Burgoyne Consultants Ltd., Burgoyne House, Chantry Drive, Ilkley, West Yorkshire, U.K.
|
|
|
- Jason Spencer
- 5 years ago
- Views:
Transcription
1 A SIMPLE METHOD OF ESTIMATING EXOTHERMICITY BY AVERAGE BOND ENERGY SUMMATION ARTHUR D. CRAVEN A simple method is described whereby the approximate exothermicity of a chemical reaction or decomposition can be predicted from the atomization energies of the reactants and the assumed products. The atomization energies of many compounds can be determined by average bond energy summation using published data. Keywords: Thermodynamics; Calculation; Exethermicity; Combustion; Explosion; Bond Energies 1. INTRODUCTION When TNT degrades explosively about 1100 calories of chemical energy are released from every gram. This energy excursion is not the result of the decomposition into the elements carbon, hydrogen, oxygen and nitrogen. The lloocal/g results from a complete rearrangement of the chemical bonds from those originally present in the trinitrotoluene molecule to those present in the solid and gaseous products arising from the decomposition and chemical recombination of the four elements into water, carbon dioxide, carbon monoxide, carbon and nitrogen. The number of calories per gram which are released will depend upon the final temperature and the physical state of these products. There is sufficient energy to raise the temperature to several thousand centigrade degrees and the total energy release may only be truly manifest if the products are cooled down in a calorimeter when even the latent heat of the water vapour is recovered by condensation to liquid (10.52kcals/mol H? 0). The gross energy excursion is usually considered to be the result of breaking all the chemical bonds originally present in the TNT molecule (this requires the addition of energy) and then recovering this energy and more when the chemical bonds are reformed in the product molecules. Burgoyne Consultants Ltd., Burgoyne House, Chantry Drive, Ilkley, West Yorkshire, U.K. 97
2 icheme SYMPOSIUM SERIES No. 102 It so happens that both water, carbon dioxide and carbon monoxide are produced in a TNT explosion and it is well known that these compounds have relatively high heats of formation. An approximate estimate of the heat of explosion of TNT can be obtained by simply estimating the energy arising from the heat of formation of these reaction products. At first sight it would appear that parts of the TNT molecule are "burning" in the oxygen available within the molecule and the same may be deduced from the explosive properties of organic peroxides. This over simplification gives rise to references to molecules containing "their own internal oxygen for combustion" although this is a phrase which is completely devoid of any technical significance. In reality the major proportion of the combined oxygen present in many organic molecules such as alcohols, acids, esters, ketones and even ethers makes no significant contribution to any energy excursion which may result from a complete rearrangement of the chemical bonds into the decomposition product molecules. In order to assess the danger inherent in producing and handling unstable materials, a simple method of estimating the approximate exothermic potential of any chemical compound, or mixture of chemical compounds is essential. High order accuracy is not necessarily justified since we only want to know whether the material has an exothermic potential of the order of say 1000, 600, 300cal/g to act as a warning indicator in any hazard analysis. A method widely used is to subtract the heats of formation of the reactants from the heats of formation of the products to give the heat of the reaction which is often expressed in kcal/mol. Two problems arise immediately here. Since the ability to explode is related to the reaction energy per unit mass and not to the energy on a molar basis, the molar heat of reaction should really be divided by the total molecular weight of the reactants. This is often overlooked with mixtures. The second problem is rather more serious in cases where the heat of formation of one or more of the reactants is not known. More complex methods are available whereby computer programs are devised to estimate heats of formation by additive assessment of the chemical groups present in the molecule. The CHETAH ASTM project (Chemical Thermodynamic and Energy Release Evaluation Program) is one such approach (reference 1). A typical example given in reference 1 relates to the heat of formation of terephthalic acid which is derived from the sum of the heats of formation of two benzoic acids molecules minus one benzene molecule. 98
3 In my view however a very much simpler method is available which is perfectly adequate "to be used as a screening tool to help set priorities for physical testing" (reference 1). The method which I have used is probably not suitable for full computerization since it assumes that the user has a basic working knowledge of organic chemistry. On the other hand it is suitable for use with the aid of a programmable pocket calculator since it involves a relatively small data base. 2. AVERAGE BOND ENERGY SUMMATION (ABES) The average bond energy summation method does not use heats of formation at all. It is a simplification of the method described by R.T. Sanderson (reference 2) based on bond energies. The energy excursion per mol of reactant (pure compound or mixture) is calculated by subtracting the total bond energies present before the reaction from the total bond energies of the products. An alternative way of looking at it is to calculate the total atomization energy of the reactants which can be based on either bond energy summation of novel molecules or well established data from e.g., reference 2. This value is then subtracted from the atomization energy of the assumed products to give the exothermicity or so called excess enthalpy either on a molar (kcal/mol) or preferably on a mass basis (cal/g). All that is required is the structural formula of the molecule involved, a table of average bond energies, or ready summations in the form of atomization energies and a knowledge of the products. Difficulties may arise in this latter part of the input data but in most cases assumptions regarding the products can be tested and those relating to the maximum theoretical energy can be used for the purpose of hazard analysis. As with most exothermicity determination methods, no account is taken of the effect of distributing the resulting excess enthalpy into the reaction products. At 1100 cal/g the temperature could be several thousand centigrade degrees which would lead to dissociation. Thus the reaction products would change. All estimates are therefore based on the assumption that the heat is removed and that the products remain undissociated. It is assumed however for the purposes of most hazard analyses that the water vapour will not be condensed to liquid. As I have already indicated average bond energies are used since this is 99
4 usually sufficiently accurate for this purpose, it must be stressed however that this simplification is only justified for bond rearrangement energy excursions of high exothermicity. 2.1 Decomposition The decomposition energy of hydrazine is a useful example. The hydrazine molecule comprises one N-N bond and four N-H bonds. The total atomization energy of one mol of hydrazine is therefore 40.0kcal/mol (bond energy N-N) plus 4 x 92.7 (bond energy N-H) equals kcal/mol. The recombination into nitrogen 226 kcal/mol (N? ) and hydrogen (2 X H 2 ) releases kcal/mol. The net heat release per mol is therefore 23.6 kcal/mol or cal/g. This would clearly represent a very dangerous chemical to handle which is common knowledge. However it would probably not qualify hydrazine for the reputation of "super fuel" which could propel rockets without any oxidant. In actual fact, hydrazine was the super-fuel which did propel rockets without any oxidant since the main decomposition product was essentially ammonia. The recombination to one mol of ammonia (280.3 kcals) and half a mol each of hydrogen (52.1 kcals) and nitrogen (113 kcals) releases kcals which promotes hydrazine immediately to the super-fuel class with cal/g (TNT 1100 cal/g). This can be extended to cal/g by further product manipulation (all ammonia and nitrogen). The precise nature of the decomposition products seldom causes such a large difference in the estimated exothermicity. TNT is a good example. The atomization of TNT can be estimated as follows: 100
5 If it is assumed that the decomposition products are: 101
6 This is somewhat higher than the accepted value of 1100 cal/g which probably reflects the attenuation effect of the water gas reaction and the production of some carbon monoxide. If the explosion products are assumed to be the calculated exothermicity is 2.2 Combustion There is fundamentally no difference between the energy resulting from a decomposition and that associated with a combustion reaction. The energy excursion is a direct result of the rearrangement of the chemical bonds. In the case of combustion the rearrangement also involves the chemical bonds of the oxidising agent be it those holding together the oxygen molecules or similar bonds in nitrogen oxide, hydrogen peroxide or more complex oxidants. Our preoccupation with oxygen arises from the fact that only kcals/mol are required to break the two oxygen atoms apart whereas the recombination of those two atoms with either hydrogen or carbon involves the formation of chemical bonds with the resulting release of ca 200 kcals for each gram atom of oxygen. The same method of calculating the energy excursion resulting from the bond rearrangement associated with combustion can be used as that described in the previous section. Thus when hydrogen burns in oxygen the atomization of two hydrogen molecules and one oxygen molecule requires only kcal/mol whereas the recombination of the resulting atoms to form four 0-H bonds is accompanied by the release of 443.2kcal/mol. This net escape of kcal/mol is equivalent to 3211 cal/g of reactants: products. Since the two reactants are in their conventional basic state the above 57.8 kcal/mol of H 2 0 represents the heat of formation of water vapour. In this respect we are only concerned with the production of water vapour in any hazard analysis relating to exothermicity and not the kcal/mol of latent heat of evaporation which might be recovered in the classical calorimetric measurement. This applies to any heat of combustion which in most tables is usually based on gross calorific value (H 2 0 to liquid). In this respect all heats of combustion quoted in this text relate to net calorific value (H 2 O as vapour) and this usually involves the reduction of the normally quoted gross heat of combustion values by 5.26 kcal/g atom of hydrogen present in the fuel molecule. Thus, as with the CHETAH approach no account is taken of any phase change in the hazard analysis. This point is illustrated with regard to the heat of combustion of benzoic acid which is used as a calorimetric standard with a gross calorific value of 6319 cal/g. With a molecular weight of 122 this is equivalent to kcal/mol of benzoic acid (gross). If however the water vapour is not condensed to liquid the net value would be kcal/mol of benzoic acid (i.e. -6 x 5.26 : 6 hydrogen atoms per mol). 102
7 The above combustion reaction is based on the assumption that a stoichiometric amount of oxygen is involved and the bond atomization summation involves Kcal/mol Acid 0 - H bond 1 x 114 Acid C - 0 bond 1 x 110 Acid C = 0 bond 1 x Phenyl alkyl bond 1 x 84.9 Benzene ring bonds 6 x Alkyl hydrogen bonds 5 x ½ Oxygen mols 7.5 x Benzoic acid atomization energy The combustion products are:- 3H C0 2 (3 x 221.6) + (7 x 384.6) = kcal/mol Recombination ie kcals/mol (6197 cal/g) benzoic acid which 1s ca. 2% of the recognised standard value. 103
8 2.3 Heats of formation We are not concerned with heats of formation in hazard analysis since the standard state on which all heats of formation are based are seldom relevant to any process condition encountered. Nevertheless the method outlined here can be used to calculate heats of formation if only to compare with other estimation methods which usually do involve heats of formation. Thus returning to terephthalic acid in reference 1 the heat of formation can be calculated from the difference between the atomization energy of terephthalic acid and that associated with the atomic ingredients in their standard state. 104
9 3. DISCUSSION Typical values of average bond energies, atomization energies of known compounds and atomization energies of the elements in their standard state as listed in tables 1, 2 and 3. The average bond energies listed in table 1 have been taken from the values calculated by Sanderson, reference 2, and no account has been taken of the abnormal effects of highly influential neighbour bonds. This simplification tends to produce slightly lower atomization energies of more complex molecules than those which are involved which gives somewhat higher calculated values of heats of decomposition and combustion. In a hazard analysis the error is therefore on the safe side. It can be seen in reference 2 that the differences between the estimated values and experimental values of bond energies are small. The experimental values therefore have been used in table 2 which is usually the source data for the reaction products. The atomization energies of the elemental species have been taken directly from reference 2 and are given in terms of energy per gram atom. For most elements encountered this is equal to half that associated with the standard state i.e. H , N 2 etc. By adopting this convention problems associated with carbon C n and other materials with complex values do not arise. I have also found that the data is more easily programmed in this form. A number of other authors have referred to this method but using both "bond energies" and "resonant energies" in their assessment of atomization energies. Thus the carbon to carbon bond energy in hexane could be considered to be 83.2 kcals and in acetylene kcals. In benzene this could give a total carbon to carbon bond energy of (3 x 83.2) + (3 x 144.5) = This is below the estimated value for the six carbon bonds in benzene which is and the shortfall of about 50 kcals may be referred to as residual "resonant" energy. In the method described in this paper however it is accepted that the carbon to carbon bonds in benzene, TNT, benzoic acid etc. are not represented by alternate single and double bonds but are unique and requiring kcals each to break them. Any resonant energy is thereby built into the individual bonds. Using the data from tables 1, 2 and 3 the reliability of the method has been checked against standard heats of combustion (see table 4) and heats of formation (see table 5). It can be seen that the agreement between the measured values and those predicted using the readily available data is sufficient for a preliminary survey relating to either a compound or a mixture of compounds. The largest percentage errors occur with heats of formation (see table 5). With the large heats of formation of the water and carbon dioxide however, the heats of formation of the initial fuels are lost in the resulting heats of combustion. With small molecules however the errors arising from this method are greatest. It is recommended therefore that with such small molecules the bond energies should be either calculated from first principles by the method given in reference 2 or taken directly from tables. 105
10 This exploitation of Hess's Law enables the thermochemistry to be measured on an absolute zero basis. Thus if the energy available from the formation of any chemical bond is derived from the condition of the solitary atoms in space, any resulting release of chemical energy must be equal to the total bond energy in the molecule under consideration. In this respect it must be stressed that the single bond energies are not the classical dissociation energies which can be applied to kinetics. The bond energy summation method assumes that all the bonds are broken at the same instant in time to give the total atomization energy of the molecule. The same is assumed in the reverse process with the formation of the reaction products. From this simple and fundamental approach, the contribution of either molecular substitution or addition and dilation can be assessed for any process or product at the conceptual stage. ACKNOWLEDGEMENT I wish to express my thanks to my friends and colleagues Dr. J.H. Burgoyne and Dr. P.V. Rutledge for their advice during the preparation of this paper and to Dr. R.T. Sanderson who I have never met but whose interesting book (reference 2) is the source of both the data and the scientific principles which I have so briefly outlined in this paper. R E F E R E N C E S Reference 1. Carole A. Davies et al. The Thermochemical and Hazard Data of Chemicals. (Estimation Using the ASTM CHETAH Program). Chapter 9 from Chemical Process Hazard Review Proceedings of Symposia No. 274 of the American Chemical Society 1985 SBN Reference 2. R.T. Sanderson. Chemical Bond and Bond Energy. Academic Press. New York. London Reference 3. Handbook of Chemistry and Physics. 55th Edition CRC Press. 106
11 TABLE 1 SUGGESTED AVERAGE BOND ENERGIES 107
12 TABLE 2 TYPICAL MOLAR ATOHIZATION ENERGIES 108
13 TABLE 3 Examples of Experimental Atomization Energies Values in kilocalories per gram atom 109
14 110
15 111
Module 5: Combustion Technology. Lecture 32: Fundamentals of thermochemistry
 1 P age Module 5: Combustion Technology Lecture 32: Fundamentals of thermochemistry 2 P age Keywords : Heat of formation, enthalpy change, stoichiometric coefficients, exothermic reaction. Thermochemistry
1 P age Module 5: Combustion Technology Lecture 32: Fundamentals of thermochemistry 2 P age Keywords : Heat of formation, enthalpy change, stoichiometric coefficients, exothermic reaction. Thermochemistry
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Quantitative Relationships in Chemical Reactions Chapter 7
 Quantitative Relationships in Chemical Reactions Chapter 7 The burning of charcoal releases heat (thermal energy) that grills our food. But the combustion of charcoal and fossil fuels also releases CO
Quantitative Relationships in Chemical Reactions Chapter 7 The burning of charcoal releases heat (thermal energy) that grills our food. But the combustion of charcoal and fossil fuels also releases CO
SUPPLEMENTARY TOPIC 3 ENERGY AND CHEMICAL REACTIONS
 SUPPLEMENTARY TOPIC 3 ENERGY AND CHEMICAL REACTIONS Rearranging atoms. In a chemical reaction, bonds between atoms in one or more molecules (reactants) break and new bonds are formed with other atoms to
SUPPLEMENTARY TOPIC 3 ENERGY AND CHEMICAL REACTIONS Rearranging atoms. In a chemical reaction, bonds between atoms in one or more molecules (reactants) break and new bonds are formed with other atoms to
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
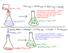 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS. Honors Chemistry Ms. Agostine
 CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS Honors Chemistry Ms. Agostine 16.1 Thermochemistry Definition: study of the transfers of energy as heat that accompany chemical reactions and
CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS Honors Chemistry Ms. Agostine 16.1 Thermochemistry Definition: study of the transfers of energy as heat that accompany chemical reactions and
Define the term enthalpy change of formation of a compound
 1. Alkanes are important hydrocarbons since they are used as fuels in homes and in industry. It is important that the enthalpy changes involved in alkane reactions are known. Define the term enthalpy change
1. Alkanes are important hydrocarbons since they are used as fuels in homes and in industry. It is important that the enthalpy changes involved in alkane reactions are known. Define the term enthalpy change
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
1. Enthalpy changes of reaction can be determined indirectly from average bond enthalpies and standard enthalpy changes.
 1. Enthalpy changes of reaction can be determined indirectly from average bond enthalpies and standard enthalpy changes. The table below shows the values of some average bond enthalpies. bond average bond
1. Enthalpy changes of reaction can be determined indirectly from average bond enthalpies and standard enthalpy changes. The table below shows the values of some average bond enthalpies. bond average bond
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
CHEMISTRY LEVEL 4C (CHM415115)
 CHEMISTRY LEVEL 4C (CHM415115) THERMOCHEMISTRY & ENERGY CHANGES THEORY SUMMARY & REVISION QUESTIONS Tasmanian TCE Chemistry Revision Guides by Jak Denny are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives
CHEMISTRY LEVEL 4C (CHM415115) THERMOCHEMISTRY & ENERGY CHANGES THEORY SUMMARY & REVISION QUESTIONS Tasmanian TCE Chemistry Revision Guides by Jak Denny are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
The table below includes some values of standard enthalpies of formation ( H ).
 1. A vessel and its contents of total heat capacity 120 J K 1 were heated using a methane burner. Calculate the maximum theoretical temperature rise when 0.10 g of methane was completely burned. The standard
1. A vessel and its contents of total heat capacity 120 J K 1 were heated using a methane burner. Calculate the maximum theoretical temperature rise when 0.10 g of methane was completely burned. The standard
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
LECTURE 4 Variation of enthalpy with temperature
 LECTURE 4 Variation of enthalpy with temperature So far, we can only work at 25 C. Like c v we define a constant pressure heat capacity, c p, as the amount of heat energy needed to raise the temperature
LECTURE 4 Variation of enthalpy with temperature So far, we can only work at 25 C. Like c v we define a constant pressure heat capacity, c p, as the amount of heat energy needed to raise the temperature
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy......... Standard enthalpy of formation............ (5) (b) Some mean bond enthalpies
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy......... Standard enthalpy of formation............ (5) (b) Some mean bond enthalpies
Chemistry 223: Thermochemistry David Ronis McGill University
 Chemistry 223: Thermochemistry David Ronis McGill University 1. Enthalpy Calculations: Chemical Reactions and Hess Law The enthalpy change for a process, H, isequal to the heat absorbed by the system if
Chemistry 223: Thermochemistry David Ronis McGill University 1. Enthalpy Calculations: Chemical Reactions and Hess Law The enthalpy change for a process, H, isequal to the heat absorbed by the system if
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
How many ml of 0.250M potassium permangenate are needed to react with 3.36 g of iron(ii) sulfate?
 115 How many ml of 0.250M potassium permangenate are needed to react with 3.36 g of iron(ii) sulfate? 1) Convert 3.36 grams iron(ii) sulfate to moles. Use FORMULA WEIGHT. 2) Convert moles iron(ii) sulfate
115 How many ml of 0.250M potassium permangenate are needed to react with 3.36 g of iron(ii) sulfate? 1) Convert 3.36 grams iron(ii) sulfate to moles. Use FORMULA WEIGHT. 2) Convert moles iron(ii) sulfate
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron.
 Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron. 1. Which number on the graph to the right represents the effect of the
Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron. 1. Which number on the graph to the right represents the effect of the
Assuming that no heat is lost from the water to the surrounding air, Conservation of energy. The terms add to zero because they have opposite signs.
 CALORIMETRY - the measurement of heat. How do we measure heat flow? 0.20 mol A 100 g water A -> B + C When we add the reactant to water, it decomposes - heating the water. 100 g water A -> B + C... what
CALORIMETRY - the measurement of heat. How do we measure heat flow? 0.20 mol A 100 g water A -> B + C When we add the reactant to water, it decomposes - heating the water. 100 g water A -> B + C... what
Unit 7 Thermochemistry Chemistry 020, R. R. Martin
 Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Science 10. Unit 2: Chemistry. Book 6: energy changes in chemical reactions. Block: Name: Zukowski
 Science 10 Unit 2: Chemistry Book 6: energy changes in chemical reactions Name: Zukowski Block: 1 How is energy involved in chemical processes? and energy are continually interacting in the world around
Science 10 Unit 2: Chemistry Book 6: energy changes in chemical reactions Name: Zukowski Block: 1 How is energy involved in chemical processes? and energy are continually interacting in the world around
Energetics. These processes involve energy exchanges between the reacting system and its surroundings.
 Energetics Chemical reactions involve: the breaking of bonds between atoms the making of new bonds between atoms These processes involve energy exchanges between the reacting system and its surroundings.
Energetics Chemical reactions involve: the breaking of bonds between atoms the making of new bonds between atoms These processes involve energy exchanges between the reacting system and its surroundings.
TERMS AND DEFINITIONS IN THERMOCHEMISTRY
 (v.2.0 16/3/90 8hrs.) TERMS AND DEFINITIONS IN THERMOCHEMISTRY (v.2.1 12/4/90 2hrs.) 1) ENTHALPY CHANGE ( H) This is the same as the heat change involved in a process (provided the initial and final states
(v.2.0 16/3/90 8hrs.) TERMS AND DEFINITIONS IN THERMOCHEMISTRY (v.2.1 12/4/90 2hrs.) 1) ENTHALPY CHANGE ( H) This is the same as the heat change involved in a process (provided the initial and final states
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS
 CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS Introduction In this chapter, we discuss the First Law of Thermodynamics (energy cannot be created or destroyed). In our discussion, we will define some important
CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS Introduction In this chapter, we discuss the First Law of Thermodynamics (energy cannot be created or destroyed). In our discussion, we will define some important
F322: Chains, Energy and Resources Enthalpy Changes
 F322: Chains, Energy and Resources 2.3.1 Enthalpy Changes 1. Some reactions of 2 O 2 are exothermic. Use ideas about the enthalpy changes that take place during bond breaking and bond making to explain
F322: Chains, Energy and Resources 2.3.1 Enthalpy Changes 1. Some reactions of 2 O 2 are exothermic. Use ideas about the enthalpy changes that take place during bond breaking and bond making to explain
Enthalpy Chapter 5.3-4,7
 Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
I PUC CHEMISTRY CHAPTER - 06 Thermodynamics
 I PUC CHEMISTRY CHAPTER - 06 Thermodynamics One mark questions 1. Define System. 2. Define surroundings. 3. What is an open system? Give one example. 4. What is closed system? Give one example. 5. What
I PUC CHEMISTRY CHAPTER - 06 Thermodynamics One mark questions 1. Define System. 2. Define surroundings. 3. What is an open system? Give one example. 4. What is closed system? Give one example. 5. What
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
(02) WMP/Jun10/CHEM2
 Energetics 2 Section A Answer all the questions in the spaces provided. 1 An equation for the equilibrium reaction between hydrogen, iodine and hydrogen iodide is shown below. H 2 (g) + I 2 (g) 2HI(g)
Energetics 2 Section A Answer all the questions in the spaces provided. 1 An equation for the equilibrium reaction between hydrogen, iodine and hydrogen iodide is shown below. H 2 (g) + I 2 (g) 2HI(g)
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Higher Chemistry Principles to Production October Revision
 igher Chemistry Principles to Production October Revision You should use your class notes, Evans2Chemweb and Scholar to help. Show your working for each question. Sections covered so far; Principles to
igher Chemistry Principles to Production October Revision You should use your class notes, Evans2Chemweb and Scholar to help. Show your working for each question. Sections covered so far; Principles to
CHEM Thermodynamics. Heat calculations
 Thermodynamics Heat calculations l Internal Energy, E The internal energy of other systems that are more complex than the ideal gas cannot be measured. But the internal energy of the system is still proportional
Thermodynamics Heat calculations l Internal Energy, E The internal energy of other systems that are more complex than the ideal gas cannot be measured. But the internal energy of the system is still proportional
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
CHEM What is Energy? Terminology: E = KE + PE. Thermodynamics. Thermodynamics
 Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
CHEM1901/ J-8 June 2013
 CHEM1901/3 2013-J-8 June 2013 The atmosphere of Venus contains 96.5 % CO 2 at 95 atm of pressure, leading to an average global surface temperature of 462 C. The energy density of solar radiation striking
CHEM1901/3 2013-J-8 June 2013 The atmosphere of Venus contains 96.5 % CO 2 at 95 atm of pressure, leading to an average global surface temperature of 462 C. The energy density of solar radiation striking
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Reacting Gas Mixtures
 Reacting Gas Mixtures Reading Problems 15-1 15-7 15-21, 15-32, 15-51, 15-61, 15-74 15-83, 15-91, 15-93, 15-98 Introduction thermodynamic analysis of reactive mixtures is primarily an extension of the principles
Reacting Gas Mixtures Reading Problems 15-1 15-7 15-21, 15-32, 15-51, 15-61, 15-74 15-83, 15-91, 15-93, 15-98 Introduction thermodynamic analysis of reactive mixtures is primarily an extension of the principles
THERMODYNAMICS. Energy can be neither created nor destroyed but it can be converted from one form to another.
 Chemical Energetics 1 TERMODYNAMICS First Law Energy can be neither created nor destroyed but it can be converted from one form to another. all chemical reactions are accompanied by some form of energy
Chemical Energetics 1 TERMODYNAMICS First Law Energy can be neither created nor destroyed but it can be converted from one form to another. all chemical reactions are accompanied by some form of energy
Energy & Chemistry. Internal Energy (E) Energy and Chemistry. Potential Energy. Kinetic Energy. Energy and Chemical Reactions: Thermochemistry or
 Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Note: 1 calorie = 4.2 Joules
 Enthalpy Changes All substances contain chemical energy, called enthalpy. Like any kind of energy it is measured in Joules (previously energy was measured in Calories). When reactions happen, energy is
Enthalpy Changes All substances contain chemical energy, called enthalpy. Like any kind of energy it is measured in Joules (previously energy was measured in Calories). When reactions happen, energy is
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
_ + Units of Energy. Energy in Thermochemistry. Thermochemistry. Energy flow between system and surroundings. 100º C heat 50º C
 Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
3.2.1 Energetics. Calorimetry. 121 minutes. 120 marks. Page 1 of 19
 3..1 Energetics Calorimetry 11 minutes 10 marks Page 1 of 19 Q1. A 50.0 cm 3 sample of a 0.00 mol dm 3 solution of silver nitrate was placed in a polystyrene beaker. An excess of powdered zinc was added
3..1 Energetics Calorimetry 11 minutes 10 marks Page 1 of 19 Q1. A 50.0 cm 3 sample of a 0.00 mol dm 3 solution of silver nitrate was placed in a polystyrene beaker. An excess of powdered zinc was added
Propose a method for measuring your results.
 System vs. Surroundings In thermodynamics, a system is defined as that part of the universe that is under consideration (the part of the universe that you are studying). A hypothetical boundary separates
System vs. Surroundings In thermodynamics, a system is defined as that part of the universe that is under consideration (the part of the universe that you are studying). A hypothetical boundary separates
Energy Changes in Chemical Reactions
 Unit 3 Energetics Unit 3-1 Section 3.1 Energy Changes in Chemical Reactions ( 1 ) Conservation of energy An object which is capable of doing work is said to possess energy. There are many forms of energy:
Unit 3 Energetics Unit 3-1 Section 3.1 Energy Changes in Chemical Reactions ( 1 ) Conservation of energy An object which is capable of doing work is said to possess energy. There are many forms of energy:
Enthalpy Change & Hess's Law
 Enthalpy hange & Hess's Law Question Paper 1 Level International Level Subject hemistry Exam oard IE Topic hemical Energetics Sub-Topic Paper Type Enthalpy hange & Hess's Law Multiple hoice ooklet Question
Enthalpy hange & Hess's Law Question Paper 1 Level International Level Subject hemistry Exam oard IE Topic hemical Energetics Sub-Topic Paper Type Enthalpy hange & Hess's Law Multiple hoice ooklet Question
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
3.2.1 Energetics. Enthalpy Change. 263 minutes. 259 marks. Page 1 of 41
 ..1 Energetics Enthalpy Change 6 minutes 59 marks Page 1 of 41 Q1. (a) Define the term standard molar enthalpy of formation, ΔH f. (b) State Hess s law. (c) Propanone, CO, burns in oxygen as shown by the
..1 Energetics Enthalpy Change 6 minutes 59 marks Page 1 of 41 Q1. (a) Define the term standard molar enthalpy of formation, ΔH f. (b) State Hess s law. (c) Propanone, CO, burns in oxygen as shown by the
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Chapter 3. Thermochemistry: Energy Flow and Chemical Change. 5.1 Forms of Energy and Their Interconversion
 Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
exothermic reaction and that ΔH c will therefore be a negative value. Heat change, q = mcδt q = m(h 2
 Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHEM J-11 June /01(a)
 CHEM1001 2014-J-11 June 2014 22/01(a) Combustion of 15.0 g of coal provided sufficient heat to increase the temperature of 7.5 kg of water from 286 K to 298 K. Calculate the amount of heat (in kj) absorbed
CHEM1001 2014-J-11 June 2014 22/01(a) Combustion of 15.0 g of coal provided sufficient heat to increase the temperature of 7.5 kg of water from 286 K to 298 K. Calculate the amount of heat (in kj) absorbed
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Is the study of the energy changes that accompany physical and chemical changes. Energy is defined as the ability to do work or the capacity to produce change. The forms of
Thermochemistry (chapter 5) Is the study of the energy changes that accompany physical and chemical changes. Energy is defined as the ability to do work or the capacity to produce change. The forms of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Thermochemical Equations
 Thermochemical Equations Enthalpy - an extensive property that measures heat absorbed or evolved in a reaction represented with an H In thermochemical equations, the enthalpy sign determines whether heat
Thermochemical Equations Enthalpy - an extensive property that measures heat absorbed or evolved in a reaction represented with an H In thermochemical equations, the enthalpy sign determines whether heat
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Chemical reactions. C2- Topic 5
 Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Enthalpy changes
 3.2.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The products have less energy than the If an enthalpy change occurs then energy is
3.2.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The products have less energy than the If an enthalpy change occurs then energy is
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
CH10007/87. Thermodynamics. Dr Toby Jenkins
 CH10007/87 Thermodynamics Dr Toby Jenkins 1 Objectives To introduce the basic concepts of thermodynamics To apply them to chemical systems To develop competence in thermodynamics calculations 2 Equilibrium
CH10007/87 Thermodynamics Dr Toby Jenkins 1 Objectives To introduce the basic concepts of thermodynamics To apply them to chemical systems To develop competence in thermodynamics calculations 2 Equilibrium
Chemistry 101 Chapter 10 Energy
 Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Section 3.0. The 1 st Law of Thermodynamics. (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities Definitions and Concepts
 Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
1.4 Energetics. N Goalby chemrevise.org 1. Standard Enthalpy Change of Formation. Standard Enthalpy Change of Combustion
 1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
COMBUSTION OF FUEL 12:57:42
 COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
Methane contains atoms of two elements, combined chemically. Methane is a mixture of two different elements.
 Q1.Methane (CH 4) is used as a fuel. (a) The displayed structure of methane is: Draw a ring around a part of the displayed structure that represents a covalent bond. (b) Why is methane a compound? Tick
Q1.Methane (CH 4) is used as a fuel. (a) The displayed structure of methane is: Draw a ring around a part of the displayed structure that represents a covalent bond. (b) Why is methane a compound? Tick
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Chemical Reactions. Writing chemical reactions Types of chemical reactions Reactions in aqueous solutions. (ionic equations and solubility rules)
 Chemical Reactions Writing chemical reactions Types of chemical reactions Reactions in aqueous solutions (ionic equations and solubility rules) Writing Equations REACTANTS PRODUCTS gold (III) sulfide is
Chemical Reactions Writing chemical reactions Types of chemical reactions Reactions in aqueous solutions (ionic equations and solubility rules) Writing Equations REACTANTS PRODUCTS gold (III) sulfide is
