Selective monomethylation reactions of methylene-active compounds with dimethylcarbonate. An example of clean synthesis*
|
|
|
- Rose Hall
- 6 years ago
- Views:
Transcription
1 Pure Appl. Chem., Vol. 72, No. 9, pp , IUPAC Selective monomethylation reactions of methylene-active compounds with dimethylcarbonate. An example of clean synthesis* Pietro Tundo Department of Environmental Science, Cà Foscari University of Venice and Interuniversity Consortium Chemistry for the Environment, Italy Abstract: Dimethylcarbonate (DMC), an environmentally friendly substitute for dimethylsulfate and methyl halides in methylation reactions, is also a very selective reagent. Under batch conditions, with potassium carbonate as the catalyst, the reactions of DMC, used as the solvent of the reactions, with methylene-active compounds (arylacetonitriles and arylacetoesters, aroxyacetonitriles and methyl aroxyacetates, benzylaryl- and alkylaryl-sulfones) produce monomethylated derivatives, with a selectivity not previously observed (i. e., >99%). These are examples of green chemistry. Dimethylcarbonate (DMC) is a nontoxic building block, used in organic syntheses as a green substitute for toxic and corrosive reagents such as phosgene, dimethylsulfate, and methyl iodide [1,2]. Depending on the experimental conditions, it may act as a methylating agent (Eq. 1), in the place of dimethylsulfate (Eq. 2) and methyl iodide (Eq. 3), or a carboxymethylating agent (Eq. 4), as a substitute of phosgene (Eq. 5). PhOH + CH 3 OCOOCH 3 PhOCH 3 + CO 2 + CH 3 OH (1) PhOH + (CH 3 O) 2 SO 2 + NaOH PhOCH 3 + CH 3 OSO 2 Na + H 2 O (2) PhOH + CH 3 I + NaOH PhOCH 3 + NaI + H 2 O (3) ROH + CH 3 OCOOCH 3 ROCOOCH 3 + CH 3 OH (4) 2 ROH + COCl NaOH ROCOOR + 2 NaCl + 2H 2 O (5) Both reactions (methylation and carboxymethylation) occur in the presence of a weak base (usually an alkaline carbonate) that provides nucleophile activation. The nucleophilic anion may react at one of the two carbon centers of DMC, the carbonyl group or the methyl group. In particular, when the reaction is performed under refluxing conditions (T = 90 C), the nucleophilic anion attacks the acyl carbon, giving the carboxymethylation product and the methoxide anion as the leaving group (B Ac 2 mechanism). The latter provides further substrate activation, so that only catalytic amounts of the base are required. * Lecture presented at the 13 th International Conference on Organic Synthesis (ICOS-13), Warsaw, Poland, 1 5 July Other presentations are published in this issue, pp Fax: ; tundop@unive.it 1793
2 1794 P. TUNDO When operating at high temperatures (T 160 C), the nucleophilic anion attacks the methyl group of the organic carbonate (B Al 2 mechanism). In such conditions, the leaving group (methoxycarbonate anion, CH 3 OCOO ) is not stable; it rapidly decomposes into methoxide anion and CO 2. Also in this case, the base is used in catalytic amounts. While carboxymethylation is an equilibrium reaction, the methylation is not. Both carboxylation with phosgene and methylation with dimethylsulfate or methyl iodide generate stoichiometric quantities of the inorganic salt as byproducts, a base being used as a reagent. Instead, the corresponding processes of DMC do not involve disposal problems since no salts are produced and the only byproduct, methanol, can be easily recycled in the DMC production plant [3]. Methylation reactions with DMC can be profitably carried out both under gas liquid phase-transfer catalysis (GL-PTC) conditions [4,5] and batchwise [6]. As DMC boils at 90 C and acts as a methylating agent only when operating at high temperature, the batch reactions are performed in a stainless steel autoclave heated by an electrical oven. The base (K 2 CO 3 ) can be used in catalytic amounts (0.05 molar equiv.). DMC can be used in large excess (10 30 molar excess), acting at the same time as the solvent and the reagent. Monomethylation reactions of methylene-active compounds are not a one-step process in the industry because of the relevant quantity of dimethyl derivatives obtained with the usual methylating agents [7]. However, under batch conditions, the reactions of DMC with methylene-active compounds produce monomethylated derivatives, with a selectivity not previously observed. The mono-methylation reactions of arylacetonitriles and arylacetoesters are noteworthy for the production of 2-arylpropionic acid, a class of intermediates for the production of anti-inflamatory drugs, such as ketoprofen, naproxen, etc. The methylation process with DMC (Eq. 6) affords the corresponding mono-c-methylderivatives with unprecedented selectivity [6]. A few examples are reported in Table 1. K 2 CO 3 ArCH 2 X + CH 3 OCOOCH 3 ArCH(CH 3 )X + CH 3 OH + CO 2 (6) X = CN, COOCH 3 Table 1 Reaction of arylacetonitriles and methyl arylacetates with DMC a. Substrate T Reaction Conv. Product ArCH 2 X ( C) time (h) (%) b Yield(%) c 1 Ar = Ph X = CN Ar = o-meoc 6 H 5 X = CN Ar = m-meo C 6 H 5 X = CN Ar = p-meoc C 6 H 5 X = CN Ar = o-mec C 6 H 5 X = CN Ar = p-mec C 6 H 5 X = CN Ar = p-cl C 6 H 5 X = CN Ar = p-f C 6 H 5 X = CN Ar = m-meo 2 C C 6 H 5 X = CN Ar = Ph X = COOMe Ar = 2-(6-MeOC 10 H 6 ) X = COOMe a Substrate, DMC and K 2 CO 3 = 1:18:2 molar ratio. b Conversions determined by GC c Yields based on distilled (entries 1-10) or recrystallized (entry 11) products.
3 Selective monomethylation with dimethylcarbonate 1795 Also, the methylation of aroxyacetonitriles and methyl aroxyacetates proceeds with a selectivity up to 99% in the mono-methyl derivatives (examples are given in Table 2): 2-aroxypropionitriles and methyl 2-aroxypropionates are the corresponding products (Eq. 7) [7]. base ArOCH 2 X + CH 3 OCOOCH 3 ArOCH(CH 3 )X + CH 3 OH + CO 2 (7) X = CN, COOCH 3 Table 2 Reactions of aryloxyacetates and aryloxyacetonitriles with DMC a. Substrate T Reaction Conv. Product ArOCH 2 X ( C) time (h) (%) b Yield(%) c 1 ArO = PhO X = COOCH ArO = p-ch 3 C 6 H 4 O X = COOCH ArO = m-clc 6 H 4 O X = COOCH ArO = PhO X = CN ArO = p-ch 3 C 6 H 4 O X = CN ArO = m-clc 6 H 4 O X = CN ArO = PhO X = COOH a Substrate, DMC and K 2 CO 3 = 1:30:2 molar ratio. b Conversions determined by GC analyses. c Yields based on distilled products. Sulfones bearing α-methylene groups (benzylaryl- and alkylaryl-sulfones: ArCH 2 SO 2 Ar and RCH 2 SO 2 Ar ) can be effectively mono-c-methylated (selectivity > 99%) by DMC (Eq. 8) [8]. A few examples are reported in Table 3. K 2 CO 3 RCH 2 SO 2 R + CH 3 OCOOCH 3 RCH(CH 3 )SO 2 R + CH 3 OH + CO 2 (8) Table 3 Reactions of benzylaryl- and alkylarylsulfones with DMC. Substrate T ( C) Product RCH2SO2R' Yield (%) a 1 R = Ph R'= Ph R = p-clc 6 H 5 R' = Ph 76 3 R = p-ch 3 C 6 H 5 R' = Ph 92 4 R = Ph R' = p-clc 6 H R = p-clc 6 H 5 R' = p-clc 6 H R = Ph R' = CH R = p-clc 6 H 5 R' = CH R = p-ch 3 C 6 H 5 R' = CH a Yields based on distilled products.
4 1796 P. TUNDO The reaction mechanism has been studied in detail for the reaction of arylacetonitriles and arylacetoesters with DMC. Experimental evidences (detection and behavior of ArCH(COOCH 3 )X and ArC(CH 3 )(COOCH 3 )X as reaction intermediates) support the hypothesis that the high monomethyl selectivity is not due to the S N 2 displacement of the nucleophile ArCH( )X on DMC [9]. Instead, DMC acts first as a carboxymethylating agent (B Ac 2 mechanism) which allows the protection of the methylene-active derivatives and permits nucleophilic displacement (B Al 2) to occur with another molecule of DMC. This pattern shows the very peculiar action of the methoxycarbonyl group, that plays a two-fold role: i) the increased acidity of ArCH(COOCH 3 )X favors the formation of the corresponding anion and ii) the methoxycorbonyl group acts as a protecting group which prevents further methylation. The key step is the attack of the anion ArC( )(COOCH 3 )X to the DMC molecule. As evidence, the reaction of the potassium salt of 2-carboxymethylphenylacetonitrile [K + PhC( )(COOCH 3 )CN] with DMC affords PhC(CH 3 )(COOCH 3 )CN as the sole product. For this reaction, the activation energy was found to be 23.4 kcal mol 1, higher than as observed with usual displacements [10]. The same mechanism, in all likelihood, operates in the reactions of aroxyacetonitriles and methyl aroxyacetates and sulfones. As a general feature, the reaction occurs faster on nitriles than on esters (Tables 1 and 2), that require higher temperatures and longer reaction times for the conversion to be completed. The higher reactivity of the nitriles may be explained with the easier formation of the corresponding carbanions ArCH( )CN and ArOCH( )CN. CONCLUSIONS One of the primary objectives for pollution prevention is the adoption of processes that reduce or eliminate the use and generation of hazardous substances. This is the challenge for the future of chemical industry, its development being mostly linked to how closely environmental needs will be coupled with new ideas in fundamental research. In this context, substitution of usual methylating agents (dimethylsulfate, methyl halides) with DMC has the following noteworthy advantages: a nontoxic reagent (DMC) and catalysts (K 2 CO 3 -PEGs) are used; no byproducts (inorganic salts) to be disposed of are formed; a very high selectivity in mono-methylation (of methylene active compounds) is obtained. ACKNOWLEDGMENTS These works were supported by the MURST (Ministero Università e Ricerca Scientifica e Tecnologica) and the Interuniversity Consortium Chemistry for the Environment and are gratefully acknowledged. REFERENCES 1. P. Tundo.Continuous Flow Methods in Organic Synthesis. Horwood, E. Pub., Chichester, UK (1991). 2. M. Lissel, A. R. Rohani-Dezfull, G. Vogt. J. Chem. Res. (M), 2434 (1989). 3. D. Delledonne, F. Rivetti, U. Romano. J. Organomet. Chem. 448, C15 C19 (1995). 4. P. Tundo. J. Org. Chem. 44, (1979). 5. P. Tundo, G. Moraglio, F. Trotta. Ind. Eng. Chem. Res. 28, (1989). 6. M. Selva, C. A. Marques, P. Tundo. J. Chem. Soc. Perkin Trans. 1, 1323 (1994). 7. A. Bomben, C. A. Marques, M. Selva, P. Tundo. Tetrahedron, 51, (1995). 8. A. Bomben, M. Selva, P, Tundo. J. Chem. Res. (S) 448 (1997).
5 Selective monomethylation with dimethylcarbonate P. Tundo, M. Selva, S. Memoli. Dimethylcarbonate as a Green Reagent. In Green Chemistry: Advances in Synthesis and Chemical Processing, ACS Symposium Series No. 767, P. Anastas, L. Bartlett, T. Williams (Eds.), American Chemical Society, Washington, DC (2000). 10. A. K. Axelson, A. Nordahl, R. Carlson. J. Chemom. 9(6), (1995).
New developments in dimethyl carbonate chemistry*
 Pure Appl. Chem., Vol. 73, No. 7, pp. 1117 1124, 2001. 2001 IUPAC New developments in dimethyl carbonate chemistry* Pietro Tundo Dipartimento di Scienze Ambientali, Università Ca Foscari Dorsoduro 2137
Pure Appl. Chem., Vol. 73, No. 7, pp. 1117 1124, 2001. 2001 IUPAC New developments in dimethyl carbonate chemistry* Pietro Tundo Dipartimento di Scienze Ambientali, Università Ca Foscari Dorsoduro 2137
Green Organic Syntheses: Organic Carbonates As Methylating Agents
 THE CHEMICAL RECORD Green Organic Syntheses: Organic Carbonates As Methylating Agents PIETRO TUNDO, ALVISE PEROSA Dipartimento di Scienze Ambientali, Università Ca Foscari, Dorsoduro 2137 30123, Venezia,
THE CHEMICAL RECORD Green Organic Syntheses: Organic Carbonates As Methylating Agents PIETRO TUNDO, ALVISE PEROSA Dipartimento di Scienze Ambientali, Università Ca Foscari, Dorsoduro 2137 30123, Venezia,
Espacenet Claims: US (A)
 Espacenet - Claims http://worldwide.espacenet.com/publicationdetails/claims?cc=us&... 1 di 1 03/07/2012 17:00 Espacenet Claims: US5278333 (A) 1994-01-11 Process for the alpha-monoalkylation of arylacetonitriles,
Espacenet - Claims http://worldwide.espacenet.com/publicationdetails/claims?cc=us&... 1 di 1 03/07/2012 17:00 Espacenet Claims: US5278333 (A) 1994-01-11 Process for the alpha-monoalkylation of arylacetonitriles,
Dimethyl Carbonate as an Ambident Electrophile
 Dimethyl Carbonate as an Ambident Electrophile Pietro Tundo,* Laura Rossi, and Alessandro Loris Dipartimento di Scienze Ambientali, Università Ca Foscari Venezia, Italy, and Consorzio Interuniversitario
Dimethyl Carbonate as an Ambident Electrophile Pietro Tundo,* Laura Rossi, and Alessandro Loris Dipartimento di Scienze Ambientali, Università Ca Foscari Venezia, Italy, and Consorzio Interuniversitario
Selective mono-n-methylation of primary aromatic amines by dimethyl carbonate over faujasite X- and Y-type zeolites
 Selective mono-n-methylation of primary aromatic amines by dimethyl carbonate over faujasite X- and Y-type zeolites Maurizio Selva,* Andrea Bomben and Pietro Tundo Dipartimento di Scienze Ambientali dell
Selective mono-n-methylation of primary aromatic amines by dimethyl carbonate over faujasite X- and Y-type zeolites Maurizio Selva,* Andrea Bomben and Pietro Tundo Dipartimento di Scienze Ambientali dell
1,2-Dimethylimidazole (DMI) and microwaves in the alkylation of carboxylic acids and phenols with dimethyl and diethyl carbonates
 General Papers AKIVC 2008 (xi) 295-306 1,2-Dimethylimidazole (DMI) and microwaves in the alkylation of carboxylic acids and phenols with dimethyl and diethyl carbonates Leticia Guerrero. and Ignacio A.
General Papers AKIVC 2008 (xi) 295-306 1,2-Dimethylimidazole (DMI) and microwaves in the alkylation of carboxylic acids and phenols with dimethyl and diethyl carbonates Leticia Guerrero. and Ignacio A.
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Summary of mechanisms. Type of reaction: Nucleophilic subsitution/hydrolysis
 S Summary of mechanisms S Summary of mechanisms electrophilic addition Electrophiles: H δ in H (Ni catalyst needed), H δ in H-X; X δ in X ; H δ in H O (g) (conc H 3 PO 4 cat needed); H δ in NH 3 ; H δ
S Summary of mechanisms S Summary of mechanisms electrophilic addition Electrophiles: H δ in H (Ni catalyst needed), H δ in H-X; X δ in X ; H δ in H O (g) (conc H 3 PO 4 cat needed); H δ in NH 3 ; H δ
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Ethers. Synthesis of Ethers. Chemical Properties of Ethers
 Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Objective 11. Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product
 Objective 11 Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product Given Reactants ----> Predict Products How to figure
Objective 11 Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product Given Reactants ----> Predict Products How to figure
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Chapter 9 Alkynes. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chapter 9 Alkynes Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9.1 Sources of Alkynes Acetylene Industrial preparation of acetylene is by dehydrogenation of
Chapter 9 Alkynes Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9.1 Sources of Alkynes Acetylene Industrial preparation of acetylene is by dehydrogenation of
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION
 CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1
 Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1 You will need to be able to show picture ID to take the test. DO NOT OPEN TIS TEST UNTIL EVERYONE AS ONE I encourage following instructions: ten
Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1 You will need to be able to show picture ID to take the test. DO NOT OPEN TIS TEST UNTIL EVERYONE AS ONE I encourage following instructions: ten
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Microwave-promoted synthesis in water
 Microwave-promoted synthesis in water icholas E. Leadbeater nicholas.leadbeater@uconn.edu Outline of what we do Synthesis / Methodology / ew techniques Pure organic synthesis eg. Baylis-Hillman eaction
Microwave-promoted synthesis in water icholas E. Leadbeater nicholas.leadbeater@uconn.edu Outline of what we do Synthesis / Methodology / ew techniques Pure organic synthesis eg. Baylis-Hillman eaction
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
PRACTICE PROBLEMS UNIT 8
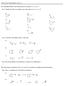 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
acetone CH 3 I + Cl _ methyl iodide (reacts rapidly) 3 C
 82 CHAPTER 9 THE CHEMSTRY OF ALKYL HALDES Some equilibria that are not too unfavorable can be driven to completion by applying Le Châtelier s principle (Sec. 4.9, p. 171). For example, alkyl chlorides
82 CHAPTER 9 THE CHEMSTRY OF ALKYL HALDES Some equilibria that are not too unfavorable can be driven to completion by applying Le Châtelier s principle (Sec. 4.9, p. 171). For example, alkyl chlorides
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Isomerism and Carbonyl Compounds
 Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 2, 2017, 8:25-9:15
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 2, 2017, 8:25-9:15
ALE 9. Equilibrium Problems: ICE Practice!
 Name Chem 163 Section: Team Number: ALE 9. Equilibrium Problems: ICE Practice! (Reference: 17.5 Silberberg 5 th edition) Equilibrium Calculations: Show all work with correct significant figures. Circle
Name Chem 163 Section: Team Number: ALE 9. Equilibrium Problems: ICE Practice! (Reference: 17.5 Silberberg 5 th edition) Equilibrium Calculations: Show all work with correct significant figures. Circle
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System
 Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison, 1101
Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison, 1101
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds
 Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Page 2. Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? alcohols C nh 2n+2O. aldehydes C nh 2n+1O
 Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
12A Lab Activity Notes
 12A Lab Activity Notes Lab Activity 12 In this experiment, RX reacts with a base. The methoxide ion (CH 3 O - ) is a small, strong base. The tert-butoxide ion ((CH 3 ) 3 CO - ) is a large, strong base.
12A Lab Activity Notes Lab Activity 12 In this experiment, RX reacts with a base. The methoxide ion (CH 3 O - ) is a small, strong base. The tert-butoxide ion ((CH 3 ) 3 CO - ) is a large, strong base.
Chapter 18: Ketones and Aldehydes. I. Introduction
 1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
Dry media reactions* M. Kidwai. Pure Appl. Chem., Vol. 73, No. 1, pp , IUPAC
 Pure Appl. Chem., Vol. 73, No. 1, pp. 147 151, 2001. 2001 IUPAC Dry media reactions* M. Kidwai Department of Chemistry, University of Delhi, Delhi-110007, India Abstract: Dry media reaction under microwaves
Pure Appl. Chem., Vol. 73, No. 1, pp. 147 151, 2001. 2001 IUPAC Dry media reactions* M. Kidwai Department of Chemistry, University of Delhi, Delhi-110007, India Abstract: Dry media reaction under microwaves
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Use your knowledge of organic reaction mechanisms to complete the mechanism for this step by drawing two curly arrows on the following equation.
 Q1.The carboxylic acid 3-methylbutanoic acid is used to make esters for perfumes. The following scheme shows some of the reactions in the manufacture of this carboxylic acid. (a) One of the steps in the
Q1.The carboxylic acid 3-methylbutanoic acid is used to make esters for perfumes. The following scheme shows some of the reactions in the manufacture of this carboxylic acid. (a) One of the steps in the
Synthesis of Amines Amine Alkylation by SN2 reaction II. Reductive Amination
 Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
H H O C C O H Carboxylic Acids and Derivatives C CH 2 C. N Goalby chemrevise.org. Strength of carboxylic acids.
 19 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending -oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
19 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending -oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
Chemical Equilibrium
 Chemical Equilibrium Many reactions are reversible, i.e. they can occur in either direction. A + B AB or AB A + B The point reached in a reversible reaction where the rate of the forward reaction (product
Chemical Equilibrium Many reactions are reversible, i.e. they can occur in either direction. A + B AB or AB A + B The point reached in a reversible reaction where the rate of the forward reaction (product
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
(a) Name the alcohol and catalyst which would be used to make X. (2)
 1 The chemical X is an ester with formula CH 3 COOC(CH 3 ) 3 which occurs in raspberries and pears. It can be prepared in the laboratory by refluxing ethanoic acid with an alcohol in the presence of a
1 The chemical X is an ester with formula CH 3 COOC(CH 3 ) 3 which occurs in raspberries and pears. It can be prepared in the laboratory by refluxing ethanoic acid with an alcohol in the presence of a
Ch 18 Ethers and Epoxides
 Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
O-methylation of natural phenolic compounds based on green chemistry using dimethyl carbonate
 IP Conference Series: Materials Science and Engineering PAPER PEN ACCESS -methylation of natural phenolic compounds based on green chemistry using dimethyl carbonate To cite this article: N I Prakoso et
IP Conference Series: Materials Science and Engineering PAPER PEN ACCESS -methylation of natural phenolic compounds based on green chemistry using dimethyl carbonate To cite this article: N I Prakoso et
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
CHEM4. General Certificate of Education Advanced Level Examination January Unit 4 Kinetics, Equilibria and Organic Chemistry
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2011 Question 1 2 Mark
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2011 Question 1 2 Mark
Review Questions for the Chem 2315 Final Exam
 Review Questions for the Chem 2315 Final Exam These questions do not have to be turned in, and will not be graded. They are intended to help you review the material we have covered in the lab so far, and
Review Questions for the Chem 2315 Final Exam These questions do not have to be turned in, and will not be graded. They are intended to help you review the material we have covered in the lab so far, and
HOMEWORK PROBLEMS: ALKYNES. 1. Provide the complete IUPAC name for the following compounds:
 CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
Kinetics Reaction and Mechanism of Thiazole with Sodium MethOxide in CSTR Reactor
 World Journal of Organic Chemistry, 2016, Vol. 4, No. 1, 8-12 Available online at http://pubs.sciepub.com/wjoc/4/1/2 Science and Education Publishing DOI:10.12691/wjoc-4-1-2 Kinetics Reaction and Mechanism
World Journal of Organic Chemistry, 2016, Vol. 4, No. 1, 8-12 Available online at http://pubs.sciepub.com/wjoc/4/1/2 Science and Education Publishing DOI:10.12691/wjoc-4-1-2 Kinetics Reaction and Mechanism
Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem reaction what is added to the C=C what kind of molecule results addition of HX HX only
 I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Topic 4.10 ORGANIC SYNTHESIS AND ANALYSIS. Organic analysis Organic synthesis
 Topic 4.10 ORGANIC SYNTHESIS AND ANALYSIS Organic analysis Organic synthesis DISTINGUISHING BETWEEN DIFFERENT ORGANIC COMPOUNDS Many of the organic compounds prepared in AS Unit 2 and in A2 Unit 4 can
Topic 4.10 ORGANIC SYNTHESIS AND ANALYSIS Organic analysis Organic synthesis DISTINGUISHING BETWEEN DIFFERENT ORGANIC COMPOUNDS Many of the organic compounds prepared in AS Unit 2 and in A2 Unit 4 can
ORGANIC - BROWN 8E CH. 22- REACTIONS OF BENZENE AND ITS DERIVATIVES
 !! www.clutchprep.com CONCEPT: ELECTROPHILIC AROMATIC SUBSTITUTION GENERAL MECHANISM Benzene reacts with very few reagents. It DOES NOT undergo typical addition reactions. Why? If we can get benzene to
!! www.clutchprep.com CONCEPT: ELECTROPHILIC AROMATIC SUBSTITUTION GENERAL MECHANISM Benzene reacts with very few reagents. It DOES NOT undergo typical addition reactions. Why? If we can get benzene to
17.3 REACTIONS INVOLVING ALLYLIC AND BENZYLIC ANIONS
 798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
Unit 2. Answers to examination-style questions. Answers Marks Examiner s tips. 1 (a) heat energy change at constant pressure
 (a) heat energy change at constant pressure This is in the spec but not so well known. Learn it. (b) N 2 (g) + ½O 2 (g) N 2 O(g) (c) (i) D = (bonds broken) (bonds made) = ½(945) + (3/2)(59) 3(278) = 23
(a) heat energy change at constant pressure This is in the spec but not so well known. Learn it. (b) N 2 (g) + ½O 2 (g) N 2 O(g) (c) (i) D = (bonds broken) (bonds made) = ½(945) + (3/2)(59) 3(278) = 23
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 5, 2015, 8:25-9:15
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 5, 2015, 8:25-9:15
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Carboxylic Acids & Their Derivatives. Organic Chemistry, 2nd Edition David R. Klein
 Carboxylic Acids & Their Derivatives Organic Chemistry, 2nd Edition David R. Klein Introduction to Carboxylic Acids Carboxylic acids are compounds with a -COOH group. These compounds are abundant in nature,
Carboxylic Acids & Their Derivatives Organic Chemistry, 2nd Edition David R. Klein Introduction to Carboxylic Acids Carboxylic acids are compounds with a -COOH group. These compounds are abundant in nature,
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Aldehydes, Ketones and Carboxylic acids
 Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Fluorous methods for synthesis and separation of organic molecules*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1649 1653, 2000. 2000 IUPAC Fluorous methods for synthesis and separation of organic molecules* Dennis P. Curran Department of Chemistry, University of Pittsburgh,
Pure Appl. Chem., Vol. 72, No. 9, pp. 1649 1653, 2000. 2000 IUPAC Fluorous methods for synthesis and separation of organic molecules* Dennis P. Curran Department of Chemistry, University of Pittsburgh,
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Chem 232. Problem Set 4. Table. Substrate Types and the Choice of S N 1 of S N 2
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 4 Does the nucleophilic substitution of substrate X proceed via an N 2 or N 1 mechanism? is a perennial question in rganic Chemistry. Use the table below
Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 4 Does the nucleophilic substitution of substrate X proceed via an N 2 or N 1 mechanism? is a perennial question in rganic Chemistry. Use the table below
The effect of phase transition of methanol on the reaction rate in the alkylation of hydroquinone
 Korean J. Chem. Eng., 26(3), 649-653 (2009) SHORT COMMUNICATION The effect of phase transition of methanol on the reaction rate in the alkylation of hydroquinone Jung Je Park*, Soo Chool Lee*, Sang Sung
Korean J. Chem. Eng., 26(3), 649-653 (2009) SHORT COMMUNICATION The effect of phase transition of methanol on the reaction rate in the alkylation of hydroquinone Jung Je Park*, Soo Chool Lee*, Sang Sung
ORGANIC CHEMISTRY II
 ORGANIC EMISTRY II. CARBOXYLIC ACIDS Saturated mono carboxylic s are called fatty s. group is COOH which is made up of carbonyl and hydroxy groups. General molecular formula is CnH n O or C n H n+1 COOH.
ORGANIC EMISTRY II. CARBOXYLIC ACIDS Saturated mono carboxylic s are called fatty s. group is COOH which is made up of carbonyl and hydroxy groups. General molecular formula is CnH n O or C n H n+1 COOH.
Section A. 1 at a given temperature. The rate was found to be first order with respect to the ester and first order with respect to hydroxide ions.
 2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
