Enumeration Method for Search of Low-energy Structures on Graphene. Joseph Lawrence
|
|
|
- Dominick Summers
- 5 years ago
- Views:
Transcription
1 Enumeration Method for Search of Low-energy Structures on Graphene Joseph Lawrence A senior thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Bachelor of Science Bret Hess, Advisor Department of Physics and Astronomy Brigham Young University August 2016 Copyright 2016 Joseph Lawrence All Rights Reserved
2 ABSTRACT Enumeration Method for Search of Low-energy Structures on Graphene Joseph Lawrence Department of Physics and Astronomy, BYU Bachelor of Science Structures of metal on graphene play important roles in batteries and hydrogen storage, and discovery of new structures could improve these applications. Through computational methods, the formation energy of a proposed structure can be calculated. Enumeration is the process of creating lists of possible structures to search for low formation energy. We present an enumeration method that allows for efficient treatment of vacancies and apply it to the case of six sites per two carbon atoms. If the separation between two sites in the lattice is less than a specified distance, the pair is referred to as forbidden. The enumeration skips structures where at least one forbidden pair has both sites non-vacant, speeding up the process exponentially by volume. This allows for enumeration of structures of high volumes that would be impossible using standard methods. The search method is applied to the case of scandium, titanium, yttrium, and zirconium on graphene. We report results of several low-energy structures, many of which do not currently appear in the literature. These structures may be useful in future applications of batteries and hydrogen storage. Keywords: graphene, enumeration, density functional theory, cluster expansion, scandium, titanium, yttrium, zirconium
3 ACKNOWLEDGMENTS I would like to thank Dr. Bret Hess for his advisement on this project. I would also like to thank the Physics and Astronomy department at Brigham Young University for the funding that made this work possible. PHSCS 416, Writing in Physics, provided considerable help in the steps of writing and revising this thesis.
4 Contents Table of Contents List of Figures iv v 1 Introduction Overview Past Results Purpose Methods Program Outline VASP Cluster Expansion Enumeration New Enumeration Method Results Enumeration Results Newly Discovered Structures Future Work Conclusion Bibliography 20 Index 21 iv
5 List of Figures 1.1 Adatom on graphene Low-energy configuration of titanium on graphene Cells and supercells Sites in the new enumeration method Examples of tiles Formation energy vs. Sc concentration Formation energy vs. Ti concentration Formation energy vs. Y concentration Formation energy vs. Zr concentration v
6 Chapter 1 Introduction 1.1 Overview Graphene has many applications in both nanotechnology and batteries. When graphene is doped with a metal, it acts as a conductor and can be used in electronics. By doping graphene with a transition metal at the right concentration and configuration, a very effective cathode or anode can be created. Additionally, metal on a graphene layer can be used to facilitate hydrogen storage. The metal atoms allow hydrogen atoms to bind to the structure, storing them for later use such as in a fuel cell. This is an alternative to storing hydrogen in liquid or gas form, and is a preferable method for its efficiency and safety [1, 2]. In fabrication of these nanostructures, the bonds between graphene and metals must be understood. Due to the large number of possible metals and their configurations, studies of graphenemetal interaction are varied and far from complete. This thesis outlines computational methods used in searching for stable, low-energy structures. By calculating energies computationally rather than experimentally, thousands or even millions of structures can be searched. An endless number of possible structures on graphene exist any combination of atoms in any 1
7 1.1 Overview 2 Figure 1.1 An adatom on graphene. configuration could be considered. To limit the search to a reasonable number of structures, I narrowed the search to structures consisting of a single metal element on graphene. (see Fig. 1.1) The search was further narrowed to consider only scandium, titanium, yttrium, or zirconium, which are the lightest of the transition metals and most useful for batteries. In determining how favorable a structure is, many characteristics could be considered. Formation energy was chosen as the primary characteristic to compare because it indicates stability of the structure. A lower formation energy indicates greater stability and thus a greater likelihood that the structure would form in the lab. Other characteristics will be referred to later to deepen the analysis of specific structures. Formation energy is often calculated using density functional theory (DFT), a computational method that is performed on one structure at a time. Depending on the complexity of the structure, this can take a few minutes to several hours per structure. In searching a large set for low-energy structures, this process can be time consuming. The search is made more efficient by the use of a predictive model called cluster expansion. Once a few hundred formation energies have been calculated, cluster expansion is used to predict which uncalculated structures will have the lowest formation energies. This makes it so only a fraction of the structures in the set need to be calculated, allowing for searches of sets including millions of structures. Previous work has been done to systematically generate all possible structures given a particular configuration. [3, 4] This process is called enumeration. The computer code developed by Hart
8 1.2 Past Results 3 Figure 1.2 A low-energy configuration of titanium on graphene as reported by Sivek [2]. Blue circles represent carbon atoms and yellow circles represent titanium atoms. and Forcade takes an input lattice of possible atom positions, and generates a complete list of possible structures, excluding any that are rotationally or translationally equivalent. This method was modified for the case of metal on graphene, and the resulting set was searched for low-energy structures. 1.2 Past Results Past work has identified a few low-energy structures involving transition metals on graphene. This work presents a comprehensive search of structures that is much more complete than the guessand-check methods previously employed. To give context, a particular low-energy structure of titanium reported by Sivek is shown in Figure 1.2 [2]. This structure is reported to have a formation energy of 0.93 ev per Ti atom, which was the lowest energy of the structures investigated by the paper. In the results section of this thesis, several other low-energy structures are shown, giving a more complete picture of the behavior of these structures.
9 1.3 Purpose Purpose The purpose of this thesis is to present a modified version of the enumeration method, applied to metal on graphene. The modifications allow the inclusion of six possible sites for metal atoms per cell. A cell is a unit of the lattice that includes two carbon atoms and any atoms added above the graphene layer. Each of the six sites in a cell may be occupied by a metal atom or vacant, which allows for a wide variety of concentrations and configurations. Normally, enumeration with that many possible sites would be lengthy. Enumeration of six or more cells with six sites each can take days and involve billions of structures. The proposed method skips a large portion of the structures by including only structures that have sufficient space between the metal atoms, using a specified distance dependent on the atom size. This allows for efficient enumeration of structures that are both large and complex. This thesis presents a search which was used to find low-energy structures of scandium, titanium, yttrium, and zirconium at varying concentrations. The following chapter of this thesis outlines the methods used in this search. The final chapter reports the results of the search, analyzing the structures of greatest interest and comparing them to the literature.
10 Chapter 2 Methods 2.1 Program Outline Our group has developed a program that performs all the steps of the low-energy search. [5] The user gives some input parameters, then the program performs the steps of enumeration, VASP calculations and cluster expansion to find the structures with the lowest formation energy. The input parameters define the positions of sites in each cell. Also defined are the atoms that can occupy these sites, including an option that allows sites to be vacant. The maximum volume is given, where volume is defined to be the number of cells in a structure. This is also where additional parameters are given for calculation accuracy and program flow. The following subsections give some detail about the key elements of the program VASP calculations, cluster expansion and enumeration VASP The formation energy of a given structure can be calculated using VASP, a computational package that implements density functional theory (DFT). VASP starts with initial atom positions and finds 5
11 2.1 Program Outline 6 the lowest possible energy state through iterative relaxation. First, the electrons in the structure are relaxed, finding the most favorable electron configuration. Then VASP relaxes the positions of the ions to find a lower energy configuration. These two types of relaxation are repeated until the process converges and the lowest possible energy state is found. VASP reports a total energy for the structure. To calculate the formation energy, the total energy is compared to reference energies for each element. For metals, this reference energy is the energy per atom of an isolated hexagonal monolayer. For carbon, the reference energy is the energy per atom of graphene. The formation energy per adatom (atom added to the graphene layer) can be calculated by FE = ( Estruct N C [ ] ) xi E re f,i Ere f,c i N C N adatoms, where E struct is the energy reported by VASP, N C is the number of carbon atoms, x i is the concentration per carbon of the ith adatom, E re f,i is the reference energy for the ith adatom, E re f,c is the reference energy for carbon, and N adatoms is the total number of adatoms Cluster Expansion Rather than using VASP to calculate the energy of each enumerated structure, cluster expansion takes a few VASP energies and predicts the energies of the remaining structures. The details of cluster expansion are explained by Rosenbrock et al. [6] and is implemented in the UNCLE code by Nelson et al. [7] Cluster expansion is analogous to the simpler case of approximation of functions. For example, imagine you have n points in xy coordinates. Any n points can be fit exactly with an nth order polynomial. If not all the points are known, a lower-order polynomial can be fit to the known points, and the polynomial can often be a good approximation for the unknown points. Cluster expansion works in a similar manner. For sites on a lattice, clusters of these sites are
12 2.1 Program Outline 7 chosen. Two sites make a 2-body cluster, three sites make a 3-body cluster, and so on. For a given structure, the occupation of these clusters is considered. Fitting coefficients are multiplied by the occupation of each of these clusters, and the sum of all the terms is the predicted formation energy. If one knows the formation energy of every structure, coefficients can be assigned to the clusters that fit the formation energies exactly. This is often a good approximation before all the structures have been calculated, and can be used to predict formation energies of uncalculated structures. Previously calculated energies are used to fit the coefficients, and the formula is then used to predict the energies of the remaining structures. After formation energies are predicted for each structure, the lowest-energy structures of each concentration are sent to VASP for accurate energy calculation. Those new energies are added to the cluster expansion fit, and the program alternates iteratively between VASP and cluster expansion until the program stops finding new low-energy structures Enumeration Before the search can begin, a list of structures to search must be generated. This process is called enumeration, and is done systematically following the approach described by Hart and Forcade [3]. A summary of the method is given here. The cell is the basic unit of a structure (see Fig. 2.1). Each cell can have one or more sites for possible atom locations. The cell has boundaries defined by three vectors coming from the origin, called parent lattice vectors. Cells are placed next to each other to repeat periodically in all directions. For the case of graphene, the layers are kept 15 ÃĚ apart so the interaction between them is negligible. The labeling of a particular cell is a string that represents the occupation of each site in the cell. A volume-1 structure is a structure with only one cell repeated periodically. The majority of structures are of higher volume. The volume indicates how many cells combine together to create
13 2.2 New Enumeration Method 8 Figure 2.1 An example cell on a graphene lattice. Blue circles represent carbon atoms. The two parent lattice vectors start at the origin of the cell and define the cell boundaries. A third parent lattice vector (not shown) points out of the page in the z direction. a supercell, and the supercell is repeated periodically to create a structure. New lattice vectors describe the size of the supercell, which is a linear combination of the parent lattice vectors. Different linear combinations of the parent lattice vectors create different sizes and shapes of supercells. Once the size of the cell, the coordinates of its sites, and the maximum volume is determined, the enumeration code generates a list of all possible supercells for each volume. This list is then reduced by symmetry of the parent lattice vectors to remove rotationally equivalent supercells. For each remaining supercell, a list of all possible structures is created. This has a length of the number of possible atoms at each site to the power of the total number of sites. This list is then reduced for translational equivalence along the parent lattice vectors, as well as rotational equivalence about the parent lattice vectors. 2.2 New Enumeration Method For enumeration of structures on graphene, we can define a cell to contain two carbon atoms in the graphene layer. To make the search as complete as possible, we choose to include six sites for adatoms above the layer. This includes one hollow site, two top sites and three bridge sites (see Fig. 2.2). Since the two carbon atoms are constant for each cell, they are not considered in
14 2.2 New Enumeration Method 9 Figure 2.2 The six sites in a unit cell. Blue circles represent carbon atoms and yellow circles represent sites for metal atoms above the graphene layer. There are three different kind of sites: One hollow site is at the cell s origin, two top sites are above the carbon atoms, and three bridge sites are between pairs of carbon atoms. the enumeration and are simply added in for VASP calculations. The cell can be represented as a six-digit label, with each digit in the label describing the content of each of the six adatom sites. Only a small number of the possible structures need to be considered. Each site can be occupied by a metal atom or be vacant. These sites are close enough together that if all of them cannot be filled with metal atoms. If two atoms are too close together, the VASP energy cannot converge. We can define a minimum distance between atoms to be 0.75 times the sum of their atomic radii. Any structures containing atoms closer than this distance is said to contain a forbidden pair and is an invalid structure. It is possible to run the standard enumeration code and remove structures with forbidden pairs afterwards. However, this is extremely time consuming since huge numbers of structures would have to generated and most of them would contain forbidden pairs. Instead, the proposed method runs the enumeration from scratch, skipping entirely any cells that contain forbidden pairs. To begin, every possible volume-1 structure is generated, including rotationally and translationally equivalent structures. For the binary case with six sites that makes 2 6 = 64 possible volume-1 structures, which are referred to as tiles. Any structure can be made up of these tiles. However, many of these tiles contain forbidden pairs, and can be dropped from the enumeration. For the tran-
15 New Enumeration Method ( a ) ( b) ( c ) Figure 2.3 Tiles that are used to create structures. (a) shows the nine tiles that are possible with metal on graphene. (b) shows some tiles combined to form a valid supercell. (c) shows a combination of tiles that creates a forbidden pair. sition metals we are considering, only nine of the sixty-four possible tiles do not contain forbidden pairs (see Fig 2.3). For each supercell, tiles are combined systematically to create structures. Since the number of valid tiles has been significantly reduced, this creates far fewer structures than standard enumeration. After each structure is created, it is tested to see if any forbidden pairs have been introduced across tile boundaries. Additionally, since the supercell is repeated periodically, tests are made for forbidden pairs across the supercell boundary. For each remaining structure, a list of translationally equivalent structures is created. The structure is only retained if its label is the lowest numerically on the list. Similarly, rotationally equivalent structures are removed, using symmetries of the lattice vectors. This ensures that only one unique structure is retained from each group of translationally or rotationally equivalent structures.
16 Chapter 3 Results 3.1 Enumeration Results Table 3.1 compares the number of structures considered by standard enumeration and forbidden pairs enumeration. The number of structures considered is a good indicator of both time and memory required for enumeration. Volume 6 could not finish standard enumeration in a few days, whereas forbidden pair enumeration could finish the same set in about an hour. The data in Table 3.1 can be approximated by a power law. The increase in speed of the forbidden pairs method is about 3.8 V, where V is the maximum volume enumerated. According to this formula, volume 6 is over 3000 times faster to enumerate using the forbidden pairs method. This exponential increase in speed by volume allows for enumerations of volume and complexity that would be impossible using standard enumeration. 3.2 Newly Discovered Structures The new enumeration method was used to perform a search of scandium, titanium, yttrium, and zirconium each on graphene. This search was complete up to volume six. The search resulted in 11
17 3.2 Newly Discovered Structures 12 Table 3.1 A comparison of number of structures considered by standard enumeration versus forbidden pair enumeration for the case of six sites per unit cell. For each volume, the total number of enumerated structures is given for three cases: First, the number of structures output by standard enumeration after all calculations; Second, the total number of structures considered by the forbidden pairs method; Third, the number of unique structures after all forbidden pairs are removed in either case. Volume Standard Enumeration Forbidden Pairs Final Usable Structures ? hundreds of low-energy structures for each element. Figures 3.1, 3.2, 3.3, and 3.4 show the results of the low-energy search for each element. The graph in each figure shows the calculated and predicted formation energies for structures of varying concentration. The reported concentration is the site concentration, meaning the fraction of the sites in the supercell are filled with a metal. Of greatest interest are the structures with the lowest VASP energies at each concentration. Though the cluster expansion energies are shifted somewhat, the ordering of the structures is usually quite accurate, leading to useful predictions of what the lowest-energy structures are. The search stopped when new low-energy structures stopped being discovered, so only about a third of the structures have VASP energies. The red line on each figure indicates the convex hull. The structures on the convex hull are the lowest-energy structures of their respective concentrations. Structures that would make the line not convex are skipped, meaning only a few structures are included. In experiment, these are the structures that are most likely to form. Images of these structures are included in each figure below
18 3.2 Newly Discovered Structures CE predict VASP calc Formation Energy (ev) Sc site concentration (a) (b) (c) (d) (e) Conc: FE: Conc: FE: Conc: FE: Conc: FE: Conc: FE: Figure 3.1 Formation energy vs. Sc site concentration for structures in the search set. The green circles indicate energies predicted by cluster expansion. Blue stars indicate energies calculated by VASP. The red line indicates the convex hull. A representation of each structure that lies on the convex hull is shown, as well as the structure s site concentration and formation energy in ev.
19 3.2 Newly Discovered Structures CE predict VASP calc Formation Energy (ev) Ti site concentration (a) (b) Conc: FE: Conc: FE: Figure 3.2 Formation energy vs. Ti site concentration for structures in the search set. The green circles indicate energies predicted by cluster expansion. Blue stars indicate energies calculated by VASP. The red line indicates the convex hull. A representation of each structure that lies on the convex hull is shown, as well as the structure s site concentration and formation energy in ev.
20 3.2 Newly Discovered Structures CE predict VASP calc Formation Energy (ev) Y site concentration (a) (b) (c) (d) (e) Conc: FE: Conc: FE: Conc: FE: Conc: FE: Conc: FE: Figure 3.3 Formation energy vs. Y site concentration for structures in the search set. The green circles indicate energies predicted by cluster expansion. Blue stars indicate energies calculated by VASP. The red line indicates the convex hull. A representation of each structure that lies on the convex hull is shown, as well as the structure s site concentration and formation energy in ev.
21 3.2 Newly Discovered Structures CE predict VASP calc Formation Energy (ev) Zr site concentration (a) (b) (c) Conc: FE: Conc: FE: Conc: FE: Figure 3.4 Formation energy vs. Zr site concentration for structures in the search set. The green circles indicate energies predicted by cluster expansion. Blue stars indicate energies calculated by VASP. The red line indicates the convex hull. A representation of each structure that lies on the convex hull is shown, as well as the structure s site concentration and formation energy in ev.
22 3.2 Newly Discovered Structures 17 the graph of formation energies. Only the initial positions are shown, so it can be expected that these structures will relax in their positions somewhat. Each metal had different low-energy structures on the convex hull, though it is interesting to note that all four metals formed the same lowest-energy structure at concentration This structure consisted of two metal atoms for every three carbon, spread evenly in a lattice of equilateral triangles. This is the same structure reported by Sivek for titanium in Section 1.2. Sivek s reported formation energy for this structure ( 0.93 ev) is considerably different than the energy calculated in this work ( ev). This can be attributed to the low-precision setting used for the search. Despite the bias error, we agree that the reported structure has the lowest formation energy for titanium. Further work could involve recalculating these low-energy structures at highprecision to get their absolute formation energies. Other work has shown that in addition to bias error, the formation energies calculated in our search may have an ordering error of about 20 mev. This is due to using supercells of different sizes and shapes in the calculations. In some cases, there are multiple structures in a concentration within 20 ev of the lowest energy. This means that any one of those structures may be the one that actually forms. However, these structures are usually very similar and often relax to the same end positions, so only one structure is shown at each concentration. These figures show some general trends. The group 3 elements, scandium and yttrium, have a minimum in the convex hull at a lower concentration than the group 4 elements, titanium and zirconium. This allows for more structures in the convex hull. Additionally, both of the group 3 elements have lower minimum formation energies, yttrium having the lowest at ev.
23 3.3 Future Work Future Work The search presented in this thesis dealt with a very limited subspace of the structures that could be considered. The search could be expanded from the binary case to include structures with three or four elements. These structures could be made up of multiple metals as well as hydrogen or oxygen. This could lead to understanding of the characteristics of the different elements in these structures. The new enumeration method quickly generates a complete list of structures, but this method could be sped up even further using another tile strategy that has yet to be implemented. Certain combinations of tiles will never work next to each other due to atoms being too close together across the boundary. The current method still generates these structures and removes them individually in testing for forbidden pairs. However, a search could be made beforehand that identifies pairs of tiles that are forbidden, and skips generation of those structures. This would create the same number of structures but drastically reduce the time required, allowing enumeration of higher volume structures. 3.4 Conclusion When used effectively, cluster expansion of adatoms on graphene can expedite the process of searching for low-energy structures, and lead to the discovery of new interesting structures. This allows for a complete search of the set of possible structures, rather than having to guess and check. As this method is utilized, unexpected low-energy structures may be discovered that would not be found without doing a complete search. This was shown to be the case with titanium. The new enumeration method allows for the creation of more complicated structures much faster than standard enumeration would require. Before the new enumeration method was implemented, the most complicated structures considered had two top sites per cell. Implementing the
24 3.4 Conclusion 19 method allowed for the addition of three bridge sites and a hollow site per cell, leading to structures that were much more varied. With this search method, low-energy structures on graphene can be found which may lead to the creation of better batteries, as well as facilitate hydrogen storage.
25 Bibliography [1] Y. Sanchez-Paisal, D. Sanchez-Portal, N. Garmendia, R. Munoz, I. Obieta, J. Arbiol, L. Calvo- Barrio, and A. Ayuela, Zr-metal adhesion on graphenic nanostructures, Applied Physics Letters 93, (2008). [2] J. Sivek, H. Sahin, B. Partoens, and F. M. Peeters, Adsorption and absorption of boron, nitrogen, aluminum, and phosphorus on silicene: Stability and electronic and phonon properties, Physical Review B 87, (2013). [3] G. L. W. Hart and R. W. Forcade, Algorithm for generating derivative structures, Physical Review B 77, (2008). [4] G. L. W. Hart and R. W. Forcade, Generating derivative structures from multilattices: Algorithm and application to hcp alloys, Physical Review B 80, (2009). [5] E. Swenson, Nanostructures formed by calcium substitution in graphane,, Senior Thesis, Brigham Young University, (2016). [6] C. W. Rosenbrock, B. Bieniek, and V. Blum, Hands-On Tutorial on Cluster Expansion IPAM Los Angeles, California, July 2014, (2014). [7] L. J. Nelson, V. Ozolins, C. S. Reese, F. Zhou, and G. L. W. Hart, Cluster expansion made easy with Bayesian compressive sensing, Physical Review B 88, (2013). 20
26 Index Cluster expansion, 6, 18 Convex hull, 12 Density functional theory, 2, 5 Enumeration, 2, 4, 7, 8, 18 Forbidden pairs, 9 Formation energy, 2, 6 Hyrdrogen storage, 1 Labeling, 7 Parent lattice vectors, 7 Scandium, 2, 11 Supercell, 7, 10 Tiles, 9 Titanium, 2, 3, 11 Vacancies, 4, 9 VASP, 5 Volume, 5, 7 Yttrium, 2, 11 Zirconium, 2, 11 21
INVESTIGATION OF THE CO-RE-TI SYSTEM AS A POTENTIAL SUPERALLOY. Johnathon M. Rackham. A senior thesis submitted to the faculty of
 INVESTIGATION OF THE CO-RE-TI SYSTEM AS A POTENTIAL SUPERALLOY by Johnathon M. Rackham A senior thesis submitted to the faculty of Brigham Young University - Idaho in partial fulfillment of the requirements
INVESTIGATION OF THE CO-RE-TI SYSTEM AS A POTENTIAL SUPERALLOY by Johnathon M. Rackham A senior thesis submitted to the faculty of Brigham Young University - Idaho in partial fulfillment of the requirements
6. Computational Design of Energy-related Materials
 6. Computational Design of Energy-related Materials Contents 6.1 Atomistic Simulation Methods for Energy Materials 6.2 ab initio design of photovoltaic materials 6.3 Solid Ion Conductors for Fuel Cells
6. Computational Design of Energy-related Materials Contents 6.1 Atomistic Simulation Methods for Energy Materials 6.2 ab initio design of photovoltaic materials 6.3 Solid Ion Conductors for Fuel Cells
PREDICTING NEW STRUCTURES: THE SIMPLE CUBIC AND PEROVSKITE CASE
 PREDICTING NEW STRUCTURES: THE SIMPLE CUBIC AND PEROVSKITE CASE by Matthew Lords advisor Dr. Gus Hart Physics 492R Capstone Project Report Department of Physics and Astronomy Brigham Young University March
PREDICTING NEW STRUCTURES: THE SIMPLE CUBIC AND PEROVSKITE CASE by Matthew Lords advisor Dr. Gus Hart Physics 492R Capstone Project Report Department of Physics and Astronomy Brigham Young University March
CITY UNIVERSITY OF HONG KONG. Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires
 CITY UNIVERSITY OF HONG KONG Ë Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires u Ä öä ªqk u{ Submitted to Department of Physics and Materials Science gkö y in Partial Fulfillment
CITY UNIVERSITY OF HONG KONG Ë Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires u Ä öä ªqk u{ Submitted to Department of Physics and Materials Science gkö y in Partial Fulfillment
Self-healing tile sets: rates of regrowth in damaged assemblies
 University of Arkansas, Fayetteville ScholarWorks@UARK Mathematical Sciences Undergraduate Honors Theses Mathematical Sciences 5-2015 Self-healing tile sets: rates of regrowth in damaged assemblies Kevin
University of Arkansas, Fayetteville ScholarWorks@UARK Mathematical Sciences Undergraduate Honors Theses Mathematical Sciences 5-2015 Self-healing tile sets: rates of regrowth in damaged assemblies Kevin
The samples used in these calculations were arranged as perfect diamond crystal of
 Chapter 5 Results 5.1 Hydrogen Diffusion 5.1.1 Computational Details The samples used in these calculations were arranged as perfect diamond crystal of a2 2 2 unit cells, i.e. 64 carbon atoms. The effect
Chapter 5 Results 5.1 Hydrogen Diffusion 5.1.1 Computational Details The samples used in these calculations were arranged as perfect diamond crystal of a2 2 2 unit cells, i.e. 64 carbon atoms. The effect
Unit 02 Review: Atomic Theory and Periodic Table Review
 Practice Multiple Choice Questions Unit 02 Review: Atomic Theory and Periodic Table Review 1. The number of neutrons in an atom of radioactive C 14 is: a) 6 c) 8 b) 12 d) 14 2. When a radioactive nucleus
Practice Multiple Choice Questions Unit 02 Review: Atomic Theory and Periodic Table Review 1. The number of neutrons in an atom of radioactive C 14 is: a) 6 c) 8 b) 12 d) 14 2. When a radioactive nucleus
Adsorption and Diffusion of Lithium on MoS 2 Monolayer: The Role of Strain and Concentration
 Int. J. Electrochem. Sci., 8 (2013) 2196-2203 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Adsorption and Diffusion of Lithium on MoS 2 Monolayer: The Role of Strain and Concentration
Int. J. Electrochem. Sci., 8 (2013) 2196-2203 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Adsorption and Diffusion of Lithium on MoS 2 Monolayer: The Role of Strain and Concentration
JUMPMath. Manitoba Curriculum Correlations. Contents
 Manitoba Curriculum Correlations JUMPMath Contents Grade 1 JUMP Math Manitoba Curriculum Correlation 1-1 Grade 2 JUMP Math Manitoba Curriculum Correlation 2-1 Grade 3 JUMP Math Manitoba Curriculum Correlation
Manitoba Curriculum Correlations JUMPMath Contents Grade 1 JUMP Math Manitoba Curriculum Correlation 1-1 Grade 2 JUMP Math Manitoba Curriculum Correlation 2-1 Grade 3 JUMP Math Manitoba Curriculum Correlation
Mutual Influence of Different Hydrogen Concentration in α-zirconium System with Vacancies
 Mutual Influence of Different Hydrogen Concentration in α-zirconium System with Vacancies Heng-Yu Li 12, Hao-Jun Jia 12, Yan-Zhen Zhao 12, L.A. Svyatkin 1, I.P. Chernov 1 a Department of General Physics,
Mutual Influence of Different Hydrogen Concentration in α-zirconium System with Vacancies Heng-Yu Li 12, Hao-Jun Jia 12, Yan-Zhen Zhao 12, L.A. Svyatkin 1, I.P. Chernov 1 a Department of General Physics,
Chemistry Review for 1st Semester Exam
 Chemistry Review for 1st Semester Exam I. Measurement and Calculations: 1. Demonstrate understanding of the use of measurements in science. You should be able to apply the rules of significant figures
Chemistry Review for 1st Semester Exam I. Measurement and Calculations: 1. Demonstrate understanding of the use of measurements in science. You should be able to apply the rules of significant figures
Solution Manual For E&M TIPERs Electricity & Magnetism Tasks by Hieggelke Link download full:
 Solution Manual For E&M TIPERs Electricity & Magnetism Tasks by Hieggelke Link download full: http://testbankcollection.com/download/solution-manual-for-e-and-m-tiperselectricity-and-magnetism-tasks-by-hieggelke
Solution Manual For E&M TIPERs Electricity & Magnetism Tasks by Hieggelke Link download full: http://testbankcollection.com/download/solution-manual-for-e-and-m-tiperselectricity-and-magnetism-tasks-by-hieggelke
First Principles Investigation of Nickel-Graphene Interfaces
 Research Experience for Undergraduates Report First Principles Investigation of Nickel-Graphene Interfaces by Andrew J. Ross (Saint Anselm College) Advisers: L. Adamska Y. Lin I. I. Oleynik Physics Department
Research Experience for Undergraduates Report First Principles Investigation of Nickel-Graphene Interfaces by Andrew J. Ross (Saint Anselm College) Advisers: L. Adamska Y. Lin I. I. Oleynik Physics Department
1. The elements on the Periodic Table are arranged in order of increasing A atomic mass C molar mass
 1. The elements on the Periodic Table are arranged in order of increasing A atomic mass C molar mass A Br, Ga, Hg C O, S, Se B atomic number D oxidation number 2. Which list includes elements with the
1. The elements on the Periodic Table are arranged in order of increasing A atomic mass C molar mass A Br, Ga, Hg C O, S, Se B atomic number D oxidation number 2. Which list includes elements with the
Binding energy of 2D materials using Quantum Monte Carlo
 Quantum Monte Carlo in the Apuan Alps IX International Workshop, 26th July to 2nd August 2014 The Apuan Alps Centre for Physics @ TTI, Vallico Sotto, Tuscany, Italy Binding energy of 2D materials using
Quantum Monte Carlo in the Apuan Alps IX International Workshop, 26th July to 2nd August 2014 The Apuan Alps Centre for Physics @ TTI, Vallico Sotto, Tuscany, Italy Binding energy of 2D materials using
Southeast University, Nanjing, China 2 Department of Applied Physics, Aalto University,
 Supplementary Information to Solubility of Boron, Carbon and Nitrogen in Transition Metals: Getting Insight into Trends from First-Principles Calculations Xiaohui Hu, 1,2 Torbjörn Björkman 2,3, Harri Lipsanen
Supplementary Information to Solubility of Boron, Carbon and Nitrogen in Transition Metals: Getting Insight into Trends from First-Principles Calculations Xiaohui Hu, 1,2 Torbjörn Björkman 2,3, Harri Lipsanen
Supplementary Information
 Supplementary Information Supplementary Figure 1: Electronic Kohn-Sham potential profile of a charged monolayer MoTe 2 calculated using PBE-DFT. Plotted is the averaged electronic Kohn- Sham potential
Supplementary Information Supplementary Figure 1: Electronic Kohn-Sham potential profile of a charged monolayer MoTe 2 calculated using PBE-DFT. Plotted is the averaged electronic Kohn- Sham potential
HALL EFFECT IN SEMICONDUCTORS
 Warsaw University of Technology Faculty of Physics Physics Laboratory I P Andrzej Kubiaczyk 30 HALL EFFECT IN SEMICONDUCTORS 1. ackground 1.1. Electron motion in electric and magnetic fields A particle
Warsaw University of Technology Faculty of Physics Physics Laboratory I P Andrzej Kubiaczyk 30 HALL EFFECT IN SEMICONDUCTORS 1. ackground 1.1. Electron motion in electric and magnetic fields A particle
Chromium Cluster on Defected Graphene
 Chromium Cluster on Defected Graphene Yuhang Liu June 29, 2017 Abstract In this work, diffusion process of Cr atoms on two types of defected graphene and structure and magnetic properties of Cr cluster
Chromium Cluster on Defected Graphene Yuhang Liu June 29, 2017 Abstract In this work, diffusion process of Cr atoms on two types of defected graphene and structure and magnetic properties of Cr cluster
CHAPTER 3 THE STRUCTURE OF CRYSTALLINE SOLIDS PROBLEM SOLUTIONS
 CHAPTER THE STRUCTURE OF CRYSTALLINE SOLIDS PROBLEM SOLUTIONS Fundamental Concepts.1 What is the difference between atomic structure and crystal structure? Atomic structure relates to the number of protons
CHAPTER THE STRUCTURE OF CRYSTALLINE SOLIDS PROBLEM SOLUTIONS Fundamental Concepts.1 What is the difference between atomic structure and crystal structure? Atomic structure relates to the number of protons
X B1 X B Quiz Fall points total. 1. Thermodynamics. (50 points)
 3.012 Quiz 4 3.012 12.15.03 Fall 2003 100 points total 1. Thermodynamics. (50 points) a. Shown below is a portion of the nickel-titanium binary phase diagram. Using the diagram, answer the questions below.
3.012 Quiz 4 3.012 12.15.03 Fall 2003 100 points total 1. Thermodynamics. (50 points) a. Shown below is a portion of the nickel-titanium binary phase diagram. Using the diagram, answer the questions below.
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. Intrinsically patterned two-dimensional materials for selective adsorption of molecules and nanoclusters X. Lin 1,, J. C. Lu 1,, Y. Shao 1,, Y. Y. Zhang
In the format provided by the authors and unedited. Intrinsically patterned two-dimensional materials for selective adsorption of molecules and nanoclusters X. Lin 1,, J. C. Lu 1,, Y. Shao 1,, Y. Y. Zhang
Electron Configurations
 Section 3 Electron Configurations Key Terms electron configuration Pauli exclusion principle noble gas Aufbau principle Hund s rule noble-gas configuration Main Ideas Electrons fill in the lowest-energy
Section 3 Electron Configurations Key Terms electron configuration Pauli exclusion principle noble gas Aufbau principle Hund s rule noble-gas configuration Main Ideas Electrons fill in the lowest-energy
Size, Shape and Composition Dependent Model for Metal Nanoparticle Stability Prediction
 Supplementary Information Size, Shape and Composition Dependent Model for Metal Nanoparticle Stability Prediction Zihao Yan, Michael G. Taylor, Ashley Mascareno, and Giannis Mpourmpakis* Department of
Supplementary Information Size, Shape and Composition Dependent Model for Metal Nanoparticle Stability Prediction Zihao Yan, Michael G. Taylor, Ashley Mascareno, and Giannis Mpourmpakis* Department of
Defects in TiO 2 Crystals
 , March 13-15, 2013, Hong Kong Defects in TiO 2 Crystals Richard Rivera, Arvids Stashans 1 Abstract-TiO 2 crystals, anatase and rutile, have been studied using Density Functional Theory (DFT) and the Generalized
, March 13-15, 2013, Hong Kong Defects in TiO 2 Crystals Richard Rivera, Arvids Stashans 1 Abstract-TiO 2 crystals, anatase and rutile, have been studied using Density Functional Theory (DFT) and the Generalized
Linker Dependent Bond Rupture Force Measurements in Single-Molecule Junctions
 Supplemental Information Linker Dependent Bond Rupture Force Measurements in Single-Molecule Junctions M. Frei 1, S Aradhya 1, M. S. Hybertsen 2, L. Venkataraman 1 1 Department of Applied Physics and Applied
Supplemental Information Linker Dependent Bond Rupture Force Measurements in Single-Molecule Junctions M. Frei 1, S Aradhya 1, M. S. Hybertsen 2, L. Venkataraman 1 1 Department of Applied Physics and Applied
Measurements of Liquid Scintillator Light Yield for Future Neutrino Experiments
 Measurements of Liquid Scintillator Light Yield for Future Neutrino Experiments Athena Ierokomos University of California, Berkeley 2013 University of California, Los Angeles REU Program Abstract Neutrinoless
Measurements of Liquid Scintillator Light Yield for Future Neutrino Experiments Athena Ierokomos University of California, Berkeley 2013 University of California, Los Angeles REU Program Abstract Neutrinoless
Radioactivity, Radioactive Dating, Band Theory of Solids, and Quantum Electronics Devices such as LEDs
 Physics 5K Lecture Friday May 11, 2012 Radioactivity, Radioactive Dating, Band Theory of Solids, and Quantum Electronics Devices such as LEDs Joel Primack Physics Department UCSC Friday, May 11, 12 Distant
Physics 5K Lecture Friday May 11, 2012 Radioactivity, Radioactive Dating, Band Theory of Solids, and Quantum Electronics Devices such as LEDs Joel Primack Physics Department UCSC Friday, May 11, 12 Distant
Copyright 2010 Sponholtz Productions, LLC Page 1
 Introduction to the Atom Key Terms: abbreviated electron configuration - combines the inert, noble core electrons with the remaining, outermost electrons, which are commonly called valence electrons. angular
Introduction to the Atom Key Terms: abbreviated electron configuration - combines the inert, noble core electrons with the remaining, outermost electrons, which are commonly called valence electrons. angular
Astronomy 102 Lab: Hubble Law
 Name: Astronomy 102 Lab: Hubble Law Part of today s lab will involve the use of laptops. If you own one, please bring it to class. Pre-Lab Assignment: In this week's lab, you will study the expansion of
Name: Astronomy 102 Lab: Hubble Law Part of today s lab will involve the use of laptops. If you own one, please bring it to class. Pre-Lab Assignment: In this week's lab, you will study the expansion of
and Polar Molecules Patrick Regan December 5, 2014
 Forcing Chemistry: Comparing Intermolecular Forces Between Ions and Polar Molecules Patrick Regan December 5, 201 1 Abstract Molecules come in varying shapes, sizes, and compositions. Consequently, different
Forcing Chemistry: Comparing Intermolecular Forces Between Ions and Polar Molecules Patrick Regan December 5, 201 1 Abstract Molecules come in varying shapes, sizes, and compositions. Consequently, different
1. The elements on the Periodic Table are arranged in order of increasing A atomic mass C molar mass
 1. The elements on the Periodic Table are arranged in order of increasing A atomic mass C molar mass A Br, Ga, Hg C O, S, Se B atomic number D oxidation number 2. Which list includes elements with the
1. The elements on the Periodic Table are arranged in order of increasing A atomic mass C molar mass A Br, Ga, Hg C O, S, Se B atomic number D oxidation number 2. Which list includes elements with the
Solutions for Assignment-8
 Solutions for Assignment-8 Q1. The process of adding impurities to a pure semiconductor is called: [1] (a) Mixing (b) Doping (c) Diffusing (d) None of the above In semiconductor production, doping intentionally
Solutions for Assignment-8 Q1. The process of adding impurities to a pure semiconductor is called: [1] (a) Mixing (b) Doping (c) Diffusing (d) None of the above In semiconductor production, doping intentionally
Lecture Outline: Spectroscopy (Ch. 4)
 Lecture Outline: Spectroscopy (Ch. 4) NOTE: These are just an outline of the lectures and a guide to the textbook. The material will be covered in more detail in class. We will cover nearly all of the
Lecture Outline: Spectroscopy (Ch. 4) NOTE: These are just an outline of the lectures and a guide to the textbook. The material will be covered in more detail in class. We will cover nearly all of the
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Two-dimensional BX (X=P, As, Sb) Semiconductors with Mobilities
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Two-dimensional BX (X=P, As, Sb) Semiconductors with Mobilities
arxiv: v2 [cond-mat.mtrl-sci] 24 Dec 2014
![arxiv: v2 [cond-mat.mtrl-sci] 24 Dec 2014 arxiv: v2 [cond-mat.mtrl-sci] 24 Dec 2014](/thumbs/93/113232647.jpg) Defect in Phosphorene arxiv:1411.6986v2 [cond-mat.mtrl-sci] 24 Dec 2014 Wei Hu 1, 2, 3, and Jinlong Yang 1 Computational Research Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
Defect in Phosphorene arxiv:1411.6986v2 [cond-mat.mtrl-sci] 24 Dec 2014 Wei Hu 1, 2, 3, and Jinlong Yang 1 Computational Research Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
Student Exploration: Electron Configuration
 Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Name: Date: Student Exploration: Electron Configuration Vocabulary: atomic number, atomic radius, Aufbau principle, chemical family, diagonal rule, electron configuration, Hund s rule, orbital, Pauli exclusion
Chapter 7: Nuclear Energy and Society
 Chapter 7: Nuclear Energy and Society Mini Investigation: Simulating Nuclear Reactions, page 317 Answers may vary. Sample answers: A. The graph is a curve showing a decline in the number of heads, approaching
Chapter 7: Nuclear Energy and Society Mini Investigation: Simulating Nuclear Reactions, page 317 Answers may vary. Sample answers: A. The graph is a curve showing a decline in the number of heads, approaching
Optimizing Solvent Blends for a Quinary System
 The University of Akron IdeaExchange@UAkron Honors Research Projects The Dr. Gary B. and Pamela S. Williams Honors College Spring 2016 Optimizing Solvent Blends for a Quinary System Thomas L. Hoy tlh125@zips.uakron.edu,
The University of Akron IdeaExchange@UAkron Honors Research Projects The Dr. Gary B. and Pamela S. Williams Honors College Spring 2016 Optimizing Solvent Blends for a Quinary System Thomas L. Hoy tlh125@zips.uakron.edu,
CBSE XII Physics 2015
 Time: 3 hours; Maximum Marks: 70 General Instructions: 1. All questions are compulsory. There are 26 questions in all. 2. This question paper has five sections: Section A, Section B, Section, Section D
Time: 3 hours; Maximum Marks: 70 General Instructions: 1. All questions are compulsory. There are 26 questions in all. 2. This question paper has five sections: Section A, Section B, Section, Section D
Review for Unit Test #2: Chemical Bonding
 Practice Multiple hoice Questions: Review for Unit Test #2: hemical Bonding 1. Atoms form chemical bonds to: a) attain a more stable electron configuration c) increase their energy b) neutralize their
Practice Multiple hoice Questions: Review for Unit Test #2: hemical Bonding 1. Atoms form chemical bonds to: a) attain a more stable electron configuration c) increase their energy b) neutralize their
Permutation Generators Based on Unbalanced Feistel Network: Analysis of the Conditions of Pseudorandomness 1
 Permutation Generators Based on Unbalanced Feistel Network: Analysis of the Conditions of Pseudorandomness 1 Kwangsu Lee A Thesis for the Degree of Master of Science Division of Computer Science, Department
Permutation Generators Based on Unbalanced Feistel Network: Analysis of the Conditions of Pseudorandomness 1 Kwangsu Lee A Thesis for the Degree of Master of Science Division of Computer Science, Department
Increasing the Computational Efficiency of Combinatoric Searches
 Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2016-09-01 Increasing the Computational Efficiency of Combinatoric Searches Wiley Spencer Morgan Brigham Young University Follow
Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2016-09-01 Increasing the Computational Efficiency of Combinatoric Searches Wiley Spencer Morgan Brigham Young University Follow
AN ACCELERATED SURFACE-HOPPING METHOD FOR COMPUTATIONAL SEMICLASSICAL MOLECULAR DYNAMICS. Laren K. Mortensen
 AN ACCELERATED SURFACE-HOPPING METHOD FOR COMPUTATIONAL SEMICLASSICAL MOLECULAR DYNAMICS by Laren K. Mortensen A senior thesis submitted to the faculty of Brigham Young University in partial fulfillment
AN ACCELERATED SURFACE-HOPPING METHOD FOR COMPUTATIONAL SEMICLASSICAL MOLECULAR DYNAMICS by Laren K. Mortensen A senior thesis submitted to the faculty of Brigham Young University in partial fulfillment
Report on Atomistic Modeling of Bonding in Carbon-Based Nanostructures
 Report on Atomistic Modeling of Bonding in Carbon-Based Nanostructures Timothy Stillings Department of Physics, Astronomy and Materials Science Missouri State University Advisor: Ridwan Sakidja Abstract
Report on Atomistic Modeling of Bonding in Carbon-Based Nanostructures Timothy Stillings Department of Physics, Astronomy and Materials Science Missouri State University Advisor: Ridwan Sakidja Abstract
Chapter 7 Ionic And Metallic Bonding Answer Key Pearson Education
 Chapter 7 Ionic And Metallic Bonding Answer Key Pearson Education CHAPTER 7 IONIC AND METALLIC BONDING ANSWER KEY PEARSON EDUCATION PDF - Are you looking for chapter 7 ionic and metallic bonding answer
Chapter 7 Ionic And Metallic Bonding Answer Key Pearson Education CHAPTER 7 IONIC AND METALLIC BONDING ANSWER KEY PEARSON EDUCATION PDF - Are you looking for chapter 7 ionic and metallic bonding answer
AP* Chapter 10. Liquids and Solids. Friday, November 22, 13
 AP* Chapter 10 Liquids and Solids AP Learning Objectives LO 1.11 The student can analyze data, based on periodicity and the properties of binary compounds, to identify patterns and generate hypotheses
AP* Chapter 10 Liquids and Solids AP Learning Objectives LO 1.11 The student can analyze data, based on periodicity and the properties of binary compounds, to identify patterns and generate hypotheses
Mat E 272 Lecture 25: Electrical properties of materials
 Mat E 272 Lecture 25: Electrical properties of materials December 6, 2001 Introduction: Calcium and copper are both metals; Ca has a valence of +2 (2 electrons per atom) while Cu has a valence of +1 (1
Mat E 272 Lecture 25: Electrical properties of materials December 6, 2001 Introduction: Calcium and copper are both metals; Ca has a valence of +2 (2 electrons per atom) while Cu has a valence of +1 (1
Boxes within boxes: discovering the atomic structure of an Al-Co-Ni quasicrystal
 Boxes within boxes: discovering the atomic structure of an Al-Co-Ni quasicrystal Nan Gu, Marek Mihalkovič, and C.L. Henley, Cornell University LASSP Pizza talk, Tues July 11, 2006 1 1. Quasicrystals Fourier
Boxes within boxes: discovering the atomic structure of an Al-Co-Ni quasicrystal Nan Gu, Marek Mihalkovič, and C.L. Henley, Cornell University LASSP Pizza talk, Tues July 11, 2006 1 1. Quasicrystals Fourier
How do Elements Combine to Form Compounds?
 How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Think about the calcium atom vs the calcium ion Compounds account for the huge variety of matter on Earth All the compounds that
How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Think about the calcium atom vs the calcium ion Compounds account for the huge variety of matter on Earth All the compounds that
= proton (positive charge) = electron (negative charge) = neutron (no charge) A Z. ,, and are notations that represent isotopes of carbon.
 ChemQuest 8 Name: Date: Hour: Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following
ChemQuest 8 Name: Date: Hour: Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following
1 Adsorption of NO 2 on Pd(100) Juan M. Lorenzi, Sebastian Matera, and Karsten Reuter,
 Supporting information: Synergistic inhibition of oxide formation in oxidation catalysis: A first-principles kinetic Monte Carlo study of NO+CO oxidation at Pd(100) Juan M. Lorenzi, Sebastian Matera, and
Supporting information: Synergistic inhibition of oxide formation in oxidation catalysis: A first-principles kinetic Monte Carlo study of NO+CO oxidation at Pd(100) Juan M. Lorenzi, Sebastian Matera, and
arxiv: v1 [cond-mat.mes-hall] 15 Aug 2014
![arxiv: v1 [cond-mat.mes-hall] 15 Aug 2014 arxiv: v1 [cond-mat.mes-hall] 15 Aug 2014](/thumbs/89/97831186.jpg) The potential applications of phosphorene as anode arxiv:1408.3488v1 [cond-mat.mes-hall] 15 Aug 2014 materials in Li-ion batteries Shijun Zhao,, and Wei Kang, HEDPS, Center for Applied Physics and Technology,
The potential applications of phosphorene as anode arxiv:1408.3488v1 [cond-mat.mes-hall] 15 Aug 2014 materials in Li-ion batteries Shijun Zhao,, and Wei Kang, HEDPS, Center for Applied Physics and Technology,
Inorganic Exam 1 Chm October 2010
 Inorganic Exam 1 Chm 451 28 October 2010 Name: Instructions. Always show your work where required for full credit. 1. In the molecule CO 2, the first step in the construction of the MO diagram was to consider
Inorganic Exam 1 Chm 451 28 October 2010 Name: Instructions. Always show your work where required for full credit. 1. In the molecule CO 2, the first step in the construction of the MO diagram was to consider
How do Elements Combine to Form Compounds?
 How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Compounds account for the huge variety of matter on Earth All the compounds that exist on Earth are built from elements 118 elements
How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Compounds account for the huge variety of matter on Earth All the compounds that exist on Earth are built from elements 118 elements
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Energetics of Hydrogen Storage Reactions: The Power of DFT. Jan Herbst Materials and Processes Laboratory GM R&D Center
 Energetics of Hydrogen Storage Reactions: The Power of DFT Jan Herbst Materials and Processes Laboratory GM R&D Center ACKNOWLEDGEMENTS Fellow explorer: Lou Hector Sometime shipmate: Wes Capehart Outline
Energetics of Hydrogen Storage Reactions: The Power of DFT Jan Herbst Materials and Processes Laboratory GM R&D Center ACKNOWLEDGEMENTS Fellow explorer: Lou Hector Sometime shipmate: Wes Capehart Outline
Quantum anomalous Hall states on decorated magnetic surfaces
 Quantum anomalous Hall states on decorated magnetic surfaces David Vanderbilt Rutgers University Kevin Garrity & D.V. Phys. Rev. Lett.110, 116802 (2013) Recently: Topological insulators (TR-invariant)
Quantum anomalous Hall states on decorated magnetic surfaces David Vanderbilt Rutgers University Kevin Garrity & D.V. Phys. Rev. Lett.110, 116802 (2013) Recently: Topological insulators (TR-invariant)
PHYSICAL REVIEW LETTERS
 PHYSICAL REVIEW LETTERS VOLUME 86 28 MAY 21 NUMBER 22 Mathematical Analysis of Coupled Parallel Simulations Michael R. Shirts and Vijay S. Pande Department of Chemistry, Stanford University, Stanford,
PHYSICAL REVIEW LETTERS VOLUME 86 28 MAY 21 NUMBER 22 Mathematical Analysis of Coupled Parallel Simulations Michael R. Shirts and Vijay S. Pande Department of Chemistry, Stanford University, Stanford,
H ψ = E ψ. Introduction to Exact Diagonalization. Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden
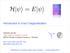 H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
Practice Paper-3. Q. 2. An electron beam projected along + X-axis, in a magnetic field along the + Z-axis. What is
 Practice Paper-3 Q. 1. An electric dipole of dipole moment 20 10 6 cm is enclosed by a closed surface. What is the net flux coming out of the surface? Q. 2. An electron beam projected along + X-axis, in
Practice Paper-3 Q. 1. An electric dipole of dipole moment 20 10 6 cm is enclosed by a closed surface. What is the net flux coming out of the surface? Q. 2. An electron beam projected along + X-axis, in
Thursday, February 26, 15
 High-throughput Data and New Representations for Models and Machine Learning Gus L. W. Hart Why am I here? Automatic-FLOW for Materials Discovery This talk is for you, not for me Automatic-FLOW for Materials
High-throughput Data and New Representations for Models and Machine Learning Gus L. W. Hart Why am I here? Automatic-FLOW for Materials Discovery This talk is for you, not for me Automatic-FLOW for Materials
Thermal transport from first-principles DFT calculations. Keivan Esfarjani MIT. Department of Mechanical Engineering. 5/23/2012 Phonon UWM 1
 Thermal transport from first-principles DFT calculations Keivan Esfarjani Department of Mechanical Engineering MIT 5/23/2012 Phonon School @ UWM 1 Classical MD simulations use an empirical potential fitted
Thermal transport from first-principles DFT calculations Keivan Esfarjani Department of Mechanical Engineering MIT 5/23/2012 Phonon School @ UWM 1 Classical MD simulations use an empirical potential fitted
Nuclear Physics Fundamentals and Application Prof. H.C. Verma Department of Physics Indian Institute of Technology, Kanpur
 Nuclear Physics Fundamentals and Application Prof. H.C. Verma Department of Physics Indian Institute of Technology, Kanpur Lecture - 34 Nuclear fission of uranium So, we talked about fission reactions
Nuclear Physics Fundamentals and Application Prof. H.C. Verma Department of Physics Indian Institute of Technology, Kanpur Lecture - 34 Nuclear fission of uranium So, we talked about fission reactions
For more information, please contact: or +1 (302)
 Introduction Graphene Raman Analyzer: Carbon Nanomaterials Characterization Dawn Yang and Kristen Frano B&W Tek Carbon nanomaterials constitute a variety of carbon allotropes including graphene, graphene
Introduction Graphene Raman Analyzer: Carbon Nanomaterials Characterization Dawn Yang and Kristen Frano B&W Tek Carbon nanomaterials constitute a variety of carbon allotropes including graphene, graphene
Simulation: Density FOR THE TEACHER
 Simulation: Density FOR THE TEACHER Summary In this simulation, students will investigate the effect of changing variables on both the volume and the density of a solid, a liquid and a gas sample. Students
Simulation: Density FOR THE TEACHER Summary In this simulation, students will investigate the effect of changing variables on both the volume and the density of a solid, a liquid and a gas sample. Students
Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene
 Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene Mohamed Hassan, Michael Walter *,,, and Michael Moseler, Freiburg
Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene Mohamed Hassan, Michael Walter *,,, and Michael Moseler, Freiburg
Calibration Routine. Store in HDD. Switch "Program Control" Ref 1/ Ref 2 Manual Automatic
 4.2 IMPLEMENTATION LABVIEW 4.2.1 LabVIEW features LabVIEW (short for Laboratory Virtual Instrument Engineering Workbench) originally released for the Apple Macintosh in 1986. It is a highly productive
4.2 IMPLEMENTATION LABVIEW 4.2.1 LabVIEW features LabVIEW (short for Laboratory Virtual Instrument Engineering Workbench) originally released for the Apple Macintosh in 1986. It is a highly productive
Understanding and Measuring Urban Expansion
 VOLUME 1: AREAS AND DENSITIES 21 CHAPTER 3 Understanding and Measuring Urban Expansion THE CLASSIFICATION OF SATELLITE IMAGERY The maps of the urban extent of cities in the global sample were created using
VOLUME 1: AREAS AND DENSITIES 21 CHAPTER 3 Understanding and Measuring Urban Expansion THE CLASSIFICATION OF SATELLITE IMAGERY The maps of the urban extent of cities in the global sample were created using
Student Handout: Unit 1 Lesson 1. Diagnostic/Introductory Activity
 Diagnostic/Introductory Activity Examine the pictures below and identify the hazard and to what degree (danger, warning, or caution) a product is dangerous SNC1PI_Unit1_Lesson1_Diagnostic Student Instruction
Diagnostic/Introductory Activity Examine the pictures below and identify the hazard and to what degree (danger, warning, or caution) a product is dangerous SNC1PI_Unit1_Lesson1_Diagnostic Student Instruction
Experimental designs for multiple responses with different models
 Graduate Theses and Dissertations Graduate College 2015 Experimental designs for multiple responses with different models Wilmina Mary Marget Iowa State University Follow this and additional works at:
Graduate Theses and Dissertations Graduate College 2015 Experimental designs for multiple responses with different models Wilmina Mary Marget Iowa State University Follow this and additional works at:
Designing Graphene for Hydrogen Storage
 Designing Graphene for Hydrogen Storage Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Introduction to Hydrogen Storage Epitaxial Graphene Hydrogen Storage
Designing Graphene for Hydrogen Storage Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Introduction to Hydrogen Storage Epitaxial Graphene Hydrogen Storage
Hello. The form at /genedchecklist/welcome.cfm was submitted on Monday, September 30, 2013 at 2:13 PM. The data is below:
 Hello. The form at /genedchecklist/welcome.cfm was submitted on Monday, September 30, 2013 at 2:13 PM. The data is below: Course information: ------------------- 1. Course name: Chemistry of the Elements
Hello. The form at /genedchecklist/welcome.cfm was submitted on Monday, September 30, 2013 at 2:13 PM. The data is below: Course information: ------------------- 1. Course name: Chemistry of the Elements
SEQUENTIAL AND SIMULTANEOUS LIFTING IN THE NODE PACKING POLYHEDRON JEFFREY WILLIAM PAVELKA. B.S., Kansas State University, 2011
 SEQUENTIAL AND SIMULTANEOUS LIFTING IN THE NODE PACKING POLYHEDRON by JEFFREY WILLIAM PAVELKA B.S., Kansas State University, 2011 A THESIS Submitted in partial fulfillment of the requirements for the degree
SEQUENTIAL AND SIMULTANEOUS LIFTING IN THE NODE PACKING POLYHEDRON by JEFFREY WILLIAM PAVELKA B.S., Kansas State University, 2011 A THESIS Submitted in partial fulfillment of the requirements for the degree
cha1873x_p02.qxd 3/21/05 1:01 PM Page 104 PART TWO
 cha1873x_p02.qxd 3/21/05 1:01 PM Page 104 PART TWO ROOTS OF EQUATIONS PT2.1 MOTIVATION Years ago, you learned to use the quadratic formula x = b ± b 2 4ac 2a to solve f(x) = ax 2 + bx + c = 0 (PT2.1) (PT2.2)
cha1873x_p02.qxd 3/21/05 1:01 PM Page 104 PART TWO ROOTS OF EQUATIONS PT2.1 MOTIVATION Years ago, you learned to use the quadratic formula x = b ± b 2 4ac 2a to solve f(x) = ax 2 + bx + c = 0 (PT2.1) (PT2.2)
Lecture 6 - Bonding in Crystals
 Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
CHAPTER 5. Electrons in Atoms. Rutherford Model. Bohr Model. Plum Pudding Model. 5.1 Atomic Models
 CHAPTER 5 Electrons in Atoms 5.1 Atomic Models The Chemical properties of atoms, ions, and molecules are related to the arrangement of the electrons within them. The first model of the atom was Dalton
CHAPTER 5 Electrons in Atoms 5.1 Atomic Models The Chemical properties of atoms, ions, and molecules are related to the arrangement of the electrons within them. The first model of the atom was Dalton
SIMULATION-BASED APPROXIMATE GLOBAL FAULT COLLAPSING
 SIMULATION-BASED APPROXIMATE GLOBAL FAULT COLLAPSING Hussain Al-Asaad and Raymond Lee Computer Engineering Research Laboratory Department of Electrical & Computer Engineering University of California One
SIMULATION-BASED APPROXIMATE GLOBAL FAULT COLLAPSING Hussain Al-Asaad and Raymond Lee Computer Engineering Research Laboratory Department of Electrical & Computer Engineering University of California One
DFT modeling of novel materials for hydrogen storage
 DFT modeling of novel materials for hydrogen storage Tejs Vegge 1, J Voss 1,2, Q Shi 1, HS Jacobsen 1, JS Hummelshøj 1,2, AS Pedersen 1, JK Nørskov 2 1 Materials Research Department, Risø National Laboratory,
DFT modeling of novel materials for hydrogen storage Tejs Vegge 1, J Voss 1,2, Q Shi 1, HS Jacobsen 1, JS Hummelshøj 1,2, AS Pedersen 1, JK Nørskov 2 1 Materials Research Department, Risø National Laboratory,
Taylor series. Chapter Introduction From geometric series to Taylor polynomials
 Chapter 2 Taylor series 2. Introduction The topic of this chapter is find approximations of functions in terms of power series, also called Taylor series. Such series can be described informally as infinite
Chapter 2 Taylor series 2. Introduction The topic of this chapter is find approximations of functions in terms of power series, also called Taylor series. Such series can be described informally as infinite
Defects activated photoluminescence in two-dimensional semiconductors: interplay between bound, charged, and free excitons.
 Supplementary Information for Defects activated photoluminescence in two-dimensional semiconductors: interplay between bound, charged, and free excitons Sefaattin Tongay 1, 2 +, Joonki Suh 1, 2 +, Can
Supplementary Information for Defects activated photoluminescence in two-dimensional semiconductors: interplay between bound, charged, and free excitons Sefaattin Tongay 1, 2 +, Joonki Suh 1, 2 +, Can
Unit Two Test Review. Click to get a new slide. Choose your answer, then click to see if you were correct.
 Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Phase Space Tomography of an Electron Beam in a Superconducting RF Linac
 Phase Space Tomography of an Electron Beam in a Superconducting RF Linac Tianyi Ge 1, and Jayakar Thangaraj 2, 1 Department of Physics and Astronomy, University of California, Los Angeles, California 90095,
Phase Space Tomography of an Electron Beam in a Superconducting RF Linac Tianyi Ge 1, and Jayakar Thangaraj 2, 1 Department of Physics and Astronomy, University of California, Los Angeles, California 90095,
Document Version Publisher s PDF, also known as Version of Record (includes final page, issue and volume numbers)
 Parametrization of modified embedded-atom-method potentials for Rh, Pd, Ir, and Pt based on density functional theory calculations, with applications to surface properties Beurden, van, P.; Kramer, G.J.
Parametrization of modified embedded-atom-method potentials for Rh, Pd, Ir, and Pt based on density functional theory calculations, with applications to surface properties Beurden, van, P.; Kramer, G.J.
Chapter 4: Bonding in Solids and Electronic Properties. Free electron theory
 Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
CHEM 130 Exp. 8: Molecular Models
 CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
TiC 2 : A New Two Dimensional Sheet beyond MXenes
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supplementary Information (SI) TiC 2 : A New Two Dimensional Sheet beyond MXenes Tianshan Zhao,
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supplementary Information (SI) TiC 2 : A New Two Dimensional Sheet beyond MXenes Tianshan Zhao,
SCIENCE & TECHNOLOGY
 Pertanika J. Sci. & Technol. 25 (S): 205-212 (2017) SCIENCE & TECHNOLOGY Journal homepage: http://www.pertanika.upm.edu.my/ Effect of Boron and Oxygen Doping to Graphene Band Structure Siti Fazlina bt
Pertanika J. Sci. & Technol. 25 (S): 205-212 (2017) SCIENCE & TECHNOLOGY Journal homepage: http://www.pertanika.upm.edu.my/ Effect of Boron and Oxygen Doping to Graphene Band Structure Siti Fazlina bt
Outline. Introduction: graphene. Adsorption on graphene: - Chemisorption - Physisorption. Summary
 Outline Introduction: graphene Adsorption on graphene: - Chemisorption - Physisorption Summary 1 Electronic band structure: Electronic properties K Γ M v F = 10 6 ms -1 = c/300 massless Dirac particles!
Outline Introduction: graphene Adsorption on graphene: - Chemisorption - Physisorption Summary 1 Electronic band structure: Electronic properties K Γ M v F = 10 6 ms -1 = c/300 massless Dirac particles!
Team H2 Final Report ENMA490. Norena Beaty, Nick Faenza, Tanner Hamann, Owen McGovern, Santiago Miret, and Mark Reese
 Team H2 Final Report ENMA490 Norena Beaty, Nick Faenza, Tanner Hamann, Owen McGovern, Santiago Miret, and Mark Reese Current Energy System Unsustainable Fossil fuels vs. hydrogen Nanoparticle Catalysts
Team H2 Final Report ENMA490 Norena Beaty, Nick Faenza, Tanner Hamann, Owen McGovern, Santiago Miret, and Mark Reese Current Energy System Unsustainable Fossil fuels vs. hydrogen Nanoparticle Catalysts
California CCSS Mathematics Grades 1-3
 Operations and Algebraic Thinking Represent and solve problems involving addition and subtraction. 1.OA.1. Use addition and subtraction within 20 to solve word problems involving situations of adding to,
Operations and Algebraic Thinking Represent and solve problems involving addition and subtraction. 1.OA.1. Use addition and subtraction within 20 to solve word problems involving situations of adding to,
Unique phenomena of tungsten associated with fusion reactor: uncertainties of stable hydrogen configuration tapped in tungsten vacancy
 Unique phenomena of tungsten associated with fusion reactor: uncertainties of stable hydrogen configuration tapped in tungsten vacancy Kyushu University Kazuhito Ohsawa Technical Meeting of the International
Unique phenomena of tungsten associated with fusion reactor: uncertainties of stable hydrogen configuration tapped in tungsten vacancy Kyushu University Kazuhito Ohsawa Technical Meeting of the International
1 Development of the Atomic Theory
 CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
4R & 4A Math Pacing Guides
 GRADING PERIOD: 1st Nine Weeks Getting to Know You - Community Building 4.14- Data a. Collect data, using observations, surveys, measurement, polls, or questionnaires. b. Organize data into a chart or
GRADING PERIOD: 1st Nine Weeks Getting to Know You - Community Building 4.14- Data a. Collect data, using observations, surveys, measurement, polls, or questionnaires. b. Organize data into a chart or
Phenomenology of a Toy Model Inspired by the Spontaneous Reduction of the Spectral Dimension in Quantum Gravity. Patrick Greene
 the Phenomenology of a Toy Model Inspired by the Spontaneous Reduction of the Spectral Dimension in Quantum Gravity Department of Physics University of New Hampshire Senior Thesis Presentations, 2015 Outline
the Phenomenology of a Toy Model Inspired by the Spontaneous Reduction of the Spectral Dimension in Quantum Gravity Department of Physics University of New Hampshire Senior Thesis Presentations, 2015 Outline
Faculté Polytechnique
 Faculté Polytechnique The use of heat transfer functions for heat flow computation through multilayer walls Master Thesis Xavier Botey i Bassols Under the supervision of teachers Mr. Eric DUMONT Mr. Renato
Faculté Polytechnique The use of heat transfer functions for heat flow computation through multilayer walls Master Thesis Xavier Botey i Bassols Under the supervision of teachers Mr. Eric DUMONT Mr. Renato
Topic 6a Electrode Potentials Revision Notes
 Topic 6a Electrode Potentials Revision Notes 1) Redox Redox reactions involve the transfer of electrons e.g. in the reaction between zinc metal and copper (II) sulphate, electrons are transferred from
Topic 6a Electrode Potentials Revision Notes 1) Redox Redox reactions involve the transfer of electrons e.g. in the reaction between zinc metal and copper (II) sulphate, electrons are transferred from
Extrinsic Defect Reactions in
 Chapter 5 Extrinsic Defect Reactions in Perovskite Materials The work presented in this Chapter has been published in Solid State Ionics [203]. 5.1 Introduction With dwindling fossil fuel reserves [204]
Chapter 5 Extrinsic Defect Reactions in Perovskite Materials The work presented in this Chapter has been published in Solid State Ionics [203]. 5.1 Introduction With dwindling fossil fuel reserves [204]
OPEN CLUSTER PRELAB The first place to look for answers is in the lab script!
 NAME: 1. Define using complete sentences: Globular Cluster: OPEN CLUSTER PRELAB The first place to look for answers is in the lab script! Open Cluster: Main Sequence: Turnoff point: Answer the following
NAME: 1. Define using complete sentences: Globular Cluster: OPEN CLUSTER PRELAB The first place to look for answers is in the lab script! Open Cluster: Main Sequence: Turnoff point: Answer the following
5.03 In-Class Exam 2
 5.03 In-Class Exam 2 Christopher C. Cummins March 12, 2010 Instructions Clearly write your name at the top of this front page, but otherwise do not write on this front page as it will be used for scoring.
5.03 In-Class Exam 2 Christopher C. Cummins March 12, 2010 Instructions Clearly write your name at the top of this front page, but otherwise do not write on this front page as it will be used for scoring.
