Michael F Reid Department of Physics and Astronomy and MacDiarmid Institute for Advanced Materials and Nanotechnology University
4/21/2010. Schrödinger Equation For Hydrogen Atom. Spherical Coordinates CHAPTER 8
 CHAPTER 8 Hydrogen Atom 8.1 Spherical Coordinates 8.2 Schrödinger's Equation in Spherical Coordinate 8.3 Separation of Variables 8.4 Three Quantum Numbers 8.5 Hydrogen Atom Wave Function 8.6 Electron Spin
CHAPTER 8 Hydrogen Atom 8.1 Spherical Coordinates 8.2 Schrödinger's Equation in Spherical Coordinate 8.3 Separation of Variables 8.4 Three Quantum Numbers 8.5 Hydrogen Atom Wave Function 8.6 Electron Spin
Electronic Structure and Transition Intensities in Rare-Earth Materials
 Electronic Structure and Transition Intensities in Rare-Earth Materials Michael F Reid School of Physical and Chemical Sciences Te Kura Matū University of Canterbury Christchurch, New Zealand http://www.phys.canterbury.ac.nz
Electronic Structure and Transition Intensities in Rare-Earth Materials Michael F Reid School of Physical and Chemical Sciences Te Kura Matū University of Canterbury Christchurch, New Zealand http://www.phys.canterbury.ac.nz
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.76 Modern Topics in Physical Chemistry Spring, Problem Set #2
 Reading Assignment: Bernath Chapter 5 MASSACHUSETTS INSTITUTE O TECHNOLOGY 5.76 Modern Topics in Physical Chemistry Spring 994 Problem Set # The following handouts also contain useful information: C &
Reading Assignment: Bernath Chapter 5 MASSACHUSETTS INSTITUTE O TECHNOLOGY 5.76 Modern Topics in Physical Chemistry Spring 994 Problem Set # The following handouts also contain useful information: C &
Nucleon Transfer within Distorted Wave Born Approximation
 Nucleon Transfer within Distorted Wave Born Approximation N R V Project Flerov Laboratory of Nuclear Reactions, 141980, Dubna, Russian Federation Abstract. The finite range Distorted Wave Born Approximation
Nucleon Transfer within Distorted Wave Born Approximation N R V Project Flerov Laboratory of Nuclear Reactions, 141980, Dubna, Russian Federation Abstract. The finite range Distorted Wave Born Approximation
Experiment 10. Zeeman Effect. Introduction. Zeeman Effect Physics 244
 Experiment 10 Zeeman Effect Introduction You are familiar with Atomic Spectra, especially the H-atom energy spectrum. Atoms emit or absorb energies in packets, or quanta which are photons. The orbital
Experiment 10 Zeeman Effect Introduction You are familiar with Atomic Spectra, especially the H-atom energy spectrum. Atoms emit or absorb energies in packets, or quanta which are photons. The orbital
The Hydrogen Atom. Dr. Sabry El-Taher 1. e 4. U U r
 The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
26 Group Theory Basics
 26 Group Theory Basics 1. Reference: Group Theory and Quantum Mechanics by Michael Tinkham. 2. We said earlier that we will go looking for the set of operators that commute with the molecular Hamiltonian.
26 Group Theory Basics 1. Reference: Group Theory and Quantum Mechanics by Michael Tinkham. 2. We said earlier that we will go looking for the set of operators that commute with the molecular Hamiltonian.
Chm 331 Fall 2015, Exercise Set 4 NMR Review Problems
 Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
LS coupling. 2 2 n + H s o + H h f + H B. (1) 2m
 LS coupling 1 The big picture We start from the Hamiltonian of an atomic system: H = [ ] 2 2 n Ze2 1 + 1 e 2 1 + H s o + H h f + H B. (1) 2m n e 4πɛ 0 r n 2 4πɛ 0 r nm n,m Here n runs pver the electrons,
LS coupling 1 The big picture We start from the Hamiltonian of an atomic system: H = [ ] 2 2 n Ze2 1 + 1 e 2 1 + H s o + H h f + H B. (1) 2m n e 4πɛ 0 r n 2 4πɛ 0 r nm n,m Here n runs pver the electrons,
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Introduction to Hartree-Fock calculations in Spartan
 EE5 in 2008 Hannes Jónsson Introduction to Hartree-Fock calculations in Spartan In this exercise, you will get to use state of the art software for carrying out calculations of wavefunctions for molecues,
EE5 in 2008 Hannes Jónsson Introduction to Hartree-Fock calculations in Spartan In this exercise, you will get to use state of the art software for carrying out calculations of wavefunctions for molecues,
Electronic Structure and Transition Intensities in Rare-Earth Materials
 Electronic Structure and Transition Intensities in Rare-Earth Materials Michael F Reid School of Physical and Chemical Sciences Te Kura Matū University of Canterbury Christchurch, New Zealand http://www.phys.canterbury.ac.nz
Electronic Structure and Transition Intensities in Rare-Earth Materials Michael F Reid School of Physical and Chemical Sciences Te Kura Matū University of Canterbury Christchurch, New Zealand http://www.phys.canterbury.ac.nz
Modeling of Er in ceramic YAG and comparison with single-crystal YAG
 Modeling of Er in ceramic YAG and comparison with single-crystal YAG Bahram Zandi a, John B. Gruber b, Dhiraj K. Sardar c, Toomas H. Allik d a ARL/Adelphi Laboratory Center, 2800 Powder Mill RoadAdelphi,
Modeling of Er in ceramic YAG and comparison with single-crystal YAG Bahram Zandi a, John B. Gruber b, Dhiraj K. Sardar c, Toomas H. Allik d a ARL/Adelphi Laboratory Center, 2800 Powder Mill RoadAdelphi,
Qualifying Exam. Aug Part II. Please use blank paper for your work do not write on problems sheets!
 Qualifying Exam Aug. 2015 Part II Please use blank paper for your work do not write on problems sheets! Solve only one problem from each of the four sections Mechanics, Quantum Mechanics, Statistical Physics
Qualifying Exam Aug. 2015 Part II Please use blank paper for your work do not write on problems sheets! Solve only one problem from each of the four sections Mechanics, Quantum Mechanics, Statistical Physics
CHEM3023: Spins, Atoms and Molecules
 CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
Tutorial for PhET Sim Quantum Bound States
 Tutorial for PhET Sim Quantum Bound States J. Mathias Weber * JILA and Department of Chemistry & Biochemistry, University of Colorado at Boulder With this PhET Sim, we will explore the properties of some
Tutorial for PhET Sim Quantum Bound States J. Mathias Weber * JILA and Department of Chemistry & Biochemistry, University of Colorado at Boulder With this PhET Sim, we will explore the properties of some
Lectures about Python, useful both for beginners and experts, can be found at (http://scipy-lectures.github.io).
 Random Matrix Theory (Sethna, "Entropy, Order Parameters, and Complexity", ex. 1.6, developed with Piet Brouwer) 2016, James Sethna, all rights reserved. This is an ipython notebook. This hints file is
Random Matrix Theory (Sethna, "Entropy, Order Parameters, and Complexity", ex. 1.6, developed with Piet Brouwer) 2016, James Sethna, all rights reserved. This is an ipython notebook. This hints file is
Particle Physics. Michaelmas Term 2011 Prof. Mark Thomson. Handout 2 : The Dirac Equation. Non-Relativistic QM (Revision)
 Particle Physics Michaelmas Term 2011 Prof. Mark Thomson + e - e + - + e - e + - + e - e + - + e - e + - Handout 2 : The Dirac Equation Prof. M.A. Thomson Michaelmas 2011 45 Non-Relativistic QM (Revision)
Particle Physics Michaelmas Term 2011 Prof. Mark Thomson + e - e + - + e - e + - + e - e + - + e - e + - Handout 2 : The Dirac Equation Prof. M.A. Thomson Michaelmas 2011 45 Non-Relativistic QM (Revision)
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours.
 Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Particle Physics Dr. Alexander Mitov Handout 2 : The Dirac Equation
 Dr. A. Mitov Particle Physics 45 Particle Physics Dr. Alexander Mitov µ + e - e + µ - µ + e - e + µ - µ + e - e + µ - µ + e - e + µ - Handout 2 : The Dirac Equation Dr. A. Mitov Particle Physics 46 Non-Relativistic
Dr. A. Mitov Particle Physics 45 Particle Physics Dr. Alexander Mitov µ + e - e + µ - µ + e - e + µ - µ + e - e + µ - µ + e - e + µ - Handout 2 : The Dirac Equation Dr. A. Mitov Particle Physics 46 Non-Relativistic
Chapter 1. Magnetism of the Rare-Earth Ions in Crystals
 Chapter Magnetism of the Rare-Earth Ions in Crystals 3 . Magnetism of the Rare-Earth ions in Crystals In this chapter we present the basic facts regarding the physics of magnetic phenomena of rare-earth
Chapter Magnetism of the Rare-Earth Ions in Crystals 3 . Magnetism of the Rare-Earth ions in Crystals In this chapter we present the basic facts regarding the physics of magnetic phenomena of rare-earth
Basic Physical Chemistry Lecture 2. Keisuke Goda Summer Semester 2015
 Basic Physical Chemistry Lecture 2 Keisuke Goda Summer Semester 2015 Lecture schedule Since we only have three lectures, let s focus on a few important topics of quantum chemistry and structural chemistry
Basic Physical Chemistry Lecture 2 Keisuke Goda Summer Semester 2015 Lecture schedule Since we only have three lectures, let s focus on a few important topics of quantum chemistry and structural chemistry
2.4. Quantum Mechanical description of hydrogen atom
 2.4. Quantum Mechanical description of hydrogen atom Atomic units Quantity Atomic unit SI Conversion Ang. mom. h [J s] h = 1, 05459 10 34 Js Mass m e [kg] m e = 9, 1094 10 31 kg Charge e [C] e = 1, 6022
2.4. Quantum Mechanical description of hydrogen atom Atomic units Quantity Atomic unit SI Conversion Ang. mom. h [J s] h = 1, 05459 10 34 Js Mass m e [kg] m e = 9, 1094 10 31 kg Charge e [C] e = 1, 6022
A Computer Study of Molecular Electronic Structure
 A Computer Study of Molecular Electronic Structure The following exercises are designed to give you a brief introduction to some of the types of information that are now readily accessible from electronic
A Computer Study of Molecular Electronic Structure The following exercises are designed to give you a brief introduction to some of the types of information that are now readily accessible from electronic
Department of Physics and Astronomy 2 nd Year Laboratory. G4 Quinckes method
 nd year laboratory script G4 Quincke s methods Department of Physics and Astronomy nd Year Laboratory G4 Quinckes method Scientific aims and objectives To determine the volume magnetic susceptibility of
nd year laboratory script G4 Quincke s methods Department of Physics and Astronomy nd Year Laboratory G4 Quinckes method Scientific aims and objectives To determine the volume magnetic susceptibility of
Potential energy, from Coulomb's law. Potential is spherically symmetric. Therefore, solutions must have form
 Lecture 6 Page 1 Atoms L6.P1 Review of hydrogen atom Heavy proton (put at the origin), charge e and much lighter electron, charge -e. Potential energy, from Coulomb's law Potential is spherically symmetric.
Lecture 6 Page 1 Atoms L6.P1 Review of hydrogen atom Heavy proton (put at the origin), charge e and much lighter electron, charge -e. Potential energy, from Coulomb's law Potential is spherically symmetric.
The Hydrogen Atom. Thornton and Rex, Ch. 7
 The Hydrogen Atom Thornton and Rex, Ch. 7 Applying Schrodinger s Eqn to the Hydrogen Atom The potential: -1 e 2 V(r) = 4p e0 r Use spherical polar coordinates (with y(x,y,z) => y(r,q,f) ): 1 y 1 y ( r
The Hydrogen Atom Thornton and Rex, Ch. 7 Applying Schrodinger s Eqn to the Hydrogen Atom The potential: -1 e 2 V(r) = 4p e0 r Use spherical polar coordinates (with y(x,y,z) => y(r,q,f) ): 1 y 1 y ( r
Problem 1: Spin 1 2. particles (10 points)
 Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
20 The Hydrogen Atom. Ze2 r R (20.1) H( r, R) = h2 2m 2 r h2 2M 2 R
 20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
Addition of Angular Momenta
 Addition of Angular Momenta What we have so far considered to be an exact solution for the many electron problem, should really be called exact non-relativistic solution. A relativistic treatment is needed
Addition of Angular Momenta What we have so far considered to be an exact solution for the many electron problem, should really be called exact non-relativistic solution. A relativistic treatment is needed
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q.
 1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
Multielectron Atoms.
 Multielectron Atoms. Chem 639. Spectroscopy. Spring 00 S.Smirnov Atomic Units Mass m e 1 9.109 10 31 kg Charge e 1.60 10 19 C Angular momentum 1 1.055 10 34 J s Permittivity 4 0 1 1.113 10 10 C J 1 m 1
Multielectron Atoms. Chem 639. Spectroscopy. Spring 00 S.Smirnov Atomic Units Mass m e 1 9.109 10 31 kg Charge e 1.60 10 19 C Angular momentum 1 1.055 10 34 J s Permittivity 4 0 1 1.113 10 10 C J 1 m 1
Calculating NMR Chemical Shifts for beta-ionone O
 Calculating NMR Chemical Shifts for beta-ionone O Molecular orbital calculations can be used to get good estimates for chemical shifts. In this exercise we will calculate the chemical shifts for beta-ionone.
Calculating NMR Chemical Shifts for beta-ionone O Molecular orbital calculations can be used to get good estimates for chemical shifts. In this exercise we will calculate the chemical shifts for beta-ionone.
St Hugh s 2 nd Year: Quantum Mechanics II. Reading. Topics. The following sources are recommended for this tutorial:
 St Hugh s 2 nd Year: Quantum Mechanics II Reading The following sources are recommended for this tutorial: The key text (especially here in Oxford) is Molecular Quantum Mechanics, P. W. Atkins and R. S.
St Hugh s 2 nd Year: Quantum Mechanics II Reading The following sources are recommended for this tutorial: The key text (especially here in Oxford) is Molecular Quantum Mechanics, P. W. Atkins and R. S.
Model Building An Introduction to Atomistic Simulation
 Materials and Modelling MPhil 2006-07 COURSE MP3: MONTE CARLO AND MOLECULAR DYNAMICS COMPUTING CLASS 1 Model Building An Introduction to Atomistic Simulation Wednesday 22 nd November 2006 14.00 16.00 1
Materials and Modelling MPhil 2006-07 COURSE MP3: MONTE CARLO AND MOLECULAR DYNAMICS COMPUTING CLASS 1 Model Building An Introduction to Atomistic Simulation Wednesday 22 nd November 2006 14.00 16.00 1
Applied Nuclear Physics (Fall 2006) Lecture 8 (10/4/06) Neutron-Proton Scattering
 22.101 Applied Nuclear Physics (Fall 2006) Lecture 8 (10/4/06) Neutron-Proton Scattering References: M. A. Preston, Physics of the Nucleus (Addison-Wesley, Reading, 1962). E. Segre, Nuclei and Particles
22.101 Applied Nuclear Physics (Fall 2006) Lecture 8 (10/4/06) Neutron-Proton Scattering References: M. A. Preston, Physics of the Nucleus (Addison-Wesley, Reading, 1962). E. Segre, Nuclei and Particles
Exercises for Windows
 Exercises for Windows CAChe User Interface for Windows Select tool Application window Document window (workspace) Style bar Tool palette Select entire molecule Select Similar Group Select Atom tool Rotate
Exercises for Windows CAChe User Interface for Windows Select tool Application window Document window (workspace) Style bar Tool palette Select entire molecule Select Similar Group Select Atom tool Rotate
Electric Fields and Equipotentials
 OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
Hyperfine interaction
 Hyperfine interaction The notion hyperfine interaction (hfi) comes from atomic physics, where it is used for the interaction of the electronic magnetic moment with the nuclear magnetic moment. In magnetic
Hyperfine interaction The notion hyperfine interaction (hfi) comes from atomic physics, where it is used for the interaction of the electronic magnetic moment with the nuclear magnetic moment. In magnetic
A = 3 B = A 1 1 matrix is the same as a number or scalar, 3 = [3].
![A = 3 B = A 1 1 matrix is the same as a number or scalar, 3 = [3]. A = 3 B = A 1 1 matrix is the same as a number or scalar, 3 = [3].](/thumbs/78/77913594.jpg) Appendix : A Very Brief Linear ALgebra Review Introduction Linear Algebra, also known as matrix theory, is an important element of all branches of mathematics Very often in this course we study the shapes
Appendix : A Very Brief Linear ALgebra Review Introduction Linear Algebra, also known as matrix theory, is an important element of all branches of mathematics Very often in this course we study the shapes
Phys 622 Problems Chapter 5
 1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
Crystal field effect on atomic states
 Crystal field effect on atomic states Mehdi Amara, Université Joseph-Fourier et Institut Néel, C.N.R.S. BP 66X, F-3842 Grenoble, France References : Articles - H. Bethe, Annalen der Physik, 929, 3, p.
Crystal field effect on atomic states Mehdi Amara, Université Joseph-Fourier et Institut Néel, C.N.R.S. BP 66X, F-3842 Grenoble, France References : Articles - H. Bethe, Annalen der Physik, 929, 3, p.
Using web-based Java pplane applet to graph solutions of systems of differential equations
 Using web-based Java pplane applet to graph solutions of systems of differential equations Our class project for MA 341 involves using computer tools to analyse solutions of differential equations. This
Using web-based Java pplane applet to graph solutions of systems of differential equations Our class project for MA 341 involves using computer tools to analyse solutions of differential equations. This
Chapter 10: Multi- Electron Atoms Optical Excitations
 Chapter 10: Multi- Electron Atoms Optical Excitations To describe the energy levels in multi-electron atoms, we need to include all forces. The strongest forces are the forces we already discussed in Chapter
Chapter 10: Multi- Electron Atoms Optical Excitations To describe the energy levels in multi-electron atoms, we need to include all forces. The strongest forces are the forces we already discussed in Chapter
Time Independent Perturbation Theory Contd.
 Time Independent Perturbation Theory Contd. A summary of the machinery for the Perturbation theory: H = H o + H p ; H 0 n >= E n n >; H Ψ n >= E n Ψ n > E n = E n + E n ; E n = < n H p n > + < m H p n
Time Independent Perturbation Theory Contd. A summary of the machinery for the Perturbation theory: H = H o + H p ; H 0 n >= E n n >; H Ψ n >= E n Ψ n > E n = E n + E n ; E n = < n H p n > + < m H p n
Angular momentum and spin
 Luleå tekniska universitet Avdelningen för Fysik, 007 Hans Weber Angular momentum and spin Angular momentum is a measure of how much rotation there is in particle or in a rigid body. In quantum mechanics
Luleå tekniska universitet Avdelningen för Fysik, 007 Hans Weber Angular momentum and spin Angular momentum is a measure of how much rotation there is in particle or in a rigid body. In quantum mechanics
Problem Set 2 Due Tuesday, September 27, ; p : 0. (b) Construct a representation using five d orbitals that sit on the origin as a basis: 1
 Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
B7 Symmetry : Questions
 B7 Symmetry 009-10: Questions 1. Using the definition of a group, prove the Rearrangement Theorem, that the set of h products RS obtained for a fixed element S, when R ranges over the h elements of the
B7 Symmetry 009-10: Questions 1. Using the definition of a group, prove the Rearrangement Theorem, that the set of h products RS obtained for a fixed element S, when R ranges over the h elements of the
Ethene. Introduction. The ethene molecule is planar (i.e. all the six atoms lie in the same plane) and has a high degree of symmetry:
 FY1006 Innføring i kvantefysikk og TFY4215 Kjemisk fysikk og kvantemekanikk Spring 2012 Chemical Physics Exercise 1 To be delivered by Friday 27.04.12 Introduction Ethene. Ethylene, C 2 H 4, or ethene,
FY1006 Innføring i kvantefysikk og TFY4215 Kjemisk fysikk og kvantemekanikk Spring 2012 Chemical Physics Exercise 1 To be delivered by Friday 27.04.12 Introduction Ethene. Ethylene, C 2 H 4, or ethene,
Total Angular Momentum for Hydrogen
 Physics 4 Lecture 7 Total Angular Momentum for Hydrogen Lecture 7 Physics 4 Quantum Mechanics I Friday, April th, 008 We have the Hydrogen Hamiltonian for central potential φ(r), we can write: H r = p
Physics 4 Lecture 7 Total Angular Momentum for Hydrogen Lecture 7 Physics 4 Quantum Mechanics I Friday, April th, 008 We have the Hydrogen Hamiltonian for central potential φ(r), we can write: H r = p
Quantum Physics II (8.05) Fall 2002 Assignment 12 and Study Aid
 Quantum Physics II (8.05) Fall 2002 Assignment 12 and Study Aid Announcement This handout includes 9 problems. The first 5 are the problem set due. The last 4 cover material from the final few lectures
Quantum Physics II (8.05) Fall 2002 Assignment 12 and Study Aid Announcement This handout includes 9 problems. The first 5 are the problem set due. The last 4 cover material from the final few lectures
Electronic structure of correlated electron systems. G.A.Sawatzky UBC Lecture
 Electronic structure of correlated electron systems G.A.Sawatzky UBC Lecture 6 011 Influence of polarizability on the crystal structure Ionic compounds are often cubic to maximize the Madelung energy i.e.
Electronic structure of correlated electron systems G.A.Sawatzky UBC Lecture 6 011 Influence of polarizability on the crystal structure Ionic compounds are often cubic to maximize the Madelung energy i.e.
I. CSFs Are Used to Express the Full N-Electron Wavefunction
 Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
G : Quantum Mechanics II
 G5.666: Quantum Mechanics II Notes for Lecture 5 I. REPRESENTING STATES IN THE FULL HILBERT SPACE Given a representation of the states that span the spin Hilbert space, we now need to consider the problem
G5.666: Quantum Mechanics II Notes for Lecture 5 I. REPRESENTING STATES IN THE FULL HILBERT SPACE Given a representation of the states that span the spin Hilbert space, we now need to consider the problem
Using Microsoft Excel
 Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
For example, in one dimension if we had two particles in a one-dimensional infinite potential well described by the following two wave functions.
 Identical particles In classical physics one can label particles in such a way as to leave the dynamics unaltered or follow the trajectory of the particles say by making a movie with a fast camera. Thus
Identical particles In classical physics one can label particles in such a way as to leave the dynamics unaltered or follow the trajectory of the particles say by making a movie with a fast camera. Thus
COMPUTATIONAL TOOL. Fig. 4.1 Opening screen of w2web
 CHAPTER -4 COMPUTATIONAL TOOL Ph.D. Thesis: J. Maibam CHAPTER: 4 4.1 The WIEN2k code In this work, all the calculations presented are performed using the WIEN2k software package (Blaha et al., 2001). The
CHAPTER -4 COMPUTATIONAL TOOL Ph.D. Thesis: J. Maibam CHAPTER: 4 4.1 The WIEN2k code In this work, all the calculations presented are performed using the WIEN2k software package (Blaha et al., 2001). The
charges q r p = q 2mc 2mc L (1.4) ptles µ e = g e
 APAS 5110. Atomic and Molecular Processes. Fall 2013. 1. Magnetic Moment Classically, the magnetic moment µ of a system of charges q at positions r moving with velocities v is µ = 1 qr v. (1.1) 2c charges
APAS 5110. Atomic and Molecular Processes. Fall 2013. 1. Magnetic Moment Classically, the magnetic moment µ of a system of charges q at positions r moving with velocities v is µ = 1 qr v. (1.1) 2c charges
Surrey R-matrix mini-school: AZURE2 tutorial
 Surrey R-matrix mini-school: AZURE2 tutorial May 9, 2013 School: AZURE: JINA: NNDC: TUNL: IBANDL: EXFOR: UNIX: http://www.nucleartheory.net/npg/minischool_r-matrix/index.htm http://www.nd.edu/~azure http://www.jinaweb.org/events/matrix/lectures.html
Surrey R-matrix mini-school: AZURE2 tutorial May 9, 2013 School: AZURE: JINA: NNDC: TUNL: IBANDL: EXFOR: UNIX: http://www.nucleartheory.net/npg/minischool_r-matrix/index.htm http://www.nd.edu/~azure http://www.jinaweb.org/events/matrix/lectures.html
CHEMISTRY 4021/8021 MIDTERM EXAM 1 SPRING 2014
 CHEMISTRY 4021/8021 Q1) Propose a simple, united-atom molecular mechanics force-field needed to generate a potential energy surface for an isolated molecule of acetone (Me 2 CO). I.e., provide an energy
CHEMISTRY 4021/8021 Q1) Propose a simple, united-atom molecular mechanics force-field needed to generate a potential energy surface for an isolated molecule of acetone (Me 2 CO). I.e., provide an energy
Electronic structure of correlated electron systems. Lecture 2
 Electronic structure of correlated electron systems Lecture 2 Band Structure approach vs atomic Band structure Delocalized Bloch states Fill up states with electrons starting from the lowest energy No
Electronic structure of correlated electron systems Lecture 2 Band Structure approach vs atomic Band structure Delocalized Bloch states Fill up states with electrons starting from the lowest energy No
Quantum Physics 130A. April 1, 2006
 Quantum Physics 130A April 1, 2006 2 1 HOMEWORK 1: Due Friday, Apr. 14 1. A polished silver plate is hit by beams of photons of known energy. It is measured that the maximum electron energy is 3.1 ± 0.11
Quantum Physics 130A April 1, 2006 2 1 HOMEWORK 1: Due Friday, Apr. 14 1. A polished silver plate is hit by beams of photons of known energy. It is measured that the maximum electron energy is 3.1 ± 0.11
Alkali metals show splitting of spectral lines in absence of magnetic field. s lines not split p, d lines split
 Electron Spin Electron spin hypothesis Solution to H atom problem gave three quantum numbers, n,, m. These apply to all atoms. Experiments show not complete description. Something missing. Alkali metals
Electron Spin Electron spin hypothesis Solution to H atom problem gave three quantum numbers, n,, m. These apply to all atoms. Experiments show not complete description. Something missing. Alkali metals
Quantum Theory of Angular Momentum and Atomic Structure
 Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Atomic Structure, Periodic Table, and Other Effects: Chapter 8 of Rex and T. Modern Physics
 Atomic Structure, Periodic Table, and Other Effects: Chapter 8 of Rex and T Modern Physics 11/16 and 11/19/2018 1 Introduction In Chapter 7, we studied the hydrogen atom. What about other elements, e.g.,
Atomic Structure, Periodic Table, and Other Effects: Chapter 8 of Rex and T Modern Physics 11/16 and 11/19/2018 1 Introduction In Chapter 7, we studied the hydrogen atom. What about other elements, e.g.,
CHAPTER 8 The Quantum Theory of Motion
 I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
Diatomics in Molecules - DIM
 Chapter 4 Diatomics in Molecules - DIM In this chapter it is shown how the Diatomic in molecules method is applied to systems of dihalogens in a rare gas environment. irst, the construction of the amiltonian
Chapter 4 Diatomics in Molecules - DIM In this chapter it is shown how the Diatomic in molecules method is applied to systems of dihalogens in a rare gas environment. irst, the construction of the amiltonian
Page 712. Lecture 42: Rotations and Orbital Angular Momentum in Two Dimensions Date Revised: 2009/02/04 Date Given: 2009/02/04
 Page 71 Lecture 4: Rotations and Orbital Angular Momentum in Two Dimensions Date Revised: 009/0/04 Date Given: 009/0/04 Plan of Attack Section 14.1 Rotations and Orbital Angular Momentum: Plan of Attack
Page 71 Lecture 4: Rotations and Orbital Angular Momentum in Two Dimensions Date Revised: 009/0/04 Date Given: 009/0/04 Plan of Attack Section 14.1 Rotations and Orbital Angular Momentum: Plan of Attack
Determining C-H Connectivity: ghmqc and ghmbc (VnmrJ-2.2D Version: For use with the new Software)
 Determining C-H Connectivity: ghmqc and ghmbc (VnmrJ-2.2D Version: For use with the new Software) Heteronuclear Multiple Quantum Coherence (HMQC) and Heteronuclear Multiple Bond Coherence (HMBC) are 2-dimensional
Determining C-H Connectivity: ghmqc and ghmbc (VnmrJ-2.2D Version: For use with the new Software) Heteronuclear Multiple Quantum Coherence (HMQC) and Heteronuclear Multiple Bond Coherence (HMBC) are 2-dimensional
Nuclear Shell Model. Experimental evidences for the existence of magic numbers;
 Nuclear Shell Model It has been found that the nuclei with proton number or neutron number equal to certain numbers 2,8,20,28,50,82 and 126 behave in a different manner when compared to other nuclei having
Nuclear Shell Model It has been found that the nuclei with proton number or neutron number equal to certain numbers 2,8,20,28,50,82 and 126 behave in a different manner when compared to other nuclei having
! Except for a fully filled subshell, we rarely presume to know which of the two possible spin states individual electrons have (m s = ±½).
 Terms of Free Ions with d n Configurations! The usual notation for electronic configurations (e.g., 3d 2 ) does not tell us which specific orbitals are occupied, except when a degenerate set of orbitals
Terms of Free Ions with d n Configurations! The usual notation for electronic configurations (e.g., 3d 2 ) does not tell us which specific orbitals are occupied, except when a degenerate set of orbitals
Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found.
 Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found. In applying quantum mechanics to 'real' chemical problems, one is usually faced with a Schrödinger
Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found. In applying quantum mechanics to 'real' chemical problems, one is usually faced with a Schrödinger
Quantum simulation program instructions
 Quantum simulation program instructions Introduction Model: This program follows the evolution of a system of coupled spins, using a quantum mechanical density matrix method. Each spin included has a location
Quantum simulation program instructions Introduction Model: This program follows the evolution of a system of coupled spins, using a quantum mechanical density matrix method. Each spin included has a location
Lecture #13 1. Incorporating a vector potential into the Hamiltonian 2. Spin postulates 3. Description of spin states 4. Identical particles in
 Lecture #3. Incorporating a vector potential into the Hamiltonian. Spin postulates 3. Description of spin states 4. Identical particles in classical and QM 5. Exchange degeneracy - the fundamental problem
Lecture #3. Incorporating a vector potential into the Hamiltonian. Spin postulates 3. Description of spin states 4. Identical particles in classical and QM 5. Exchange degeneracy - the fundamental problem
Magnetism in low dimensions from first principles. Atomic magnetism. Gustav Bihlmayer. Gustav Bihlmayer
 IFF 10 p. 1 Magnetism in low dimensions from first principles Atomic magnetism Gustav Bihlmayer Institut für Festkörperforschung, Quantum Theory of Materials Gustav Bihlmayer Institut für Festkörperforschung
IFF 10 p. 1 Magnetism in low dimensions from first principles Atomic magnetism Gustav Bihlmayer Institut für Festkörperforschung, Quantum Theory of Materials Gustav Bihlmayer Institut für Festkörperforschung
EXPERIMENT 15. USING CONDUCTIVITY TO LOOK AT SOLUTIONS: DO WE HAVE CHARGED IONS OR NEUTRAL MOLECULES? rev 7/09
 EXPERIMENT 15 USING CONDUCTIVITY TO LOOK AT SOLUTIONS: DO WE AVE CARGED IONS OR NEUTRAL MOLECULES? rev 7/09 GOAL After you complete this experiment, you should have a better understanding of aqueous solutions
EXPERIMENT 15 USING CONDUCTIVITY TO LOOK AT SOLUTIONS: DO WE AVE CARGED IONS OR NEUTRAL MOLECULES? rev 7/09 GOAL After you complete this experiment, you should have a better understanding of aqueous solutions
Quantum Physics II (8.05) Fall 2002 Assignment 11
 Quantum Physics II (8.05) Fall 00 Assignment 11 Readings Most of the reading needed for this problem set was already given on Problem Set 9. The new readings are: Phase shifts are discussed in Cohen-Tannoudji
Quantum Physics II (8.05) Fall 00 Assignment 11 Readings Most of the reading needed for this problem set was already given on Problem Set 9. The new readings are: Phase shifts are discussed in Cohen-Tannoudji
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department. Experiment 03: Work and Energy
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01 Fall Term 2010 Experiment 03: Work and Energy Purpose of the Experiment: In this experiment you allow a cart to roll down an inclined
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01 Fall Term 2010 Experiment 03: Work and Energy Purpose of the Experiment: In this experiment you allow a cart to roll down an inclined
A VERY BRIEF LINEAR ALGEBRA REVIEW for MAP 5485 Introduction to Mathematical Biophysics Fall 2010
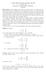 A VERY BRIEF LINEAR ALGEBRA REVIEW for MAP 5485 Introduction to Mathematical Biophysics Fall 00 Introduction Linear Algebra, also known as matrix theory, is an important element of all branches of mathematics
A VERY BRIEF LINEAR ALGEBRA REVIEW for MAP 5485 Introduction to Mathematical Biophysics Fall 00 Introduction Linear Algebra, also known as matrix theory, is an important element of all branches of mathematics
QM and Angular Momentum
 Chapter 5 QM and Angular Momentum 5. Angular Momentum Operators In your Introductory Quantum Mechanics (QM) course you learned about the basic properties of low spin systems. Here we want to review that
Chapter 5 QM and Angular Momentum 5. Angular Momentum Operators In your Introductory Quantum Mechanics (QM) course you learned about the basic properties of low spin systems. Here we want to review that
Vibrations of Carbon Dioxide and Carbon Disulfide
 Vibrations of Carbon Dioxide and Carbon Disulfide Purpose Vibration frequencies of CO 2 and CS 2 will be measured by Raman and Infrared spectroscopy. The spectra show effects of normal mode symmetries
Vibrations of Carbon Dioxide and Carbon Disulfide Purpose Vibration frequencies of CO 2 and CS 2 will be measured by Raman and Infrared spectroscopy. The spectra show effects of normal mode symmetries
BCMB/CHEM 8190 Lab Exercise Using Maple for NMR Data Processing and Pulse Sequence Design March 2012
 BCMB/CHEM 8190 Lab Exercise Using Maple for NMR Data Processing and Pulse Sequence Design March 2012 Introduction Maple is a powerful collection of routines to aid in the solution of mathematical problems
BCMB/CHEM 8190 Lab Exercise Using Maple for NMR Data Processing and Pulse Sequence Design March 2012 Introduction Maple is a powerful collection of routines to aid in the solution of mathematical problems
Nuclear Shell Model. P461 - Nuclei II 1
 Nuclear Shell Model Potential between nucleons can be studied by studying bound states (pn, ppn, pnn, ppnn) or by scattering cross sections: np -> np pp -> pp nd -> nd pd -> pd If had potential could solve
Nuclear Shell Model Potential between nucleons can be studied by studying bound states (pn, ppn, pnn, ppnn) or by scattering cross sections: np -> np pp -> pp nd -> nd pd -> pd If had potential could solve
Application Note. Capillary Zone Electrophoresis
 Application Note Capillary Zone Electrophoresis i Application Note: Capillary Zone Electrophoresis Version 8/PC Part Number 30-090-101 September 2008 Copyright IntelliSense Software Corporation 2004, 2005,
Application Note Capillary Zone Electrophoresis i Application Note: Capillary Zone Electrophoresis Version 8/PC Part Number 30-090-101 September 2008 Copyright IntelliSense Software Corporation 2004, 2005,
NAG Library Routine Document F02HDF.1
 F02 Eigenvalues and Eigenvectors NAG Library Routine Document Note: before using this routine, please read the Users Note for your implementation to check the interpretation of bold italicised terms and
F02 Eigenvalues and Eigenvectors NAG Library Routine Document Note: before using this routine, please read the Users Note for your implementation to check the interpretation of bold italicised terms and
MAT300/500 Programming Project Spring 2019
 MAT300/500 Programming Project Spring 2019 Please submit all project parts on the Moodle page for MAT300 or MAT500. Due dates are listed on the syllabus and the Moodle site. You should include all neccessary
MAT300/500 Programming Project Spring 2019 Please submit all project parts on the Moodle page for MAT300 or MAT500. Due dates are listed on the syllabus and the Moodle site. You should include all neccessary
Multi-Electron Atoms II
 Multi-Electron Atoms II LS Coupling The basic idea of LS coupling or Russell-Saunders coupling is to assume that spin-orbit effects are small, and can be neglected to a first approximation. If there is
Multi-Electron Atoms II LS Coupling The basic idea of LS coupling or Russell-Saunders coupling is to assume that spin-orbit effects are small, and can be neglected to a first approximation. If there is
Particle Physics. Michaelmas Term 2009 Prof Mark Thomson. Handout 7 : Symmetries and the Quark Model. Introduction/Aims
 Particle Physics Michaelmas Term 2009 Prof Mark Thomson Handout 7 : Symmetries and the Quark Model Prof. M.A. Thomson Michaelmas 2009 205 Introduction/Aims Symmetries play a central role in particle physics;
Particle Physics Michaelmas Term 2009 Prof Mark Thomson Handout 7 : Symmetries and the Quark Model Prof. M.A. Thomson Michaelmas 2009 205 Introduction/Aims Symmetries play a central role in particle physics;
129 Lecture Notes More on Dirac Equation
 19 Lecture Notes More on Dirac Equation 1 Ultra-relativistic Limit We have solved the Diraction in the Lecture Notes on Relativistic Quantum Mechanics, and saw that the upper lower two components are large
19 Lecture Notes More on Dirac Equation 1 Ultra-relativistic Limit We have solved the Diraction in the Lecture Notes on Relativistic Quantum Mechanics, and saw that the upper lower two components are large
Linear Motion with Constant Acceleration
 Linear Motion 1 Linear Motion with Constant Acceleration Overview: First you will attempt to walk backward with a constant acceleration, monitoring your motion with the ultrasonic motion detector. Then
Linear Motion 1 Linear Motion with Constant Acceleration Overview: First you will attempt to walk backward with a constant acceleration, monitoring your motion with the ultrasonic motion detector. Then
ECEN 5005 Crystals, Nanocrystals and Device Applications Class 20 Group Theory For Crystals
 ECEN 5005 Crystals, Nanocrystals and Device Applications Class 20 Group Theory For Crystals Laporte Selection Rule Polarization Dependence Spin Selection Rule 1 Laporte Selection Rule We first apply this
ECEN 5005 Crystals, Nanocrystals and Device Applications Class 20 Group Theory For Crystals Laporte Selection Rule Polarization Dependence Spin Selection Rule 1 Laporte Selection Rule We first apply this
Instructions for use of molly to prepare files for Doppler or Modmap
 Instructions for use of molly to prepare files for Doppler or Modmap In the following instructions, computer output is in small type, prompts end with : or >, comments are in boldface and that which the
Instructions for use of molly to prepare files for Doppler or Modmap In the following instructions, computer output is in small type, prompts end with : or >, comments are in boldface and that which the
Physics 212E Spring 2004 Classical and Modern Physics. Computer Exercise #2
 Physics 212E Spring 2004 Classical and Modern Physics Chowdary Computer Exercise #2 Launch Mathematica by clicking on the Start menu (lower left hand corner of the screen); from there go up to Science
Physics 212E Spring 2004 Classical and Modern Physics Chowdary Computer Exercise #2 Launch Mathematica by clicking on the Start menu (lower left hand corner of the screen); from there go up to Science
Login -the operator screen should be in view when you first sit down at the spectrometer console:
 Lab #2 1D 1 H Double Resonance (Selective Decoupling) operation of the 400 MHz instrument using automated sample insertion (robot) and automated locking and shimming collection of 1D 1 H spectra retrieving
Lab #2 1D 1 H Double Resonance (Selective Decoupling) operation of the 400 MHz instrument using automated sample insertion (robot) and automated locking and shimming collection of 1D 1 H spectra retrieving
ARTICLE IN PRESS. Journal of Alloys and Compounds xxx (2008) xxx xxx. Contents lists available at ScienceDirect. Journal of Alloys and Compounds
 Journal of Alloys and Compounds xxx (2008) xxx xxx Contents lists available at ScienceDirect Journal of Alloys and Compounds journal homepage: www.elsevier.com/locate/jallcom Intensity parametrizations
Journal of Alloys and Compounds xxx (2008) xxx xxx Contents lists available at ScienceDirect Journal of Alloys and Compounds journal homepage: www.elsevier.com/locate/jallcom Intensity parametrizations
Chem 673, Problem Set 5 Due Thursday, November 29, 2007
 Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
CHM Physical Chemistry II Chapter 9 - Supplementary Material. 1. Constuction of orbitals from the spherical harmonics
 CHM 3411 - Physical Chemistry II Chapter 9 - Supplementary Material 1. Constuction of orbitals from the spherical harmonics The wavefunctions that are solutions to the time independent Schrodinger equation
CHM 3411 - Physical Chemistry II Chapter 9 - Supplementary Material 1. Constuction of orbitals from the spherical harmonics The wavefunctions that are solutions to the time independent Schrodinger equation
