A new method for thermal dehydration studies of clay minerals.
|
|
|
- Virgil Goodman
- 6 years ago
- Views:
Transcription
1 53 A new method for thermal dehydration studies of clay minerals. By Georges KULBICKI, 1 D.Sc., and Ralph E. GR~, Ph.D. Department of Geology, University of Illinois, Urbana, Illinois. [Taken as read 29 January 1959.] Summary. The water vapour released upon heating the specimen is driven through a water absorber. The intensity of the exothermic reaction produced by absorption of the water is recorded against the temperature of the specimen. A series of curves obtained with various clay minerals are presented to illustrate the method. The significance of results in the identification of the clay minerals and the determination of their structure is considered. T HE present equipment originated in a search for a sensitive procedure able to detect the possible release of small amounts of water by clay minerals above 800 ~ C. For example, it was desired to verify whether any water-loss is connected with the endothermic reaction of montmorillonites around 900 ~ C., and whether the slight endothermic feature sometimes preceding the exothermic reaction of kaolinites at about 950 ~ C. is accompanied by any dehydration. The curves obtained by thermogravimetric and differential-thermal methods do not provide completely convincing information on these matters, and it was thought that a more sensitive procedure might be helpful. Also it was desired to study the rate of evolution of water vapour versus temperature. Apparatus and method. The principle of the new method is as follows : The water vapour evolved from the specimen is carried by a flow of nitrogen, previously dried, through a water absorber; the absorption of water causes an exothermic reaction, which causes a slight increase in the temperature of the carrying gas, which is detected by means of a differential thermocouple and recorded as a function of the temperature of the specimen. Details of the apparatus are shown in fig. 1. The furnace is made of a tube of Vycor glass, 40 cm. long and 12 ram. inside diameter. Five feet of Nichrome wire, of 0"02 in. diameter, is wound in a double layer around the central part of the tube with a sheet of asbestos for insulation. The ends of the tube remain cool so that rubber stoppers can be used. The 1 On leave from the University of Toulouse, Toulouse, France.
2 54 GEORGES KULBICKI AND RALPH E. GRIM ON applied voltage is automatically raised by a 'Variac' transformer driven by an electric motor through a speed reducer; the temperature of 1100 ~ C. was reached with 40 volts and 5 amps. An approximate heating rate of 15 ~ C. per minute was choseu for the curves reported herein. Aplatinum, platinum-rhodium thermocouple indicates the temperature of the specimen, which is placed in a small flat porcelain crucible. hermocoupie ecofder ~Somple Nitrogen I water w FURNAGE GOI L FIG. 1. Sketch of apparatus used in obtaining vapour-absorption curves. Dried nitrogen gas is directed through the furnace at a constant rate of 25 ml. per minute to sweep out any water vapour. The nitrogen with any water vapour is cooled to room temperature in a small coil and then directed through the absorber. The absorber is a vertical glass tube with a silvered vacuum jacket for thermal protection. A grid holds some pellets of a dehydrating agent and a differential thermocouple records the changes in temperature of the gas before and after absorption of water. In the first series of experiments, the thermal sensitivity of crystal diodes was used for detecting the temperature variations of the gas flow. Two germanium diodes mounted on a Wheatstone bridge were utilized as differential thermal gauges; however, the extreme sensitivity of the device created many experimental difficulties. For example, the weight of the specimen had to be kept at a fraction of ~ milligram and very complete thermal insulation was necessary. Finally, differential thermocouples of chromel and alumel wires were adopted. Several water absorber compounds were tried. Calcium hydride gave the best results, but silica gel was also used successfully. The curves were recorded on a Brown electronic potentiometer. Operating under
3 THERMAL DEHYDRATION OF CLAY MINERALS 55 the above conditions, the dehydration of 40 mg. of kaolinite produced a deflection of about 15 cm. Analytical ~esults. The curves in fig. 2, for ha]loysite with gibbsite, for kaolinite, and for dickite, show peaks for loss of water of a size and shape that would be anticipated from D.T.A. (differential thermal analysis) curves (Grim, 1953). It is not expected that the vapour curves should be precisely the same as the D.T.A. curves since any structural changes accompanying dehydration are not reflected in the vapour curves. Each of the vapour curves, fig. 2, shows a faint depression at about 950~ ~ C. D.T.A. curves for these minerals frequently show a slight endothermic reaction at this temperature which has been interpreted (Grim, 1953) as being caused by the destruction of the dehydrated kaolinite structures. The present data suggest that, at least during very rapid heating, a small amount of water is present in the so-called metakaolin structure and is expelled when this structure is destroyed. In this conclusion we agree with V. StubiSan (this issue, p. 38), who presents infra-red absorption spectrum evidence for the presence of hydroxyl groups in metakaolin. The fireclay sample, fig. 3, which has a clay mineral composition of about 75 ~o kaolinite and 25 ~ illite, produces the vapour-loss curve expected from its mineral composition and D.T.A. curve. The illite samples, fig. 3, show the expulsion of adsorbed water at low temperatures. The curves show that the hydroxyl water is driven off gradually over a considerable temperature interval, at least at the rapid heating rates of the experiments. There is no suggestion of any water loss at high temperatures corresponding to the third endothermie reaction shown on D.T.A. curves (Grim, 1953) of these minerals. The nontronite sample shows a vapour-loss curve that corresponds closely to the D.T.A. curve of the mineral (Grim, 1953). The hectorite sample shows the loss of adsorbed water at low temperatures, and the expulsion of hydroxyl water between about 650 ~ and 950 ~ C. The dioctahedral montmorillonites studied, fig. 4, show the expulsion of the hydroxyl water at a somewhat lower temperature and often more abruptly than this trioctahedral montmorillonite. The talc sample, fig. 3, shows only the Ross of hydroxyl water over a wide temperature interval extending from about 700 ~ C. to above 1000 ~ C. The curves for the three montmorillonite samples, fig. 4, show peaks for the loss of adsorbed water corresponding to those on the D.T.A. curves, fig. 5. The peaks for the expulsion of hydroxyl water also
4 56 GEORGES KULBI(:KI AND RALPH E. GRIM ON Y! f 1 I ~ I O io00 Degrees- C Fm. 2. Vapour-absorption curves:a, Halloysitc(with gibbsi~), Bed~rd, Indiana;B Kaolini~ Mclntvre, Georgia c, Dickite, Baraboo, Wisconsin.
5 THERMAL DEHYDRATION OF CLAY MINERALS 57 A B D E I I I -- I I I IOqO Degrees -C :FIe. 3. Vapour-absorption curves: )~, 75% kaolinite, 25% illite (fireclay), Grundy County, Illinois; J*, ]llitc, Vermilion County, Illinois; c, Illite, Grundy County, Illinois; D, Nontronite, Manito, Washington; ~, Hectorire, Hector, California; F, Talc, New York.
6 58 GEORGES KULBICKI AND RALPH E. GI~IM ON A E I r l i [ I I, IO0O Degrees - C FIo. 4. Vapour-absorption curves: A, Montmorillonite, Colony, Wyoming; ~, Montmorillonite, Amory, Mississippi; c, Montmorillonite, Mendoza, Argentina; D, Sepiolite, Vallecas, Spain; ~:, Palygorskite, Attapulgus, Georgia.
7 THERMAL DEHYDRATION OF CLAY MINERALS 59 A B k._ I I I I I !000 Degrees -C ~Io. 5. Differential thermal analysis curves: A, Montmorillonite, Colony, Wyoming; ~, Montmorillonite, Amory, Mississippi; c, Montmorillonite, Mendoza, Argentina.
8 60 GEORGES KULBICKI AND RALPH E. GRIM ON L I! 1 I I I I000 Degrees-C :FIG. 6. Differential thermal analysis curves : D, Palygorskite, Attapulgus, Georgia; ]~, Halloysite (with gibbsite), Bedford, Indiana; F, Sepiolite, Vallecas, Spain.
9 THERMAL DEHYDRATION OF CLAY MINERALS 61 correlate in the two types of curves. When the hydroxyl dehydration peak is a doublet on the D.T.A. curve, it is also a dual peak on the vapourloss curve indicating that both parts of the doublet are related to water loss. The vapour-ioss curves are at least as sensitive in detecting such doublets as are the D.T.A. curves. The vapour-loss curves do not indicate any expulsion of water corresponding to the high-temperature endothermic peaks of the montmorillonites on the D.T.A. curves, fig. 5. The vapour-loss curve for the palygorskite (attapulgite) sample, fig. 4, corresponds to the D.T.A. curve, fig. 6, except that the vapour-loss curve resolves the higher-temperature endothermic peak into a doublet. The low temperature portion of the vapour-loss curve for the sepiolite sample, fig. 4, corresponds to endothermic reactions on the D.T.A. curve for the same sample, fig. 6. The vapour-loss curve shows a definite vapour expulsion at 500o-600 ~ C. which is only suggested on the D.T.A. curve. Also the vapour-loss curve shows that for the sepiolite the endothermic reaction at about 800 ~ C. is accompanied by a loss of water. Martin-Vivaldi and Cano-Ruiz (1956) have recently published a detailed study of the dehydration of the same palygorskite (attapulgite) and sepiolite. Their data correlate exactly with the vapour-absorption curves. In fact the correlation is far superior to that with the D.T.A. curves. Discussion of results. It is believed that the analytical method presented herein provides a simple means of obtaining additional definitive characteristics of hydrous minerals. Using other absorbents, similar curves could undoubtedly be obtained to record the expulsion of other gaseous materials produced on heating minerals, for example, carbon dioxide from carbonates. The new method can contribute to structural studies by showing whether or not loss of water accompanies a thermal reaction. In the temperature range for the loss of hydroxyl water, the vapour-loss method appears to be extremely sensitive. In this interval the vapour-loss curves may indicate reactions shown only very slightly on the D.T.A. curve (e.g. montmorillonite from Mississippi), or not at all (e.g. palygorskite). In the temperature interval of the loss of the adsorbed water the vapour-loss curve appears to be less sensitive than the D.T.A. curve. Thus the double peak of the Mississippi montmorillonite is shown as a single peak in the vapour-loss curve. It should be pointed out that this apparent difference in sensitivity between the vapour-loss and D.T.A. curves may have another explanation, namely, that these thermal reactions shown on D.T.A. curves are a consequence of structural changes to a greater degree than has heretofore been considered. Thus the double
10 62 C. KULBICKI AND R. E. GRIM ON THERMAL :DEHYDRATION nature of the peak for loss of adsorbed water from calcium montmorillonites as shown on D.T.A. curves may be due to a change in water structure after a certain amount of moisture is lost rather than to any periodicity in the water loss. Further work is necessary to resolve this matter. It must be remembered that some variations in the vapour-loss curves would result from different heating rates, different particle size of the sample, &c. References. Gram (Ralph E.), Clay Mineralogy, McGraw-Hill Co., New York. )/~ARTIN-VIVALDI (J. IJ.) and CANO-RUIZ {J.), Clay and Clay Minerals, Pub. 456, U.S. :Nat. Acad. Sci., p. 177.
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION
 THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
Thermal Methods of Analysis
 Thermal Methods of Analysis Calorie-something we know What is calorie? Can you see or touch a calorie? How is it measured? Working out in gym Change in weight Loss of calories-burning of fat? (10 km=500calories/9cal
Thermal Methods of Analysis Calorie-something we know What is calorie? Can you see or touch a calorie? How is it measured? Working out in gym Change in weight Loss of calories-burning of fat? (10 km=500calories/9cal
CHEM*3440. Thermal Methods. Thermogravimetry. Instrumental Components. Chemical Instrumentation. Thermal Analysis. Topic 14
 Thermal Methods We will examine three thermal analytical techniques: Thermogravimetric Analysis (TGA) CHEM*3440 Chemical Instrumentation Topic 14 Thermal Analysis Differential Thermal Analysis (DTA) Differential
Thermal Methods We will examine three thermal analytical techniques: Thermogravimetric Analysis (TGA) CHEM*3440 Chemical Instrumentation Topic 14 Thermal Analysis Differential Thermal Analysis (DTA) Differential
THE THERMAL DECOMPOSITION OF NH4-MONTMORILLONITES. PART II
 (?lay Min. Bull. (1964), 5, 401. THE THERMAL DECOMPOSITION OF NH4-MONTMORILLONITES. PART II J. L. MARTIN VIVALDI, F. GIRELA VILCHEZ AND P. FENOLL HACH-ALI Estaeion Experimental del Zaidin, Avenida de Cervantes,
(?lay Min. Bull. (1964), 5, 401. THE THERMAL DECOMPOSITION OF NH4-MONTMORILLONITES. PART II J. L. MARTIN VIVALDI, F. GIRELA VILCHEZ AND P. FENOLL HACH-ALI Estaeion Experimental del Zaidin, Avenida de Cervantes,
Effect of EcSS 3000 on Expansive Clays
 Effect of EcSS 3000 on Expansive Clays R. Malek Director, Particle Characterization Laboratory, Materials Research Institute, Pennsylvania State University, University Park, PA 16802. RQM@PSU.EDU (814)
Effect of EcSS 3000 on Expansive Clays R. Malek Director, Particle Characterization Laboratory, Materials Research Institute, Pennsylvania State University, University Park, PA 16802. RQM@PSU.EDU (814)
SOLID PHASE REACTIONS WITH TUNELLITE MINERAL
 SOLID PHASE REACTIONS WITH TUNELLITE MINERAL Hüseyin GÜLENSOY and T. TEBERDAR İstanbul University, Faculty of Chemistry SUMMARY. The solid phase reactions between tunellite mineral (SrO 3B 2 O 3 4H 2 O)
SOLID PHASE REACTIONS WITH TUNELLITE MINERAL Hüseyin GÜLENSOY and T. TEBERDAR İstanbul University, Faculty of Chemistry SUMMARY. The solid phase reactions between tunellite mineral (SrO 3B 2 O 3 4H 2 O)
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
CLAY MINERALS BULLETIN
 CLAY MINERALS BULLETIN JULY, 196 Vol. 4, No. 23 CHANGES EFFECTED IN LAYER SILICATES BY HEATING BELOW 55~ * By C. M. WARSHAW, P. E. ROSENBERG and R. RoY. The Pennsylvania State University, University Park,
CLAY MINERALS BULLETIN JULY, 196 Vol. 4, No. 23 CHANGES EFFECTED IN LAYER SILICATES BY HEATING BELOW 55~ * By C. M. WARSHAW, P. E. ROSENBERG and R. RoY. The Pennsylvania State University, University Park,
Calcimeter. Meet the difference. Manual. T E I
 Calcimeter Manual Meet the difference Eijkelkamp Soil & Water Nijverheidsstraat 30 NL-6987 EM Giesbeek T +31 313 880 200 E info@eijkelkamp.com I www.eijkelkamp.com 1 2018-05 M-0853E Contents On these operating
Calcimeter Manual Meet the difference Eijkelkamp Soil & Water Nijverheidsstraat 30 NL-6987 EM Giesbeek T +31 313 880 200 E info@eijkelkamp.com I www.eijkelkamp.com 1 2018-05 M-0853E Contents On these operating
Resistivity and Temperature Coefficients (at 20 C)
 Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermal Methods of Analysis Theory, General Techniques and Applications. Prof. Tarek A. Fayed
 Thermal Methods of Analysis Theory, General Techniques and Applications Prof. Tarek A. Fayed 1- General introduction and theory: Thermal analysis (TA) is a group of physical techniques in which the chemical
Thermal Methods of Analysis Theory, General Techniques and Applications Prof. Tarek A. Fayed 1- General introduction and theory: Thermal analysis (TA) is a group of physical techniques in which the chemical
Identification of an Unknown Compound through Mass Correlations
 EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
Chapter 31. Thermal Methods
 Chapter 31. Thermal Methods Thermal analysis: Physical property of a substance or its reaction products is measured as a function of temperature. * TGA: Thermogravimetric Analysis ( 熱重分析法 ) * DTA: Differential
Chapter 31. Thermal Methods Thermal analysis: Physical property of a substance or its reaction products is measured as a function of temperature. * TGA: Thermogravimetric Analysis ( 熱重分析法 ) * DTA: Differential
HETEROGENEITY IN MONTMORILLONITE. JAMES L. MCATEE, JR. Baroid Division, National Lead Co., Houston, Texas
 HETEROGENEITY IN MONTMORILLONITE By JAMES L. MCATEE, JR. Baroid Division, National Lead Co., Houston, Texas ABSTRACT X-ray diffraction patterns and cation-exchange data are presented for centrifuged Wyoming
HETEROGENEITY IN MONTMORILLONITE By JAMES L. MCATEE, JR. Baroid Division, National Lead Co., Houston, Texas ABSTRACT X-ray diffraction patterns and cation-exchange data are presented for centrifuged Wyoming
Department of Mechanical Engineering ME 96. Free and Forced Convection Experiment. Revised: 25 April Introduction
 CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE LEARNING OBJECTIVES
 Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
LONG-TERM SETTLING CHARACTERISTICS OF SOME CLAYS by
 LONG-TERM SETTLING CHARACTERISTICS OF SOME CLAYS by T. J. TOBIAS and A. P. RUOTSALA Michigan Technological University, Houghton, Michigan ABSTRACT A SERIES of experiments was set up, the purpose of which
LONG-TERM SETTLING CHARACTERISTICS OF SOME CLAYS by T. J. TOBIAS and A. P. RUOTSALA Michigan Technological University, Houghton, Michigan ABSTRACT A SERIES of experiments was set up, the purpose of which
STUDIES ON THE BASIC CARBONATES OF NICKEL
 STUDIES ON THE BASIC CARBONATES OF NICKEL Part VJ : Thermal Decomposition of Basic Nickel Carbonates in Vacuum and the nature of the surfaces BY R. M. MALLYA AND A. R. VASUDBVA MURTHY (Department of Inorganic
STUDIES ON THE BASIC CARBONATES OF NICKEL Part VJ : Thermal Decomposition of Basic Nickel Carbonates in Vacuum and the nature of the surfaces BY R. M. MALLYA AND A. R. VASUDBVA MURTHY (Department of Inorganic
PRETREATMENT OF SOILS AND CLAYS FOR MEASUREMENT OF EXTERNAL SURFACE AREA BY GLYCEROL RETENTION
 PRETREATMENT OF SOILS AND CLAYS FOR MEASUREMENT OF EXTERNAL SURFACE AREA BY GLYCEROL RETENTION by EARL B. Kn~TEB AND SIDNEY D~_MO~D Division of Physical Research, Bureau of Public Roads, Washington, D.C.
PRETREATMENT OF SOILS AND CLAYS FOR MEASUREMENT OF EXTERNAL SURFACE AREA BY GLYCEROL RETENTION by EARL B. Kn~TEB AND SIDNEY D~_MO~D Division of Physical Research, Bureau of Public Roads, Washington, D.C.
A Method, tor Determining the Slope. or Neutron Moisture Meter Calibration Curves. James E. Douglass
 Station Paper No. 154 December 1962 A Method, tor Determining the Slope or Neutron Moisture Meter Calibration Curves James E. Douglass U.S. Department of Agriculture-Forest Service Southeastern Forest
Station Paper No. 154 December 1962 A Method, tor Determining the Slope or Neutron Moisture Meter Calibration Curves James E. Douglass U.S. Department of Agriculture-Forest Service Southeastern Forest
Unit 6 Current Electricity and Circuits
 Unit 6 Current Electricity and Circuits 2 Types of Electricity Electricity that in motion. Electricity that in motion. Occurs whenever an moves through a. 2 Types of Current Electricity Electricity that
Unit 6 Current Electricity and Circuits 2 Types of Electricity Electricity that in motion. Electricity that in motion. Occurs whenever an moves through a. 2 Types of Current Electricity Electricity that
Apparatus to measure high-temperature thermal conductivity and thermoelectric power of small specimens
 Apparatus to measure high-temperature thermal conductivity and thermoelectric power of small specimens T. Dasgupta and A. M. Umarji a Materials Research Centre, Indian Institute of Science, Bangalore-560012,
Apparatus to measure high-temperature thermal conductivity and thermoelectric power of small specimens T. Dasgupta and A. M. Umarji a Materials Research Centre, Indian Institute of Science, Bangalore-560012,
DSC AND TG/DTA AS PROBLEM-SOLVING TOOLS: CHARACTERIZATION OF PHARMACEUTICAL COMPOUNDS
 DSC AND TG/DTA AS PROBLEM-SOLVING TOOLS: CHARACTERIZATION OF PHARMACEUTICAL COMPOUNDS Problem A scientist working for a major pharmaceutical R&D center is having difficulties in interpreting the DSC results
DSC AND TG/DTA AS PROBLEM-SOLVING TOOLS: CHARACTERIZATION OF PHARMACEUTICAL COMPOUNDS Problem A scientist working for a major pharmaceutical R&D center is having difficulties in interpreting the DSC results
Objective of Lecture Discuss resistivity and the three categories of materials Chapter 2.1 Show the mathematical relationships between charge,
 Objective of Lecture Discuss resistivity and the three categories of materials Chapter 2.1 Show the mathematical relationships between charge, current, voltage, and energy. Chapter 2.2-2.4 Define resistance
Objective of Lecture Discuss resistivity and the three categories of materials Chapter 2.1 Show the mathematical relationships between charge, current, voltage, and energy. Chapter 2.2-2.4 Define resistance
STABILITIES OF THREE-LAYER PHYLLOSILICATES RELATED TO THEIR IONIC-COVALENT BONDING by
 STABILITIES OF THREE-LAYER PHYLLOSILICATES RELATED TO THEIR IONIC-COVALENT BONDING by JOHN W. TLAPEK The California Company, Jackson, Miasissippi and W. D. KELLER University of Missouri, Columbia, Missouri
STABILITIES OF THREE-LAYER PHYLLOSILICATES RELATED TO THEIR IONIC-COVALENT BONDING by JOHN W. TLAPEK The California Company, Jackson, Miasissippi and W. D. KELLER University of Missouri, Columbia, Missouri
X-RAY AND INFRARED DATA ON HECTORITE-GUANlDINES AND MONTMORILLONITE-GUANIDINESi
 X-RAY AND INFRARED DATA ON HECTORITE-GUANlDINES AND MONTMORILLONITE-GUANIDINESi hy CABL W. BECK2 AND GEORGE BBTJNTON The Pure Oil Company, Research Center, Crystal Lake, Illinois ABSTRACT Clay-organic
X-RAY AND INFRARED DATA ON HECTORITE-GUANlDINES AND MONTMORILLONITE-GUANIDINESi hy CABL W. BECK2 AND GEORGE BBTJNTON The Pure Oil Company, Research Center, Crystal Lake, Illinois ABSTRACT Clay-organic
THERMOGRAVIMETRIC ANALYSIS OF' CLAY AND CLAY-LIKE MINERALS By
 THERMOGRAVMETRC ANALYSS OF' CLAY AND CLAY-LKE MNERALS By RCHARD C. MELENZ, N. CYRL SCHELTZ and MYRLE E. KNG Petrographic Laboratory, Bureau of Reclamation, Denver Federal Center, Denver, Colorado ABSTRACT
THERMOGRAVMETRC ANALYSS OF' CLAY AND CLAY-LKE MNERALS By RCHARD C. MELENZ, N. CYRL SCHELTZ and MYRLE E. KNG Petrographic Laboratory, Bureau of Reclamation, Denver Federal Center, Denver, Colorado ABSTRACT
CHEM 254 EXPERIMENT 7. Phase Diagrams - Liquid Vapour Equilibrium for two component solutions
 pressure CHEM 254 EXPERIMENT 7 Phase Diagrams - Liquid Vapour Equilibrium for two component solutions The partial pressures of the components of an ideal solution of two volatile liquids are related to
pressure CHEM 254 EXPERIMENT 7 Phase Diagrams - Liquid Vapour Equilibrium for two component solutions The partial pressures of the components of an ideal solution of two volatile liquids are related to
COPYRIGHT FOUNTAINHEAD PRESS
 Water of Hydration Objectives To calculate the percent water by mass in several hydrated compounds; to dehydrate an unknown solid sample and identify it by comparing its percent water with known hydrated
Water of Hydration Objectives To calculate the percent water by mass in several hydrated compounds; to dehydrate an unknown solid sample and identify it by comparing its percent water with known hydrated
THE ROLE OF WATER VAPOUR IN THE DEHYDROXYLATION OF CLAY MINERALS
 THE ROLE OF WATER VAPOUR IN THE DEHYDROXYLATION OF CLAY MINERALS By G. W. BRI~qDLE~C and M. NAtOa~RA Department of Ceramic Technology, The Pennsylvania State University, University Park, Pa., U.S.A. [MS.
THE ROLE OF WATER VAPOUR IN THE DEHYDROXYLATION OF CLAY MINERALS By G. W. BRI~qDLE~C and M. NAtOa~RA Department of Ceramic Technology, The Pennsylvania State University, University Park, Pa., U.S.A. [MS.
Clays and Clay Minerals
 Clays and Clay Minerals Fields of interest for clays Various definitions Acients: Earths in the earth-air-fire-water system Definition of clay depends on discipline: Geologist grain size
Clays and Clay Minerals Fields of interest for clays Various definitions Acients: Earths in the earth-air-fire-water system Definition of clay depends on discipline: Geologist grain size
Simultaneous Thermal Analyzer (DTA/TGA)
 Simultaneous Thermal Analyzer (DTA/TGA) Common term Thermal analysis: A technique of measuring the changes of physical properties of substances with program controlling temperature. All kinds of thermal
Simultaneous Thermal Analyzer (DTA/TGA) Common term Thermal analysis: A technique of measuring the changes of physical properties of substances with program controlling temperature. All kinds of thermal
Matter & Energy: Temperature & Heat in Physical Processes
 Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Working in the Chemistry Laboratory
 Working in the Chemistry Laboratory Accelerated Chemistry I Introduction: One of the most important components of your chemistry course is the laboratory experience. Perhaps you have done experiments in
Working in the Chemistry Laboratory Accelerated Chemistry I Introduction: One of the most important components of your chemistry course is the laboratory experience. Perhaps you have done experiments in
WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS?
 WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS? David Bish and David Vaniman Indiana University Los Alamos National Laboratory Products of Mineralogical Studies Mars surface mineralogy
WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS? David Bish and David Vaniman Indiana University Los Alamos National Laboratory Products of Mineralogical Studies Mars surface mineralogy
Salts are compounds composed of a metal ion plus a non-metal (or polyatomic) ion, e.g., sodium chloride (NaCl), and sodium phosphate (Na 3 PO 4 ).
 Experiment 4 Water of Hydration Objective - Determine the percent of water in a hydrate. Introduction Salts are compounds composed of a metal ion plus a non-metal (or polyatomic) ion, e.g., sodium chloride
Experiment 4 Water of Hydration Objective - Determine the percent of water in a hydrate. Introduction Salts are compounds composed of a metal ion plus a non-metal (or polyatomic) ion, e.g., sodium chloride
Specific Heat of a Metal
 Specific Heat of a Metal Purpose The objective of this experiment is to determine the specific heat of zinc sample using coffeecup calorimeter. Theory In a chemical reaction, the quantity of heat that
Specific Heat of a Metal Purpose The objective of this experiment is to determine the specific heat of zinc sample using coffeecup calorimeter. Theory In a chemical reaction, the quantity of heat that
Put sufficient ice cubes into water (1 M) and wait for equilibrium (both exist) (1 M)
 NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
I. CHEM. E. SYMPOSIUM SERIES No. 49
 FLAMMABILITY AND EXPLOSIBILITY OF AMMONIA G.F.P.Harris, P.E.MacDermott Research Dept. ICI Organics Division, Blackley, Manchester The flammability limits of oxygen/nitrogen/ammonia mixtures have been determined
FLAMMABILITY AND EXPLOSIBILITY OF AMMONIA G.F.P.Harris, P.E.MacDermott Research Dept. ICI Organics Division, Blackley, Manchester The flammability limits of oxygen/nitrogen/ammonia mixtures have been determined
Carbonate content. SCAN-N 32:98 Revised White, green and black liquors and burnt lime sludge
 Revised 1998 White, green and black liquors and burnt lime sludge Carbonate content 0 Introduction This SCAN-test Method replaces SCAN-N 32:88 from which it differs in that it, in addition to white and
Revised 1998 White, green and black liquors and burnt lime sludge Carbonate content 0 Introduction This SCAN-test Method replaces SCAN-N 32:88 from which it differs in that it, in addition to white and
Thermal Analysis Premium
 Thermal Analysis Premium HP DSC 2+ STAR e System Innovative Technology Versatile Modularity Swiss Quality DSC Measurements under Pressure for Accelerated Materials Testing Double Safety System The Right
Thermal Analysis Premium HP DSC 2+ STAR e System Innovative Technology Versatile Modularity Swiss Quality DSC Measurements under Pressure for Accelerated Materials Testing Double Safety System The Right
NATIONAL ACADEMY OF SCIENCES
 PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES Volume 15 November 15, 1929 Number I I ON THE POLARIZATION OF X-RADIATION By WILLIAM DuANn DZ3PARTMZNT OF PHYSICS, HARVARD UNIVZRSITY Communicated October
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES Volume 15 November 15, 1929 Number I I ON THE POLARIZATION OF X-RADIATION By WILLIAM DuANn DZ3PARTMZNT OF PHYSICS, HARVARD UNIVZRSITY Communicated October
Thermal Beneficiation of Kaolin Clay in the Removal of Potassium Ion
 International Journal of Material Science Innovations (IJMSI) 2(5): 124-131, 2014 ISSN 2289-4063 Academic Research Online Publisher Research Article Thermal Beneficiation of Kaolin Clay in the Removal
International Journal of Material Science Innovations (IJMSI) 2(5): 124-131, 2014 ISSN 2289-4063 Academic Research Online Publisher Research Article Thermal Beneficiation of Kaolin Clay in the Removal
Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR)
 Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
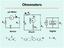 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
J. PETROVIČ. Institute of Inorganic Chemistry, Slovak Academy of Sciences, Bratislava 9. Received March 13, 1969
 Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. II. The Mixtures Prepared from Different Starting Materials and from Gels Containing Aluminium Ion J. PETROVIČ Institute of
Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. II. The Mixtures Prepared from Different Starting Materials and from Gels Containing Aluminium Ion J. PETROVIČ Institute of
PHYS 2212L - Principles of Physics Laboratory II
 PHYS 2212L - Principles of Physics Laboratory II Laboratory Advanced Sheet Resistors 1. Objectives. The objectives of this laboratory are a. to verify the linear dependence of resistance upon length of
PHYS 2212L - Principles of Physics Laboratory II Laboratory Advanced Sheet Resistors 1. Objectives. The objectives of this laboratory are a. to verify the linear dependence of resistance upon length of
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
J. U. ANDERSON. Agronomy Department, New Mexico State University, University Park, New Mexico ABSTRACT
 Page - 380 - AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS OF SAMPLES CONTAINING ORGANIC MATTER by J. U. ANDERSON Agronomy Department, New Mexico
Page - 380 - AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS AN IMPROVED PRETREATMENT FOR MINERALOGICAL ANALYSIS OF SAMPLES CONTAINING ORGANIC MATTER by J. U. ANDERSON Agronomy Department, New Mexico
1 Written and composed by: Prof. Muhammad Ali Malik (M. Phil. Physics), Govt. Degree College, Naushera
 CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
Superconductivity. Never store liquid nitrogen in a container with a tight fitting lid.
 Superconductivity 1 Introduction In this lab we will do some very simple experiments involving superconductors. You will not have to take much data; much of what you do will be qualitative. However, in
Superconductivity 1 Introduction In this lab we will do some very simple experiments involving superconductors. You will not have to take much data; much of what you do will be qualitative. However, in
THERMAL EXPANSION OF FLUORSPAR AND IRON PYRITE BY D. C. PRESS
 THERMAL EXPANSION OF FLUORSPAR AND IRON PYRITE BY D. C. PRESS (From the Department of Physics, Indian Institute of Science, Bangalore) Received October 26, 1949 (Communicated by Prof. R. S. Krishnan, F.A.SC.)
THERMAL EXPANSION OF FLUORSPAR AND IRON PYRITE BY D. C. PRESS (From the Department of Physics, Indian Institute of Science, Bangalore) Received October 26, 1949 (Communicated by Prof. R. S. Krishnan, F.A.SC.)
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
A601. Milwaukie HS Chemistry Linman. Period Date / / In each of the following chemicals, determine the oxidation states of each element:
 A601 Assigning Oxidation Numbers In each of the following chemicals, determine the oxidation states of each element: Example: sodium nitrate NaNO 3 12) N 2 O 3 +1 +5-2 2) ammonia 13) H 2 SO 4 3) zinc oxide
A601 Assigning Oxidation Numbers In each of the following chemicals, determine the oxidation states of each element: Example: sodium nitrate NaNO 3 12) N 2 O 3 +1 +5-2 2) ammonia 13) H 2 SO 4 3) zinc oxide
Experiment 3. Electrical Energy. Calculate the electrical power dissipated in a resistor.
 Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS
 50 CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS 4.1 INTRODUCTION In the development of any energy-efficient heat transfer fluids for enhanced heat transfer performance, in practical applications,
50 CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS 4.1 INTRODUCTION In the development of any energy-efficient heat transfer fluids for enhanced heat transfer performance, in practical applications,
Specific Heat Measurement of High Temperature Thermal Insulations by Drop Calorimeter Method
 International Journal of Thermophysics, Vol 24, No 2, March 23 ( 23) Specific Heat Measurement of High Temperature Thermal Insulations by Drop Calorimeter Method T Ohmura, 1,2 M Tsuboi, 1 M Onodera, 1
International Journal of Thermophysics, Vol 24, No 2, March 23 ( 23) Specific Heat Measurement of High Temperature Thermal Insulations by Drop Calorimeter Method T Ohmura, 1,2 M Tsuboi, 1 M Onodera, 1
CSUS Department of Chemistry Experiment 2 Chem. 1A EXPERIMENT 2: HYDRATE PRE-LABORATORY ASSIGNMENT
 Name: Lab Section: EXPERIMENT 2: HYDRATE PRE-LABORATORY ASSIGNMENT 1. A student obtains the following data: Mass of test tube: Mass of test tube and hydrate: Mass of test tube and anhydrous residue after
Name: Lab Section: EXPERIMENT 2: HYDRATE PRE-LABORATORY ASSIGNMENT 1. A student obtains the following data: Mass of test tube: Mass of test tube and hydrate: Mass of test tube and anhydrous residue after
CATION EXCHANGE BETWEEN MIXTURES OF CLAY MINERALS AND BETWEEN A ZEOLITE AND A CLAY MINERAL*
 CATION EXCHANGE BETWEEN MIXTURES OF CLAY MINERALS AND BETWEEN A ZEOLITE AND A CLAY MINERAL* by PATRICK J. DENNY and RUSTUM ROY Pennsylvania State University, University Park, Pa. ABSTRACT The electron
CATION EXCHANGE BETWEEN MIXTURES OF CLAY MINERALS AND BETWEEN A ZEOLITE AND A CLAY MINERAL* by PATRICK J. DENNY and RUSTUM ROY Pennsylvania State University, University Park, Pa. ABSTRACT The electron
National 5 Physics. Electricity and Energy. Notes
 National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
EXPERIMENTAL SETUP AND PROCEDURE
 CHAPTER 3 EXPERIMENTAL SETUP AND PROCEDURE 3.1 Determination of vapour-liquid equilibria Isobaric Vapour-Liquid Equilibria date have been obtained, using a Smith and Bonner [39] type still which is a modified
CHAPTER 3 EXPERIMENTAL SETUP AND PROCEDURE 3.1 Determination of vapour-liquid equilibria Isobaric Vapour-Liquid Equilibria date have been obtained, using a Smith and Bonner [39] type still which is a modified
University of Illinois Urbana-Champaign
 URBANA Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/effectsofsoapdet12whit 'IMP t - > cijf ENVIRONMENTAL GEOLOGY NOTES
URBANA Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/effectsofsoapdet12whit 'IMP t - > cijf ENVIRONMENTAL GEOLOGY NOTES
J. PETROVIÖ, V. RUSNÁK and L. ŠTEVULA. Institute of Inorganic Chemistry, Slovak Academy of Sciences, Bratislava 9 Received July 2, 1968
 Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. I. The Mixtures of ifferent Forms of Silicon ioxide and of ifferent Compounds of Aluminium J. PETROVIÖ, V. RUSNÁK and L. ŠTEVULA
Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. I. The Mixtures of ifferent Forms of Silicon ioxide and of ifferent Compounds of Aluminium J. PETROVIÖ, V. RUSNÁK and L. ŠTEVULA
Instrumental Characterization of Montmorillonite Clay by FT-IR and XRD from J.K.U.A.T Farm, in the Republic of Kenya
 Instrumental Characterization of Montmorillonite Clay by FT-IR and XRD from J.K.U.A.T Farm, in the Republic of Kenya Maina,E.W. Wanyika, H.J. Gacanja, A.N. Department of Chemistry, Faculty of Science,
Instrumental Characterization of Montmorillonite Clay by FT-IR and XRD from J.K.U.A.T Farm, in the Republic of Kenya Maina,E.W. Wanyika, H.J. Gacanja, A.N. Department of Chemistry, Faculty of Science,
T 282. WORKING GROUP CHAIRMAN Junyong Zhu SUBJECT
 NOTICE: This is a DRAFT of a TAPPI Standard in ballot. Although available for public viewing, it is still under TAPPI s copyright and may not be reproduced or distributed without permission of TAPPI. This
NOTICE: This is a DRAFT of a TAPPI Standard in ballot. Although available for public viewing, it is still under TAPPI s copyright and may not be reproduced or distributed without permission of TAPPI. This
Calorimetry: differential scanning calorimetry (DSC), isothermal titration calorimetry (ITC)
 Calorimetry: differential scanning calorimetry (DSC), isothermal titration calorimetry (ITC) Dr. Yin Li Department of Biophysics, Medical School University of Pecs Thermal Analysis IUPAC definition - a
Calorimetry: differential scanning calorimetry (DSC), isothermal titration calorimetry (ITC) Dr. Yin Li Department of Biophysics, Medical School University of Pecs Thermal Analysis IUPAC definition - a
UPGRADING AND CHARACTERIZATION OF SMECTITE FROM SELECTED VOLCANIC SEDIMENTS OF EASTERN UGANDA
 UPGRADING AND CHARACTERIZATION OF SMECTITE FROM SELECTED VOLCANIC SEDIMENTS OF EASTERN UGANDA *Mukasa-Tebandeke, I.Z 1.; Ssebuwufu, P.J.M 1.; Schumann, A 3 Ntale, M 1. and Lugolobi F 2 1 Makerere University,
UPGRADING AND CHARACTERIZATION OF SMECTITE FROM SELECTED VOLCANIC SEDIMENTS OF EASTERN UGANDA *Mukasa-Tebandeke, I.Z 1.; Ssebuwufu, P.J.M 1.; Schumann, A 3 Ntale, M 1. and Lugolobi F 2 1 Makerere University,
Unit 3, Lesson 02: Enthalpy Changes in Chemical Reactions
 Unit 3, Lesson 02: Enthalpy Changes in Chemical Reactions Chemical Potential Energy refers to the energy that is stored within an atom or molecule because of electrostatic attraction and repulsion between
Unit 3, Lesson 02: Enthalpy Changes in Chemical Reactions Chemical Potential Energy refers to the energy that is stored within an atom or molecule because of electrostatic attraction and repulsion between
Chemistry Stoichiometry and Heat Exam (ver.1) Mr. Thaler. Please do not write on this exam. Mark your answers on the scantron only.
 1. Identify from the unbalanced equations below the one that does not represent a redox reaction. a. H 2O 2(aq) + MnO 4 - (aq) O 2(g) + Mn 2+ (aq) b. H 2(g) + N 2(g) NH 3(g) c. NaCl (aq) + AgNO 3(aq) NaNO
1. Identify from the unbalanced equations below the one that does not represent a redox reaction. a. H 2O 2(aq) + MnO 4 - (aq) O 2(g) + Mn 2+ (aq) b. H 2(g) + N 2(g) NH 3(g) c. NaCl (aq) + AgNO 3(aq) NaNO
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation
 Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect
 Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Chapter 3: Electric Current And Direct-Current Circuits
 Chapter 3: Electric Current And Direct-Current Circuits 3.1 Electric Conduction 3.1.1 Describe the microscopic model of current Mechanism of Electric Conduction in Metals Before applying electric field
Chapter 3: Electric Current And Direct-Current Circuits 3.1 Electric Conduction 3.1.1 Describe the microscopic model of current Mechanism of Electric Conduction in Metals Before applying electric field
DSC AS PROBLEM-SOLVING TOOL: BETTER INTERPRETATION OF Tg USING CYCLIC DSC
 DSC AS PROBLEM-SOLVING TOOL: BETTER INTERPRETATION OF Tg USING CYCLIC DSC Problem A scientist is having difficulty in interpreting DSC results on a sample of polystyrene film. The sample exhibits a complex
DSC AS PROBLEM-SOLVING TOOL: BETTER INTERPRETATION OF Tg USING CYCLIC DSC Problem A scientist is having difficulty in interpreting DSC results on a sample of polystyrene film. The sample exhibits a complex
#30 Thermochemistry: Heat of Solution
 #30 Thermochemistry: Heat of Solution Purpose: You will mix different salts with water and note any change in temperature. Measurements using beakers will be compared to measurements using polystyrene
#30 Thermochemistry: Heat of Solution Purpose: You will mix different salts with water and note any change in temperature. Measurements using beakers will be compared to measurements using polystyrene
Trilogy Quantitative chemistry
 Trilogy Quantitative chemistry Foundation revision questions Name: Class: Date: Time: 6 minutes Marks: 6 marks Comments: Page of 23 (a) Formulae and equations are used to describe chemical reactions. Aluminium
Trilogy Quantitative chemistry Foundation revision questions Name: Class: Date: Time: 6 minutes Marks: 6 marks Comments: Page of 23 (a) Formulae and equations are used to describe chemical reactions. Aluminium
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
SALICYLATE. CXLIII. INVESTIGATIONS ON GELATIN. PART VIII. THE OSMOTIC PRESSURE OF GELATIN IN SOLUTIONS OF SODIUM. Scientific and Industrial Research.
 CXLIII. INVESTIGATIONS ON GELATIN. PART VIII. THE OSMOTIC PRESSURE OF GELATIN IN SOLUTIONS OF SODIUM SALICYLATE. BY ELEANOR VIOLET HORNE. From the Biochemical Department of the Imperial College of Science
CXLIII. INVESTIGATIONS ON GELATIN. PART VIII. THE OSMOTIC PRESSURE OF GELATIN IN SOLUTIONS OF SODIUM SALICYLATE. BY ELEANOR VIOLET HORNE. From the Biochemical Department of the Imperial College of Science
Measurement of small sample thermal conductivity by parallel thermal conductance technique
 Measurement of small sample thermal conductivity by parallel thermal conductance technique Bartosz M. Zawilski 1, Roy T. Littleton IV 2, and Terry M. Tritt 1, 2 1 Department of Physics and Astronomy 2
Measurement of small sample thermal conductivity by parallel thermal conductance technique Bartosz M. Zawilski 1, Roy T. Littleton IV 2, and Terry M. Tritt 1, 2 1 Department of Physics and Astronomy 2
University of Illinois Urbana-Champaign
 SMSfij«]J»TE j GEOLOGICAL SURVEY 3 3051 00005 8770 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/surveyofsomeilli12whit
SMSfij«]J»TE j GEOLOGICAL SURVEY 3 3051 00005 8770 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/surveyofsomeilli12whit
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Resistance : R = ρ( ) units are Ohms ( 14 ) Resistor 100 ohms
 Resistance : If we look a little more closely into how charge flows in a conductor, we see that the electron is essentially free to move about the metal conductor material. The electron roams about the
Resistance : If we look a little more closely into how charge flows in a conductor, we see that the electron is essentially free to move about the metal conductor material. The electron roams about the
Complex Compounds Background of Complex Compound Technology
 Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
Chapter 3. Experimental Details
 Chapter 3 Experimental Details This chapter deals with the details of materials used and the experimental procedures adopted in the present investigation. 3.1 BLACK LIQUOR SAMPLES Black liquor samples
Chapter 3 Experimental Details This chapter deals with the details of materials used and the experimental procedures adopted in the present investigation. 3.1 BLACK LIQUOR SAMPLES Black liquor samples
One of the most important physical properties that differs over approximately 26 orders of
 Verification of Kirchhoff s Law and Resistivity as a Material Property and its Temperature Dependence of Al Wire and Carbon Resistor Eman Mousa Alhajji North Carolina State University Department of Materials
Verification of Kirchhoff s Law and Resistivity as a Material Property and its Temperature Dependence of Al Wire and Carbon Resistor Eman Mousa Alhajji North Carolina State University Department of Materials
Thermal energy. Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules.
 Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
THE USE OF DEWAR CALORIMETRY IN THE ASSESSMENT OF CHEMICAL REACTION HAZARDS
 THE USE OF DEWAR CALORIMETRY IN THE ASSESSMENT OF CHEMICAL REACTION HAZARDS R.L. ROGERS* Dewar Calorimetry is one of the simplest and most useful techniques used in the assessment of chemical reaction
THE USE OF DEWAR CALORIMETRY IN THE ASSESSMENT OF CHEMICAL REACTION HAZARDS R.L. ROGERS* Dewar Calorimetry is one of the simplest and most useful techniques used in the assessment of chemical reaction
Macromolecules on Nano-Outlets Responding to Electric Field and ph for Dual-Mode Drug Delivery
 Supporting Information for: Macromolecules on Nano-Outlets Responding to Electric Field and ph for Dual-Mode Drug Delivery Fang Li, Yingchun Zhu*, Zhiyong Mao, Yunli Wang, Qichao Ruan, Jianlin Shi, Congqin
Supporting Information for: Macromolecules on Nano-Outlets Responding to Electric Field and ph for Dual-Mode Drug Delivery Fang Li, Yingchun Zhu*, Zhiyong Mao, Yunli Wang, Qichao Ruan, Jianlin Shi, Congqin
EXPERIMENT 2 DEHYDRATION OF 1- & 2-BUTANOL & DEHYDROBROMINATION OF 1 & 2-BROMOBUTANE: ANALYSIS OF GASEOUS PRODUCTS BY GAS CHROMATOGRAPHY
 EXPERIMENT 2 DEYDRATION OF 1- & 2-BUTANOL & DEYDROBROMINATION OF 1 & 2-BROMOBUTANE: ANALYSIS OF GASEOUS PRODUTS BY GAS ROMATOGRAPY A part of this procedure is adopted from an article published by.m. Gilow
EXPERIMENT 2 DEYDRATION OF 1- & 2-BUTANOL & DEYDROBROMINATION OF 1 & 2-BROMOBUTANE: ANALYSIS OF GASEOUS PRODUTS BY GAS ROMATOGRAPY A part of this procedure is adopted from an article published by.m. Gilow
COPYRIGHT FOUNTAINHEAD PRESS
 Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Heat can be thought of as the flow of energy between two bodies because of a difference in temperature
 Why? The amount of heat energy released or absorbed by a chemical or physical change can be measured using an instrument called a calorimeter. This measurement is based on the law of energy conservation.
Why? The amount of heat energy released or absorbed by a chemical or physical change can be measured using an instrument called a calorimeter. This measurement is based on the law of energy conservation.
Chemistry 151 Last Updated: Dec Lab 5: Hydrated Compounds
 Chemistry 151 Last Updated: Dec. 2013 Lab 5: Hydrated Compounds Introduction When ionic compounds form, there are sometimes gaps or cavities within the crystal lattice that are large enough to trap water
Chemistry 151 Last Updated: Dec. 2013 Lab 5: Hydrated Compounds Introduction When ionic compounds form, there are sometimes gaps or cavities within the crystal lattice that are large enough to trap water
Chemistry 213. Electrochemistry I
 1 Chemistry 213 Electrochemistry I Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
1 Chemistry 213 Electrochemistry I Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
Chemistry. Understanding Water V. Name: Suite 403, 410 Elizabeth St, Surry Hills NSW 2010 (02)
 Chemistry Understanding Water V Name: Suite 403, 410 Elizabeth St, Surry Hills NSW 2010 (02) 9211 2610 info@keystoneeducation.com.au keystoneeducation.com.au Water has a higher heat capacity than many
Chemistry Understanding Water V Name: Suite 403, 410 Elizabeth St, Surry Hills NSW 2010 (02) 9211 2610 info@keystoneeducation.com.au keystoneeducation.com.au Water has a higher heat capacity than many
PC1142 Physics II. The Ideal Gas Law
 PC114 Physics II The Ideal Gas Law 1 Objectives Determine experimentally the ideal gas law governing the pressure P, volume V and temperature T of an ideal gas. Determine an experimental value for the
PC114 Physics II The Ideal Gas Law 1 Objectives Determine experimentally the ideal gas law governing the pressure P, volume V and temperature T of an ideal gas. Determine an experimental value for the
TGA Thermal Gravimetric Analysis
 TGA Thermal Gravimetric Analysis General Thermogravimetry is a technique in which the mass of the sample is monitored against time or temperature while the temperature of the sample, in a specified atmosphere,
TGA Thermal Gravimetric Analysis General Thermogravimetry is a technique in which the mass of the sample is monitored against time or temperature while the temperature of the sample, in a specified atmosphere,
UNIT 11 DIFFERENTIAL THERMAL ANALYSIS, SCANNING CALORIMETRY AND THERMOMETRIC TITRATIONS
 UNIT 11 DIFFERENTIAL THERMAL ANALYSIS, SCANNING CALORIMETRY AND THERMOMETRIC TITRATIONS Differential Thermal Structure 11.1 Introduction Objectives 11.2 Differential Thermal Analysis (DTA) Principle Characteristics
UNIT 11 DIFFERENTIAL THERMAL ANALYSIS, SCANNING CALORIMETRY AND THERMOMETRIC TITRATIONS Differential Thermal Structure 11.1 Introduction Objectives 11.2 Differential Thermal Analysis (DTA) Principle Characteristics
