Construction of Adjacent Quaternary and Tertiary Stereocenters via an Organocatalytic Allylic Alkylation of Morita Baylis Hillman Carbonates
|
|
|
- Cecily Eaton
- 5 years ago
- Views:
Transcription
1 DOI: /adsc Construction of Adjacent Quaternary and Tertiary Stereocenters via an Organocatalytic Allylic Alkylation of Morita Baylis Hillman Carbonates Dirk Jan V. C. van Steenis, a Tommaso Marcelli, a Martin Lutz, b Anthony L. Spek, b Jan H. van Maarseveen, a andhenk Hiemstra a, * a b Van t Hoff Institute for Molecular Sciences, University of Amsterdam, Nieuwe Achtergracht 129, 1018 WS Amsterdam, The Netherlands Fax: (+ 31) ; hiemstra@science.uva.nl Bijvoet Center for Biomolecular Research, Crystal andstructural Chemistry, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands Received: September 12, 2006 Supporting information for this article is available on the WWW under Abstract: Racemic Baylis Hillman carbonates can be convertedin densely functionalizedproducts by reaction with cyano esters in the presence of a catalytic amount of a modified Cinchona alkaloidin high enantioselectivities andfair diastereoselectivities. A rational for the observedstereoselectivity is presented. Keywords: allylic substitution; asymmetric catalysis; Baylis Hillman reaction; Cinchona alkaloids; hydrogen bond The enantioselective construction of all-carbon quaternary stereocenters still represents a major challenge in synthetic organic chemistry. [1] The simultaneous generation of two adjacent quaternary and tertiary stereocenters is even more problematic and, to date, only few examples have been reported, all of them relying on conjugate additions. [2] The Morita Baylis Hillman (MBH) reaction has attractedthe attention of many research groups, due to the possibility of accessing densely functionalized products in one step. [3] In the last decade, these efforts have resultedin great progress being made in terms of rate, scope andenantioselectivity. Upon conversion of the alcohol to a leaving group, MBH adducts become substrates for asymmetric allylic substitution. Trost andco-workers successfully appliedthis strategy to the synthesis of natural products employing chiral palladium catalysts in the dynamic kinetic resolution of MBH acetates. [4] More recently, other groups investigatedthe feasibility of this reaction with an (asymmetric) nucleophilic organocatalyst, [5] relying on a tandem S N 2 /S N 2 substitution sequence (Scheme 1). DABCO [6] andtriphenylphosphine [7] were shown to catalyze the allylic substitution of MBH adducts with goodregioselectivity. Krische et al. reportedthat commercially available Cl-OMe-BIPHEP promotes the amination of MBH acetates with phthalimide in 56 % ee. [8] Cinchona derivatives were also employed as catalysts for relatedtransformations: (DHQD) 2 PHAL was usedin the asymmetric hydrolysis of MBH acetates with sodium bicarbonate, giving medium to high levels of asymmetric induction (up to 84 % ee). [9] Lu andco-workers investigatedthe reaction of MBH carbonates with a variety of nucleophiles in the presence of a catalytic amount of b-isocupreidine (b-icpd), [10] a strained quinidine derivative that was earlier used by Hatakeyama et al. to achieve excellent levels of enantioselection in the parent MBH reaction of aldehydes and activated acrylates. [11] Remarkably, besides Scheme 1. Organocatalytic vs. Pd-catalyzed allylic substitution. Adv. Synth. Catal. 2007, 349, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 281
2 Dirk Jan V. C. van Steenis et al. Table 1. Substrate scope for the organocatalytic allylic alkylation. [a] Entry Product T [8C] t [h] Yield[%] [b] dr [c] ee [%] [d] : : : : [e] 4: [f] 3:1 80 [a] [b] [c] [d] [e] [f] Reaction conditions: 0.6 mmol 1, 0.3 mmol 2, 0.06 mmol b-icpd in 6 ml toluene. Isolatedyield(mixture of diastereomers). Determinedby 1 H NMR analysis of the crude reaction mixture. The ee value of the major diastereoisomer was determined by chiral HPLC. Compound 3e was obtainedas a pure diasteromer. Reaction performedon 9.0 mmol of 1f Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Adv. Synth. Catal. 2007, 349,
3 Construction of Adjacent Quaternary and Tertiary Stereocenters COMMUNICATIONS Scheme 2. Reaction of an MBH carbonate with dimethyl malonate. nitrogen andoxygen nucleophiles, the authors reportedthe reaction of an MBH carbonate with dimethyl malonate. Although the enantioselectivity was moderate (51 % ee), Lu andco-workers establishedfor the first time the possibility of using carbon nucleophiles for an organocatalytic allylic alkylation. In this communication we report our investigations on the same reaction using prochiral tertiary nucleophiles thus leading to products with two adjacent quaternary/tertiary stereocenters. In our preliminary experiments, we re-investigated the reaction of carbonate 1a with dimethyl malonate in the presence of b-icpd: although screening of different reaction conditions (solvent, concentration, temperature) did not improve the results reported by Lu, we foundthat the employment of a methyl ester as the electrophile resultedin slightly higher levels of asymmetric induction (63 vs. 51 % ee, Scheme 2). Next, we examinedthe reaction of compound1a with highly reactive ethyl a,a-cyanophenylacetate (2a) in toluene at room temperature. The reaction went to completion in less than 24 h with high regioselectivity yet moderate stereoselectivity (dr 3:1, 68% ee for the major diastereomer). Employment of an excess of compound 1a gave a strong rate enhancement, probably due to a kinetic resolution effect (see later), allowing the reaction to be carriedout at a lower temperature. Gratifyingly, performing the reaction at 208C with 20 mol% of b-icpd resultedin full conversion to the adduct 3a in 83% ee (dr 4:1). We then examineddifferent combinations of reaction partners (Table 1). In general, compounds 3 were obtainedin high yields andgoodenantioselectivities. Substrate 1d gave disappointing results, possibly due to its high reactivity towards conjugate addition. Hindered substrates (1b and 1e, entries 2 and5) required slightly higher temperatures to obtain satisfying reaction rates; fortunately, the level of enantioselection was not negatively affected. Figure 1. Displacement ellipsoidplot of compound3f in the crystal, drawn at the 50 % probability level. Scheme 3. Kinetic resolution of MBH carbonate 1f. As some adducts were obtained as crystalline solids after chromatography, we ran a reaction on a gram scale with the aim to upgrade the optical purity of the product via recrystallization. Hence, compound 1f (3.3 g, 9.0 mmol) was reactedwith cyanoacetate 2a (0.78 ml, 4.5 mmol) to afford, after chromatography, adduct 3f in 80 % ee and95 % yield(entry 7). A single recrystallization from cyclohexane afforded compound 3f (59 % yield, calculated on cyanoacetate 2a) with 98 % ee as a pure diastereomer. [12] X-ray analysis of these crystals allowedan unambiguous assignment of the absolute andrelative configuration (Figure 1). [13] Interestingly, HPLC analysis of the recoveredstarting material (1f) gave evidence for a kinetic resolution effect (Scheme 3). Compound 1f was isolated from the crude mixture after column chromatography in 90% ee. Recrystallization afforded enantiopure crystals (47 % yieldbasedon 2a) whose absolute con- Adv. Synth. Catal. 2007, 349, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 283
4 Dirk Jan V. C. van Steenis et al. Figure 2. Displacement ellipsoidplot of compound1f in the crystal, drawn at the 50 % probability level. figuration couldbe determinedwith X-ray spectroscopy (Figure 2). As suggestedby Kim andco-workers, [9] who observeda similar effect, this can be explainedin terms of a kinetic resolution in the first step. This process, however, does not affect the stereoselectivity of the second conjugate addition generating the C C bond. To confirm that we examinedthe reaction of 1 equivalent of 1a with 2 equivalents of 2a at room temperature (hence reversing the reagents ratio): after 24 h, 1a was completely convertedto 3a with similar stereoselectivity (68 % ee, 3:1 dr), showing that both enantiomers of the MBH carbonate undergo Michael addition although with different rates, followed by elimination of the leaving group leading to the same intermediate. Next, we investigatedthe role of the C-6 -OH of b- ICPD, which was shown to be fundamental in the Baylis Hillman reaction. [11] When b-isoquinidine (b- IQD) was usedas the catalyst, less than 5 % conversion was observedafter two weeks. Furthermore, substrate 4, featuring a cyano group (a poorer hydrogen bondacceptor) insteadof a methyl ester, underwent allylic substitution giving adduct 5 in high yieldbut no asymmetric induction was observed (Scheme 4). On the basis of all these observations, we propose a tentative hypothesis to rationalize our experimental results (Scheme 5). First, substrate 1a undergoes a conjugate addition to form adduct A. Subsequently, elimination of the OBoc group, leading to the formation of CO 2 and tert-butoxide anion provides Michael acceptor B. We believe that this irreversible reaction step is responsible for the observedkinetic resolution. As shown in the experiment with b-isoquinidine, the presence of the C-6 -OH in the catalyst is requiredfor activation of the ester prior to conjugate addition. As already proposed by Lu, [10] the tert-butoxide anion de- Scheme 4. Effects of the structure of catalyst andsubstrate. protonates cyanoacetate 2a which, in turn, attacks the a,b-unsaturatedintermediate B, present as the E isomer, as previously shown by Kim andco-workers. [9] Probably, an intramolecular hydrogen bond between the phenolic OH andthe ester moiety is responsible for the face shielding conferring stereoselectivity to the reaction; a DFT-minimizedstructure of B supporting this hypothesis is depicted in Figure 3. Elimination of the catalyst from intermediate C liberates alkylatedproduct 3a, closing the catalytic cycle. In conclusion, we have developed a new methodology to access highly functionalizedcompounds featuring two adjacent stereocenters, one of them bearing four carbon substituents. We also demonstrated for one example that the same reaction can be exploited as a kinetic resolution to obtain highly enantioenrichedmbh carbonates. Experimental Section General Procedure for the Asymmetric Allylic Alkylation of MBH Carbonates (1a f and 4) b-icpd (20 mol %) was added to the total volume of toluene andthe resulting suspension was heatedandstirred until formation of a clear solution. Subsequently, the mixture was allowedto cool to the indicatedtemperature. The MBH carbonate (1 a f or 4) was added to the reaction mixture andthe mixture was stirreduntil complete dissolution (~5 10 min). Cyanoacetate (2a or b) was added in one portion andthe mixture was kept at the indicatedtemperature for the described reaction time. The experiments were stop Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Adv. Synth. Catal. 2007, 349,
5 Construction of Adjacent Quaternary and Tertiary Stereocenters COMMUNICATIONS Scheme 5. Proposedmechanism. Figure 3. Minimizedstructure of intermediate B (B3LYP/6-31G**). pedby filtration of the reaction mixture over a short padof silica gel andelution with Et 2 O. Solvents were removedby rotary evaporation andthe crude mixture was purifiedby column chromatography. Acknowledgements The Dutch National Research School Combination: Catalysis (NRSC-C) is gratefully acknowledged for financial support. Adv. Synth. Catal. 2007, 349, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 285
6 References [1] For recent reviews, see: a) B. M. Trost, C. Jiang, Synthesis 2006, ; b) J. Christoffers, A. Baro, Adv. Synth. Cat. 2005, 347, ; c) C. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, [2] a) F. Wu, R. Hong, J. Khan, X. Liu, L. Deng, Angew. Chem. Int. Ed. 2006, 45, ; b) F. Lu, H. Li, R. Hong, L. Deng, Angew. Chem. Int. Ed. 2006, 45, ; c) H. Li, Y. Wang, L. Tang, F. Wu, X. Liu, C. Guo, B. M. Foxman, L. Deng, Angew. Chem. Int. Ed. 2005, 44, ; d) J. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao, D. W. C. MacMillan, Proc. Natl. Acad. Sci. USA 2004, 101, 5482; e) M. S. Taylor, E. N. Jacobsen, J. Am. Chem. Soc. 2003, 125, 11204; f) T. Okino, Y. Hoashi, T. Furukawa, X. Xu, Y. Takemoto, J. Am. Chem. Soc. 2005, 127, [3] For an authoritative review, see: a) D. Basavaiah, A. J. Rao, T. Satyanarayana, Chem. Rev. 2003, 103, ; for a short review on manipulations of MBH adducts, see: b) K. Y. Lee, S. Gowrisankar, J. N. Kim, Bull. Korean Chem. Soc. 2005, 26, [4] a) B. M. Trost, M. R. Machacek, H.-C. Tsui, J. Am. Chem. Soc. 2005, 127, ; b) O. R. Thiel, B. M. Trost, H.-C. Tsui, J. Am. Chem. Soc. 2003, 125, ; c) B. M. Trost, O. R. Thiel, H.-C. Tsui, J. Am. Chem. Soc. 2002, 124, [5] For reviews, see: a) S. France, D. J. Guerin, S. J. Miller, T. Lectka, Chem. Rev. 2003, 103, (nucleophilic amines); b) J. L. Methot, W. R. Roush, Adv. Dirk Jan V. C. van Steenis et al. Synth. Cat. 2004, 346, (nucleophilic phosphines). [6] a) J. H. Gong, H. R. Kim, E. K. Ryu, J. N. Kim, Bull. Korean Chem. Soc. 2002, 23, 789; b) J.-N. Kim, H.-J. Lee, K.-Y. Lee, J.-H. Gong, Synlett 2002, 173. [7] C.-W. Cho, M. J. Krische, Angew. Chem. Int. Ed. 2004, 43, [8] C.-W. Cho, J.-R. Kong, M. J. Krische, Org. Lett. 2004, 6, [9] J.-N. Kim, H.-J. Lee, J.-H. Gong, Tetrahedron Lett. 2002, 43, [10] Y. Du, X. Han. X. Lu, Tetrahedron Lett. 2004, 45, [11] a) Y. Iwabuchi, M. Nakatani, N. Yokoyama, S. Hatakeyama, J. Am. Chem. Soc. 1999, 121, ; b) A. Nakano, S. Kawahara, S. Akamatsu, K. Morokuma, M. Nakatani, Y. Iwabuchi, K. Takahashi, J. Ishihara, S. Hatakeyama, Tetrahedron 2005, 62, [12] Similar results were obtainedwith carbonate 1a: adduct 3a was obtainedas a single diastereomer in 63 % yieldand99 % ee after recrystallization; see Supporting Information for details. [13] Experimental details of the crystal structure determinations for 3f, 1f, and 3a are given in the Supporting Information. CCDC (compound 3f), CCDC (1f) andccdc (3 ) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via ac.uk/data request/cif Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Adv. Synth. Catal. 2007, 349,
The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts. Larry Wolf SED Group Meeting
 The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts Larry Wolf SED Group Meeting 04-10-07 Outline Brief historical account and Utility Mechanism Different methods for asymmetric
The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts Larry Wolf SED Group Meeting 04-10-07 Outline Brief historical account and Utility Mechanism Different methods for asymmetric
Morita Baylis Hillman Reaction. Aaron C. Smith 11/10/04
 Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
Phosphine-Catalyzed Formation of Carbon-Sulfur Bonds: Catalytic Asymmetric Synthesis of gamma-thioesters
 Phosphine-Catalyzed Formation of Carbon-Sulfur Bonds: Catalytic Asymmetric Synthesis of gamma-thioesters The MIT Faculty has made this article openly available. Please share how this access benefits you.
Phosphine-Catalyzed Formation of Carbon-Sulfur Bonds: Catalytic Asymmetric Synthesis of gamma-thioesters The MIT Faculty has made this article openly available. Please share how this access benefits you.
Bifunctional Asymmetric Catalysts: Design and Applications. Junqi Li CHEM Sep 2010
 Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
Stereodivergent Catalysis. Aragorn Laverny SED Group Meeting July
 Stereodivergent Catalysis Aragorn Laverny SED Group Meeting July 31 2018 1 Stereodivergent Catalysis In the context of asymmetric synthesis, a stereodivergent process is one that allows access to any given
Stereodivergent Catalysis Aragorn Laverny SED Group Meeting July 31 2018 1 Stereodivergent Catalysis In the context of asymmetric synthesis, a stereodivergent process is one that allows access to any given
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Development of Small Organic Molecules as Catalysts for Asymmetric
 Development of Small Organic Molecules as Catalysts for Asymmetric Organic Transformations The development of new and efficient catalysts capable of catalyzing enantioselective transformation in a controlled
Development of Small Organic Molecules as Catalysts for Asymmetric Organic Transformations The development of new and efficient catalysts capable of catalyzing enantioselective transformation in a controlled
Mechanistic Implications in the Morita Baylis Hillman Alkylation: Isolation and Characterization of an Intermediate
 Mechanistic Implications in the Morita Baylis Hillman Alkylation: Isolation and Characterization of an Intermediate M. E. Krafft,* T. F. N. Haxell, K. A. Seibert, and K. A. Abboud Department of Chemistry
Mechanistic Implications in the Morita Baylis Hillman Alkylation: Isolation and Characterization of an Intermediate M. E. Krafft,* T. F. N. Haxell, K. A. Seibert, and K. A. Abboud Department of Chemistry
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions
 Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
The Synthesis of Molecules Containing Quaternary Stereogenic Centers via the Intramolecular Asymmetric Heck Reaction
 The Synthesis of Molecules Containing Quaternary Stereogenic Centers via the Intramolecular Asymmetric Heck Reaction Reported by Eric P. Gillis April 19, 2007 INTRODUCTION The enantioselective synthesis
The Synthesis of Molecules Containing Quaternary Stereogenic Centers via the Intramolecular Asymmetric Heck Reaction Reported by Eric P. Gillis April 19, 2007 INTRODUCTION The enantioselective synthesis
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Organocatalytic Doubly Annulative Approach to 3,4-Dihydrocoumarins Bearing a Fused Pyrrolidine Scaffold. Dorota Kowalczyk, and Łukasz Albrecht*
 Organocatalytic Doubly Annulative Approach to 3,4-Dihydrocoumarins Bearing a Fused Pyrrolidine Scaffold Dorota Kowalczyk, and Łukasz Albrecht* Institute of Organic Chemistry, Chemistry Department, Lodz
Organocatalytic Doubly Annulative Approach to 3,4-Dihydrocoumarins Bearing a Fused Pyrrolidine Scaffold Dorota Kowalczyk, and Łukasz Albrecht* Institute of Organic Chemistry, Chemistry Department, Lodz
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Supporting information. Direct Enantioselective Aldol Reactions catalyzed by a Proline-Thiourea Host- Guest Complex
 Supporting information Direct Enantioselective Aldol Reactions catalyzed by a Proline-Thiourea Host- Guest Complex Ömer Reis, Serkan Eymur, Barbaros Reis, Ayhan S. Demir* Department of Chemistry, Middle
Supporting information Direct Enantioselective Aldol Reactions catalyzed by a Proline-Thiourea Host- Guest Complex Ömer Reis, Serkan Eymur, Barbaros Reis, Ayhan S. Demir* Department of Chemistry, Middle
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet Spengler Reaction of Indolyl Dihydropyridines
 Literature Report V Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet Spengler Reaction of Indolyl Dihydropyridines Reporter Checker Date : Xiao-Yong Zhai : Xin-Wei Wang : 2018-04-02 You,
Literature Report V Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet Spengler Reaction of Indolyl Dihydropyridines Reporter Checker Date : Xiao-Yong Zhai : Xin-Wei Wang : 2018-04-02 You,
Chiral Ionic Liquids (CILs) in Asymmetric Synthesis: The story so far.
 Chiral Ionic Liquids (CILs) in Asymmetric Synthesis: The story so far. Literature Presentation Aman Desai 06.16.06 1. Angew. Chem. Int. Ed. 2006, 45, 3689 2. Angew. Chem. Int. Ed. 2006, 45, 3093 3. Tetrahedron:
Chiral Ionic Liquids (CILs) in Asymmetric Synthesis: The story so far. Literature Presentation Aman Desai 06.16.06 1. Angew. Chem. Int. Ed. 2006, 45, 3689 2. Angew. Chem. Int. Ed. 2006, 45, 3093 3. Tetrahedron:
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chiral phosphine Lewis bases in catalytic, asymmetric aza-morita Baylis Hillman reaction*
 Pure Appl. Chem., Vol. 77, No. 12, pp. 2105 2110, 2005. DOI: 10.1351/pac200577122105 2005 IUPAC Chiral phosphine Lewis bases in catalytic, asymmetric aza-morita Baylis Hillman reaction* Min Shi and Lian-Hui
Pure Appl. Chem., Vol. 77, No. 12, pp. 2105 2110, 2005. DOI: 10.1351/pac200577122105 2005 IUPAC Chiral phosphine Lewis bases in catalytic, asymmetric aza-morita Baylis Hillman reaction* Min Shi and Lian-Hui
Efficient enantioselective hydrogenation of quinolines catalyzed by conjugated microporous polymers with embedded chiral BINAP ligand
 Chinese Journal of Catalysis 36 (2015) 1170 1174 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/chnjc Communication Efficient enantioselective hydrogenation of quinolines
Chinese Journal of Catalysis 36 (2015) 1170 1174 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/chnjc Communication Efficient enantioselective hydrogenation of quinolines
Hai-Bin Yang, Xing Fan, Yin Wei,* Min Shi*
 Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2015 Solvent-controlled Nucleophilic Trifloromethylthiolation of Morita- Baylis-Hillman
Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2015 Solvent-controlled Nucleophilic Trifloromethylthiolation of Morita- Baylis-Hillman
Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide
 General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
Chiral Brønsted Acid Catalysis
 another. 1 One interesting aspect of chiral Brønsted acid catalysis is that the single s orbital of hydrogen Chiral Brønsted Acid Catalysis Reported by Matthew T. Burk December 3, 2007 INTRODUCTION The
another. 1 One interesting aspect of chiral Brønsted acid catalysis is that the single s orbital of hydrogen Chiral Brønsted Acid Catalysis Reported by Matthew T. Burk December 3, 2007 INTRODUCTION The
An Efficient Synthesis of Poly-Substituted Phenols and Pyridines from Morita-Baylis-Hillman Acetates and Diethyl Oxalacetate
 An Efficient Synthesis of Poly-Substituted Phenols and Pyridines Bull. Korean Chem. Soc. 2013, Vol. 34, No. 10 3027 http://dx.doi.org/10.5012/bkcs.2013.34.10.3027 An Efficient Synthesis of Poly-Substituted
An Efficient Synthesis of Poly-Substituted Phenols and Pyridines Bull. Korean Chem. Soc. 2013, Vol. 34, No. 10 3027 http://dx.doi.org/10.5012/bkcs.2013.34.10.3027 An Efficient Synthesis of Poly-Substituted
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones and Aldehydes
 A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Tsuji Trost N-Allylation with Allylic Acetates by Using a Cellulose Palladium Catalyst
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Environmental Protection Agency Papers U.S. Environmental Protection Agency 2012 Tsuji Trost N-Allylation with Allylic
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Environmental Protection Agency Papers U.S. Environmental Protection Agency 2012 Tsuji Trost N-Allylation with Allylic
Supporting Information
 Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information for Chiral Brönsted Acid Catalyzed Asymmetric Baeyer-Villiger Reaction of 3-Substituted Cyclobutanones Using Aqueous
Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information for Chiral Brönsted Acid Catalyzed Asymmetric Baeyer-Villiger Reaction of 3-Substituted Cyclobutanones Using Aqueous
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Supporting Information 1. Rhodium-catalyzed asymmetric hydroalkoxylation and hydrosufenylation of diphenylphosphinylallenes
 Supporting Information 1 Rhodium-catalyzed asymmetric hydroalkoxylation and hydrosufenylation of diphenylphosphinylallenes Takahiro Kawamoto, Sho Hirabayashi, Xun-Xiang Guo, Takahiro Nishimura,* and Tamio
Supporting Information 1 Rhodium-catalyzed asymmetric hydroalkoxylation and hydrosufenylation of diphenylphosphinylallenes Takahiro Kawamoto, Sho Hirabayashi, Xun-Xiang Guo, Takahiro Nishimura,* and Tamio
Asymmetric Catalysis by Chiral Hydrogen-Bond Donors
 Asymmetric Catalysis by Chiral Hydrogen-Bond Donors Angew. Chem. Int. Ed., 2006, 45, 1520~1543 Mark S. Taylor and Eric N. Jacobsen* Current Literature Presentation Zhenglai Fang Wipf s Group at Pitt Zhenglai
Asymmetric Catalysis by Chiral Hydrogen-Bond Donors Angew. Chem. Int. Ed., 2006, 45, 1520~1543 Mark S. Taylor and Eric N. Jacobsen* Current Literature Presentation Zhenglai Fang Wipf s Group at Pitt Zhenglai
Dual enantioselective control by heterocycles of (S)-indoline derivatives*
 Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown.
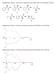 Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Table of Contents 1. General procedure for the chiral phosphoric acid catalyzed asymmetric reductive amination using benzothiazoline
 Enantioselective Organocatalytic Reductive Amination of Aliphatic Ketones by Benzothiazoline as Hydrogen Donor Kodai Saito, Takahiko Akiyama* Department of Chemistry, Faculty of Science, Gakushuin University,
Enantioselective Organocatalytic Reductive Amination of Aliphatic Ketones by Benzothiazoline as Hydrogen Donor Kodai Saito, Takahiko Akiyama* Department of Chemistry, Faculty of Science, Gakushuin University,
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes*
 Pure Appl. Chem., Vol. 80, No. 5, pp. 1161 1166, 2008. doi:10.1351/pac200880051161 2008 IUPAC Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes* Naofumi Tsukada, Satoshi
Pure Appl. Chem., Vol. 80, No. 5, pp. 1161 1166, 2008. doi:10.1351/pac200880051161 2008 IUPAC Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes* Naofumi Tsukada, Satoshi
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Experiment #3: Asymmetric Synthesis Use of a Chiral Manganese Catalyst for the Enantioselective Epoxidation of Alkenes
 Experiment #3: Asymmetric Synthesis Use of a Chiral Manganese Catalyst for the Enantioselective Epoxidation of Alkenes INTRODUCTION Chemists have discovered and developed many elegant and synthetically
Experiment #3: Asymmetric Synthesis Use of a Chiral Manganese Catalyst for the Enantioselective Epoxidation of Alkenes INTRODUCTION Chemists have discovered and developed many elegant and synthetically
Department of Chemistry, University of Saskatchewan Saskatoon SK S7N 4C9, Canada. Wipf Group. Tyler E. Benedum Current Literature February 26, 2005
 Ward, D.E; Jheengut, V.; Akinnusi, O.T. Enantioselective Direct Intermolecular Aldol Reactions with Enantiotopic Group Selectivity and Dynamic Kinetic Resolution, Organic Letters 2005, ASAP. Department
Ward, D.E; Jheengut, V.; Akinnusi, O.T. Enantioselective Direct Intermolecular Aldol Reactions with Enantiotopic Group Selectivity and Dynamic Kinetic Resolution, Organic Letters 2005, ASAP. Department
International Journal of Scientific Research and Reviews
 esearch article Available online www.ijsrr.org ISSN: 2279 0543 International Journal of Scientific esearch and eviews ecent Advances in ate Acceleration of Baylis-Hillman eaction Dandamudi V. Lenin* School
esearch article Available online www.ijsrr.org ISSN: 2279 0543 International Journal of Scientific esearch and eviews ecent Advances in ate Acceleration of Baylis-Hillman eaction Dandamudi V. Lenin* School
Career Review of Dean Toste I. 2015/9/9 Zhi Ren
 Career Review of Dean Toste I 2015/9/9 Zhi Ren Introduction F. Dean Toste, now in UC Berkeley Career: Full Professor since 2009-now Associate Professor 2006-2009 Assistant Professor 2002-2006 Faculty Scientist
Career Review of Dean Toste I 2015/9/9 Zhi Ren Introduction F. Dean Toste, now in UC Berkeley Career: Full Professor since 2009-now Associate Professor 2006-2009 Assistant Professor 2002-2006 Faculty Scientist
Chiral Supramolecular Catalyst for Asymmetric Reaction
 Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Planar-Chiral Phosphine-Olefin Ligands Exploiting a (Cyclopentadienyl)manganese(I) Scaffold to Achieve High Robustness and High Enantioselectivity
 Planar-Chiral Phosphine-Olefin Ligands Exploiting a (Cyclopentadienyl)manganese(I) Scaffold to Achieve High Robustness and High Enantioselectivity Reporter: Cong Liu Checker: Hong-Qiang Shen Date: 2017/02/27
Planar-Chiral Phosphine-Olefin Ligands Exploiting a (Cyclopentadienyl)manganese(I) Scaffold to Achieve High Robustness and High Enantioselectivity Reporter: Cong Liu Checker: Hong-Qiang Shen Date: 2017/02/27
Supporting Information for: Using a Lipase as a High Throughput Screening Method for Measuring the Enantiomeric. Excess of Allylic Acetates
 Supporting Information for: Using a Lipase as a High Throughput Screening Method for Measuring the Enantiomeric Excess of Allylic Acetates M. Burak Onaran and Christopher T. Seto* Department of Chemistry,
Supporting Information for: Using a Lipase as a High Throughput Screening Method for Measuring the Enantiomeric Excess of Allylic Acetates M. Burak Onaran and Christopher T. Seto* Department of Chemistry,
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
CATALYSIS MULTICATALYST SYSTEM IN ASYMMETRIC. Wiley. Department of Chemistry
 MULTICATALYST SYSTEM IN ASYMMETRIC CATALYSIS JIAN ZHOU Shanghai Key Laboratory of Green Chemistry and Chemical Processes Department of Chemistry East China Normal University Shanghai, China Wiley Preface
MULTICATALYST SYSTEM IN ASYMMETRIC CATALYSIS JIAN ZHOU Shanghai Key Laboratory of Green Chemistry and Chemical Processes Department of Chemistry East China Normal University Shanghai, China Wiley Preface
Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers)
 Suggested Reading: Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers) Jones Section 4.9 Physical Properties of Diastereomers: Optical Resolution pages 176-178 Introduction
Suggested Reading: Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers) Jones Section 4.9 Physical Properties of Diastereomers: Optical Resolution pages 176-178 Introduction
Supporting Information. for. Angew. Chem. Int. Ed. Z Wiley-VCH 2003
 Supporting Information for Angew. Chem. Int. Ed. Z53001 Wiley-VCH 2003 69451 Weinheim, Germany 1 Ordered Self-Assembly and Electronic Behavior of C 60 -Anthrylphenylacetylene Hybrid ** Seok Ho Kang 1,
Supporting Information for Angew. Chem. Int. Ed. Z53001 Wiley-VCH 2003 69451 Weinheim, Germany 1 Ordered Self-Assembly and Electronic Behavior of C 60 -Anthrylphenylacetylene Hybrid ** Seok Ho Kang 1,
A Novel Bis-Thiourea Organocatalyst for the Asymmetric Aza-HenryReaction
 DOI: 10.1002/adsc.200800214 A Novel Bis-Thiourea Organocatalyst for the Asymmetric Aza-HenryReaction Constantinos Rampalakos a and William D. Wulff a, * a Department of Chemistry, Michigan State University,
DOI: 10.1002/adsc.200800214 A Novel Bis-Thiourea Organocatalyst for the Asymmetric Aza-HenryReaction Constantinos Rampalakos a and William D. Wulff a, * a Department of Chemistry, Michigan State University,
C H Activated Trifluoromethylation
 Literature report C H Activated Trifluoromethylation Reporter:Yan Fang Superior:Prof. Yong Huang Jun. 17 th 2013 Contents Background Trifluoromethylation of sp-hybridized C-H Bonds Trifluoromethylation
Literature report C H Activated Trifluoromethylation Reporter:Yan Fang Superior:Prof. Yong Huang Jun. 17 th 2013 Contents Background Trifluoromethylation of sp-hybridized C-H Bonds Trifluoromethylation
Mild and Efficient Oxidation of Primary and Secondary Alcohols Using NiO 2 /Silica Gel System (Solvent Free)
 ISSN: 0973-4945; CDEN ECJA E- Chemistry http://www.e-journals.net 2011, 8(2), 491-494 Mild and Efficient xidation of Primary and Secondary Alcohols Using Ni 2 /Silica Gel System (Solvent Free) MAMMAD KTI
ISSN: 0973-4945; CDEN ECJA E- Chemistry http://www.e-journals.net 2011, 8(2), 491-494 Mild and Efficient xidation of Primary and Secondary Alcohols Using Ni 2 /Silica Gel System (Solvent Free) MAMMAD KTI
Chiral Ru/PNNP complexes in catalytic and stoichiometric electrophilic O- and F-atom transfer to 1,3-dicarbonyl compounds*
 Pure Appl. Chem., Vol. 78, No. 2, pp. 391 396, 2006. doi:10.1351/pac200678020391 2006 IUPAC Chiral Ru/PNNP complexes in catalytic and stoichiometric electrophilic O- and F-atom transfer to 1,3-dicarbonyl
Pure Appl. Chem., Vol. 78, No. 2, pp. 391 396, 2006. doi:10.1351/pac200678020391 2006 IUPAC Chiral Ru/PNNP complexes in catalytic and stoichiometric electrophilic O- and F-atom transfer to 1,3-dicarbonyl
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Organocatalytic asymmetric synthesis of 3,3-disubstituted oxindoles featuring two heteroatoms at C3 position
 Organocatalytic asymmetric synthesis of 3,3-disubstituted oxindoles featuring two heteroatoms at C3 position Feng Zhou, Xing-Ping Zeng, Chao Wang, Xiao-Li Zhao, and Jian Zhou* [a] Shanghai Key Laboratory
Organocatalytic asymmetric synthesis of 3,3-disubstituted oxindoles featuring two heteroatoms at C3 position Feng Zhou, Xing-Ping Zeng, Chao Wang, Xiao-Li Zhao, and Jian Zhou* [a] Shanghai Key Laboratory
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Chiral Amplification. Literature Talk Fabian Schneider Konstanz, Universität Konstanz
 Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
ummary Manipulating Radicals
 Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
Supporting information. Ni-catalyzed the efficient conversion of phenols protected with 2, 4, 6-trichloro-1, 3, 5- triazine (TCT) to olefins
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting information Ni-catalyzed the efficient conversion of phenols protected with 2, 4, 6-trichloro-1,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting information Ni-catalyzed the efficient conversion of phenols protected with 2, 4, 6-trichloro-1,
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Diphenylprolinol Silyl Ether in Enantioselective, Catalytic Tandem Michael-Henry Reaction for the Control of Four Stereocenters Yujiro Hayashi*,
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Diphenylprolinol Silyl Ether in Enantioselective, Catalytic Tandem Michael-Henry Reaction for the Control of Four Stereocenters Yujiro Hayashi*,
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supporting Information Enantioselective Synthesis of Axially Chiral Vinyl arenes through Palladium-catalyzed
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supporting Information Enantioselective Synthesis of Axially Chiral Vinyl arenes through Palladium-catalyzed
Supporting Information
 Supporting Information Enantioselective Synthesis of 3-Alkynyl-3-Hydroxyindolin-2-ones by Copper-Catalyzed Asymmetric Addition of Terminal Alkynes to Isatins Ning Xu, Da-Wei Gu, Jing Zi, Xin-Yan Wu, and
Supporting Information Enantioselective Synthesis of 3-Alkynyl-3-Hydroxyindolin-2-ones by Copper-Catalyzed Asymmetric Addition of Terminal Alkynes to Isatins Ning Xu, Da-Wei Gu, Jing Zi, Xin-Yan Wu, and
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 208 Supporting Information Cobalt-Catalyzed Regioselective Syntheses of Indeno[2,-c]pyridines
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 208 Supporting Information Cobalt-Catalyzed Regioselective Syntheses of Indeno[2,-c]pyridines
Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
 Pure Appl. Chem., Vol. 76, No. 3, pp. 651 656, 2004. 2004 IUPAC Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
Pure Appl. Chem., Vol. 76, No. 3, pp. 651 656, 2004. 2004 IUPAC Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
SUPPORTING INFORMATION. A simple asymmetric organocatalytic approach to optically active cyclohexenones
 SUPPRTING INFRMATIN A simple asymmetric organocatalytic approach to optically active cyclohexenones Armando Carlone, Mauro Marigo, Chris North, Aitor Landa and Karl Anker Jørgensen* Danish National Research
SUPPRTING INFRMATIN A simple asymmetric organocatalytic approach to optically active cyclohexenones Armando Carlone, Mauro Marigo, Chris North, Aitor Landa and Karl Anker Jørgensen* Danish National Research
Chapter 14. Principles of Catalysis
 Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Theses of PhD dissertation
 Theses of PhD dissertation Reactivity and bifunctionality in organocatalysis. Experimental and theoretical chemistry investigations of the reaction mechanism Eszter Varga Supervisor: Dr. Tibor Soós PhD,
Theses of PhD dissertation Reactivity and bifunctionality in organocatalysis. Experimental and theoretical chemistry investigations of the reaction mechanism Eszter Varga Supervisor: Dr. Tibor Soós PhD,
1. Theoretical Investigation of Mechanisms and Stereoselectivities of Synthetic Organic Reactions
 1. Theoretical Investigation of Mechanisms and Stereoselectivities of Synthetic Organic Reactions 2. Copper Catalyzed One-Pot Synthesis of Multisubstituted Quinolinones Hao Wang Denmark Group Presentation
1. Theoretical Investigation of Mechanisms and Stereoselectivities of Synthetic Organic Reactions 2. Copper Catalyzed One-Pot Synthesis of Multisubstituted Quinolinones Hao Wang Denmark Group Presentation
Organocatalytic asymmetric biomimetic transamination of aromatic ketone to optically active amine
 Organocatalytic asymmetric biomimetic transamination of aromatic ketone to optically active amine Ying Xie, a Hongjie Pan, a Xiao Xiao, a Songlei Li a and Yian Shi* a,b a Beijing National Laboratory for
Organocatalytic asymmetric biomimetic transamination of aromatic ketone to optically active amine Ying Xie, a Hongjie Pan, a Xiao Xiao, a Songlei Li a and Yian Shi* a,b a Beijing National Laboratory for
Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Recent applications of chiral binaphtholderived phosphoric acid in catalytic asymmetric reactions
 Recent applications of chiral binaphtholderived phosphoric acid in catalytic asymmetric reactions 1. Seayad, J.; Seayad, A. M.; List, B. J. Am. Chem. Soc. 2006, ASAP. 2. Storer, R. L.; Carrera, D. E.;
Recent applications of chiral binaphtholderived phosphoric acid in catalytic asymmetric reactions 1. Seayad, J.; Seayad, A. M.; List, B. J. Am. Chem. Soc. 2006, ASAP. 2. Storer, R. L.; Carrera, D. E.;
Supporting information. Enantioselective synthesis of 2-methyl indoline by palladium catalysed asymmetric C(sp 3 )-H activation/cyclisation.
 Supporting information Enantioselective synthesis of 2-methyl indoline by palladium catalysed asymmetric C(sp 3 )-H activation/cyclisation Saithalavi Anas, Alex Cordi and Henri B. Kagan * Institut de Chimie
Supporting information Enantioselective synthesis of 2-methyl indoline by palladium catalysed asymmetric C(sp 3 )-H activation/cyclisation Saithalavi Anas, Alex Cordi and Henri B. Kagan * Institut de Chimie
Supporting Information
 Supporting Information Synthesis of 2-Benzazepines from Benzylamines and MBH Adducts Under Rhodium(III) Catalysis via C(sp 2 ) H Functionalization Ashok Kumar Pandey, a Sang Hoon Han, a Neeraj Kumar Mishra,
Supporting Information Synthesis of 2-Benzazepines from Benzylamines and MBH Adducts Under Rhodium(III) Catalysis via C(sp 2 ) H Functionalization Ashok Kumar Pandey, a Sang Hoon Han, a Neeraj Kumar Mishra,
Enantioselective Borylations. David Kornfilt Denmark Group Meeting Sept. 14 th 2010
 Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Chromium Arene Complexes
 Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
PhD research with Prof. Lutz H. Gade at the Univ. of Strasbourg. Postdoc in 2003 with Andreas Pfaltz (Basel Switzerland)
 idium-catalyzed Asymmetric Isomerization of rimary Allylic Alcohols Mantilli, L., Gerard, D., Torche, S., Besnard, C., Mazet * C. Angew. Chem. Int. Ed., 2009, 48, 1-6 1,n-Glycols as Dialdehyde Equivalents
idium-catalyzed Asymmetric Isomerization of rimary Allylic Alcohols Mantilli, L., Gerard, D., Torche, S., Besnard, C., Mazet * C. Angew. Chem. Int. Ed., 2009, 48, 1-6 1,n-Glycols as Dialdehyde Equivalents
Measuring enzyme (enantio)selectivity
 Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Supporting Information
 Supporting Information Organocatalytic Enantioselective Formal Synthesis of Bromopyrrole Alkaloids via Aza-Michael Addition Su-Jeong Lee, Seok-Ho Youn and Chang-Woo Cho* Department of Chemistry, Kyungpook
Supporting Information Organocatalytic Enantioselective Formal Synthesis of Bromopyrrole Alkaloids via Aza-Michael Addition Su-Jeong Lee, Seok-Ho Youn and Chang-Woo Cho* Department of Chemistry, Kyungpook
Asymmetric Organocatalytic Strecker-Type Reactions of Aliphatic N,N- Dialkylhydrazones
 Asymmetric Organocatalytic Strecker-Type Reactions of Aliphatic N,N- Dialkylhydrazones Aurora Martínez-Muñoz, David Monge,* Eloísa Martín-Zamora, Eugenia Marqués-López, Eleuterio Álvarez, Rosario Fernández,*
Asymmetric Organocatalytic Strecker-Type Reactions of Aliphatic N,N- Dialkylhydrazones Aurora Martínez-Muñoz, David Monge,* Eloísa Martín-Zamora, Eugenia Marqués-López, Eleuterio Álvarez, Rosario Fernández,*
Supporting Information: Regioselective esterification of vicinal diols on monosaccharide derivatives via
 Supporting Information: Regioselective esterification of vicinal diols on monosaccharide derivatives via Mitsunobu reactions. Guijun Wang,*Jean Rene Ella-Menye, Michael St. Martin, Hao Yang, Kristopher
Supporting Information: Regioselective esterification of vicinal diols on monosaccharide derivatives via Mitsunobu reactions. Guijun Wang,*Jean Rene Ella-Menye, Michael St. Martin, Hao Yang, Kristopher
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates
 Pure & Appl. Chern., Vol. 70, No. 5, pp. 11 17-1 122, 1998. Printed in Great Britain. (0 1998 IUPAC Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates Mogens
Pure & Appl. Chern., Vol. 70, No. 5, pp. 11 17-1 122, 1998. Printed in Great Britain. (0 1998 IUPAC Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates Mogens
Enantioselective Conjugate Addition of 3-Fluoro-Oxindoles to. Vinyl Sulfone: An Organocatalytic Access to Chiral. 3-Fluoro-3-Substituted Oxindoles
 Enantioselective Conjugate Addition of 3-Fluoro-Oxindoles to Vinyl Sulfone: An Organocatalytic Access to Chiral 3-Fluoro-3-Substituted Oxindoles Xiaowei Dou and Yixin Lu * Department of Chemistry & Medicinal
Enantioselective Conjugate Addition of 3-Fluoro-Oxindoles to Vinyl Sulfone: An Organocatalytic Access to Chiral 3-Fluoro-3-Substituted Oxindoles Xiaowei Dou and Yixin Lu * Department of Chemistry & Medicinal
Supporting Information:
 Supporting Information: An rganocatalytic Asymmetric Sequential Allylic Alkylation/Cyclization of Morita-Baylis-Hillman Carbonates and 3-Hydroxyoxindoles Qi-Lin Wang a,b, Lin Peng a, Fei-Ying Wang a, Ming-Liang
Supporting Information: An rganocatalytic Asymmetric Sequential Allylic Alkylation/Cyclization of Morita-Baylis-Hillman Carbonates and 3-Hydroxyoxindoles Qi-Lin Wang a,b, Lin Peng a, Fei-Ying Wang a, Ming-Liang
Asymmetric Palladium Catalyzed Cross-Coupling Reactions. Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1
 Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
I. Introduction. Peng Zhao. Liu lab
 Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Copper-mediated asymmetric transformations*
 Pure Appl. Chem., Vol. 74, No. 1, pp. 37 42, 2002. 2002 IUPAC Copper-mediated asymmetric transformations* Alexandre Alexakis Department of Organic Chemistry, University of Geneva, 30 quai Ernest Ansermet,
Pure Appl. Chem., Vol. 74, No. 1, pp. 37 42, 2002. 2002 IUPAC Copper-mediated asymmetric transformations* Alexandre Alexakis Department of Organic Chemistry, University of Geneva, 30 quai Ernest Ansermet,
Glyoxylic acid derivatives in asymmetric synthesis*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1589 1596, 2000. 2000 IUPAC Glyoxylic acid derivatives in asymmetric synthesis* Janusz Jurczak 1,2 and Tomasz Bauer 1 1 Department of Chemistry, University of Warsaw,
Pure Appl. Chem., Vol. 72, No. 9, pp. 1589 1596, 2000. 2000 IUPAC Glyoxylic acid derivatives in asymmetric synthesis* Janusz Jurczak 1,2 and Tomasz Bauer 1 1 Department of Chemistry, University of Warsaw,
Oxidative couplings of two nucleophiles
 Oxidative Couplings of Hydrocarbons Oxidative couplings of two nucleophiles Oxidants involved: O 2 H 2 O 2 high h valent metals(copper salts) halides(iodine(Ⅲ) oxidants) Lei, A. W. Chem. Rev., 2011, 111,
Oxidative Couplings of Hydrocarbons Oxidative couplings of two nucleophiles Oxidants involved: O 2 H 2 O 2 high h valent metals(copper salts) halides(iodine(Ⅲ) oxidants) Lei, A. W. Chem. Rev., 2011, 111,
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
